Abstract
Rationale
Enhancement of N-methyl-d-aspartate receptor (NMDAR) activity through its glycine modulatory site (GMS) is a novel therapeutic approach in schizophrenia. Brain concentrations of endogenous GMS agonist d-serine and antagonist N-acetyl-aspartylglutamate are regulated by serine racemase (SR) and glutamic acid decarboxylase 2 (GCP2), respectively. Using mice genetically, under-expressing these enzymes may clarify the role of NMDAR-mediated neurotransmission in schizophrenia.
Objectives
We investigated the behavioral effects of two psychotomimetic drugs, the noncompetitive NMDAR antagonist, phencyclidine (PCP; 0, 1.0, 3.0, or 6.0 mg/kg), and the indirect dopamine receptor agonist, amphetamine (AMPH; 0, 1.0, 2.0, or 4.0 mg/kg), in SR −/− and GCP2 −/+ mice. Outcome measures were locomotor activity and prepulse inhibition (PPI) of the acoustic startle reflex. Acute effects of an exogenous GMS antagonist, gavestinel (0, 3.0, or 10.0 mg/kg), on PCP-induced behaviors were examined in wild-type mice for comparison to the mutants with reduced GMS activity.
Results
PCP-induced hyperactivity was increased in GCP2 −/+ mice, and PCP-enhanced startle reactivity was increased in SR −/− mice. PCP disruption of PPI was unaffected in either mutant. In contrast, gavestinel attenuated PCP-induced PPI disruption without effect on baseline PPI or locomotor activity. AMPH effects were similar to controls in both mutant strains.
Conclusions
The results of the PCP experiments demonstrate that convergence of pharmacological and genetic manipulations at NMDARs may confound the predictive validity of these preclinical assays for the effects of GMS activation in schizophrenia. The AMPH data provide additional evidence that hyperdopaminergia in schizophrenia may be distinct from NMDAR hypofunction.
Keywords: NMDA receptor, Glutamate, Schizophrenia, Serine racemase, Phencyclidine, Amphetamine, Glutamate carboxypeptidase 2, Glycine modulatory site, Gavestinel
Introduction
The N-methyl-d-aspartate subtype of ionotropic glutamate receptors (NMDAR) plays a critical role in functional and structural synaptic plasticity. In addition to membrane depolarization, activation of these heterotetrameric receptors requires glutamate binding to NR2 subunits and co-agonist binding to the glycine modulatory site (GMS) on NR1 subunits (Laube et al. 1998). The GMS can be activated by glycine or d-serine (Matsui et al. 1995; Mothet et al. 2000). The overlap of high d-serine concentrations with NMDAR expression suggests that it may be the dominant endogenous ligand in forebrain regions (Hashimoto et al. 1993). D-Serine levels in the brain are chiefly determined by the synthesis enzyme serine racemase (SR) and the catabolic enzyme d-amino acid oxidase (DAAO; Schell et al. 1995; Wolosker et al. 1999). Endogenous N-acetyl-aspartylglutamate (NAAG) also plays a role in ligand dynamics at NMDARs, acting as an allosteric inhibitor of GMS agonist binding (Bergeron et al. 2005; Bergeron et al. 2007). Extracellular NAAG is catabolized to N-acetyl aspartate and glutamate by the astrocytic enzyme glutamate carboxypeptidase 2 (GCP2; Robinson et al. 1987; Berger et al. 1999).
Hypofunction of NMDARs has been hypothesized as a factor in the etiology and/or pathophysiology of schizophrenia (Coyle 1996; Krystal et al. 2003; Lisman et al. 2008). The argument for the role of NMDAR hypofunction is supported by molecular findings of altered NMDAR expression in postmortem studies (Akbarian et al. 1996; Kristiansen et al. 2006; Beneyto and Meador-Woodruff 2008). Meta-analyses of genetic data from patient populations have reported significant association of schizophrenia with allelic variants of genes relevant to NMDAR activity, including DAAO, G72, and NR2B (Li and He 2007a, b; Allen et al. 2008). Moreover, this hypothesis is supported by the psychotomimetic effects of the dissociative anesthetics, phencyclidine (PCP) and ketamine. These noncompetitive inhibitors of NMDARs block the pore of the channel in an activity-dependent manner. In humans, these drugs induce a syndrome that is reminiscent of schizophrenia (Krystal et al. 1994; Vollenweider and Geyer 2001) and exacerbate symptoms in schizophrenia (Lahti et al. 2001). These observations have encouraged the development of mouse models of NMDAR hypofunction by different strategies of genetic manipulation, notably reduced NR1 expression (Mohn et al. 1999; Shimizu et al. 2000; Belforte et al. 2010), reduced affinity of NR1 for GMS agonists (Kew et al. 2000; Ballard et al. 2002), and altered endogenous GMS agonist or antagonist levels (Basu et al. 2009; Han et al. 2009). The latter studies employ constitutive deletions in SR and GCP2, respectively. Homozygous deletion of SR (−/−) caused a 90% reduction in brain d-serine levels, a resultant reduction in NMDAR signaling, hyperactivity, and spatial memory deficits (Basu et al. 2009). GCP2 hypomorphs (GCP2 −/+), with a presumed increase in extracellular NAAG, exhibit hyperactivity and social withdrawal (Han et al. 2009).
This study examines the behavioral effects of the psychotomimetics PCP and amphetamine (AMPH) in SR −/− and GCP2 −/+ mutant mouse lines. Investigation of these drugs in mutant mouse strains can provide behavioral indices of the state of glutamatergic (via PCP) and dopaminergic (via AMPH) neurotransmission and potentially reveal behavioral phenotypes not observable in a drug-free state because of compensatory changes resulting from constitutive mutations (van den Buuse 2010). Pharmacological enhancement of GMS activity can reverse NMDAR blocker-induced behaviors (Javitt et al. 1997; Nilsson et al. 1997). Subjects with schizophrenia display enhanced AMPH-induced neurochemical changes in imaging studies, consistent with a hyperdopaminergic state (Abi-Dargham et al. 2009). Consequently, we hypothesized that NMDAR hypofunction in the SR and GCP2 mutant mice would result in increased sensitivity to PCP and/or AMPH. These genetic models of reduced GMS function were then compared to an acute, pharmacologically induced GMS hypofunction with systemic gavestinel (selective GMS antagonist) to address the role of compensation.
Methods and materials
Subjects
The SR mutant construct consists of a targeted deletion of exon 1, which encodes the catalytic domain of the enzyme (Basu et al. 2009). The GCP2 mutant construct is a targeted deletion of exons 1 and 2 (Han et al. 2009). Both mutant constructs were backcrossed onto a C57BL/6J background for >10 generations. Experimental subjects were derived from SR heterozygote (−/+) crosses to produce SR null mutant (−/−) and wild-type (WT) littermate controls. Due to homozygote lethality of the GCP2 null mutant, experiments were conducted using heterozygote mutant (−/+) mice. Experimental mice were derived from GCP2 heterozygote mutant (−/+) crosses to WT C57BL/6J mice. All C57BL/6J mice used for breeding were derived from an in-house colony of WT mice, which were no more than one generation removed from mice imported from the Jackson Laboratory (Bar Harbor, ME, USA). Experimental subjects were housed four per cage (two mutants, two WT) in a vivarium on a 12:12 h light/ dark cycle with food and water provided ad libitum. A total of 320 male animals (age 9–13 weeks of age) were used for these studies (76 SR −/−, 76 WT; 84 GCP2 −/+, 84 WT). All animals were tested in each of the behavioral assays (AMPH-induced activity, AMPH disruption of prepulse inhibition (PPI), PCP-induced activity, PCP disruption of PPI). Drug treatments were assigned pseudo-randomly and counterbalanced in a manner such that each animal received each level of drug dose (0, low, mid, high) only once to control for repeated drug treatment effects. Testing was performed at 1 week intervals, with the order of assay presentation randomized to control for order and repeat testing effects. For gavestinel experiments, WT mice (n= 90; age 9–12 weeks) derived from in-house breeding were used. All mice were within two generations of C57BL/6J mice purchased from the Jackson Laboratory. All mice were tested in both activity and PPI assays, with 1 week intervals between test days. Subjects were treated with PCP only once, either in the activity or the PPI assay. Gavestinel treatment was assigned randomly. The principles of laboratory animal care were followed, and all experiments were performed in accordance with the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003).
Drugs
PCP (phencyclidine hydrochloride, Sigma Aldrich, St. Louis, MO, USA) and AMPH (d-amphetamine sulfate, Sigma Aldrich) were dissolved in sterile water. Gavestinel {4,6-dichloro-3-[(1E)-3-oxo-3-(phenylamino)-1-propenyl]-1H-indole-2-carboxylic acid sodium salt} (GV150526A, Tocris Bioscience; Ellisville, MO, USA) was dissolved in 5% β-cyclodextrin. PCP and AMPH injections were performed via a subcutaneous (s.c.) route, and gavestinel was injected via intraperitoneal (i.p.) route. All injections were administered at a volume of 10 mL/kg.
Assessment of locomotor activity in SR −/−, GCP2 −/+, and WT littermates
Apparatus
Activity was monitored using open-field chambers (27×27×20 cm; ENV-510, Med Associates Inc., St. Albans, VT, USA). Locomotion was detected with two sets (x- and y-axes) of 16 horizontal infrared photobeams and vertical movement with a single set of 16 horizontal infrared photobeams fixed at a higher elevation. Chambers were contained in sound-attenuating cubicles, illuminated by two 28 V, 0.1 A lights. Photobeam breaks were recorded and converted to distance traveled using activity monitoring software (Activity Monitor 5.0; Med Associates Inc.) The threshold for detection of ambulation was set at three consecutive beam breaks in 500 ms. Test sessions were 120 min in length, with data collected in 5-min bins.
Drug-induced activity
All animals were placed in the activity chambers for a 30-min habituation period during which locomotor activity was recorded. At t=30 min, the program was paused and animals (n=19–21/dose×geno-type) were removed from the chambers and injected with either AMPH (vehicle, 1.0, 2.0, or 4.0 mg/kg, s.c.) or PCP (vehicle, 1.0, 3.0, or 6.0 mg/kg, s.c.). Mice were returned to the chambers and activity was monitored for 90 additional min. Locomotion was expressed as distance traveled (in centimeters), either in 5-min bins or summarized for the entire period following drug treatment (t=30–120 min).
Prepulse inhibition of the acoustic startle reflex in SR −/−, GCP2 −/+, and WT littermates
Apparatus
Subjects were placed in a nonrestraining cylindrical enclosure attached to a platform fitted with a motion transducer contained within a sound-attenuating chamber (Startle Response System, San Diego Instruments; San Diego, CA, USA). Stimuli were delivered from a PC and the motion signal of the animal in the 65 ms following stimulus presentation was recorded (SR-LAB, San Diego Instruments). Test sessions consisted of a 5-min acclimation period during which only background noise (70 dB white noise) was present, followed by 72 stimulus trials. Trials were presented at random intervals of 10–20 s with an average of 15 s. The first and last six trials of the session were acoustic startle stimuli (120 dB white noise, 20 ms in duration) presented alone. The intervening 60 trials were of five different types in equal proportions: no startle stimulus (baseline activity), startle stimulus alone, or startle stimulus preceded (50 ms) by a prepulse (white noise 3, 6, or 12 dB over background, 20 ms in duration). Reactivity in the intervening trials is used to calculate PPI.
Drug-induced disruption of PPI
Animals (n=18–21/dose× genotype) were injected, 25 min prior to the test session, with either AMPH (vehicle, 1.0, 2.0, or 4.0 mg/kg, s.c.) or PCP (vehicle, 1.0, 3.0, or 6.0 mg/kg, s.c.). Animals were retained in their home cages prior to testing. The effect of AMPH and PCP on three outcome measures was analyzed: startle stimulus reactivity, baseline activity, and PPI. Startle stimulus reactivity and baseline activity are expressed in millivolts (movement is translated in voltage change by a piezoelectric motion transducer). PPI is expressed as percentage for each prepulse intensity: % PPI=100×[(startle stimulus reactivity–startle stimulus reactivity in the presence of prepulse)/(startle stimulus reactivity)]. Subjects for which startle stimulus reactivity was not significantly greater than baseline (no stimulus) activity (p<0.05, one-tailed Student's t test or Mann–Whitney t test) were excluded from PPI analysis.
Effect of gavestinel on PCP-induced behaviors in WT mice
The ability of gavestinel to alter PCP-induced hyperactivity or PCP disruption of PPI was tested using WT mice. Activity and PPI were assessed using instruments and methods of analysis as previously described. The drug treatment conditions were as follows: In activity experiments, subjects (n=15/dose group) were removed from test chambers at t=30 min and injected with gavestinel (vehicle, 3.0, or 10.0 mg/kg, i.p.), immediately followed by PCP (vehicle or 6.0 mg/kg, s.c) and returned to the test chambers for 90 min of activity monitoring. In the PPI experiments, subjects were treated with gavestinel (vehicle, 3.0, or 10.0 mg/kg, i.p.) immediately followed by PCP (vehicle or 3.0 mg/kg, s.c.) 25 min prior to testing.
Statistical analysis
In all experiments, the performance of the mutant strain was compared to strain-specific WT littermates in a between-subjects design. Drug-induced activity, baseline activity, and startle stimulus reactivity were analyzed using two-way ANOVA, with genotype and drug dose as the main effects. Analysis of locomotor data presented in 5-min bins included time as a repeated measure main effect. Bonferroni-corrected post hoc tests were used to assess genotype effects at specific drug doses. PPI was analyzed using a standard least squares mixed model; subject was included as a random effect and prepulse intensity, genotype, and drug dose as fixed effects. Least squared means of the PPI response averaged across all prepulse intensities were examined with a post hoc Student's t test to find treatment-specific gavestinel effects.
Results
PCP disruption of PPI in SR −/− and GCP2 −/+ mice
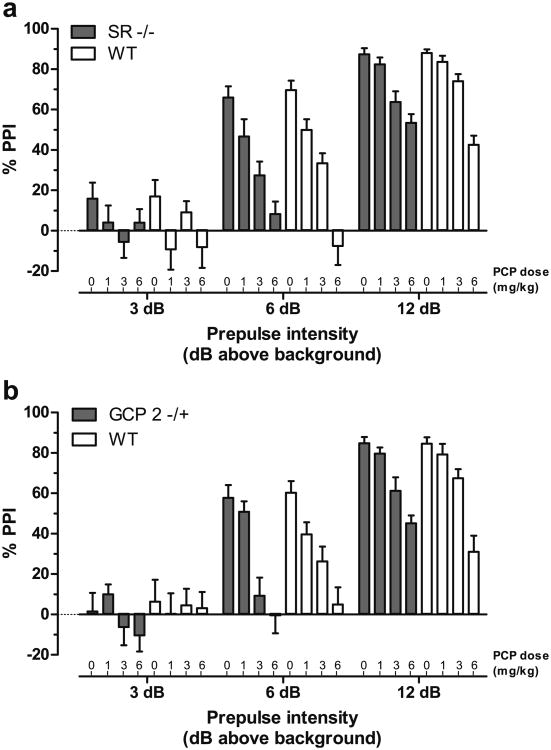
PPI of the acoustic startle reflex is a behavioral manifestation of sensorimotor gating, a form of precognitive processing that is deficient in patients with schizophrenia (for review, see Swerdlow et al. 2008). Given the similar baseline PPI response in the SR −/− and GCP2 −/+ animals (Basu et al. 2009; Han et al. 2009), drug-induced disruption of PPI was studied. Regardless of genotype, all subjects exhibited robust, dose-dependent reductions in PPI in response to treatment with PCP (1.0, 3.0, or 6.0 mg/kg; Fig. 1). The effect of PCP was most apparent at the intermediate prepulse intensity (+6 dB), reducing PPI response from ∼60% down to nearly zero. PCP-induced disruption of PPI in SR −/− mice was indistinguishable from that in WT littermates (Fig. 1a), with statistically significant main effects of prepulse intensity (F(2, 280)=309.2 p<0.0001) and PCP dose (F(3, 133)=24.1, p<0.0001). Likewise, the effects of PCP in the GCP2 −/+ mice paralleled those in WT littermates (Fig. 1b), with statistically significant main effects of prepulse intensity (F(2, 312)=236.3, p<0.0001) and PCP dose (F(3, 149)=16.2, p<0.0001).
Fig. 1.
PCP dose-dependent blockade of PPI is unaltered in SR −/− and GCP2 −/+ mice. Mean PPI (± SEM) is shown at each prepulse intensity level; 3, 6, or 12 dB over 70 dB background noise. a PPI in SR −/− (solid bars) and WT littermate (open bars) mice (n=16–19) following PCP treatment (0, 1, 3, or 6 mg/kg, s.c.). b PPI in GCP2 −/+ (solid bars) and WT littermate (open bars) mice (n=18–21) following PCP treatment
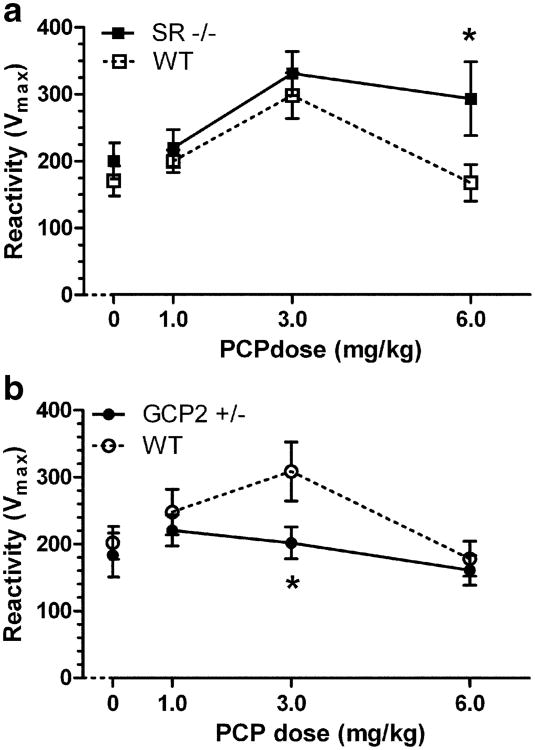
PCP not only influenced PPI of the startle reflex but had effects on startle reactivity itself. Consistent with previous findings (Swerdlow et al. 2004; Yee et al. 2004), WT littermates of either mutant strain displayed the same inverted U-shaped dose–response curve, with PCP causing a maximum enhancement of startle reactivity at the 3.0 mg/kg dose (Fig. 2; Table 1). PCP-enhanced startle stimulus response was greater in SR −/− mice than in their WT littermates (Fig. 2a). Two-way ANOVA revealed main effects of genotype (F(1, 143)=5.393, p<0.05) and PCP dose (F(3, 143)=6.262, p<0.01), and post hoc comparison indicated a significant difference in response between SR −/− and WT littermates at 6.0 mg/kg PCP. GCP2 −/+ mice displayed virtually no sensitivity to the startle-enhancing effects of PCP, thus exhibiting the opposite direction of effect to the SR mutants (Fig. 2b). Two-way ANOVA revealed main effects of genotype (F(1, 158)=4.063, p<0.05) and PCP dose (F(3, 158)=3.421, p<0.05), and post hoc comparisons indicated a significant difference between the genotypes in response at 3.0 mg/kg PCP dose. Notably, PCP caused a modest increase in baseline activity in all mouse strains, resulting in dose-dependent increases in reactivity scores that were unaffected by genotype (Table 1).
Fig. 2.
PCP dose-dependent effects on startle reactivity are differently altered in SR −/− and GCP2 −/+ mice. Mean startle reactivity (Vmax ± SEM) is shown at each PCP dose. a Increased reactivity to a 120 dB startle stimulus in SR −/− (solid squares) as compared to WT littermate (open squares) mice (n=18–19) following PCP treatment (0, 1, 3, or 6 mg/kg, s.c.). *p<0.05 indicates significant difference between WT and SR −/− at that dose of PCP (Bonferroni post hoc test). b Decreased reactivity to a 120-dB startle stimulus in GCP2 −/+ (solid circles) as compared to WT littermate (open circles) mice (n= 20–21) following PCP treatment. *p<0.05 indicates significant difference between WT and GCP2 −/+ at that dose of PCP (Bonferroni post hoc test)
Table 1. Psychotomimetic induced startle and baseline reactivity in SR −/− and GCP2 −/+ mice.
| Strain | PCP | AMPH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Dose (mg/kg) | Mutant | WT | Dose (mg/kg) | Mutant | WT | |||||
|
|
|
|
|
|||||||
| Reactivity (± SEM) | Reactivity (± SEM) | Reactivity (± SEM) | Reactivity (± SEM) | |||||||
|
|
|
|
|
|||||||
| Startle (120 dB) | Baseline | Startle (120 dB) | Baseline | Startle (120 dB) | Baseline | Startle (120 dB) | Baseline | |||
| SR −/− (n=18–19) | 0 | 201 (27) | 11 (2.1) | 171 (23) | 12 (2.5) | 0 | 172 (33) | 9 (2.0) | 194 (23) | 11 (2.8) |
| 1.0 | 220 (28) | 11 (2.4) | 200 (17) | 13 (2.5) | 1.0 | 136 (29) | 20 (4.5) | 103 (15) | 13 (2.5) | |
| 3.0 | 331 (38) | 34 (4.4) | 298 (34) | 21 (3.1) | 2.0 | 120 (26) | 21 (3.4) | 86 (17) | 26 (3.8) | |
| 6.0 | 294 (55)* | 28 (4.2) | 168 (27) | 33 (5.5) | 4.0 | 167 (26) | 39 (6.1) | 150 (29) | 48 (5.7) | |
| GCP2 −/+ (n=20–21) | 0 | 184 (33) | 16 (2.3) | 202 (23) | 11 (2.0) | 0 | 180 (28) | 13 (2.6) | 245 (45) | 15 (2.9) |
| 1.0 | 221 (23) | 21 (4.1) | 248 (34) | 11 (1.9) | 1.0 | 101 (19) | 21 (4.3) | 107 (14) | 24 (4.4) | |
| 3.0 | 202 (24)* | 33 (4.5) | 309 (44) | 28 (4.4) | 2.0 | 130 (26) | 37 (6.7) | 93 (17) | 35 (6.7) | |
| 6.0 | 161 (22) | 30 (4.4) | 178 (26) | 34 (4.6) | 4.0 | 115 (15) | 47 (5.6) | 111 (17) | 52 (5.3) | |
p<0.05 (significantly different than WT; Bonferroni post hoc t test)
AMPH disruption of PPI in SR −/− and GCP2 −/+ mice
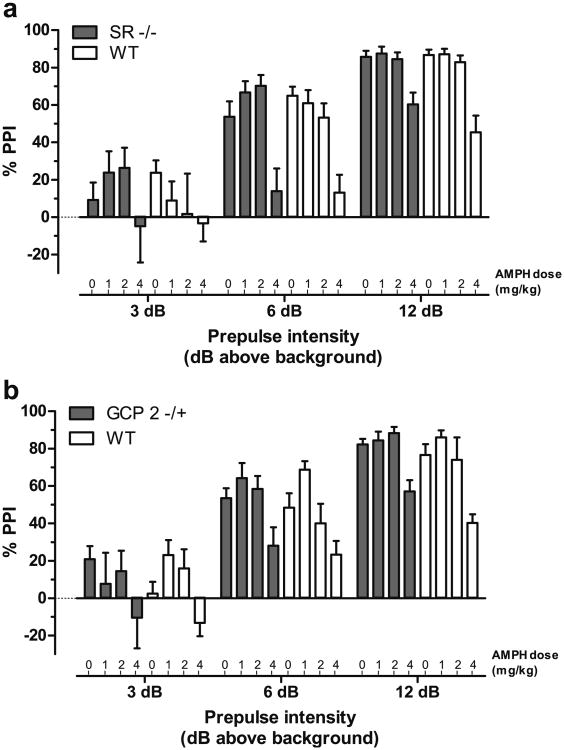
Both direct (apomorphine) and indirect (AMPH) dopamine agonists elicit reductions in PPI. In the present study, AMPH (1.0, 2.0, or 4.0 mg/kg) elicited dose-dependent reductions in PPI (Fig. 3). While the maximal efficacy achieved by AMPH was similar to that of PCP (compare AMPH at 4.0 mg/kg to PCP at 6.0 mg/kg), AMPH showed a much steeper dose–response curve, with the intermediate dose (2.0 mg/kg) showing minimal effect on PPI. PPI was virtually identical between SR −/− and WT littermate mice (Fig. 3a), with significant main effects of prepulse intensity (F(2, 222)=173.3, p<0.0001) and AMPH treatment (F(3, 104)=13.1, p<0.0001). AMPH disruption of PPI was similarly unaffected by genotype in the GCP2 −/+ and WT littermate mice. Statistical analysis confirmed the main effects of prepulse (F(2, 220)=227.7, p<0.0001) and AMPH treatment (F(3, 103)=10.7, p<0.0001).
Fig. 3.
AMPH dose-dependent blockade of PPI is unaltered in SR −/− and GCP2 −/+ mice. Mean PPI (± SEM) is shown at each prepulse intensity level; 3, 6, or 12 dB over 70 dB background noise. a PPI in SR −/− (solid bars) and WT littermate (open bars) mice (n=11–17) following AMPH treatment (0, 1, 2, or 46 mg/kg, s.c.). b PPI in GCP2 −/+ (solid bars) and WT littermate (open bars) mice (n=10–19) following AMPH treatment
AMPH also produced dose-dependent effects on startle reactivity and baseline activity, which were in opposite directions (Table 1). AMPH decreased startle reactivity and increased baseline activity. These effects confounded the assessment of PPI, causing numerous mice to be excluded from the PPI analysis because their startle reactivity levels when presented with the startle stimulus alone were not statistically distinguishable from their baseline activity levels in the testing apparatus. There were no significant differences between genotype in the proportion of animals excluded at each AMPH dose. Unlike the effect of PCP on startle reactivity, the effects of AMPH on startle reactivity and baseline activity occurred independently of genotype, as two-way ANOVA failed to detect a main effect of genotype for either mutant.
PCP-induced hyperactivity in SR −/− and GCP2 −/+ mice
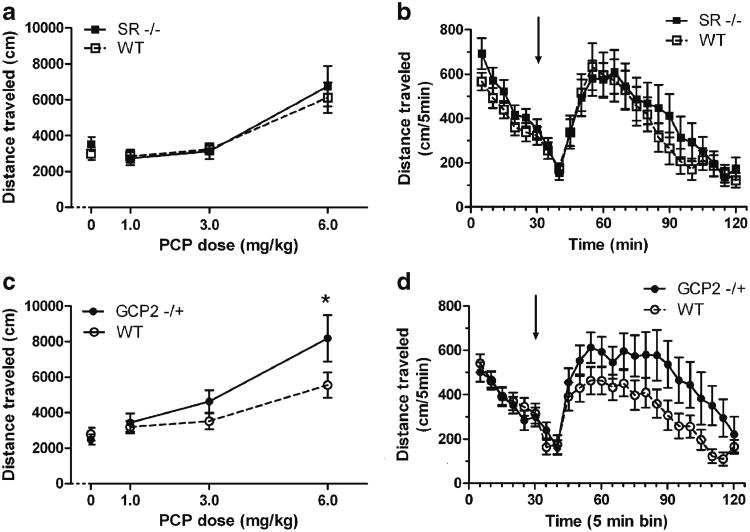
Hyperactivity, assessed in rodents as increases in horizontal locomotion, vertical movements such as rearing, and/or stereotypies are often the simplest measures in the study of the behavioral effects of psychotomimetic drugs. Subjects were given 30 min of exploration time in the test chamber prior to administration of PCP, for assessment of baseline activity and habituation. Consistent with our previous findings (Basu et al. 2009), SR −/− mice were significantly, but modestly, more active than WT littermates at baseline, with the largest differences in the first 15 min (data not shown). PCP caused dose-dependent increases in locomotor activity with similar potency in both SR −/− and WT littermate mice (Fig. 4a). Two-way ANOVA confirmed the main effect of drug dose (F(3, 143)=16.26, p<0.001). At the highest dose of PCP tested (6.0 mg/kg), the onset of action was rapid, reaching peak effect within 30 min, and waning by the end of the test session (Fig. 4b). This time course of efficacy (observed in preliminary studies) was used to dictate the pretreatment interval for PCP in the PPI experiments so as to measure disruption of PPI under optimal conditions (25–50 min post-injection).
Fig. 4.
Increased efficacy of PCP to induce hyperactivity in GCP2 −/+ mice, but not in SR −/− mice. Data depicted are total distance traveled (mean ± SEM) in 90 min following PCP (0, 1, 3, or 6 mg/kg, s.c.) treatment (a and c) or distance traveled (mean ± SEM) in each 5-min time bin throughout the 120-min test session before and after 6.0 mg/kg PCP treatment (b and d). a PCP dose-dependent induction of activity in SR −/− (solid squares) and WT littermate (open squares) mice (n=19). b Activity in SR −/− and WT mice before and after treatment with 6.0 mg/kg PCP. c PCP dose-dependent induction of activity in GCP2 −/+ (solid circles) and WT littermate (open circles) mice (n=21). d Activity in GCP2 −/+ and WT mice before and after treatment with 6.0 mg/kg PCP. Arrows indicate time of PCP treatment. *p<0.05 indicates significant difference between WT and GCP2 −/+ at that dose of PCP (Bonferroni post hoc test)
Unlike SR mutants, GCP2 −/+ mice displayed increased sensitivity to PCP-induced hyperlocomotion compared to WT littermates (Fig. 4c). Two-way ANOVA revealed main effects of PCP dose (F(3, 158)=16.1, p<0.001) and genotype (F(1, 158)=4.01, p<0.05), with post hoc analysis highlighting a significant difference between GCP2 −/+ and WT at 6.0 mg/kg PCP. The lack of significant statistical interaction between dose and genotype suggests that the data represent a vertical shift (increase in efficacy) rather than a rightward shift (increase in potency) in the dose– response curve for PCP. The locomotion induced by 6.0 mg/kg PCP in GCP2 −/+ and WT littermates showed that PCP-induced activity followed a similar time course in each group, with only the GCP2 −/+ showing more activity in each time bin (Fig. 4d). These data suggest that pharmacodynamic differences between the genotypes were probably not responsible for the activity difference when summarized over the 90-min period post-injection. The overlapping activity levels of the two genotypes during the 30-min test chamber habituation period suggest that the difference in drug-induced activity is not due to an idiosyncratic interaction between baseline activity and drug-induced activity. The overlap in baseline activity was consistently evident in all the drug treatment groups. In contrast, hyperactivity was observed in our previous work, but in those studies was measured in a 12-h session during the dark phase of the subjects’ circadian cycle (Han et al. 2009).
AMPH-induced hyperactivity in SR −/− and GCP2 −/+ mice
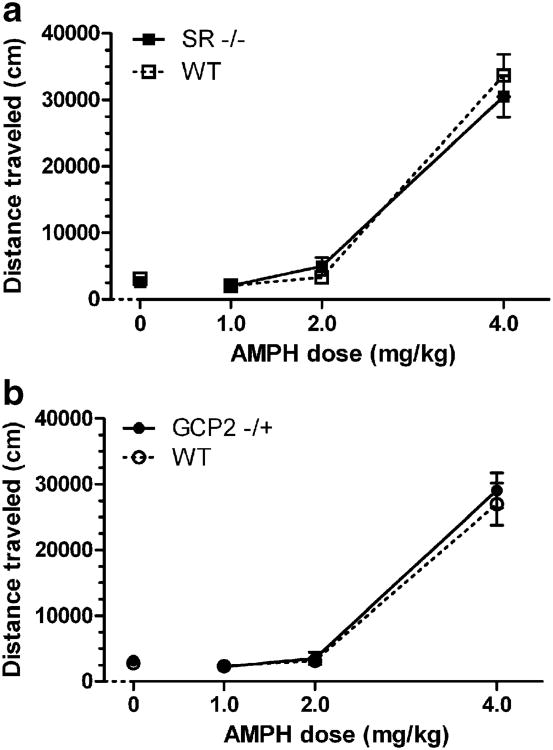
Psychomotor stimulants such as AMPH and cocaine are potent inducers of locomotor activity. As in the PPI experiment, activation of locomotor activity by AMPH followed steep dose–response curves that did not interact with genotype (Fig. 5). Marked hyperactivity was elicited at the highest dose (4.0 mg/kg) while minimal activity was apparent at the intermediate dose (2.0 mg/kg) in all groups of subjects. AMPH efficacy was overlapping in SR −/− and WT mice (Fig. 5a), with two-way ANOVA confirming only a main effect of AMPH dose (F(3, 128)=149.9, p<0.001). Likewise in the GCP2 −/+ mutants (Fig. 5b), statistical analysis of the dose–response data found only a main effect of AMPH dose (F(3, 160)=128.8, p<0.001).
Fig. 5.
Unaltered efficacy of AMPH to induce hyperactivity in SR −/− and GCP2 −/+ mice. Data depicted are total distance traveled (mean ± SEM) in the 90 min following AMPH (0, 1, 2, or 4 mg/kg, s.c.) treatment. a AMPH dose-dependent induction of activity in SR −/− (solid squares) and WT littermate (open squares) mice (n=16–17). b AMPH dose-dependent induction of activity in GCP2 −/+ (solid circles) and WT littermate (open circles) mice (n=20–21). Arrows indicate time of AMPH treatment
Behavioral profile of the GMS antagonist gavestinel
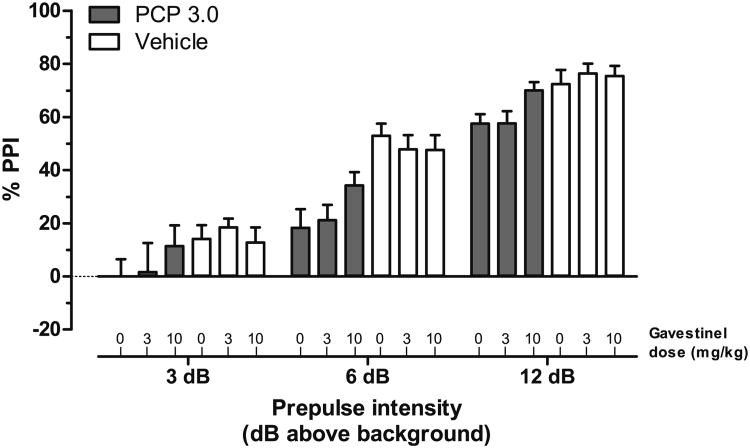
Gavestinel has been shown to be a selective and in vivo efficacious antagonist of the GMS (Di Fabio et al. 1997). We investigated behavioral interactions between gavestinel and PCP in light of the differing results obtained in the studies with SR −/− and GCP2 −/+ mice. In principle, acute gavestinel treatment was presumed to have a similar net effect to the GCP2 −/+ mutation, increased GMS antagonism. PCP (3.0 mg/kg, s.c.) disruption of PPI was blocked by the co-administration of gavestinel (vehicle, 3.0, or 10.0 mg/kg, i.p.; Fig. 6). A standard least squares mixed model of the PPI data showed significant main effects of prepulse intensity (F(2, 160)=261.9, p<0.0001) and PCP dose (F(1, 75)=15.2, p<0.001), with an interaction between PCP dose and gavestinel dose that approached statistical significance (F(2, 75)=2.7, p=0.075). Table 2 details the least squared means (determined from averaged PPI response under each drug treatment condition). Tukey HSD post hoc tests showed that only PCP treatment alone (group 4) or PCP in the presence of 3.0 mg/kg gavestinel (group 5) produced significant reduction in PPI response (p<0.05). Student's post hoc tests revealed a significant difference between the gavestinel (0)–PCP (3.0) and gavestinel (10.0)–PCP (3.0) treatment groups (group 4 versus 6; p<0.05). These post hoc tests confirm that there was a significant reversal of the PCP-induced disruption of PPI by the 10.0-mg/kg dose of gavestinel. The increased PPI (on average 13.2% PPI; Table 2) in the presence of this dose of gavestinel represents a 62.5% reversal of the total PCP-induced deficit. Gavestinel did not have any effect on PPI in the absence of PCP (Fig. 6). Analysis of startle reactivity measures showed that gavestinel resulted in lower mean PCP-enhanced startle reactivity, but this reduction was not statistically significant (data not shown). Unlike the effect of gavestinel on PCP disruption of PPI, gavestinel was found to have no effect on PCP-induced hyperactivity (data not shown).
Fig. 6.
Gavestinel reverses PCP-induced blockade of PPI, without affecting baseline PPI (vehicle treatment). Mean PPI (± SEM) is shown at each prepulse intensity level; 3, 6, or 12 dB over 70 dB background noise. The dose-dependent effects of gavestinel (0, 3, or 10 mg/kg, i.p.) were investigated in the presence of 3.0 mg/kg PCP (solid bars) or vehicle treatment (open bars) in WT mice (n=13–15)
Table 2. Reversal of PCP-induced disruption of PPI by gavestinel.
| Treatment group | Gavestinal (mg/kg) | PCP (mg/kg) | LSM | SEM |
|---|---|---|---|---|
| 1 | 0 | 0 | 46.438 | 4.699** |
| 2 | 3 | 0 | 47.515 | 4.375** |
| 3 | 10 | 0 | 40.748 | 4.699** |
| 4 | 0 | 3 | 25.285 | 4.528* |
| 5 | 3 | 3 | 26.809 | 4.699* |
| 6 | 10 | 3 | 38.516 | 4.699** |
LSM least squared mean, SEM standard error of the mean
p<0.05 (significantly different than control; group 1; Tukey HSD post hoc t test and Student's t test);
p<0.05 (significantly different than PCP treatment alone; group 4; Student's post hoc t test)
Discussion
Examination of changes in locomotor activity and sensorimotor gating is a common first-line analysis of behavioral phenotypes in animal models of schizophrenia (Crawley 2003). When alterations are apparent, further study aimed at the pharmacological reversal of these behavioral changes can provide insight into the molecular underpinnings of these behaviors and can aid in the preclinical stage of novel antipsychotic drug development. In many genetic animal model studies of psychiatric illnesses, baseline locomotor activity and/or sensorimotor gating have been minimally affected (van den Buuse 2010). One possible explanation, in light of the constitutive nature of many of these genetic manipulations, is that compensatory changes mask abnormalities in neuro-transmission at baseline, which become apparent only under challenged conditions. Pharmacological challenges with psychotropic agents such as AMPH and PCP may provide insight into alterations in dopaminergic and glutamatergic neurotransmission, respectively. In the present study, while many of the primary outcome measures of drug activity were unaffected by NMDAR hypofunction caused by genetic deletion of SR or GCP2, some subtle differences were observed. Most prominent were the alteration of PCP-induced activity in GCP2 −/+ mice and the differences in PCP-mediated enhancement of startle reactivity, with the SR −/− mice showing increased sensitivity to PCP and GCP2 −/+ mice displaying a lack of sensitivity to PCP by this measure. These findings suggest that while both of these mutants may achieve a common endpoint (i.e., NMDAR hypofunction), the different modes of achieving this effect (decreasing GMS agonism versus increasing GMS antagonism) likely have different consequences for neurotransmission. These consequences may be due to primary effects of the mutations as well as downstream/compensatory mechanisms. Furthermore, the SR −/− genotype results only in a d-serine loss without directly affecting the availability of the other GMS agonist, glycine, whereas elevated NAAG would block the effects of both d-serine and glycine at the GMS. An important confound of interpreting the GCP2 −/+ data in terms of GMS antagonism is the potential for NAAG to signal through mGlu3 receptors (Wroblewska et al. 1997). The lack of dramatically altered sensitivity to PCP and AMPH in the GCP2 mutant mouse is further confounded by our use of a heterozygous mutant. While the adult constitutive homozygous mutant is unobtainable due to embryonic lethality (Han et al. 2009), the heterozygous mutant may have suboptimal blockade of the GMS to observe effects. Physiological and neurochemical data are needed to elucidate the synaptic concentrations of NAAG and degree of NMDAR activity.
The relative lack of, or minimal effect of, SR and GCP2 mutations on PCP-induced activity or disruption of PPI is in contrast to findings using other genetically induced NMDAR alterations. The development of a NR1 hypomorphic allele (expresses <10% of WT NR1 levels) as well as conditional NR1 deletion mutants have proven useful tools for investigating NMDAR function, particularly in learning and memory (Mohn et al. 1999; Shimizu et al. 2000). The global NR1 hypomorphic mice are insensitive to both PCP and MK801 (Mohn et al. 1999), as are cell-type specific NR1 knockouts with NMDAR expression ablated in parvalbumin-positive GABAergic interneurons (Belforte et al. 2010). Observations of the NR1 mutants are consistent with the interpretation that removing the target for PCP eliminates its behavioral effect. Likewise, an NR1 point mutation that dramatically reduces affinity of agonists for the GMS results in insensitivity to the locomotor activating effects the NMDA blocker MK801 (Ballard et al. 2002). Perhaps comparison across models is complicated by differences in the severity and nature of the NMDAR hypofunction. The SR and GCP2 mutants indirectly affect NMDAR activity via availability of a subset of GMS ligands, as opposed to the NR1 hypomorphs which have a loss of the receptor. The point mutation in NR1 results directly in a loss of binding for all GMS ligands, in contrast to the SR and GCP2 mutants in which other endogenous ligands (glycine, d-aspartate, etc.) remain available to interact with the receptor. The complex regulation of NMDAR signaling, particularly GMS binding, contains redundancies that could compensate for the loss/gain of one component. These caveats make both the development of hypotheses and interpretation of results difficult and suggest that a nuanced approach should be taken when studying the effects of manipulations that are convergent upon NMDARs.
GMS activation has become a major area of interest for the development of therapeutic interventions in schizophrenia. A recent meta-analysis of existing clinical trial data demonstrated the efficacy of d-serine and the glycine transporter blocker sarcosine (which causes elevated extracellular glycine) to reduce symptoms in schizophrenia, particularly negative and cognitive symptoms (Tsai and Lin 2010). Also of note was the demonstration that both d-serine and sarcosine were more efficacious than the GMS partial agonist d-cycloserine (Tsai and Lin 2010). The clinical potentials of glycine and d-serine were initially supported by preclinical findings that demonstrated the ability of these compounds to reverse MK801/PCP-induced behaviors (Nilsson et al. 1997; Javitt et al. 1997). Recent studies expand on these findings indicating that glycine transporter subtype 1 (GlyT1) and DAAO blockers possess similar preclinical therapeutic profiles in their ability to reverse MK801/PCP-induced behavioral deficits (Hashimoto et al. 2009; Boulay et al. 2010). However, the present findings with gavestinel should give pause to the interpretation that inhibition of MK801/PCP-induced behaviors is predictive of therapeutic effects in schizophrenia. The observation that gavestinel reverses PCP-induced deficits in PPI suggests that modulation of the GMS site in either direction, antagonism or agonism, can disrupt the in vivo effects of activity-dependent NMDAR pore blockade. The present study failed to show an effect of gavestinel on PCP-induced hyperactivity although other GMS antagonists do reverse PCP-induced locomotion (Karcz-Kubicha et al. 1999; Bristow et al. 1993).
Excessive dopaminergic neurotransmission has been a core hypothesis of schizophrenia dating back to the observations that clinically efficacious antipsychotic therapies involve dopamine receptor antagonism and that AMPH produces a psychotic state (Snyder 1976). Imaging studies demonstrated increased dopaminergic neurotransmission in schizophrenia following an AMPH challenge that is correlated with baseline hyperdopaminergia (Laruelle et al. 1996; Abi-Dargham et al. 2009). The present study suggests that an impairment of NMDAR function at the GMS does not give rise to hypersensitive dopaminergic signaling, in contrast to the more severe receptor subunit-level NMDAR hypofunction models. The global NR1 hypomorphs display enhanced sensitivity to the PPI-disrupting effects of AMPH (Moy et al. 2006). Elevated striatal dopamine is observed in mice carrying the NR1 point mutation (Ballard et al. 2002). Consistent with the present findings is the demonstration that GMS activation with either glycine or a GlyT1 inhibitor fails to attenuate AMPH-induced hyperactivity (Javitt et al. 1997; Boulay et al. 2010). The GlyT1 inhibitor, SSR103800, also failed to reverse hyperactivity induced by a genetic deletion of the dopamine transporter, while reversing MK801-induced hyperactivity and the hyperactivity of a NR1 hypomorphic mouse (Boulay et al. 2010).
While the acute PCP model has face validity for the study of acute NMDAR hypofunction, the pharmacological validity of this model for determining the efficacy of therapeutic interventions targeted at the NMDAR is debatable. This caveat could likely extend to the investigation of other pharmacological targets that are predicted to directly affect NMDAR activity (e.g., mGlu5 receptor, Erb4, M1/M4 receptors), as opposed to interventions downstream in the circuitry (e.g., mGlu2/3 receptors). Characterizations of the behavioral impact of targeted mutations are inevitably confounded by compensatory changes, and such effects might explain the most discordant results that came from the two most mechanistically comparable manipulations, GCP2 −/+ and gavestinel treatment; both increase GMS antagonism yet produce opposing effects on PCP-induced behavior. Nonetheless, compensatory changes in response to NMDAR hypofunction may be informative of the underlying pathophysiology of schizophrenia.
Acknowledgments
This research was supported by NIH Grants MH05129 (JTC) and P50 MH060450 (JTC) and the Andrew P. Merrill Memorial Research Fellowship (MAB). JTC holds a patent on the use of d-serine to treat serious mental illness that is owned by the Massachusetts General Hospital but could yield royalties. We thank Jonathan Picker and William Carlezon for the use of behavioral testing equipment. We thank Jiamin Feng for genotyping and maintaining our animal colony.
Contributor Information
Michael A. Benneyworth, Laboratory for Psychiatric and Molecular Neuroscience, McLean Hospital, 115 Mill St., Belmont, MA 02478, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA
Alo C. Basu, Laboratory for Psychiatric and Molecular Neuroscience, McLean Hospital, 115 Mill St., Belmont, MA 02478, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA
Joseph T. Coyle, Email: joseph_coyle@hms.harvard.edu, Laboratory for Psychiatric and Molecular Neuroscience, McLean Hospital, 115 Mill St., Belmont, MA 02478, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA.
References
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naïve schizophrenic subjects. Biol Psychiatry. 2009;65(12):1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE, Jr, Jones EG. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16(1):19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40(7):827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Ballard TM, Pauly-Evers M, Higgins GA, Ouagazzal AM, Mutel V, Borroni E, Kemp JA, Bluethmann H, Kew JN. Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. J Neurosci. 2002;22(15):6713–6723. doi: 10.1523/JNEUROSCI.22-15-06713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R, Coyle JT. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2009;14(7):719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33(9):2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Berger UV, Luthi-Carter R, Passani LA, Elkabes S, Black I, Konradi C, Coyle JT. Glutamate carboxypeptidase II is expressed by astrocytes in the adult rat nervous system. J Comp Neurol. 1999;415(1):52–64. doi: 10.1002/(sici)1096-9861(19991206)415:1<52::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Coyle JT, Tsai G, Greene RW. NAAG reduces NMDA receptor current in CA1 hippocampal pyramidal neurons of acute slices and dissociated neurons. Neuropsychopharmacology. 2005;30(1):7–16. doi: 10.1038/sj.npp.1300559. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Imamura Y, Frangioni JV, Greene RW, Coyle JT. Endogenous N-acetylaspartylglutamate reduced NMDA receptor-dependent current neurotransmission in the CA1 area of the hippocampus. J Neurochem. 2007;100(2):346–357. doi: 10.1111/j.1471-4159.2006.04253.x. [DOI] [PubMed] [Google Scholar]
- Boulay D, Bergis O, Avenet P, Griebel G. The glycine transporter-1 inhibitor SSR103800 displays a selective and specific antipsychotic-like profile in normal and transgenic mice. Neuropsychopharmacology. 2010;35(2):416–427. doi: 10.1038/npp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow LJ, Hutson PH, Thorn L, Tricklebank MD. The glycine/NMDA receptor antagonist, R-(+)-HA-966, blocks activation of the mesolimbic dopaminergic system induced by phencyclidine and dizocilpine (MK-801) in rodents. Br J Pharmacol. 1993;108(4):1156–1163. doi: 10.1111/j.1476-5381.1993.tb13520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3(5):241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of rodents. Comp Med. 2003;53(2):140–146. [PubMed] [Google Scholar]
- Di Fabio R, Capelli AM, Conti N, Cugola A, Donati D, Feriani A, Gastaldi P, Gaviraghi G, Hewkin CT, Micheli F, Missio A, Mugnaini M, Pecunioso A, Quaglia AM, Ratti E, Rossi L, Tedesco G, Trist DG, Reggiani A. Substituted indole-2-carboxylates as in vivo potent antagonists acting as the strychnine-insensitive glycine binding site. J Med Chem. 1997;40(6):841–850. doi: 10.1021/jm960644a. [DOI] [PubMed] [Google Scholar]
- Han L, Picker JD, Schaevitz LR, Tsai G, Feng J, Jiang Z, Chu HC, Basu AC, Berger-Sweeney J, Coyle JT. Phenotypic characterization of mice heterozygous for a null mutation of glutamate carboxypeptidase II. Synapse. 2009;63(8):625–635. doi: 10.1002/syn.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Oka T, Takahashi K. Endogenous D-serine in rat brain: N-methyl-D-aspartate receptor-related distribution and aging. J Neurochem. 1993;60(2):783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Horio M, Kunitachi S, Iyo M, Ferraris D, Tsukamoto T. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol Psychiatry. 2009;65(12):1103–1106. doi: 10.1016/j.biopsych.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Sershen H, Hashim A, Lajtha A. Reversal of phencyclidine-induced hyperactivity by glycine and the glycine uptake inhibitor glycyldodecylamide. Neuropsychopharmacology. 1997;17(3):202–204. doi: 10.1016/S0893-133X(97)00047-X. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Wedzony K, Zajaczkowski W, Danysz W. NMDA receptor antagonists acting at the glycineB site in rat models for antipsychotic-like activity. J Neural Transm. 1999;106(11– 12):1189–1204. doi: 10.1007/s007020050233. [DOI] [PubMed] [Google Scholar]
- Kew JN, Koester A, Moreau JL, Jenck F, Ouagazzal AM, Mutel V, Richards JG, Trube G, Fischer G, Montkowski A, Hundt W, Reinscheid RK, Pauly-Evers M, Kemp JA, Bluethmann H. Functional consequences of reduction in NMDA receptor glycine affinity in mice carrying targeted point mutations in the glycine binding site. J Neurosci. 2000;20(11):403. doi: 10.1523/JNEUROSCI.20-11-04037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11(8):737–747. 705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendo-crine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169(3–4):215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18(8):2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, He L. Association study between the NMDA receptor 2B subunit gene (GRIN2B) and schizophrenia: a HuGE review and meta-analysis. Genet Med. 2007a;9(1):4–8. doi: 10.1097/01.gim.0000250507.96760.4b. [DOI] [PubMed] [Google Scholar]
- Li D, He L. G72/G30 genes and schizophrenia: a systematic meta-analysis of association studies. Genetics. 2007b;175(2):917–922. doi: 10.1534/genetics.106.061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K. Functional comparison of D-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J Neurochem. 1995;65(1):454–458. doi: 10.1046/j.1471-4159.1995.65010454.x. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98(4):427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-Serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000;97(9):4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Perez A, Koller BH, Duncan GE. Amphetamine-induced disruption of prepulse inhibition in mice with reduced NMDA receptor function. Brain Res. 2006;1089(1):186–194. doi: 10.1016/j.brainres.2006.03.073. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academy Press; 2003. [PubMed] [Google Scholar]
- Nilsson M, Carlsson A, Carlsson ML. Glycine and D-serine decrease MK-801-induced hyperactivity in mice. J Neural Transm. 1997;104(11–12):1195–1205. doi: 10.1007/BF01294720. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Blakely RD, Couto R, Coyle JT. Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate. Identification and characterization of a novel N-acetylated alpha-linked acidic dipeptidase activity from rat brain. J Biol Chem. 1987;262(30):14498–14506. [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-Serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92(9):3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290(5494):1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. Am J Psychiatry. 1976;133(2):197–202. doi: 10.1176/ajp.133.2.197. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Crain S, Goins J, Onozuka K, Auerbach PP. Sensitivity to drug effects on prepulse inhibition in inbred and outbred rat strains. Pharmacol Biochem Behav. 2004;77(2):291–302. doi: 10.1016/j.pbb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199(3):331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36(2):246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull. 2001;56(5):495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA. 1999;96(23):13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-Acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69(1):174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- Yee BK, Chang DL, Feldon J. The effects of dizocilpine and phencyclidine on prepulse inhibition of the acoustic startle reflex and on prepulse-elicited reactivity in C57BL6 mice. Neuropsychopharmacology. 2004;29(10):1865–1877. doi: 10.1038/sj.npp.1300480. [DOI] [PubMed] [Google Scholar]