Abstract
The involvement of brain 2-arachidonoylglycerol (2-AG) in a number of critical physiological and pathophysiological regulatory mechanisms highlights the importance for an accurate brain 2-AG determination. In the present study, we validated head-focused microwave irradiation (MW) as a method to prevent postmortem brain 2-AG alterations before analysis. We have compared MW to freezing to prevent 2-AG induction and estimated exogenous and endogenous 2-AG stability upon exposure to MW. Using MW, we have measured, for the first time, true 2-AG brain levels under basal conditions, 30 sec after brain removal from the cranium, and upon exposure to 5 min of brain global ischemia. Our data indicate that brain 2-AG levels are instantaneously and dramatically increased ~ 60 fold upon brain removal from the cranium. Upon 5 min of brain global ischemia 2-AG levels are also, but less dramatically, increased 3.5 fold. Our data indicate that brain tissue fixation with MW is a required technique to measure both true basal 2-AG levels and 2-AG alterations under different experimental conditions including global ischemia, and 2-AG is stable upon exposure to MW.
Keywords: Endocannabinoids, 2-arachidonoylglycerol, brain, stroke, ischemia, injury, mass spectrometry, analysis, microwave
Introduction
2-Arachidonoylglycerol (2-AG), the major cannabinoid type 1 receptor (CB1) agonist, has an important role in synaptic plasticity, sensation, and behavioral responses, but is also involved in the development of a number of pathophysiological conditions. The major physiological effect of endocannabinoids is analgesia [1, 2], but 2-AG is also implicated in food intake [3], as well as in anxiety, stress and fear responses, and movement disorders [4–6]. In addition, 2-AG levels are altered under ischemia and stroke [7–10], traumatic brain injury [11], obesity [12], Parkinson’s disease [13], and multiple sclerosis [14], indicating a possible role for 2-AG under these conditions. The molecular mechanism of 2-AG action is usually associated with fine-tune regulation of synaptic transmission through retrograde short-term plasticity[15–17]. In this mechanism, post-synaptically released 2-AG is involved in the synaptic regulation of GABA and glutamate release [18, 19], and allosteric potentiation of GABA [20, 21]. Intriguingly, endocannabinoids including 2-AG are substrates for oxidation by cyclooxygenases, lipoxygenases, and cytochromes P450 [22], and 2-AG is a source of arachidonic acid for eicosanoid production in brain [23], indicating possible cross-talk between the endocannabinoid and eicosanoid signaling pathways [22].
The involvement of brain 2-AG in a number of critical physiological and pathophysiological regulatory mechanisms highlights the importance for an accurate brain 2-AG determination. However, the values reported by different laboratories for basal brain 2-AG levels are inconsistent and vary ~280 fold from 90 to 24,600 ng/gww in rat brain [13, 24–34], and ~80 fold from 190 to 15,000 ng/gww in mouse brain [7, 10, 11, 14, 35–39]. Similarly, the reported data are inconsistent for brain 2-AG alterations under pathophysiological conditions. Some studies have reported an increase in 2-AG upon cerebral ischemia in rodents [7, 10, 11], although it was reported to be unchanged or have a trend to be decreased in other studies [34, 39–41].
A number of variables may contribute to the broad range of the reported brain 2-AG levels including post-mortem 2-AG alterations. It is well known that brain 2-AG levels depend upon the rate of enzymatic synthesis and hydrolysis. 2-AG is produced on-demand at the site of need from phosphatidylinositol (PtdIns) hydrolysis by a phospholipase C (PLC) to diacylglycerols (DAG), and subsequent DAG hydrolysis by a DAG lipase [28, 33], or through PtdIns hydrolysis by PLA1 to lyso- PtdIns intermediate, and then by lysophosphatidylinositol-selective PLC (lyso-PLC) [42]. Once produced, 2-AG is rapidly hydrolyzed by mono-acylglycerol lipase (MAGL) [43–46] and other esterases [47]. Because a number of lipases and phospholipases are rapidly and dramatically activated under acute brain ischemia-injury conditions [48–51], a rapid increase in 2-AG production and hydrolysis upon animal euthanasia and/or brain removal from the cranium may contribute to the inconsistency in the reported 2-AG levels. In fact, a rapid ~10 fold increase in brain 2-AG has been reported 1 min after decapitation as determined in brains extracted from heads soaked in liquid nitrogen [27]. Thus, a fast enzyme inactivation prior to 2-AG analysis may be required to measure true 2-AG basal levels and effect of different conditions on the brain endogenous 2-AG concentrations.
One of the methods to prevent post-mortem tissue alterations is high-energy focused microwave (MW) irradiation to heat-denature tissue enzymes [52–60]. We, and others, have demonstrated a safe use of MW to prevent brain and kidney eicosanoid postmortem alterations [52–55, 57, 58]. The measurement of another endocannabinoid, N-arachidonoylethanolamine (AEA), in rat brain also requires tissue fixation with MW [61]. However, to the best of our knowledge, this technique has not been previously validated and/or applied to measure 2-AG endogenous basal levels and alterations under brain global ischemia/hypoxia.
In the present study, we validated MW as a method to prevent postmortem brain 2-AG alterations. We have compared MW to freezing to prevent 2-AG induction, estimated exogenous and endogenous 2-AG stability upon exposure to MW, and measured true 2-AG brain levels under basal conditions and upon exposure to a 5 min of brain global ischemia. Our data indicate that brain tissue fixation with head focused MW is a required technique to measure both basal 2-AG levels and 2-AG alteration under different experimental conditions, and 2-AG is stable upon exposure to MW.
Methods
Animals
All animal use was in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals 8th Addition and approved by the University of North Dakota IACUC (protocol #1111-3). Sprague Dawley rats (225–300g) were provided standard laboratory chow and water ad libitum.
Materials
2-Arachidonoylglycerol (2-AG) and 2-arachidonoylglycerol-d8 containing deuterium atoms at the 5, 6, 8, 9, 11, 12, 14, and 15 positions of the arachidonic acid moiety (2-AGd8) were purchased from Cayman Chemicals (Ann Arbor, MI). All solvents of LC-MS grade or higher were purchased from Fisher Scientific (Waltham, MA).
Tissue Collection and Sample Preparation
Rats were anaesthetized with 3% isoflurane and euthanized either by decapitation to collect non-fixed tissue (non-MW), or by MW to denature enzymes [52–55, 57, 58]. A head-focused microwave machine (Cober Electronics, Inc., Norwalk, CT) with a rat adaptor was set at 70% power (5 kW measured output power) for 1.6 sec. The final brain temperature was 75–80 °C. In some experiments, decapitated but not exposed to MW intact heads were immediately (within 1–3 sec) placed in liquid nitrogen (non-MW fast frozen). In the experiments to validate stability of exogenous 2-AG upon exposure to head-focused microwave irradiation in biological matrix, 2-AGd8 was infused stereotexically (10 µg/animal, i.c.i., Bregma:-4 mm, Midline: 3 mm, Depth: 3.5 mm) into microwaved brain, and samples were collected 10 min after infusion (MW +infusion) or 10 min after immediate re-exposure to microwave irradiation (MW+infusion+MW). To validate endogenous 2-AG stability under MW conditions, we microwaved brains a second time (MW+MW). For both microwave and non-microwave irradiated animals, the brains were removed and frozen in liquid nitrogen. Time between decapitation and brain freezing was kept at 30 sec for all experiments except for freezing intact heads. The brains were pulverized to a homogenous powder under liquid nitrogen before analysis.
Tissue incubation with 2-AGd8
To validate endogenous and exogenous 2-AG alteration in tissue homogenate, 10 mg of tissue powder from frozen non-MW brains was spiked with 1µg of 2-AGd8 in 50 µL of artificial cerebral-spinal fluid (aCSF, Harvard Apparatus, MA, catalog# 59-7316) and incubated for 30 min at 40°C and 100% humidity in 2 mL centrifuge tubes placed in the heated water bath.
2-AG extraction
The tissue 2-AG was extracted using an acetone liquid/liquid extraction protocol as we previously described for eicosanoids [53, 62, 63] with the modification that the hexane fraction was collected for MS analysis. Briefly, tissue (approximately 10 mg of homogeneous brain powder) was homogenized in a Tenbroeck tissue grinder(Kontes Glass Co., Vineland, NJ) containing 3 mL of 2:1 acetone:saline with 100 ng of 2-AGd8 (added with 10 µL of ethanol) as an internal standards. In the tissue incubation experiments, the incubation mixture was transferred into a tissue grinder using acetone followed by saline washes. The 2-AGd8 internal standard was not used when 2-AGd8 was injected into the brain or used as a substrate during tissue incubation. The homogenate was transferred to a silanized (Sigmacote, Sigma Chemical Co., St. Louis, MO) screw top tube and centrifuged at 2000g for 10 min. The supernatant was transferred into a new silanized tube and extracted 3 times with 2 mL of hexane. The hexane extracts of 2-AG were combined in a new silanized glass tube. Our preliminary studies indicated a ~95% of recovery for 2-AGd8 in the hexane fraction.
The hexane extracts were dried under nitrogen, re-dissolved in 1 mL of acetonitrile, an aliquot was transferred to silanized microvial inserts (Micosolv, Eatontown, NJ, part #9502S-02ND) and mixed with an equal volume of water.
UPLC separation and MS Analysis
2-AG were resolved on Waters ACUITY UPLC HSS T3 column (1.8 µM, 100 Å pore diameter, 2.1x150mm, Waters, Milford, MA) with an ACUITY UPLC HSS T3 precolumn (1.8 µM, 100 Å pore diameter, 2.1x5mm, Waters) at a temperature of 55°C. The LC system consisted of a Waters ACUITY Class1 UPLC pump with a FTN sampler manager operated at 8 °C. Ten µL of sample was injected on column.
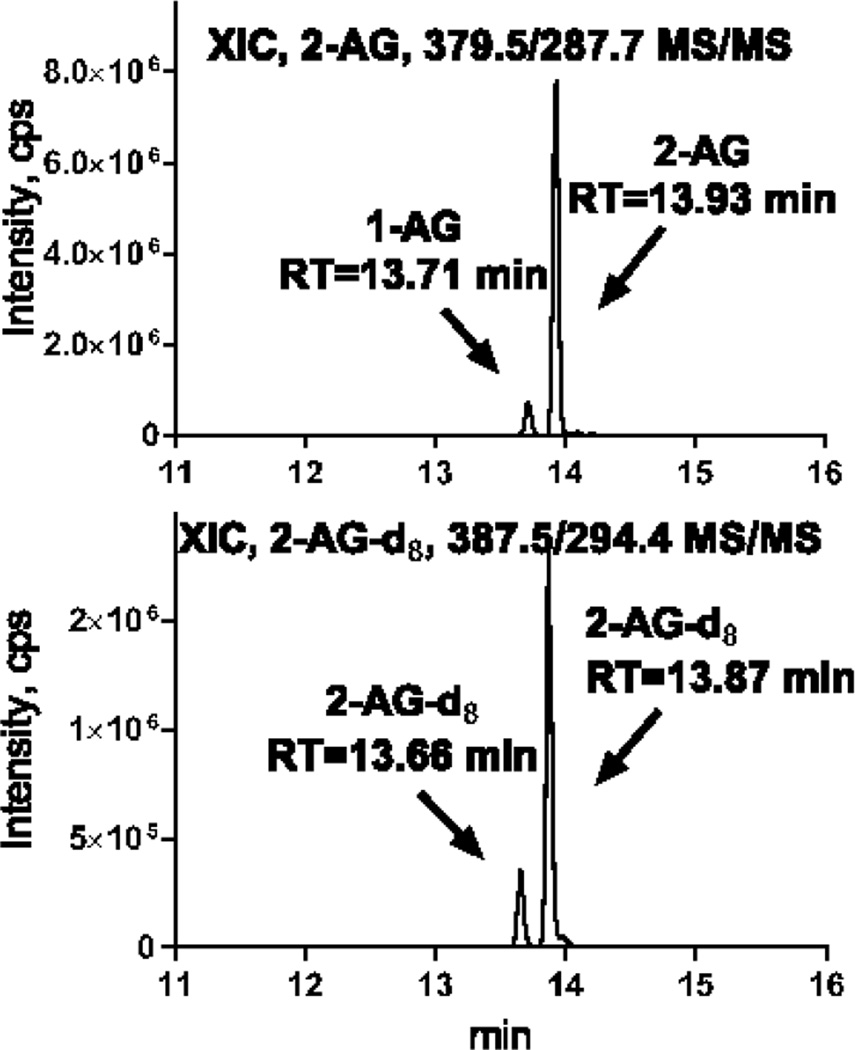
The separation was based on the UPLC method described previously [58, 64]. Solvent A consisted of water containing 0.1% formic acid and solvent B was acetonitrile with 0.1% formic acid. The flow rate was 0.45 mL/min. The initial conditions of 39% B were held for 0.5 min. Solvent B was increased to 40.5% over 6.88 min, followed by an increase to 70% over 1.62 min, then increased to 75% over 3 min, and further increased to 98% over 1.5 min where it was held for 5.3 min. Finally, solvent B was returned to initial conditions over 0.2 minutes to re-equilibrate the column for 2min. This method allows for 1-AG and 2-AG separation (Fig. 1). It is well known that 2-monoacylglycerols and 1-monoacylglycerols undergo inter-conversion when the acyl group migrates to a more stable sn-1 position. In the current study, we assumed that endogenous 2-AG and exogenous 2-AGd8 standard undergo a similar conversion, allowing to correct for this artifact by quantifying against 2-AGd8 internal standard.
Fig. 1.
Extracted ion chromatograms for 2-AG and 2-AGd8 analyzed using UPLC-MS/MS
2-AG were extracted from 10 mg of brain tissue with 100 ng of 2-AGd8 added as an internal standard before extraction as described in the Methods. 0.5% of the extract was loaded on the column for UPLC-MS/MS analysis.
For MS/MS analysis, a triple quadrupole mass spectrometer (Xevo TQ-S, Waters) with electrospray ionization operated in positive ion mode was used. The capillary voltage was 3.29 kV and the cone voltage was 61V. The desolvation temperature was 500 °C and the source temperature was 150 °C. The desolvation gas flow was 1000 L/hr, the cone gas flow was 150 L/hr, and the nebulizer gas was at 5.0 Bar. MassLynx V4.1 software (Waters) was used for instrument control, acquisition, and sample analysis.
The analytes were monitored in MRM mode using 379.3/287.7 mass transition for 2-AG, and 387.5/294.4 for 2-AGd8. The collision energy was 13V. 2-AG was quantified using 2-AGd8 as an internal standard except for experiments where 2-AGd8 was injected into the brain or used as a substrate during tissue incubation, and the results are expressed as peak areas when analyzed without 2-AGd8.
Statistics
Statistical comparisons were performed using a two-way, unpaired Student’s t-test or ANOVA with Tukey post-hoc test using a GraphPad Prism 6 software (GraphPad, San Diego, CA). Statistical significance was defined as p<0.05. All values are expressed as mean ± SD.
Results and Discussion
A dramatic postmortem 2-AG increase is prevented by MW but not by fast freezing of intact head in liquid nitrogen
A number of bioactive lipids, including brain and kidney eicosanoids and ethanolamides, are immediately and dramatically increased upon tissue removal from the animal body [52–61]. One method to prevent these post-mortem tissue alterations is MW to heat-denature enzymes by raising the tissue temperature to 70–80 °C [52–60]. We, and others, have demonstrated that focused MW is a safe method to prevent a rapid, 30-fold increase in brain and 150-fold increase in kidney prostaglandin mass within seconds upon tissue extraction from the body [52–55, 57, 58]. In addition, MW is required to measure another endocannabinoid, N-arachidonoylethanolamine concentration in rat brain [61]. However, to the best of our knowledge this technique has not been previously validated and/or applied to measure endogenous levels of 2-AG. Thus, we first measured 2-AG levels in fixed with MW brains and compared the levels to non-MW brains or brains removed from intact heads frozen in liquid nitrogen.
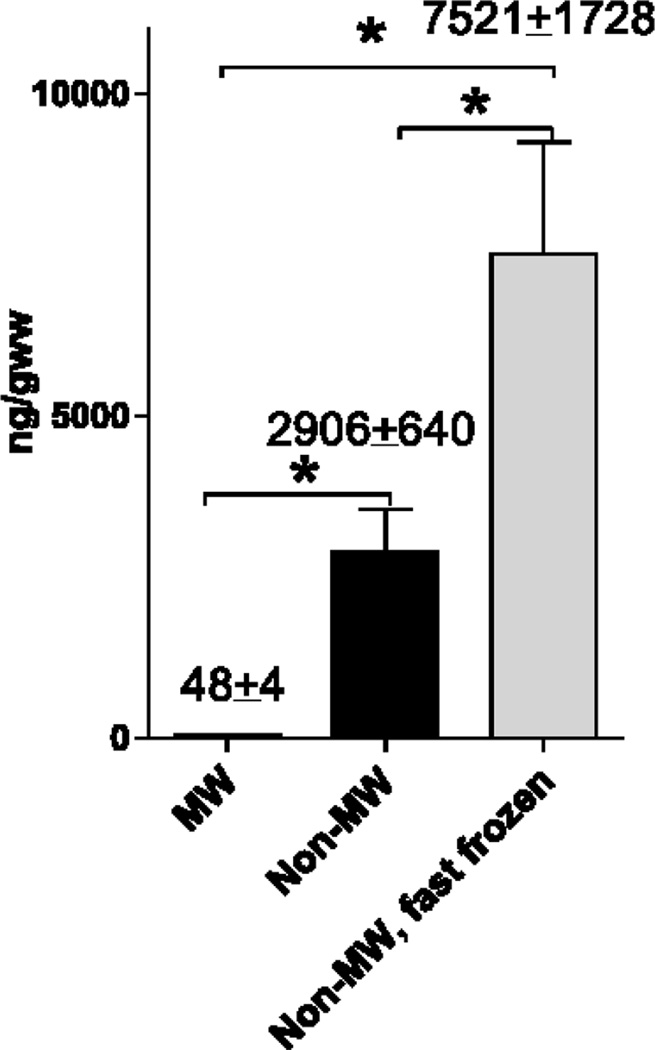
As compared to MW brains, 2-AG levels in non-MW tissue were ~60 fold higher (Fig. 2). Importantly, 30 sec were required to remove brain tissue from the body before freezing the samples in liquid nitrogen for further analysis. Thus, this data demonstrate a dramatic and almost instantaneous postmortem induction of brain 2-AG, similarly to other bioactive lipids [52–61]. Brain removal from the cranium mimics a traumatic brain injury, thus suggesting similar 2-AG alterations under clinically relevant conditions. Consistent with this assumption, 2-AG levels are increased 10 fold 4 h after closed head injury [11]. Importantly, 2-AG concentrations measured in MW rat brains are significantly lower compared to the previously reported values ranging from 90 to 24,600 ng/gww in the rat brain [13, 24–34] and from 190 to 15,000 ng/gww in the mouse brain [7, 10, 11, 14, 35–39] where non-fixed brain tissue was analyzed.
Fig. 2.
A dramatic postmortem 2-AG increase is prevented by MW but not by fast freezing of intact head in liquid nitrogen
Brain samples were analyzed from fixed with microwave irradiation brains (MW), from non-MW brains frozen within 30 sec after removing from the heads (non-MW), or from non-MW brains frozen in situ in the animal heads in liquid nitrogen within 3 sec after decapitation (non-MW, fast frozen). Samples were analyzed using UPLC-MS/MS analysis as described in the Methods. Values (also indicated above the bars) are mean ± SD, n=4. * - Statistically different (p<0.05) as compared to MW using ANOVA with Tukey post-hoc test. The data is representative of two independent sets of experiments.
Surprisingly, a fast (within 3 sec) freezing of the whole non-MW head with an intact brain did not prevent a rapid 2-AG increase and resulted in even higher (~2.5 fold) 2-AG levels compared to the non-MW brains frozen after removal from the heads (Fig. 2), and was ~150 fold higher compared to MW brains. This data may be explained by activation of enzymes producing 2-AG during tissue handling and extraction after removal of the brain sample from the frozen head, or by 2-AG induction upon decapitation. Although we immediately (within 3 sec) froze the heads in the liquid nitrogen, the heads do not freeze instantaneously when a thin layer of boiling nitrogen around the head may serve as an insulator.
Alternatively, lower levels of 2-AG in the non-MW brains removed from heads before freezing may be explained by an activation of both 2-AG producing and degrading enzymes with a higher contribution of 2-AG hydrolyzing enzymes as compared to the frozen intact heads. Obviously, brain 2-AG levels depend upon the rate of enzymatic synthesis and hydrolysis. Two pathways may be involved in 2-AG production from PtdIns including hydrolysis by PLC and subsequent DAG lipase [28, 33], or through hydrolysis by PLA1 and subsequent lyso-PLC [42]. Produced 2-AG is rapidly hydrolyzed by MAGL [43–46] and other esterases [47]. Because a number of lipases and phospholipases are rapidly and dramatically activated under acute brain ischemia-injury conditions that are mimicked by brain removal from the body [48–51], rapid alterations in 2-AG in both direction may be expected.
Our results are contradictory to the previously published study [27] where 2-AG levels were decreased in the frozen intact rat heads. However, we also report ~60 fold higher levels of 2-AG levels in the frozen brains. This may be explained by different methods of analysis (direct LC-MS analysis in our study versus fluorescence detection after two thin layer chromatography and extraction steps in [27]) or by different tissue handling techniques that may also affect the results in non-MW tissue as discussed above. Independently from the mechanism, this data demonstrates that MW is a safer and more consistent approach to prevent brain 2-AG postmortem induction compared to fast freezing techniques.
MW does not degrade endogenous or exogenous 2-AG in the biological matrix
Alternatively, a dramatic ~60 fold difference in 2-AG levels between MW and non-MW brains (Fig. 2) may be explained by 2-AG degradation in the biological matrix upon exposure to high temperatures caused by MW. Consistent with previously reported data [53, 57, 58, 62, 64], the applied MW settings raised the brain tissue temperature to 75–85°C in 1.6 sec as measured using a thermocouple. To validate stability of 2-AG under these conditions in biological matrix, we used two alternative approaches.
First, we validated exogenous 2-AGd8 stability. In this experiment, we exposed rats to head-focused MW to heat denature lipases to prevent enzymatic degradation of 2-AG, and then injected (i.c.i.) deuterium labeled 2-AGd8. One group was extracted immediately, and another was subjected to the second MW exposure to test 2-AGd8 stability. The extracted 2-AGd8 was analyzed with LC-MS/MS method and peak areas were compared between groups (Table. 1). The 2-AGd8 peak area was not different between groups, indicating that exogenous 2-AG was not destroyed in the biological matrix upon exposure to high energy MW.
Table 1.
Exogenous and endogenous 2-AG stability in biological matrix upon exposure to microwave irradiation
| Exogenous 2-AGd8
recovery upon MW, % |
Endogenous 2-AG, ng/gww | |||||
|---|---|---|---|---|---|---|
| AV | SD | Significance | AV | SD | Significance | |
| MW+infusion+MW | 102.1 | 22.7 | N/S | |||
| MW | 91.9 | 13.4 | ||||
| MW+MW | 85.8 | 8.4 | N/S | |||
To validate stability of exogenous 2-AG upon exposure to head-focused microwave irradiation in biological matrix, 2-AGd8 was infused stereotexically (i.c.i., 10 ug/animal) into microwaved brain. 2-AGd8 was extracted 10 min after infusion (MW+infusion) or 10 min after re-exposure to microwave irradiation to validate 2-AG stability (MW+infusion+MW). The 2-AGd8 recovery upon MW was calculated as a ratio of peak areas between MW+infusion+MW and MW+infusion. Statistical significance was calculated for peak areas (data not shown). The stability of endogenous 2-AG was validated by comparison of 2-AG levels in microwaved tissue before (MW) and after (MW+MW) second exposure to MW irradiation. Abbreviations are: AV-average; SD-standard deviation; N/S – not statistically different, n=4.
Second, we validated endogenous 2-AG stability upon MW. In this experiment, we analyzed endogenous 2-AG levels in the rat brain after the first (to prevent endogenous 2-AG induction during tissue handling) and second exposure to MW (Table. 1). The basal 2-AG levels were unchanged after the second MW, once again supporting the 2-AG stability under exposure to MW.
Together, these data indicate that MW does not destroy 2-AG and is a safe approach to fix tissue before 2-AG analysis.
2-AG is rapidly produced and hydrolyzed in non-MW brain tissue
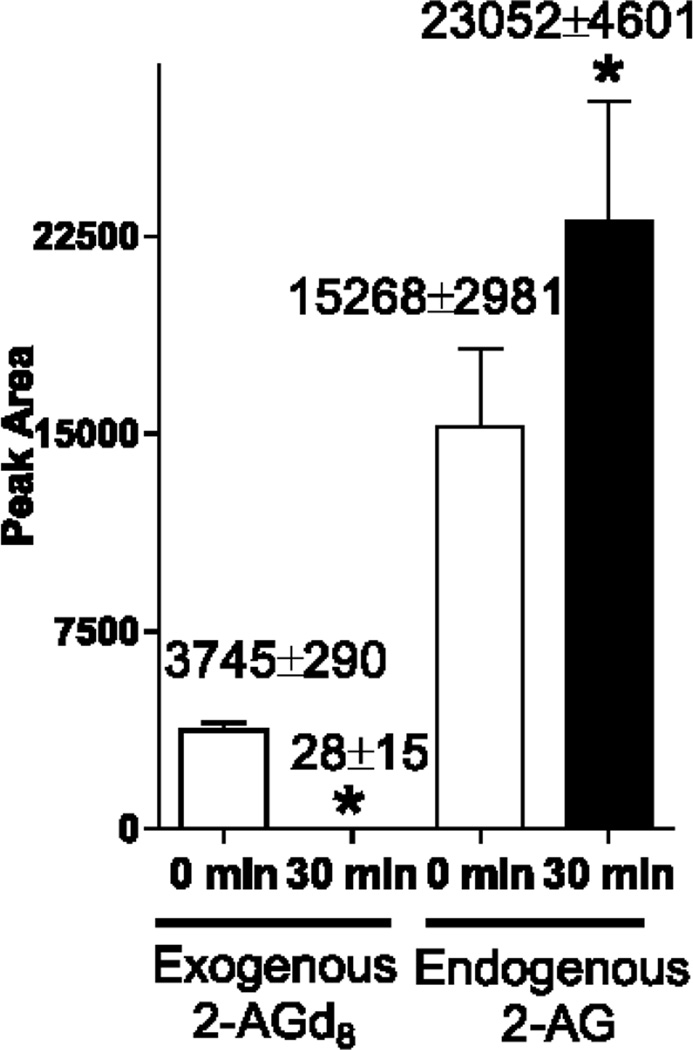
To further confirm activation of both induction and degradation of 2-AG in the non-MW postmortem brains, we spiked 2-AGd8 (10 µg per 10 mg of non-MW brain tissue powder) and analyzed 2-AG before or after incubation of brain tissue at 40°C, 100% humidity for 30 min (Fig. 3). The levels of exogenous 2-AGd8 were ~130 fold decreased upon incubation, indicating an activation of 2-AG hydrolyzing enzymes. A number of cytosolic hydrolyzes can degrade 2-acylglycerols, including monoacylglycerol lipase [46] and other esterases [47]. However, the endogenous 2-AG levels were significantly 51% increased upon incubation, probably through activation of phospholipases, triacylglycerol, and DG lipases [28, 33, 42]. These data clearly indicate two independent enzymatic activities in a non fixed brain tissue that is difficult to account for, and both processes may have impact on the quantitative results both in vitro and in vivo.
Fig. 3.
2-AG is rapidly produced and hydrolyzed upon incubation of non-MW brain tissue
Frozen non-microwaved brains were pulverized and 10 mg of brain powder was spiked with 2-AGd8 (10 µg). 2-AG and 2-AGd8 were extracted immediately or after incubation at 40°C for 30 min and quantified using UPLC-MS/MS analysis as described in the Methods. Values (also indicated above the bars) are Mean ± SD, n=4. Because labeled 2-AGd8 was used to account for hydrolysis of exogenous 2-AG, we could not use it as an internal standard. Thus, the results are reported as peak area for 2-AGd8 and unlabeled 2-AG.* - Statistically different (p<0.05).
Head-focused microwave irradiation is required to measure brain 2-AG alterations under brain global ischemia
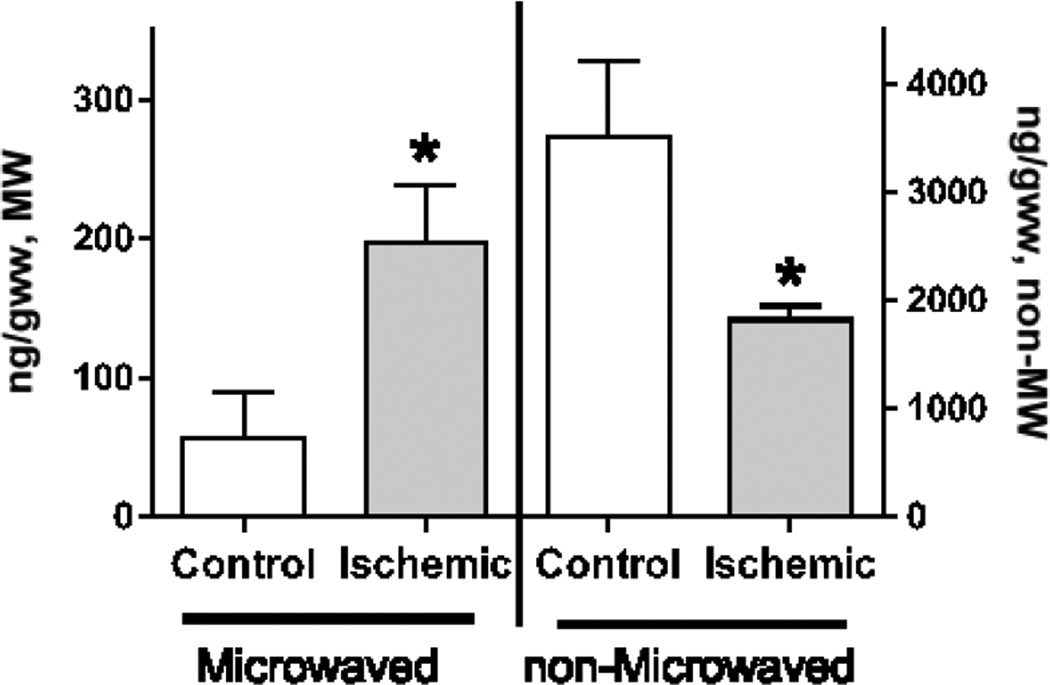
Because both 2-AG induction and degradation pathways are activated in the non-MW postmortem brain tissue removed from the cranium (Fig. 3), the measured 2-AG levels may be increased or decreased in the ischemic non-MW brains depending upon the handling time and extraction conditions. Indeed, brain 2-AG levels were reported to be increased upon cerebral ischemia in rodents [7, 10, 11], although it was reported to be unchanged or have a trend to be decreased in other rodent studies [34, 39–41]. To address this contradiction, we analyzed 2-AG levels in ischemic brains with or without MW fixation (Fig. 4). To model brain global ischemia, mouse brains were analyzed 5 min after decapitation [52, 53, 55, 64, 65]. Significantly, 2-AG levels were increased ~3.5 fold in the ischemic brains analyzed in the fixed tissue, while they were ~2 fold decreased when analyzed without fixation. Importantly, the basal levels were ~60 fold lower in the fixed tissue (Fig. 2 and 4). These data clearly indicate the importance of brain tissue fixation with head focused microwave irradiation not only for basal 2-AG analysis, but also for measurement of true 2-AG alteration under different experimental conditions including global brain ischemia.
Fig. 4.
MW is required to measure 2-AG alterations under ischemia/injury
Rat brain 2-AG levels were analyzed immediately after decapitation (control) or after incubation of decapitated heads at 40°C for 5 min (ischemic). Before decapitation, brain tissue was fixed with head focused microwave irradiation (Microwaved) or processed without fixation (non-Microwaved). 2-AG was analyzed using UPLC-MS/MS analysis as described in the Methods. Values are mean ± SD, n=3. * - Statistically different (p<0.05).
In summary, our data indicate a significant activation of both 2-AG producing and degrading pathways in the brain upon ischemia/injury mimicked by brain removal from cranium that is prevented by MW; and the importance for brain tissue MW fixation to measure true basal 2-AG levels and alterations under different experimental conditions including global brain ischemia. Applying MW brain tissue fixation, we demonstrated, for the first time, an instantaneous and dramatic ~ 60 fold increased in brain 2-AG levels upon brain ischemia/injury mimicked by brain removal from the cranium, and 3.5 fold induction under global brain ischemia.
Acknowledgments
This publication was made possible by NIH Grant 5R01AG042819-04 (MG), NIH funded COBRE Mass Spec Core Facility Grant 5P30GM103329-04 (MG), and UND Office of the Vice President for Research Research Enhancement Award. We thank Ms. Amanda Marquardt for her excellent assistance with sample preparation.
Glossary
- 2-AG
2-arachidonoylglycerol
- 2-AGd8
2-arachidonoylglycerol containing deuterium atoms at the 5, 6, 8, 9, 11, 12, 14, and 15 positions of the arachidonic acid moiety
- AEA
N-arachidonoylethanolamine
- aCSF
artificial cerebral-spinal fluid
- DAG
diacylglycerols
- Lyso-PLC
lysophosphatidylinositol-selective PLC
- MAGL
mono-acylglycerol lipase
- MW
microwave irradiation
- non-MW
non-fixed tissue
- PLC
phospholipase C
- PtdIns
phosphatidylinositol
- Lyco-PLC
lysophosphatidylinositol-selective PLC
Footnotes
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 2.Nyilas R, Gregg LC, MacKie K, Watanabe M, Zimmer A, Hohmann AG, Katona I. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur J Neurosci. 2009;29:1964–1978. doi: 10.1111/j.1460-9568.2009.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores Á, Maldonado R, Berrendero F. Cannabinoid-hypocretin cross-talk in the central nervous system: what we know so far. Frontiers in Neuroscience. 2013;7 doi: 10.3389/fnins.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 5.Ruehle S, Rey AA, Remmers F, Lutz B. The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol (Oxf) 2012;26:23–39. doi: 10.1177/0269881111408958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenniches I, Ternes S, Albayram O, Otte DM, Bach K, Bindila L, Michel K, Lutz B, Bilkei-Gorzo A, Zimmer A. Anxiety, Stress, and Fear Response in Mice with Reduced Endocannabinoid Levels. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Melis M, Pillolla G, Bisogno T, Minassi A, Petrosino S, Perra S, Muntoni AL, Lutz B, Gessa GL, Marsicano G, Di Marzo V, Pistis M. Protective activation of the endocannabinoid system during ischemia in dopamine neurons. Neurobiol Dis. 2006;24:15–27. doi: 10.1016/j.nbd.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrini-Giampietro DE, Mannaioni G, Bagetta G. Post-ischemic brain damage: the endocannabinoid system in the mechanisms of neuronal death. FEBS Journal. 2009;276:2–12. doi: 10.1111/j.1742-4658.2008.06765.x. [DOI] [PubMed] [Google Scholar]
- 9.Amantea D, Spagnuolo P, Bari M, Fezza F, Mazzei C, Tassorelli C, Morrone LA, Corasaniti MT, Maccarrone M, Bagetta G. Modulation of the endocannabinoid system by focal brain ischemia in the rat is involved in neuroprotection afforded by 17β-estradiol. FEBS Journal. 2007;274:4464–4775. doi: 10.1111/j.1742-4658.2007.05975.x. [DOI] [PubMed] [Google Scholar]
- 10.Degn M, Lambertsen KL, Petersen G, Meldgaard M, Artmann A, Clausen BH, Hansen SH, Finsen B, Hansen HS, Lund TM. Changes in brain levels of N-acylethanolamines and 2-arachidonoylglycerol in focal cerebral ischemia in mice. J Neurochem. 2007;103:1907–1916. doi: 10.1111/j.1471-4159.2007.04892.x. [DOI] [PubMed] [Google Scholar]
- 11.Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 12.Cristino L, Busetto G, Imperatore R, Ferrandino I, Palomba L, Silvestri C, Petrosino S, Orlando P, Bentivoglio M, Mackie K, Di Marzo V. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc Natl Acad Sci U S A. 2013;110:E2229–E2238. doi: 10.1073/pnas.1219485110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson's disease. FASEB J. 2000;14:1432–1438. doi: 10.1096/fj.14.10.1432. [DOI] [PubMed] [Google Scholar]
- 14.Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno T, Di Marzo V. Endocannabinoids control spasticity in a multiple sclerosis model. The FASEB Journal. 2000 doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- 15.Földy C, Neu A, Jones MV, Soltesz I. Presynaptic, Activity-Dependent Modulation of Cannabinoid Type 1 Receptor-Mediated Inhibition of GABA Release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 17.Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS and Neurological Disorders - Drug Targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rea K, Roche M, Finn DP. Supraspinal modulation of pain by cannabinoids: The role of GABA and glutamate. Br J Pharmacol. 2007;152:633–648. doi: 10.1038/sj.bjp.0707440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baur R, Kielar M, Richter L, Ernst M, Ecker GF, Sigel E. Molecular analysis of the site for 2-arachidonylglycerol (2-AG) on the β2 subunit of GABAA receptors. J Neurochem. 2013;126:29–36. doi: 10.1111/jnc.12270. [DOI] [PubMed] [Google Scholar]
- 21.Sigel E, Baur R, Rácz I, Marazzi J, Smart TG, Zimmer A, Gertsch J. The major central endocannabinoid directly acts at GABA A receptors. Proc Natl Acad Sci U S A. 2011;108:18150–18155. doi: 10.1073/pnas.1113444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouzer CA, Marnett LJ. Endocannabinoid Oxygenation by Cyclooxygenases, Lipoxygenases, and Cytochromes P450: Cross-Talk between the Eicosanoid and Endocannabinoid Signaling Pathways. Chem Rev. 2011;111:5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MCG, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid Hydrolysis Generates Brain Prostaglandins That Promote Neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maccarrone M, Attinà M, Cartoni A, Bari M, Finazzi-Agrò A. Gas chromatography–mass spectrometry analysis of endogenous cannabinoids in healthy and tumoral human brain and human cells in culture. J Neurochem. 2001;76:594–601. doi: 10.1046/j.1471-4159.2001.00092.x. [DOI] [PubMed] [Google Scholar]
- 25.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylgylcerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 26.Sugiura T, Yoshinaga N, Kondo S, Waku K, Ishima Y. Generation of 2-Arachidonoylglycerol, an Endogenous Cannabinoid Receptor Ligand, in Picrotoxinin-Administered Rat Brain. Biochem Biophys Res Commun. 2000;271:654–658. doi: 10.1006/bbrc.2000.2686. [DOI] [PubMed] [Google Scholar]
- 27.Sugiura T, Yoshinaga N, Waku K. Rapid generation of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, in rat brain after decapitation. Neurosci Lett. 2001;297:175–178. doi: 10.1016/s0304-3940(00)01691-8. [DOI] [PubMed] [Google Scholar]
- 28.Stella N, Schweitzer P, Plomelli D. A second endogenous' cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 29.Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, Fernandez-Ruiz JJ, Di Marzo V. Brain Regional Distribution of Endocannabinoids: Implications for Their Biosynthesis and Biological Function. Biochem Biophys Res Commun. 1999;256:377–380. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- 30.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, Rana GL, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 31.Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18:1607–1614. doi: 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed] [Google Scholar]
- 32.Hansen HH, Schmid PC, Bittigau P, Lastres-Becker I, Berrendero F, Manzanares J, Ikonomidou C, Schmid HHO, Fernández-Ruiz JJ, Hansen HS. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J Neurochem. 2001;78:1415–1427. doi: 10.1046/j.1471-4159.2001.00542.x. [DOI] [PubMed] [Google Scholar]
- 33.Kondo S, Kondo H, Nakane S, Kodaka T, Tokumura A, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: Identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through Ca2+-dependent and - independent mechanisms. FEBS Lett. 1998;429:152–156. doi: 10.1016/s0014-5793(98)00581-x. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Nie H, Tian L, Tong L, Yang L, Lao N, Dong H, Sang H, Xiong L. Nicotine-Induced Neuroprotection Against Ischemic Injury Involves Activation of Endocannabinoid System in Rats. Neurochem Res. 2013;38:364–370. doi: 10.1007/s11064-012-0927-6. [DOI] [PubMed] [Google Scholar]
- 35.Kingsley PJ, Marnett LJ. Analysis of endocannabinoids by Ag+ coordination tandem mass spectrometry. Anal Biochem. 2003;314:8–15. doi: 10.1016/s0003-2697(02)00643-7. [DOI] [PubMed] [Google Scholar]
- 36.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe S, Doshi M, Hamazaki T. n-3 Polyunsaturated fatty acid (PUFA) deficiency elevates and n-3 PUFA enrichment reduces brain 2-arachidonoylglycerol level in mice. Prostaglandins Leukot Essent Fatty Acids. 2003;69:51–59. doi: 10.1016/s0952-3278(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 38.Hanuš L, amp x, Avraham Y, Ben-Shushan D, Zolotarev O, Berry EM, Mechoulam R. Short-term fasting and prolonged semistarvation have opposite effects on 2-AG levels in mouse brain. Brain Res. 2003;983:144–151. doi: 10.1016/s0006-8993(03)03046-4. [DOI] [PubMed] [Google Scholar]
- 39.Franklin A, Parmentier-Batteur S, Walter L, Greenberg DA, Stella N. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J Neurosci. 2003;23:7767–7775. doi: 10.1523/JNEUROSCI.23-21-07767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Der Stelt M, Veldhuis WB, Van Haaften GW, Fezza F, Bisogno T, Bär PR, Veldink GA, Vliegenthart JFG, Di Marzo V, Nicolay K. Exogenous anandamide protects rat brain against acute neuronal injury in vivo. J Neurosci. 2001;21:8765–8771. doi: 10.1523/JNEUROSCI.21-22-08765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muthian S, Rademacher DJ, Roelke CT, Gross GJ, Hillard CJ. Anandamide content is increased and CB1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience. 2004;129:743–750. doi: 10.1016/j.neuroscience.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 42.Ueda H, Kobayashi T, Kishimoto M, Tsutsumi T, Okuyama H. A possible pathway of phosphoinositide metabolism through EDTA-insensitive phospholipase A1 followed by lysophosphoinositide-specific phospholipase C in rat brain. J Neurochem. 1993;61:1874–1881. doi: 10.1111/j.1471-4159.1993.tb09829.x. [DOI] [PubMed] [Google Scholar]
- 43.Blankman JL, Simon GM, Cravatt BF. A Comprehensive Profile of Brain Enzymes that Hydrolyze the Endocannabinoid 2-Arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vila A, Rosengarth A, Piomelli D, Cravatt B, Marnett LJ. Hydrolysis of Prostaglandin Glycerol Esters by the Endocannabinoid-Hydrolyzing Enzymes,. Monoacylglycerol Lipase and Fatty Acid Amide Hydrolase. Biochemistry. 2007;46:9578–9585. doi: 10.1021/bi7005898. [DOI] [PubMed] [Google Scholar]
- 45.Blankman JL, Cravatt BF. Chemical Probes of Endocannabinoid Metabolism. Pharmacol Rev. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nithipatikom K, Endsley MP, Isbell MA, Wheelock CE, Hammock BD, Campbell WB. A new class of inhibitors of 2-arachidonoylglycerol hydrolysis and invasion of prostate cancer cells. Biochem Biophys Res Commun. 2005;332:1028–1033. doi: 10.1016/j.bbrc.2005.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikeda M, Yoshida S, Busto R, Santiso M, Ginsberg MD. Polyphosphoinositides as a probable source of brain free fatty acids accumulated at the onset of ischemia. J Neurochem. 1986;47:123–132. doi: 10.1111/j.1471-4159.1986.tb02839.x. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida S, Ideda M, Busto R, Santiso M, Martinez E, Ginsberg MD. Cerebral phosphoinositide, triacylglycerol, and energy metabolism in reversible ischemia: Origin and fate of free fatty acids. J Neurochem. 1986;47:744–757. doi: 10.1111/j.1471-4159.1986.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 50.Lin TN, Liu TH, Xu J, Hsu CY, Sun GY. Brain polyphosphoinositide metabolism during focal ischemia in rat cortex. Stroke. 1991;22:495–498. doi: 10.1161/01.str.22.4.495. [DOI] [PubMed] [Google Scholar]
- 51.Murphy EJ, Haun SE, Rosenberger TA, Horrocks LA. Altered lipid metabolism in the presence and absence of extracellular Ca 2+ during combined oxygen-glucose deprivation in primary astrocyte cell cultures. J Neurosci Res. 1995;42:109–116. doi: 10.1002/jnr.490420112. [DOI] [PubMed] [Google Scholar]
- 52.Golovko MY, Murphy EJ. Brain prostaglandin formation is increased by α-synuclein gene-ablation during global ischemia. Neurosci Lett. 2008;432:243–247. doi: 10.1016/j.neulet.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golovko MY, Murphy EJ. An improved LC-MS/MS procedure for brain prostanoid analysis using brain fixation with head-focused microwave irradiation and liquid-liquid extraction. J Lipid Res. 2008;49:893–902. doi: 10.1194/jlr.D700030-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Anton RF, Wallis C, Randall CL. In vivo regional levels of PGE and thromboxane in mouse brain: Effect of decapitation, focused microwave fixation, and indomethacin. Prostaglandins. 1983;26:421–429. doi: 10.1016/0090-6980(83)90177-6. [DOI] [PubMed] [Google Scholar]
- 55.Farias SE, Basselin M, Chang L, Heidenreich KA, Rapoport SI, Murphy RC. Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J Lipid Res. 2008;49:1990–2000. doi: 10.1194/jlr.M800200-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy EJ. Brain fixation for analysis of brain lipid-mediators of signal transduction and brain eicosanoids requires head-focused microwave irradiation: An historical perspective. Prostaglandins Other Lipid Mediat. 2010;91:63–67. doi: 10.1016/j.prostaglandins.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Galli C, Racagni G, Lands WEM, Smith WL. Use of microwave techniques to inactivate brain enzymes rapidly. In: Colowick SP, Kaplan NO, editors. Methods Enzymol. New York: Academic Press; 1982. [DOI] [PubMed] [Google Scholar]
- 58.Brose SA, Golovko MY. Eicosanoid post-mortem induction in kidney tissue is prevented by microwave irradiation. Prostaglandins Leukot Essent Fatty Acids. 2013;89:313–318. doi: 10.1016/j.plefa.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cenedella RJ, Galli C, Paoletti R. Brain free fatty acid levels in rats sacrificed by decapitation versus focused microwave irradiation. Lipids. 1975;10:290–293. doi: 10.1007/BF02532702. [DOI] [PubMed] [Google Scholar]
- 60.Kingsley PJ, Marnett LJ. Analysis of endocannabinoids, their congeners and COX-2 metabolites. Journal of Chromatography B. 2009;877:2746–2754. doi: 10.1016/j.jchromb.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bazinet RP, Lee H-J, Felder CC, Porter AC, Rapoport SI, Rosenberger TA. Rapid High-Energy Microwave Fixation is Required to Determine the Anandamide (N-arachidonoylethanolamine) Concentration of Rat Brain. Neurochem Res. 2005;30:597–601. doi: 10.1007/s11064-005-2746-5. [DOI] [PubMed] [Google Scholar]
- 62.Brose SA, Thuen BT, Golovko MY. LC/MS/MS method for analysis of E2 series prostaglandins and isoprostanes. J Lipid Res. 2011;52:850–859. doi: 10.1194/jlr.D013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raatz SK, Golovko MY, Brose SA, Rosenberger TA, Burr GS, Wolters WR, Picklo MJ. Baking Reduces Prostaglandin, Resolvin, and Hydroxy-Fatty Acid Content of Farm-Raised Atlantic Salmon (Salmo salar) J Agric Food Chem. 2011;59:11278–11286. doi: 10.1021/jf202576k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brose S, Baker A, Golovko M. A Fast One-Step Extraction and UPLC–MS/MS Analysis for E2/D2 Series Prostaglandins and Isoprostanes. Lipids. 2013;48:411–419. doi: 10.1007/s11745-013-3767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Golovko M, Barceló-Coblijn G, Castagnet P, Austin S, Combs C, Murphy E. The role of α-synuclein in brain lipid metabolism: a downstream impact on brain inflammatory response. Mol Cell Biochem. 2009;326:55–66. doi: 10.1007/s11010-008-0008-y. [DOI] [PubMed] [Google Scholar]