Abstract
Background
Socioeconomic status (SES) is a significant determinant of health outcomes and may be an important component of the causal chain surrounding racial disparities in kidney transplantation (KTX). The social adaptability index (SAI) is a validated and quantifiable measure of SES, with a lack of studies analyzing this measure longitudinally or between races.
Methods
Longitudinal cohort study in adult KTX transplanted at a single-center between 2005 and 2012. The SAI score includes 5 domains (employment, education, marital status, substance abuse and income), each with a minimum of 0 and maximum of 3 for an aggregate of 0 to 15 (higher score → better SES).
Results
1,171 patients were included; 624 (53%) were African American (AA) and 547 were non-AA. AAs had significantly lower mean baseline SAI scores (AAs 6.5, vs. non-AAs 7.8; p<0.001). Cox regression analysis demonstrated that there was no association between baseline SAI and acute rejection in non-AAs (HR 0.92, 95% CI 0.81–1.05), while it was a significant predictor of acute rejection in AAs (HR 0.89, 95% CI 0.80–0.99). Similarly, A two stage approach to joint modelling of time to graft loss and longitudinal SAI did not predict graft loss in non-AAs (HR 1.01, 95% 0.28–3.62), while it was a significant predictor of graft loss in AAs (HR 0.23, 95% 0.06–0.93).
Conclusion
After controlling for confounders, SAI scores were associated with a lower risk of acute rejection and graft loss in AA kidney transplant recipients; while neither baseline nor follow up SAI predicted outcomes in non-AA kidney transplant recipients.
INTRODUCTION
Although race is predominantly a social construct with some biologic differences, it has substantial real life implications across a wide variety of health outcomes.1 Within kidney transplantation, African American (AA) race is a major risk factor for reduced access to transplant and subpar graft survival.2,3 This health outcomes disparity has been extensively researched over the past four decades, yet little has changed in its importance or magnitude.4–6 Socioeconomic factors have been studied, as potential explanatory variables that may account for a substantial amount of the risk associated between AA race and graft survival.7,8 Within AA disparities research, education,9 income7 and insurance10 have all been separately identified as factors that are likely to impact graft outcomes.
In the past, concurrently studying the impact of a wide array of SES factors on health outcomes has been difficult due to a limited ability to quantify these factors. The social adaptability index (SAI) has been developed as a scoring mechanism that quantifies a patient’s SES based on five domains, including education level, employment status, marital status, income level and substance abuse.11 Research has demonstrated that the SAI is a major risk determinant for health outcomes for diabetes,12 CKD,13 ESRD14 and access to transplant;15 more recently, one study demonstrated that a low baseline SAI score increased the risk of graft loss post-transplant.16 However, to date, there is a lack of studies analyzing the impact of follow up SAI score on post-transplant outcomes and assessing if these associations differ by race. Thus, the aim of this single-center longitudinal analysis was to determine the influence of baseline and follow-up SAI scores on the rates of acute rejection and graft loss in adult kidney transplant recipients, while assessing whether race impacts any of these associations.
MATERIALS AND METHODS
Study Design
This was a single-center longitudinal cohort study encompassing a population of racially diverse adult kidney transplant recipients with long-term follow up. Data was retrospectively collected, starting at the time of transplant and following the patients through graft loss, death, or end of follow up, which was July 2013. The predominant exposure of interest was the SAI score, which was calculated as described below and measured at the time of transplant (baseline) and at yearly intervals thereafter (longitudinal component). Cohorts were assigned, first based on the baseline SAI, and secondly based on the slope of the patients’ follow-up SAI. For the baseline SAI cohort assignment, a cut point SAI score of 6 was used, as this was the median baseline score for the entire cohort of patients. The change in follow-up SAI was calculated for each patient as the slope of the linear mixed model (LMM) for follow up SAI scores available for each individual patient. Cohorts for the follow up SAI score were assigned into one of three categories for comparison: increase in score (positive slope) no change in score (slope of zero) or decrease in score (negative slope). For both cohort comparisons (baseline SAI and follow up SAI), groups were stratified by race (AA or non-AA) to asses if this influenced any significant associations between SAI on outcomes.
Patients
Patients were considered for inclusion in this study if they received a kidney transplant from 2005 to 2012 and were adults at the time of transplant (≥ 18 years of age). Patients were excluded if they received a non-renal organ transplant prior, simultaneous or subsequent to the kidney transplant or were lost to follow-up and did not have baseline data to analyze. Patients with early graft loss (defined as within one year of transplant) were included in the baseline SAI analysis but excluded from the follow-up SAI analysis, because SAI slopes could not be calculated from the one baseline score.
Outcomes
The two outcome measurements of interest for this study included the rates of acute rejection and graft loss, both of which were assessed in a time dependent fashion. Time to these events was computed as the number of years starting at the time of transplant to the development of the event or end of follow up (July 2013).
Calculation of SAI
SAI was calculated in a similar fashion to a previous published study in kidney transplant recipients.16 It was calculated based on the five SES domains, including employment status, marital status, education level, substance abuse and income. Each is scored from 0 to 3 based on the criteria below, added together in equal weighting to get the total SAI, which ranges from 0 to 15. Because income status was difficult to find in the medical record, insurance status was substituted in a similar fashion to the previous study.16 Also, because education level is well documented at baseline for transplant recipients, but change in education status is not documented in the medical record, this SAI score domain was assumed to be constant in the longitudinal assessment.
Employment status: 0=unemployed or not working due to medical condition or by choice, 1=retired, 2=working part time and 3=working full time.
Marital status: 0=never married or widowed, 1=divorced or separated, 2=married without children and 3=married with children.
Education level: 0=below high school, 1=high school graduate, 2=college graduate, 3=post-graduate education.
Substance abuse: 0=abusing drugs, alcohol and tobacco, 1=abusing two substances, 2=abusing one substance, 3=abusing none.
Income (primary insurance used as a surrogate: 0=no insurance, 1=Medicaid, 2=Medicare, 3=private insurance.
Study Definitions
The following clinical definitions were utilized within this study: graft failure was defined as a documented return to chronic dialysis, retransplantation or death. Acute rejection was defined as biopsy proven and at least Banff grade of 1A per the 1997 staging criteria.17 Delayed graft function was defined as the need for dialysis within 7 days following transplant
Immunosuppression Regimens and Follow-Up Schedule
All patients received induction therapy with either thymoglobulin 1.5 mg/kg IV daily for 3 to 5 doses, daclizumab 1 mg/kg IV on day 0 and day 7 post-transplant, or basiliximab 20 mg IV on day 0 and day 4 post-transplant. Choice of induction therapy was based on protocols that utilized thymoglobulin for high immunologic risk patients (re-transplantation, CIT >24 hours, or PRA >20%), and an IL2 receptor antibody in all other patients. Maintenance immunosuppression consisted of tacrolimus as the first-line calcineurin inhibitor of choice, with protocols that specify dose adjustments made to maintain target trough whole blood concentrations between 8 and 12 ng/mL for weeks 1 through 6, 6 to 10 ng/mL for weeks 7 through month 12, and > 5 ng/mL after 1 year. In addition, our adjunct agent of choice is mycophenolate mofetil 1 gram twice daily and all patients received prednisone titrated to 5 mg daily by day 60 post-transplant. Supplemental Table 1 displays our standard clinic and follow-up schedule for usual care of stable kidney transplant recipients. This plan is the minimum expected follow-up and can become more intense based on acute care events, hospitalizations, rejections or infections.
Statistical Analysis
Unless otherwise stated, univariate summary results are reported in means ± standard deviations (SD), with categorical data presented in percentages. Adjusted estimates from multivariable models are presented as hazard ratios (HR) with 95% confidence intervals (CI).
For the univariate analyses, continuous variables were compared using t-tests (two groups) or 1-way ANOVA (three groups), while categorical variables were compared using the Pearson chi square test. Kaplan-Meier curves were used to depict the survival time of the cohort with unadjusted group comparisons conducted using the Log Rank test stratified by race. For the multivariate analyses, we used a two-stage approach to jointly model the longitudinal time-varying SAI and time to graft loss. In the first stage, a longitudinal model for SAI is assumed to follow a parametric linear mixed model (LMM), where both covariate effects and subject-specific random effects are modeled parametrically (Proc MIXED, SAS 9.4). In the second stage, a Cox model (Proc PHREG, SAS 9.4) for time to graft loss is assumed. The mixed model for the longitudinal process and the Cox model for the survival process are associated through common covariates in both models and the stochastic dependence between the random-effects terms in both models. This approach is widely accepted to model longitudinal data when the predictor (SAI) and outcome (graft loss) are associated, as it leads to less bias and improved accuracy of the inferences.18–23 Covariates that were included in the models include age, gender, baseline comorbidities (diabetes, hypertension, and cardiovascular disease), time on dialysis, re-transplant, PRA, HLA mismatches, cold time, warm time, donor type, donor age, induction therapy, delayed graft function and acute rejection (only in the graft survival analysis). In the Cox models, robust variance was used when testing the significance of the HR estimates. Baseline and follow up missing data were minimal (<10%) and the results of the joint modeling are valid under the assumption of missing at random. Statistical significance was based on a p-value of less than 0.05. Statistical analyses were performed using SPSS (version 22, IBM Corp, Armonk, NY) and SAS (version 9.4, SAS Institute Inc, Cary, NC).
RESULTS
Patients
Between 2005 and 2012, a total of 1,501 kidney transplants were conducted at the study institution and screened for potential inclusion; 185 were excluded for receiving non-kidney transplants, 79 patients were excluded for being <18 years of age and 66 were excluded for missing data/lost to follow up, leaving 1,171 patients included in the final analysis. Mean follow-up time for the entire cohort was 5.0±2.3 years; which was similar across race (non-AA 4.9±2.3 vs. AA 5.0±2.3 years, p=0.445) and baseline SAI group (SAI ≤ 6 5.0±2.3 vs. SAI >6 4.9±2.3 years, p=0.330).
SAI Comparison by Race
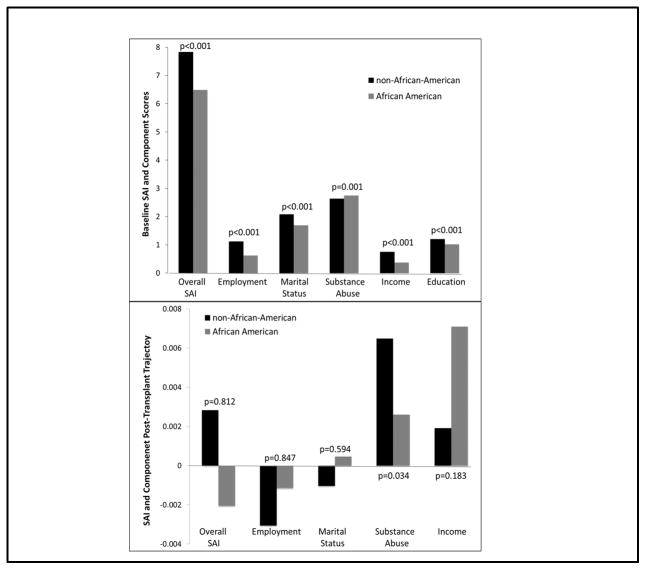
The top of Figure 1 displays the baseline SAI and component scores, comparing these across race. AA patients had significantly lower mean baseline SAI scores (non-AA 7.8 vs. AA 6.5, p<0.001), which was driven by lower mean employment (non-AA 1.1 vs. AA 0.6, p<0.001), marital status (non-AA 2.1 vs. AA 1.7, p<0.001), income (non-AA 0.8 vs. AA 0.4, p<0.001) and education scores (non-AA 1.2 vs. AA 1.0, p<0.001); mean baseline substance abuse scores were higher in AA recipients (non-AA 2.6 vs. AA 2.8, p=0.001). The post-transplant follow-up SAI data is displayed in the bottom of Figure 1. AA patients had a decrease in their post-transplant SAI slope, while non-AAs had an increase (AA −0.002 vs. non-AA 0.003, p=0.812), which was not statistically significant. Follow-up SAI component slopes were similar between AAs and non-AAs, except for substance abuse, which increased to a greater degree in non-AAs (0.006 vs. 0.003, p=0.034, respectively).
Figure 1.
Baseline and follow-up SAI and component scores across racial cohorts
The baseline recipient, donor and transplant characteristics, stratified by race and compared across baseline and follow-up SAI slopes, are displayed in Tables 1 and 2, respectively. Groups were divided by baseline SAI (≤ 6 vs. >6) and follow-up SAI (increase, no change or decrease in SAI slope) and compared accordingly. For both non-AAs and AAs, those with a low baseline SAI (≤6) were younger, more likely to be female, less likely to receive a preemptive transplant and spent longer time on dialysis. Low baseline SAI was also associated with receiving a younger donor organ and having a PRA >20%. Within non-AAs, low baseline SAI was associated with a lower incidence of hypertension, higher rates of retransplant, receiving a deceased donor and receiving cytolytic induction therapy; this was not demonstrated in AAs.
Table 1.
Baseline characteristics compared across baseline SAI exposure cohorts and stratified by race
| Variables | African American | Non-African American | ||
|---|---|---|---|---|
| Baseline SAI >6 (n=311) | Baseline SAI ≤ 6 (n=313) | Baseline SAI >6 (n=388) | Baseline SAI ≤ 6 (n=159) | |
| Recipient Characteristics | ||||
| Mean Age (±SD) | 53±12 | 48±14* | 55±13 | 46±16* |
| Female Gender | 37% | 48%* | 36% | 47%* |
| History of Diabetes | 42% | 37% | 30% | 24% |
| History of Hypertension | 94% | 95% | 92% | 85%* |
| History of Heart Disease | 14% | 17% | 24% | 17% |
| Preemptive | 14% | 7%* | 38% | 24%* |
| Mean Years on Dialysis (±SD) | 3.2±2.6 | 4.0±3.2* | 1.6±2.0 | 2.2±2.2* |
| Previous Kidney Transplant | 7% | 7% | 11% | 18%* |
| Donor Characteristics | ||||
| Deceased Donor | 90% | 93% | 70% | 80%* |
| Mean Age (±SD) | 38±15 | 33±16* | 39±15 | 34±15* |
| AA Race | 29% | 36% | 14% | 20% |
| Extended Criteria Donor | 15% | 9%* | 18% | 10% |
| Transplant Characteristics | ||||
| Cytolytic Induction | 45% | 46% | 38% | 50%* |
| Mean PRA (±SD) | 15±28 | 19±30 | 14±28 | 24±35* |
| PRA >20% | 23% | 33%* | 21% | 31%* |
| PRA >80% | 8% | 10% | 7% | 16%* |
| Mean HLA MM (±SD) | 4.6±1.3 | 1.6±1.2 | 3.9±1.6 | 3.6±1.7 |
| Mean Cold Time, hrs (±SD) | 17.7±8.9 | 17.7±8.3 | 14±10 | 16±10 |
| Mean Warm Time, min (±SD) | 37±8 | 38±8 | 36±9 | 36±7 |
| Delayed Graft Function | 17% | 19% | 10% | 8% |
p<0.05, as compared to the estimate in the same racial cohort
Table 2.
Baseline outcomes compared across SAI slope exposure cohorts and stratified by race
| African American SAI Post-Transplant Slope | Non-African American SAI Post-Transplant Slope | |||||
|---|---|---|---|---|---|---|
| Variables | Increase (n=74) | No Change (n=461) | Decrease (n=43) | Increase (n=89) | No Change (n=362) | Decrease (n=66) |
| Recipient Characteristics | ||||||
| Mean Age (±SD) | 52±13 | 51±13 | 50±12 | 50±13 | 53±15 | 50±14 |
| Female Gender | 32% | 45% | 39% | 37% | 41% | 36% |
| History of Diabetes* | 36% | 41% | 35% | 15% | 30% | 28% |
| History of Hypertension | 97% | 95% | 94% | 90% | 89% | 87% |
| History of Heart Disease | 16% | 15% | 15% | 15% | 24% | 19% |
| Preemptive | 14% | 9% | 13% | 38% | 35% | 32% |
| Mean Years on Dialysis (±SD) | 3.3±2.5 | 3.7±3.0 | 3.1±3.0 | 1.4±1.9 | 1.8±2.1 | 1.8±2.3 |
| Previous Kidney Transplant^ | 8% | 5% | 15% | 17% | 12% | 12% |
| Donor Characteristics | ||||||
| Deceased Donor*^ | 91% | 93% | 80% | 64% | 74% | 82% |
| Mean Age (±SD) | 36±16 | 35±16 | 35±13 | 36±14 | 37±15 | 36±16 |
| AA Race | 45% | 33% | 38% | 30% | 18% | 19% |
| Extended Criteria Donor | 12% | 12% | 8% | 15% | 16% | 13% |
| Transplant Characteristics | ||||||
| Cytolytic Induction* | 45% | 47% | 41% | 44% | 38% | 54% |
| Mean PRA (±SD) | 15±29 | 19±30 | 14±25 | 16±30 | 17±31 | 16±30 |
| PRA >20%^ | 21% | 32% | 20% | 20% | 25% | 25% |
| PRA >80% | 9% | 9% | 7% | 9% | 10% | 9% |
| Mean HLA MM (±SD) | 4.4±1.5 | 4.6±1.3 | 4.6±1.1 | 3.4±1.6 | 3.8±1.7 | 3.8±1.6 |
| Mean Cold Time, hrs (±SD)* | 19±9 | 18±8 | 16±11 | 13±11 | 15±10 | 17±9 |
| Mean Warm Time, min (±SD) | 37±8 | 36±8 | 36±12 | 35±10 | 36±9 | 36±7 |
| Delayed Graft Function* | 12% | 18% | 13% | 1% | 10% | 10% |
p<0.05 for comparison within African Americans
p<0.05 for comparison within non-African Americans
In terms of baseline comparisons of recipient, donor and transplant characteristics across follow-up SAI slope categories, the groups were similar for both AAs and non-AAs for the majority of variables (Table 2). Diabetes, deceased donors, cytolytic induction, mean cold ischemic time and DGF were significant different across SAI slope categories in non-AAs, while re-transplant and deceased donor were different across SAI slope categories for AAs.
Impact of SAI on Outcomes
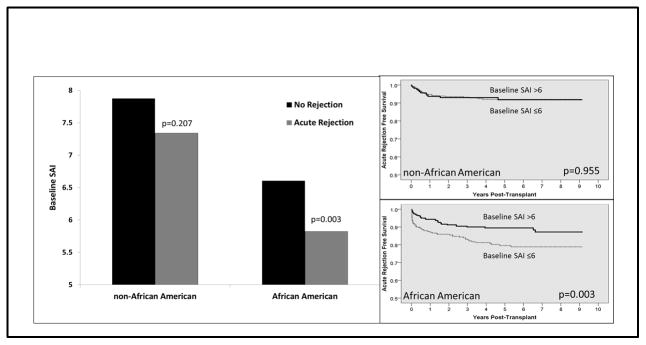
There was no appreciable association between baseline SAI score and acute rejection rates for non-AAs, both when analyzed by mean score (7.9±2.7 non-rejecters vs 7.3±2.2 rejecters, p=0.207) and when rejection free survival was compared by baseline SAI score (≤6: 92.6% vs. >6: 92.8%, p=0.955). Within AAs, mean baseline SAI scores were significantly lower in those that developed acute rejection (6.6±2.3 non-rejecters vs. 5.8±2.3 rejecters, p=0.003) and rejection free survival was significantly lower in the low baseline SAI group (≤6: 81.4% vs. >6: 89.7%, p=0.003). These data are displayed in Figure 2. There was no association in follow-up SAI scores on acute rejection for both non-AAs and AAs (data not shown).
Figure 2.
Baseline SAI score is significantly associated with acute rejection in AAs, while no such association was demonstrated in non-AAs
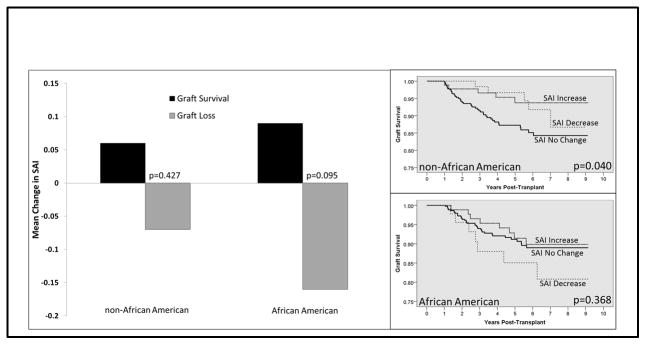
Figure 3 displays the association between follow-up SAI slopes and graft survival rates, with the data again stratified by race. AAs with graft loss had a downward SAI slope (slope change per year), while those with graft survival had an increasing SAI slope (-0.09 vs. 0.16, p=0.095); however, this failed to reach statistical significance in univariate analysis. Non-AAs had similar trends in SAI slope based on graft status, but these were not as distinct as compared to AAs (graft loss −0.07 vs graft survival 0.06, p=0.427). Kaplan-Meier graft survival estimates, compared across SAI slope categories and stratified by race (right side of Figure 3) demonstrated that non-AAs with an increase in SAI slope had significantly higher graft survival rates, as compared to those with no change or a decrease in SAI slope (94.4% vs. 88.8%, respectively, p=0.047). Within AAs, those with a decrease in SAI slope had reduced graft survival, as compared to those with no net change or with an increase (84.4% vs. 91.5%, respectively; p=0.368); however, this failed to reach statistical significance in univariate analysis.
Figure 3.
Univariate analyses assessing the association between post-transplant SAI slope and graft survival, stratified by race
Multivariable Modelling
Due to differences in a number of baseline characteristics known to influence the measured outcomes (acute rejection and graft survival) and because of the inherent association between the longitudinal SAI data and graft loss outcome, two-stage joint models, using LMM and Cox regression were employed. These models were stratified by race, with the results displayed in Table 3. After adjustment, the baseline SAI failed to be associated with acute rejection in non-AAs (adjusted HR 0.92, 95% CI 0.81–1.05), while it remained a significant predictor of acute rejection in AAs (adjusted HR 0.89, 95% CI 0.80–0.99). Cox regression modelling using the longitudinal data demonstrated that the post-transplant SAI slope failed to predict graft loss in non-AAs (adjusted HR 1.01, 95% CI 0.28–3.62), while it was a significant predictor of graft loss in AAs (adjusted hazard ratio, 0.23 95% CI 0.06–0.93), such that an AA patient with a one point increase per year in SAI score was associated with a 77% lower risk of graft loss. For both acute rejection and graft loss, an increasing SAI score was associated with reduced risk in AAs, but not in in non-AAs.
Table 3.
Multivariable modeling for acute rejection and graft loss, stratified by race*
| Exposure | Outcome | Non-African American | African American | ||
|---|---|---|---|---|---|
| Hazard Ratio (95%CI) | p-Value | Hazard Ratio (95%CI) | p-Value | ||
| Baseline SAI Score | Time to Acute Rejection | 0.92 (0.81–1.05) | 0.215 | 0.89 (0.80–0.99) | 0.027 |
| Post-Transplant SAI Slope (Change per Year) | Time to Graft Loss | 1.01 (0.28–3.62) | 0.993 | 0.23 (0.06–0.93) | 0.039 |
Multivariable models adjusted for: age, gender, baseline comorbidities (diabetes, hypertension, and cardiovascular disease), time on dialysis, re-transplant, PRA, HLA mismatches, cold time, warm time, donor type, donor age, induction therapy, delayed graft function and acute rejection (only in the graft survival analysis)
DISCUSSION
The results of this analysis demonstrate that the impact of SES on outcomes in kidney transplantation significantly differs by race. AAs with higher SES at the time of transplant are substantially less likely to develop acute rejection, such that for a one point increase in the SAI, AAs have an 11% reduction in the risk of acute rejection. Concurrently, AAs that demonstrate a one point increase in their SAI score over 4 post-transplant years have 31% lower risk of graft loss. In non-AAs, after adjusting for confounders, the influence of SAI on clinical outcomes was not significant. These results demonstrate the substantial influence SES has on clinical outcomes within AA kidney transplant recipients and highlights the importance of focusing on interventions designed to mitigate this risk. If successful, these interventions may go a long way in reducing the racial disparity that has existed in kidney transplant since its inception.2,8,11
Previous studies have analyzed the influence of different baseline SES components on outcomes across racial cohorts, but none have done so by analyzing the change in post-transplant SES (slope change per year). Golfarb-Rumyantzev et al utilized the USRDS registry to determine the influence of baseline education, citizenship, and insurance on post-transplant outcomes across race. The authors demonstrated that education and insurance were significant predictors of graft loss and patient death, with the magnitude of the association being stronger in AA recipients.24 This study did not analyze acute rejection rates, but did find contrary results to our study in that baseline SES factors were associated with graft loss; we found that only follow up SAI slope was associated with graft loss. However, this comparison should be taken in context, since our analysis was conducted within single-center, while the Golfarb-Rumyantzev study was conducted with national data.
Butkus et al demonstrated that graft loss due to immunologic etiologies was most prominent in young AA recipients, which was also more common in those with lower education levels and non-adherence to transplant medications. In multivariable analysis, non-adherence was the strongest risk factor for immunologic graft loss, which was associated with pre-transplant substance abuse.7 These results are largely similar to the findings of our study, and may support biologic plausibility. It is likely that SES risk factors exert its impact on transplant outcomes through medication errors and non-adherence as intermediaries. It is interesting to note that our study found that baseline SAI was the strongest predictor of acute rejection, while post-transplant SAI slope predicted graft loss. We theorize that baseline SAI is a risk factor for acute rejection because it likely identifies patients that are disposed to developing medication safety events (errors and non-adherence), with these events leading to acute rejection. Post-transplant medication regimens are exceptionally complex, usually include the treatment of new or worsening comorbidities (such as diabetes or hypertension) and are often adjusted several times each week based on laboratory results.25 Thus, patients with lower educational status or without strong social support networks are at substantial risk for inadvertent non-adherence.26 These events are particularly troublesome in patients at higher immunologic risk, such as those that are younger, sensitized, have more HLA mismatches, develop DGF or receive marginal donors. As AA recipients are over-represented in these risk groups, it is rational that these patients are at higher risk to develop deleterious clinical sequelae as a result of medication errors or inadvertent non-adherence.27–29 Therefore, our finding that baseline SAI is a risk factor for acute rejection only within AA recipients is plausible in this context. Within AA recipients, immunosuppressant efficacy is more dose or concentration sensitive, thus making these patients prone to adverse events related to medication non-adherence.28,30
Post-transplant SAI slope may identify patients predisposed to non-adherence due to financial issues (can’t afford the medication or loss of insurance), loss of social support, loss of motivation or lack of understanding the importance of continued adherence to immunosuppression therapy. Thus, similar to baseline SAI, the impact of post-transplant SAI on outcomes is likely partially mediated by medication errors or non-adherence. Concordantly, AA patients are likely at higher risk for developing clinical issues secondary to non-adherence, due to having more immunologic risk factors.27 It is likely that the influence of SAI on non-adherence is similar across race; yet the magnitude of non-adherence risk on adverse clinical events is more significant in AA patients.
When these results are compared to those from other countries, there are several interesting differences that are worthy of discussion. A study by Pallet et al demonstrated a lack of racial disparities, when comparing 953 Caucasians to 140 African Europeans at a single French transplant center. The incidence of acute rejection (31% vs. 30%) and 5 year graft survival (83% in both) was similar across racial cohorts. The authors suggest that SES differences may be an important factor contributing to the racial disparities demonstrated in the U.S., as SES was similar across groups in this study.31 Our results also support this conclusion. However, Rudge et al demonstrated significant racial disparities within renal transplant recipients within the UK, with Black patients having significantly lower 3-year graft survival.32 Within Canada, recent studies demonstrate similar post-transplant outcomes between Caucasians and those of African descent33, while data from Australia and New Zealand demonstrate significant racial disparities comparing indigenous and non-indigenous transplant recipients.34 Importantly, universal healthcare is available across all of these countries; thus, the questions as to whether components of SES influence racial disparities, at a global level, have yet to be fully answered.35
There are a number of limitations with this study that should be addressed. First, it is a retrospective analysis, and reviewing medical records for SES data is a difficult task, as this type of information is not traditionally well-documented in the medical record. However, our transplant program focuses efforts to assess these socioeconomic factors during the evaluation of potential transplant recipients. Thus, baseline SAI data is easily retrievable. Post-transplant, this data may be missing or not well-documented. Consequently, misclassification of follow-up SAI scores is a concern. We attempted to minimize this by reviewing all available data in the medical record during the entire follow up period, including registration information (which documents insurance, employment and marital status at every encounter) and progress notes. The study is also single-center, and given the differences seen in these results as compared to the Garg study16, these data may only be applicable to transplant programs with large numbers of AA patients or those with a similar distribution of socioeconomic factors. This is also the case for non-U.S. based transplant centers, as outcomes in patients of African descent are quite heterogeneous across different regions of the globe. Additional limitations to this retrospective study include the potential for bias and confounding. There are a large number of potential confounders that can impact acute rejection and graft loss. We attempted to account for these through the novel use of two-stage multivariable modelling, but there are likely other variables, not in the models that influence the outcome. In particular, follow up immunosuppression regimens, doses and levels are likely important.
In summary, after multivariable adjustment via joint modeling, AAs with low baseline SAI lower risk of graft loss; this was also not demonstrated in non-AAs. These data suggest that socioeconomic factors likely represent components of the causal sequence that help to explain racial disparities in kidney transplantation. Intervening to modify the impact of SES on outcomes represents a potentially promising mechanism to mitigate these disparities.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers K23DK099440 and T35 DK007431.
Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers K23DK099440 and T35 DK007431.
Abbreviations
- AA
African American
- CI
Confidence interval
- CIT
Cold ischemic time
- CKD
Chronic kidney disease
- ESRD
End stage renal disease
- HLA MM
Human leukocyte antigen mismatch
- HR
Hazard ratio
- IL2
Interleukin-2
- KTX
Kidney transplant
- LR
Linear regression
- PRA
Panel reactive antibody
- SAI
Social adaptability index
- SD
Standard deviation
- SES
Socioeconomic status
Footnotes
Disclosure
The authors have no conflicts of interest to disclose as it relates to the content of this manuscript.
Authorship Contributions:
David J Taber: Participated in the research design, gathering of data, data analysis, and writing of paper. Dr. Taber’s efforts were supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers K23DK099440. Dr. Taber has no conflicts of interest to disclose.
Mahsa Hamedi: Participated in the research design, gathering of data and review and editing of the final paper. Ms. Hamedi has no conflicts of interest to disclose.
James Rodrigue: Participated in the research design and review and editing of the final paper. Dr. Rodrigue has no conflicts of interest to disclose
Mulugeta G Gebregziabher: Participated in the research design, data analysis and writing of paper. Dr. Gebregziabher has no conflicts of interest to disclose.
Titte R Srinivas: Participated in the research design, data analysis and writing of paper. Dr. Srinivas has no conflicts of interest to disclose.
Prabhakar K Baliga: Participated in the research design and writing and editing of paper. Dr. Baliga has no conflicts of interest to disclose.
Leonard E Egede: Participated in the research design and writing and editing of paper. Dr. Egede has no conflicts of interest to disclose.
Contributor Information
David J Taber, Email: taberd@musc.edu.
Mahsa Hamedi, Email: hamedi@musc.edu.
James R Rodrigue, Email: jrrodrig@bidmc.harvard.edu.
Mulugeta G Gebregziabher, Email: gebregz@musc.edu.
Titte R Srinivas, Email: srinivat@musc.edu.
Prabhakar K Baliga, Email: baligap@musc.edu.
Leonard E Egede, Email: egedel@musc.edu.
References
- 1.Penner LA, Blair IV, Albrecht TL, Dovidio JF. Reducing racial health care disparities: a social psychological analysis. Policy Insights from the Behavioral and Brain Sciences. 2014;1(1):204–212. doi: 10.1177/2372732214548430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young CJ, Kew C. Health disparities in transplantation: Focus on the complexity and challenge of renal transplantation in African Americans. Med Clin North Am. 2005;89(5):1003–1031. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Epstein AM, Ayanian JZ, Keogh JH, et al. Racial disparities in access to renal transplantation--clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343(21):1537–1544. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opelz G, Mickey MR, Terasaki PI. Influence of race on kidney transplant survival. Transplant Proc. 1977;9(1):137–142. [PubMed] [Google Scholar]
- 5.Scantlebury V, Gjertson D, Eliasziw M, et al. Effect of HLA mismatch in African-Americans. Transplantation. 1998;65(4):586. doi: 10.1097/00007890-199802270-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matas A, Smith J, Skeans M, et al. OPTN/SRTR 2012 annual data report: Kidney. American Journal of Transplantation. 2014;14(S1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 7.Butkus DE, Dottes AL, Meydrech EF, Barber WH. Effect of poverty and other socioeconomic variables on renal allograft survival. Transplantation. 2001;72(2):261–266. doi: 10.1097/00007890-200107270-00017. [DOI] [PubMed] [Google Scholar]
- 8.Garg PP, Diener-West M, Powe NR. Reducing racial disparities in transplant activation: Whom should we target? American Journal of Kidney Diseases. 2001;37(5):921–931. doi: 10.1016/s0272-6386(05)80007-1. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb-Rumyantzev AS, Sandhu GS, Barenbaum A, et al. Education is associated with reduction in racial disparities in kidney transplant outcome. Clin Transplant. 2012;26(6):891–899. doi: 10.1111/j.1399-0012.2012.01662.x. [DOI] [PubMed] [Google Scholar]
- 10.Butkus DE, Meydrech EF, Raju SS. Racial differences in the survival of cadaveric renal allografts. overriding effects of HLA matching and socioeconomic factors. N Engl J Med. 1992;327(12):840–845. doi: 10.1056/NEJM199209173271203. [DOI] [PubMed] [Google Scholar]
- 11.Hod T, Goldfarb-Rumyantzev AS. The role of disparities and socioeconomic factors in access to kidney transplantation and its outcome. Ren Fail. 2014;36(8):1193–1199. doi: 10.3109/0886022X.2014.934179. [DOI] [PubMed] [Google Scholar]
- 12.Goldfarb-Rumyantzev AS, Rout P, Sandhu GS, et al. Social adaptability index predicts overall mortality in patients with diabetes. J Diabetes Complications. 2012;26(1):44–49. doi: 10.1016/j.jdiacomp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Goldfarb-Rumyantzev AS, Rout P, Sandhu GS, Khattak M, Tang H, Barenbaum A. Association between social adaptability index and survival of patients with chronic kidney disease. Nephrol Dial Transplant. 2010;25(11):3672–3681. doi: 10.1093/ndt/gfq177. [DOI] [PubMed] [Google Scholar]
- 14.Sandhu GS, Khattak M, Rout P, et al. Social adaptability index: Application and outcomes in a dialysis population. Nephrol Dial Transplant. 2011;26(8):2667–2674. doi: 10.1093/ndt/gfq789. [DOI] [PubMed] [Google Scholar]
- 15.Goldfar-bRumyantzev AS, Sandhu GS, Baird BC, Khattak M, Barenbaum A, Hanto DW. Social adaptability index predicts access to kidney transplantation. Clin Transplant. 2011;25(6):834–842. doi: 10.1111/j.1399-0012.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 16.Garg J, Karim M, Tang H, et al. Social adaptability index predicts kidney transplant outcome: A single-center retrospective analysis. Nephrol Dial Transplant. 2012;27(3):1239–1245. doi: 10.1093/ndt/gfr445. [DOI] [PubMed] [Google Scholar]
- 17.Racusen LC, Solez K, Colvin RB, et al. The banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 18.Gebregziabher M, Egede LE, Lynch CP, Echols C, Zhao Y, et al. Effect of trajectories of glycemic control on mortality in type 2 diabetes: a semiparametric joint modeling approach. Am J Epidemiol. 2010;171(10):1090–8. doi: 10.1093/aje/kwq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye W, Lin X, Taylor JM. Semiparametric modeling of longitudinal measurements and time-to-event data—a two-stage regression calibration approach. Biometrics. 2008;64(4):1238–46. doi: 10.1111/j.1541-0420.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Hu B, Greene T. A semiparametric joint model for longitudinal and survival data with application to hemodialysis study. Biometrics. 2009;65(3):737–45. doi: 10.1111/j.1541-0420.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 21.Guo X, Carlin BP. Separate and joint modeling longitudinal and event time data using standard computer packages. Am Stat. 2004;58(1):16–24. [Google Scholar]
- 22.Wang Y, Taylor JMG. Jointly modeling longitudinal and event time data with application to acquired immunodeficiency syndrome. J Am Stat Assoc. 2001;96(455):895–905. [Google Scholar]
- 23.Taylor JM, Yu M, Sandler HM. Individualized predictions of disease progression following radiation therapy for prostate cancer. J Clin Oncol. 2005;23(4):816–825.18. doi: 10.1200/JCO.2005.12.156. [DOI] [PubMed] [Google Scholar]
- 24.Goldfarb-Rumyantzev AS, Koford JK, Baird BC, et al. Role of socioeconomic status in kidney transplant outcome. Clin J Am Soc Nephrol. 2006;1(2):313–322. doi: 10.2215/CJN.00630805. [DOI] [PubMed] [Google Scholar]
- 25.Musgrave C, Pilch N, Taber D, et al. Improving transplant patient safety through pharmacist discharge medication reconciliation. American Journal of Transplantation. 2013;13(3):796–801. doi: 10.1111/ajt.12070. [DOI] [PubMed] [Google Scholar]
- 26.Arthur T. The role of social networks: A novel hypothesis to explain the phenomenon of racial disparity in kidney transplantation. American Journal of Kidney Diseases. 2002;40(4):678–681. doi: 10.1053/ajkd.2002.35672. [DOI] [PubMed] [Google Scholar]
- 27.Taber DJ, Douglass K, Srinivas T, et al. Significant racial differences in the key factors associated with early graft loss in kidney transplant recipients. Am J Nephrol. 2014;40(1):19–28. doi: 10.1159/000363393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malat GE, Culkin C, Palya A, Ranganna K, Kumar MS. African american kidney transplantation survival: The ability of immunosuppression to balance the inherent pre- and post-transplant risk factors. Drugs. 2009;69(15):2045–2062. doi: 10.2165/11318570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Taber DJ, Pilch NA, Bratton CF, McGillicuddy JW, Chavin KD, Baliga PK. Medication errors and adverse drug events in kidney transplant recipients: Incidence, risk factors, and clinical outcomes. Pharmacotherapy. 2012;32(12):1053–1060. doi: 10.1002/phar.1145. [DOI] [PubMed] [Google Scholar]
- 30.Neylan JF. Immunosuppressive therapy in high-risk transplant patients: dose-dependent efficacy of mycophenolate mofetil in African-American renal allograft Recipients1. Transplantation. 1997;64(9):1277–1282. doi: 10.1097/00007890-199711150-00008. [DOI] [PubMed] [Google Scholar]
- 31.Pallet N, Thervet E, Alberti C, Emal-Algae V, Bedrossian J, et al. Kidney transplant in Black recipients: are African Europeans different from African Americans? American Journal of Transplantation. 2005;5:2682–87. doi: 10.1111/j.1600-6143.2005.01057.x. [DOI] [PubMed] [Google Scholar]
- 32.Rudge C, Johnson RJ, Fuggle SV, Forsythe JLR. Renal transplantation in the United Kingdom for patients from ethnic minorities. Transplantation. 2007;83:1169–73. doi: 10.1097/01.tp.0000259934.06233.ba. [DOI] [PubMed] [Google Scholar]
- 33.Yeates KE, Wiebe N, Gill J, et al. Similar outcomes among black and white renal allograft recipients. American Journal of Kidney Disease. 2009;20:172–9. doi: 10.1681/ASN.2007070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald SP, Russ GR. Current incidence, treatment patterns and outcome of end stage renal disease among indigenous groups in Australia and New Zealand. Nephrology. 2003;8:42–8. doi: 10.1046/j.1440-1797.2003.00131.x. [DOI] [PubMed] [Google Scholar]
- 35.Malek SK, Keys BJ, Kumar S, Milford E, Tullius SG. Racial and ethnic disparities in kidney transplantation. Transplant International. 2011;24:419–24. doi: 10.1111/j.1432-2277.2010.01205.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.