Abstract
Over the past 20 years, considerable advances have been made toward our understanding of how post-translational modifications affect a wide variety of biological processes, including morphology and virulence, in medically important fungi. Phosphorylation stands out as a key molecular switch and regulatory modification that plays a critical role in controlling these processes. In this article, we first provide a comprehensive and up-to-date overview of the regulatory roles that both Ser/Thr and non-Ser/Thr kinases and phosphatases play in model and pathogenic fungi. Next, we discuss the impact of current global approaches that are being used to define the complete set of phosphorylation targets (phosphoproteome) in medically important fungi. Finally, we provide new insights and perspectives into the potential use of key regulatory kinases and phosphatases as targets for the development of novel and more effective antifungal strategies.
Keywords: phosphorylation, kinases, phosphatases, human fungal pathogens, phosphoproteome
Introduction
Protein phosphorylation is the most common type of post-translational modification in eukaryotes, including medically important fungi. Protein kinases and phosphatases mediate cellular homeostasis by the continual adjustment of complex signal transduction events in response to various internal and external environmental cues.1–4 Protein phosphorylation is a reversible modification that is crucial for the regulation of diverse cellular processes, including metabolism, cell cycle, transcription, mating, filamentation, cell wall synthesis, maintenance of cellular integrity in stress situations (eg, in the presence of high-osmolarity and heat stresses), and virulence.1,5–13
The concept of protein phosphorylation was first introduced by Edmond Fischer and Edwin Krebs in the mid-1950s through studies of a special muscle system. Fischer and Krebs demonstrated a dual requirement for adenosine triphosphate (ATP) and what they described as “converting enzyme” (later called phosphorylase kinase) in the conversion of phosphorylase b to phosphorylase a in vitro.14,15 Protein phosphatases, on the other hand, are important for the counterbalance mechanism that removes phosphate groups from various phosphorylated amino acids. Dephosphorylation mainly occurs on the hydroxyl-group-containing amino acid residues by a hydrolysis reaction. In eukaryotic cells these amino acids are typically serine, threonine and tyrosine residues.16–18 Phosphorylation-dephosphorylation cycles serve as “on-off” switches that can trigger conformational changes of target proteins and alter their properties. 19–22
Protein phosphorylation has been extensively studied in the model yeast Saccharomyces cerevisiae. A sequence search analysis of the S. cerevisiae genome indicated the presence of 113 genes encoding putative protein kinases.23 Interestingly, while a similar number of protein phosphatases was expected to counteract and maintain reversible protein phosphorylation, only 31 putative phosphatases were identified.24 In addition, the human genome encodes about 500 protein kinases, whereas phosphatases comprise only 150 members.25,26 Therefore, many researchers have concluded that protein phosphatases are likely to display a wider range of substrate specificity than that of protein kinases. Consistent with this notion, the structural diversity of phosphatases can be mainly attributed to an alternative regulatory subunit, which results in a diverse set of enzymes with a vast array of substrate specificities.24,27
In medically important fungi, the study of phosphorylation takes on an added importance since a variety of key virulence processes are controlled by this modification. These fungi include Candida species, which represent the 4th leading cause of hospital-acquired bloodstream infections in the United States.28 Approximately 50% of infections can be attributed to the major human fungal pathogen Candida albicans, which is capable of causing a wide variety of mucosal and systemic infections in immunocompromised individuals.29,30 Major virulence properties of C. albicans include phenotypic switching, biofilm formation, adhesion to host cells, secretion of degradative enzymes and the ability to undergo a reversible morphological transition from yeast to filamentous form in response to numerous host inducing signals.31–33 Cryptococcus neoformans represents another major human fungal pathogen that can typically be found in a number of environmental reservoirs including the soil, compost piles and pigeon droppings.34,35 C. neoformans spores are inhaled by the host, eventually leading to systemic infections that particularly target the central nervous system and can result in cryptococcal meningitis.36,37 C. neoformans virulence properties include thermotolerance and melanin formation as well as formation of a protective capsule.35,38–40 Another medically important fungus, Histoplasma capsulatum, is predominantly found in the soil in endemic regions such as the Ohio river valley;41 major virulence properties include a mycelia-to-yeast transition, melanin and thermotolerance.35,42 Aspergillus fumigatus also represents a major fungal pathogen that can be found in the soil as well as on decaying plant material. As a mould, A. fumigatus grows in the mycelial form and inhalation of conidia can lead to life-threatening pulmonary and disseminated aspergillosis.43 A. fumigatus virulence properties include thermotolerance, angioinvasion, nutrient acquisition, protease secretion and gliotoxin production.44–46 Importantly, many of the virulence properties of fungal pathogens discussed above are at least partly controlled by phosphorylation. Here, we will provide a broad and comprehensive overview of the various classes of kinases and phosphatases in pathogenic fungi and their regulatory roles, with an emphasis on enzymes that target serine (Ser)/threonine (Thr) residues (since greater than 98% of protein phosphorylation occurs on these residues).47 Specific kinases and phosphatases of interest in model and pathogenic fungi are listed in Tables S1 and S2, respectively. We will also discuss new global approaches and efforts that have been made to define the phosphoproteome of pathogenic fungi as well as the potential that kinases and phosphatases may hold for serving as antifungal targets.
Ser/Thr Protein Kinases
Protein kinases in model and medically important fungi can be classified into several major groups, based on the amino acid residues that they phosphorylate, as shown in Figure 1. Ser/Thr kinases are the predominant kinase superfamily in fungi and other eukaryotes. Tyrosine kinases, by contrast, are responsible for only 0.1% of total phosphorylation events.48 As a consequence, most research efforts have centered on describing Ser/Thr kinases in medically important fungi, particularly with respect to their roles in cell cycle, morphogenesis and pathogenicity and we will therefore focus our discussion on this family.
Figure 1.
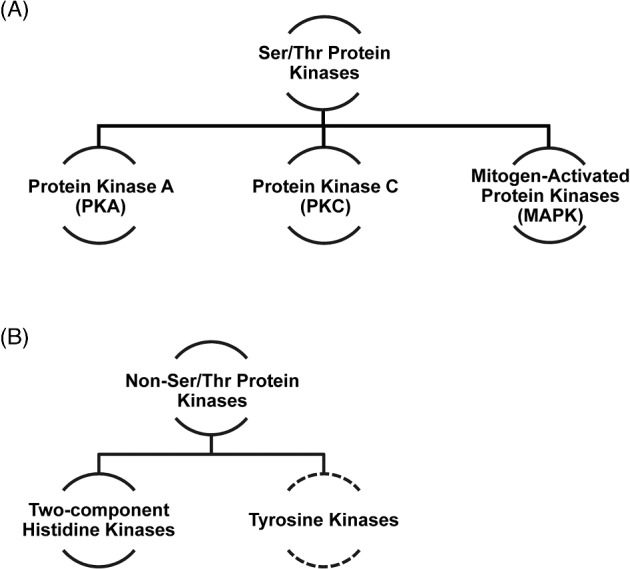
Major classes of kinases in model and pathogenic fungi. Major classes of Ser/Thr (A) and Non-Ser/Thr (B) protein kinases discussed in this review. Dashed line indicates that putative tyrosine kinases have been identified in several major human fungal pathogens.
1. Cyclic AMP-dependent protein kinase A
Protein kinase A (PKA) is a Ser/Thr protein kinase that serves as the main intracellular target of cAMP in all eukaryotes.49 PKA has been well-characterized in S. cerevisiae as well as several pathogenic fungi and was also the first protein kinase to have its crystal structure resolved.50 In fungal cells, cAMP levels are controlled by the interplay between a membrane-associated adenylate cyclase for synthesis, and a cAMP-specific phosphodiesterase for degradation. Activation of adenylate cyclase is usually mediated by heterotrimeric GTP-binding proteins in most fungi.51 The regulatory subunit of PKA normally functions to inhibit the activity of the catalytic subunit. However, upon binding to cAMP, the regulatory subunit dissociates from the catalytic subunit, leading to its activation. As a consequence, specific downstream transcription factor targets of the cAMP-PKA signaling pathway are activated, leading to the induction of genes required for many aspects of fungal growth and differentiation processes.51–53 Genes encoding the catalytic subunit of PKA have been characterized in several filamentous fungi, including Ustilago maydis, Aspergillus niger, Blastocladiella emersonii and Colletotrichum trifolii.54–57 In Neurospora crassa, the regulatory subunit of PKA, encoded by the mcb gene, was shown to be important for polarized growth.58 Interestingly, expression of the C. trifolii regulatory subunit of PKA was able to complement the N. crassa mcb mutant defect,57 suggesting that the structure and function of genes encoding fungal PKAs are highly conserved.
In the major human fungal pathogen Candida albicans, the cAMP-PKA signaling cascade is very important for morphogenesis and many components of this pathway are required for filamentous growth under a variety of different conditions, including serum and body temperature (37°C).59–63 The C. albicans PKA complex consists of two catalytic subunits, Tpk1 and Tpk2, as well as the regulatory subunit Bcy1. cAMP inhibits Bcy1, allowing Tpk1 and Tpk2 to promote filamentation by phosphorylating downstream transcription factors, which regulate the expression of filament-specific genes. Tpk1 and Tpk2 have distinct roles in promoting filamentation. Tpk1 is important for hyphal growth on solid media, whereas Tpk2 is more important for filamentation in liquid media.64 Interestingly, while Tpk1 is not required for adherence, invasion and damage of oral epithelial cells in vitro, both Tpk2 and Efg1, the downstream transcription factor target of the cAMP-PKA pathway, were shown to play important roles in these processes.65 Both tpk2Δ/Δ and efg1Δ/Δ mutants were shown to be significantly attenuated for virulence in a murine model of oropharyngeal candidiasis, although only the efg1Δ/Δ mutant was attenuated in a mouse systemic model. These results suggest that hyphal formation directed by cAMP-PKA-mediated signaling represents an important virulence mechanism in oropharyngeal candidiasis and that Tpk2 is more important for oral vs. systemic infections. While a role for Tpk1 in virulence remains elusive, its distinct ability to promote filamentation under solid conditions in vitro may suggest a more niche-specific role during infection. The highly specific roles that Tpk1 and Tpk2 play in filamentation and/or virulence also suggest that the C. albicans cAMP-PKA signaling pathway possesses a level of plasticity that can adapt to multiple host filament-inducing conditions.
The cAMP-PKA pathway is also important for regulating a variety of processes in another human fungal pathogen, A. fumigatus. The A. fumigatus PKA complex consists of a type II regulatory subunit and two catalytic subunits.66,67 The catalytic subunits (PkaC1 and PkaC2) play redundant functions with respect to conidial germination and work together to control the carbon catabolic pathway. While the pkaC1 mutant is defective for virulence in a mouse model of invasive aspergillosis, overexpression of pkaC2 in this mutant can rescue the virulence defect. In addition, the pkaC1 single mutant appeared to be less attenuated for virulence in this model than the pkaC1 pkaC2 double mutant.66 While the complementary roles of pkaC1 and pkaC2 in conidial germination and carbon catabolism may contribute to virulence, these results also suggest that PkaC1 and PkaC2 may have independent functions associated with pathogenesis. Deletion of the gene encoding the regulatory subunit of protein kinase A, pkaR, resulted in reduced A. fumigatus growth and germination rates, morphological abnormalities in conidiophores and reduced conidiation.68 Consistent with findings for the A. fumigatus PKA catalytic subunit mutants, the pkaR mutant was also found to be significantly attenuated for virulence when conidia were administered intranasally in an immunosuppressed mouse model.
In the human fungal pathogen C. neoformans, the cAMP-PKA pathway has been found to be important for regulating many cellular processes, including capsule production, melanin formation, mating, and virulence.69 Mutant strains lacking conserved components of the cAMP-signaling cascade such as Gα protein (Gpa1) and adenylyl cyclase (Cac1) are attenuated for virulence, most likely as a result of not showing an increase in capsule or melanin production in response to normal inducing conditions.70–72 In addition, mutants lacking the C. neoformans PKA catalytic subunit, Pka1, are unable to mate, fail to produce melanin or capsule, and show reduced virulence in animal models, whereas mutants lacking the PKA regulatory subunit, Pkr1, are hyper-virulent and overproduce capsule.73 Interestingly, hyperactivation of the PKA signaling pathway leads to enhanced virulence in C. neoformans72 whereas in the plant fungal pathogen U. maydis, PKA hyperactivation results in defects in tumor (gall) formation and thus reduced virulence.74,75 These studies illustrate how a conserved phosphorylation signaling pathway has been exploited to serve related, but distinct, virulence functions in two different fungal pathogens as they evolved to adapt to different host environments.
2. Protein kinase C
Protein kinase C (PKC) is a calcium/phospholipid-dependent Ser/Thr kinase, which acts as a transmitter and amplifies signal transduction pathways. PKC is a key component of the phosphoinositide cascade, which stimulates a wide variety of responses in various cell types, including cell proliferation, gene expression, membrane transport and organization of the cytoskeleton.76,77 In fungi, genes predicted to encode PKCs have been characterized in S. cerevisiae,78 Schizosaccharomyces pombe,79 C. albicans,80 Trichoderma reesei, and A. niger.81 PKC orthologs are well-conserved among these fungi and appear to function as regulators of cell wall biosynthesis.82 In S. cerevisiae, Pkc1 has multiple targets and is important for maintaining cell wall integrity in response to stress during growth and morphogenesis.83–85 Interestingly, S. cerevisiae Pkc1 has been shown to function independently of both Ca2+ and phospholipids, but is regulated by autophosphorylation.78 However, in S. pombe and T. reesei PKC activity is phospholipid-dependent, but Ca2+-independent.79,81
In C. neoformans, the PKC signaling pathway is important for fluconazole tolerance as well as invasion of human brain microvascular endothelial cells.86,87 C. neoformans strains deleted for PKC1, encoding a key component of this pathway, show altered capsule formation, reduced melanin production and are hypersensitive to oxidative and nitrosative stress, cell wall-inhibiting agents and temperature.88 The PKC signal transduction pathway has also been shown to play a key role in controlling C. neoformans cell wall integrity.89 In the filamentous fungus A. nidulans, the pkcA gene (encoding PKC) is essential and important for establishment of polarity and suppression of apoptosis under thermal stress.90,91 In C. albicans, homozygous pkc1 deletion mutants are viable and can undergo the yeast-to-hypha transition, but both yeast and hyphal cells show increased lysis defects.92 The C. albicans Pkc1-activated mitogen-activated protein kinase (MAPK) cascade is conserved and has been implicated in the up-regulation of chitin synthase (CHS) genes in response to antifungals such as echinocandins.92 These studies support a role for PKCs in maintaining cell wall integrity during growth and morphogenesis of both pathogenic and nonpathogenic fungi.
3. Mitogen-activated protein kinases
Mitogen-activated protein (MAP) kinase cascades are evolutionarily conserved among all eukaryotes and have been identified in a variety of organisms from fungi to humans.93,94 MAP kinases have been shown to participate in transducing a diverse array of extracellular signals and regulating vital cellular processes such as cell differentiation, cell movement, cell division, and cell death.95–97 MAP kinases are usually activated by dual phosphorylation of tyrosine and threonine residues by MAP kinase kinases (MAPKK), which in turn, are activated by MAP kinase kinase kinases (MAPKKK). The sequential activation of the MAPK cascade eventually results in the activation of transcription factors and the expression of specific sets of genes in response to environmental stimuli.93
In S. cerevisiae, MAP kinase signal transduction pathways have been extensively studied and shown to be involved in many cellular processes including mating, high osmolarity responses, cell wall remodeling, filamentation, and sporulation.98,99 Adaptation to osmotic stress mainly occurs through the high osmolarity glycerol (HOG) MAP kinase pathway.98 The S. cerevisiae MAP kinase pathway associated with mating is triggered by pheromones and involved in shmoo formation as well as subsequent diploid formation.100 The S. cerevisiae filamentous growth MAPK pathway functions through Kss1 and is activated by the Ras2-Cdc42-Bmh1-Ste11 cascade.101–103
MAP kinases have also been shown to be important for virulence and/or virulence-related processes of several fungal pathogens, including Botrytis cinerea,104 Cochliobolus heterostrophus,105 Fusarium oxysporum106 and Ustilago maydis.107 In C. albicans, MAPK signal transduction pathways that regulate the yeast-to-hypha transition, virulence and white-opaque switching have been well-studied and characterized.108–110 The Cek1 (homolog of S. cerevisiae Kss1) MAPK cascade plays an important role in the C. albicans yeast-hypha transition and virulence.111 Several components of the Cek1-MAPK pathway, including STE2, CST20, HST7, CEK1, and CPH1, are also involved in C. albicans mating responses.108,110 In addition, the C. albicans cell wall integrity pathway, important for virulence, is controlled by the Mkc1-MAPK pathway.112,113
In C. neoformans, the Cpk1-MAPK signaling cascade plays important roles in mating and monokaryotic fruiting, and shares many features with the well-characterized pheromone response pathway in S. cerevisiae described above. Both mating and monokaryotic fruiting in C. neoformans are mediated by Gbp1, which activates Ste20, a p21-activated protein kinase (PAK) homolog in the Cpk1-MAPK cascade.114 Interestingly, in contrast to the case of S. cerevisiae, disruption of C. neoformans STE12, encoding a downstream pheromone response target of the Cpk1-MAPK pathway, does not abolish pheromone sensing or mating,115,116 and additional downstream effectors for Cpk1 in this cascade have been identified.117 A conserved Pbs2-Hog1 MAP kinase pathway has also been shown to control morphological differentiation as well as virulence properties (eg, thermotolerance, response to oxidative stress) in the highly virulent serotype A but not a less virulent laboratory-generated serotype D, strain of C. neoformans.118 Interestingly these findings suggest that fungal pathogens such as C. neoformans have evolved specialized MAP kinase signal transduction pathways to control virulence-related properties in more pathogenic strains. Not surprisingly, C. neoformans serotype A hog1Δ mutants were found to be attenuated for virulence in a mouse model of disseminated cryptococcosis.
In Pneumocystis carinii, a gene encoding a putative MAPKKKK, PCSTE20, has been shown to be strongly up-regulated in response to binding of the pathogen to extracellular matrix proteins.119 In A. fumigatus there are four known MAPKs: SakA is closely related to the HOG-MAPKs of other fungi, MpkB is similar to MAPKs involved in pheromone signaling, MpkA is similar to MAPKs involved in cell wall integrity and MpkC appears to be involved in conidial germination.120 SakA and MpkA are both associated with A. fumigatus morphogenesis.121,122 Deletion of the gene encoding the HOG-MAPK pathway component SakA results in abnormal conidial germination under different environmental conditions.122 Overall, these studies indicate the important role that Ser/Thr signaling kinases play in morphology and mediating additional virulence-related processes in A. fumigatus and other pathogenic fungi.
Non-Ser/Thr protein kinases
While considerably less abundant than Ser/Thr kinases, several non-Ser/Thr protein kinases, in particular two-component histidine kinases, play important regulatory roles in human fungal pathogens. Two component histidine kinase systems are composed of a histidine kinase (HK) and a response regulator (RR) protein. In S. cerevisiae, histidine kinase phosphorelay systems transmit signals to activate the HOG1-MAPK pathway in response to osmotic stress.123 In C. albicans, Cos1, a two-component histidine kinase, is important for hyphal development under both solid and liquid filament-inducing conditions. 124 Nik-1, a homolog of Cos1 in the filamentous fungus N. crassa, was also found to be important for filamentation, especially under increased osmotic pressure during growth on solid medium.125,126 In addition, SRR1, a putative two-component response regulator gene in C. albicans, was found to be important for oxidative and osmotic stress adaptation, morphogenesis, and virulence.127 Two-component histidine kinase systems have also been reported to be essential for stress adaptation and virulence in other pathogenic fungi, including A. fumigatus, C. neoformans, and B. dermatitidis.42,128–130
Tyrosine kinases represent a second class of non-Ser/Thr kinases. A general comparative genetic analysis of over 30 different fungal species determined that the overall representation of the tyrosine kinase group is very small.131 While tyrosine kinases have been shown to be important for cell cycle control in S. cerevisiae132 and mitotic entry/DNA damage checkpoint control in S. pombe,133,134 considerably little is known about the role of these enzymes in pathogenic fungi. However, putative tyrosine kinases have been identified in several major human fungal pathogens including C. albicans and C. neoformans (http://www.candidagenome.org/, http://genome.jgi-psf.org/pages/search-for-genes.jsf?organism=Cryne_JEC21_1).
Ser/Thr protein phosphatases
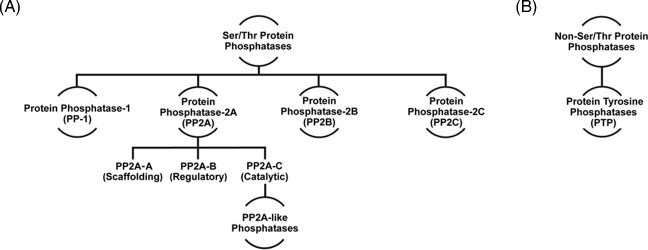
Ser/Thr protein phosphatases represent more than 90% of all phosphatases and play essential regulatory roles in all eukaryotes.135 An increasing number of Ser/Thr protein phosphatases have been discovered and characterized in fungi, several of which play important cellular functions including cell cycle regulation, growth, protein synthesis, filamentation and maintenance of cellular integrity.19,135,136 Interestingly, biochemical analyses of Ser/Thr protein phosphatases in certain filamentous fungi have provided evidence for functional similarities with those studied in higher eukaryotes.137–139 For example, in the presence of calmodulin, a highly conserved catalytic subunit of a N. crassa calmodulin-dependent protein phosphatase showed equivalent phosphatase activity to that of bovine brain calcineurin.138 In addition, a protein phosphatase-1 (PP1) inhibitor has also been shown to effectively inhibit both mammalian and N. crassa PP1.137 While Ser/Thr protein phosphatase catalytic domains are remarkably similar, enzyme structural diversity within subfamilies is mainly attributed to regulatory subunit specificities.23,25 Ser/Thr protein phosphatase complexes consist of multiple combinations of the conserved catalytic subunit and numerous regulatory subunits that control a broad spectrum of signaling pathways.18,22,139–143 Due to the ‘eccentric' functionality of these enzymes, relatively few Ser/Thr phosphatases control the specific dephosphorylation of thousands of phosphoprotein substrates.144 Ser/Thr protein phosphatases are classified biochemically based on substrate specificity and sensitivity to endogenous inhibitors141 and are divided into two broad groups, type-l and type-2. The type-2 enzymes are further separated into three subgroups, 2A, 2B, and 2C, based on their structure and regulation19,23 as illustrated in Figure 2. Next, we will discuss the role of specific Ser/Thr phosphatase subfamilies in controlling a variety of biological processes in model and medically important fungi.
Figure 2.
Major classes of phosphatases in model and pathogenic fungi. Major classes of Ser/Thr (A) and Non-Ser/Thr (B) protein phosphatases discussed in this review.
1. Protein phosphatase 1
Protein phosphatase 1 is one of the major eukaryotic Ser/Thr protein phosphatase classes that regulates an enormous variety of cellular functions. This is believed to occur by interaction of the catalytic subunit of this enzyme with multiple regulatory subunits.145–147 In contrast to the protein Ser/Thr kinases,148 PP-1 does not display obvious consensus sequence selectivity, dephosphorylating multiple substrates both in vivo and in vitro.149
From fungi to mammals, PP-1 has been shown to play an evolutionarily conserved role in controlling cell cycle progression.150–153 S. pombe DIS2, which encodes PP-1, is required for chromosome disjoining during mitosis.154 In S. cerevisiae, multiple studies have also suggested that PP-1 is important for reversing phosphorylation of aurora kinases, a family of mitotic Ser/Thr kinases, during mitosis and meiosis.155–159 In A. nidulans, BIMG11, which encodes PP-1, is required for completion of anaphase in the cell cycle.160 More recent work in S. cerevisiae has suggested that PP-1 is also important for regulating the spindle checkpoint during chromosome segregation in the cell cycle.153,161
In addition, PP-1 is known to control protein synthesis in a wide range of eukaryotes.162,163 Phosphorylation of eIF2α is the principal mechanism yeast cells use to inhibit protein synthesis under a variety of stress conditions including amino acid starvation. PP-1, however, restores protein synthesis by dephosphorylating eIF2α.164 PP-1 has also been shown to be important for controlling glycogen accumulation in yeast as well as metabolism and glucose regulation in mammals.165–167
In C. albicans, few PP-1 enzymes have been identified and characterized. A study has determined that the PP-1/Glc7 regulator, Shp1, plays important roles in C. albicans morphogenesis, cell cycle progression and DNA damage response.168 In S. cerevisiae Bni4 represents the PP-1/Glc7 phosphatase targeting subunit and is involved in bud-neck localization of chitin synthase.169,170 A C. albicans strain deleted for the BNI4 homolog formed lemon-shaped yeast cells, had a 30 % reduction in cell-wall chitin, and showed reduced hyphal formation under filament-inducing conditions. 171 These results suggest an important role for PP-1 in C. albicans cell wall maintenance and filamentation. A C. albicans mutant for Sal6, a PP-1-related phosphatase, has been reported to have a slight to moderate virulence defect in a silkworm infection model.172 Overall, PP-1 plays critical roles in dephosphorylating substrates to control a variety of cellular processes in fungi, including mitosis, meiosis, cell division, filamentous growth, protein synthesis and glycogen metabolism.24
2. Protein phosphatase-2B
Protein phosphatase 2B (PP2B), also known as calcineurin (CaN), is a highly conserved Ca2+/calmodulin-regulated Ser/Thr protein phosphatase present in many organisms from yeast to humans.173 Calcineurin is typically composed of a catalytic calmodulin-binding A subunit and a regulatory Ca2+-binding B subunit.174 The regulatory B subunit functions to promote the activity of the catalytic A subunit. Like other Ser/Thr protein phosphatases, calcineurin also has broad substrate specificity. Calcineurin functions in many pathogenic fungi to control a broad spectrum of cellular processes, including cation homeostasis, morphogenesis, and virulence175,176 and is considered to be a key regulator of cellular stress responses in eukaryotes.176
In S. cerevisiae, both genes encoding calcineurin catalytic subunits (CNA1 and CNA2) are not essential for viability.177 However, calcineurin is required for cellular adaptation under a variety of environmental stresses. Once activated, calcineurin dephosphorylates the transcription factor Crz1, which, in turn, activates genes involved in a wide variety of processes, including signal transduction and cell wall integrity.178 In C. albicans, calcineurin is not essential. However, this phosphatase is critical for mediating cell survival during membrane stress.179 Calcineurin can be pharmacologically inhibited in C. albicans by the combination of either cyclosporine A or tacrolimus (FK506) with fluconazole.179 Homozygous deletion of C. albicans CMP1, which encodes the calcineurin A (CNA) subunit, resulted in hypersensitivity to serum and antifungal agents that target ergosterol biosynthesis in vitro, as well as attenuated virulence in a mouse model of systemic candidiasis.180,181 These findings suggest that calcineurin plays a key role in the ability of C. albicans to adapt to serum and stress conditions in the host environment and the observed virulence defect may be attributed to a reduced ability to respond to environmental stresses during infection. Deletion of the calcineurin target, CRZ1, in C. albicans results in hypersensitivity to membrane stress conditions. Interestingly, crz1 homozygous deletion mutants are not defective for virulence in a mouse model of systemic candidiasis.182,183 Deletion of CRZ1 only partially reduces azole resistance in S. cerevisiae, whereas deletion of CNB1, a regulatory subunit of calcineurin, completely blocks resistance.182,184 These results suggest that additional downstream effector(s) of the calcineurin-signaling cascade, besides Crz1, regulate azole resistance.
In C. neoformans, calcineurin plays a central role in regulating virulence and morphogenesis.185,186 Pharmacological inhibition of calcineurin by FK506 renders cells unable to mate. Calcineurin is also required for C. neoformans hyphal elongation in diploid strains and asexual monokaryotic fruiting of MATα cells in response to nitrogen limitation.187 Indeed, calcineurin is required for virulence in both a rabbit model of cryptococcal meningitis as well as a murine systemic model.185,188 These virulence defects can most likely be attributed to the inability of C. neoformans calcineurin mutant strains to survive under in vitro conditions similar to those of the host environment (alkaline pH, high temperature, 5% CO2). Cbp1, a calcineurin-binding protein in C. neoformans, functions as a targeting subunit to regulate mating-dependent filamentation.186,189 However, cbp1 mutants show no defects during haploid fruiting and only a modest virulence defect in mice, suggesting that additional targeting proteins(s) interact with calcineurin to regulate these processes.
The calcineurin pathway is also important for morphology in A. fumigatus. Both pharmacological and genetic inhibition of A. fumigatus calcineurin impairs filamentation, resulting in delayed hypha production.190 Strains bearing mutations in cnaA, which encodes the A. fumigatus calcineurin catalytic subunit, display improper polarized growth, reduced filamentation, and decreased virulence in a mouse model of invasive aspergillosis;191,192 virulence defects are most likely at least partly attributed to reduced filamentation. Similar defects are observed upon mutation of A. fumigatus crzA (the CRZ1 homolog).193,194
Interestingly, recent studies have demonstrated that calcineurin plays a key role in the dimorphic transition and virulence of Mucor circinelloides.195,196 M. circinelloides is a causative agent of mucormycosis, a frequently lethal, but uncommon human fungal infection.197 Deletion of the gene encoding the calcineurin regulatory B subunit of M. circinelloides resulted in a mutant locked in yeast phase growth.195 Similar results were also observed when M. circinelloides was grown in the presence of the calcineurin inhibitor FK506. The calcineurin regulatory B subunit gene deletion mutant was also attenuated for virulence in a wax moth larvae model, suggesting that the M. circinelloides yeast-hyphal dimorphic transition is important for this process. More recent work with this yeast-locked mutant has showed that phagosome maturation occurs in the presence of yeast but not spores.196 Surprisingly, M. circinelloides mutants for cnaA, encoding the calcineurin A catalytic subunit A, showed larger size spores and increased virulence in the wax moth larvae model.195 One possible explanation for this unexpected finding is that calcineurin phosphatase negatively regulates other kinases in the cell that are important for virulence. Consistent with this notion, in U. maydis and S. cerevisiae there is an established antagonistic relationship between calcineurin and PKA.198,199 Interestingly, mutants in the M. circinelloides calcineurin A catalytic subunit B showed several functional differences when compared to cnaA mutants (eg, greater sensitivity to cyclosporine A and inability to produce hyphae in the presence of this compound) and were not attenuated for virulence in the wax moth larvae model.196 As in the case of PKA, these findings suggest that M. circinelloides calcineurin catalytic subunits play related, but distinct, roles with respect to morphology, virulence and/or response to the host environment.
3. Protein phosphatase-2C
Protein phosphatase-2C (PP2C) is a class of Mg2+-dependent Ser/Thr phosphatases that are highly conserved, present in both prokaryotes and eukaryotes and involved in a wide variety of key cellular processes, including proliferation, metabolism, and cell death.200–202 In contrast to other Ser/Thr phosphatases, PP2C phosphatases are monomeric enzymes and share no structural homology with PP-1, PP2A, or PP2B.202 PP2C phosphatases are not associated with multiple regulatory subunits and their function is usually achieved by multiple catalytic isoforms. For example, more than 14 genes encoding PPC phosphatases were identified in humans, and up to 80 PP2C proteins have been predicted in Arabidopsis thaliana.203,204 These multiple catalytic isoforms are likely to provide the structural basis for functional specificity. In S. cerevisiae there are seven identified PP2C-encoding genes (PTC1-7),205,206 which are involved in diverse cellular functions. Homologs of several of these genes play important roles in medically important fungi.
Ptc1 phosphatase is the best-characterized of the PP2C isoforms in yeast. Several genetic studies have demonstrated that Ptc1 is a negative regulator of the HOG pathway and associates with components of this pathway in S. cerevisiae.207–209 Ptc1 phosphatase has also been linked to the yeast MAPK cell wall integrity (CWI) pathway (Slt2/Mpk1) via interaction with Pck1 kinase.210 Ptc1 plays an important role in the regulation of mating in S. cerevisiae211 and is likely to be involved in controlling numerous additional processes in yeast since ptc1 mutant strains are hypersensitive to heavy metals, alkaline pH, calcium ions, and exhibit fragmented vacuoles, a random budding pattern, as well as defects in both vacuolar and cortical ER inheritance.210,212–214 Ptc2 and Ptc3 have been implicated in regulating progression through the yeast cell cycle.205,215 S. cerevisiae Ptc6 has been shown to be necessary for survival of stationary phase cells and is also involved in the mitochondrial degradation process known as mitophagy.216,217
The C. albicans homozygous null ptc1 mutant is more resistant than a wild-type strain to the cell wall stressor Congo red and the antifungal terbinafine.172 However, this mutant also shows hypersensitivity to the echinocandin-derived antifungal micafungin, reduced hyphal growth both in vitro and in vivo and a significant attenuation in virulence in both silkworm and mouse models of disseminated candidiasis.172 C. albicans cells deleted for PTC2 are sensitive to azole antifungals and SDS, as well as the DNA synthesis inhibitor hydroxyurea and the DNA methylation agent methylmethane sulphonate (MMS).218 Ptc2 is also associated with mitochondria and these findings suggest that this phosphatase has multiple functions in C. albicans, including checkpoint recovery from DNA damage and the control of mitochondrial physiology. Disruption of other Ptc isoforms, such as PTC7, does not affect growth or filament development in C. albicans.219 A new member of the PP2C family, Ptc8, has also been characterized in C. albicans.220 PTC8 is induced in response to growth in the presence of high osmolarity as well as serum at 37°C. The ptc8Δ/Δ mutant is defective for hyphal formation220 but has not been linked to any known filamentous growth signaling pathways.
In Fusarium graminearum, the major causal agent of Fusarium head blast disease on barley and wheat, PTC1 was found to play an important role in the ability of mycelial growth to resist lithium toxicity.221 Deletion of PTC1 attenuates F. graminearum virulence on wheat coleoptiles but not on wheat heads.221,222 While these results may suggest that mycelial growth mediated by Ptc1 plays a specialized role in directing virulence against specific niches on wheat, another possible explanation is that independently constructed versions of the ptc1 deletion strain were used to test for virulence in the wheat coleoptile vs. head models. We conclude that PP2C phosphatases play an integral role in an array of key cellular processes in pathogenic and non-pathogenic fungi, including cell wall integrity, filamentous growth, and virulence.
4. Protein phosphatase-2A
Type 2A protein phosphatases (PP2A) constitute a diverse family of Ser/Thr phosphatases that are ubiquitously expressed in eukaryotic cells and perform multiple functions in cellular signaling.141 PP2A is a multiprotein complex composed of three distinct subunits. The A subunit (PP2A-A) is the structural subunit that serves as a scaffold to accommodate the other two subunits. The C subunit (PP2A-C) is the catalytic subunit and the B subunit (PP2A-B) is the regulatory subunit which dictates substrate specificity and intracellular localization of the enzyme.141 It is considered the most structurally diverse subunit. To date, four unrelated protein families of PP2A regulatory subunits have been identified: B, B′, B″, and B″′.223–225 In higher eukaryotes (eg, mammals) each family is encoded by multiple genes and some transcripts of these genes undergo alternative splicing to generate an even greater number of isoforms.226,227 The functional involvement of PP2A in so many diverse biological processes can be largely attributed to the B subunit. PP2A exists in two different forms: dimeric form (PP2AD) and trimeric form (PP2AT). The dimeric form is known as the core enzyme and is composed of the catalytic and scaffold subunits, while the trimeric form is an active heterotrimeric holoenzyme complex which consists of all three subunits.139,141,228
Regulation by PP2A and PP2A catalytic, scaffold and regulatory subunits
PP2A phosphatases are highly conserved from fungi to humans and involved in a variety of functions in multiple species, including cell differentiation, cell cycle, oncogenic transformation, signal transduction, and filamentous growth.139,229 Not surprisingly, PP2A phosphatases are also tightly regulated by post-translational modifications. These modifications mainly involve methylation at the carboxyl terminus of the catalytic subunit230,231 and phosphorylation.232
In S. cerevisiae, loss of both PP2A catalytic subunits (PPH21 and PPH22) impairs growth, but is not lethal.233,234 In comparison, PPA1 and PPA2, which encode PP2A catalytic subunits, are essential for growth in S. pombe.235 In A. nidulans, deletion of PPHA, a PP2A homolog, leads to slow growth, delayed germ tube emergence and mitotic defects at low temperature.236 The S. cerevisiae PP2A scaffolding subunit is encoded by the gene TPD3. Deletion of TPD3 is not lethal but renders yeast cells cold-sensitive. Following a shift to 13°C, tpd3 mutant cells become multibudded and multinucleate, suggesting a defect in cytokinesis.237 The tpd3 deletion mutants are also sensitive to high temperature (eg, 37°C), and this temperature sensitivity phenotype is most likely attributed to a defect in RNA polymerase III transcription.237 A recent BLAST search has indicated the presence of orthologs for Tpd3 in major human fungal pathogens, including C. albicans, A. fumigatus, H. capsulatum, and C. neoformans (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
The PP2A regulatory subunit (PP2A-B) is encoded by one gene in S. cerevisiae, CDC55. S. cerevisiae strains deleted for CDC55 showed multi-budded and multi-nucleated yeast cells (similar to the tpd3 phenotype), suggesting a role for PP2A in cell cytokinesis.238 Genetic analysis indicates that Cdc55 is involved in at least two steps during the cell cycle: the metaphase-anaphase transition and mitotic exit.239–241 Orthologs for Cdc55 are present in multiple human fungal pathogens such as H. capsulatum, A. fumigatus, C. neoformans and C. albicans (http://blast.ncbi.nlm.nih.gov/Blast.cgi). In N. crassa, PP2A-B is required for completion of macroconidiation. A study has found that RGB-1, encoding a putative PP2A-B regulatory subunit, is a regulator of budding during the macroconidiation process; rgb-1 mutants, which are defective for macroconidiation budding, instead undergo arthroconidiation.242 In A. nidulans, two PP2A-B regulatory subunit homologs, parA and pabA, have been recently identified and characterized.243 Deletion of parA causes hyper-septation, while overexpression of parA abolishes septum formation. Interestingly, this study also showed that parA deletion is capable of suppressing septation defects in pabA mutants,243 suggesting that ParA counteracts PabA during the septation process. However, both PP2A-B regulatory subunits act synergistically during hyphal growth, since a double mutation of parA and pabA led to synthetic defects in colony growth at 42°C.243
PP2A-like phosphatases
PP2A-like phosphatases show a degree of sequence identity to PP2A enzymes but are not sufficiently identical to be classified as homologs.233,234,244,245 In addition, in yeast, a PP2A-like protein has been shown to only partially complement defects of strains deleted for PP2A.233 PP2A-like phosphatases can form complexes with regulatory subunits and are highly conserved from yeast to humans.246–249 Three different PP2A-like phosphatases have been identified in fungi: Sit4, Pph3 and Ppg1.245
Sit4 plays a key role in cell growth and proliferation in S. cerevisiae.233,245,250 Deletion of SIT4 also causes cell cycle arrest at late G1, suggesting that the Sit4 phosphatase is required for the G1/S transition.244 Another study has suggested that Sit4 is required for expression of the G1 cyclins, CLN1 and CLN2.251 S. cerevisiae SIT4 has also been shown to be involved in the Pkc1-MAPK signaling pathway, which is important for the transcriptional response to stresses that alter cell wall integrity.84,252 In C. albicans, disruption of SIT4 causes a significant reduction in growth rate, hyphal formation and virulence in a mouse model of systemic candidiasis.253 Consistent with these findings, a more recent study has indicated that the C. albicans sit4 null mutant is defective for morphogenesis on solid Spider medium.254 C. albicans sit4 cells also displayed reduced transcript levels for genes encoding HOG1-MAPK pathway components in a DNA microarray experiment.253
A second PP2A-like phosphatase is Pph3. In C. albicans, Pph3 and its regulatory subunit, Psy2, control dephosphorylation of Rad53, a putative component of the cell cycle checkpoint, and cell morphogenesis during recovery from DNA damage.246,255 Deletion of PPH3 or PSY2 results in hypersensitivity to DNA-damaging agents, such as cisplatin and MMS.246 In addition, pph3Δ/Δ and psy2Δ/Δ mutant cells show robust filamentation under genotoxic stress.246 Interestingly, more recent studies in S. cerevisiae have linked the activity of Pph3 to both the nonhomologous end-joining (NHEJ) pathway as well as cell cycle progression.256 Consistent with the later finding, a recent study has determined that the C. albicans Pph3–Psy2 phosphatase complex is important for Rfa2 dephosphorylation during G1-phase and under DNA replication stress.257 Rfa2 is a key subunit of the replication protein A (RPA) heterotrimeric complex, which functions in DNA replication, repair and recombination pathways in eukaryotes.258,259 Altogether, these studies suggest that the Pph3/Psy2 complex plays key roles in cell morphogenesis, cell cycle progression and/or recovery from DNA damage in S. cerevisiae and C. albicans. However, little is known about the function of Pph3/Psy2 complexes in other fungal systems.
The final PP2A-like phosphatase that we will discuss is Ppg1. PPG1 was first cloned and identified in S. cerevisiae based on sequence similarity to other Ser/Thr phosphatases.245 S. cerevisiae ppg1 deletion mutants are viable, but show a decrease in glycogen accumulation.245 Recently, a transposon mutagenesis screen has suggested a role for Ppg1 in ethanol and heat tolerance in S. cerevisiae.260 In S. pombe Ppa3, a Ppg1 ortholog, was found to be involved in regulating two of the SIN (septation initiation network) pathway kinases, Cdc7 and Sid1, important for actomyosin ring maturation and stability.261 A more recent study, however, demonstrated that the S. pombe ppg1Δ strain shows normal cell morphology.262 C. albicans Ppg1 was first identified in a screen of orthologs of previously annotated S. cerevisiae protein phosphatases.172 A systematic screen of a C. albicans homozygous deletion library has demonstrated that the ppg1Δ/Δ mutant strain is defective for morphogenesis and shows reduced kidney fungal burden in a mouse model of systemic candidiasis.254 A recent study has also demonstrated that both a ppg1Δ/Δ mutant as well as a mutant specifically defective for Ppg1 phosphatase activity show reduced filament extension and invasion as well as highly attenuated virulence in the mouse systemic model.136 In addition, C. albicans Ppg1 appears to function via the cAMP/PKA filamentous growth pathway. While the ppg1Δ/Δ virulence defect is most likely attributed to defects in filamentation and invasion, Ppg1 may control other virulence-related processes which have yet to be elucidated. Within C. albicans, the Ppg1 catalytic subunit is highly conserved among other PP2A and PP2A-like phosphatases.
Non-Ser/Thr protein phosphatases
Three protein tyrosine phosphatases (PTPs) have been identified in S. cerevisiae: Ptp1, Ptp2, and Ptp3.263,264 Ptp2 and Ptp3, but not Ptp1,265 are involved in regulation of various MAPK cascades. Ptp2 and Ptp3, however, differ in their ability to dephosphorylate yeast MAP kinases. Ptp2 preferentially dephosphorylates Hog1 and Mpk1 (involved in the cell wall integrity pathway), whereas Ptp3 preferentially dephosphorylates Fus3 (involved in the pheromone response pathway).264,266,267 A ptp2Δ/Δ ptp3Δ/Δ double mutant shows significantly decreased sporulation efficiency in S. cerevisiae.21 In human fungal pathogens, the role of PTPs in controlling virulence and virulence-related properties is poorly understood. A recent study, however, has found that PTP1 and PTP2 are important for both C. neoformans differentiation and pathogenicity.268 Consistent with results in S. cerevisiae, C. neoformans Ptp2 suppressed the hyperphosphorylation of Hog1. C. neoformans Ptp2 was also found to be involved in mediating vegetative growth, sexual differentiation, stress responses, and antifungal drug resistance. In contrast, C. neoformans Ptp1 was not essential for Hog1 regulation. However, PTP1 overexpression could rescue or partially rescue ptp2 mutant defects in thermotolerance, as well as resistance to H2O2, flucytosine and CdSO4. Importantly, this study also determined that Ptp2 is important for virulence in a murine model of systemic cryptococcosis. The observed virulence defect can most likely at least partly be attributed to one or more of the in vitro ptp2 mutant defects listed above. It is hoped that future studies will identify and characterize PTPs in other human fungal pathogens. While protein histidine phosphatases (PHPs) represent an important class of non-Ser/Thr phosphatases, their potential role in medically important fungi remains elusive.
Global analyses of fungal pathogen phosphoproteomes
Recent advances in proteomics have made it possible to define the complete set of proteins in human fungal pathogens which are phosphorylated (phosphoproteome). Typically, proteins isolated from cultures grown in vitro are digested with trypsin, subjected to titanium dioxide-based enrichment and analyzed by mass spectrometry. A recent phosphoproteomic study in the model filamentous fungus A. nidulans269 identified 1801 phosphosites corresponding to 1637 unique phosphorylated peptides. Further analysis indicated an enrichment among the phosphoproteins for gene ontology (GO) terms related to fungal morphogenesis, including “site of polarized growth,” “vesicle-mediated transport,” and “cytoskeleton organization.” The majority of phosphoproteins were targets of the CDK and CK2 kinase families. A significant number of substrates for kinases that control hydrolytic enzyme secretion were also identified by this analysis.269
A recent phosphoproteomic analysis of C. neoformans270 has identified 1089 phosphopeptides from 648 protins, including 45 kinases. Similar to the case of A. nidulans, most CDK substrates were phosphorylated, as indicated by a motif enrichment analysis. Among the phosphoproteins, enriched GO terms included “metabolism,” “transport,” “signal transduction,” “transcription,” “cell cycle progression,” and “stress response.” Phosphorylated kinases identified by this study were known to control the cell cycle, metabolic processes and virulence. Kinases included components of the cAMP/PKA and MAPK pathways. Phosphorylation of cAMP/PKA components is known to be important for controlling C. neoformans capsule size and melanin biosynthesis.271 Additional phosphoproteins included components of the PKC MAPK signaling pathway, important for cell wall integrity and thermotolerance.88,89,270 Four phosphopeptides corresponding to Sp1, a transcription factor important for resistance to nitrosative stress, maintenance of cell wall integrity and virulence,272 were also identified in this study.270 In addition, two members of the p21-activated protein kinase (PAK) family, important for mating, cytokinesis and virulence in serotypes A and D,273 were shown to be phosphorylated. Finally, phosphopeptides corresponding to Ypk1, important for the ability of C. neoformans to tolerate fluconazole treatment were also identified.270 Altogether, results from this study strongly suggest that a wide variety of processes important for C. neoformans virulence appear to be controlled by phosphorylation.
A comprehensive analysis of the C. albicans phosphoproteome in hyphal form cells has also recently been carried out.274 In sum, 15,906 unique phosphosites were identified on a total of 2,896 proteins. Serine and threonine phosphosites were highly represented (80.01% and 18.11%, respectively) and, as expected, tyrosine phosphosites were a small minority (1.81%) of the total. Interestingly, several differences were noted in GO enrichment for Tyr vs. Ser/Thr phosphorylated proteins. For example, a greater fraction of Tyr-phosphorylated proteins were enriched for “kinase,” “DNA-binding,” and “signal transducer” Molecular Function categories. Proteins important for maintaining and establishing cytoskeletal polarity, as well proteins associated with hyphal growth, were among the most highly phosphorylated. These proteins included Gin4, a Ser/Thr protein kinase involved in septum formation, and the related kinase Hsl1. Bud neck and septin ring formation proteins, including Spa2, Bni3, and Bud4 were also highly phosphorylated. As expected, numerous components and targets of the C. albicans Ras cAMP/PKA filamentous growth pathway were also found to be phosphorylated. In addition, the Mediator complex, important for RNA polymerase II transcription, was highly phosphorylated. Several of these phosphorylation events were found to be mediated by Cbk8, a kinase component of Mediator important for stress resistance, metabolism and hyphal growth.274
Although relatively few phosphoproteomic analyses have been performed to date in pathogenic fungi, these studies have highlighted the importance of phosphorylation for controlling multiple virulence-related processes and are beginning to provide new insights into the global impact of phosphorylation on fungal pathogenesis. The recent emergence of new and more quantitative proteomic techniques should facilitate this process. Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC) involves metabolic incorporation of stable isotope-labeled amino acids, such as 15N-arginine, into the proteome of cells grown in culture.275–277 Equal quantities of protein extracts from cells grown in both “light” medium, containing natural isotope amino acids, and “heavy” medium, containing labeled isotope amino acids, are mixed, digested into peptides and analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS). The advantage of this technique is that the ratio of signal intensities from “light” and “heavy” samples provides a highly accurate quantitation of relative protein abundance. In addition, unlike previous techniques which involve examining 32P-labeled bands on 1D or 2D gels or Westerns with phosphosite-specific antibodies, SILAC can be used to very accurately and quantitatively detect novel phosphosites and dynamic changes in phosphorylation events that occur on a global scale across the whole proteome.16,277,278 In addition to SILAC, high-accuracy MS technology, phosphopeptide enrichment techniques based on affinity chromatography and recently developed bioinformatics tools277,279 are likely to greatly facilitate the identification and analysis of fungal pathogen phosphoproteomes. While experiments utilizing many of these techniques should provide a wealth of new information about global phosphorylation events associated with fungal pathogen virulence properties that can be assessed in vitro (eg, filamentation and biofilm formation), greater challenges are likely to be encountered in the assessment of phosphorylation patterns during infection in vivo. For example, it may be difficult to obtain sufficient quantities of fungal pathogen proteins for phosphoproteomic analysis from infected tissues. In addition, in the case of SILAC it may be difficult to obtain a sufficiently high level of stable isotope labeling for fungal pathogen proteins during an infection. Overall, however, future phosphoproteomic studies in medically important fungi are likely to provide valuable information and could lead to the identification of important kinase/phosphatase substrates, interacting partners and potential targets for the development of new and more effective antifungal strategies.
Perspectives and future directions: targeting kinases and phosphatases for the development of new antifungal strategies
Given the variety of key functions that kinases and phosphatases play in controlling morphology, virulence and a variety of virulence-related processes in pathogenic fungi, could these enzymes serve as effective antifungal drug targets? Probably the best example of such a potential drug target is the calcium/calmodulin-dependent protein phosphatase calcineurin. As discussed previously, calcineurin plays an important role in filamentation, virulence, stress response, antifungal drug tolerance and/or mating of a variety of medically important fungi, such as C. neoformans, A. fumigatus, and M. circinelloides and multiple Candida species (including C. albicans).175,176,179–181,185–187,190–192,195 Two pharmacological inhibitors of calcineurin, cyclosporin A and FK506, are effective against a variety of fungal pathogens, especially when combined with azole or echinocandin treatments.179,186,280–283 However, these inhibitors also have immunosuppressive effects and are unlikely to serve as viable antifungals in the clinic. Future studies to identify cyclosporin A or FK506 analogs, or other inhibitors of the calcium-calcineurin signaling pathway, with fewer side effects may hold promise.282,283 As previously discussed, the calcineurin target, Crz1, controls virulence-related processes in multiple fungal pathogens182,183,193,194 and has also been regarded as a promising antifungal target. However, Crz1 does not appear to be universally required for virulence in human fungal pathogens and is thus less likely to serve as a more broad-spectrum antifungal target.282 It is hoped that future phosphoproteomic studies, mentioned above, will elucidate more promising calcineurin targets for antifungal development.
A recent BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) search has also indicated the presence of homologs for several important kinases (PKC, Cek1-MAPK, PKA) and phosphatases (calcineurin, Ptc1, and Ppg1) in additional major human fungal pathogens including Coccidiodes immitis, H. capsulatum, and Blastomyces dermatitidis (homologs for the kinases listed above were also identified in the more distantly related fungal pathogen P. carinii). Several of these enzymes are likely to play roles in pathogenicity.
In particular, C. albicans Ppg1 has recently been shown to play a critical role in filamentation and virulence136 and could serve as a promising drug target. Importantly, the catalytic activity of Ppg1 has specifically been shown to be required for pathogenesis and future studies to screen small molecule libraries for inhibitors of Ppg1 phosphatase activity may hold promise. One concern, however, is that the Ppg1 catalytically active binuclear center is highly conserved in mammalian PP2A phosphatases, raising the possibility that such inhibitors may have detrimental side effects if used as therapeutics. Ultimately, future studies to solve the crystal structures of Ppg1, and other critical phosphatases/kinases in medically important fungi may allow for the identification of fungal-specific active site subregions that could be targeted by small molecule inhibitors. However, an easier approach may be to target kinases and phosphatases which are entirely fungal-specific and not conserved in mammalian hosts. A recent large-scale comparative genomics study has identified 222 C. albicans proteins with catalytic activity which are unique when compared to the human proteome.284 Crk1, a Cdc2-related protein kinase important for C. albicans hyphal development and virulence,285 was among these proteins and could eventually serve as a novel antifungal target. Additional fungal-specific kinases and phosphatases involved in metabolic processes which are essential for viability are also likely to represent promising targets. Future studies, which focus on the identification and characterization of fungal-specific kinases and phosphatases (or kinase/phosphatase substrates) that are critical for viability and/or virulence in human fungal pathogens are therefore likely to hold significant therapeutic potential.
Acknowledgments
We thank Brian Wickes for useful comments on the manuscript. M.T.A. was supported by a National Institute of Dental and Craniofacial Research Ruth L. Kirschstein National Research Service Award for Individual Postdoctoral Fellows (F32DE023471). D.K. was supported by a Voelcker Young Investigator Award from the Max and Minnie Tomerlin Voelcker Fund as well as a grants 5R01AI083344 and 1R21AI117299 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, National Institute of Allergy and Infectious Diseases or National Institute of Dental and Craniofacial Research.
Footnotes
Current address: Oshsner Health System, New Orleans, LA 70121
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
Supplementary Material
Supplementary material is available at Medical Mycology online (http://www.mmy.oxfordjournals.org/).
References
- 1.Manning G, Plowman GD, Hunter T, et al. Evolution of protein kinase signaling from yeast to man. Trends Biochem Sci. 2002;27:514520. doi: 10.1016/s0968-0004(02)02179-5. [DOI] [PubMed] [Google Scholar]
- 2.Manning G, Whyte DB, Martinez R, et al. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 3.Andreeva AV, Kutuzov MA. Protozoan protein tyrosine phosphatases. Int J Parasitol. 2008;38:1279–1295. doi: 10.1016/j.ijpara.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Brown AJ, Gow NA. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7:333–338. doi: 10.1016/s0966-842x(99)01556-5. [DOI] [PubMed] [Google Scholar]
- 5.Borgia PT. Roles of the Orla, Tse, and Bimg Genes of Aspergillus nidulans in chitin synthesis. J Bacteriol. 1992;174:384–389. doi: 10.1128/jb.174.2.384-389.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csank C, Makris C, Meloche S, et al. Derepressed hyphal growth and reduced virulence in a VH1 family-related protein phosphatase mutant of the human pathogen Candida albicans. Mol Biol Cell. 1997;8:2539–2551. doi: 10.1091/mbc.8.12.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler A, Arkind C, Mattison CP, et al. Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryot Cell. 2002;1:163–173. doi: 10.1128/EC.1.2.163-173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews PD, Stark MJR. Type 1 protein phosphatase is required for maintenance of cell wall integrity, morphogenesis and cell cycle progression in Saccharomyces cerevisiae. J Cell Sci. 2000;113:507–520. doi: 10.1242/jcs.113.3.507. [DOI] [PubMed] [Google Scholar]
- 9.Leach MD, Brown AJ. Posttranslational modifications of proteins in the pathobiology of medically relevant fungi. Eukaryot Cell. 2012;11:98–108. doi: 10.1128/EC.05238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da-Silva AM, Zapella PD, Andrioli LP, et al. Searching for the role of protein phosphatases in eukaryotic microorganisms. Braz J Med Biol Res. 1999;32:835–839. doi: 10.1590/s0100-879x1999000700006. [DOI] [PubMed] [Google Scholar]
- 11.Madhani HD, Fink GR. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 1998;8:348–353. doi: 10.1016/s0962-8924(98)01298-7. [DOI] [PubMed] [Google Scholar]
- 12.Yatzkan E, Szoor B, Feher Z, et al. Protein phosphatase 2A is involved in hyphal growth of Neurospora crassa. Mol Gen Genet. 1998;259:523–531. doi: 10.1007/s004380050844. [DOI] [PubMed] [Google Scholar]
- 13.Suresh K, Subramanyam C. A putative role for calmodulin in the activation of Neurospora crassa chitin synthase. FEMS Microbiol Lett. 1997;150:95–100. doi: 10.1111/j.1574-6968.1997.tb10355.x. [DOI] [PubMed] [Google Scholar]
- 14.Krebs EG, Fischer EH. The phosphorylase b to a converting enzyme of rabbit skeletal muscle. Biochim Biophys Acta. 1956;20:150–157. doi: 10.1016/0006-3002(56)90273-6. [DOI] [PubMed] [Google Scholar]
- 15.Krebs EG, Kent AB, Fischer EH. The muscle phosphorylase b kinase reaction. J Biol Chem. 1958;231:73–83. [PubMed] [Google Scholar]
- 16.Olsen JV, Blagoev B, Gnad F, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Szoor B. Trypanosomatid protein phosphatases. Mol Biochem Parasitol. 2010;173:53–63. doi: 10.1016/j.molbiopara.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoker AW. Protein tyrosine phosphatases and signalling. J Endocrinol. 2005;185:19–33. doi: 10.1677/joe.1.06069. [DOI] [PubMed] [Google Scholar]
- 19.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 20.Cohen P. The structure and regulation of protein phosphatases. Adv Second Messenger Phosphoprotein Res. 1990;24:230–235. [PubMed] [Google Scholar]
- 21.Zhan XL, Hong Y, Zhu T, et al. Essential functions of protein tyrosine phosphatases PTP2 and PTP3 and RIM11 tyrosine phosphorylation in Saccharomyces cerevisiae meiosis and sporulation. Mol Biol Cell. 2000;11:663–676. doi: 10.1091/mbc.11.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mustelin T. Protein tyrosine phosphatases in human disease. Adv Exp Med Biol. 2006;584:53–72. doi: 10.1007/0-387-34132-3_5. [DOI] [PubMed] [Google Scholar]
- 23.Hunter T, Plowman GD. The protein kinases of budding yeast: six score and more. Trends Biochem Sci. 1997;22:18–22. doi: 10.1016/s0968-0004(96)10068-2. [DOI] [PubMed] [Google Scholar]
- 24.Stark MJ. Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast. 1996;12:1647–1675. doi: 10.1002/(SICI)1097-0061(199612)12:16%3C1647::AID-YEA71%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Barford D. Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem Sci. 1996;21:407–412. doi: 10.1016/s0968-0004(96)10060-8. [DOI] [PubMed] [Google Scholar]
- 26.Kobor MS, Archambault J, Lester W, et al. An unusual eukaryotic protein phosphatase required for transcription by RNA polymerase II and CTD dephosphorylation in S. cerevisiae. Mol Cell. 1999;4:55–62. doi: 10.1016/s1097-2765(00)80187-2. [DOI] [PubMed] [Google Scholar]
- 27.Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Curr Opin Genet Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Edmond MB, Wallace SE, McClish DK, et al. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 29.Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 30.Pfaller MA, Andes D, Diekema DJ, et al. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat. 2010;13:180–195. doi: 10.1016/j.drup.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Odds FC. Candida and Candidosis. 2nd edn. London: Baillière Tindall; 1988. [Google Scholar]
- 32.Calderone RA, Clancy CJ, editors. Candida and Candidiasis. 2nd edn. Washington, DC: ASM Press; 2012. [Google Scholar]
- 33.Brown AJ. Expression of growth form-specific factors during morphogenesis in Candida albicans. In: Calderone RA, editor. Candida and Candidiasis. Washington, DC: ASM Press; 2002. pp. 87–93. [Google Scholar]
- 34.Cogliati M. Global molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii: an atlas of the molecular types. Scientifica (Cairo) 2013;2013:675213. doi: 10.1155/2013/675213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polvi EJ, Li X, O'Meara TR, et al. Opportunistic yeast pathogens: reservoirs, virulence mechanisms, and therapeutic strategies. Cell Mol Life Sci. 2015;72:2261–2287. doi: 10.1007/s00018-015-1860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idnurm A, Bahn YS, Nielsen K, et al. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat Rev Microbiol. 2005;3:753–764. doi: 10.1038/nrmicro1245. [DOI] [PubMed] [Google Scholar]
- 37.Sabiiti W, May RC. Mechanisms of infection by the human fungal pathogen Cryptococcus neoformans. Future Microbiol. 2012;7:1297–1313. doi: 10.2217/fmb.12.102. [DOI] [PubMed] [Google Scholar]
- 38.Coelho C, Bocca AL, Casadevall A. The tools for virulence of Cryptococcus neoformans. Adv Appl Microbiol. 2014;87:1–41. doi: 10.1016/B978-0-12-800261-2.00001-3. [DOI] [PubMed] [Google Scholar]
- 39.Nosanchuk JD, Valadon P, Feldmesser M, et al. Melanization of Cryptococcus neoformans in murine infection. Mol Cell Biol. 1999;19:745–750. doi: 10.1128/mcb.19.1.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casadevall A, Rosas AL, Nosanchuk JD. Melanin and virulence in Cryptococcus neoformans. Curr Opin Microbiol. 2000;3:354–358. doi: 10.1016/s1369-5274(00)00103-x. [DOI] [PubMed] [Google Scholar]
- 41.Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 42.Nemecek JC, Wuthrich M, Klein BS. Global control of dimorphism and virulence in fungi. Science. 2006;312:583–588. doi: 10.1126/science.1124105. [DOI] [PubMed] [Google Scholar]
- 43.McCormick A, Loeffler J, Ebel F. Aspergillus fumigatus: contours of an opportunistic human pathogen. Cell Microbiol. 2010;12:1535–1543. doi: 10.1111/j.1462-5822.2010.01517.x. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Ami R, Lewis RE, Kontoyiannis DP. Enemy of the (immunosuppressed) state: an update on the pathogenesis of Aspergillus fumigatus infection. Br J Haematol. 2010;150:406–417. doi: 10.1111/j.1365-2141.2010.08283.x. [DOI] [PubMed] [Google Scholar]
- 45.Hohl TM, Feldmesser M. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot Cell. 2007;6:1953–1963. doi: 10.1128/EC.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honkanen RE, Golden T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr Med Chem. 2002;9:2055–2075. doi: 10.2174/0929867023368836. [DOI] [PubMed] [Google Scholar]
- 48.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 49.Robertson LS, Fink GR. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc Natl Acad Sci USA. 1998;95:13783–13787. doi: 10.1073/pnas.95.23.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knighton DR, Zheng JH, Ten Eyck LF, et al. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- 51.Borges-Walmsley MI, Walmsley AR. cAMP signalling in pathogenic fungi: control of dimorphic switching and pathogenicity. Trends Microbiol. 2000;8:133–41. doi: 10.1016/s0966-842x(00)01698-x. [DOI] [PubMed] [Google Scholar]
- 52.Kronstad JW, Staben C. Mating type in filamentous fungi. Annu Rev Genet. 1997;31:245–276. doi: 10.1146/annurev.genet.31.1.245. [DOI] [PubMed] [Google Scholar]
- 53.Mehrabi R, M'Barek S B, Saidi A, et al. MAP kinase phosphorylation and cAMP assessment in fungi. Methods Mol Biol. 2012;835:571–583. doi: 10.1007/978-1-61779-501-5_35. [DOI] [PubMed] [Google Scholar]
- 54.Durrenberger F, Wong K, Kronstad JW. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc Natl Acad Sci U S A. 1998;95:5684–5689. doi: 10.1073/pnas.95.10.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bencina M, Panneman H, Ruijter GJ, et al. Characterization and overexpression of the Aspergillus niger gene encoding the cAMP-dependent protein kinase catalytic subunit. Microbiology. 1997;143(Pt 4):1211–1220. doi: 10.1099/00221287-143-4-1211. [DOI] [PubMed] [Google Scholar]
- 56.de Oliveira JC, Borges AC, Marques Mdo V, et al. Cloning and characterization of the gene for the catalytic subunit of cAMP-dependent protein kinase in the aquatic fungus Blastocladiella emersonii. Eur J Biochem. 1994;219:555–562. doi: 10.1111/j.1432-1033.1994.tb19971.x. [DOI] [PubMed] [Google Scholar]
- 57.Yang Z, Dickman MB. Colletotrichum trifolii mutants disrupted in the catalytic subunit of cAMP-dependent protein kinase are nonpathogenic. Mol Plant Microbe Interact. 1999;12:430–439. doi: 10.1094/MPMI.1999.12.5.430. [DOI] [PubMed] [Google Scholar]
- 58.Bruno KS, Aramayo R, Minke PF, et al. Loss of growth polarity and mislocalization of septa in a Neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 1996;15:5772–5782. [PMC free article] [PubMed] [Google Scholar]
- 59.Lengeler KB, Davidson RC, D'souza C, et al. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev. 2000;64:746–785. doi: 10.1128/mmbr.64.4.746-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rocha CRC, Schroppel K, Harcus D, et al. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell. 2001;12:3631–3643. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindsay AK, Deveau A, Piispanen AE, et al. Farnesol and cyclic AMP signaling effects on the hypha-to-yeast transition in Candida albicans. Eukaryot Cell. 2012;11:1219–1225. doi: 10.1128/EC.00144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stoldt VR, Sonneborn A, Leuker CE, et al. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu H. Transcriptional control of dimorphism in Candida albicans. Curr Opin Microbiol. 2001;4:728–735. doi: 10.1016/s1369-5274(01)00275-2. [DOI] [PubMed] [Google Scholar]
- 64.Bockmuhl DP, Krishnamurthy S, Gerads M, et al. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol Microbiol. 2001;42:1243–1257. doi: 10.1046/j.1365-2958.2001.02688.x. [DOI] [PubMed] [Google Scholar]
- 65.Park H, Myers CL, Sheppard DC, et al. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol. 2005;7:499–510. doi: 10.1111/j.1462-5822.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 66.Fuller KK, Richie DL, Feng X, et al. Divergent Protein Kinase A isoforms co-ordinately regulate conidial germination, carbohydrate metabolism and virulence in Aspergillus fumigatus. Mol Microbiol. 2011;79:1045–1062. doi: 10.1111/j.1365-2958.2010.07509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oliver BG, Panepinto JC, Fortwendel JR, et al. Cloning and expression of pkaC and pkaR, the genes encoding the cAMP-dependent protein kinase of Aspergillus fumigatus. Mycopathologia. 2002;154:85–91. doi: 10.1023/a:1015533406565. [DOI] [PubMed] [Google Scholar]
- 68.Zhao W, Panepinto JC, Fortwendel JR, et al. Deletion of the regulatory subunit of protein kinase A in Aspergillus fumigatus alters morphology, sensitivity to oxidative damage, and virulence. Infect Immun. 2006;74:4865–4874. doi: 10.1128/IAI.00565-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pukkila-Worley R, Gerrald QD, Kraus PR, et al. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryotic Cell. 2005;4:190–201. doi: 10.1128/EC.4.1.190-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alspaugh JA, Perfect JR, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alspaugh JA, Pukkila-Worley R, Harashima T, et al. Adenylyl cyclase functions downstream of the G alpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot Cell. 2002;1:75–84. doi: 10.1128/EC.1.1.75-84.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D'Souza CA, Alspaugh JA, Yue C, et al. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21:3179–3191. doi: 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hicks JK, D'Souza CA, Cox GM, et al. Cyclic AMP-dependent protein kinase catalytic subunits have divergent roles in virulence factor production in two varieties of the fungal pathogen Cryptococcus neoformans. Eukaryot Cell. 2004;3:14–26. doi: 10.1128/EC.3.1.14-26.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gold SE, Brogdon SM, Mayorga ME, et al. The Ustilago maydis regulatory subunit of a cAMP-dependent protein kinase is required for gall formation in maize. Plant Cell. 1997;9:1585–1594. doi: 10.1105/tpc.9.9.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kruger J, Loubradou G, Wanner G, et al. Activation of the cAMP pathway in Ustilago maydis reduces fungal proliferation and teliospore formation in plant tumors. Mol Plant Microbe Interact. 2000;13:1034–1040. doi: 10.1094/MPMI.2000.13.10.1034. [DOI] [PubMed] [Google Scholar]
- 76.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 77.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 78.Antonsson B, Montessuit S, Friedli L, et al. Protein-Kinase-C in yeast - characteristics of the Saccharomyces cerevisiae Pkc1 gene product. J Biol Chem. 1994;269:16821–16828. [PubMed] [Google Scholar]
- 79.Matsusaka T, Hirata D, Yanagida M, et al. A novel protein kinase gene Ssp1(+) is required for alteration of growth polarity and actin localization in fission yeast. EMBO J. 1995;14:3325–3338. doi: 10.1002/j.1460-2075.1995.tb07339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paravicini G, Mendoza A, Antonsson B, et al. The Candida albicans PKC1 gene encodes a protein kinase C homolog necessary for cellular integrity but not dimorphism. Yeast. 1996;12:741–756. doi: 10.1002/(sici)1097-0061(19960630)12:8<741::aid-yea967>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 81.Lendenfeld T, Kubicek CP. Characterization and properties of protein kinase C from the filamentous fungus Trichoderma reesei. Biochem J. 1998;330(Pt 2):689–694. doi: 10.1042/bj3300689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cabib E, Drgonova J, Drgon T. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu Rev Biochem. 1998;67:307–333. doi: 10.1146/annurev.biochem.67.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fuchs BB, Mylonakis E. Our paths might cross: the role of the fungal cell wall integrity pathway in stress response and cross talk with other stress response pathways. Eukaryot Cell. 2009;8:1616–1625. doi: 10.1128/EC.00193-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao X, Mehrabi R, Xu JR. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot Cell. 2007;6:1701–1714. doi: 10.1128/EC.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jong A, Wu CH, Prasadarao NV, et al. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells requires protein kinase C-alpha activation. Cell Microbiol. 2008;10:1854–1865. doi: 10.1111/j.1462-5822.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee H, Khanal Lamichhane A, Garraffo HM, et al. Involvement of PDK1, PKC and TOR signalling pathways in basal fluconazole tolerance in Cryptococcus neoformans. Mol Microbiol. 2012;84:130–146. doi: 10.1111/j.1365-2958.2012.08016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerik KJ, Bhimireddy SR, Ryerse JS, et al. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot Cell. 2008;7:1685–1698. doi: 10.1128/EC.00146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gerik KJ, Donlin MJ, Soto CE, et al. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol Microbiol. 2005;58:393–408. doi: 10.1111/j.1365-2958.2005.04843.x. [DOI] [PubMed] [Google Scholar]
- 90.Ichinomiya M, Uchida H, Koshi Y, et al. A protein kinase C-encoding gene, pkcA, is essential to the viability of the filamentous fungus Aspergillus nidulans. Biosci Biotechnol Biochem. 2007;71:2787–2799. doi: 10.1271/bbb.70409. [DOI] [PubMed] [Google Scholar]
- 91.Katayama T, Uchida H, Ohta A, et al. Involvement of protein kinase C in the suppression of apoptosis and in polarity establishment in Aspergillus nidulans under conditions of heat stress. PLoS One. 2012;7:e50503. doi: 10.1371/journal.pone.0050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paravicini G, Mendoza A, Antonsson B, et al. The Candida albicans PKC1 gene encodes a protein kinase C homolog necessary for cellular integrity but not dimorphism. Yeast. 1996;12:741–756. doi: 10.1002/(sici)1097-0061(19960630)12:8<741::aid-yea967>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 93.Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 94.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peter M, Sanghera JS, Pelech SL, et al. Mitogen-activated protein kinases phosphorylate nuclear lamins and display sequence specificity overlapping that of mitotic protein kinase P34 cdc2. Eur J Biochem. 1992;205:287–294. doi: 10.1111/j.1432-1033.1992.tb16779.x. [DOI] [PubMed] [Google Scholar]
- 96.Dickman MB, Yarden O. Serine/threonine protein kinases and phosphatases in filamentious fungi. Fungal Genet Biol. 1999;26:99–117. doi: 10.1006/fgbi.1999.1118. [DOI] [PubMed] [Google Scholar]
- 97.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 98.Gustin MC, Albertyn J, Alexander M, et al. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1264–1300. doi: 10.1128/mmbr.62.4.1264-1300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kronstad J, De Maria AD, Funnell D, et al. Signaling via cAMP in fungi: interconnections with mitogen-activated protein kinase pathways. Arch Microbiol. 1998;170:395–404. doi: 10.1007/s002030050659. [DOI] [PubMed] [Google Scholar]
- 100.Bardwell L. A walk-through of the yeast mating pheromone response pathway. Peptides. 2005;26:339–350. doi: 10.1016/j.peptides.2004.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee BN, Elion EA. The MAPKKK Ste11 regulates vegetative growth through a kinase cascade of shared signaling components. Proc Natl Acad Sci USA. 1999;96:12679–12684. doi: 10.1073/pnas.96.22.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan XW, Heitman J. Protein kinase A operates a molecular switch that governs yeast pseudohyphal differentiation. Mol Cell Biol. 2002;22:3981–3993. doi: 10.1128/MCB.22.12.3981-3993.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin R, Dobry CJ, McCown PJ, et al. Large-scale analysis of yeast filamentous growth by systematic gene disruption and overexpression. Mol Biol Cell. 2008;19:284–296. doi: 10.1091/mbc.E07-05-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han L, Li GJ, Yang KY, et al. Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J. 2010;64:114–127. doi: 10.1111/j.1365-313X.2010.04318.x. [DOI] [PubMed] [Google Scholar]
- 105.Lev S, Sharon A, Hadar R, et al. A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: Diverse roles for mitogen-activated protein kinase homologs in foliar pathogens. Proc Natl Acad Sci USA. 1999;96:13542–13547. doi: 10.1073/pnas.96.23.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Di Pietro A, Garcia-Maceira FI, Meglecz E, et al. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol Microbiol. 2001;39:1140–1152. [PubMed] [Google Scholar]
- 107.Andrews DL, Egan JD, Mayorga ME, et al. The Ustilago maydis ubc4 and ubc5 genes encode members of a MAP kinase cascade required for filamentous growth. Mol Plant Microbe Interact. 2000;13:781–786. doi: 10.1094/MPMI.2000.13.7.781. [DOI] [PubMed] [Google Scholar]
- 108.Monge RA, Roman E, Nombela C, et al. The MAP kinase signal transduction network in Candida albicans. Microbiology. 2006;152(Pt 4):905–912. doi: 10.1099/mic.0.28616-0. [DOI] [PubMed] [Google Scholar]
- 109.Bennett RJ, Johnson AD. Mating in Candida albicans and the search for a sexual cycle. Annu Rev Microbiol. 2005;59:233–255. doi: 10.1146/annurev.micro.59.030804.121310. [DOI] [PubMed] [Google Scholar]
- 110.Chen JY, Chen J, Lane S, et al. A conserved mitogen-activated protein kinase pathway is required for mating in Candida albicans. Mol Microbiol. 2002;46:1335–1344. doi: 10.1046/j.1365-2958.2002.03249.x. [DOI] [PubMed] [Google Scholar]
- 111.Csank C, Schroppel K, Leberer E, et al. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Navarrogarcia F, Sanchez M, Pla J, et al. Functional-characterization of the Mkc1 gene of Candida albicans, which encodes a mitogen-activated protein-kinase homolog related to cell integrity. Mol Cell Biol. 1995;15:2197–2206. doi: 10.1128/mcb.15.4.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diez-Orejas R, Molero G, Navarro-Garcia F, et al. Reduced virulence of Candida albicans MKC1 mutants: a role for mitogen-activated protein kinase in pathogenesis. Infect Immun. 1997;65:833–837. doi: 10.1128/iai.65.2.833-837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang P, Perfect JR, Heitman J. The G-protein beta subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol Cell Biol. 2000;20:352–362. doi: 10.1128/mcb.20.1.352-362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chang YC, Wickes BL, Miller GF, et al. Cryptococcus neoformans STE12 alpha regulates virulence but is not essential for mating. J Exp Med. 2000;191:871–881. doi: 10.1084/jem.191.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yue CL, Cavallo LM, Alspaugh JA, et al. The STE12 alpha homolog is required for haploid filamentation but largely dispensable for mating and virulence in Cryptococcus neoformans. Genetics. 1999;153:1601–1615. doi: 10.1093/genetics/153.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin X, Jackson JC, Feretzaki M, et al. Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite- and same-sex mating in Cryptococcus neoformans. PLoS Genet. 2010;6:e1000953. doi: 10.1371/journal.pgen.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bahn YS, Kojima K, Cox GM, et al. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol Biol Cell. 2005;16:2285–2300. doi: 10.1091/mbc.E04-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kottom TJ, Kohler JR, Thomas CF, Jr, et al. Lung epithelial cells and extracellular matrix components induce expression of Pneumocystis carinii STE20, a gene complementing the mating and pseudohyphal growth defects of STE20 mutant yeast. Infect Immun. 2003;71:6463–6471. doi: 10.1128/IAI.71.11.6463-6471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.May GS, Xue T, Kontoyiannis DP, et al. Mitogen activated protein kinases of Aspergillus fumigatus. Med Mycol. 2005;43:S83–S86. doi: 10.1080/13693780400024784. [DOI] [PubMed] [Google Scholar]
- 121.Valiante V, Heinekamp T, Jain R, et al. The mitogen-activated protein kinase MpkA of Aspergillus fumigatus regulates cell wall signaling and oxidative stress response. Fungal Genet Biol. 2008;45:618–627. doi: 10.1016/j.fgb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 122.Xue T, Nguyen CK, Romans A, et al. A mitogen-activated protein kinase that senses nitrogen regulates conidial germination and growth in Aspergillus fumigatus. Eukaryot Cell. 2004;3:557–560. doi: 10.1128/EC.3.2.557-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Posas F, WurglerMurphy SM, Maeda T, et al. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 ‘'two-component'’ osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 124.Alex LA, Korch C, Selitrennikoff CP, et al. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc Natl Acad Sci U S A. 1998;95:7069–7073. doi: 10.1073/pnas.95.12.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Srikantha T, Tsai L, Daniels K, et al. The two-component hybrid kinase regulator CaNIK1 of Candida albicans. Microbiology. 1998;144:2715–2729. doi: 10.1099/00221287-144-10-2715. [DOI] [PubMed] [Google Scholar]
- 126.Alex LA, Borkovich KA, Simon MI. Hyphal development in Neurospora crassa: involvement of a two-component histidine kinase. Proc Natl Acad Sci U S A. 1996;93:3416–3421. doi: 10.1073/pnas.93.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Desai C, Mavrianos J, Chauhan N. Candida albicans SRR1, a putative two-component response regulator gene, is required for stress adaptation, morphogenesis, and virulence. Eukaryot Cell. 2011;10:1370–1374. doi: 10.1128/EC.05188-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clemons KV, Miller TK, Selitrennikoff CP, et al. fos-1, a putative histidine kinase as a virulence factor for systemic aspergillosis. Med Mycol. 2002;40:259–262. doi: 10.1080/mmy.40.3.259.262. [DOI] [PubMed] [Google Scholar]
- 129.Wormley FL, Jr., Heinrich G, Miller JL, et al. Identification and characterization of an SKN7 homologue in Cryptococcus neoformans. Infect Immun. 2005;73:5022–5030. doi: 10.1128/IAI.73.8.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kruppa M, Calderone R. Two-component signal transduction in human fungal pathogens. FEMS Yeast Res. 2006;6:149–159. doi: 10.1111/j.1567-1364.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 131.Kosti I, Mandel-Gutfreund Y, Glaser F, et al. Comparative analysis of fungal protein kinases and associated domains. BMC Genomics. 2010;11:133. doi: 10.1186/1471-2164-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sia RA, Herald HA, Lew DJ. Cdc28 tyrosine phosphorylation and the morphogenesis checkpoint in budding yeast. Mol Biol Cell. 1996;7:1657–1666. doi: 10.1091/mbc.7.11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gould KL, Nurse P. Tyrosine phosphorylation of the fission yeast Cdc2+ protein-kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- 134.Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- 135.Arino J. Novel protein phosphatases in yeast. Eur J Biochem. 2002;269:1072–1077. doi: 10.1046/j.0014-2956.2002.02753.x. [DOI] [PubMed] [Google Scholar]
- 136.Albataineh MT, Lazzell A, Lopez-Ribot JL, et al. Ppg1, a PP2A-type protein phosphatase, controls filament extension and virulence in Candida albicans. Eukaryot Cell. 2014;13:1538–1547. doi: 10.1128/EC.00199-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zapella PDA, daSilva AM, daCostaMaia JC, et al. Serine/threonine protein phosphatases and a protein phosphatase 1 inhibitor from Neurospora crassa. Braz J Med Biol Res. 1996;29:599–604. [PubMed] [Google Scholar]
- 138.Higuchi S, Tamura J, Giri PR, et al. Calmodulin-dependent protein phosphatase from Neurospora crassa - molecular cloning and expression of recombinant catalytic subunit. J Biol Chem. 1991;266:18104–18112. [PubMed] [Google Scholar]
- 139.Cohen PT. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem Sci. 1997;22:245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- 140.Stark MJR. Yeast protein serine/threonine phosphatases: Multiple roles and diverse regulation. Yeast. 1996;12:1647–1675. doi: 10.1002/(SICI)1097-0061(199612)12:16%3C1647::AID-YEA71%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 141.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 142.Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods Enzymol. 1991;201:389–398. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- 143.Andersen JN, Elson A, Lammers R, et al. Comparative study of protein tyrosine phosphatase-epsilon isoforms: membrane localization confers specificity in cellular signalling. Biochem J. 2001;354:581–590. doi: 10.1042/0264-6021:3540581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu Y, Fisher GJ. Receptor type protein tyrosine phosphatases (RPTPs)—roles in signal transduction and human disease. J Cell Commun Signal. 2012;6:125–138. doi: 10.1007/s12079-012-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen YH, Chen MX, Alessi DR, et al. Molecular cloning of cDNA encoding the 110 kDa and 21 kDa regulatory subunits of smooth muscle protein phosphatase 1 M. FEBS Lett. 1994;356:51–55. doi: 10.1016/0014-5793(94)01231-8. [DOI] [PubMed] [Google Scholar]
- 146.Cohen PT. Protein phosphatase 1–targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 147.Chen YH, Hansen L, Chen MX, et al. Sequence of the human glycogen-associated regulatory subunit of type 1 protein phosphatase and analysis of its coding region and mRNA level in muscle from patients with NIDDM. Diabetes. 1994;43:1234–1241. doi: 10.2337/diabetes.43.10.1234. [DOI] [PubMed] [Google Scholar]
- 148.Pinna LA, Ruzzene M. How do protein kinases recognize their substrates? Biochim Biophys Acta. 1996;1314:191–225. doi: 10.1016/s0167-4889(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 149.Pinna LA, Donelladeana A. Phosphorylated synthetic peptides as tools for studying protein phosphatases. Biochim Biophys Acta. 1994;1222:415–431. doi: 10.1016/0167-4889(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 150.Reed SI. The Role of P34 kinases in the G1 to S-phase transition. Annu Rev Cell Biol. 1992;8:529–661. doi: 10.1146/annurev.cb.08.110192.002525. [DOI] [PubMed] [Google Scholar]
- 151.Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 152.Elledge SJ, Harper JW. Cdk inhibitors: on the threshold of checkpoints and development. Curr Opin Cell Biol. 1994;6:847–852. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 153.Kang J, Yu H. Kinase signaling in the spindle checkpoint. J Biol Chem. 2009;284:15359–15363. doi: 10.1074/jbc.R900005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ohkura H, Kinoshita N, Miyatani S, et al. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989;57:997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- 155.Francisco L, Chan CSM. Regulation of yeast chromosome segregation by Ipl1 protein kinase and type-1 protein phosphatase. Cell Mol Biol Res. 1994;40:207–213. [PubMed] [Google Scholar]
- 156.Pinsky BA, Kotwaliwale CV, Tatsutani SY, et al. Glc7/protein phosphatase 1 regulatory subunits can oppose the Ipl1/aurora protein kinase by redistributing Glc7. Mol Cell Biol. 2006;26:2648–2660. doi: 10.1128/MCB.26.7.2648-2660.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Emanuele MJ, Lan W, Jwa M, et al. Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J Cell Biol. 2008;181:241–254. doi: 10.1083/jcb.200710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Murnion ME, Adams RR, Callister DM, et al. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J Biol Chem. 2001;276:26656–26665. doi: 10.1074/jbc.M102288200. [DOI] [PubMed] [Google Scholar]
- 159.Bharucha JP, Larson JR, Gao L, et al. Ypi1, a positive regulator of nuclear protein phosphatase type 1 activity in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19:1032–1045. doi: 10.1091/mbc.E07-05-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Doonan JH, Morris NR. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989;57:987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- 161.Pinsky BA, Nelson CR, Biggins S. Protein phosphatase 1 regulates exit from the spindle checkpoint in budding yeast. Curr Biol. 2009;19:1182–1187. doi: 10.1016/j.cub.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dever TE, Feng L, Wek RC, et al. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 163.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 164.Rojas M, Gingras AC, Dever TE. Protein phosphatase PP1/GLC7 interaction domain in yeast eIF2 gamma bypasses targeting subunit requirement for eIF2 alpha dephosphorylation. Proc Natl Acad Sci USA. 2014;111:E1344–E1353. doi: 10.1073/pnas.1400129111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ramaswamy NT, Li L, Khalil M, et al. Regulation of yeast glycogen metabolism and sporulation by Glc7p protein phosphatase. Genetics. 1998;149:57–72. doi: 10.1093/genetics/149.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Suzuki Y, Lanner C, Kim JH, et al. Insulin control of glycogen metabolism in knockout mice lacking the muscle-specific protein phosphatase PP1G/RGL. Mol Cell Biol. 2001;21:2683–2694. doi: 10.1128/MCB.21.8.2683-2694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Printen JA, Brady MJ, Saltiel AR. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science. 1997;275:1475–1478. doi: 10.1126/science.275.5305.1475. [DOI] [PubMed] [Google Scholar]
- 168.Hu KD, Li WJ, Wang HT, et al. Shp1, a regulator of protein phosphatase 1 Glc7, has important roles in cell morphogenesis, cell cycle progression and DNA damage response in Candida albicans. Fungal Genet Biol. 2012;49:433–442. doi: 10.1016/j.fgb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 169.DeMarini DJ, Adams AE, Fares H, et al. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1997;139:75–93. doi: 10.1083/jcb.139.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kozubowski L, Panek H, Rosenthal A, et al. A Bni4-Glc7 phosphatase complex that recruits chitin synthase to the site of bud emergence. Mol Biol Cell. 2003;14:26–39. doi: 10.1091/mbc.E02-06-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rowbottom L, Munro CA, Gow NA. Candida albicans mutants in the BNI4 gene have reduced cell-wall chitin and alterations in morphogenesis. Microbiology. 2004;150:3243–3252. doi: 10.1099/mic.0.27167-0. [DOI] [PubMed] [Google Scholar]
- 172.Hanaoka N, Takano Y, Shibuya K, et al. Identification of the putative protein phosphatase gene PTC1 as a virulence-related gene using a silkworm model of Candida albicans infection. Eukaryot Cell. 2008;7:1640–1648. doi: 10.1128/EC.00129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kincaid R. Calmodulin-dependent protein phosphatases from microorganisms to man. A study in structural conservatism and biological diversity. Adv Second Messenger Phosphoprotein Res. 1993;27:1–23. [PubMed] [Google Scholar]
- 174.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 175.Yakel JL. Calcineurin regulation of synaptic function: from ion channels to transmitter release and gene transcription. Trends Pharmacol Sci. 1997;18:124–134. doi: 10.1016/s0165-6147(97)01046-8. [DOI] [PubMed] [Google Scholar]
- 176.Fox DS, Heitman J. Good fungi gone bad: the corruption of calcineurin. Bioessays. 2002;24:894–903. doi: 10.1002/bies.10157. [DOI] [PubMed] [Google Scholar]
- 177.Cyert MS, Kunisawa R, Kaim D, et al. Yeast has homologs (Cna1 and Cna2 Gene-Products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci USA. 1991;88:7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yoshimoto H, Saltsman K, Gasch AP, et al. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J Biol Chem. 2002;277:31079–31088. doi: 10.1074/jbc.M202718200. [DOI] [PubMed] [Google Scholar]
- 179.Cruz MC, Goldstein AL, Blankenship JR, et al. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 2002;21:546–559. doi: 10.1093/emboj/21.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Blankenship JR, Wormley FL, Boyce MK, et al. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot Cell. 2003;2:422–430. doi: 10.1128/EC.2.3.422-430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sanglard D, Ischer F, Marchetti O, et al. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol. 2003;48:959–976. doi: 10.1046/j.1365-2958.2003.03495.x. [DOI] [PubMed] [Google Scholar]
- 182.Onyewu C, Wormley FL, Perfect JR, et al. The calcineurin target, crz1, functions in azole tolerance but is not required for virulence of Candida albicans. Infect Immun. 2004;72:7330–7333. doi: 10.1128/IAI.72.12.7330-7333.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Santos M, de Larrinoa IF. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr Genet. 2005;48:88–100. doi: 10.1007/s00294-005-0003-8. [DOI] [PubMed] [Google Scholar]
- 184.Cowen LE, Carpenter AE, Matangkasombut O, Fink GR, Lindquist S. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot Cell. 2006;5:2184–2188. doi: 10.1128/EC.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Odom A, Muir S, Lim E, et al. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Steinbach WJ, Reedy JL, Cramer RA, et al. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nature Rev Microbiol. 2007;5:418–430. doi: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]
- 187.Cruz MC, Fox DS, Heitman J. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 2001;20:1020–1032. doi: 10.1093/emboj/20.5.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cruz MC, Sia RA, Olson M, et al. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect Immun. 2000;68:982–985. doi: 10.1128/iai.68.2.982-985.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gorlach J, Fox DS, Cutler NS, et al. Identification and characterization of a highly conserved calcineurin binding protein, CBP1/calcipressin, in Cryptococcus neoformans. EMBO J. 2000;19:3618–3629. doi: 10.1093/emboj/19.14.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Steinbach WJ, Schell WA, Blankenship JR, et al. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob Agents Chemother. 2004;48:1664–1669. doi: 10.1128/AAC.48.5.1664-1669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.da Silva Ferreira ME, Capellaro JL, dos Reis Marques E, et al. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrob Agents Chemother. 2004;48:4405–4413. doi: 10.1128/AAC.48.11.4405-4413.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Steinbach WJ, Cramer RA, Jr., Perfect BZ, et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006;5:1091–1103. doi: 10.1128/EC.00139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Soriani FM, Malavazi I, da Silva Ferreira ME, et al. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol Microbiol. 2008;67:1274–1291. doi: 10.1111/j.1365-2958.2008.06122.x. [DOI] [PubMed] [Google Scholar]
- 194.Cramer RA, Jr., Perfect BZ, Pinchai N, et al. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot Cell. 2008;7:1085–1097. doi: 10.1128/EC.00086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee SC, Li A, Calo S, et al. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 2013;9:e1003625. doi: 10.1371/journal.ppat.1003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lee SC, Li A, Calo S, et al. Calcineurin orchestrates dimorphic transitions, antifungal drug responses, and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol Microbiol. 2015;97:844–865. doi: 10.1111/mmi.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ibrahim AS, Spellberg B. Zygomycetes as agents of infectious disease in humans. In: Heitman J, Filler SG, Edwards JE Jr, Mitchell AP, editors. Molecular Principles of Fungal Pathogenesis. Washington, DC: ASM Press; 2006. pp. 429–440. [Google Scholar]
- 198.Egan JD, Garcia-Pedrajas MD, Andrews DL, et al. Calcineurin is an antagonist to PKA protein phosphorylation required for postmating filamentation and virulence, while PP2A is required for viability in Ustilago maydis. Mol Plant Microbe Interact. 2009;22:1293–1301. doi: 10.1094/MPMI-22-10-1293. [DOI] [PubMed] [Google Scholar]
- 199.Kafadar KA, Cyert MS. Integration of stress responses: modulation of calcineurin signaling in Saccharomyces cerevisiae by protein kinase A. Eukaryot Cell. 2004;3:1147–1153. doi: 10.1128/EC.3.5.1147-1153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lammers T, Lavi S. Role of type 2C protein phosphatases in growth regulation and in cellular stress signaling. Crit Rev Biochem Mol Biol. 2007;42:437–461. doi: 10.1080/10409230701693342. [DOI] [PubMed] [Google Scholar]
- 201.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 202.Schweighofer A, Hirt H, Meskiene I. Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci. 2004;9:236–243. doi: 10.1016/j.tplants.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 203.Kerk D, Bulgrien J, Smith DW, et al. The complement of protein phosphatase catalytic subunits encoded in the genome of Arabidopsis. Plant Physiol. 2002;129:908–925. doi: 10.1104/pp.004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xue T, Wang D, Zhang S, et al. Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC Genomics. 2008;9:550. doi: 10.1186/1471-2164-9-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cheng A, Ross KE, Kaldis P, et al. Dephosphorylation of cyclin-dependent kinases by type 2C protein phosphatases. Genes Dev. 1999;13:2946–2957. doi: 10.1101/gad.13.22.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ruan H, Yan Z, Sun H, et al. The YCR079w gene confers a rapamycin-resistant function and encodes the sixth type 2C protein phosphatase in Saccharomyces cerevisiae. FEMS Yeast Res. 2007;7:209–215. doi: 10.1111/j.1567-1364.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 207.Maeda T, Tsai AYM, Saito H. Mutations in a protein tyrosine phosphatase gene (PTP2) and a protein serine/threonine phosphatase gene (PTC1) cause a synthetic growth defect in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:5408–5417. doi: 10.1128/mcb.13.9.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jiang B, Ram AFJ, Sheraton J, et al. Regulation of cell wall beta-glucan assembly Ptc1 negatively affects Pbs2 action in a pathway that includes modulation of Exg1 transcription. Mol Gen Genet. 1995;248:260–269. doi: 10.1007/BF02191592. [DOI] [PubMed] [Google Scholar]
- 209.Ito T, Chiba T, Ozawa R, et al. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc Natl Acad Sci U S A. 2001;98:4569–4574. doi: 10.1073/pnas.061034498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gonzalez A, Ruiz A, Serrano R, et al. Transcriptional profiling of the protein phosphatase 2C family in yeast provides insights into the unique functional roles of Ptc1. J Biol Chem. 2006;281:35057–35069. doi: 10.1074/jbc.M607919200. [DOI] [PubMed] [Google Scholar]
- 211.Malleshaiah MK, Shahrezaei V, Swain PS, et al. The scaffold protein Ste5 directly controls a switch-like mating decision in yeast. Nature. 2010;465:101–105. doi: 10.1038/nature08946. [DOI] [PubMed] [Google Scholar]
- 212.Du Y, Walker L, Novick P, et al. Ptc1p regulates cortical ER inheritance via Slt2p. EMBO J. 2006;25:4413–4422. doi: 10.1038/sj.emboj.7601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sen Gupta S, Ton VK, Beaudry V, et al. Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J Biol Chem. 2003;278:28831–28839. doi: 10.1074/jbc.M303300200. [DOI] [PubMed] [Google Scholar]
- 214.Jin Y, Eves PT, Tang F, et al. PTC1 is required for vacuole inheritance and promotes the association of the myosin-V vacuole-specific receptor complex. Mol Biol Cell. 2009;20:1312–1323. doi: 10.1091/mbc.E08-09-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cheng A, Kaldis P, Solomon MJ. Dephosphorylation of human cyclin-dependent kinases by protein phosphatase type 2C alpha and beta 2 isoforms. J Biol Chem. 2000;275:34744–34749. doi: 10.1074/jbc.M006210200. [DOI] [PubMed] [Google Scholar]
- 216.Tal R, Winter G, Ecker N, et al. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J Biol Chem. 2007;282:5617–5624. doi: 10.1074/jbc.M605940200. [DOI] [PubMed] [Google Scholar]
- 217.Journo D, Mor A, Abeliovich H. Aup1-mediated regulation of Rtg3 during mitophagy. J Biol Chem. 2009;284:35885–35895. doi: 10.1074/jbc.M109.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Feng J, Zhao J, Li J, et al. Functional characterization of the PP2C phosphatase CaPtc2p in the human fungal pathogen Candida albicans. Yeast. 2010;27:753–764. doi: 10.1002/yea.1778. [DOI] [PubMed] [Google Scholar]
- 219.Wang JH, Yan ZH, Shen SH, Whiteway M, Jiang LH. Expression of CaPTC7 is developmentally regulated during serum-induced morphogenesis in the human fungal pathogen Candida albicans. Can J Microbiol. 2007;53:237–244. doi: 10.1139/w06-125. [DOI] [PubMed] [Google Scholar]
- 220.Fan J, Wu M, Jiang L, et al. A serine/threonine protein phosphatase-like protein, CaPTC8, from Candida albicans defines a new PPM subfamily. Gene. 2009;430:64–76. doi: 10.1016/j.gene.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 221.Jiang LH, Yang JR, Fan FY, et al. The type 2C protein phosphatase FgPtc1p of the plant fungal pathogen Fusarium graminearum is involved in lithium toxicity and virulence. Mol Plant Pathol. 2010;11:277–282. doi: 10.1111/j.1364-3703.2009.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jiang JH, Yun YZ, Yang QQ, et al. A type 2C protein phosphatase FgPtc3 is involved in cell wall integrity, lipid metabolism, and virulence in Fusarium graminearum. PLoS One. 2011;6:e25311. doi: 10.1371/journal.pone.0025311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Price NE, Mumby MC. Effects of regulatory subunits on the kinetics of protein phosphatase 2A. Biochemistry. 2000;39:11312–11318. doi: 10.1021/bi0008478. [DOI] [PubMed] [Google Scholar]
- 224.Zolnierowicz S, Csortos C, Bondor J, et al. Diversity in the regulatory B-subunits of protein phosphatase 2A: identification of a novel isoform highly expressed in brain. Biochemistry. 1994;33:11858–11867. doi: 10.1021/bi00205a023. [DOI] [PubMed] [Google Scholar]
- 225.Strack S, Chang D, Zaucha JA, et al. Cloning and characterization of B delta, a novel regulatory subunit of protein phosphatase 2A. FEBS Lett. 1999;460:462–466. doi: 10.1016/s0014-5793(99)01377-0. [DOI] [PubMed] [Google Scholar]
- 226.Moorhead GB, De Wever V, Templeton G, et al. Evolution of protein phosphatases in plants and animals. Biochem J. 2009;417:401–409. doi: 10.1042/BJ20081986. [DOI] [PubMed] [Google Scholar]
- 227.Tung HY, Alemany S, Cohen P. The protein phosphatases involved in cellular regulation. 2. Purification, subunit structure and properties of protein phosphatases-2A0, 2A1, and 2A2 from rabbit skeletal muscle. Eur J Biochem. 1985;148:253–263. doi: 10.1111/j.1432-1033.1985.tb08833.x. [DOI] [PubMed] [Google Scholar]
- 228.Kamibayashi C, Estes R, Lickteig RL, et al. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J Biol Chem. 1994;269:20139–20148. [PubMed] [Google Scholar]
- 229.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wei HJ, Ashby DG, Moreno CS, et al. Carboxymethylation of the PP2A catalytic subunit in Saccharomyces cerevisiae is required for efficient interaction with the B-type subunits CDC55p and RTS1p. J Biol Chem. 2001;276:1570–1577. doi: 10.1074/jbc.M008694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tolstykh T, Lee J, Vafai S, et al. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Brautigan DL. Flicking the switches: phosphorylation of serine/threonine protein phosphatases. Semin Cancer Biol. 1995;6:211–217. doi: 10.1006/scbi.1995.0028. [DOI] [PubMed] [Google Scholar]
- 233.Ronne H, Carlberg M, Hu GZ, et al. Protein phosphatase 2A in Saccharomyces cerevisiae: effects on cell growth and bud morphogenesis. Mol Cell Biol. 1991;11:4876–4884. doi: 10.1128/mcb.11.10.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sneddon AA, Cohen PT, Stark MJ. Saccharomyces cerevisiae protein phosphatase 2A performs an essential cellular function and is encoded by two genes. EMBO J. 1990;9:4339–4346. doi: 10.1002/j.1460-2075.1990.tb07883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kinoshita N, Ohkura H, Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990;63:405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- 236.Kosmidou E, Lunness P, Doonan JH. A type 2A protein phosphatase gene from Aspergillus nidulans is involved in hyphal morphogenesis. Curr Genet. 2001;39:25–34. doi: 10.1007/s002940000177. [DOI] [PubMed] [Google Scholar]
- 237.van Zyl W, Huang W, Sneddon AA, et al. Inactivation of the protein phosphatase 2A regulatory subunit A results in morphological and transcriptional defects in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:4946–4959. doi: 10.1128/mcb.12.11.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jiang Y. Regulation of the cell cycle by protein phosphatase 2A in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2006;70:440–449. doi: 10.1128/MMBR.00049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang YC, Burke DJ. Cdc55p, the B-type regulatory subunit of protein phosphatase 2A, has multiple functions in mitosis and is required for the kinetochore/spindle checkpoint in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:620–626. doi: 10.1128/mcb.17.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yellman CM, Burke DJ. The role of Cdc55 in the spindle checkpoint is through regulation of mitotic exit in Saccharomyces cerevisiae. Mol Biol Cell. 2006;17:658–666. doi: 10.1091/mbc.E05-04-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang Y, Ng TY. Phosphatase 2A negatively regulates mitotic exit in Saccharomyces cerevisiae. Mol Biol Cell. 2006;17:80–89. doi: 10.1091/mbc.E04-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yatzkan E, Yarden O. The B regulatory subunit of protein phosphatase 2A is required for completion of macrocondiation and other developmental processes in Neurospora crassa. Mol Microbiol. 1999;31:197–209. doi: 10.1046/j.1365-2958.1999.01161.x. [DOI] [PubMed] [Google Scholar]
- 243.Zhong G, Jiang P, Qiao WR, et al. Protein phosphatase 2A (PP2A) regulatory subunits ParA and PabA orchestrate septation and conidiation and are essential for PP2A activity in Aspergillus nidulans. Eukaryot Cell. 2014;13:1494–1506. doi: 10.1128/EC.00201-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sutton A, Lin F, Sarabia MJF, et al. The Sit4 protein phosphatase is required in late G(1) for progression into S-phase. Cold Spring Harb Symp Quant Biol. 1991;56:75–81. doi: 10.1101/sqb.1991.056.01.011. [DOI] [PubMed] [Google Scholar]
- 245.Posas F, Clotet J, Muns MT, et al. The gene PPG encodes a novel yeast protein phosphatase involved in glycogen accumulation. J Biol Chem. 1993;268:1349–1354. [PubMed] [Google Scholar]
- 246.Sun LL, Li WJ, Wang HT, et al. Protein phosphatase Pph3 and its regulatory subunit Psy2 regulate Rad53 dephosphorylation and cell morphogenesis during recovery from DNA damage in Candida albicans. Eukaryot Cell. 2011;10:1565–1573. doi: 10.1128/EC.05042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Van Hoof C, Martens E, Longin S, et al. Specific interactions of PP2A and PP2A-like phosphatases with the yeast PTPA homologues, Ypa1 and Ypa2. Biochem J. 2005;386:93–102. doi: 10.1042/BJ20040887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bastians H, Ponstingl H. The novel human protein serine/threonine phosphatase 6 is a functional homologue of budding yeast Sit4p and fission yeast ppe1, which are involved in cell cycle regulation. J Cell Sci. 1996;109:2865–2874. doi: 10.1242/jcs.109.12.2865. [DOI] [PubMed] [Google Scholar]
- 249.Mann DJ, Dombradi V, Cohen PTW. Drosophila protein phosphatase-V functionally complements a Sit4 mutant in Saccharomyces cerevisiae and its amino-terminal region can confer this complementation to a heterologous phosphatase catalytic domain. EMBO J. 1993;12:4833–4842. doi: 10.1002/j.1460-2075.1993.tb06173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sutton A, Immanuel D, Arndt KT. The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol Cell Biol. 1991;11:2133–2148. doi: 10.1128/mcb.11.4.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fernandezsarabia MJ, Sutton A, Zhong T, et al. Sit4 protein phosphatase is required for the normal accumulation of Swi4, Cln1, Cln2, and Hcs26 RNAs during late G1. Genes Dev. 1992;6:2417–2428. doi: 10.1101/gad.6.12a.2417. [DOI] [PubMed] [Google Scholar]
- 252.de la Torre-Ruiz MA, Torres J, Arino J, et al. Sit4 is required for proper modulation of the biological functions mediated by Pkc1 and the cell integrity pathway in Saccharomyces cerevisiae. J Biol Chem. 2002;277:33468–33476. doi: 10.1074/jbc.M203515200. [DOI] [PubMed] [Google Scholar]
- 253.Lee CM, Nantel A, Jiang L, et al. The serine/threonine protein phosphatase SIT4 modulates yeast-to-hypha morphogenesis and virulence in Candida albicans. Mol Microbiol. 2004;51:691–709. doi: 10.1111/j.1365-2958.2003.03879.x. [DOI] [PubMed] [Google Scholar]
- 254.Noble SM, French S, Kohn LA, et al. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet. 2010;42:590–598. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Shi QM, Wang YM, Zheng XD, et al. Critical role of DNA checkpoints in mediating genotoxic-stress-induced filamentous growth in Candida albicans. Mol Biol Cell. 2007;18:815–826. doi: 10.1091/mbc.E06-05-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Omidi K, Hooshyar M, Jessulat M, et al. Phosphatase complex Pph3/Psy2 is involved in regulation of efficient non-homologous end-joining pathway in the yeast Saccharomyces cerevisiae. PLoS One. 2014;9:e87248. doi: 10.1371/journal.pone.0087248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang HT, Gao JX, Wong AHH, et al. Rfa2 is specifically dephosphorylated by Pph3 in Candida albicans. Biochem J. 2013;449:673–681. doi: 10.1042/BJ20120952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Brill SJ, Stillman B. Replication Factor-a from Saccharomyces cerevisiae is encoded by 3 essential genes coordinately expressed at S-Phase. Genes Dev. 1991;5:1589–1600. doi: 10.1101/gad.5.9.1589. [DOI] [PubMed] [Google Scholar]
- 259.Fanning E, Klimovich V, Nager AR. A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res. 2006;34:4126–4137. doi: 10.1093/nar/gkl550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kim HS, Kim NR, Yang J, et al. Identification of novel genes responsible for ethanol and/or thermotolerance by transposon mutagenesis in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2011;91:1159–1172. doi: 10.1007/s00253-011-3298-z. [DOI] [PubMed] [Google Scholar]
- 261.Singh NS, Shao N, McLean JR, et al. SIN-inhibitory phosphatase complex promotes Cdc11p dephosphorylation and propagates SIN asymmetry in fission yeast. Curr Biol. 2011;21:1968–1678. doi: 10.1016/j.cub.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hayles J, Wood V, Jeffery L, et al. A genome-wide resource of cell cycle and cell shape genes of fission yeast. Open Biol. 2013;3:130053. doi: 10.1098/rsob.130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Guan KL, Deschenes RJ, Qiu H, et al. Cloning and expression of a yeast protein tyrosine phosphatase. J Biol Chem. 1991;266:12964–12970. [PubMed] [Google Scholar]
- 264.Zhan XL, Deschenes RJ, Guan KL. Differential regulation of FUS3 MAP kinase by tyrosine-specific phosphatases PTP2/PTP3 and dual-specificity phosphatase MSG5 in Saccharomyces cerevisiae. Genes Dev. 1997;11:1690–1702. doi: 10.1101/gad.11.13.1690. [DOI] [PubMed] [Google Scholar]
- 265.Saito H, Tatebayashi K. Regulation of the osmoregulatory HOG MAPK cascade in yeast. J Biochem. 2004;136:267–272. doi: 10.1093/jb/mvh135. [DOI] [PubMed] [Google Scholar]
- 266.WurglerMurphy SM, Maeda T, Witten EA, et al. Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol Cell Biol. 1997;17:1289–1297. doi: 10.1128/mcb.17.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Jacoby T, Flanagan H, Faykin A, et al. Two protein tyrosine phosphatases inactivate the osmotic stress response pathway in yeast by targeting the mitogen-activated protein kinase, Hog1. J Biol Chem. 1997;272:17749–17755. doi: 10.1074/jbc.272.28.17749. [DOI] [PubMed] [Google Scholar]
- 268.Lee KT, Byun HJ, Jung KW, et al. Distinct and redundant roles of protein tyrosine phosphatases Ptp1 and Ptp2 in governing the differentiation and pathogenicity of Cryptococcus neoformans. Eukaryot Cell. 2014;13:796–812. doi: 10.1128/EC.00069-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ramsubramaniam N, Harris SD, Marten MR. The phosphoproteome of Aspergillus nidulans reveals functional association with cellular processes involved in morphology and secretion. Proteomics. 2014;14:2454–2459. doi: 10.1002/pmic.201400063. [DOI] [PubMed] [Google Scholar]
- 270.Selvan LD, Renuse S, Kaviyil JE, et al. Phosphoproteome of Cryptococcus neoformans. J Proteomics. 2014;97:287–295. doi: 10.1016/j.jprot.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 271.O'Meara TR, Norton D, Price MS, et al. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog. 2010;6:e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ubersax JA, Ferrell , Jr JE. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 273.Wang P, Nichols CB, Lengeler KB, et al. Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryot Cell. 2002;1:257–272. doi: 10.1128/EC.1.2.257-272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Willger SD, Liu Z, Olarte RA, et al. Analysis of the Candida albicans phosphoproteome. Eukaryot Cell. 2015;14:474–485. doi: 10.1128/EC.00011-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Grønborg M, Kristiansen TZ, Iwahori A, et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5:157–171. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 276.Mann M. Functional and quantitative proteomics using SILAC. Nat Rev Mol Cell Biol. 2006;7:952–958. doi: 10.1038/nrm2067. [DOI] [PubMed] [Google Scholar]
- 277.Chen X, Wei S, Ji Y, et al. Quantitative proteomics using SILAC: Principles, applications, and developments. Proteomics. 2015;15:3175–3192. doi: 10.1002/pmic.201500108. [DOI] [PubMed] [Google Scholar]
- 278.Rigbolt KT, Prokhorova TA, Akimov V, et al. System-wide temporal characterization of the proteome and phosphoproteome of human embryonic stem cell differentiation. Sci Signal. 2011;4:rs3. doi: 10.1126/scisignal.2001570. [DOI] [PubMed] [Google Scholar]
- 279.Humphrey SJ, Yang G, Yang P, et al. Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 2013;17:1009–1020. doi: 10.1016/j.cmet.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Uppuluri P, Nett J, Heitman J, et al. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother. 2008;52:1127–1132. doi: 10.1128/AAC.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kontoyiannis DP, Lewis RE, Alexander BD, et al. Calcineurin inhibitor agents interact synergistically with antifungal agents in vitro against Cryptococcus neoformans isolates: correlation with outcome in solid organ transplant recipients with cryptococcosis. Antimicrob Agents Chemother. 2008;52:735–738. doi: 10.1128/AAC.00990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yu SJ, Chang YL, Chen YL. Calcineurin signaling: lessons from Candida species. FEMS Yeast Res. 2015;15:fov016. doi: 10.1093/femsyr/fov016. [DOI] [PubMed] [Google Scholar]
- 283.Liu S, Hou Y, Liu W, et al. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot Cell. 2015;14:324–334. doi: 10.1128/EC.00271-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tripathi H, Luqman S, Meena A, et al. Genomic identification of potential targets unique to Candida albicans for the discovery of antifungal agents. Curr Drug Targets. 2014;15:136–149. doi: 10.2174/138945011501140115112242. [DOI] [PubMed] [Google Scholar]
- 285.Chen J, Zhou S, Wang Q, et al. Crk1, a novel Cdc2-related protein kinase, is required for hyphal development and virulence in Candida albicans. Mol Cell Biol. 2000;20:8696–8708. doi: 10.1128/mcb.20.23.8696-8708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]