Abstract
Objective
To describe the endoscopic characteristics of renal papillae in struvite stone formers (SF).
Materials and Methods
From 2009 to 2014, patients undergoing percutaneous nephrolithotomy were prospectively enrolled in our study. Endoscopic analysis and biopsy of papillae was performed to demonstrate the presence and percentage surface area (SA) of Randall's plaque or ductal plug. Comparison with idiopathic calcium oxalate (CaOx) SF and non-SF controls was performed.
Results
We identified 29 struvite SF to compare with 90 idiopathic CaOx SF and 17 controls. On endoscopic mapping, 28 struvite SF (97%) demonstrated Randall's plaque and 9 (31%) had plugging. The average mean SA of Randall's plaque in struvite SF (1.5±1.4%) was less than CaOx SF (3.7 ±4.3%, p=0.0018) and similar to controls (1.7 ±2.7%, p=0.76). Average mean plug SA was similar between struvite SF, CaOx SF, and controls. On metabolic assessment, 83% of struvite SF had at least one urine abnormality, with urinary uric acid and oxalate levels significantly higher among struvite SFs compared to controls (p=0.002 ). Despite lack of active UTI, interstitial inflammation was more prevalent in struvite SF compared to CaOx SF (43.5% vs 7.3%, p=0.0001).
Conclusions
Our findings suggest a limited role for Randall's plaque in struvite stone formation. Struvite SF have less plaque formation than CaOx SF, but demonstrate evidence of severe parenchymal inflammation compared to other stone formers. The role of this prominent interstitial inflammation requires further study.
Keywords: kidney calculi, kidney, nephrolithiasis, struvite
INTRODUCTION
With kidney stone disease affecting a significant proportion of the population, methods to decrease the incidence and recurrence are vital.1 Struvite stones constitute only a small minority of all stones, but carry greater morbidity and even mortality risk when in staghorn form.2,3 Due to improved minimally-invasive technology such as percutaneous nephrolithotomy (PCNL) and ureteroscopy, the intraoperative identification of stone precursor lesions, namely Randall's plaque and duct of Bellini plugs, is possible.4 Randall's plaque, calcifications arising from the basement membrane of the thin loops of Henle, has been suggested to contribute to idiopathic calcium oxalate (CaOx) nephrolithiasis.5-8 Additionally, hydroxyapatite plugs have been identified in the ducts of Bellini of patients with enteric hyperoxaluria,9 brushite stone disease,10 renal tubular acidosis,11 and hyperparathyroidism,12 suggesting a potential progenitor role.
While candidate precursor lesions have been identified in some specific stone disease states, there is a paucity of data regarding endoscopically-visible precursors of struvite stone disease. Urease is known to play a pivotal role allowing triple phosphate crystals to grow in a urinary milieu by generating abundant ammonium and an alkaline pH. However, it is unclear if other elements of the baseline urine chemistry and/or anatomical features favor growth or retention of struvite crystals.
Thus, the goal of this study was to endoscopically and histologically characterize the papillary pathology of struvite stone formers (SF). Moreover, we sought to assess clinical risk factors and define urinary metabolic abnormalities found in this patient population.
MATERIALS AND METHODS
Study sample
After Institutional Review Board approval and informed consent, we prospectively enrolled all adults undergoing elective PCNL for nephrolithiasis at our institution from September 2009 to November 2014. All patients had CT imaging preoperatively to assess stone burden. Patients with a history of renal tubular acidosis, significant bowel resection, medullary sponge kidney, hyperparathyroidism, or primary hyperoxaluria were excluded from analysis. Only patients with struvite or calcium oxalate stones (without the above secondary causes) were included in the current analysis. Patients with upper tract urothelial carcinoma or other endoscopic upper tract procedures unrelated to nephrolithiasis served as non-stone forming controls for comparison, and underwent mapping and biopsy similar to the stone patients.
Papillary pathology
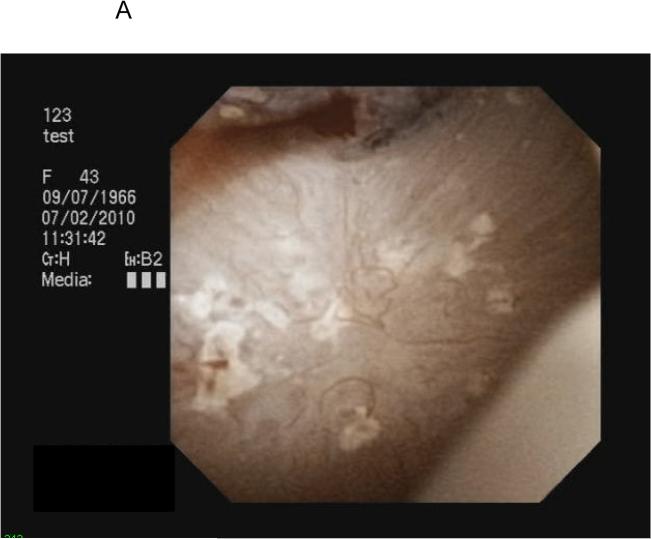
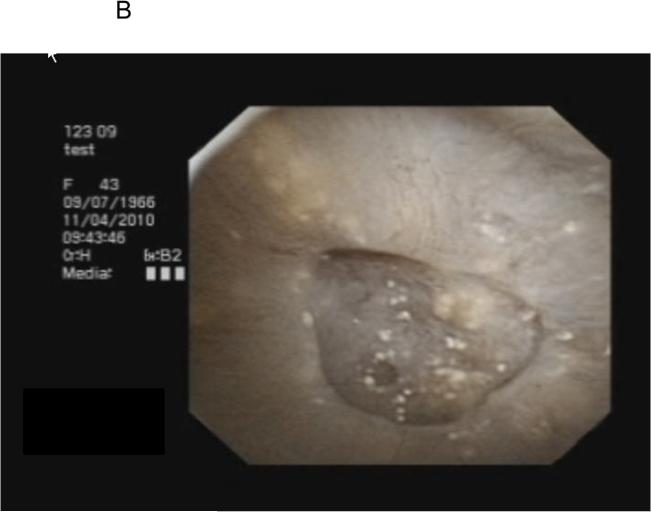
All PCNL procedures were performed by a single surgeon (A.E.K.) with endoscopic mapping and papillary biopsy obtained as previously described.13 Stones were removed with separate portions sent for micro-CT directed infrared spectrophotometry and stone culture. Following stone removal, endoscopic mapping utilizing digital nephroscopy with video capture was performed. Findings suggestive of Randall's plaque included white areas under the papillary epithelium, while plugs appeared as yellow protrusions from the papillary surface (Figure 1). Using video clips merged with fluoroscopic still image data as a reference, the papillary location, Randall's plaque, and plugs were recorded. Images of individual papillae with associated plaque and plugs were outlined utilizing Mayo Clinic Analyze image processing software (Rochester, MN) and the percentage surface area (SA) of Randall's plaque and plug present within each papilla (Figure 1) was calculated.
FIGURE 1.
Endoscopic view of Randall's plaque and ductal plugging (A,B) and quantification utilizing image processing software (C).
Papillary biopsy was performed to confirm endoscopic visual findings. After fixation and hematoxylin and eosin and Yassue staining, specimens were read by a single renal pathologist (L.H.H.) blinded to clinical details of the cases and scored as none, mild (1-25% SA), moderate (26-75% SA), or marked (>75% SA) in relation to the following pathologies: 1) punctate tubular basement membrane (BM) on Yassue staining; 2) dense tubular basement with adjacent interstitial Yassue staining; 3) intratubular Yassue positive calcifications; and 4) interstitial inflammation. The presence or absence of polarizing crystals was also noted.
Infectious and metabolic evaluations
Medical records were reviewed for urine studies during the clinical care preceding PCNL. Urine 24-hour metabolic analysis was performed at least six weeks following stone manipulation. Specifically in the struvite SF, all patients had completed at least one month of stone culture specific antibiotics based on stone culture prior to metabolic evaluation. Due to a lack of laboratory data in a comparable size of endoscopically-mapped non-SF controls, a group of age and gender controlled non-SF in the community, collected as part of a parallel protocol, were matched in a 2:1 fashion to the UA SF. The Mayo Renal Laboratory reference range values (25-75% for 24 hour urine supersaturation studies used in this analysis were as follows: sodium 41-227 mmol/24 hours; calcium males 159-291, females 123-202 mg/specimen; magnesium males 73-118, females 73-118 mg/specimen; citrate males 492-911, females 572-967 mg/specimen; oxalate males 0.25-0.35, females 0.20-0.28 mmol/specimen; uric acid males 565-833, females 400-590 mg/specimen; and volume males 0.9-1.7, females 1.1-2.0 liters/specimen.
Statistical analysis
Descriptive statistics were used to summarize the demographic and clinical characteristics of the study population. Differences were considered statistically significant at p <0.05. Continuous variables are expressed as a mean ± standard deviation (SD) and categorical variables are expressed as frequency and percentages. Characteristics of struvite SF, CaOx SF, and controls were compared using Kruskal-Wallis or Chi-square test as appropriate. The statistical analysis was performed using SAS version 9.3 computer software (Cary, NC).
RESULTS
Patient demographics are listed in Table 1 for the 29 struvite SF, 90 CaOx SF, and 17 controls. Of the 29 struvite stone formers, the mean age was 55.4 years (±15.5) and only 10 were males (35%). Patients were considered struvite stone formers if they had any degree of struvite present on stone analysis. All 29 struvite stones (100%) contained hydroxyapatite, while 16 stones (55%) continued calcium oxalate, and 2 stones (7%) contained brushite. There was a history of previous stone surgery in 14 patient, diabetes mellitus in 7 patients, and hypertension in 8 patients. Eight (28%) struvite SFs had a history of neurogenic bladder, and 4 (14%) had prior bowel interposition. Seven (24%) patients had an indwelling catheter or intermittently catheterized. Stone culture demonstrated microbial or fungal organism growth in 27 of 29 (93%) patients with struvite stones, with the most common being Proteus (7 patients), Enterococcus (7), and Candida (4). Polymicrobial growth was present in 9 stone cultures. In contrast, 12 of 90 (13%) patients with calcium oxalate stones had positive stone cultures (p<0.0001).
Table 1.
Patient characteristics.
| Variable | Struvite N=29 | CaOx N=90 | P* | PCNL Controls N=17 | P* |
|---|---|---|---|---|---|
| Mean age (SD) | 55.4 (±15.5) | 60.5 (±12.2) | 0.21 | 67.6 (±11.1) | 0.01 |
| Male (%) | 10 (34.5) | 40 (44.4) | 0.35 | 12 (70.6) | 0.02 |
| Mean BMI (SD) | 32.5 (±10.0) | 30.1 (±6.6) | 0.56 | 28.8 (6.0) | 0.54 |
| Diabetes mellitus (%) | 7 (24.1) | 21 (23.3) | 0.93 | 3 (17.6) | 0.61 |
| Hypertension (%) | 8 (27.6) | 40 (44.4) | 0.11 | 9 (52.9) | 0.09 |
| Previous stone surgery (%) | 14 (48.3) | 44 (48.9) | 0.95 | 1 (5.9) | 0.003 |
| Previous bowel surgery (%) | 4 (13.8) | 0 | 0.003 | 2 (11.8) | 0.84 |
compared to struvite group
Endoscopic mapping was performed in all 29 patients with struvite stone disease at the time of PCNL (Table 2). Randall's plaque was present in 28 patients (97%), with an average mean surface area of 1.5±1.4%, which was similar to the average mean surface area of Randall's plaque observed in the controls (1.7±2.7%, p=0.76). Among the 90 idiopathic CaOx SF, a larger mean Randall's plaque surface area of 3.7% (±4.3) was noted compared to the struvite SF group (p=0.002). Plugs were present endoscopically in 9 struvite SF (31%) with an overall average mean surface area of 0.3% (±0.8), which did not significantly differ from the mean % surface area occupied by plug in CaOx SF (mean 0.8±2.8%), p=0.14) or controls (mean 0.0% (±0.1), p=0.12).
TABLE 2.
Endoscopic mapping data.
| Struvite | CaOx | P | PCNL Controls | P | |
|---|---|---|---|---|---|
| Endoscopic mapping, N | 29 | 90 | 17 | ||
| Plaque, N (%) | 28 (97) | 90 (100) | 15 (88) | ||
| Mean SA, % (SD) | 1.5 (±1.4) | 3.7 (±4.3) | 0.0018 | 1.7 (±2.7) | 0.76 |
| Max SA, % (SD) | 3.6 (±4.4) | 8.4 (±11.2) | 0.0028 | 3.1 (±4.1) | 0.61 |
| Plug, N (%) | 9 (31) | 40 (44) | 2 (12) | ||
| Mean SA, % (SD) | 0.3 (±0.8) | 0.8 (±2.8) | 0.14 | 0 (±0.1) | 0.13 |
| Max SA, % (SD) | 0.8 (±1.8) | 2.1 (±5.5) | 0.16 | 0.1 (±0.4) | 0.14 |
CaOx = calcium oxalate stone formers
SA = surface area
Comparative endoscopic biopsy data is presented in Table 3. While the presence versus absence of interstitial inflammation was similar in struvite and CaOx SFs, marked inflammation was more prevalent in struvite SFs compared to CaOx SFs (43.5% vs 7.3%, p=0.0001). Within the calcium oxalate cohort there was no difference in the presence of marked inflammation in patients with and without positive stone cultures. An example of severe interstitial inflammation is shown in Supplementary Figure 1. On biopsy the struvite SF also demonstrated less punctate or dense basement membrane calcifications compared to the CaOx group.
TABLE 3.
Endoscopic biopsy data.
| Struvite | CaOx | P | |
|---|---|---|---|
| Endoscopic biopsy, N (%) | 29 | 83 | |
| Any punctate calcifications | 2 (9.1) | 29 (52.7) | 0.0004 |
| Rare | 2 (9.1) | 24 (43.6) | 0.004 |
| Moderate | 0 | 3 (5.5) | 0.26 |
| Marked | 0 | 2 (3.6) | 0.36 |
| Any dense BM calcifications | 2 (9.1) | 30 (54.5) | 0.0003 |
| Rare | 1 (4.5) | 21 (38.2) | 0.003 |
| Moderate | 0 | 2 (3.6) | 0.36 |
| Marked | 1 (4.5) | 7 (12.7) | 0.29 |
| Any interstitial inflammation | 19 (82.6) | 52 (94.5) | 0.09 |
| Rare | 3 (13) | 42 (76.4) | <0.0001 |
| Moderate | 6 (26.1) | 6 (10.9) | 0.09 |
| Marked | 10 (43.5) | 4 (7.3) | 0.0001 |
| Any intraluminal crystals | 14 (63.6) | 45 (80.4) | 0.12 |
| Rare | 11 (50) | 42 (75) | 0.03 |
| Moderate | 2 (9.1) | 3 (5.4) | 0.54 |
| Marked | 1 (4.5) | 0 | 0.11 |
| Any polarizing crystals | 21 (91.3) | 52 (92.9) | 0.81 |
BM = basement membrane
Metabolic evaluation results, including 24-hour urinary supersaturation studies, are presented in Supplementary Table 1. Specific abnormalities in 24-hour urine supersaturation components were noted in 20 of 24 (83%) struvite SF, the most common being hypocitraturia (11 patients) followed by low urine volume (10). Compared to the CaOx group, a higher urine pH (6.4 (±0.6) vs 6.0 (±0.6), p=0.005) and lower mean urine citrate (420.0 (±294.0) vs. 655.2 (±357.9) mg/24hr, p=0.007) were noted. When compared to the non-stone forming control group, significantly higher urine uric acid (532.3 (±174.2) vs 391.2 (±178.2) mg/24h, p=0.001) and oxalate levels (0.4 (±0.2) vs 0.3 (±0.1) mmol/24h, p=0.002) were noted in struvite SFs, while urinary citrate levels were similar between struvite (420 mg/24hr) versus control (559 mg/24hr) individuals (p=0.15).
DISCUSSION
This prospective study of patients undergoing PCNL, endoscopic mapping and papillary biopsy identified frequent duct of Bellini plugs and Randall's plaque in struvite SF. As expected, most struvite SF had UTIs, unlike the CaOx SF and controls. However, struvite SF (and controls) demonstrated less than half as much Randall's plaque coverage than CaOx SF patients. Struvite SF also had less microscopic plug on biopsy analysis than CaOx SF patients, although more than controls. These findings suggest different stone pathogenetic steps in struvite versus idiopathic CaOx SF. Similarly, struvite SF had more ductal plugging on endoscopic mapping than CaOx SF. Although the latter did not achieve statistical significance, it is possible that ductal plugs may play a role in the pathogenesis of struvite stones. Similar to the CaOx group, nearly all struvite SF demonstrated an underlying metabolic abnormality, suggesting that in addition to infection, urinary abnormalities that favor crystallization may play a role in struvite stone formation. Finally, struvite stone formers more commonly demonstrated severe papillary inflammation, and had higher serum creatinine (and hence lower eGFR) than idiopathic CaOx SF.
Struvite calculi are well known to associate with urease-producing bacterial infection.14 Urease acts to break down urea to create free ammonium ions and an alkaline pH. These are requisite conditions for precipitation of the ammonium and phospate ions with calcium and magnesium to create the triple phosphate crystals of struvite stones. Indeed, urease-producing bacteria were cultured from most stones in this series, which is likely an essential feature. Chronic bacterial infection may also contribute to biofilm production.15,16 Other risk factors in the literature for struvite stone formation relate to risk of urinary tract infection, including collecting system obstruction, presence of a chronic indwelling catheter, urinary diversion, medullary sponge kidney, and neurogenic bladder dysfunction.17
While the role of a urease positive infection and resulting changes in urine composition in the pathogenesis of struvite stones is well-established, little data exists regarding the potential for other contributing factors. Can struvite crystals nucleate and grow free in solution, or is an anchoring site required? In our study, Randall's plaque was less common in the struvite patients than in the CaOx SF group, and did not differ from controls. Interestingly, ductal plugging was also observed at a similar frequency as the CaOx group. Thus, we cannot discern the relative role of plugs or plaques as an anchoring site. However, it is notable that urease-producing bacterial urinary infections are not uncommon and most do not lead to struvite stones, suggesting other mechanisms for stone initiation besides infection could be important.
Marked interstitial inflammation was notable in 10 of the papillary biopsies of the struvite group, significantly more so than in the CaOx patients (43.5% vs 7.3%, p=0.0001). On the surface this might not seem surprising, but the current is one of the largest cohorts of well-characterized struvite SF with concurrent papillary pathology to vividly demonstrate this. The serum creatinine of the struvite patients in our cohort was also higher than that in the sex and age-matched urine controls and idiopathic CaOx SF group. Indeed, frequent urinary tract infection appears to be an independent risk factor for chronic kidney disease amongst patients with stones.18 Furthermore, infection stones appear more common than expected among patients whose end-stage renal failure is attributed to kidney stones.19 We know that it is important to remove infection stones and eradicate infection. Among 177 patients with staghorn calculi that were followed for 7.7 years, CKD developed in 67% of those monitored conservatively but in none who were surgically rendered stone free.20 In our cohort even though all patients had received prolonged antibiotics prior to surgery, microscopic inflammation persisted in many. Thus it is possible that the intense inflammation (as noted in our cohort of struvite stone formers) contributes to the higher rate of chronic kidney disease by direct destruction of nephrons and/or promulgation of interstitial fibrosis.
Specific stone forming-populations have been studied utilizing endoscopic mapping and papillary biopsy to determine incidence and distribution of candidate precursor lesions such as Randall's plaque and ductal plugs, which may contribute to crystal retention and subsequent renal stone formation. To our knowledge, no prior study has specifically looked at endoscopic precursor lesions in struvite SF as has been done in other patients with both metabolic abnormalities and specific stone types, namely idiopathic and enteric calcium oxalate,6,21 brushite,10 and primary hyperparathyroidism and calcium phosphate.12 Low and Stoller first reported a potential relationship between endoscopically-identified Randall's plaque and calcium-based nephrolithiasis.22 In their study, endoscopic evidence of Randall's plaque was found at a higher incidence in SF compared to non-SF controls. Randall's plaque presence varied depending on stone type, with plaque found in 100% of calcium phosphate SF and 87% of CaOx SF but only 20% of struvite SF (2 of 10 patients). While prior studies have shown a common link between both calcium-based nephrolithiasis and urinary parameters and Randall's plaque, there is minimal evidence that this translates to infection SF as very few struvite patients have been included in prior endoscopic mapping studies.
Prior work has identified an underlying anatomic and metabolic abnormality in ~75% of patients with infection stones.23,24 Lack of metabolic abnormities appears more common in women (~30%) than men (~10%) with infection stones.23 However, recurrence after successful surgery and antibiotic regimens is relatively uncommon, consistent with infection being the dominant factor.25 In our current study all but four patients who completed their metabolic evaluation demonstrated at least one abnormality on their urine supersaturation study. However, when the overall mean urine values for the struvite SF group were compared to matched non-stone forming controls, higher urinary oxalate and uric acid levels were the only differing factors, and their role in the formation of the struvite stones is unclear. Additionally, when compared to the mapped CaOx SF group, there was a lower mean citrate level and higher pH. Perhaps this reflects, in part, a long lasting effect of the ongoing interstitial inflammation on renal acidification, since all samples were obtained postoperatively when patients were infection free. While these findings should be interpreted with caution, it may suggest that underlying metabolic abnormalities play a role in struvite stone formation. Hypocitraturia is a risk factor for stone formation, and was more profound in the struvite SF group than the idiopathic CaOx SF group. Notably, despite the 24-hour urine studies being performed 6 weeks after stone removal and treatment of infection, we still observed a higher urine pH and lower urine citrate in the struvite SF compared to the CaOx SF. Overall, given the high incidence of metabolic abnormalities, we advocate for metabolic testing in all struvite SF at follow-up.
In our series, Randall's plaque was present in a majority of struvite SF. However, there was a lower amount compared to CaOx SF. Due to the lack of significant papillary Randall's plaque in struvite SF, it is unlikely that struvite forms on Randall's plaque as is theorized in CaOx stone formation, and an alternative pathogenic mechanism is thus implicated. Although almost all the struvite patients demonstrated metabolic abnormalities, the lack of significant Randall's plaque is not entirely surprising as few patients had hypercalciuria or low mean urine volume, both of which have been correlated with increased papillary coverage by Randall's plaque.26
While pathways detailing CaOx stone formation on Randall's plaque have been suggested, the remaining categories of nephrolithiasis have less clear mechanisms.5,8 One alternative process involves a propensity for crystal deposition in tubules, or plugging. Prior studies have shown elevated urine pH seems to play a role in ductal plugging and subsequent growth of brushite and calcium phosphate stone.10,11 Such intratubular deposits are also found in hyperparathyroid SF, another population with elevated urinary pH.12 Other studies have shown plugging in struvite SF, but not severe.4 The exact mechanism linking stone formation with tubular plugging is not currently known and remains hypothetical. Based on our study results, this pathway to struvite stone growth would seem possible as 31% of struvite SF had evidence of ductal plugging on endoscopic mapping. However, this should be interpreted with caution as plugging was found in both CaOx SF and in two control patients. Thus, plug formation is most likely not the sole factor in crystal retention during struvite stone formation. Unfortunately, the composition of the plugs present in the struvite SF in this study is unknown, but based on endoscopic appearance they are similar to those noted in calcium phosphate stone formers.
The marked interstitial inflammation observed was somewhat unexpected, since all patients received prolonged (at least one month) antibiotic courses preoperatively. Thus it appears that the inflammatory response may take some time to resolve and there may be prolonged effect on specific renal function measures. It is also unclear what effect the papillary inflammation might have on the collecting ducts and/or urothelium, and whether this in turn might affect crystal retention or stone pathogenesis. This is an area worthy of further study.
There are limitations to our study. First, our overall cohort sizes are rather small and may not be large enough to identify differences between cohorts, especially regarding the mapped control group, since only 17 patients were available and many did not have urine studies. Thus, we also compared struvite SF to CaOx SF and a matched non-stone forming control group with urine data. Finally, we were unable to retrieve plugs for stone analysis and therefore plug composition in these patients remains unknown and will require further prospective investigation.
CONCLUSION
In conclusion, this group of struvite SF had minimal Randall's plaque formation by endoscopic mapping and significantly less than a group of CaOx SF. A third of these struvite SF demonstrated ductal plugging, similar to the cohort of CaOx SF. A majority of struvite SF demonstrated metabolic abnormalities, most notably hypocitraturia. Additionally, biopsy data suggested a marked inflammatory response in struvite SFs that could further contribute to stone formation and/or chronic kidney disease risk. These data suggest a complex interaction of urease producing pathogens and urinary metabolites leading to struvite SF in a manner not dependent on the presence of Randall's plaque.
Supplementary Material
Acknowledgements
We thank Dr. James Williams, Indiana University School of Medicine, for micro-CT directed analysis of stone specimens. We also thank Dr. Eric Bergstralh, Mayo Clinic, Rochester, MN, for statistical support.
FUNDING SUPPORT
This study was supported by the Mayo Clinic O’Brien Urology Research Center (U54 DK100227), funded by the NIDDK and the Mayo Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
Amy E. Krambeck, M.D. serves as a DSMB member for HistoSonics, Inc. No other competing financial interests exist regarding this study or its authors.
REFERENCES
- 1.Scales CD, Jr., Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012 Jul;62(1):160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieske JC, Rule AD, Krambeck AE, et al. Stone composition as a function of age and sex. Clin J Am Soc Nephrol. 2014 Dec 5;9(12):2141–2146. doi: 10.2215/CJN.05660614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blandy JP, Singh M. The case for a more aggressive approach to staghorn stones. The Journal of urology. 1976 May;115(5):505–506. doi: 10.1016/s0022-5347(17)59258-7. [DOI] [PubMed] [Google Scholar]
- 4.Linnes MP, Krambeck AE, Cornell L, et al. Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int. 2013 Oct;84(4):818–825. doi: 10.1038/ki.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coe FL, Evan AP, Worcester EM, Lingeman JE. Three pathways for human kidney stone formation. Urol Res. 2010 Jun;38(3):147–160. doi: 10.1007/s00240-010-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evan A, Lingeman J, Coe FL, Worcester E. Randall's plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006 Apr;69(8):1313–1318. doi: 10.1038/sj.ki.5000238. [DOI] [PubMed] [Google Scholar]
- 7.Matlaga BR, Williams JC, Jr., Kim SC, et al. Endoscopic evidence of calculus attachment to Randall's plaque. The Journal of urology. 2006 May;175(5):1720–1724. doi: 10.1016/S0022-5347(05)01017-7. discussion 1724. [DOI] [PubMed] [Google Scholar]
- 8.Miller NL, Gillen DL, Williams JC, Jr., et al. A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall's plaque. BJU Int. 2009 Apr;103(7):966–971. doi: 10.1111/j.1464-410X.2008.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evan AP, Lingeman JE, Coe FL, et al. Intra-tubular deposits, urine and stone composition are divergent in patients with ileostomy. Kidney Int. 2009 Nov;76(10):1081–1088. doi: 10.1038/ki.2009.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005 Feb;67(2):576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 11.Evan AP, Lingeman J, Coe F, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int. 2007 Apr;71(8):795–801. doi: 10.1038/sj.ki.5002113. [DOI] [PubMed] [Google Scholar]
- 12.Evan AE, Lingeman JE, Coe FL, et al. Histopathology and surgical anatomy of patients with primary hyperparathyroidism and calcium phosphate stones. Kidney Int. 2008 Jul;74(2):223–229. doi: 10.1038/ki.2008.161. [DOI] [PubMed] [Google Scholar]
- 13.Krambeck AE, Lieske JC, Li X, et al. Current computed tomography techniques can detect duct of Bellini plugging but not Randall's plaques. Urology. 2013 Aug;82(2):301–306. doi: 10.1016/j.urology.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Healy KA, Ogan K. Pathophysiology and management of infectious staghorn calculi. Urol Clin North Am. 2007 Aug;34(3):363–374. doi: 10.1016/j.ucl.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Rahman NU, Meng MV, Stoller ML. Infections and urinary stone disease. Curr Pharm Des. 2003;9(12):975–981. doi: 10.2174/1381612033455125. [DOI] [PubMed] [Google Scholar]
- 16.Choong S, Whitfield H. Biofilms and their role in infections in urology. BJU Int. 2000 Nov;86(8):935–941. doi: 10.1046/j.1464-410x.2000.00949.x. [DOI] [PubMed] [Google Scholar]
- 17.Bichler KH, Eipper E, Naber K, Braun V, Zimmermann R, Lahme S. Urinary infection stones. Int J Antimicrob Agents. 2002 Jun;19(6):488–498. doi: 10.1016/s0924-8579(02)00088-2. [DOI] [PubMed] [Google Scholar]
- 18.Saucier NA, Sinha MK, Liang KV, et al. Risk factors for CKD in persons with kidney stones: a case-control study in Olmsted County, Minnesota. Am J Kidney Dis. 2010 Jan;55(1):61–68. doi: 10.1053/j.ajkd.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambaro G, Favaro S, D'Angelo A. Risk for renal failure in nephrolithiasis. Am J Kidney Dis. 2001 Feb;37(2):233–243. doi: 10.1053/ajkd.2001.21285. [DOI] [PubMed] [Google Scholar]
- 20.Teichman JM, Long RD, Hulbert JC. Long-term renal fate and prognosis after staghorn calculus management. The Journal of urology. 1995 May;153(5):1403–1407. [PubMed] [Google Scholar]
- 21.Evan AP, Lingeman JE, Coe FL, et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003 Mar;111(5):607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low RK, Stoller ML. Endoscopic mapping of renal papillae for Randall's plaques in patients with urinary stone disease. The Journal of urology. 1997 Dec;158(6):2062–2064. doi: 10.1016/s0022-5347(01)68153-9. [DOI] [PubMed] [Google Scholar]
- 23.Resnick MI, Boyce WH. Bilateral staghorn calculi--patient evaluation and management. The Journal of urology. 1980 Mar;123(3):338–341. doi: 10.1016/s0022-5347(17)55924-8. [DOI] [PubMed] [Google Scholar]
- 24.Amaro CR, Goldberg J, Agostinho AD, et al. Metabolic investigation of patients with staghorn calculus: is it necessary? Int Braz J Urol. 2009 Nov-Dec;35(6):658–661. doi: 10.1590/s1677-55382009000600004. discussion 662-653. [DOI] [PubMed] [Google Scholar]
- 25.Silverman DE, Stamey TA. Management of infection stones: the Stanford experience. Medicine (Baltimore) 1983 Jan;62(1):44–51. doi: 10.1097/00005792-198301000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Kuo RL, Lingeman JE, Evan AP, et al. Urine calcium and volume predict coverage of renal papilla by Randall's plaque. Kidney Int. 2003 Dec;64(6):2150–2154. doi: 10.1046/j.1523-1755.2003.00316.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.