Abstract
Rationale
Sympathetic nervous system (SNS) hyperactivity is associated with poor prognosis in patients with HF, yet routine assessment of SNS activation is not recommended for clinical practice. Myocardial G protein-coupled receptor kinase 2 (GRK2) is up-regulated in heart failure (HF) patients, causing dysfunctional β-adrenergic receptor signaling. Importantly, myocardial GRK2 levels correlate with levels found in peripheral lymphocytes of HF patients.
Objective
The independent prognostic value of blood GRK2 measurements in HF patients has never been investigated, thus, the purpose of the present study was to evaluate whether lymphocyte GRK2 levels predict clinical outcome in HF patients.
Methods and Results
We prospectively studied 257 HF patients with mean left ventricular ejection fraction (LVEF) of 31.4±8.5%. At the time of enrollment, plasma norepinephrine, serum NT-proBNP and lymphocyte GRK2 levels, as well as clinical and instrumental variables were measured. The prognostic value of GRK2 to predict cardiovascular (CV) death and all-cause mortality was assessed using the Cox proportional hazard model including demographic, clinical, instrumental and laboratory data. Over a mean follow-up period of 37.5±20.2 months (range: 3–60 months) there were 102 CV deaths. Age, LVEF, NYHA class, Chronic Obstructive Pulmonary Disease, Chronic Kidney Disease, N-terminal-pro Brain Natriuretic Peptide, and lymphocyte GRK2 protein levels were independent predictors of CV mortality in HF patients. GRK2 levels showed an additional prognostic and clinical value over demographic and clinical variables. The independent prognostic value of lymphocyte GRK2 levels was also confirmed for all-cause mortality.
Conclusion
Lymphocyte GRK2 protein levels can independently predict prognosis in patients with HF.
Keywords: G protein-coupled receptor kinase 2, brain natriuretic peptide, beta adrenergic receptor, heart failure, prognosis
INTRODUCTION
Heart failure (HF) is a leading cause of morbidity and mortality worldwide, with a 2-year mortality rate approaching 50% in patients with NYHA class III-IV symptoms [1,2]. Sympathetic nervous system (SNS) hyperactivity is a salient characteristic of HF [3–5]. Although it represents an early compensatory response aimed at enhancing cardiac contractility, sustained SNS activation exerts detrimental effects on the failing heart in the long term [3–5] and it is correlated with increased mortality in HF patients [4]. Consistently, β-adrenergic receptor (βAR) blockers have been shown to improve quality of life and to reduce re-hospitalization and mortality in HF patients [6–8]. Thus, measurement of SNS activity has been suggested to help assessment of prognosis and clinical management of HF patients [9–11]. However, although SNS hyperactivity measured through plasma circulating norepinephrine (NE) levels [12], cardiac or renal NE spillover [13], heart rate variability [14,15], and iodine-123-metaiodobenzylguanidine (123I-MIBG) myocardial scintigraphy [10,16–18] has prognostic value in HF patients, none of these approaches is routinely used in clinical practice or recommended by guidelines [11,19].
It has been repeatedly reported that HF-related SNS hyperactivity is responsible for enhanced cardiac G protein-coupled receptor (GPCR) kinase 2 (GRK2) levels [20–22], that is critically involved in the processes of cardiac βAR down-regulation/desensitization, which occurs in HF [23–25]. GRK2 is a serine-threonine kinase that by phosphorylating agonist-bound GPCRs, including βARs, leads to the recruitment of arrestins and attenuates intracellular G protein-dependent signaling [21,22]. We and others have reported that enhanced GRK2 activity plays a key role in the pathogenesis of HF [21,22]. Moreover, preclinical studies have shown that GRK2 inhibition in HF results in improved cardiac function, reverse remodeling, restoration of HF-related βAR abnormalities, and attenuation of SNS activity and neuro-hormonal responses [26–31].
Of interest for the present study, GRK2 expression measured in peripheral lymphocytes of HF patients correlates directly with levels of this kinase in failing myocardium and reflects the loss of hemodynamic function, the disease severity, and the degree of left ventricular (LV) dysfunction [32–34]. Additionally, GRK2 is comparably and significantly reduced in both lymphocytes and myocardium of HF patients following LV unloading and exercise training [35,36]. However, the prognostic value of lymphocyte GRK2 levels has never been investigated. The present study aims to assess the value of lymphocyte GRK2 to predict outcome in patients with systolic HF. Our results do indeed suggest that blood GRK2 offers advantages over existing biomarkers and provide valuable data to predict HF outcomes.
METHODS
Study design and population
The study was conducted at the Department of Translational Medical Sciences of the University Federico II (Naples, Italy), and at Salvatore Maugeri Foundation, Scientific Institute of Telese Terme (Telese Terme, BN, Italy) and was approved by the Local Ethical Committee. All patients gave written informed consent. We prospectively enrolled 303 consecutive patients with HF between January 2007 and July 2010. Of these 303 subjects, 21 subjects had to be excluded because of poor quality of lymphocyte extracts and 25 patients because of lack of serum N-terminal pro-brain natriuretic peptide (NT-proBNP) values, leaving a final study population of 257 patients. To be included in the study, patients needed to fulfill the following criteria: diagnosis of HF due to ischemic or non-ischemic etiology; LV ejection fraction (LVEF) ≤ 45%; clinical stability of symptoms for at least 1 month prior to inclusion and guidelines-based optimal pharmacotherapy. Exclusion criteria were: cardiac revascularization or acute myocardial infarction within 3 months before study entry, uncontrolled hypertension (>180 mm Hg systolic or >110 mm Hg diastolic on measurements made on at least 3 separate dates during the preceding 3 months), severe chronic kidney disease (Glomerular Filtration Rate (GFR) <30ml/min), comorbidity conditions associated with lymphocyte activation (i.e. cancer, severe chronic diseases, ongoing infection, and excessive alcohol intake) and a life expectancy of less than one year, based on non-cardiac reasons. At the time of enrollment, all subjects underwent a complete clinical examination (including NYHA functional class assessment and echocardiography) and blood draw for serum N-Terminal-pro Brain Natriuretic Peptide (NT-proBNP), lymphocyte GRK2 and plasma NE levels. Demographic data including age, sex, HF medications, cardiovascular risk factors and presence of comorbidities were also collected.
NT-proBNP measurements
Level of NT-proBNP was determined by chemoluminescence (Elecsys 2010, Roche). The analytical range of the NT pro-BNP assay extends from 1 to 25 000 pg/ml [37].
Lymphocyte GRK2 immunoblotting
Blood samples were collected from all patients in EDTA tubes. Lymphocytes were isolated by ficoll gradient using HISTOPAQUE-1077 (Sigma), frozen, and stored at −80°C until the day of the assay. Immunodetection of GRK2 was performed using detergent-solubilized lymphocyte extracts after immunoprecipitation (IP) as previously described [32]. IPs were done using a monoclonal anti-GRK2/3 antibody (C5/1, Upstate) followed by Western blotting with a GRK2 polyclonal antibody (C-20, Santa Cruz Biotechnology). All IPs were done in protein lysates of the same quantity (i.e. same starting amount in micrograms of protein, 1000 μg). As previously reported [32], post-IP lysates have been blotted for residual GRK2 amounts, and typically none has been found demonstrating the quantitative nature of these experiments. Each IP was performed in duplicate. To secure the linearity between OD and GRK2 levels a standard curve was performed. The sample containing the protein to be quantified plus a set of standards were used. As standards we used 5 different dilutions of total lysates from lymphocytes of control patients that were used for GRK2-IPs. The dilution range was in an established order of magnitude (from 2000 μg to 200 μg). The 80 kDa GRK2 protein was visualized using standard enhanced chemiluminescence (ECL Kit, Amersham). For an accurate quantification it was important that the light produced was in the linear range of the film. This was achieved by making several exposures of different lengths of time. Quantitation of immunoreactive GRK2 was done by scanning the autoradiography film and using ImageQuant software (Molecular Dynamics). The concentration of the protein was quantified and read off a graph. In order to normalize data between different blots a reference sample was run in all immunoblots and data from each individual patient were normalized to this sample. Intra- and inter-assay coefficient of variation were 5% and 15%, respectively.
Assessment of outcomes and follow-up
The primary end point of the study was cardiovascular (CV) death and all-cause mortality, was a secondary end point. Causes of cardiac death were established after a review of hospital records, death certificates, and interviews with family members and/or family doctors. The patients either visited the outpatient unit of the reference hospitals or were contacted by telephone to determine their survival status. All causes of death were adjudicated by two physicians with disagreements resolved by referring to a third physician (Supplemental Table I). Follow-up period was terminated at the end of the study period (on 30th July 2012) or in the case of death.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) and compared by the use of Student t test (normally distributed) or as median ± interquartile range value and compared by the use of Mann-Whitney U test (not normally distributed), as appropriate. Normality of data distribution was evaluated using the Kolmogorov-Smirnov test. Not normally distributed continuous variables were natural log transformed (ln NE, ln GRK2 and ln NT-proBNP). Categorical variables are expressed as proportion and compared by use of χ2 test. Pearson’s correlation coefficient is calculated to assess correlation between data. The Cox proportional hazard analysis was used to identify the factors associated with CV and all cause mortality. Using parsimonious criteria and taking into account the study sample size, 19 potentially prognostic independent variables have been selected (at least 10 patients were available for each prognostic factor tested). These factors were representative of several characteristics: basic demographic data (age and sex), presence of comorbidities (chronic kidney disease [CKD], defined as Glomerular Filtration Rate ≤ 50 ml/min, diabetes, hypertension and chronic obstructive pulmonary disease [COPD]), resting systolic blood pressure (SBP) and heart rate, presence of left bundle branch block (LBBB), presence of low serum sodium (≤130 mEq/L), presence of low serum cholesterol (≤130 mg/dL), presence of hyperuricemia (≥ 6.7 mg/dL), medications (angiotensin-converting-enzyme inhibitor [ACE-I]/angiotensin receptor blockers [ARBs] and β-blockers), functional classification by NYHA class, LVEF, circulating norepinephrine (NE), serum NTpro-BNP and lymphocyte GRK2 protein levels. Only class NYHA II and III were present in the study population, therefore, the NYHA class was coded as a binary variable. Binary code was adopted for medications use and comorbidity presence.
The model-building strategy was centered on the multivariable fractional polynomials (MFP) algorithm [38,39] and was oriented to:
select the factors significantly associated with the survival outcomes;
assess the functional form (linearity or non-linearity) of the associations;
verify the proportional hazard assumption, using the MFPT algorithm [40];
assess the calibration (goodness of fit) and the discrimination of the Cox models, the first by the Gronnesby and Borgan calibration test [41] (comparing the observed vs the estimated number of deaths in 5 risk score groups) and the second by both the global explained variation (R2) and by the Harrel’s C index.
assess the potential incremental prognostic value of each factor by measuring the partial contribution of each variable to the global R2 of the linear combination (prognostic index) of the Cox model’s factors [42] and by considering the hazard ratios relative to clinically meaningful factor’s variations;
evaluate the consistency (stability) and hence the internal validity of the results obtained, using the “bootstrap” technique [43].
have an estimation of the potential additive clinical value of the GRK2 levels over the established factors by both the Net Reclassification Improvement and by comparing the “clinical net benefit” curves obtained with Decision Curve Analysis [44] (see model building strategy on supplemental material).
The stability attained in each final model in which a given prognostic factor was included as significant was measured by the number of times that the given variable was included as significant in a large (5000) number of bootstrap replications, applying the same MFP selection procedure (bootstrap inclusion frequency [BIF]). The stability of the relationship between each variable and the survival was measured by the frequency (in the bootstrap subset) of a significant linear vs. non-linear association.
In order to have a comprehensive view of the relative weight of each factor on the survival curve, the hazard ratios (HR) of the significant continuous variables included in the final Cox models were related to clinically meaningful variations (ten years period for age and 5% unit for LVEF) or to the interquartile range (75°-25° percentile difference) in case of NTpro-BNP and lymphocyte GRK2 levels (since these factors lack of a range with a definite clinical correlate).
“Directly adjusted” survival plots obtained averaging the Cox survival curves estimated using the covariate values of each subject are utilized to compare the survivals at specific factors values to the overall Kaplan Meier [45]. Data were analyzed by Stata version 13.0 (StataCorp LP, College Station, Texas). Statistical significance was accepted at p≤0.05.
RESULTS
Patient characteristics
The final study group consisted of 257 patients (71.6% male) with mean age of 70.5±10.7, mean LVEF of 31.4±8.5% and mean NT-proBNP of 1310±852 pg/dL. Only NYHA class II and III were present in the studied population (NYHA class II frequency of 18.3%). Demographic data of the overall study population and differences in the outcome groups are reported in Table 1. To note, after patient stratification according to lymphocyte GRK2 median value (1.31 D.U.), beta-blockers use and doses (low, medium, high) were equally distributed among patients with high and low lymphocyte GRK2 levels (Supplemental Table II). Moreover, there were no differences in lymphocyte GRK2 levels between patients assuming different doses or patients not taking beta-blockers (ANOVA p=0.46) (Supplemental Figure I). There were no differences in gender and medication usage between survivors and non-survivors. Subjects who died were more likely to be older, diabetic and to have NYHA functional class III, worse kidney function, lower LVEF, and higher levels of NT-proBNP, NE and lymphocyte GRK2 protein levels. Mean lymphocyte GRK2 protein levels of HF patients was 1.42±0.71 Densitometric Units (D.U.), significantly higher (over 2.5 fold, p≤0.01) compared to that of 37 healthy subjects (0.51±0.13 D.U.). Interestingly, lymphocyte GRK2 showed a significant correlation with Age, LVEF, NE, and NT proBNP levels. NT-proBNP presented a similar correlation profile (Table 2).
Table 1.
Characteristic of patients in the overall study population and in patients stratified in survivors and non survivors.
| All (n=257) | Survivors (n=126) | Non Survivors (n=131) | p value | |
|---|---|---|---|---|
| Age, y | 70.5±10.7 | 67.3±10.5 | 73.6±9.9 | <0.0001 |
| Gender, % Male (n) | 71.6 (184) | 74.6 (94) | 68.7 (90) | 0.29 |
| LVEF, % | 31.4±8.5 | 35.5±7.4 | 27.5±7.6 | <0.0001 |
| NYHA class, % (n) | II = 18.3 (47) III = 81.7 (210) |
II = 25.4 (32) III = 74.6 (94) |
II = 11.5 (15) III = 88.5 (116) |
0.004 |
| SBP (mmHg) | 132.1±15.2 | 130.6±16.4 | 133.7±14.0 | 0.11 |
| Resting HR (bpm) | 73.5±9.6 | 73.1±10.1 | 73.9±9.2 | 0.48 |
| LBBB, % (n) | 14.0 (36) | 13.5 (17) | 14.5 (19) | 0.92 |
| Low serum sodium (<130 meq/L), % (n) | 15.9 (41) | 15.8 (20) | 16.0 (21) | 0.972 |
| Low serum cholesterol (<130 mg mg/dL), % (n) | 8.5 (22) | 8.7 (11) | 8.3 (11) | 0.7 |
| Hyperuricemia (>9.5 mg/dL), % (n) | 21.7 (56) | 21.4 (27) | 22.1 (29) | 0.89 |
| Comorbidity | ||||
| Hypertension, % (n) | 76.3% (196) | 77.0% (97) | 75.6% (99) | 0.88 |
| Diabetes, % (n) | 45.1% (116) | 38.9% (49) | 51.1% (67) | 0.03 |
| COPD, % (n) | 29.2% (75) | 27.0% (34) | 31.3% (41) | 0.49 |
| GFR <50 ml/mg, % (n) | 35.8% (92) | 25.4% (32) | 45.8% (60) | 0.001 |
| Drugs | ||||
| ACE I / ARBs, % (n) | 82.1% (211) | 81.0% (102) | 83.2% (109) | 0.75 |
| BetaBlockers, % (n) | 51.8% (133) | 54.8% (69) | 48.9% (64) | 0.38 |
| Biochemical determinations | ||||
| Serum NT-proBNP*, pg/dl | 1310±852 (1169/664-1767) | 868±567 (809/425-1191) | 1736±864 (1642/1028-2295) | <0.0001 |
| Plasma Norepinephrine*, pg/dl | 606.7±246.4 (624/391-763) | 551.7±227.3 (547/377-709) | 659.5±253.3 (684/491-824) | <0.0001 |
| Lymphocyte GRK2*, D.U. | 1.42±0.71 (1,31/0.89-1.74) | 1.17±0.62 (1,01/0.77-1.52) | 1.66±0.71 (1,6/1.23-1.89) | <0.0001 |
Normally distributed variables are expressed as mean±SD, binary data as percentage and not normally distributed variables(*) are expressed as mean±SD (median/interquartile range value). LVEF= left ventricular ejection fraction; NYHA= New York Heart Association; COPD= chronic obstructive pulmonary disease; GFR= Glomerural Filtration Rate; ACE I= angiotensin-converting-enzyme inhibitor; ARBs= angiotensin receptor blockers; NT-proBNP= N-terminal pro-brain natriuretic peptide; GRK2= G coupled-receptor Kinase. P value refers to the survivors/non-survivors comparisons; LBBB= left bundle branch block.
Table 2.
Correlation matrix of continuous variables.
| Age | LVEF | Norepinephrine | Lymphocyte GRK2 | NT-proBNP | Heart Rate | SBP | |
|---|---|---|---|---|---|---|---|
| Age | 1 | ||||||
| LVEF | −0.21* | 1 | |||||
| Norepinephrine | 0.15* | −0.05 | 1 | ||||
| lymphocyte GRK2 | 0.22* | −0.36* | 0.16* | 1 | |||
| NT-proBNP | 0.47* | −0.37* | 0.40* | 0.27* | 1 | ||
| Heart Rate | 0.12* | 0.01 | −0.03 | 0.03 | 0.07 | 1 | |
| SBP | 0.01 | −0.03 | −0.03 | 0.12 | −0.01 | 0.06 | 1 |
LVEF= left ventricular ejection fraction; NE= norepinephrine; GRK2= G protein-coupled receptor kinase 2; SBP= systolic blood pressure.
=significant coefficient (p≤0.05).
Analysis of outcomes
Over a mean follow up of 37.5 ± 20.2 months (range: 3–60 months) CV and all cause deaths were 102 and 131, respectively (final cumulative year mortality rate of 47.3±3.9%, and 61.5±3.8%, with 60.3% and 49.0% censoring for CV and all-cause deaths, respectively). Specific causes of deaths are reported in Supplemental Table I. Table 3 shows the rate of CV and all cause deaths for quartiles of age, LVEF, lymphocyte GRK2, and NT-proBNP levels. Both CV and all cause death rate show a significant constant increase along the quartiles of these 4 factors. Of note, the 4th GRK2 quartile show a slight decrease in death rate compared to the 3rd quartile. The more advanced age and the higher NT-proBNP values in the 3rd respect to the 4th quartile of GRK2 could account for this observation (mean age of the four GRK2 quartiles = 67.0±9.6, 69.4±11.3, 73.0±11.0 and 72.4±9.8 respectively; mean NT-proBNP log of the four GRK2 quartiles= 6.50±0.7, 6.86±0.8, 7.13±0.9 and 7.06±1.0 respectively). To overcome these confounding effects, we performed a multivariate Cox analysis and the resulting adjusted values are reported in bold in table 3. Cox proportional hazard assumption was verified and hold for all variables in all final models. The stability of the results was documented by the high frequency of significant inclusion (above 90% for both NT-proBNP and GRK2) over 5000 bootstrap study sample replications (Table 4A and 4B), with the linear relationships being the most frequent functional form.
Table 3.
Cardiovascular and all cause death rate stratified by quartile of age, LVEF, lymphocyte GRK2 and NT-proBNP.
| Cardiac Death rate
| |||||
|---|---|---|---|---|---|
| Quartile
|
P | ||||
| 1st | 2nd | 3rd | 4th | ||
|
|
|||||
| Age | 20.6% (26.8%) | 27.9% (39.9%) | 39.7% (45.9%) | 67.1% (54.2%) | ≤0.0001 |
| LVEF | 66.1% (50.9%) | 59.5% (47.5%) | 28.0% (42.7%) | 23.9% (37.1%) | ≤0.0001 |
| GRK2 | 15.9% (28.3%) | 26.6% (39.9%) | 59.1% (48.2%) | 56.3% (58.0%) | ≤0.0001 |
| NT-proBNP | 9.23% (20.7%) | 23.3% (38.8%) | 47.1% (47.7%) | 78.1% (58.3%) | ≤0.0001 |
| All Cause Death rate
| |||||
|---|---|---|---|---|---|
| Quartile
|
P | ||||
| 1st | 2nd | 3rd | 4th | ||
|
|
|||||
| Age | 30.2% (39.8%) | 44.3% (52.2%) | 52.4% (57.5%) | 74.3% (64.4%) | ≤0.0001 |
| LVEF | 89.3% (65.4%) | 62.2% (60.6%) | 37.6% (53.6%) | 32.4% (45.0%) | ≤0.0001 |
| GRK2 | 23.8% (39.1%) | 34.4% (52.0%) | 75.8% (60.5%) | 68.8% (69.9%) | ≤0.0001 |
| NT-proBNP | 21.5% (32.4%) | 36.8% (52.1%) | 58.8% (60.6%) | 85.9% (70.1%) | ≤0.0001 |
Boldface data refer to Cox adjusted cumulative death rate at 60 months. LVEF= left ventricular ejection fraction; GRK2= G protein-coupled receptor kinase 2; NT-proBNP= N terminal pro-Brain Natriuretic Peptide
Table 4.
Cox proportional hazard models for cardiac death (A) and all cause mortality (B).
|
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A - Cardiovascular Death | B - All Cause Mortality | |||||||||
| Global R2= 0.48 - Haller’s C=0.79±0.02
|
Global R2= 0.45 - Haller’s C=0.77±0.02
|
|||||||||
| Hazard Ratio | P | Percent fraction of global R2 (%) | Bootstrap Inclusion Frequency (%) | Linearity Stability (%) | Hazard Ratio | P | Percent fraction of global R2 (%) | Bootstrap Inclusion Frequency (%) | Linearity Stability (%) | |
|
|
|
|||||||||
| Age (10 years) | 1.51 | 0.001 | 17.5% | 78.6% | 76.30% | 1.41 | 0.002 | 15.9% | 80.0% | 82.00% |
| Gender | 0.96 | 0.86 | NA | 13.5% | NA | 1.09 | 0.67 | NA | 7.3% | NA |
| LVEF (5% units) | 0.86 | 0.03 | 13.2% | 62.2% | 87.60% | 0.83 | 0.003 | 19.3% | 76.1% | 91.30% |
| NYHA | 2.45 | 0.02 | 8.7% | 62.7% | NA | 1.56 | 0.12 | NA | 40.2% | NA |
| ln NTpro-BNP (IQ units) | 2.23 | ≤ 0.0001 | 27.5% | 95.7% | 97.7% * | 2.05 | ≤ 0.0001 | 29.6% | 96.2% | 99.2% * |
| ln GRK2 (IQ units) | 2.01 | ≤ 0.0001 | 18.9% | 89.1% | 85.8% * | 1.94 | ≤ 0.0001 | 21.7% | 98.1% | 75.9% * |
| Norepinephrine | 0.90 | 0.53 | NA | 53.5% | NA | 0.99 | 0.40 | NA | 26.4% | NA |
| Systolic Blood Pressure | 1.01 | 0.13 | NA | 29.4% | NA | 1.01 | 0.11 | NA | 46.5% | NA |
| Resting heart rate | 1 | 0.08 | NA | 4.3% | NA | 1.00 | 0.99 | NA | 3.0% | NA |
| LBBB | 0.89 | 0.67 | NA | 15.2% | NA | 0.80 | 0.68 | NA | 14.3% | NA |
| Low serum sodium | 1.69 | 0.06 | NA | 55.0% | NA | 1.41 | 0.39 | NA | 35.6% | NA |
| Low serum cholesterol | 1.41 | 0.32 | NA | 16.4% | NA | 1.12 | 0.94 | NA | 11.3% | NA |
| Hyperruricemia | 0.89 | 0.64 | NA | 19.3% | NA | 0.78 | 0.56 | NA | 20.1% | NA |
| Comorbidity | ||||||||||
| Diabetes | 1.16 | 0.46 | NA | 24.3% | NA | 1.10 | 0.59 | NA | 24.5% | NA |
| Hypertension | 0.87 | 0.60 | NA | 28.8% | NA | 0.86 | 0.48 | NA | 40.5% | NA |
| COPD | 1.81 | 0.008 | 3.0% | 73.7% | NA | 1.94 | 0.001 | 4.8% | 84.0% | NA |
| CKD | 1.86 | 0.003 | 11.4% | 70.3% | NA | 1.54 | 0.02 | 8.8% | 60.7% | NA |
| Drugs | ||||||||||
| ACE-I ARBs | 1.61 | 0.14 | NA | 55.6% | NA | 1.15 | 0.56 | NA | 17.9% | NA |
| Beta Blockers | 0.87 | 0.55 | NA | 16.8% | NA | 0.97 | 0.88 | NA | 9.0% | NA |
|
|
|
|||||||||
Linearity stability for NTpro-BNP and GRK2 refers to the stability of the logarithm form of these factors.
Abbreviations: NA= not applicable, IQ= interquartile (25°<->75° percentile), COPD= chronic obstructive pulmonary disease, LVEF= left ventricular ejection fraction, NYHA= New York Heart Association, CKD= chronic kidney disease (glomerular filtration rate ≤ 50ml/min), ACE-I= angiotensin-converting-enzyme inhibitor, ARBs= angiotensin receptor blockers, LBBB=left bundle branch block. Bold face= statistically significant factors.
CV death
Multivariate Cox proportional hazards regression analysis (Table 4A) revealed that age, LVEF, NYHA class, CKD, COPD, serum NT-proBNP and lymphocyte GRK2 protein levels were all independent and significant factors associated with CV mortality (full model). The model shows a global R2 of 0.48 and a Haller’s C=0.79±0.02, denoting that a good fraction of the outcome variability is explained and, hence, denoting an adequate discrimination. Other potential prognostic factors in HF, such as heart rate, systolic blood pressure, low serum cholesterol, low serum sodium, hyperuricemia and presence of LBBB, did not show a significant association with CV death. However, low serum sodium presence was associated with a 69% greater hazard of CV death, just above the significant threshold (p=0.06). Good agreement between observed and Cox estimated death rate in 5 risk groups was acknowledged by a non-significant Gronnesby and Borgan calibration test (p=0.70). NYHA functional class III, NT-proBNP and blood GRK2 showed the greatest impact on cardiac mortality, as documented by their hazard ratios (>2.0). Interestingly, the partial R2 contributions was 27.5%, 18.9% and 8.7% for NT-proBNP, GRK2 and NYHA class, respectively, indicating that the 2 circulating biomarkers (NT-proBNP first) had the strongest contribution to the variation of the “prognostic index” (the linear combination of all factors in the Cox model). For the NYHA class, the apparent inconsistency between the hazard ratio and the R2 contribution could be explained by its skewed distribution (81.7% prevalence of the NYHA class III), considered that the actual distribution of a factor is one of the contributing determinants of the partial R2 other than its intrinsic weight (i.e. hazard ratio).
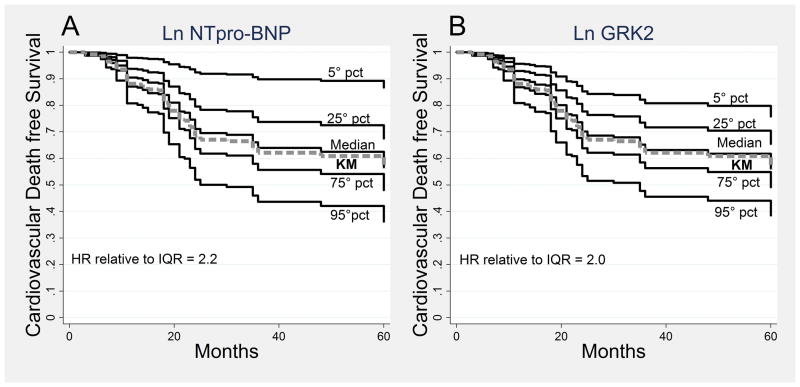
The independent prognostic value of NT-proBNP and lymphocyte GRK2 on CV survival is graphically represented in Figure 1A and 1B. These curves were generated from the Cox parameters and showed the survival of the population at the same percentile value of the 2 markers compared to the overall Kaplan Meier. The overall trend of these survival curves was comparable, depicting the similar impact of NT-proBNP and GRK2 on cardiac survival.
Figure 1.
Cardiovascular death free survival curves at different percentiles (5°, 25°, 50° (median), 75° and 95°) of ln NTpro-BNP (A) and ln GRK2 (B). The curves were obtained from the full Cox model, fixing the ln NTpro-BNP in A and the ln GRK2 in B at the specific percentile, and adjusting for the other covariates at the values observed in the study population (Directly Adjusted Curves). In panel A the adjusting covariates include also the ln GRK2, while, in panel B, ln NTpro-BNP was included. Abbreviations: pct= percentile, KM= Kaplan Meier, HR= Hazard Ratio, IQR= interquartile range, 25°<->75° percentile.
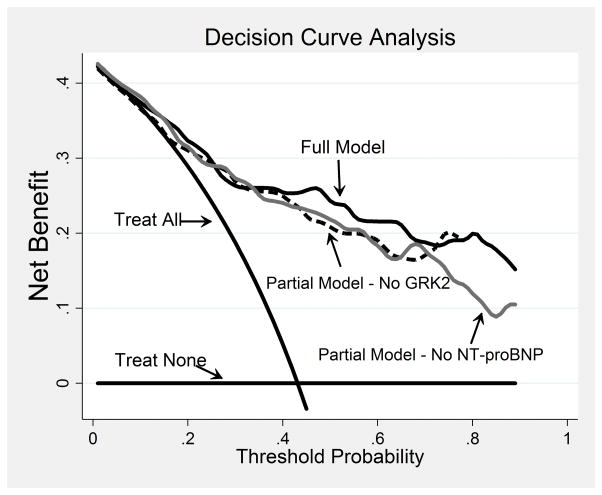
Figure 2 shows the clinical net benefit profiles of the full model and of the 2 partial models obtained excluding alternatively NT-proBNP or GRK2. The full model shows a clinical net benefit higher than the 2 partial models that, in turn, show practically overlapping net benefit profiles. Consistent results have been obtained with NRI analysis as reported in table 5.
Figure 2.
Decision curve analysis for 60 months cardiac survival. The treat none and treat all curves are compared to the net benefit curves of 3 Cox models: Full Model (continuous black line), Partial Model – No NT-proBNP (continuous gray line) and Partial Model - No GRK2 (dashed black line). The Full Model profile is higher than the 2 Partial Model profiles across the critical range of the survival threshold probabilities (40%–70%). The 2 Partial Models profiles overlap across the threshold span. All 3 models show curves well above that of the treat none and treat all.
Table 5.
Net Reclassification Improvement of clinical model relative to NT-proBNP or lymphocyte GRK2 inclusion.
| NT-proBNP | lymphocyte GRK2 | |
|---|---|---|
| P_up in Deceased | 69.60% | 67.70% |
| P_down in Survived | 68.80% | 63.90% |
| Event NRI | 39.20% | 35.30% |
| Non Event NRI | 31.60% | 27.70% |
| NRI | 70.8%±12.8% | 63.1%±12.8% |
P_up= Proportion of subjects increasing probabilities of death. P_down= Proportion of subjects decreasing probabilities of death. NRI= Net Reclassification Improvement.
All cause death
Multivariate Cox model, shown in Table 2B, indicated that age, LVEF, CKD, COPD, serum NT-proBNP and lymphocyte GRK2 protein levels were significantly associated with all cause mortality, showing a global R2 of 0.45, a Haller’s C=0.77±0.02 and a good calibration (non-significant Gronnesby and Borgan calibration test, p=0.98). Differently from cardiac mortality, NYHA class did not result an independent predictor of all cause mortality. As for CV mortality, heart rate, systolic blood pressure, low serum cholesterol, hyperuricemia and presence of LBBB, did not show a significant association with all cause death. Similarly to the results obtained for cardiac death, NT-proBNP and GRK2 were the strongest factors with an equivalent impact on survival (hazard ratios 2.1 and 1.94 and partial R2 contributions 29.6% and 21.7%, respectively).
DISCUSSION
The findings of the present study demonstrate, for the first time, that peripheral lymphocyte levels of GRK2 can independently predict mortality in patients with HF. The prognostic power of GRK2 measurement appears incremental to that provided by clinical, functional and biohumoral parameters commonly used for risk stratification of HF patients, with an impact on both cardiac and all cause mortality similar to that exerted by the NT-proBNP in our studied population.
GRK2 signaling in heart failure
Increased cardiac sympathetic activity is associated with progressive myocardial remodeling, decline in LV contractility, worsening symptoms and increase mortality in patients with chronic HF [3,4]. Thus, SNS hyperactivity has been clinically investigated with the aim to improve risk stratification, and, potentially, management of HF patients [4,11]. Importantly, increased NE concentration induces down-regulation and desensitization of myocardial βARs via GRK activity and cardiac GRK2 up-regulation has been recognized as one of the major responsible mechanisms of catecholamine-dependent βAR dysregulation and HF progression [23–25]. Notably, increased lymphocyte and cardiac GRK2 levels have been detected in HF patients with lower LVEF and more severe symptoms [32–35]. Moreover, mechanical unloading of failing human hearts is associated with decreased peripheral and cardiac GRK2 levels [32–35]. Despite this evidence no clinical studies have assessed the prognostic power of peripheral GRK2 measurements, although this information would be relevant to establish the clinical value of this novel biomarker in HF.
GRK2 as a marker of prognosis in HF
In the current study, lymphocyte GRK2 protein levels were independently associated, at multivariate analysis, with CV mortality and all cause mortality. Lower lymphocyte GRK2 levels identified patients at low-risk mortality. In particular, patients with lymphocyte GRK2 protein levels at 25th percentile showed a lower incidence rate of CV death and all cause mortality compared to patients with lymphocyte GRK2 protein levels at 75th percentile (Figure 2B). Notably, the prognostic information provided by GRK2 values proved to be additional and comparable to that obtained by the most common prognostic parameters used for risk stratification of HF patients, i.e., LVEF and NT-proBNP. Moreover, assessment of lymphocyte GRK2 might have a role in the clinical practice, as demonstrated by the improvement of the clinical net benefit derived from its introduction in the decision curve analysis (Figure 2) and by NRI analysis (Table 5). Even in this condition, NT-proBNP and GRK2 contribution resulted to be equivalent.
These findings are consistent with those reported in the ADMIRE trial [10] that assessed the prognostic value of 123I-MIBG cardiac imaging for risk stratification of patients with severe systolic HF. In that study, enrolling 961 patients with mean LVEF of 27.1%, patients with 123I-MIBG heart to mediastinum ratio below the median (<1.60), reflecting impaired cardiac innervations, showed a 6.22 fold increase in the occurrence of cardiac death, with a prognostic value additional to that of LVEF and NT-proBNP, as reported in the current study [10]. As 123I-MIBG ratio parallels the status of βAR density in patients with HF [23,46], altogether the findings from the ADMIRE trial and the current findings similarly indicate that indexes of cardiac adrenergic derangement provide independent prognostic information on top of commonly used prognostic parameters in HF patients. Therefore, GRK2 levels in white blood cells have the potential to add, over the currently available biomarkers, important information on cardiac βAR signaling and function, whose status is progressively impaired in HF patients with relevant patho-physiological consequences.
Of note, despite a significant albeit weak (R2=5.7%, p=0.001) correlation between lymphocyte GRK2 protein levels and circulating plasma NE concentrations, NE did not independently predict CV death or all cause mortality at multivariate analysis. This result might have at least two plausible explainations: 1) the low reproducibility and sensitivity of plasma NE values [47], while, GRK2 protein levels, more closely reflecting sustained hyperactivation of βAR by catecholamines, may represent a more stable surrogate of SNS hyperactivity than circulating NE concentration; 2) lymphocyte GRK2 exerts a relevant effect on the mortality that captures the NE association with outcome and actually fully overcomes its prognostic information.
Finally, in our study, variables with an established relevant effect on survival, such as LVEF, age, NT-proBNP and NYHA class, resulted to be associated with outcomes, along with lymphocyte GRK2 levels. Regarding the lack of significance for factors, such as the presence of diabetes, beta-blocker use, heart rate, systolic blood pressure, low serum cholesterol, hyperuricemia and presence of LBBB, we can only speculate that it might be ascribed to the capture of a weak effect by the different and more prognosis-impacting factors present in the model.
Study limitations
The current study reports a two center experience in a relatively small group of patients and, therefore, deserves further confirmation in a multi-center study enrolling larger number of patients, thus allowing an external validation of the present results both from a prognostic and clinical utility point of view. Our study population was at particularly high cardiovascular risk, cautioning to extrapolation of the current findings to other categories of HF patients. Thus, our results are certainly preliminary and must be confirmed in less severe HF populations. In addition, the definitive clinical relevance of our findings can only be assessed in future studies testing whether improvement of the sympathetic innervation apparatus evaluated through monitoring of GRK2 levels is associated with changes in outcomes of HF patients.
We did not verify the relationship between lymphocyte GRK2 and other measures of SNS activity, such as heart rate variability (HRV) and cardiac 123I-MIBG. Although this might represent a study limitation for a study addressing the predictive power of a new biomarker reflecting SNS activity in HF, it is important to underline that: 1) there is still debate about the value of low frequency power of HRV as a valuable measure of cardiac sympathetic tone (48); 2) cardiac 123I-MIBG was not available for a key fraction of our study population, since the present study has been planned before the demonstration of the clinical usefulness of this cardiac imaging technique.
Conclusions
Measurement of GRK2 protein levels in circulating lymphocytes provides additional and independent prognostic information on all cause and CV mortality in HF patients, over and above commonly used prognostic markers. Of noted importance, GRK2, differently from other biomarkers, is strictly related to cardiac adrenergic receptor function, whose dysregulation is a key point of HF pathophysiology and is the target of the most relevant therapy in this syndrome. Future important steps have to assess the clinical value of GRK2 as a new HF biomarker and for its potential introduction in the clinical practice, such as validation of the present results in larger HF populations, assessment of potential specific advantages offered by lymphocyte GRK2 in the clinical management of HF patients with ‘ad hoc’ planned studies, and, finally, development of a more high-throughput GRK2 quantification assay for future potential clinical use.
Supplementary Material
Novelty and Significance.
What Is Known?
Upregulation of G protein-coupled receptor kinase 2 (GRK2) in heart failure (HF) causes dysfunctional β-adrenergic receptor signaling.
GRK2 protein levels in circulating lymphocytes of HF patients correlate with cardiac levels and are reduced in response to HF effective therapies (i.e. exercise training and left ventricular assist device therapy).
What New Information Does This Article Contribute?
Levels of GRK2 protein in lymphocytes predict prognosis (all cause and cardiovascular mortality) in patients with HF.
Prognostic information provided by lymphocyte GRK2 is additional, independent and of comparable extent to commonly used prognostic markers in HF (i.e. NT-proBNP and left ventricular ejection fraction).
HF-related sympathetic nervous system hyperactivity is responsible for enhanced cardiac GRK2 levels, which in turn causes dysfunctional cardiac β-adrenergic receptor signaling. GRK2 expression in peripheral lymphocytes correlates with the levels of this kinase in failing myocardium, reflecting the loss of hemodynamic function and the extent of the disease severity. In a cohort of 257 HF patients, we demonstrate that peripheral lymphocyte levels of GRK2 is associated with all cause and cardiovascular mortality. This prognostic value of blood GRK2 levels is additional and independent to other biomarkers currently used in the clinical practice, such as NT-proBNP and left ventricular ejection fraction. However, in addition, GRK2 levels reflect the status of cardiac β-adrenergic receptor dysfunction, which plays a crucial role in HF pathophysiology and progression. These findings pave the way for future investigations testing the clinical value of GRK2 in larger and different HF populations.
Acknowledgments
SOURCES OF FUNDING
This study was supported by the Italian Ministry of University and Scientific Research, P.R.I.N. 2009 (Progetto di Ricerca di Interesse Nazionale) to Dr. Dario Leosco. Dr. Koch is supported by NIH grants R37 HL061690, R01 HL085503, P01 HL075443, P01 HL091799 and P01 HL108806.
Nonstandard Abbreviations and Acronyms
- ACE-I
Angiotensin Converting Enzyme Inhibitor
- ARBs
Angiotensin Receptor Blockers
- βAR
β-Adrenergic Receptor
- BIF
Bootstrap Inclusion Frequency
- CKD
Chronic Kidney Disease
- COPD
Chronic Obstructive Pulmonary Disease
- CV
Cardiovascular
- GPCR
G protein-coupled receptor
- GRK2
G protein-coupled receptor kinase 2
- HF
Heart Failure
- HR
Hazard Ratio
- 123I-MIBG
Iodine-123-metaiodobenzylguanidine
- KM
Kaplan Meier
- LBBB
Left bundle branch bloch
- LV
Left Ventricular
- LVEF
Left Ventricular Ejection Fraction
- MFP
Multivariable Fractional Polynomial
- MFPT
Multivariable Fractional Polynomial-time
- NE
Norepinephrine
- NT-proBNP
N-terminal-pro Brain Natriuretic Peptide
- NYHA
New York Heart Association
- SD
Standard Deviation
- SNS
Sympathetic Nervous System
Footnotes
DISCLOSURES
None.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A ESC Committee for Practice Guidelines. ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 20112: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 2.Rengo F, Leosco D, Iacovoni A, Rengo G, Golino L, Borgia F, De Lisa G, Beneduce F, Senni M. Epidemiology and risks factor for heart failure in the elderly. Ital Heart J. 2004;5:9S–16S. [PubMed] [Google Scholar]
- 3.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–53. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–62. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Lymperopoulos A, Rengo G, Koch WJ. Adrenal adrenoceptors in heart failure: Finetuning cardiac stimulation. Trends Mol Med. 2007;13:503–11. doi: 10.1016/j.molmed.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. JAMA. 2002;287:883–889. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR. β-Adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 8.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–94. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Simões MV, Barthel P, Matsunari I, Nekolla SG, Schömig A, Schwaiger M, Schmidt G, Bengel FM. Presence of sympathetically denervated but viable myocardium and its electrophysiologic correlates after early revascularised, acute myocardial infarction. Eur Heart J. 2004;25:551–7. doi: 10.1016/j.ehj.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J ADMIRE-HF Investigators. Myocardial iodine-123 metaiodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–21. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–85. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 12.Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, Barlera S, Maggioni AP, Tognoni G, Cohn JN Val-HeFT Investigators. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur Heart J. 2004;25:292–9. doi: 10.1016/j.ehj.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Tsutamoto T1, Nishiyama K, Sakai H, Tanaka T, Fujii M, Yamamoto T, Yamaji M, Horie M. Transcardiac increase in norepinephrine and prognosis in patients with chronic heart failure. Eur J Heart Fail. 2008;10:1208–14. doi: 10.1016/j.ejheart.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Fauchier L, Babuty D, Cosnay P, Fauchier JP. Prognostic value of heart rate variability for sudden death and major arrhythmic events in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1999;33:1203–1207. doi: 10.1016/s0735-1097(99)00021-2. [DOI] [PubMed] [Google Scholar]
- 15.La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 16.Imamura Y, Fukuyama T. Prognostic value of myocardial MIBG scintigraphy findings in patients with cardiomyopathy--importance of background correction for quantification of MIBG activity. Ann Nucl Med. 2002;16:387–93. doi: 10.1007/BF02990076. [DOI] [PubMed] [Google Scholar]
- 17.Paolillo S, Rengo G, Pagano G, Pellegrino T, Savarese G, Femminella GD, Tuccillo M, Boemio A, Attena E, Formisano R, Petraglia L, Scopacasa F, Galasso G, Leosco D, Trimarco B, Cuocolo A, Perrone-Filardi P. Impact of diabetes on cardiac sympathetic innervation in patients with heart failure: a 123I meta-iodobenzylguanidine (123I MIBG) scintigraphic study. Diabetes Care. 2013;36:2395–401. doi: 10.2337/dc12-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrone-Filardi P, Paolillo S, Dellegrottaglie S, Gargiulo P, Savarese G, Marciano C, Casaretti L, Cecere M, Musella F, Pirozzi E, Parente A, Cuocolo A. Assessment of cardiac sympathetic activity by MIBG imaging in patients with heart failure: a clinical appraisal. Heart. 2011;97:1828–33. doi: 10.1136/heartjnl-2011-300343. [DOI] [PubMed] [Google Scholar]
- 19.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 20.Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–9. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- 21.Rengo G, Lymperopoulos A, Leosco D, Koch WJ. GRK2 as a novel gene therapy target in heart failure. J Mol Cell Cardiol. 2011;57:356–65. doi: 10.1016/j.yjmcc.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rengo G, Lymperopoulos A, Koch WJ. Future G protein-coupled receptor targets for treatment of heart failure. Curr Treat Options Cardiovasc Med. 2009;11:328–38. doi: 10.1007/s11936-009-0033-5. [DOI] [PubMed] [Google Scholar]
- 23.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–11. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 24.Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: -adrenergic receptors in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med. 2005;2:475–483. doi: 10.1038/ncpcardio0309. [DOI] [PubMed] [Google Scholar]
- 25.Tilley DG, Rockman HA. Role of b-adrenergic receptor signaling and desensitization in heart failure: new concepts and prospects for treatment. Exp Rev Cardiovasc Ther. 2006;4:417–432. doi: 10.1586/14779072.4.3.417. [DOI] [PubMed] [Google Scholar]
- 26.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial AAV6-βARKct Gene Therapy Improves Cardiac Function and Normalizes the Neurohormonal Axis in Chronic Heart Failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rengo G, Lymperopoulos A, Zincarelli C, Femminella G, Liccardo D, Pagano G, de Lucia C, Cannavo A, Gargiulo P, Ferrara N, Perrone Filardi P, Koch W, Leosco D. Blockade of β-adrenoceptors restores the GRK2-mediated adrenal α(2) -adrenoceptor-catecholamine production axis in heart failure. Br J Pharmacol. 2012;166:2430–40. doi: 10.1111/j.1476-5381.2012.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rengo G, Zincarelli C, Femminella GD, Liccardo D, Pagano G, de Lucia C, Altobelli GG, Cimini V, Ruggiero D, Perrone-Filardi P, Gao E, Ferrara N, Lymperopoulos A, Koch WJ, Leosco D. Myocardial β(2) -adrenoceptor gene delivery promotes coordinated cardiac adaptive remodelling and angiogenesis in heart failure. Br J Pharmacol. 2012;166:2348–61. doi: 10.1111/j.1476-5381.2012.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salazar NC, Vallejos X, Siryk A, Rengo G, Cannavo A, Liccardo D, De Lucia C, Gao E, Leosco D, Koch WJ, Lymperopoulos A. GRK2 blockade with betaARKct is essential for cardiac beta2-adrenergic receptor signaling towards increased contractility. Cell Commun Signal. 2013;11:64. doi: 10.1186/1478-811X-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR, Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW, 2nd, Koch WJ. G Protein-Coupled Receptor Kinase 2 Ablation in Cardiac Myocytes Before or After Myocardial Infarction Prevents Heart Failure. Circ Res. 2008;103:413–22. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, Pleger S, Mier W, Haberkorn U, Koch WJ, Katus HA, Most P, Müller OJ. AAV6-βARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal model of heart failure. Eur Heart J. 2013;34:1437–47. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J. 2005;26:1752–1758. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- 33.Rengo G, Perrone-Filardi P, Femminella GD, Liccardo D, Zincarelli C, de Lucia C, Pagano G, Marsico F, Lymperopoulos A, Leosco D. Targeting the β-adrenergic receptor system through G-protein-coupled receptor kinase 2: a new paradigm for therapy and prognostic evaluation in heart failure: from bench to bedside. Circ Heart Fail. 2012;5:385–91. doi: 10.1161/CIRCHEARTFAILURE.112.966895. [DOI] [PubMed] [Google Scholar]
- 34.Bonita RE, Raake PW, Otis NJ, Chuprun JK, Spivack T, Dasgupta A, Whellan DJ, Mather PJ, Koch WJ. Dynamic changes in lymphocyte GRK2 levels in cardiac transplant patients: A biomarker for left ventricular function. Clin Trans Sci. 2010;3:14–18. doi: 10.1111/j.1752-8062.2010.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hata JA, Williams ML, Schroder JN, Lima B, Keys JR, Blaxall BC, Petrofski JA, Jakoi A, Milano CA, Koch WJ. Lymphocyte levels of GRK2 (betaARK1) mirror changes in the LVAD-supported failing human heart: lower GRK2 associated with improved beta adrenergic signaling after mechanical unloading. J Card Fail. 2006;12:360–8. doi: 10.1016/j.cardfail.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Rengo G, Galasso G, Femminella GD, Parisi V, Zincarelli C, Pagano G, De Lucia C, Cannavo A, Liccardo D, Marciano C, Vigorito C, Giallauria F, Ferrara N, Furgi G, Filardi PP, Koch WJ, Leosco D. Reduction of lymphocyte G protein-coupled receptor kinase-2 (GRK2) after exercise training predicts survival in patients with heart failure. Eur J Prev Cardiol. 2014;21:4–11. doi: 10.1177/2047487313491656. [DOI] [PubMed] [Google Scholar]
- 37.Karl J, Borgya A, Gallusser A, Huber E, Krueger K, Rollinger W, Schenk J. Development of a novel, N-terminal-proBNP (NT-proBNP) assay with a low detection limit. Scand J Clin Lab Invest Suppl. 1999;230:177–181. [PubMed] [Google Scholar]
- 38.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 39.Royston P, Sauerbrei W. A pragmatic approach to regression analysis based on fractional polynomials for modeling continuous variables. Chichester, UK: Wiley; 2008. Multivariate model building. [Google Scholar]
- 40.Royston P, Sauerbrei W. A new approach to modelling interactions between treatment and continuous covariates in clinical trials by using fractional polynomials. Stat Med. 2004;23:2509–25. doi: 10.1002/sim.1815. [DOI] [PubMed] [Google Scholar]
- 41.Gronnesby JK, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–28. doi: 10.1007/BF00127305. [DOI] [PubMed] [Google Scholar]
- 42.Royston P, Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med. 2004;23:723–48. doi: 10.1002/sim.1621. [DOI] [PubMed] [Google Scholar]
- 43.Royston P, Sauerbrei W. Bootstrap assessment of the stability of multivariable models. Stata J. 2009;9:547–570. [Google Scholar]
- 44.Vickers AJ. Decision analysis for the evaluation of diagnostic tests, prediction models and molecular markers. Am Stat. 2008;62:314–320. doi: 10.1198/000313008X370302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol. 1996 May 15;143:1059–68. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 46.Mardon K, Montagne O, Elbaz N, Malek Z, Syrota A, Dubois-Randé JL, Meignan M, Merlet P. Uptake-1 carrier downregulates in parallel with the beta-adrenergic receptor desensitization in rat hearts chronically exposed to high levels of circulating norepinephrine: implications for cardiac neuroimaging in human cardiomyopathies. J Nucl Med. 2003;44:1459–66. [PubMed] [Google Scholar]
- 47.Grassi G, Bolla GB, Seravalle G, Turri C, Lanfranchi A, Mancia G. Comparison between reproducibility and sensitivity of muscle sympathetic nerve traffic and plasma noradrenaline in man. Clin Sci. 1997;92:285–289. doi: 10.1042/cs0920285. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein DS, Bentho O, Park MY, Sharaby Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011;96:1255–61. doi: 10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.