Abstract
Chitosan (CHI), PCEP (poly{[(cholesteryl oxocarbonylamido ethyl) methyl bis(ethylene) ammonium iodide] ethyl phosphate}), and magnetic (MNP) nanoparticles were evaluated for the safe delivery of genes in the eye. Rabbits were injected with nanoparticles either intra-vitreally (IV) or sub-retinally (SR) and sacrificed 7 days later. Eyes were grossly evaluated for retinal pigment epithelium (RPE) abnormalities, retinal degeneration, and inflammation. All eyes were cryopreserved and sectioned for analysis of toxicity and expression of either enhanced green (GFP) or red (DsRed) fluorescent proteins. All of the nanoparticles were able to transfect cells in vitro and in vivo. IV chitosan showed inflammation in 12/13 eyes while IV PCEP and IV MNP were not inflammatory and did not induce retinal pathology. SR PCEP was nontoxic in the majority of cases but yielded poor transfection while SR MNPs were nontoxic and yielded good transfection. Therefore, we conclude that the best nanoparticle evaluated in vivo was the least toxic nanoparticle tested, the MNP.
Keywords: chitosan, magnetic nanoparticle, gene delivery, retina, toxicity
INTRODUCTION
Nanoparticle-mediated gene delivery is an alternative to viral gene delivery. Nanoparticles offer the potential for safe, targeted, and efficient gene delivery in a variety of organs. Before this potential can be realized, basic studies evaluating toxicity and transfection efficiency need to be done in vivo. This study evaluated three different nanoparticles (chitosan, PCEP, and magnetic nanoparticles) for gene delivery and toxicity in the eye.
The eye is useful for this type of study because there are multiple tissue types in a confined structure that can be easily accessed. The retina is extremely sensitive to inflammation and toxic materials. Also, the eye has the potential to be non-invasively imaged and thereby followed over time. There are a multitude of ocular diseases that have the potential to be treated by the nanoparticles used in this study. Diseases of the retina like diabetic retinopathy and macular edema can be treated by injecting therapeutic agents into the vitreous gel in the chamber between the lens and the retina, which is called an intra-vitreal injection (IV). This route is currently being used with anti-VEGF reagents for diabetic macular edema and age-related macular degeneration (AMD) [1, 2]. Another route of delivery for AMD is to inject substances under the retina or sub-retinally (SR), since AMD involves photoreceptors in posterior retina, retinal pigment epithelial cells that separate retina and choroid, and the choroidal vasculature from which neovascularization will emanate in wet or exudative AMD.
Only one of the three nanoparticles used in this study, chitosan, has a history of ocular use. Chitosan is a deacetylated form of chitin, which is the second most abundant polymer in nature after cellulose. Chitosan has been reported to be a useful controlled release agent for topical drug delivery in the eye [3]. There have also been several reports of chitosan nanoparticles being successfully used to deliver genes in systems other than the eye like liver and gut [4–8]. Chitosan has also been used to deliver genes to multiple cell types in vitro [9, 10], including retinal endothelial cells (unpublished results). All of these studies provide evidence that chitosan is a promising gene/drug delivery agent. Indeed chitosan appears to be a relatively non-toxic material that is useful in delivering both drugs and genes to multiple sites including topical eye treatment.
Another promising material for in vivo gene delivery is poly{[(cholesteryl oxocarbonylamido ethyl) methyl bis(ethylene) ammonium iodide] ethyl phosphate} (PCEP). Like chitosan, it is a DNA condensing agent. This biodegradable polymer has been shown to be able to deliver genes to muscle and intestinal mucosa in vivo [11]. Although this polymer has promise, it is relatively new and nothing is known about its tolerance or application in the eye. However, the results reported for this material to date show that it has potential for gene delivery [11].
Magnetic nanoparticles (MNP) could also be useful for gene delivery in the eye. Although largely untested in the eye, these nanoparticles are derived from an FDA approved magnetic resonance imaging (MRI) contrast agent that is being used in clinics around the world [12]. Previous in vitro studies have shown that these DNA-tethered nanoparticles are capable of efficient and non-toxic gene delivery [13, 14]. Additionally, the presence of these nanoparticles can be detected by basic histochemical techniques employed in this study. These nanoparticles are unique in this study, because Chitosan and PCEP is DNA condensing agents, whereas the MNPs have the DNA tethered to a magnetic core. The MNP cores are expected to be non-toxic and stable due to their component materials, as shown by years of use as a contrast agent, and our results on endothelial cells in vitro [13].
From our prior in vitro studies with endothelial cells, Chitosan, PCEP, and MNP are promising gene delivery agents (unpublished results). However, the location of their use in the body needs to be carefully considered. Chitosan has been successfully used in a variety of organ systems in previous studies including the surface of the eye, but this molecule had never been used in neuronal tissue like the retina. Our data shows that the use of chitosan within the eye may not be feasible. The following experiments show that this natural polymer, or its contaminants, can induce a massive immune response when injected into the eye. Neither PCEP nor MNP-induced a significant immune response. The MNP was the only nanoparticle that showed noteworthy transfection. MNP transfection was seen in individual cells in vitreous that may be hyalocytes, the dendritic cells of the vitreous, when the MNP were injected in the vitreous. Sub-retinal injection lead to a variety of transfected cells including, but not limited to, retinal pigment epithelium (RPE). Immuno-transmission electron microscopy (immuno-TEM) was used to locate MNP in RPE and photoreceptors. In RPE, MNP were seen primarily in the cytosol, but some were within the nuclei and RPE reporter gene expression was observed. Overall, PCEP and the MNP were the best tolerated intraocular nanoparticles, while the MNP was the only nanoparticle capable of efficient transfection in the eye.
MATERIALS AND METHODS
Animals, surgery, and tissue preparation
New Zealand white and Dutch Belted rabbits were purchased from Covance, Inc. (Cumberland, VA). Rabbits were 6 to 8 months of age at the time of injection. All procedures were performed in accordance with the ARVO statement for use of animals in ophthalmologic and vision research and after approval by the Johns Hopkins IACUC. New Zealand white rabbits were used for intra-vitreal injections and Dutch Belted rabbits for sub-retinal injections. Prior to injection, the animals were anesthetized with a 1:1 dilution of 20 mg/ml xylazine and 100 mg/ml ketamine (Phoenix Scientific, Inc. St. Louis, MO, US). For surgery, the pupils were dilated with 1% Tropicamide and 2.5% phenylephrine HCl (Akorn, Inc. Somerset, NJ, US) and 0.5% Tetracaine HCl (Phoenix Pharmaceutical, Inc., Burlingame, CA, US) was applied for topical anesthesia. Once anesthetized, 50 ul of vehicle (sterile phosphate buffered saline, PBS), plasmid DNA, or nanoparticles were injected through a 30-gauge needle into the vitreous space near the optic nerve head. Sub-retinal injections were done using a 1 cc syringe with a blunt, plastic, 30 gauge tube attached. Once the retina was penetrated, 50 ul was injected slowly resulting in a bleb-shaped elevation in retina. Sub-retinal injections were given 2 disk diameters inferior to the optic nerve. For both kinds of injections, the eyes were entered at the pars plana near the ora seratta. Bacitracin Zinc and Polymyxin B Sulfate ophthalmic ointment (Akorn, Inc. Somerset, NJ, US) was applied to both eyes immediately after surgery. The animals were sacrificed seven days after intra-vitreal or sub-retinal injection by a lethal intra-venous injection of 1 ml Beuthanasia (Schering-Plough, Inc., Kenilworth, NJ, US). In this initial study of these three nanoparticles used for the first time intra-ocularly, we chose to evaluate the eyes seven days after injection. Seven days was felt to be sufficient time for uptake of nanoparticles, transfection, and long enough to suggest if there were toxic effects from the nanoparticle or that expression was prolonged enough to suggest that there might be acute therapeutic efficacy with a certain nanoparticle strategy.
The eyes were then enucleated, injected with 1 ml of 2% paraformaldehyde in 0.1 M phosphate buffer and placed in 30 ml of 2% paraformaldehyde in 0.1 M phosphate buffer for one hour. After fixation, the anterior segment was removed, and gross examination and photography was done on the anterior segment and the posterior eye cup. The vitreous was examined for haze, usually a sign of mild inflammation or precipitation of material, and for membranous opacity, which can indicate severe inflammation or formation of a proliferative vitreoretinal membrane. The retina was examined for traction from a membrane, folds or tears, or thickening or thinning of the retina, which would suggest edema or retinal degeneration respectively.
After photographic documentation and gross examination, the eyes were cryopreserved. The eyes were cryopreserved with an increasing sucrose gradient and frozen in Tissue-Tek OCT (Miles, Elkart, Indiana) and 20% sucrose (1:2) as previously reported [15]. Cryoblocks were sectioned in areas with gross changes and normal appearing areas for each eye and, in sub-retinally injected eyes, we attempted to serial section through the injection site and also in areas outside the bleb. At least 10 slides from each area of each block were taken: 3 of each were evaluated for histopathological changes and the rest used for histochemistry and immunohistochemistry
Nanoparticle synthesis
Chitosan (Biomedical grade, Protosan™ CL 113, MW 110 kDa, deacetylation degree 87%) was purchased from Pronova Biomedical (Oslo, Norway). Chitosan/DNA nanoparticles were made as described previously [16]. Briefly, a stock solution of 0.02% chitosan was prepared by dissolving the chitosan in 5 mM sodium acetate buffer, pH 5.5. The chitosan stock solution was sterile filtered before use. The chitosan stock and a plasmid DNA solution, pEGFP (BD Biosciences, San Diego, CA), of 100 ug/ml in 25 mM of sodium sulfate solution were preheated to 50–55°C separately. An equal volume of both solutions were quickly mixed together and vortexed for 15–30 s. The final volume was less than 500 ul and was diluted to 1 ug DNA/50 ul of solution.
PCEP and PCEP/DNA complexes were synthesized as described previously [11]. Briefly, an emulsion of Opti-MEM (Invitrogen, Carlsbad, CA) and PCEP in chloroform was prepared by sonication at 50 watts for 1 minute. Plasmid DNA, pEGFP (BD) at 0.05g/L (w/v), was added after the chloroform was evaporated. The complexes were formed during the 20-minute incubation at room temperature.
Magnetic nanoparticles were made as previously described [13]. Streptavidin-coated magnetic nanoparticles were purchased from Miltenyi Biotech, Auburn, CA. Biotin-labeled transcriptionally active PCR products (TAPS) were generated from pDsRed-C1 (BD) and contained the full CMV promoter, DsRed coding sequence, and poly A signal. Ten micrograms of biotin-labeled TAPS in PBS, pH 7.4, was mixed with streptavidin-coated nanoparticles and incubated at room temperature for 1 hour. After binding, magnetic columns were used to remove unbound TAPS, concentrate the MNP, and then rinse them with PBS, pH 7.4. The resulting MNP were then diluted to 1 ug/50 ul in PBS, ph 7.4.
Immunohistochemistry
Cryopreserved eyes were sectioned (8 um) on a Microm cryotome (Microm International GmbH, Walldorf, Germany). The sections were then fixed with 100% methanol for 5 minutes and air dried for 10 minutes. After drying, the sections were stained with mouse anti-GFP (BD Biosciences) at 1:1000 in TBS with 1% BSA for 2 hours at room temperature. After washing in TBS three times for 10 minutes each, the secondary antibody, biotin-labeled goat anti-mouse [1:500 in TBS with 1% BSA; Kirkegaard and Perry Laboratories (KPL), Gaithersburg, MD], was added and the sections incubated for 30 minutes at room temperature. The sections were then washed three times for ten minutes each in TBS prior to the addition of streptavidin-labeled alkaline phosphatase (1:500 in TBS with 1% BSA; KPL) for 30 minutes at room temperature. The slides were then washed three times with TBS. Alkaline phosphatase was developed with the NBT/BCIP kit according to the manufacturer’s instructions (Vector Laboratories, Inc Burlingame, CA) and levamisole was included in the incubation solution to prevent endogenous APase from being detected. The slides were then washed three times in TBS prior to bleaching. The pigment in RPE and choroidal melanocytes was bleached as described previously [17]. This was necessary because RPE cells are a target for transfection after sub-retinal injection. After bleaching, the slides were washed in deinonized (DI) water and mounted in Kaiser’s glycerol gel. The above procedure was used for all vehicle, pEGFP, Chitosan, and PCEP-treated eyes. Eyes injected with Adenovirus-GFP were used as positive controls. The same procedure was used to label streptavidin on the MNP, except that the anti-GFP primary was not used; instead, biotinylated APase was used. The DsRed construct was used on MNPs and the only antibody for DsRed was made in rabbit, so immunolocalization could not be used for DsRed expression. Additionally, 8 um cryosections were stained with hematoxylin and eosin for histopathologic analysis.
Immuno-Transmission Electron Microscopy
Slides with two 8 um cryosections, were immunolabeled as described above except that streptavidin-labeled 10 nm colloidal gold (1:10 in TBS, Sigma, St. Louis, MO) was used in place of the streptavidin-labeled alkaline phosphatase. After washing to remove unbound gold, the tissue was fixed for one hour in 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M cacodylate at 4°C. The sections were then dehydrated in increasing concentrations of ethanol and finally treated with 1% uranyl acetate in absolute ethanol. The sections were then washed with propylene oxide and then infiltrated with a 1:1 mixture of propylene oxide and resin (LX112, Ladd, Williston, VT) followed by 100% LX112 resin for 4–5 hours under vacuum. A resin filled Beam capsule was inverted on top of the tissue section. The resin was allowed to polymerize for 48 hours at 60°C. The polymerized resin was scored with a razor blade around the Beam capsule prior to being immersed in boiling water for 1–2 minutes to allow removal of the beam capsule. The free beam capsule contained the tissue section and was hand trimmed immediately before sectioning. Thin sections were cut at 65–70 nm thick and analyzed on a Hitachi H7600 TEM.
Confocal Microscopy
Cryosections were fixed in methanol as described above and then mounted in VectaShield media with or without DAPI (Vector Labs). The sections were then viewed with a Zeiss 510META (Carl Zeiss, Inc. Thornwood, NY). DsRed was excited with a 543 nm emitting laser and emission detected with a 560 long pass filter. Determination of GFP expression by fluorescence analysis was hindered by the overlapping GFP and autofluorescence spectra. So spectral separation was accomplished with the Zeiss LSM software. For spectral scanning microscopy, the excitation wavelength remained the same as above but emission images were taken from 450–700 nm.
RESULTS
Post-mortem gross evaluations
Post-mortem evaluations (Figure 1) were conducted on all eyes (Table 1). All abnormalities were noted in eye cups (i.e. posterior eye) and not in the anterior segments. Figure 1 shows data that represents both intra-vitreal (A) and sub-retinal (B) gross characteristics. Several characteristics were evaluated on the gross specimens. These characteristics can be divided into several broad categories including signs of inflammation (hazy vitreous, fibrosis, and membranous opacity), physical injury from the surgery (hemorrhage, retinal folding, retinal traction, and retinal tear), and toxicity (retinal degeneration, RPE changes, and thickened retina). The vehicle and DNA alone groups had very few abnormalities in all categories. Most of the chitosan eyes showed signs of inflammation (Figure 1A). The severe vitreous haze and membranous opacities that were observed in chitosan-injected eyes were often associated with inflammation. Because of this, chitosan was not evaluated sub-retinally. The PCEP and MNP-treated eyes did not exhibit signs of inflammation whether injected IV or SR. Hemorrhage was observed in 15–30% of the total eyes used probably because of the entry site at ora seratta, since the needle must traverse the choroidal vasculature before it enters the vitreous cavity. These data show that at the gross level, chitosan appears to induce an obvious immune response, while PCEP and MNP does not. In fact, there were very few signs of toxicity or inflammation in PCEP or MNP injected eyes at the macroscopic level.
Figure 1.
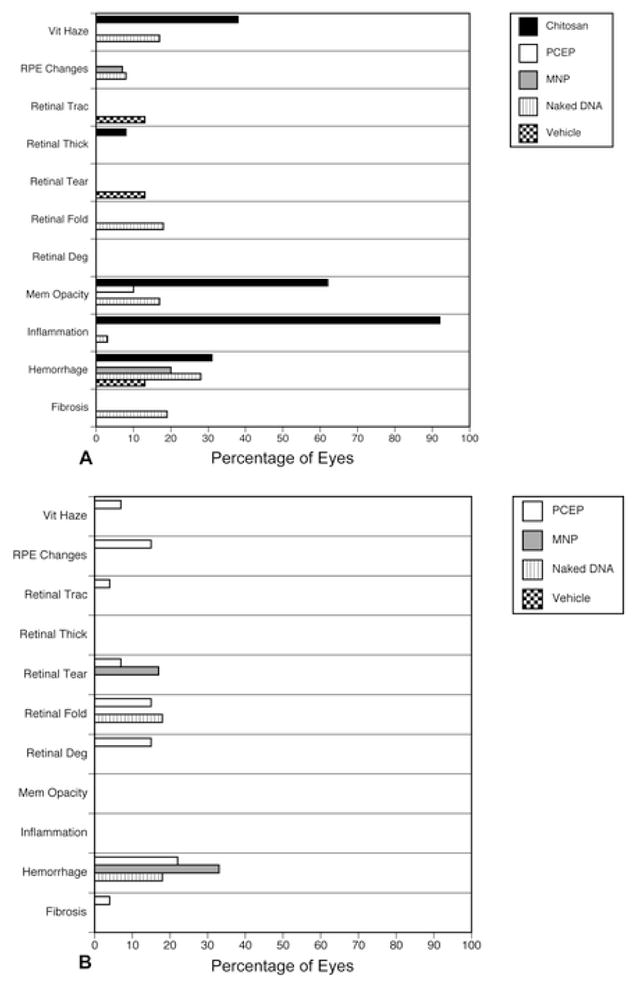
Gross post-mortem evaluations were done on all eyes for signs of toxicity, inflammation, and physical trauma. The x-axis represents the percentage of all eyes where the characteristic was noted. (A) Results of gross observations of eyes injected via the intra-vitreal route. (B) Characteristics noted in posterior eyes injected by the sub-retinal method. Inflammation refers to apparent inflammation anywhere in the eye whereas haze and membranous opacity suggest inflammatory infiltrates in vitreous.
Table 1.
Number of Eyes and Route of Administration
| Group | Intravitreal | Subretinal | Total |
|---|---|---|---|
| Vehicle | 8 | 4 | 12 |
| DNA | 36 | 11 | 47 |
| Chitosan | 13 | 0 | 13 |
| PCEP | 4 | 27 | 31 |
| MNP | 15 | 6 | 21 |
Microscopic evaluation of nanoparticle-treated eyes
Injection of 50 ul of PBS into vitreous caused no gross or histological changes in retina or vitreous (Figure 2A–B). One significant finding of this study was that chitosan nanoparticles appeared to consistently induce an immune response when injected into vitreous. This gross finding was supported by microscopic evidence of a severe cellular infiltrate (Figure 2C–E). White blood cells (WBCs), mostly monocytes (Figure 2E), were found in large numbers within the vitreous diffusely and in large clumps or mats (Figure 2D), representing vitreous haze and membranous opacity observed grossly, respectively. In the retina, the monocytes were both perivascular and located within retinal blood vessels. The infiltrating cells appear to have been attracted to the injected chitosan and the phagocytic cells may have been attempting to clear the nanoparticles from the vitreous. There is a dendritic cell of monocytic origin in the vitreous called a hyalocyte that clears foreign particles from vitreous, but the number of hyalocytes in vitreous is very small compared to the number of monocytes observed in chitosan-injected eyes (Figure 2C–E). The majority of hyalocytes normally reside in vitreous between the ciliary processes. The retinas and RPE cells were also evaluated histopathological changes. There was some retinal degeneration evident in chitosan-treated eyes, especially at sites of severe inflammation. Sub-retinal injections with chitosan were not pursued due to the inflammation observed in eyes injected with intra-vitreal chitosan. Neither PCEP (Figure 3) nor the MNP (Figure 4) induced WBC infiltrate whether injected IV or SR. Some PCEP-treated eyes (15%, Figure 1) had RPE changes (pigmentary changes) after SR injection (Figure 3C). PCEP also caused retinal degeneration in 15% of the eyes (Figure 1) when injected SR (Figure 3C–D, F). The MNP-treated eyes were relatively normal; however, occasional areas with vacuolated RPE were observed in sub-retinal injected eyes (Figure 4A). Overall, the MNP was the least toxic nanoparticle in this study.
Figure 2.
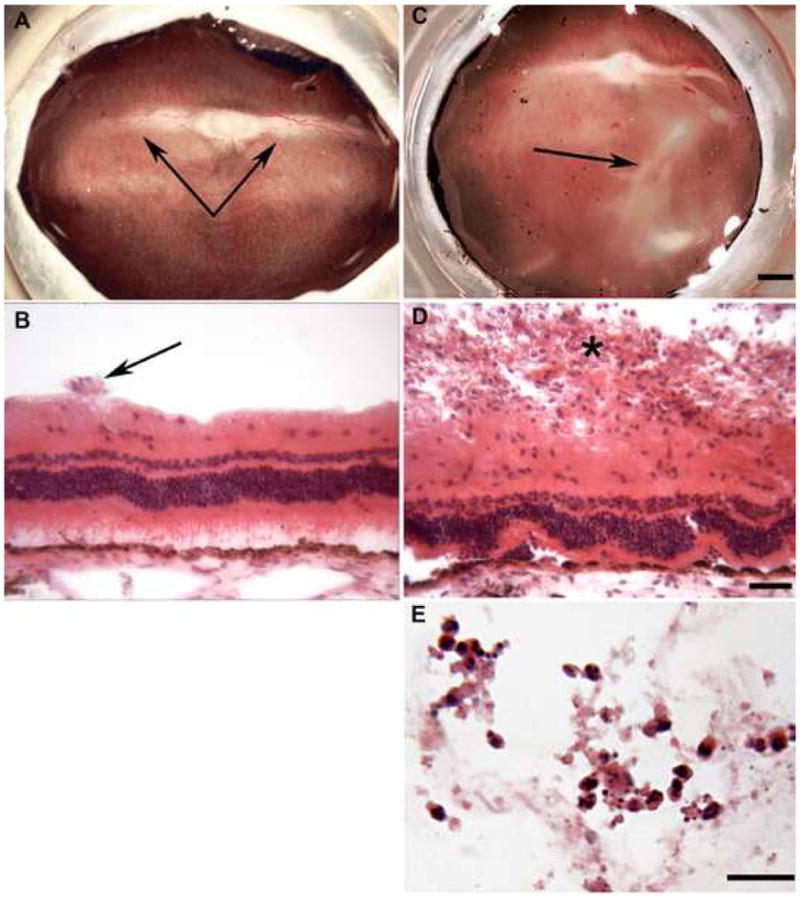
Gross photographs of eyecups (A, B) and cryosections (B, D, E) from eyes injected intra-vitreally with PBS (A, B) or chitosan nanoparticles (C, D, E). (A) The rabbit retina has blood vessels only in the medullary rays (double arrow) that emanate from the optic nerve head while the rest of retina is avascular. (B) The retinal blood vessels are on the surface of the retina (arrow) and the nuclei of neurons are in three layers: monolayer of ganglion cells inner most, the inner nuclear layer in the middle, and the outer nuclear layer, which contains the nuclei of the photoreceptors. Chitosan-induced inflammation was characterized by a large white blood cell infiltrate in the vitreous, which has the appearance of a membranous opacity in the gross photograph (Arrow in C). Inflammatory cells were apparent on the surface of the retina in cryosections (Panel D, asterisk, and E). (Bars indicate: 1 mm in Panel A and B; 20 um in Panels B and D; 50 um in Panel E. B, D, and E are hematoxylin and eosin stained sections.)
Figure 3.
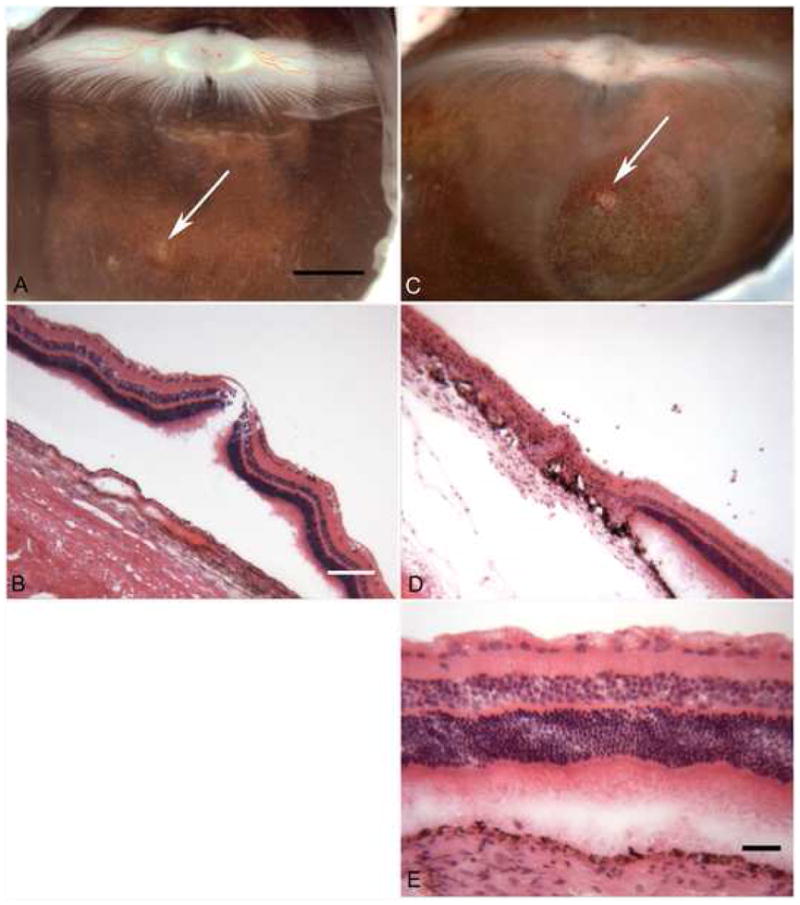
Sub-retinal injection of PBS (A, B) and PCEP (C, D, and E). (A) The sub-retinal injection of PBS caused no apparent injury in the gross photograph ((arrow indicates injection site). (B) A cryosection through the eye at the PBS injection site shows normal lamination of retina and an intact RPE monolayer under retina. The retina became artifactually detached during cryopreservation. The injection of PCEP sub-retinally induced ocular pathology in 15% of the eyes. (C) In the gross photograph of an eye injected with PCEP via a sub-retinal route demonstrates slight hypopigmentation (light areas) at the injection site (arrow). PCEP caused retina degeneration or thinning of retina in the circular bleb area. (D) Retina within the bleb site is thin and degenerated (loss of normal lamination). (E) Outside of the bleb site, the retina appears normal. [Bars indicate: 2 mm in Panels A and C; 20 um in Panels B and D; 40 um in Panel E. Panels B, D, and E are hematoxylin and eosin (H&E) stained sections.]
Figure 4.
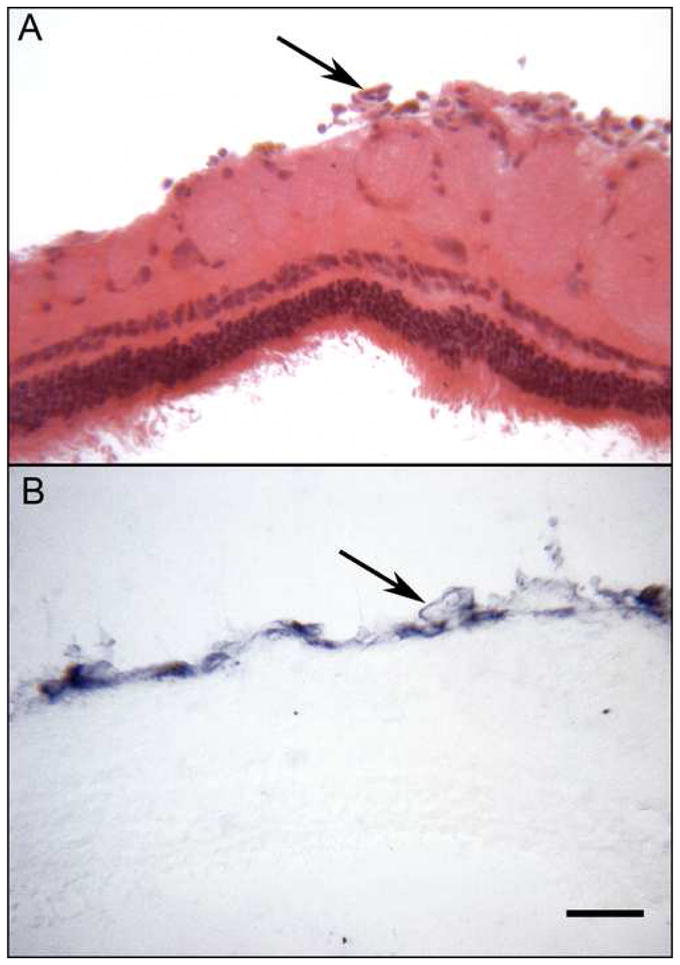
Magnetic nanoparticles were delivered via intra-vitreal injection (Panels A-B). (A) Hematoxylin and eosin (H&E) stained section demonstrates blood vessels on the surface of the retina (arrow). Histochemistry using biotinylated alkaline phosphatase shows blue purple reaction product (B) in cells associated with blood vessels that have taken up the MNP. (Bars indicate 40 um.)
Immunohistochemical evaluation of MNP localization
The eyes treated with MNP were stained for streptavidin, which is on the surface of the MNP, using biotinylated APase. This technique revealed the location of the injected MNP within the retina and vitreous (Figure 4). In eyes injected intra-vitreally, MNPs were detected immunohistochemically in blood vessels, which are on the surface of the retina in rabbit (top, Figure 4) and in a few cells in vitreous. APase reaction product was not present in PBS injected eyes (data not shown). Eyes injected via the sub-retinal route showed MNP within in a variety of retinal cell types near the injection site, but the majority of the staining was within the RPE (Figure 5B). In SR MNP eyes, blood vessels and cells in vitreous were not APase positive. Once the MNP were detected within the retina and RPE, their sub-cellular localization was determined with immuno-TEM. These specimens revealed the presence of a small number of MNP in RPE nuclei (Figure 6) and a large number of MNP within the RPE cytoplasm. The tissue was not preserved for TEM, so more detailed studies regarding the presence of MNP within vesicles was not possible.
Figure 5.
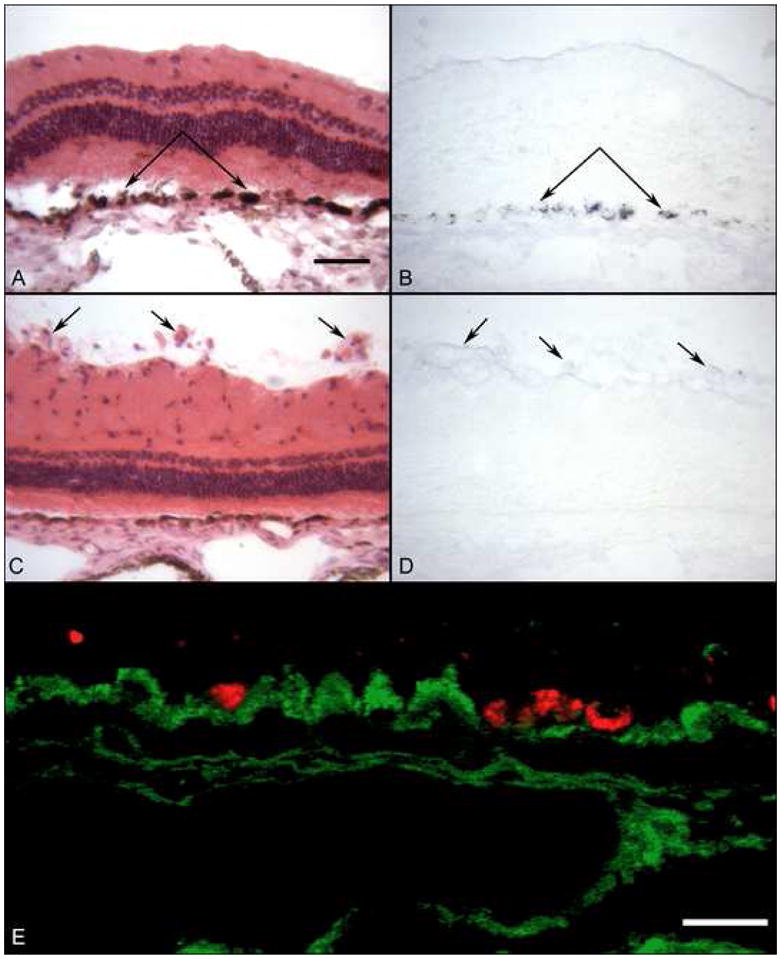
Uptake of MNP (B, D) and transfection with DsRed (E) after sub-retinal injection of MNP. Section through the bleb site stained with H&E (A) show minimal disruption of the retina and RPE cells. (B) A serial section processed for histochemistry using biotinylated APase shows that RPE cells in the bleb have taken up streptavidin-coated MPN. An area near optic nerve in the same eye appears normal with H&E staining (C) but there is no uptake of MONP by the blood vessels on top of retina (arrows) with APase histochemistry (D). (E) Some RPE in the bleb site were transfected with DsRed when spectral scanning confocal microscopy was used to differentiate between autofluorescence at GFP emission wavelength (green) and bona fide DsRed fluorescence (red) in this pseudocolored picture of the GFP image overlaid on the DsRed image. (Bars indicate 50 um in panels A–D and 20 um in panel E)
Figure 6.
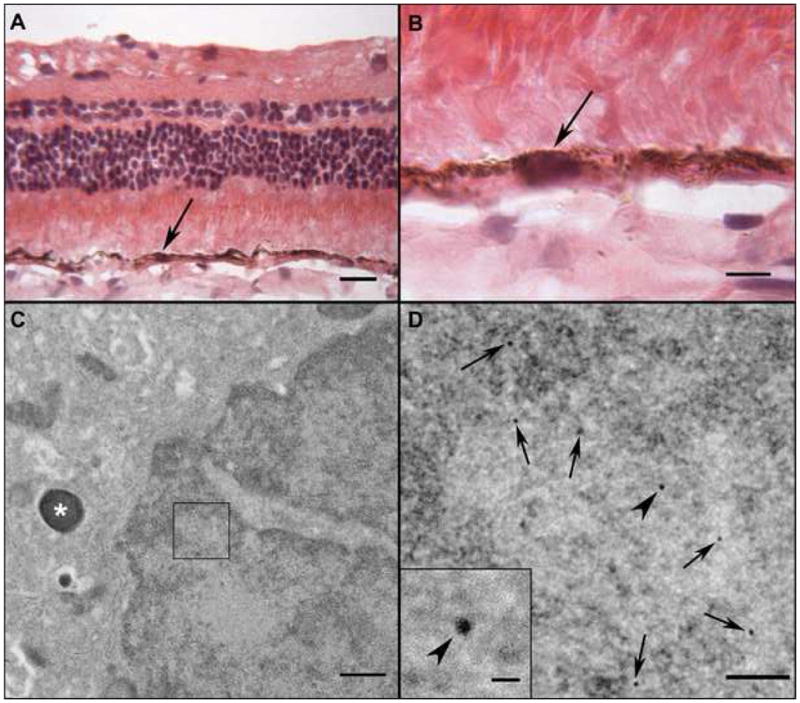
Transmission electron microscopy of MNP-transfected RPE in an eye that received a sub-retinal injection. Section stained with H&E through the bleb site at low (A) and high (B) magnification showing normal appearing RPE cells. A serial section was incubated with biotinylated gold particles. (C) An area within the nucleus (box) of an RPE cell has gold particles. The cell can be identified as RPE because of the melanin granule (asterisk) in its cytoplasm. The area within the box is magnified in (D) to show MNPs decorated with 10 nm gold particles (black arrows). The inset is even higher magnification of uppermost particle to show size of the gold particles. (Bars indicate 20 um in A, 10 um in B, 500 nm in C, 100 nm in D. Bar in inset is 20 nm)
Transfection efficiency of chitosan, PCEP, and MNP
Initial attempts to analyse transfection using GFP expression were misleading and confounding due to autofluorescence of the tissue. Only spectral analysis could differentiate between GFP and the naturally occurring autofluorescence in the adult rabbit eye (Figure 5E). Therefore, a colorimetric method with anti-GFP and APase reaction product that withstood the pigment bleaching procedure was undertaken to determine exact location of protein. Adenovirus-GFP injected eyes were used as positive controls for immunodetection and run in tandem for APase immunohistochemistry of GFP. From the APase analysis, chitosan was able to transfect a few cells in vitreous after intra-vitreal injection, albeit at low levels (data not shown). We could not distinguish between infiltrating white blood cells and hyalocytes with our analysis, so the actual origin of cells is not clear. There were very few cells in the retina that were positive for GFP in the chitosan-injected eyes. We found no PCEP transfected cells after intra-vitreal injection as detected by immunohistochemistry with rabbit anti-GFP and APase detection system (data not shown). Sub-retinal injection of PCEP yielded a few transfected cells in retina at the injection site, which may have been microglia since they had an amoeboid morphology (data not shown). Overall, neither the chitosan nor the PCEP were able to transfect ocular cells efficiently in vivo.
Later studies utilizing the MNP were done using DsRed to minimize the confounding effects of autofluorescence. Unfortunately, there was not an acceptable antibody for immunodetection of DsRed, i.e. all antibodies were made in rabbit. The confocal microscope with spectral scanning capabilities was utilized to differentiate between true DsRed expression and autofluorescence. MNP injected into the vitreous transfected large individual cells in vitreous that could be hyalocytes or invading leukocytes. Their low numbers suggested that these were hyalocytes and not a leukocytic infiltrate as seen in chitosan-injected eyes, but we do not have proof of their identity. MNP-DsRed transfected cells were verified by spectral scanning, which demonstrated that the peak emission from these cell was at the DsRed emission wavelength, 560 nm (data not shown). When MNPs were injected SR, spectral analysis demonstrated that many RPE cells were transfected with DsRed (Figure 5E), whereas all RPE cells and choroidal blood vessel walls had autofluorescence at the GFP emission wavelength (Figure 5E). Additionally, an entire sub-retinal injection bleb site was mapped using spectral analysis and DsRed positive cells were seen throughout the bleb; most transfected cells were RPE (Figure 7). These data reveal that there are several different cell types transfected by the MNP, mostly phagocytic cells like hyalocytes and RPE. These results also show that the MNP have the highest transfection efficiency, primarily RPE, of the nanoparticles evaluated.
Figure 7.
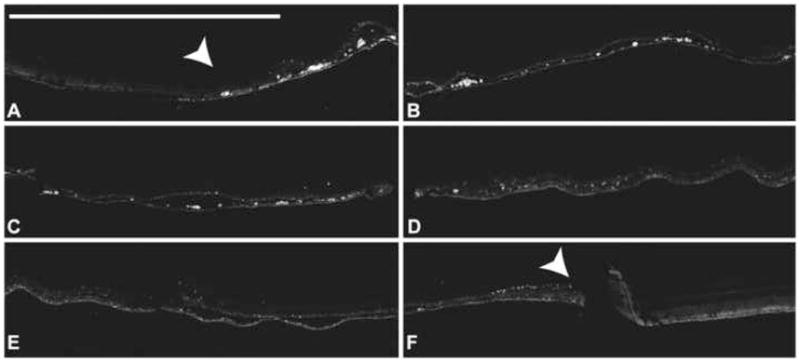
A sub-retinal injection of MNP-DsRed lead to the transfection of a variety of cell types seen in this montage of the entire bleb area of the eye (Panels A to F) The bleb site is flanked by white arrowheads. Most of the transfected cells are RPE. These images were captured at the DsRed wavelength using spectral analysis.(Bar indicates 1 mm)
DISCUSSION
The use of nanotechnology in biology and medicine has been feverishly pursued in recent years. As a result, there have been a multitude of in vitro studies that focus on the optimization of transfection efficiency in one cell line or another. In our lab, the practice of optimizing transfection efficiency in vitro has largely been in vain, since all of the nanoparticles in this study gave reasonable transfection in vitro (data not shown) but that was not always true in vivo. As logic dictates, if the nanoparticle is to be used in vivo, its use must be optimized in vivo. Another facet of this area of research that has not been pursued to any great extent is the toxicity of these novel biological/nanomaterials. Again, it seems that in vitro experiments can yield false confidence in terms of toxicity since leukocytes are not present and that in vivo models are the best way to determine toxicity and identify complications.
The immune response that was induced in the eye by chitosan was a complication that was not anticipated prior to these studies. In fact, chitosan gene delivery has been used in studies for many years without any mention of this complication [4–8]. Prior to these experiments, we regarded chitosan as having the most potential for gene delivery because it was considered nontoxic, yielded 20% transfection efficiency on retinal endothelial cells (unpublished results), and it had the desirable characteristic of being biodegradable. However, this was not the case with intraocular delivery. The mechanism of the immune induction is not known, but one hypothesis was that there are contaminants in the chitosan itself that cannot be removed. There were low levels of LPS (1.69 EU) detected in the stock solution of the biomedical grade chitosan used in this study (unpublished data) and LPS binds avidly to polysaccharides. Although this level is slightly above the limit imposed by the FDA for agents delivered into the eye, this solution was diluted by at least 50% prior to use. This analysis was done on the source of chitosan reported in the Methods section, however, chitosan from several other suppliers yielded the same inflammatory result as well. The inflammation observed in these studies appears to be limited to the eye that was injected and not in the contralateral eye. There have been many publications on the safe use of chitosan in vivo [4–8] but it appears that the vitreous chamber of the eye is a site where chitosan induces inflammation.
PCEP was reported to be relatively harmless in vivo [11] and our studies in the eye were no exception. This novel biodegradable polymer has many potential applications in drug and gene delivery. This nanomaterial is unique because it can be shaped into sheets, pellets, or nanoparticles. The results from the experiments reported herein characterize PCEP as a relatively safe nanomaterial in the eye. The only complications came from PCEP nanoparticles that had contaminants from the synthesis, which caused the severe retinal degeneration (unpublished data). Once this was addressed, the nanomaterial itself was inert and nontoxic. This highlights the need for an effective purification strategy for nanomaterials that are to be used in biological systems. The only negative characteristic of PCEP was the low transfection efficiency in vivo.
One of the better purification techniques used in these studies was the magnetic column from MNP processing. These columns allowed for the removal of free DNA and even facilitated buffer exchange when preparing MNPs. Using the magnetic technology also allowed for quick and easy synthesis, purification, and concentration of the MNP. The MNP did not induce inflammation nor did these nanoparticles show any major toxic effects on the retina. However, there were some areas with hypertrophic RPE in some of the sub-retinal injected eyes, which may have been a result of mechanical trauma. It is interesting that cells associated with blood vessels (Figure 4B) took up the MNP after intravitreal injection yet were not transfected while RPE that took up the MNP were transfected with the DsRed gene (Figure 5B, E and 7). This was the only nanoparticle of the three tested that was capable of efficiently delivering a reporter gene to the retina. These characteristics make the MNP the nanoparticle with the least toxicity and complications, while delivering genes with the greatest efficiency to the retina.
This study illustrates the importance of in vivo testing for novel nanomaterials or known nanomaterials being used in new organs or systems. There is much to be learned from even basic in vivo experiments on nanomaterials. One of the most important considerations in this area is the purification of the materials to be tested. Biological organisms are very sensitive to particular compounds. These simple experiments have resulted in several novel findings. Although chitosan is a relatively safe material for use in some tissues, it should not be injected into the eye, where it induces inflammation. PCEP was nontoxic when injected intra-vitreally, but did not efficiently deliver genes to the retina with either route of administration. MNPs could safely and efficiently deliver genes to cells in the vitreous and retina. After appropriate in vivo evaluation of toxicity and transfection efficiency, nanoparticles offer a unique approach to non-viral gene therapy.
Acknowledgments
SOURCES OF SUPPORT: This work was supported by the National Eye Institute grant R03EY013744 (GL), R01EY09357 (GL), EY01765 (Wilmer), the Johns Hopkins Hematology Training grant T32HL007525 (TP), the Altsheler Durell Foundation (GL), and unrestricted funds from a Research to Prevent Blindness grant (Wilmer).
Footnotes
Conflict of Interest: The authors have no commercial associations current or in the past five years, that might pose potential, perceived or real conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Ann Pharmacother. 2007;41:614–625. doi: 10.1345/aph.1H316. [DOI] [PubMed] [Google Scholar]
- 2.Ng EW, Adamis AP. Anti-VEGF aptamer (pegaptanib) therapy for ocular vascular diseases. Ann N Y Acad Sci. 2006;1082:151–171. doi: 10.1196/annals.1348.062. [DOI] [PubMed] [Google Scholar]
- 3.Enriquez de Salamanca A, Diebold Y, Calonge M, Garcia-Vazquez C, Callejo S, Vila A, et al. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: toxicity, uptake mechanism and in vivo tolerance. Invest Ophthalmol Vis Sci. 2006;47(4):1416–25. doi: 10.1167/iovs.05-0495. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X, Dai H, Leong KW, Goh SH, Mao HQ, Yang YY. Chitosan-g-PEG/DNA complexes deliver gene to the rat liver via intrabiliary and intraportal infusions. J Gene Med. 2006;8(4):477–87. doi: 10.1002/jgm.868. [DOI] [PubMed] [Google Scholar]
- 5.Lee D, Zhang W, Shirley SA, Kong X, Hellermann GR, Lockey RF, et al. Thiolated Chitosan/DNA Nanocomplexes Exhibit Enhanced and Sustained Gene Delivery. Pharm Res. 2007;24(1):157–167. doi: 10.1007/s11095-006-9136-9. [DOI] [PubMed] [Google Scholar]
- 6.Leong KW, Mao HQ, Truong-Le VL, Roy K, Walsh SM, August JT. DNA-polycation nanospheres as non-viral gene delivery vehicles. J Control Release. 1998;53(1–3):183–93. doi: 10.1016/s0168-3659(97)00252-6. [DOI] [PubMed] [Google Scholar]
- 7.Wong K, Sun G, Zhang X, Dai H, Liu Y, He C, et al. PEI-g-chitosan, a novel gene delivery system with transfection efficiency comparable to polyethylenimine in vitro and after liver administration in vivo. Bioconjug Chem. 2006;17(1):152–8. doi: 10.1021/bc0501597. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Chen J, Zhang Y, Pan Y, Zhao J, Ren L, et al. A novel PEGylation of chitosan nanoparticle for gene delivery. Biotechnol Appl Biochem. 2006 doi: 10.1042/BA20060163. [DOI] [PubMed] [Google Scholar]
- 9.Sander SV, Bondarchuk OI, Pentiuk AA. Effect of polysorb on adhesion of a gauze dressing to the wound surface. Klin Khir. 1992;(1):22–3. [PubMed] [Google Scholar]
- 10.Dang JM, Sun DD, Shin-Ya Y, Sieber AN, Kostuik JP, Leong KW. Temperature-responsive hydroxybutyl chitosan for the culture of mesenchymal stem cells and intervertebral disk cells. Biomaterials. 2006;27(3):406–18. doi: 10.1016/j.biomaterials.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Wen J, Mao HQ, Li W, Lin KY, Leong KW. Biodegradable polyphosphoester micelles for gene delivery. J Pharm Sci. 2004;93(8):2142–57. doi: 10.1002/jps.20121. [DOI] [PubMed] [Google Scholar]
- 12.Thorek DL, Chen AK, Czupryna J, Tsourkas A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng. 2006;34(1):23–38. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- 13.Prow T, Smith JN, Grebe R, Salazar JH, Wang N, Kotov N, et al. Construction, gene delivery, and expression of DNA tethered nanoparticles. Mol Vis. 2006;12:606–15. [PubMed] [Google Scholar]
- 14.Prow T, Grebe R, Merges C, Smith JN, McLeod DS, Leary JF, et al. Nanoparticle tethered antioxidant response element as a biosensor for oxygen induced toxicity in retinal endothelial cells. Mol Vis. 2006;12:616–25. [PubMed] [Google Scholar]
- 15.Lutty GA, Merges C, Threlkeld AB, Crone S, McLeod DS. Heterogeneity in localization of isoforms of TGF-b in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci. 1993;34:477–487. [PubMed] [Google Scholar]
- 16.Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, et al. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70(3):399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 17.Bhutto IA, Kim SY, McLeod DS, Merges C, Fukai N, Olsen BR, et al. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(5):1544–52. doi: 10.1167/iovs.03-0862. [DOI] [PubMed] [Google Scholar]


