Abstract
Purpose
Chronic lymphocytic leukemia (CLL) cells depend on microenvironmental interactions for proliferation and survival that are at least partially mediated through B cell receptor (BCR) signaling. Ibrutinib, a Bruton’s tyrosine kinase inhibitor, disrupts BCR signaling and leads to the egress of tumor cells from the microenvironment. While the on-target effects on CLL cells are well defined, the impact on the microenvironment is less well studied. We therefore sought to characterize the in vivo effects of ibrutinib on the tumor microenvironment.
Experimental Design
Patients received single agent ibrutinib on an investigator-initiated phase 2 trial. Serial blood and tissue samples were collected pre-treatment and during treatment. Changes in cytokine levels, cellular subsets and microenvironmental interactions were assessed.
Results
Serum levels of key chemokines and inflammatory cytokines decreased significantly in patients on ibrutinib. Further, ibrutinib treatment decreased circulating tumor cells and overall T cell numbers. Most notably, a reduced frequency of the Th17 subset of CD4+ T cells was observed concurrent with reduced activation markers and expression of PD-1 on T cells. Consistent with direct inhibition of T cells, ibrutinib inhibited Th17 differentiation of murine CD4+ T cells in vitro. Lastly, in the bone marrow microenvironment, we found that ibrutinib disaggregated the interactions of macrophages and CLL cells, inhibited secretion of CXCL13 and decreased the chemoattraction of CLL cells.
Conclusions
In conjunction with inhibition of BCR signaling, these changes in the tumor microenvironment likely contribute to the anti-tumor activity of ibrutinib and may impact the efficacy of immunotherapeutic strategies in patients with CLL.
Keywords: Ibrutinib, microenvironment, CLL, T cell, macrophage
Introduction
Chronic lymphocytic leukemia (CLL) cells depend on interactions with the microenvironment to such a degree that they may be considered “addicted to the host.”(1, 2) The lack of a defined oncogenic driver, thus absence of “oncogene addiction”, and the apparent importance of B cell receptor (BCR) signaling emphasizes the importance of tumor-microenvironment interactions in the CLL pathogenesis. Without support from the microenvironment, exemplified by culturing CLL cells together with monocyte derived nurse like cells (NLC) or on bone marrow stroma, CLL cells die in culture.(3) Even in in vivo models, such as in the NSG CLL xenograft model, CLL cells depend on a functional microenvironment, in particular the presence of T cells.(2, 4) Our group has previously shown that CLL cells in the lymph nodes have activated BCR and NF-κB signaling resulting in their activation and proliferation. In contrast, circulating cells in the blood tend to be resting and quiescent.(1) The recent clinical success of targeting kinases essential for BCR signaling with small molecule inhibitors underscores the importance of this pathway. The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib and the PI3K inhibitor idelalisib have been shown to extend survival of patients with relapsed or refractory CLL and have obtained regulatory approval.(5, 6) Notably, both ibrutinib and idelalisib, to varying degrees, also affect a number of signaling pathways and cell types that play a role in the tissue microenvironment.(7)
The interactions between CLL cells and microenvironmental cells are bidirectional. CLL cells secrete cytokines that attract accessory cells, such as macrophages and T cells, and modulates their anti-tumor activity.(8–12) For instance, T cells from CLL patients have been shown to be in a pseudoexhausted state.(13) In addition, subset changes in T helper cells have also been reported in CLL, with increased Th17 cells being linked to autoimmune cytopenias, although the prognostic significance of this remains unclear. These changes in T helper subsets may reflect immune dysfunction in CLL, leading to a disruption of the balance between a protective immune response and autoimmunity.(14) Similarly, interactions between microenvironmental cells and the tumor cells create an environment that is supportive of tumor cell growth via direct cell contact and through secretion of additional cytokines from the accessory cells.(15–18) As an example, monocytes secrete CXCL12 and CXCL13 leading to chemotaxis, aggregation and activation of CLL cells, via ERK1/2 and STAT3 signaling (19). Thus, targeting these bidirectional interactions between CLL cells and the microenvironment are of therapeutic interest.
Ibrutinib is an orally bioavailable small molecule that covalently binds to Cys481 in BTK, thereby irreversibly inhibiting the kinase.(20) In vitro, in addition to BTK, ibrutinib can potentially inhibit a limited number of other kinases, which have a cysteine residue aligning with Cys481 in BTK, including the TEC family kinases BLK, BMX, ITK, and TEC, as well as EGFR, ErbB2, and JAK3.(20) ITK, which is expressed in T cells and NK cells, has been shown to be inhibited at clinically achievable concentrations.(21) For other kinases, in vivo inhibition has not been reported. In CLL patients treated with ibrutinib, BCR and NF-κB signaling in the tumor cells are greatly reduced and proliferation is virtually abolished.(22) We have previously shown that ibrutinib-induced lymphocytosis is due to egress of tissue resident CLL cells into the blood.(23) Inhibition of VLA-4 dependent adhesion and reduced ability of CLL cells to migrate towards chemokines may be the key factors for the development of lymphocytosis.(24, 25) However, only a minority of tumor cells egress from the tissue sites, and persistent disease in blood, lymph nodes, and bone marrow is typically demonstrable even after years of single agent therapy.(23, 26, 27) This residual disease appears to be quiescent, with down-regulation of intracellular signaling pathways and inhibited proliferation,(22) and, at least for persistent leukemic disease, does not predict inferior durability of response.(27) However, continued therapy with ibrutinib is required, and some patients, especially those with adverse disease features may eventually develop resistance to ibrutinib.(28)
While on-target effects of ibrutinib on tumor cells are well characterized, the effects on the host microenvironment are less investigated. In vitro, ibrutinib inhibits the secretion of cytokines from activated T cells, and through inhibition of ITK has been reported to exert a Th1-selective pressure in T lymphocytes.(21, 29) Subsequently, mice with deleted ITK were shown to lack differentiation of Th17 T cells, as identified by intracellular IL17A.(30) Thus, ibrutinib may affect T cell subsets indirectly through alteration of the tumor cells and by direct inhibition of T cell pathways. More recently ibrutinib has been shown to interfere with Fc-receptor signaling, which reduced initiation of phagocytosis and NK-cell activation by rituximab bound CLL cells in vitro.(31, 32) Despite the data on ibrutinib mediated effects in different cell systems in vitro, little is known about how ibrutinib, directly or indirectly, affects the composition of the tumor microenvironment in vivo. As the microenvironment is critical in the pathogenesis of CLL, studies of the in vivo effects of ibrutinib on the microenvironment are needed. We thus set forth to describe the impact of ibrutinib on the cells that shape the tumor microenvironment in patients with CLL enrolled on an investigator-initiated phase 2 trial of ibrutinib monotherapy.(26)
Materials and Methods
Patients and clinical data
We report correlative analyses on 80 CLL patients treated on an investigator-initiated phase 2 study of single agent ibrutinib (NCT01500733). Six additional patients on the study are not included in this analysis due to incomplete data. For most experiments, only a subgroup of the patients is represented due to availability of samples. The study was approved by the local ethics committee and informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Briefly, both treatment naïve and relapsed/refractory patients with either TP53 aberrations or age ≥65 years were eligible (Table 1) and were treated with ibrutinib 420 mg orally once daily until disease progression or the occurrence of intolerable side effects. Clinical baseline data and laboratory results were retrieved from electronic records at the Clinical Center, National Institutes of Health.
Table 1.
Patient Characteristics
| Trial arm | subgroup | n= | sex (M), % | Rai ≥3, % | Unmut, % | CD38+, % |
|---|---|---|---|---|---|---|
| ≥65 | TN | 17 | 53 | 59 | 53 | 24 |
| ≥65 | RR | 16 | 50 | 81 | 81 | 56 |
| TP53 | TN | 33 | 64 | 61 | 64 | 27 |
| TP53 | RR | 14 | 50 | 79 | 71 | 36 |
| Both | all | 80 | 56 | 68 | 66 | 34 |
≥65: 65 years of age or older, TP53: TP53 aberrations, TN: treatment naïve, RR: relapsed or refractory, M: male, Unmut: unmutated IGHV (>98% homology to germline), CD38+: >30% of CLL cells express CD38 above isotype control.
Bone marrow specimens
Bone marrow biopsies performed at pre-specified time points for assessment of clinical response during treatment were evaluated by two independent hematopathologists. Immunohistochemistry was performed for CD79a, CD3 and CD68 as part of standard pathology work up. The percentage of CD79a and CD3 positive cells was recorded and the degree of CD68 positive macrophage extensions in contact with CLL cells were semiquantitatively assessed on a scale from 0 (no extensions) to 4 (max number of extensions). Microphotographs were performed with an Olympus BX51 microscope and Olympus DP72 camera (Olympus) at original enlargements as indicated in figures.
Flow cytometry
Peripheral blood mononuclear cells (PBMC) were prepared by density-gradient centrifugation (Lymphocyte Separation Media; ICN Biomedicals). Viably frozen cell suspensions were analyzed on a LSRFortessa or a FACSCanto II flow cytometer (BD Biosciences). Cell populations were gated on forward scatter and side scatter. For T cell analyses, additional CD3, CD4 and CD8 gating was used with a minimum of 50,000 CD4+ T events being collected. For analysis of proliferation, intracellular staining for Ki67 was performed. Additionally, surface staining for the activation markers HLA-DR and CD39 as well as PD-1 was performed (all antibodies from BD biosciences). Cell populations were identified as previously described and expressed as percentages of the parent population.(33) The human Th17 subset of CD4+ T cells was identified as CXCR3 negative and CCR6 positive.(34)
Cytokine and chemokine measurements in serum and bone marrow supernatants
For cytokine measurements, serum samples and bone marrow supernatants were kept at -80°C until analysis. Serum samples were analyzed by the HCYTMAG-60K-PX38 (Millipore) assay and bone marrow samples by a HCYP2MAG-62K custom designed assay (Millipore) according to instructions from the manufacturer with a Luminex 200 instrument (Luminex) for determination of individual cytokine concentrations. For serum samples, 36 cytokines were analyzed (all included in Supplementary Table 1 for completeness), only the 10 chemokines and 10 inflammatory cytokines are discussed in details.
In vitro murine Th17 differentiation
Murine CD4+ T cells were differentiated as previously described.(30) Briefly, naive CD4+ T cells from C57BL/6 mice were co-cultured at a ratio of 1:5 with mitomycin-treated T-depleted splenocytes as antigen presenting cells with 1 μg/ml anti-CD3 plus 3 μg/mL anti-CD28 and 20 ng/ml IL-6 (Peprotech), 5 ng/mL TGF-β1 (R&D), 10 μg/mL of each anti–IL-4, anti–IFN-γ, and anti–IL-12 antibodies (BioXcell). Ibrutinib (Selleckchem) was added to the culture medium at the indicated concentrations during the whole differentiation period. Murine Th17 cells were identified by intracellular staining for IL17A (eBioscience).(30)
Nurse like cells (NLC) co-cultures
NLC were allowed to grow out from fresh CLL PBMCs for 2 weeks as previously described.(3) Non-adherent cells were harvested and replated on autologous NLC at desired concentration. Ibrutinib at 1 μM or control media was added. Viability of CLL and NLC cells was assessed with the LIVE/DEAD Viability/Cytotoxicity Kit (Life Technologies) according to the manufacturer’s protocol.
Migration assays
PBMCs collected pre-treatment were evaluated for their ability to migrate towards patient matched BM supernatant collected either pre-treatment or on ibrutinib. Chemotaxis assays across polycarbonate Transwell inserts were performed as previously described.(35) 106 pre-treatment CLL PBMC cells in AIM-V medium (Gibco) were transferred into the top chambers of Transwell culture insert (Corning Incorporated) with a diameter of 6.5 mm and a pore size of 5 μm. Filters were then placed onto wells containing medium (control) or a mixture of 50% medium and 50% bone marrow supernatant collected either pre-treatment or after eight weeks on ibrutinib. CLL PBMC were allowed to migrate for 3 hours at 37°C. Migrated cells in the lower chamber were then collected and counted in triplicates using AccuCount blank particles (Spherotech) to quantify the absolute number of input and migrated B and T cells stained with CD19 and CD3 as previously described.(2) The migration index was determined by subtracting the medium control percentage migration from the chemoattractant percentage migration and normalizing.
Statistics
Statistical comparisons were performed as Student’s paired t-test except for cytokine levels, where Wilcoxon matched-pairs signed rank test was used due to non-parametric data distribution, and dose-response effect, where 2-way ANOVA was used (graphpad prism v. 6, Graphpad software).
Results
Ibrutinib decreases serum levels of multiple inflammatory cytokines and chemokines
In this study we focused on in vivo changes in tumor-microenvironment interactions during treatment of CLL with ibrutinib. We have previously reported the clinical results and correlative analyses from our investigator-initiated phase 2 single agent ibrutinib trial, including the on-target effects of BTK inhibition in CLL cells.(22, 23, 26) Of 86 patients enrolled, 80 are included in this analysis. In six patients, data or samples for analysis were not available; due mostly to early discontinuation of study in these patients. Of the 80 patients included, 50 (62.5%) received ibrutinib as first line therapy and 47 (58.8%) had a TP53 aberration (either deletion of chromosomal arm 17p or TP53 mutation). For most experiments, only a subset of patients is represented due to availability of samples. The baseline characteristics of the patients are provided in Table 1.
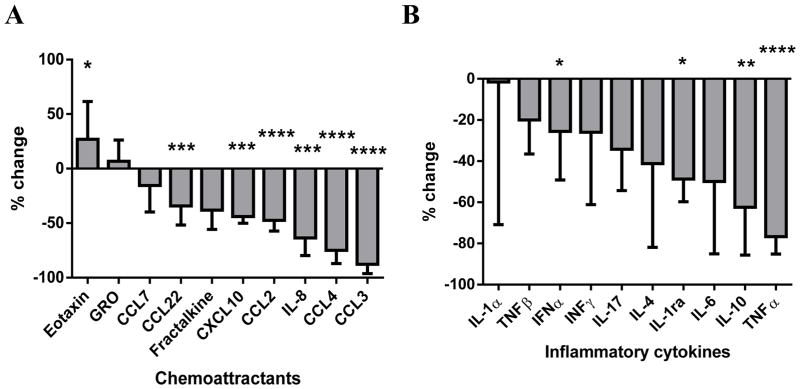
The chemokines CCL3 and CCL4 are upregulated by BCR signaling in CLL cells,(1, 36) and an on-target effect of ibrutinib is the rapid inhibition of expression and secretion of both chemokines.(22, 37) In addition, Herman et al. previously showed that ibrutinib inhibits cytokine secretion from T cells in vitro.(29) Here, we systematically measured an array of cytokines and chemokines (see Supplementary Table 1). Levels of six (CCL22, IL-8, CXCL10, CCL2, CCL3, CCL4, and CCL7) out of 10 chemokines tested showed a rapid (within 24 hours), significant and sustained decrease upon ibrutinib treatment. One additional chemokine (Fractalkine) showed a decrease only at later time points. One out of 10 of the evaluated chemokines (Eotaxin) showed a significant increase after four weeks on ibrutinib (Figure 1A and Supplementary Table S1). Supplementary Figure S1A shows the absolute levels pre-treatment and after eight weeks on ibrutinib for the 10 evaluated chemokines.
Figure 1. Ibrutinib alters the expression of inflammatory cytokines and chemokines in serum from patients with CLL.
A, The percent changes in the evaluated chemokines after eight weeks on ibrutinib treatment compared to pre-treatment levels. B, The percent changes in evaluated inflammatory cytokines after eight weeks on ibrutinib treatment compared to pre-treatment levels. In both panels, median with interquartile range is shown. See supplementary table for complete list of tested cytokines/chemokines and changes over time. Statistical analyses were by Wilcoxon matched-pairs signed rank test. Asterisks indicate degree of statistical significance compared to pre-treatment levels: * P < .05, ** P < .01, *** P < .001, and **** P < .0001.
Inflammatory cytokines such as IL10, IFNα, IFNγ, TNFα, IL6 and IL4 are reported to be upregulated in patients with CLL compared to healthy volunteers.(15, 38–40) Levels of four (IL-10, IL-1ra, TNFα, IFNα) out of 10 inflammatory cytokines detectable at baseline showed a significant decrease during the first four weeks of ibrutinib treatment and an additional two (IFNγ, IL-4) were decreased only after 24 weeks; the rest (40%) showed no significant change (Figure 1B and Supplementary Table 1). Supplementary Figure S1B shows the absolute levels pre-treatment and after eight weeks on ibrutinib for the 10 evaluated inflammatory cytokines. Overall, serum levels of 13 out of 20 (65%) chemokines and inflammatory cytokines decreased during treatment with ibrutinib suggesting widespread changes in cellular elements of the tissue microenvironment, not only in CLL cells (CCL3, CCL44),(37) but also T cells (IL6, IL10, TNFα, IFNγ),(29) and macrophages (IL8, IL10).(11)
Ibrutinib decreases cell numbers, activation, proliferation and pseudoexhaustion of T cells
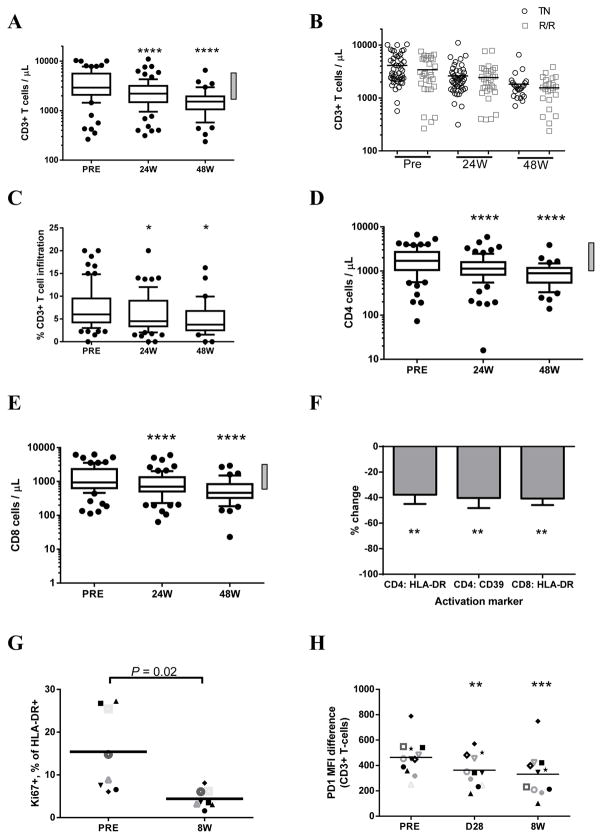
At the time of treatment initiation, most patients had elevated circulating T cell numbers (CD3+) (Figure 2A). Upon ibrutinib treatment, a significant decrease in the number of circulating T cells was observed at 24 weeks with normalization of T cell counts in most patients by 48 weeks (Figures 2A, P < .0001). A significant decrease in T cell counts was seen for treatment naïve as well as relapsed or refractory patients and the initial T cell counts were in the same range for these two patient groups (Figure 2B). A similar decrease in T cell numbers was seen in the bone marrow (Figure 2C, P < .05). The reduction in T cell numbers paralleled the progressive decrease in CD19+ tumor cells in circulation (Supplementary Figure S2A) and in the bone marrow (Supplementary Figures S2B–C). We next evaluated changes in CD4+ and CD8+ T cell populations. For most patients, both CD4+ and CD8+ T cell numbers were elevated at baseline and normalized upon ibrutinib treatment (Figures 2D–E, P < .0001). Interestingly, we noted that patients with an unmutated IGHV displayed a significantly larger reduction in all T cell populations (data not shown). The CD4/CD8 ratio in peripheral blood remained stable over the course of ibrutinib treatment (Supplementary Figure S3A). Similarly, the CD4/CD8 ratio in the bone marrow remained constant on ibrutinib (Supplementary figure S3B–D).
Figure 2. Ibrutinib decreases T cell numbers and T cell activation and reverses pseudoexhaustion.
A, Absolute CD3+ T cell counts in the peripheral blood (PB). The normal range (615–2348/μL) is indicated by the right-sided grey bar. Only patients (n=78) with data available both pre-treatment (PRE) and at 24 weeks (24W) were included, of these 47 had reached 48 weeks of treatment (48W). Whiskers indicate 10–90 percentiles. B, The relative decrease in T cell count in the PB is shown as a percentage of baseline measurements; absolute numbers shown in panel A. Mean with SEM is shown. C, Assessment of CD3+ T cells in bone marrow, n=71 at PRE and 24W, n=31 at 48W. Whiskers indicate 10–90 percentiles. D, Absolute CD4+ T cell counts in peripheral blood, normal range 334–1556/μL indicated by left-sided grey bar, n=78 (n=47 at 48W), only patients with samples available at both PRE and 24W were included. E, CD8+ T cell counts in peripheral blood, normal range 149–787/μL indicated by left-sided grey bar, n=78 (n=47 at 48W), only patients with samples available at both PRE and 24W were included. F, The mean (±SEM) percent change in CD4+ cells that express HLA-DR or CD39 and CD8+ cells that express HLA-DR after 24 weeks of ibrutinib treatment is shown, n=10. G, The percentage of activated (HLA-DR+) T cells that express Ki67 pre-treatment (PRE) and after eight weeks (8W) of ibrutinib treatment, n=8. Each symbol represents a patient, line represents mean. H, PD-1 surface staining of peripheral blood T cells pre-treatment (PRE) and on ibrutinib, n=10. Each symbol represents a patient, line represents mean. W: weeks on treatment, D: days on treatment, PRE: baseline. Statistical analyses were by Student’s paired t-test. Asterisks indicate degree of statistical significance compared to PRE: * P < .05, ** P < .01, *** P < .001, and **** P < .0001.
It has previously been shown that CLL cells cause chronic activation of T cells leading to their exhaustion.(13) Therefore, we next evaluated whether ibrutinib affects activation and pseudoexhaustion of T cells. The proportion of CD4+ T cells that expressed HLA-DR or CD39, two commonly used activation markers, decreased on ibrutinib treatment (Figure 2F, n=10, P < .01). The proportion of CD8+ cells expressing HLA-DR was also decreased during treatment (Figure 2F, n=10, P < .01). Further, proliferation of activated T cells (HLA-DR+), as indicated by the expression of Ki67, decreased as early as eight weeks on treatment (Figure 2G, n=8, P = .02). Lastly, as a measure of pseudoexhaustion, we evaluated the expression of PD-1 on T cells.(13) In vivo, PD-1 expression on T cells decreased with time on treatment; with a significant decrease as early as at four weeks and a further decrease by eight weeks of treatment (Figure 2H, n=10, P < .01). Taken together, T cell activation, proliferation, and pseudoexhaustion decreased during treatment with ibrutinib.
Ibrutinib treatment reduces Th17 T cells and inhibits in vitro differentiation of Th17 cells
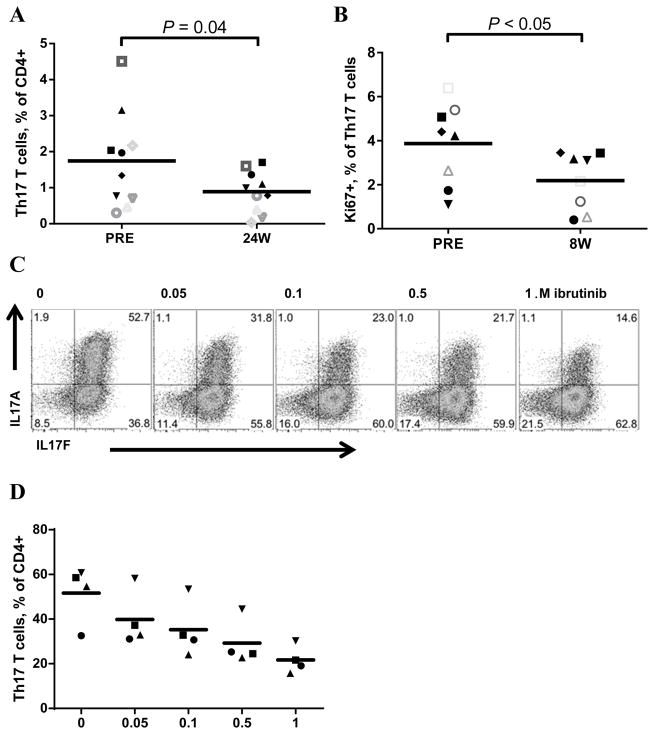
Dubovsky and colleagues reported that ibrutinib irreversibly inhibits ITK and drives a Th1-selective pressure in T lymphocytes.(21) Further, ITK knock-out mice show a striking decrease in Th17 differentiation.(30) Whether ibrutinib affects Th17 differentiation has not been reported. We therefore evaluated the effect of ibrutinib on Th17 cells in the blood, and found a mean decrease of 22% in the frequency of CD4+ Th17 T cells (CXCR3neg, CCR6+)(34) after 24 weeks of ibrutinib treatment (Figure 3A, n=10, P = .04). Consistently, reduced proliferation of Th17 cells, as reflected in a mean decrease in Ki67 positive cells of 25%, was detected in the blood already at eight weeks from treatment start (Figure 3B, n=8, P < .05). Next, we chose a well-established Th17 differentiation assay using murine CD4+ T cells to investigate the in vitro effect of ibrutinib on Th17 differentiation.(30) Ibrutinib significantly reduced the differentiation of CD4+ T cells into IL17A+ Th17 cells in a dose dependent manner, reaching half maximal effect at 0.1 μM (Figures 3C–D, P < .001). Thus, in agreement with the phenotype of the ITK knock-out mouse,(30) ibrutinib inhibits Th17 differentiation in vitro, and reduces the number of Th17 cells in CLL patients in vivo through ITK dependent and/or independent pathways.
Figure 3. Ibrutinib treatment leads to a reduction in the Th17 T-cell subset and inhibits in vitro differentiation of murine Th17 cells.
A, The percentage of Th17 cells (CXCR3neg, CCR6+) of total CD4+ T cells pre-treatment (PRE) and after 24 weeks of ibrutinib treatment (24W), n=10. Line represents mean. B, The percentage of Th17 T cells that express Ki67 pre-treatment (PRE) and after eight weeks (8W) of ibrutinib treatment, n=8. Line represents mean. C, In vitro differentiation of murine CD4+ Th17 cells, Th17 phenotype assessed by staining for IL17A expression, representative dot plots also including IL17F staining from one of four experiments shown, concentration of ibrutinib in the culture medium indicated on top of each panel, 0 represents vehicle. D, The percentage of total CD4+ T cells differentiating into IL17A positive Th17 cells upon in vitro ibrutinib. Each symbol represents an individual experiment, line represents mean. Drug effect assessed by 2-way ANOVA analysis (P = .005), statistical analyses (A and B) were determined by Student’s paired t-test.
Ibrutinib treatment disrupts macrophage-CLL interaction in bone marrow
Tumor associated macrophages are recognized as important components of the tumor supporting microenvironment. Monocyte-derived NLCs that grow out from CLL peripheral blood samples in culture, support the survival of CLL cells in vitro.(18) This pro-survival effect of NLCs, and by extension of macrophages, appears to be dependent on direct cell-cell contact. Cell-cell contact between CLL cells and macrophages in the bone marrow has been reported and is thought to play a pathogenic role.(12, 17) Notably, the CLL cell derived cytokines CCL3 and CCL4 are potent chemoattractants that could recruit macrophages to CLL infiltrates in the bone marrow. Since these chemokines rapidly decrease on ibrutinib, as shown here and reported by others,(1, 36) we sought to assess changes in the interactions between CLL cells and macrophages during treatment.
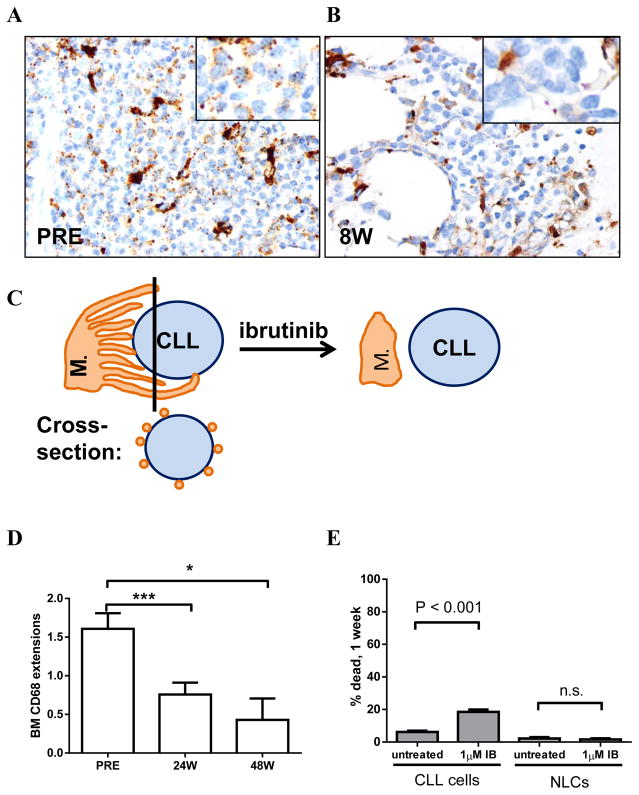
We evaluated bone marrow specimens for macrophages by immunohistochemistry. As previously reported, macrophages (CD68+) were interspersed among CLL cells in the bone marrow(12) and remained in this distribution on ibrutinib treatment (Figures 4A–B). However, the dotted appearance of CD68 positive cellular extensions in close proximity to CLL cells disappeared during ibrutinib treatment (Figures 4A–B inserts; schematically depicted in Figure 4C). These findings were semi-quantitatively graded on serial bone marrow biopsies during ibrutinib treatment (Supplementary Figures S4A–E for grading key). A significant decrease was seen over time (Figure 4D, P < .001). To further understand the in vivo disruption of CLL-macrophage interactions in the bone marrow, we utilized an in vitro system of CLL cells co-cultured with autologous NLCs.(3, 18) The viability of CLL cells but not NLCs was significantly reduced with the in vitro addition of ibrutinib to this co-culture system (Figure 4E, n=5, P<0.001). Thus, ibrutinib disrupts the tight association of CLL cells and macrophages in vivo and inhibits the supportive effect of NLCs in vitro without directly affecting the survival of the microenvironmental cells.
Figure 4. Macrophage-CLL cell interactions in the bone marrow are decreased on ibrutinib treatment.
A–B, Immunohistochemistry (original magnification 20 x) showing CD68+ (brown) macrophages in bone marrow specimens, inserts at 100 x magnification with macrophage extensions as CD68+ dots in close proximity of CLL cells, before start of treatment (PRE, A) and after eight weeks of ibrutinib treatment (8W, B). C, Schematic representation of macrophage-CLL interaction before and on ibrutinib. D, Semiquantitative assessment of CD68+ extensions among CLL cells in bone marrow specimens, 0 (no extensions) – 4 (max extensions), n=37 at PRE and 24W, n=7 at 48W, only patients with samples available at both PRE and 24W were included. See supplementary figure 3 for information on grading. Mean (±SEM). E, Mean (±SEM) percent of dead NLCs and CLL cells in co-culture after one week in 1 μM ibrutinib or control media, n=5. W: weeks on treatment, PRE: baseline. All statistical analyses were determined by Student’s paired t-test. Asterisks indicate degree of statistical significance compared to PRE: * P < .05 and *** P < .001.
Ibrutinib decreases CXCL13 and CLL cell migration towards bone marrow supernatant
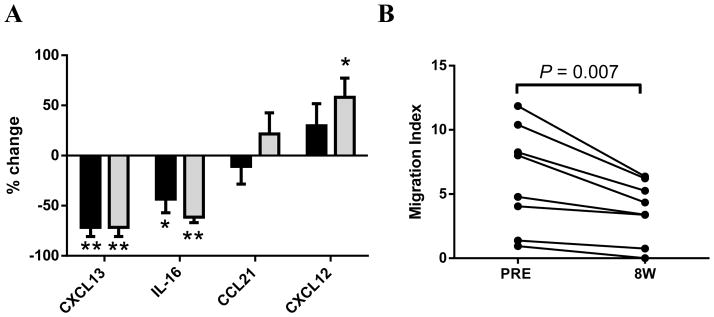
We next evaluated changes in chemokines secreted by accessory cells in the supernatant of bone marrow aspirates pre-treatment and on ibrutinib. Of all the tested chemokines (Supplementary Table 2), CXCL13, secreted predominantly by macrophages,(41) showed the most significant and sustained decrease in the bone marrow supernatant obtained from patients on ibrutinib compared to matching pre-treatment samples (Figure 5A, n=11, P ≤ .01). IL-16 levels were also significantly decreased on ibrutinib, while CXCL12 levels increased in a time-dependent manner. The latter perhaps reflects the decrease of tumor infiltration and consequently less internalization of the chemokine by CLL cells. Supplementary Figures S5A–D shows the absolute levels pre-treatment, at eight weeks and at 24 weeks on ibrutinib for the evaluated chemokines. To evaluate the impact of the chemokine content in bone marrow on CLL cells, we analyzed the migration of CLL cells collected pre-treatment towards autologous bone marrow supernatant collected either at baseline or after eight weeks of ibrutinib treatment. For all patients evaluated, a decreased migratory response was seen for bone marrow supernatant collected during ibrutinib treatment as compared to paired baseline samples (Figure 5B, n=8, P = .007).
Figure 5. Ibrutinib decreases migration of CLL cells to bone marrow supernatant.
A, The percent changes in the evaluated chemokines in bone marrow supernatant at eight weeks (black) and 24 weeks (grey) of ibrutinib treatment compared to baseline, n=11. Shown is the mean with SEM. B, PBMCs collected pre-ibrutinib evaluated for migration towards autologous BM supernatant collected pre-treatment and after eight weeks on ibrutinib, n=8. W: weeks on treatment, PRE: baseline. Statistical analyses were determined by Wilcoxon matched-pairs signed rank test for A, by Student’s paired t-test for B. Asterisks indicate degree of statistical significance compared to PRE: * P < .05 and ** P < .01.
Discussion
CLL cells critically depend on signals from the tissue microenvironment; while simultaneously altering their surrounding microenvironment, through both the secretion of cytokines/chemokines and direct cell-cell interactions. These effects create a microenvironment favorable to tumor growth by co-opting macrophages and T cells, and inhibiting anti-tumor immune responses.(8, 12) Consequently, targeting tumor-microenvironment interactions is a promising area for therapeutic intervention; yet few agents are available to specifically target such pathways. With more widespread use of BCR inhibitors it is becoming clear that these agents, directly and/or indirectly, affect not only the tumor cells but also more broadly impact the immune microenvironment. This may have important consequences for putative combination therapies and the safety of long term administration of such agents.(21, 31, 32, 42) Here we aimed to characterize the extent of these changes by systematically assessing the effect of ibrutinib on 1) cytokines and chemokines secreted in the peripheral blood and bone marrow, 2) the subset distribution and activation state of T cells and 3) the cellular composition of the bone marrow microenvironment in patients with CLL (summarized in Figure 6).
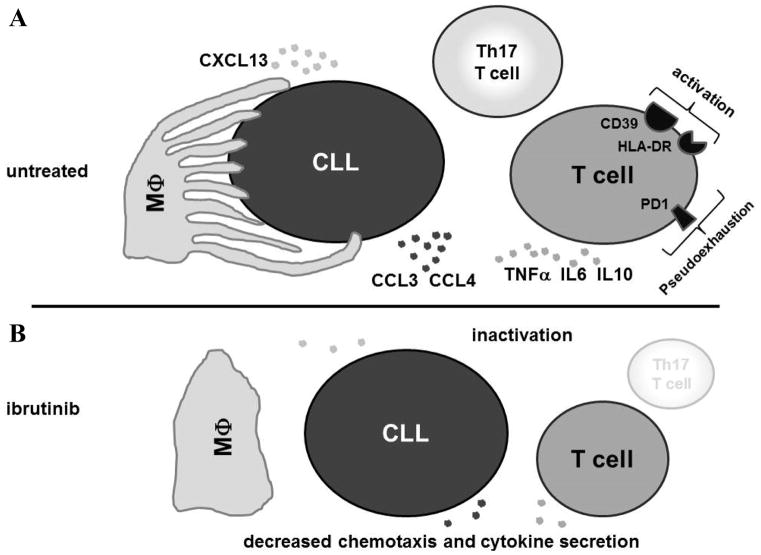
Figure 6. Changes in the tumor-microenvironment in CLL patients treated with ibrutinib.
A, CLL cells in patients before starting ibrutinib interact with T cells and macrophages (MΦ), and are surrounded by a multitude of soluble factors. Macrophages form extensions and maintain multiple sites of contact with the CLL cells and secrete CXCL13 that functions as a chemoattractant for CLL cells.(41, 49) Activated CLL cells in turn secrete CCL3 and CCL4 among other chemoattractants that stimulate macrophages and T cells.(10, 17, 37) T cells and macrophages secrete TNFα, IL8 and IL10 among other inflammatory cytokines.(11, 29) These cytokines in turn cause an inflammatory state and support CLL proliferation.(29, 50) T cells express markers of cellular activation like CD39, HLA-DR and PD-1, which has been linked to pseudoexhaustion and inhibition of immune surveillance.(13) The Th17 subset of T cells is expanded and implicated in the inflammatory drive in CLL and the development of autoimmune cytopenias.(14) B, On ibrutinib treatment, the tumor-microenvironment undergoes major changes. CLL cell activation, proliferation and cytokine/chemokine secretion is reduced.(22) In parallel, T cell activation and pseudoexhaustion is decreased, and T cell subsets shift with a marked reduction in Th17 cells. Cytokine and chemokine secretion from T cells and macrophages is decreased. Macrophage extensions detach from CLL cells and there is decreased CXCL13 secretion and consequently less migration of CLL cells. Taken together, the microenvironment on ibrutinib is reset from a hyperstimulated inflammatory state that supports tumor growth and inhibits anti-tumor T cell responses into a more resting state.
Notably, the levels of most chemokines and inflammatory cytokines decreased during treatment with ibrutinib. Several of the chemokines having reduced serum levels in patients treated with ibrutinib, including IL8, CCL3, CCL4, and CCL22, are secreted by CLL cells in response to, among others, BCR- and CD40-signaling.(10, 37) Thus, the decrease in the serum levels of these chemokines is considered an on-target effect of BCR inhibitors.(22) Other chemokines that decreased upon ibrutinib treatment may be primarily secreted by cells in the microenvironment; including CXCL10, the levels of which correlate with time to treatment of disease, and CCL2, which enhances the viability of CLL cells in vitro.(15, 16) The decrease in chemokine secretion from host cells in the tissue microenvironment may be a consequence of the treatment-induced changes in the CLL cells. For example, the serum levels of IL8 and IL10, which are secreted by monocytes exposed to CLL conditioned medium in vitro, decreased during treatment.(11) However, ibrutinib may also inhibit secretion of cytokines through a direct effect on immune cells other than CLL cells, as exemplified by the decreased secretion of TNFα and IL10 from activated T cells treated with ibrutinib in vitro.(29) Furthermore, some effects on the microenvironment may be due to inhibition of kinases other than BTK, in particular ITK, and possibly TEC by ibrutinib.(20, 21, 43) Other BTK inhibitors in preclinical and clinical development, for example CC-292 and ACP-196, may more selectively inhibit BTK, and thus be expected to have a partially different impact on microenvironmental cells.
Chronic activation of T cells decreased during ibrutinib treatment as demonstrated by reduced expression of the activation markers HLA-DR and CD39 and a decrease in T cell derived cytokines such as TNFα and IL10. T cell inactivation and cytokine changes during ibrutinib treatment are significant because cancer in general, and CLL in particular, is associated with a chronic inflammatory drive.(44) A decrease of PD-1 surface expression was also demonstrated that, along with decreased TNFα levels, indicates the potential normalization of the T-cell exhaustion previously described as a hallmark of CLL.(13) Notably, ibrutinib can also alter the composition of T cell subsets by exerting a Th1-selective pressure as described previously (21) and inhibit differentiation of the Th17 T cell subset as shown here. The latter effect is in line with data from ITK knock-out mice in which Th17 differentiation is impaired.(30) We confirmed that ibrutinib inhibits Th17 differentiation in vitro, choosing a murine system because of the more established nature of the assay. The observed shifts in T cell subsets, based on direct and indirect effects, and the decrease in chronic T cell activation could have clinical implications as they may contribute to the reported efficacy of ibrutinib in graft versus host disease.(45) Additionally, these T cell effects could enhance T cell mediated anti-tumor responses, especially in conjunction with intratumoral CpG injection or PD-1L blockade.(42, 46) Further, as a skewing of T cells towards Th17 subset differentiation in CLL may reflect disruption of a protective immune response and increased autoimmunity,(14) the here described T cell changes may indicate a normalized T cell state.
Macrophages play a pivotal role for CLL cells in the microenvironment. This is exemplified by the ability of macrophage-like NLCs to extend survival of CLL cells in in vitro cultures,(3, 18) and the importance of macrophages for the development of leukemia in the TCL-1 mouse model.(47) CD68 positive macrophages are found in CLL infiltrates in the bone marrow and are thought to be recruited by CLL cell derived cytokines such as CCL3 and CCL4.(12, 17) The reduction in macrophage pseudopodia interacting with CLL cells despite the persistence of both cell types in the bone marrow, supports the conclusion that ibrutinib disrupts tumor-microenvironmental interactions rather than the viability of the microenvironmental cells. Consistently, in vitro CLL cell death increased with ibrutinib treatment even in the presence of NLCs, while NLC viability was unaffected. As macrophage differentiation is reported to be dependent on both BTK and TEC kinases,(48) it is likely that disruption of macrophage/NLC support of CLL cells is, at least in part, mediated through direct effects of ibrutinib. In addition to changes in cell-cell contact, levels of macrophage-derived CXCL13, also known as B cell-attracting chemokine 1,(41) decreased in the supernatant from bone marrow aspirates upon ibrutinib treatment. CXCL13 has been reported to exert chemoattractant effects on CLL cells through the CXCR5 chemokine receptor.(8) Consistent with these observations, migration of CLL cells towards bone marrow supernatant collected during ibrutinib therapy decreased compared to pre-treatment samples. Thus, ibrutinib disrupts direct cell-cell interactions as well as secretion of soluble factors from macrophages.
CLL cells shape their microenvironment in ways that support tumor growth and inhibit immune surveillance.(8) Several of these cell-cell interactions have been considered as targets of therapy. As reported here, the combined effects of ibrutinib on the tumor-microenvironment are so extensive that it may not be possible to extrapolate observations made in absence of ibrutinib to ibrutinib-treated patients. On one hand, inhibition of BCR signaling in the tumor cells is likely potentiated by the concurrent inhibition of microenvironmental support. For example, changes in tumor-associated macrophages in vivo and the inhibitory effects in co-culture experiments with NLCs in vitro(37) suggest that ibrutinib may be sufficient to abrogate the supportive role of macrophages in the pathogenesis of CLL. On the other hand, the effects exerted by ibrutinib may have a major impact on other therapies. For example, cellular effector functions of monoclonal antibodies are inhibited by ibrutinib,(31, 32) raising the question whether such combinations are worthwhile. In contrast, ibrutinib may improve T cell function and could thereby enhance immunotherapeutic strategies such as CAR T-cell therapy, bispecific antibodies, or immune checkpoint inhibitors. While further studies are necessary to detail exactly how ibrutinib modulates the microenvironment, our data provide an overview of changes occurring in the tumor microenvironment that can inform the rationale design of combination therapy. The implications of these findings, especially on duration or extent of response, should be evaluated in future studies.
Supplementary Material
Statement of Translational Relevance.
The introduction of ibrutinib and other agents targeting the B cell receptor pathway has significantly improved the prognosis for patients with CLL. In addition to directly targeting the tumor cells, BCR-directed kinase inhibitors disrupt tumor-microenvironment interactions that are critical for CLL progression. Given that understanding changes in the tumor-microenvironment is essential for discerning possible synergistic and antagonistic effects of future combination regimens, we sought to characterize the in vivo changes in the microenvironment during ibrutinib treatment. On ibrutinib, a global reduction of chemoattractants and inflammatory cytokines were observed in patient serum and bone marrow supernatant. Further, we found distinct changes in overall T cell numbers, T cell subsets, activation and pseudoexhaustion. Additionally, interactions between tumor cells and macrophages decreased on treatment. These microenvironmental changes provide the basis for design of future combination therapies that may target several pathways in both the CLL cell and the tumor microenvironment.
Acknowledgments
Research support: This research was supported by the Intramural Research Program of the National, Heart, Lung and Blood Institute and CUN was supported by the Danish Cancer Society.
We thank our patients for participating and donating samples to make this research possible. We thank Susan Soto and Ajunae Wells for assistance in the clinic and Stephanie Housel, Adrian Byrnes, and Allison Wise for protocol support. We acknowledge Pharmacyclics for providing study drug and for helpful comments from employees of Pharmacyclics, who reviewed a draft of this manuscript. This work was supported by the Intramural Research Program of NHLBI, NIH. CUN was supported by The Danish Cancer Society.
Footnotes
Authors’ Contributions
Concept and design: AW, MF, CUN and SEMH
Development of methodology: JGR, AB and BC
Acquisition of data: IM, MSS, CY, KRC, RCB, GM, CUN, SM, GM, JV, YSL, DW and JJ
Analysis and interpretation of data: IM, MSS, CY, KRC, RCB, GM, CUN, SEMH, SM, GM, JV, YSL
Writing, review and/or revision of manuscript: CUN, SEMH, MF, CS, and AW, all authors approved the final version of the manuscript.
Study supervision: AW
Conflicts of Interest: AW and MZHF received research funding from Pharmacyclics Inc. CUN received grants from the Danish Cancer Society during the study; personal fees from Roche, Janssen and Novartis, and travel grants from Roche and Novartis, outside the submitted work; and is the principal investigator for clinical trials sponsored by Roche. BC is an employee of Pharmacyclics. All other authors declare no competing interests.
References
- 1.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman SE, Sun X, McAuley EM, Hsieh MM, Pittaluga S, Raffeld M, et al. Modeling tumor-host interactions of chronic lymphocytic leukemia in xenografted mice to study tumor biology and evaluate targeted therapy. Leukemia. 2013;27:1769–73. doi: 10.1038/leu.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–63. [PubMed] [Google Scholar]
- 4.Bagnara D, Kaufman MS, Calissano C, Marsilio S, Patten PE, Simone R, et al. A novel adoptive transfer model of chronic lymphocytic leukemia suggests a key role for T lymphocytes in the disease. Blood. 2011;117:5463–72. doi: 10.1182/blood-2010-12-324210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2014 doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood. 2012;120:4684–91. doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herishanu Y, Katz BZ, Lipsky A, Wiestner A. Biology of chronic lymphocytic leukemia in different microenvironments: clinical and therapeutic implications. Hematol Oncol Clin North Am. 2013;27:173–206. doi: 10.1016/j.hoc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dal Bo M, Tissino E, Benedetti D, Caldana C, Bomben R, Del Poeta G, et al. Microenvironmental interactions in chronic lymphocytic leukemia: the master role of CD49d. Semin Hematol. 2014;51:168–76. doi: 10.1053/j.seminhematol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Ghia P, Strola G, Granziero L, Geuna M, Guida G, Sallusto F, et al. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. Eur J Immunol. 2002;32:1403–13. doi: 10.1002/1521-4141(200205)32:5<1403::AID-IMMU1403>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Maffei R, Bulgarelli J, Fiorcari S, Bertoncelli L, Martinelli S, Guarnotta C, et al. The monocytic population in chronic lymphocytic leukemia shows altered composition and deregulation of genes involved in phagocytosis and inflammation. Haematologica. 2013;98:1115–23. doi: 10.3324/haematol.2012.073080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zucchetto A, Tripodo C, Benedetti D, Deaglio S, Gaidano G, Del Poeta G, et al. Monocytes/macrophages but not T lymphocytes are the major targets of the CCL3/CCL4 chemokines produced by CD38(+)CD49d(+) chronic lymphocytic leukaemia cells. British journal of haematology. 2010;150:111–3. doi: 10.1111/j.1365-2141.2010.08152.x. [DOI] [PubMed] [Google Scholar]
- 13.Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–21. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lad DP, Varma S, Varma N, Sachdeva MU, Bose P, Malhotra P. Regulatory T-cell and T-helper 17 balance in chronic lymphocytic leukemia progression and autoimmune cytopenias. Leukemia & lymphoma. 2015:1–5. doi: 10.3109/10428194.2014.986479. [DOI] [PubMed] [Google Scholar]
- 15.Yan XJ, Dozmorov I, Li W, Yancopoulos S, Sison C, Centola M, et al. Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood. 2011;118:5201–10. doi: 10.1182/blood-2011-03-342436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess M, Cheung C, Chambers L, Ravindranath K, Minhas G, Knop L, et al. CCL2 and CXCL2 enhance survival of primary chronic lymphocytic leukemia cells in vitro. Leukemia & lymphoma. 2012;53:1988–98. doi: 10.3109/10428194.2012.672735. [DOI] [PubMed] [Google Scholar]
- 17.Zucchetto A, Benedetti D, Tripodo C, Bomben R, Dal Bo M, Marconi D, et al. CD38/CD31, the CCL3 and CCL4 chemokines, and CD49d/vascular cell adhesion molecule-1 are interchained by sequential events sustaining chronic lymphocytic leukemia cell survival. Cancer research. 2009;69:4001–9. doi: 10.1158/0008-5472.CAN-08-4173. [DOI] [PubMed] [Google Scholar]
- 18.Filip AA, Cisel B, Wasik-Szczepanek E. Guilty bystanders: nurse-like cells as a model of microenvironmental support for leukemic lymphocytes. Clinical and experimental medicine. 2013 doi: 10.1007/s10238-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ten Hacken E, Burger JA. Microenvironment dependency in Chronic Lymphocytic Leukemia: The basis for new targeted therapies. Pharmacol Ther. 2014;144:338–48. doi: 10.1016/j.pharmthera.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13075–80. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–49. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herman SE, Mustafa RZ, Gyamfi JA, Pittaluga S, Chang S, Chang B, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123:3286–95. doi: 10.1182/blood-2014-02-548610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herman SE, Niemann CU, Farooqui M, Jones J, Mustafa RZ, Lipsky A, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia. 2014 doi: 10.1038/leu.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–4. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 25.Herman SE, Mustafa RZ, Jones J, Wong DH, Farooqui M, Wiestner A. Treatment with ibrutinib inhibits BTK and VLA-4 dependent adhesion of chronic lymphocytic leukemia cells in vivo. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farooqui MZ, Valdez J, Martyr S, Aue G, Saba N, Niemann CU, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. The Lancet Oncology. 2015;16:169–76. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. The New England journal of medicine. 2014;370:2286–94. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Rodriguez J, Wohlfert EA, Handon R, Meylan F, Wu JZ, Anderson SM, et al. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J Exp Med. 2014;211:529–43. doi: 10.1084/jem.20131459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohrt HE, Sagiv-Barfi I, Rafiq S, Herman SE, Butchar JP, Cheney C, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood. 2014;123:1957–60. doi: 10.1182/blood-2014-01-547869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Da Roit F, Engelberts PJ, Taylor RP, Breij EC, Gritti G, Rambaldi A, et al. Ibrutinib interferes with the cell-mediated anti-tumor activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica. 2015;100:77–86. doi: 10.3324/haematol.2014.107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biancotto A, Dagur PK, Fuchs JC, Wiestner A, Bagwell CB, McCoy JP., Jr Phenotypic complexity of T regulatory subsets in patients with B-chronic lymphocytic leukemia. Mod Pathol. 2012;25:246–59. doi: 10.1038/modpathol.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nature reviews Immunology. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94:3658–67. [PubMed] [Google Scholar]
- 36.Sivina M, Hartmann E, Kipps TJ, Rassenti L, Krupnik D, Lerner S, et al. CCL3 (MIP-1alpha) plasma levels and the risk for disease progression in chronic lymphocytic leukemia. Blood. 2011;117:1662–9. doi: 10.1182/blood-2010-09-307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahadevan D, Choi J, Cooke L, Simons B, Riley C, Klinkhammer T, et al. Gene Expression and Serum Cytokine Profiling of Low Stage CLL Identify WNT/PCP, Flt-3L/Flt-3 and CXCL9/CXCR3 as Regulators of Cell Proliferation, Survival and Migration. Human genomics and proteomics: HGP. 2009;2009:453634. doi: 10.4061/2009/453634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon JY, Lafarge S, Dawe D, Lakhi S, Kumar R, Morales C, et al. Association of interleukin-6 and interleukin-8 with poor prognosis in elderly patients with chronic lymphocytic leukemia. Leukemia & lymphoma. 2012;53:1735–42. doi: 10.3109/10428194.2012.666662. [DOI] [PubMed] [Google Scholar]
- 40.Fayad L, Keating MJ, Reuben JM, O’Brien S, Lee BN, Lerner S, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001;97:256–63. doi: 10.1182/blood.v97.1.256. [DOI] [PubMed] [Google Scholar]
- 41.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104:3021–7. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 42.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E966–72. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiestner A. BCR pathway inhibition as therapy for chronic lymphocytic leukemia and lymphoplasmacytic lymphoma. Hematology Am Soc Hematol Educ Program. 2014;2014:125–34. doi: 10.1182/asheducation-2014.1.125. [DOI] [PubMed] [Google Scholar]
- 44.Niemann CU, Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Seminars in cancer biology. 2013 doi: 10.1016/j.semcancer.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubovsky JA, Flynn R, Du J, Harrington BK, Zhong Y, Kaffenberger B, et al. Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. The Journal of clinical investigation. 2014 doi: 10.1172/JCI75328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sagiv-Barfi I, Kohrt HE, Burckhardt L, Czerwinski DK, Levy R. Ibrutinib enhances the antitumor immune response induced by intratumoral injection of a TLR9 ligand in mouse lymphoma. Blood. 2015;125:2079–86. doi: 10.1182/blood-2014-08-593137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinart N, Nguyen PH, Boucas J, Rosen N, Kvasnicka HM, Heukamp L, et al. Delayed development of chronic lymphocytic leukemia in the absence of macrophage migration inhibitory factor. Blood. 2013;121:812–21. doi: 10.1182/blood-2012-05-431452. [DOI] [PubMed] [Google Scholar]
- 48.Melcher M, Unger B, Schmidt U, Rajantie IA, Alitalo K, Ellmeier W. Essential roles for the Tec family kinases Tec and Btk in M-CSF receptor signaling pathways that regulate macrophage survival. J Immunol. 2008;180:8048–56. doi: 10.4049/jimmunol.180.12.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burkle A, Niedermeier M, Schmitt-Graff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 2007;110:3316–25. doi: 10.1182/blood-2007-05-089409. [DOI] [PubMed] [Google Scholar]
- 50.Ghamlouch H, Ouled-Haddou H, Damaj G, Royer B, Gubler B, Marolleau JP. A combination of cytokines rescues highly purified leukemic CLL B-cells from spontaneous apoptosis in vitro. PloS one. 2013;8:e60370. doi: 10.1371/journal.pone.0060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.