Abstract
There is great interest in using stem cells (SC) to regenerate a deficient urethral sphincter in patients with urinary incontinence. The smooth muscle component of the sphincter is a significant contributor to sphincter function. However, current translational efforts for sphincter muscle restoration focus only on skeletal muscle regeneration because they rely on adult mesenchymal SC as cell source. These adult SC do not yield sufficient smooth muscle cells (SMCs) for transplantation. We may be able to overcome this limitation by using pluripotent stem cell (PSC) to derive SMCs. Hence, we sought to investigate whether smooth muscle precursor cells (pSMCs) derived from human PSCs can restore urethral function in an animal model generated by surgical urethrolysis and ovariectomy. Rats were divided into four groups: control (no intervention), sham saline (surgery + saline injection), bladder SMC (surgery + human bladder SMC injection), and treatment (surgery + pSMC injection, which includes human embryonic stem cell (hESC) H9-derived pSMC, episomal reprogrammed induced pluripotent stem cells (iPSCs)-derived pSMC, or viral reprogrammed iPSC-derived pSMC). pSMCs (2 × 106 cells/rat) were injected periurethrally 3 weeks postsurgery. Leak point pressure (LPP) and baseline external urethral sphincter electromyography were measured 5 weeks postinjection. Both iPSC-derived pSMC treatment groups showed significantly higher LPP compared to the sham saline group, consistent with restoration of urethral sphincter function. While the difference between the H9-derived pSMC treatment and sham saline group was not significant, it did show a trend toward restoration of the LPP to the level of intact controls. Our data indicate that pSMCs derived from human PSCs (hESC and iPSC) can restore sphincter function.
Introduction
Stress urinary incontinence (SUI) is a major health problem with significant social and economic impact [1]. SUI is associated with a deficient urethral sphincter that cannot generate sufficient closure pressure to resist the increase in abdominal pressure during physical activity. Current treatment options include pelvic floor exercise [2], bulking agent injections into the urethral tissues, or surgery with placement of permanent mesh [3–5]. The injection of bulking agents is less invasive than surgery, but achieves variable short-term results. The need for repeat injections and persistent leakage limits its overall success. The surgical midurethral sling is efficacious for the majority of patients, but not everyone is responsive and recurrent leakage can occur years after the initial surgery, leaving a population of patients with few other options.
The urethral sphincter relies on mechanical integrity of striated and smooth muscle cells (SMCs) for effective urethral closure and continence [6]. The internal urethral sphincter, consisting of primarily SMCs, is a significant contributor to overall continence. Currently there is no therapy to restore function to this portion of the urethra. Stem cell (SC)-based therapy is attractive because it is thought that SCs can replace the specific cell types necessary to restore function. Various sources of SCs have been studied. Mesenchymal stem cells utilized in several studies were obtained from a variety of adult and fetal tissues [5–8]. So far, these adult mesenchymal stem cells have yielded promising results in preclinical and animal studies [9–15]. The SC-like cells for transplantation obtained from these adult tissues contain a heterogeneous mixture of mesenchymal cells, such as myoblasts at various stages of differentiation originating from satellite cells, mesenchymal stem cells, striated muscle cells, and fibroblasts. Some studies show that only a small fraction of the injected mesenchymal stem cell mixture ultimately differentiate into SMCs in situ [12,16]. Therefore, there are insufficient cells destined specifically to restore the smooth muscle component of the sphincter. Furthermore, all these cells possess senescence and epigenetic changes from the adult donor, which limits the total number of cells that can be harvested and expanded from the tissue biopsies.
Human embryonic stem cells (hESCs) are pluripotent stem cells (PSCs) capable of self-renewal and differentiation into all three embryonic (ectoderm, mesoderm, and endoderm) lineages. They are also devoid of senescence and have minimal epigenetic changes, suggesting that they may be an improved SC source for regenerative therapies. Evolving technologies have enabled the genetic reprogramming of somatic cells into induced pluripotent stem cells (iPSC), which possess many of the PSC properties. This has opened up a new cell source for autologous SC therapies. Therefore, in this study, we sought to investigate whether PSCs such as hESCs and patient-specific iPSCs can be used as a SC source for derivation of a pure population of smooth muscle precursor cells (pSMCs) and whether transplantation of these precursor cells can restore urethral function in a SUI rat model.
Materials and Methods
The Institutional Review Board of the Stanford University School of Medicine and the Stanford University Stem Cell Research Oversight Committee approved this study. The animal protocols were approved by the Stanford Animal Research Committee. A total of 108 adult female Virgin, Rowett Nude (RNU) rats, ages 11–12 weeks were used for this study.
Bilateral ovariectomy (bilateral salpingo-oophorectomy)
A 1.5–2 cm low-vertical abdominal incision is made in the rat. The ovaries are exteriorized through the abdominal incision by grasping the periovarian fat. A 4-0 absorbable suture is used to ligate the junction between the fallopian tube and the ovary together with all the accompanying blood vessels and fat. The ovaries are transected and the fallopian tubes are returned into the abdominal cavity.
Urethrolysis
Urethrolysis is performed as described by Rodriguez et al. [17], except with a minor modification. The fatty tissue in front of the bladder neck, rather than the bladder, is pulled during the urethral dissection. We introduced this modification to minimize trauma to the bladder. The proximal and distal urethra is detached circumferentially by incising the endopelvic fascia and separating the urethra from the anterior vaginal wall and pubic bone by sharp dissection.
Cell preparation
Human bladder smooth cells (passage 3; LONZA) are used as terminally differentiated cell treatment for comparison with pSMC treatment. They are cultured in 90% Dulbecco's modified Eagle's medium and 10% fetal bovine serum (Fisher Scientific) at 37°C in an atmosphere of 95% air and 5% CO2. Cells are transplanted into the animals at passage 7.
Human pSMCs are differentiated from hESCs [luciferase (Fluc)-tagged H9 line, a gift from Dr. Joseph Wu, Stanford University], retrovirus reprogrammed PSCs (iPSCs), and episomal vector reprogrammed PSCs (Epi-iPSCs). The iPSC (a gift from Dr. Michele Pamela Calos, Stanford University) and Epi-iPSC lines were reprogrammed from fibroblasts from two separate donors. The reprogramming methodology is as described by Takahashi et al. and Nguyen et al. [18,19] and by Yu et al. [20], respectively. G-banded karyotyping of iPSC was performed as previously described by WiCell [21]. To track cells by imaging techniques, a fraction of the pSMCs is differentiated from SCs transfected with modified attB donor plasmid encoding luciferase gene. Luciferase (Luc)-tagged progenitor smooth cells were used for in vivo tracking.
Human pSMCs are differentiated from iPSCs and ESCs (H9) using a modified, feeder cell-free, vascular progenitor protocol as previously described [22,23]. Briefly, the SCs are cultured in chemically defined media [RPMI 1640 with 1 mM Glutamax, 1% Nonessential Amino Acids, 0.1 mM β-mercaptoethanol, 1% penicillin and streptomycin (Life Science Technology, Inc.)], 1% ITS (Sigma-Aldrich) supplemented with Activin A (50 ng/mL), BMP4 (50 ng/mL, Sigma), then basic fibroblast growth factor (50 ng/mL), and vascular endothelial growth factor (40 ng/mL; Invitrogen). The cells are then sorted for CD31+/CD34+ vascular progenitors by FACS. Sorted cells are expanded in SMC growth medium (Invitrogen) to yield pSMCs. The subset of these precursor cells are allowed to terminally differentiate to mature SMCs for further characterization and functional tests. pSMC are characterized by staining for α-SMA, CNN1, SM22α, smoothelin, and desmin. Ki67 staining is used to show proliferation [24]. Terminal SMCs are characterized by staining for myosin heavy chain and elastin and also by contraction assay. Contractions are induced by treating the cells with 100 mM Carbachol (Sigma-Aldrich). Images are recorded every minute for 10 min on a Nikon BioStation IM (Nikon; www.nikon.com) [22].
Implantation of human pSMCs into rodents
The animals are anesthetized with 1%–3% isoflurane. To examine human pSMC survival in vivo, 1 million luciferase-tagged pSMCs in 50 μL of Matrigel (50%) are injected directly into the adductor longus muscles of five SCID mice and imaged for 3 months. To examine the effect of pSMCs on the restoration of urethral function in SUI rats, cells suspended in 100 μL of smooth muscle growth supplement (Life Technologies) are injected periurethrally three weeks after urethrolysis. We use a commercial, terminally differentiated human SMC line (passage 7; LONZA) for comparison with the pSMC treatment. A total of 2 × 106 cells suspended in the respective medium are injected into each urethra. The sham treatment SUI rat (urethrolysis plus ovariectomy) is injected with saline only.
Two experiments were performed, one testing pSMCs derived from hESCs H9 line (H9-pSMC) and another testing pSMCs derived from human iPSC (iPSC-pSMC) and Epi-iPSC lines (Epi-iPSC-pSMC). Rodent experimental groups are shown in Table 1.
Table 1.
Rodent Groups in Each Experiment
| Experiment | Control (N) | Sham saline (N) | H9-pSMC (N) | Bladder-SMC (N) |
|---|---|---|---|---|
| H9-pSMC experiment | 13 | 12 | 22 | 11 |
| Control (N) | Sham saline (N) | Epi-iPSC-pSMC (N) | iPSC-pSMC (N) | |
| iPSC-pSMC experiment | 15 | 16 | 10 | 9 |
Epi-iPSC, episomal vector reprogrammed PSC; pSMC, smooth muscle precursor cell; iPSC, induced pluripotent stem cell.
In vivo bioluminescence imaging of transplanted pSMCs
Luc-tagged pSMCs (2 × 106) are injected into the urethral sphincter region of the RNU rats or the hind leg of SCID mice for in vivo cell tracking. Transplanted cell survival is longitudinally monitored through bioluminescence imaging (BLI) using the Xenogen In Vivo Imaging System (Caliper Life Sciences). In brief, d-luciferin (BIOSYNTH INTERNATIONAL, Inc.) is administered intraperitoneally at a dose of 375 mg/kg of body weight 15 min before image acquisition. Animals are placed in a light-tight chamber, and photons emitted from luciferase-expressing cells are collected with integration times of 2 min. BLI signal is quantified in maximum photons/s/cm per steradian and presented as Log10 (photons/s). To examine the viability of transplanted cells in the sphincter urethra of rats or the hind leg of mice, the images are obtained every 2 days until disappearance of signals, then once per week for 5 weeks or 3 months, respectively.
Double immunofluorescence staining
The cryostat sections of mouse hind leg muscle are prepared and fixed as described previously [25]. The slides are treated with 0.25% Triton X-100 in phosphate-buffered saline (PBS) for 10 min at room temperature. After washing with phosphate-buffered saline with 0.01% Tween 20 (PBS-T)-(0.01% Tween in PBS) and blocking with 1% bovine serum albumin in PBS-T, the slides are double stained with 1/50 rabbit anti- smoothelin (Santa Cruz Biotechnology) and 1/50 mouse anti-human nuclei (HuNu; EMD Millipore Corporation) primary antibody at 4°C overnight. Then after washing, the slides are incubated with1/100 mouse anti-rabbit-immunoglobulin G (IgG)-fluorescein isothiocyanate and 1/100 goat anti-mouse-IgG-tetramethylrhodamineisothiocyanate (Sigma) at room temperature for 1 h in a dark chamber. The slides of non-cell injected hind leg muscle from the same mouse are used as negative controls. The 4′,6-diamidine-2-phenylidole is used to stain the nuclei. After rinsing and mounting, the slides are examined with a fluorescence microscope.
Suprapubic bladder catheter implantation
Five weeks after injection, rats are anesthetized for leak point pressure (LPP) measurements. A suprapubic incision is made and the bladder exteriorized from the abdominal cavity. A small incision is made in the dome of the bladder and a polyethylene catheter (PE-50 tube with a flared tip) (Becton Dickinson) is inserted into the bladder. The catheter is connected through a three-way stopcock to a syringe for filling with physiological saline and to a pressure transducer (TSD 104A; BIOPAC Systems, Inc.) for monitoring bladder pressure.
Baseline measurement of urethral sphincter electromyography
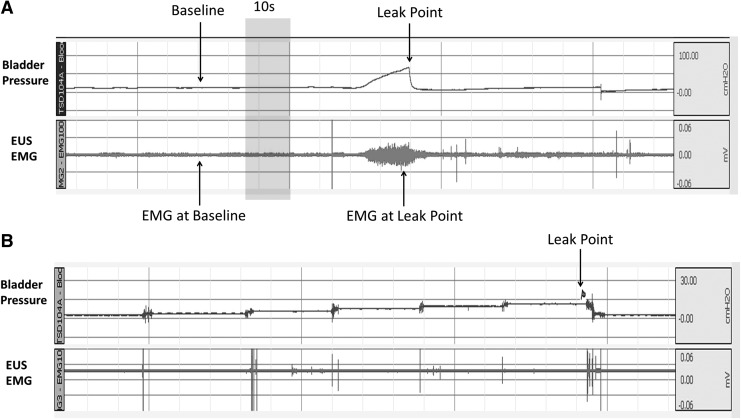
The 0.003′′ platinum–iridium wire electrodes (A-M Systems) are inserted into the mid urethra bilaterally near the urethral meatus using a 25 G 5/8′′ syringe needle (BD Bioscience; Fig. 1). Electromyography (EMG) activity is recorded with a MP150 system (BIOPAC Systems). All EMG signals are recorded at 1,000 Hz and analyzed with the AcqKnowledge 3.9.1 software (BIOPAC Systems). We calculate the average rectified EMG [average rectified value (ARV)], which is defined as a time-windowed mean of the absolute value of the signal. ARV is one of the processing methods used to construct derived signals from raw EMG data and it is a measurement that can be used to analyze EMG data [26,27]. Three randomly selected ten-second segments at baseline bladder pressure are averaged to arrive at the mean of ARV.
FIG. 1.
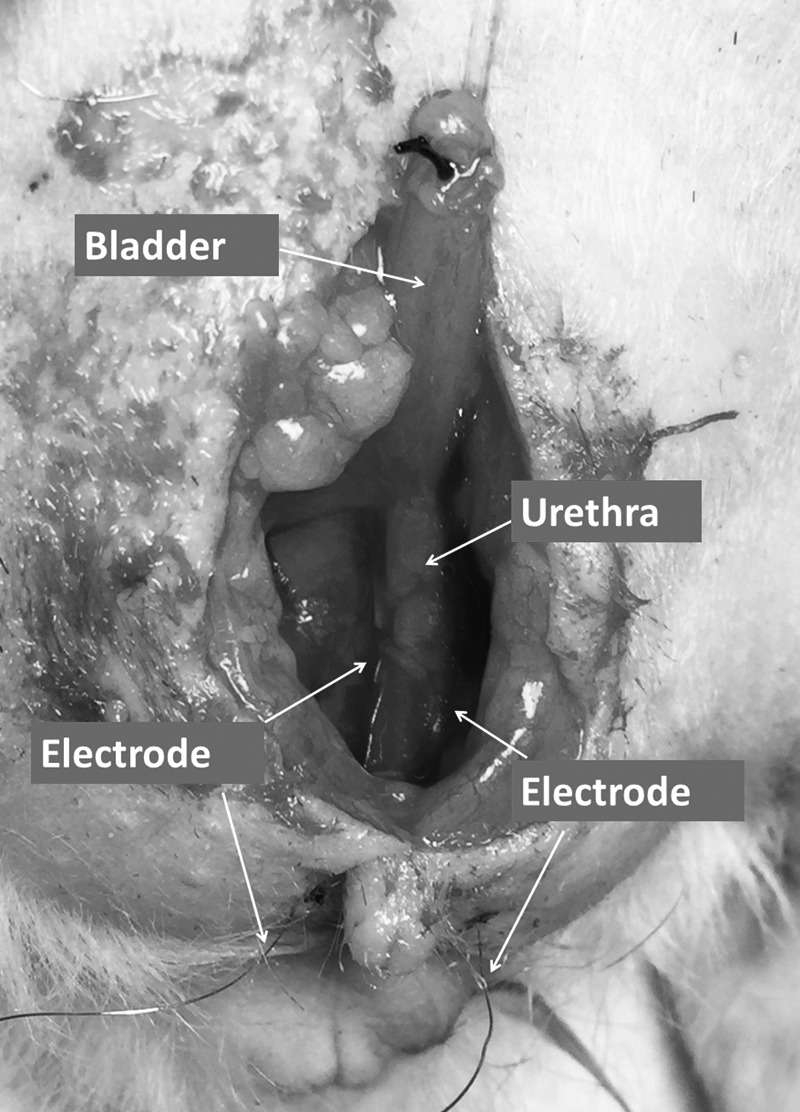
Position of the electrodes for urethral sphincter EMG measurements. Electrodes are placed at the midurethra bilaterally, but inserted externally. EMG, electromyography.
Vertical tilt table LPP measurement
Vertical tilt table LPP test is performed according to a previously described method [28]. Investigators performing LPP are blinded to the group assignment of each animal. Spinal cord transection is performed at the T8–T9 level to eliminate spontaneous bladder activity in response to increasing intravesical pressure. The rats are mounted on a tilt table and placed in a vertical position. The bladder catheter is connected to a 50-mL syringe (reservoir), which is fixed onto a metered vertical pole. Intravesical pressure is elevated by raising the height of the reservoir gradually, by 2–3 cm increments every 2 min starting from 0 cm, until urinary leakage is observed. The pressure at which leakage occurs is recorded as the LPP. The average of three consecutive LPPs from each rat is used for data analysis.
Data statistical analysis
Quantitative values are presented as the mean ± standard error. JMP pro 10.0.2 software (SAS Institute, Inc.) is used for statistical analysis. Nonparametric comparisons for each pair are performed using the Wilcoxon Method, with P < 0.05 indicating a significant difference between groups.
Results
Characterization of terminally differentiated SMC
Our differentiation protocol successfully differentiated pSMCs from human PSC lines (both hESCs and iPSCs). Immunofluorescence staining revealed robust expression of SM22α, α-SMA, CNN1, smoothelin, and desmin in pSMCs. Terminal SMCs were characterized and exhibited expression of myosin heavy chain and elastin. Over 50% of cells contracted with carbachol stimulation consistent with human aorta SMCs (data not shown). Karyotype analysis revealed normal chromosomes in both iPSC lines, confirming that the genetic reprograming did not cause significant genetic aberrations.
In vivo tracking of fluc-tagged pSMC
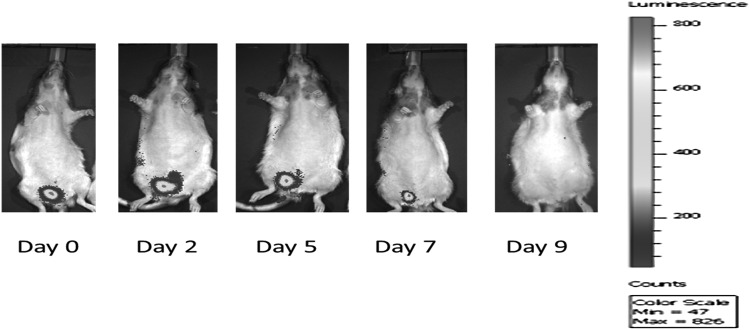
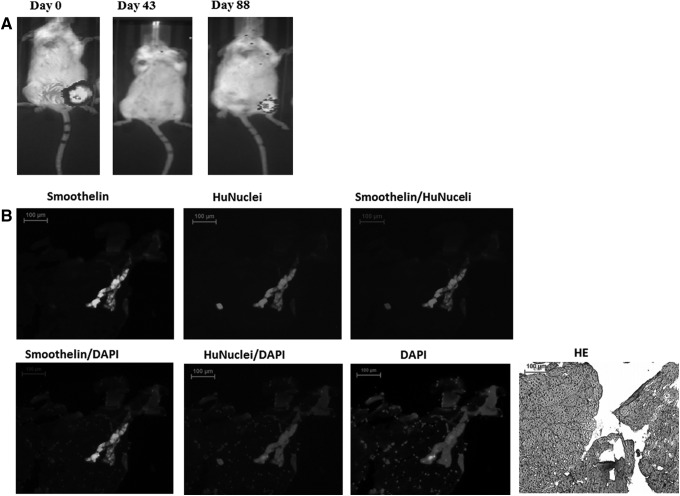
Rats with periurethral injection of 2 million Fluc-tagged pSMCs showed a strong BLI signal in the urethral region 20 min after injection confirming site-appropriate injection (Fig. 2). BLI signal in the periurethral region of the SUI rats disappeared after 9 days. This may be due to cell attrition to a level below the detection level of BLI technique. This is because the RNU rat is not completely immunodeficient, thus causing the transplanted human cells to undergo attrition over time. Therefore, we used SCID mice to further examine cell survival and integration in vivo. Additionally, the deep location of the rat urethra decreases the intensity of the BLI signals. The SCID mouse is severely immunodeficient and injection into the hind leg, which is superficial, allows for better detection of the BLI signal. BLI signal of iPSC-pSMCs injected into the hind leg of the SCID mice persisted for 20–43 days. Re-emergence of the BLI signal was observed and the signal persisted for at least 88 days when the experiment was terminated (Fig. 3). Cells in the skeletal muscle of the SCID mouse stained positive for HuNu (protein) and human smoothelin (a SMC protein) confirmed persistence of human SMCs in vivo (Fig. 3).
FIG. 2.
2 × 106 Fluc-tagged-H9-pSMC were transplanted into the urethra of the Rowett Nude rat and monitored by BLI. The BLI signal was strong on the day of transplantation and gradually became weaker until day 9, when no signal was detected. BLI, bioluminescence imaging; pSMC, smooth muscle precursor cell.
FIG. 3.
(A) The SCID mouse injected with luciferase-tagged iPSC-pSMCs (1 × 106) in the one hind leg was monitored with BLI for 88 days. The BLI signal disappeared at day 43, and then reappeared at day 88. (B) Identification of the injected iPSC-pSMCs in the hind leg of SCID mouse with immunofluorescence staining. At day 88 after cell injection, the transplanted cells, which were stained positive with human nuclei (HuNuclei) and human smoothelin, were detected focally at the injection site of the hind leg. (Scale bar: 100 μM). iPSC, induced pluripotent stem cell.
H9-pSMC efficacy experiment
Average LPP value of the sham saline injection group was significantly lower (N = 10, mean = 15.52 ± 1.09 cm H2O) than intact controls (N = 13, mean = 18.75 ± 0.96 cm H2O, P < 0.05) 8 weeks after urethrolysis, confirming persistence of SUI in the model. The average LPP value of the bladder SMC group (N = 11, mean = 18.95 ± 1.04 cm H2O) was significantly higher than that of the sham saline group (P < 0.05). LPP value of the H9-pSMC treatment group was higher than that of the saline sham group, although the difference was not significant. There was also no difference in LPP between the H9-pSMC treatment group (N = 22, mean = 16.66 ± 0.74 cm H2O) and intact controls (Fig. 4). The group treated with human SMCs showed significant improvement in LPP compared to the sham saline group. Two outlier LPP values (22 cm of H2O and 2.3 cm of H2O) were deleted in the sham saline group. It is not uncommon for RNU rats to have variable anatomy due to inbreeding [29]. Therefore, extremes in measurements are likely to reflect anatomic variance and may skew the data abnormally.
FIG. 4.
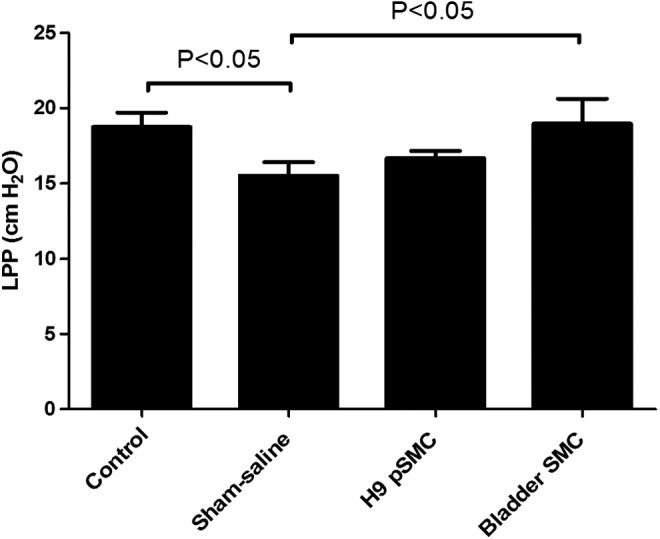
Comparison of LPP values between groups in the H9-pSMC efficacy experiment. The results are presented as the mean ± standard error of mean. LPP, leak point pressure.
iPSC-pSMC efficacy experiment
SUI models again demonstrated persistence of SUI throughout the experiment according to the LPP comparison between the intact control group (N = 13, mean = 17.67 ± 1.11 cm H2O) and sham saline group (N = 14, mean = 14.16 ± 1.07 cm H2O, P < 0.05). Epi-iPSC-pSMC treatment group had significantly higher LPP value (N = 9, mean = 19.4 ± 1.33 cm H2O) compared to sham saline group (P < 0.05). LPP value of iPSC-pSMC group was also significantly higher (N = 8, mean = 18.45 ± 1.41 cm H2O) than the sham saline group (P < 0.05, Fig. 5). Extreme outlier LPP values were also observed in each group, including in intact controls. These outliers were removed from our data analysis because of concerns related to anatomic variability.
FIG. 5.
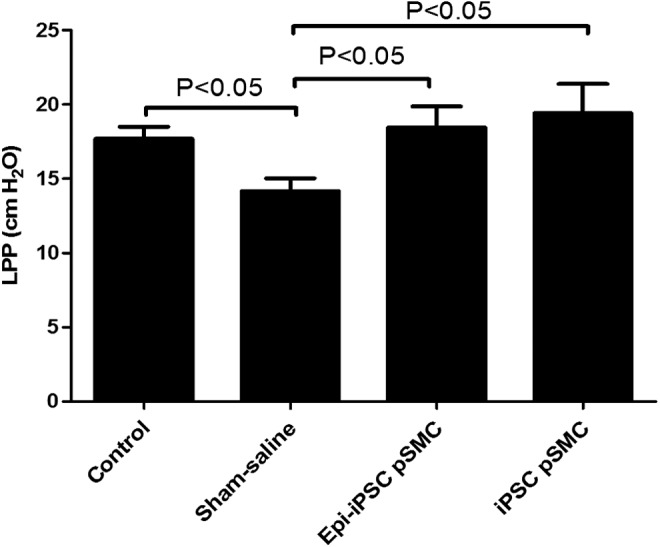
Comparison of LPP values between groups in the iPSCs-pSMC efficacy experiment. The results are presented as the mean ± standard error of mean.
Baseline EMG activities of the urethral sphincter
EMG activity represents the motor unit potential of the sphincter muscle. SMCs have resting EMG, therefore, the recorded EMG activity includes that of both skeletal and smooth muscles [30]. EMG measurements can provide information about the integrity of innervation and the condition of neuromuscular junctions and myofibers [31]. The average rectified EMG (ARV) reflects the compound action potential amplitude level. The greater the amplitude of the ARV, the greater the number of excited muscle cells. ARVs were used to compare EMG activity between the experimental groups (Fig. 6).
FIG. 6.
(A) A representative EMG activity tracing from a control rat. Baseline EMG was stable when bladder pressure was near 0 cm H2O. The arrow (EMG at baseline) indicates a randomly selected 10 s segment used for ARV analysis. The EMG at leak point arrow shows the variability of the measurement at LPP. (B) A representative LPP graph from a control rat after spinal transection. Intravesical pressure was elevated by raising the height of reservoir gradually by 2–3 cm every 2 min. The arrow indicates when urinary leakage was observed. No bladder contraction was detected. ARV, average rectified value.
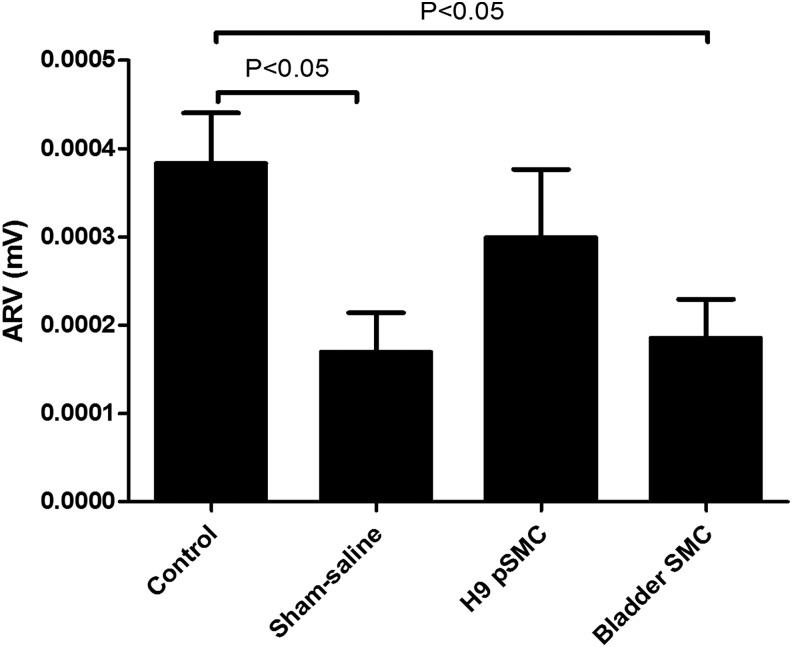
ARV in the control group (N = 11, mean = 0.000383 ± 0.00008 mV) was significantly higher than in the sham saline group (N = 12, mean = 0.00017 ± 0.000076 mV, P < 0.05) and bladder SMC group (N = 11, mean = 0.000185 ± 0.00008 mV, P < 0.05). This is consistent with the surgical neuromuscular injury the sham saline and bladder SMC groups experienced. Although the ARV for the H9-pSMC treatment group (N = 22, mean = 0.000299 ± 0.000056 mV) appeared higher than the sham saline group, the difference was not statistically significant (Fig. 7). EMG data from all but two rats in the control group were available for analysis. Data loss was due to software issues.
FIG. 7.
Comparison of EMG (ARV) between groups in the H9-pSMC efficacy experiment. The results are presented as the mean ± standard error of mean.
Both Epi-iPSC-pSMC treatment group (N = 10, mean = 0.000158 ± 0.000031 mV) and iPSC-pSMC treatment group (N = 6, mean = 0.000119 ± 0.00004 mV) showed higher ARV trends compared to the sham saline group (Fig. 8). ARV data of four rats in the sham saline group and three rats in the iPSC-pSMC group were lost because data were not archived correctly in the system.
FIG. 8.
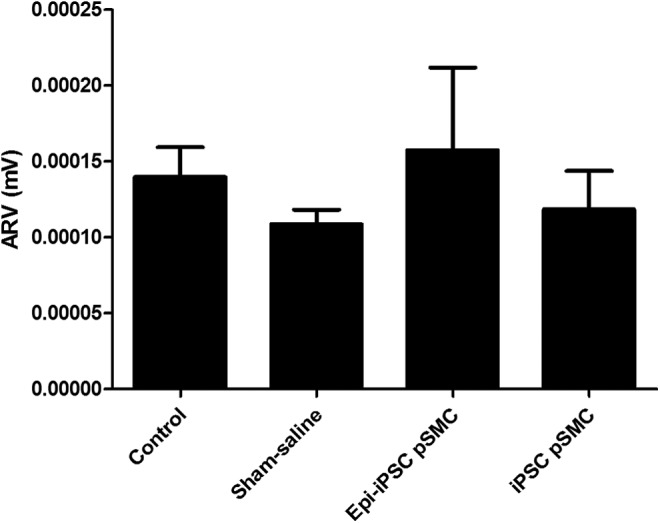
Comparison of EMG (ARV) between groups in the iPSC-pSMC efficacy experiment. The results are presented as the mean ± standard error of mean. No significant differences were found.
Overall, we found that placement of the wire electrodes in the rat periurethral region was difficult and unreliable.
Discussion
Urethral sphincter muscle, connective tissue, neural, and vascular support are responsible for maintaining continence [32]. The smooth muscle component has been documented to be an important contributor to urethral pressure recovery and leakage prevention in animal studies [33]. It is thought that the internal urethral sphincter, which consists of SMCs, is an important contributor to urinary continence. Weakening of this area due to injury or loss of cells from aging is associated with moderate to severe incontinence [34–36]. Current therapies do not address restoration of SMCs in the urethral sphincter.
In recent years, SC-based regenerative medicine has shown promising effects on the recovery of human sphincter dysfunction or injury. In these studies, implanted SCs were urine derived [13] or adult muscle biopsy derived [37], which consisted of heterogeneous cells with small numbers of SC subpopulations. Hence, the effect for SUI recovery was generated by a mixture of cells with variable differentiation and renewal potential. It is impossible to correlate the efficacy for functional regeneration of the urethral sphincter to specific cell types. In this study, we used a pure population of pSMCs differentiated from human PSCs, hESC, and iPSC, to investigate its effect on SUI recovery.
In both of our experiments (using H9- and iPSC-pSMCs), LPP was significantly lower in the sham saline (surgery + saline injection) group compared to intact controls, confirming persistence of SUI in our animal model throughout the experimental period. While there was no significant difference between the H9-pSMC treatment and the sham saline group, LPP was higher in the former and trended toward that of intact controls, suggesting restoration of urethral function. Data from the iPSC-pSMC experiment did show significant improvement in LPP for both iPSC-pSMC and Epi-iPSC-pSMC treatment groups compared to the sham saline group. Because LLP measurements are prone to observer bias, the researchers in this study were blinded to the rat treatment groups.
We note that a change in cell processing protocol may have contributed to the lack of significance in the H9-pSMC study. The H9-pSMCs were freshly thawed before transplantation. Whereas, cells for the iPSC-pSMC study were allowed to recover in culture for 2 days after thawing and before transplantation. This could have contributed to improved in vivo performance in the iPSC experiment. Another potential source of variability relates to the RNU rats. They are known to have variable anatomy due to inbreeding [29]. This can account for the extreme outlier values seen even in the control group. To minimize this effect, we deleted the extreme outliers in our analysis.
Bladder SMCs as a source of SMCs for bladder tissue regeneration has been studied [38–41]. The bladder SMCs and urothelial cells were reported to contribute to the development of bladder smooth muscle [42]. However, biopsy barriers and severe fibrosis were significant issues. We used bladder SMCs to explore whether transplantation of terminally differentiated SMCs can restore urethral function in our SUI rat model. In our study, LPP was significantly higher with bladder SMCs treatment compared to the sham saline injected rats, confirming that replacement of SMCs restores function. However, autologous bladder SMC transplantation and autologous muscle-biopsy SC therapies share similar limitations, including biopsy barriers, ability to harvest sufficient number of cells, limited cell expansion capabilities, and cell senescence.
EMG activity has been used to assess sphincter function [30,43] and evaluate its regeneration in some studies involving autologous muscle progenitor cell and periurethral skeletal myofiber treatment [44,45].
Therefore, we included EMG measurements as an adjunct to our LPP data. Our primary outcome measure is LPP. In our study, pSMC-treatment groups showed increases in ARV, but the differences were not significant compared to the sham saline group. Our LPP data does not agree with the EMG data. We believe this is due to the high variability of this methodology and the loss of data related to technical issues. The rat urethral sphincter measures roughly 1 cm in length, which makes it difficult to insert electrodes externally into the same area reproducibly (Fig. 1). Thus, inadequate EMG activity or excessive crosstalk from other muscles can cause high variability [46]. We did not use the EMG activity at leak point because that measurement is subject to even more uncertainty (Fig. 6A). The high variability makes EMG unsuitable as a measurement method for studies involving many animals. Our experience agrees with other publications, where LPP is used as the primary measurement method to assess urethral function.
There are several possible ways in which pSMCs may restore urethral function: (i) differentiation of pSMCs into functional SMCs, (ii) pSMCs may persist in the urethra acting as a bulking agent, and/or (iii) autocrine and/or paracrine effects of secreted cytokines that induce proliferation of rat urethral sphincter muscles [47] or extracellular matrix. According to the BLI tracking of pSMCs in vivo, signals disappeared in all rats after 9 days, suggesting that there was significant attrition of the transplanted pSMCs 1 week postinjection. Perhaps the pSMCs could not survive the stress of transplantation or they were eliminated by the incompletely suppressed immune system of the RNU rats. However, given the LPP results, it is possible that a small population of pSMC, too low to be detected by BLI, was able to expand and continue to differentiate into terminal SMCs. To test this hypothesis, we conducted a cell integration study using SCID mice. This type of mouse is severely immunodeficient. We also injected into a muscle area that is more superficial to increase ability to detect the BLI signal. Our data confirm survival of pSMCs in vivo up to 88 days and further in vivo differentiation into SMCs. Hence, cell integration is part of the pSMC mechanism of action post transplantation. We are currently conducting studies to examine the paracrine effects of pSMCs.
Conclusion
This is a translational study that explores a unique SC source for SMC regeneration. Currently most therapies for muscle replacement focus on skeletal muscle regeneration through adult mesenchymal stem cells. There are no treatments that target replacement of SMCs, which are a significant component of many organs. The positive trend in LLP with hESC, H9-pSMC transplant, and significant improvements in LLP with iPSC-pSMC transplant, in conjunction with evidence of long-term cell integration, suggest that human PSC-derived pSMCs can restore urethral sphincter function. These findings open up the possibility of using PSCs for urologic conditions, where large numbers of SMCs are needed to restore function.
Acknowledgments
The authors would like to express their gratitude to Fangfang Zhu, PhD (postdoctoral research fellow in Dr. Michele Pamela Calos' Laboratory, Stanford University School of Medicine) for her SC technical support. We would like to thank China Chien for her invaluable advice and help with the leak point pressure testing methodology. This research is funded by CIRM (California Institute of Regenerative Medicine), Grant No. 106180-TR3-05569.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Imamura M, Abrams P, Bain C, Buckley B, Cardozo L, Cody J, Cook J, Eustice S, Glazener C, et al. (2010). Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence. Health Technol Assess 14:1–188, iii–iv [DOI] [PubMed] [Google Scholar]
- 2.Holroyd-Leduc JM, Steinke V, Elliott D, Mullin K, Elder K, Callender S. and Hildebrand KA. (2013). Improving the quality of care for older adults using evidence-informed clinical care pathways. Can Geriatr J 16:111–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart ML, Izeta A, Herrera-Imbroda B, Amend B. and Brinchmann JE. (2015). Cell therapy for stress urinary incontinence. Tissue Eng Part B Rev 21:365–376 [DOI] [PubMed] [Google Scholar]
- 4.Sathianathen NJ, McGuigan SM. and Moon DA. (2014). Outcomes of artificial urinary sphincter implantation in the irradiated patient. BJU Int 113:636–641 [DOI] [PubMed] [Google Scholar]
- 5.Wang HJ, Chuang YC. and Chancellor MB. (2011). Development of cellular therapy for the treatment of stress urinary incontinence. Int Urogynecol J 22:1075–1083 [DOI] [PubMed] [Google Scholar]
- 6.Wallner C, Dabhoiwala NF, DeRuiter MC. and Lamers WH. (2009). The anatomical components of urinary continence. Eur Urol 55:932–943 [DOI] [PubMed] [Google Scholar]
- 7.Covas DT, Panepucci RA, Fontes AM, Silva WA, Jr., Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC. and Zago MA. (2008). Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol 36:642–654 [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B. and Ignarro LJ. (2006). Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A 103:12167–12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J. and Chancellor MB. (2003). Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology 62:958–963 [DOI] [PubMed] [Google Scholar]
- 10.Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, Erickson J, Huard J. and Chancellor MB. (2008). 1-Year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 19:881–883 [DOI] [PubMed] [Google Scholar]
- 11.Fu Q, Song XF, Liao GL, Deng CL. and Cui L. (2010). Myoblasts differentiated from adipose-derived stem cells to treat stress urinary incontinence. Urology 75:718–723 [DOI] [PubMed] [Google Scholar]
- 12.Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, Lue TF. and Lin CS. (2010). Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy 12:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G, Wang X, Sun X, Deng C, Atala A. and Zhang Y. (2013). The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials 34:8617–8629 [DOI] [PubMed] [Google Scholar]
- 14.Stangel-Wojcikiewicz K, Jarocha D, Piwowar M, Jach R, Uhl T, Basta A. and Majka M. (2014). Autologous muscle-derived cells for the treatment of female stress urinary incontinence: a 2-year follow-up of a Polish investigation. Neurourol Urodyn 33:324–330 [DOI] [PubMed] [Google Scholar]
- 15.Strasser H, Marksteiner R, Margreiter E, Mitterberger M, Pinggera GM, Frauscher F, Fussenegger M, Kofler K. and Bartsch G. (2007). Transurethral ultrasonography-guided injection of adult autologous stem cells versus transurethral endoscopic injection of collagen in treatment of urinary incontinence. World J Urol 25:385–392 [DOI] [PubMed] [Google Scholar]
- 16.De Coppi P, Callegari A, Chiavegato A, Gasparotto L, Piccoli M, Taiani J, Pozzobon M, Boldrin L, Okabe M, et al. (2007). Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol 177:369–376 [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez LV, Chen S, Jack GS, de Almeida F, Lee KW. and Zhang R. (2005). New objective measures to quantify stress urinary incontinence in a novel durable animal model of intrinsic sphincter deficiency. Am J Physiol Regul Integr Comp Physiol 288:R1332–R1338 [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed] [Google Scholar]
- 19.Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schule B, Dolmetsch RE, et al. (2011). LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8:267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II. and Thomson JA. (2009). Human induced pluripotent stem cells free of vector and transgene sequences. Science 324:797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seabright M. (1971). A rapid banding technique for human chromosomes. Lancet 2:971–972 [DOI] [PubMed] [Google Scholar]
- 22.Marchand M, Anderson EK, Phadnis SM, Longaker MT, Cooke JP, Chen B. and Reijo Pera RA. (2014). Concurrent generation of functional smooth muscle and endothelial cells via a vascular progenitor. Stem Cells Transl Med 3:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Tang X, Sun X, Miao Z, Lv Y, Yang Y, Zhang H, Zhang P, Liu Y, et al. (2012). TGFbeta inhibition enhances the generation of hematopoietic progenitors from human ES cell-derived hemogenic endothelial cells using a stepwise strategy. Cell Res 22:194–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loukovaara S, Gucciardo E, Repo P, Lohi J, Salven P. and Lehti K. (2015). A case of abnormal lymphatic-like differentiation and endothelial progenitor cell activation in neovascularization associated with hemi-retinal vein occlusion. Case Rep Ophthalmol 6:228–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Cho DB. and Yang PC. (2010). Double staining immunohistochemistry. N Am J Med Sci 2:241–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merletti R, Knaflitz M. and De Luca CJ. (1990). Myoelectric manifestations of fatigue in voluntary and electrically elicited contractions. J Appl Physiol (1985) 69:1810–1820 [DOI] [PubMed] [Google Scholar]
- 27.Lowery MM. and O'Malley MJ. (2003). Analysis and simulation of changes in EMG amplitude during high-level fatiguing contractions. IEEE Trans Biomed Eng 50:1052–1062 [DOI] [PubMed] [Google Scholar]
- 28.Conway DA, Kamo I, Yoshimura N, Chancellor MB. and Cannon TW. (2005). Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 16:359–363 [DOI] [PubMed] [Google Scholar]
- 29.Loh HS, Mohd-Lila MA, Abdul-Rahman SO. and Kiew LJ. (2006). Pathogenesis and vertical transmission of a transplacental rat cytomegalovirus. Virol J 3:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafik AA, Shafik IA. and El Sibai O. (2014). On the etiology of the electric activity of the external anal and urethral sphincters. J Invest Surg 27:267–272 [DOI] [PubMed] [Google Scholar]
- 31.Rahman SW, Rahman KM, Sarker CB, Latif SA, Ahmed S, Hossain S. and Rahman S. (2004). Neurophysiology in clinical practice. Mymensingh Med J 13:215–219 [PubMed] [Google Scholar]
- 32.Chen HY, Lin YN, Chen YH. and Chen WC. (2012). Stress urinary incontinence following vaginal trauma involves remodeling of urethral connective tissue in female mice. Eur J Obstet Gynecol Reprod Biol 163:224–229 [DOI] [PubMed] [Google Scholar]
- 33.Jiang HH, Salcedo LB. and Damaser MS. (2011). Quantification of neurological and other contributors to continence in female rats. Brain Res 1382:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clobes A, DeLancey JO. and Morgan DM. (2008). Urethral circular smooth muscle in young and old women. Am J Obstet Gynecol 198:587 e1–e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez L, Carrillo M, Sorbera LA, Zohar Y. and Zanuy S. (2004). Effects of photoperiod on pituitary levels of three forms of GnRH and reproductive hormones in the male European sea bass (Dicentrarchus labrax, L.) during testicular differentiation and first testicular recrudescence. Gen Comp Endocrinol 136:37–48 [DOI] [PubMed] [Google Scholar]
- 36.Trowbridge ER, Wei JT, Fenner DE, Ashton-Miller JA. and Delancey JO. (2007). Effects of aging on lower urinary tract and pelvic floor function in nulliparous women. Obstet Gynecol 109:715–720 [DOI] [PubMed] [Google Scholar]
- 37.Smaldone MC. and Chancellor MB. (2008). Muscle derived stem cell therapy for stress urinary incontinence. World J Urol 26:327–332 [DOI] [PubMed] [Google Scholar]
- 38.Deng K, Lin DL, Hanzlicek B, Balog B, Penn MS, Kiedrowski MJ, Hu Z, Ye Z, Zhu H. and Damaser MS. (2015). Mesenchymal stem cells and their secretome partially restore nerve and urethral function in a dual muscle and nerve injury stress urinary incontinence model. Am J Physiol Renal Physiol 308:F92-F100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hidas G, Lee HJ, Bahoric A, Kelly MS, Watts B, Liu Z, Saharti S, Lusch A, Alamsahebpour A, et al. (2015). Aerosol transfer of bladder urothelial and smooth muscle cells onto demucosalized colonic segments for bladder augmentation: in vivo, long term, and functional pilot study. J Pediatr Urol 5:260e1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oberpenning F, Meng J, Yoo JJ. and Atala A. (1999). De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol 17:149–155 [DOI] [PubMed] [Google Scholar]
- 41.Raghavan AM. and Shenot PJ. (2009). Bladder augmentation using an autologous neo-bladder construct. Kidney Int 76:236. [DOI] [PubMed] [Google Scholar]
- 42.Baskin LS, Hayward SW, Young P. and Cunha GR. (1996). Role of mesenchymal-epithelial interactions in normal bladder development. J Urol 156:1820–1827 [PubMed] [Google Scholar]
- 43.Jiang HH, Pan HQ, Gustilo-Ashby MA, Gill B, Glaab J, Zaszczurynski P. and Damaser M. (2009). Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol Urodyn 28:229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kajbafzadeh AM, Elmi A, Talab SS, Esfahani SA. and Tourchi A. (2010). Functional external anal sphincter reconstruction for treatment of anal incontinence using muscle progenitor cell auto grafting. Dis Colon Rectum 53:1415–1421 [DOI] [PubMed] [Google Scholar]
- 45.Yiou R, Hogrel JY, Loche CM, Authier FJ, Lecorvoisier P, Jouany P, Roudot-Thoraval F. and Lefaucheur JP. (2013). Periurethral skeletal myofibre implantation in patients with urinary incontinence and intrinsic sphincter deficiency: a phase I clinical trial. BJU Int 111:1105–1116 [DOI] [PubMed] [Google Scholar]
- 46.LaPallo BK, Wolpaw JR, Chen XY. and Carp JS. (2014). Long-term recording of external urethral sphincter EMG activity in unanesthetized, unrestrained rats. Am J Physiol Renal Physiol 307:F485–F497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jack GS, Almeida FG, Zhang R, Alfonso ZC, Zuk PA. and Rodriguez LV. (2005). Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol 174:2041–2045 [DOI] [PubMed] [Google Scholar]