Abstract
The ventral tegmental area (VTA) is an evolutionarily conserved structure that has roles in reward-seeking, safety-seeking, learning, motivation, and neuropsychiatric disorders such as addiction and depression. The involvement of the VTA in these various behaviors and disorders is paralleled by its diverse signaling mechanisms. Here we review recent advances in our understanding of neuronal diversity in the VTA with a focus on cell phenotypes that participate in ‘multiplexed’ neurotransmission involving distinct signaling mechanisms. First, we describe the cellular diversity within the VTA, including neurons capable of transmitting dopamine, glutamate or GABA as well as neurons capable of multiplexing combinations of these neurotransmitters. Next, we describe the complex synaptic architecture used by VTA neurons in order to accommodate the transmission of multiple transmitters. We specifically cover recent findings showing that VTA multiplexed neurotransmission may be mediated by either the segregation of dopamine and glutamate into distinct microdomains within a single axon or by the integration of glutamate and GABA into a single axon terminal. In addition, we discuss our current understanding of the functional role that these multiplexed signaling pathways have in the lateral habenula and the nucleus accumbens. Finally, we consider the putative roles of VTA multiplexed neurotransmission in synaptic plasticity and discuss how changes in VTA multiplexed neurons may relate to various psychopathologies including drug addiction and depression.
Keywords: reward, addiction, depression, aversion, co-transmission, dopamine, glutamate, GABA
Introduction
Midbrain dopamine neurons (DA) are most often associated with reward processing of both natural rewards (e.g., food, water, etc.) and drugs of abuse (Schultz, 2002; Wise, 2004; Sulzer, 2011). Over fifty years of intense research has led to the proposal that neurons belonging to the ventral tegmental area (VTA), which includes but is not limited to DA neurons, are paramount to reward processing. Many hypotheses have been put forward regarding the specific function of VTA DA neurons in reward processing, such as decision making (Salamone & Correa, 2002; Saddoris et al., 2015), flexible approach behaviors (Nicola, 2010), incentive salience (Berridge & Robinson, 1998; Berridge, 2007), and learning or the facilitation of memory formation (Adcock et al., 2006; Steinberg et al., 2013). However, several studies have also shown that VTA DA neurons are involved in the processing of aversive outcomes (Laviolette et al, 2002; Young, 2004; Pezze & Feldon, 2004; Brischoux et al., 2009; Lammel et al., 2012; Twining et al., 2014; Hennigan et al., 2015), fear (Abraham, Neve, & Lattal, 2014), aggression (Yu et al., 2014a,b), depression (Tidey & Miczek, 1996; Tye et al., 2013), and drug withdrawal (Grieder et al., 2014). Other hypotheses have proposed that VTA DA neurons play a more general role in processes such as associative learning (Brown et al., 2012), arousal (Horvitz, 2000), or general motivational salience and cognition (Bromberg-Martin et al., 2010).
The functional diversity associated with the VTA may be mediated, in part, by different VTA subpopulations of neurons. A particular advancement that may subserve the functional diversity of the VTA is the recent discovery of neurons that are capable of signaling using one or more neurotransmitters. In the present review, we cover recent literature on the diversity of VTA neuronal phenotypes as they relate to ‘multiplexed neurotransmission’. We refer the reader to recent comprehensive reviews detailing VTA cellular composition, VTA efferent and afferents, and VTA functions(Oades & Halliday, 1987; Fields et al., 2007; Ikemoto, 2007; Nair-Roberts et al., 2008; Morales & Pickel, 2012; Trudeau et al., 2014; Morales & Root 2014; Pignatelli and Bonci, 2015; Saunders et al., 2015; Luthi and Luscher, 2014). Moreover, the present review does not cover co-transmission of neurotransmitters and neuropeptides, which has long been known and recently reviewed (Morales & Pickel, 2012). Here, we use the phrase “multiplexed neurotransmission” to describe neurons that are capable of signaling using two or more neurotransmitters. In many circuits, our understanding of the specific mechanisms by which neurons utilize multiple neurotransmitters is limited. Thus, we have chosen the term multiplexed neurotransmission to encompass known and unknown mechanisms of co-release and co-transmission (e.g., Nusbaum et al., 2001; Mestikawy et al., 2011), while also allowing for the possibility of independent release of individual neurotransmitters either in time or space.
Cellular Diversity in the Ventral Tegmental Area
Following the discovery of DA as a chemical neurotransmitter in the brain (Montagu, 1957), the DAergic neurons in the “ventral tegmental area of Tsai” (Nauta, 1958) were identified by formaldehyde histofluorescence (Carlsson et al., 1962). These neurons, along with other catecholaminergic and serotonergic neurons throughout the brain were shown to comprise twelve discrete cell groups (labeled as A1-A12 groups; Dahlström & Fuxe, 1964). One feature of the A10 group, in particular, is the heterogeneous morphology among its neurons. Based on cytoarchitecture, the A10 region has been divided into two lateral nuclei [the Parabrachial Pigmented Nucleus (PBP) and Paranigral Nucleus (PN)], and three midline nuclei [the Rostral Linear Nucleus of the Raphe (RLi), Interfasicular Nucleus (IF), and Caudal Linear Nucleus (CLi)]. Traditionally, the VTA has been considered to include just the lateral nuclei (PBP, PN) (Swanson, 1982), however, modern conceptions of VTA function have often included the midline nuclei (RLi, IF, CLi) as subnuclei of the VTA (Ikemoto, 2007; Nair-Roberts et al., 2008; Morales and Root, 2014). Thus, in this review, we use the term VTA to define the midbrain A10 structure containing lateral (PBP, PN) and midline nuclei (RLi, IF, CLi). The cellular heterogeneity within the VTA subnuclei, together with findings showing that a single A10 neuron rarely innervates multiple structures (Swanson, 1982; Takada and Hattori, 1987; Lammel et al., 2008; Hosp et al. 2015), suggests that the VTA utilizes highly specific projections from different sets of neurons.
Dopamine neurons, defined by the expression of tyrosine hydroxylase (TH) protein (Figure 1), are interspersed throughout all VTA nuclei, but are most prevalent in the lateral PBP and PN (Swanson, 1982; Ikemoto, 2007; Li et al., 2013). In addition to the co-expression of TH and aromatic decarboxylase (AADC), the majority of rat lateral PBP and lateral PN neurons co-express the dopamine transporter (DAT), D2 receptor (D2R), and vesicular monoamine transporter 2 (VMAT2) mRNA (Li et al., 2013). More medially within the rat PBP and PN, as well as within the RLi, CLi, and IF, subsets of TH-expressing neurons either express or lack different combinations of DAT, VMAT2, or D2 receptor (Li et al., 2013, reviewed in Morales and Root, 2014). Our understanding of diversity among DAergic neurons in other species than the rat is less understood. However, recent studies have shown that, while all VTA neurons in the rat VTA expressing TH mRNA co-express the TH protein, some mouse VTA neurons expressing TH mRNA lack TH protein (Yamaguchi et al., 2015). In addition, ventrally to the VTA within the interpeduncular nucleus, there is in the mouse, but not in the rat, a subpopulation of neurons expressing TH mRNA, but lacking TH protein (Yamaguchi et al., 2015; Lammel et al., 2015). So far, detailed molecular characterizations of VTA neurons of nonhuman primates or humans has not been reported.
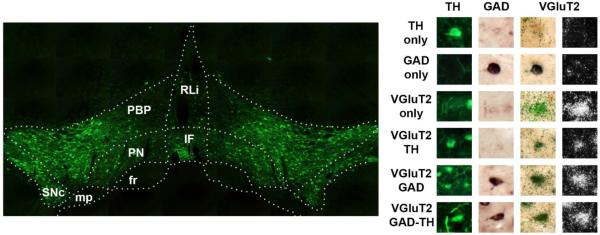
Figure 1. Neurons in the ventral tegmental area (VTA) are capable of multiplexed neurotransmission.
Detection of tyrosine hydroxylase (TH) immunoreactivity within the VTA, (low magnification, left panel). VTA combined immunohistochemistry and in situ hybridization showing at high magnification (right panel) neurons expressing TH (green cells), glutamic acid decarboxylase mRNA (GAD 65/67; purple cells), vesicular glutamate transporter 2 mRNA (VGluT2; green or white grain aggregates) or combinations of these cell markers. Abbreviations. Left: RLi- Rostral Linear Nucleus, IF- Interfasicular Nucleus, PBP- Parabrachial Pigmented Nucleus, PN- Paranigral Nucleus, SNc- Substantia Nigra Pars Compacta, fr- fasciculus retroflexus, mp- Mammillary Peduncle, Right: TH- tyrosine hydroxylase, GAD- glutamic acid decarboxylase, VGluT2- vesicular glutamate transporter 2.
Rat TH-expressing neurons within the lateral PBP and lateral PN have also been electrophysiologically characterized (so-called ‘primary’ neurons) based on their long-duration action potentials and hyperpolarization-activated cation currents (Grace & Onn, 1989). However, recent findings have shown that not all VTA TH-expressing neurons share these electrophysiological criteria (Margolis et al., 2006). In addition, although lack of direct electrophysiological responses to the mu opioid receptor agonist DAMGO has been proposed as a property shared by VTA DAergic neurons (Johnson and North, 1992), the VTA has a subpopulation of TH-expressing neurons that are directly excited or inhibited by DAMGO (Margolis et al., 2014). So far, it seems that hyperpolarization-activated cation currents, spike duration, inhibition by D2R agonist and other electrophysiological properties are unreliable predictors for the identification of all VTA DAergic neurons (Margolis et al., 2006), further supporting the heterogeneity of VTA DAergic neurons.
Along with DAergic neurons, γ-aminobutyric-acid (GABA) neurons are also present in the VTA (Nagai et al., 1983; Kosaka et al., 1987). These GABAergic neurons are relatively less prevalent than the DAergic neurons, and are identified by their expression of glutamic acid decarboxylase (GAD) 65 or 67 mRNA, isoforms of the enzyme involved in the synthesis of GABA. GABAergic VTA neurons are also identified by their expression of vesicular GABA transporter (VGaT) mRNA. Electrophysiologically, putative VTA GABAergic ‘secondary’ neurons have been characterized based on the observation that these cells are hyperpolarized by mu opioid agonists (Johnson and North, 1992). As with VTA DAergic neurons, VTA GABAergic neurons are pharmacologically and electrophysiologically heterogeneous. For example, approximately 60% of VTA GABAergic neurons are inhibited by the mu opioid receptor agonist DAMGO, but all seem to be unaffected by the GABA-B agonist baclofen (Margolis et al., 2012). GABAergic neurons in the VTA are known to establish local inhibitory connections on DAergic neurons (Johnson & North, 1992; Omelchenko & Sesack, 2009), but have also been shown to project outside of the VTA to the ventral striatum (nAcc; Van Bockstaele & Pickel, 1995) basal forebrain (Taylor et al., 2014), the prefrontal cortex (Steffensen et al., 1998; Carr & Sesack, 2000), the lateral habenula (LHb; Stamatakis et al., 2013; Root et al., 2014a; Taylor et al., 2014; Lammel et al., 2015), lateral hypothalamus, preoptic area, and amygdala, as well as to structures in the thalamus, midbrain, pons and medulla (Taylor et al., 2014). GABAergic neurons are scattered throughout the A10 region, and although a detailed subregional mapping of these neurons has not been yet reported, a dense group of GABAergic neurons has been identified in an area ventro-caudal to the VTA, referred as the ‘tail of the VTA’ (tVTA; Kaufling et al., 2009) or the rostromedial tegmental area (RMTg; Jhou, 2005, 2009a, 2009b; Geisler et al., 2008; Lavezzi & Zahm, 2011). The GABAergic neurons of the tVTA/RMTg provide a major inhibitory control to VTA DAergic neurons (Kaufling et al., 2010; Matsui et al., 2011).
In addition to VTA DAergic and GABAergic neurons, early electrophysiological studies of the midbrain suggested the possibility of glutamatergic signaling by some VTA neurons (Wilson et al., 1982; Mercuri et al., 1985; Sulzer et al 1998; Joyce & Rapport, 2000; Chuhma et al., 2004; Ungless et al., 2004; Lavin et al., 2005; Chuhma et al., 2009). Anatomical identification of glutamatergic neurons has recently become possible due to the cloning of three distinct vesicular glutamate transporters (VGluT1, VGluT2, and VGluT3; Bellocchio et al., 1998; Bai et al., 2001; Fremeau et al., 2001, 2002; Fujiyama et al., 2001; Hayashi et al., 2001; Herzog et al., 2001; Takamori et al., 2000; Varoqui et al., 2002; Gras et al., 2002). By in situ hybridization, it has been demonstrated that some neurons within the VTA (Kawano et al., 2006; Yamaguchi et al., 2007; 2011), substantia nigra and retrorubral field (Yamaguchi et al., 2013) express VGluT2 mRNA, but not VGluT1 or VGluT3. The VTA-VGluT2 neurons are present in all A10 nuclei, but are particularly prevalent within midline nuclei (Yamaguchi et al., 2007, 2011). In fact, glutamatergic neurons outnumber the DAergic neurons in the rostral and medial portions of the VTA (Yamaguchi et al., 2007, 2011). Thus, these neurons represent a major subpopulation in certain parts of the VTA. Similar to VTA GABAergic neurons, VGluT2-glutamatergic neurons in the VTA establish local and extrinsic synapses (Dobi et al., 2010; Yamaguchi et al., 2011; Zhang et al., 2015; Wang et al., 2015). Specifically, glutamatergic VTA neurons establish local asymmetric synapses with both DAergic and non-DAergic neurons (Dobi et al., 2010; Wang et al., 2015). Additionally, glutamatergic VTA neurons project to other regions of the brain including the LHb (Root et al., 2014a,b), nAcc (Zhang et al., 2015), amygdala, basal forebrain, and prefrontal cortex (Hnasko et al., 2012; Taylor et al., 2014).
Moreover, increasing evidence indicates that subpopulations of VTA neurons are capable of releasing DA and GABA, or DA and glutamate (Kosaka et al., 1987; Sulzer et al., 1998; Rayport, 2001; Dal Bo et al., 2004; Trudeau, 2004; Seutin, 2005; Lapish et al, 2006; Yamaguchi et al., 2007, 2011; Hnasko et al., 2010; Tritsch et al, 2012; Li et al., 2013; Mingote et al., 2015). Recent work from our laboratory has shown that there is a subset of VTA neurons capable of co-releasing DA and glutamate or glutamate and GABA (Zhang et al., 2015; Root et al., 2014a).
Multiplexed neurotransmission by some VTA neurons and associated circuits is a property shared by other brain structures. For instance, glutamate and GABA co-neurotransmission has been reported in epilepsy models within mossy fiber terminals (Gutiérrez et al., 2003, Gutiérrez 2003, 2005; Trudeau & Gutiérrez , 2007; Münster-Wandowski et al., 2013), developing medial trapezoid body terminals from the lateral superior olive (Gillespie et al. 2005; Noh et al., 2010), entopeduncular nucleus projection to the LHb (Shabel et al., 2014), and cortex (Fattorini et al., 2015). In addition, there is evidence for GABA and DA co-transmission in by substantia nigra pars compacta neurons as well as retinal amacrine neurons (Tritsch et al. 2012; Hirasawa et al., 2012). Other forms of neurotransmission include GABA and histamine by hypothalamic neurons (Yu et al., 2015), glutamate and acetylcholine co-transmission in striatal interneurons or medial habenula neurons (Gras et al., 2008; Ren et al., 2011; Higley et al., 2011; Nelson et al., 2014), GABA and acetylcholine co-transmission in corticopetal globus pallidus neurons (Saunders et al., 2015).
Based on the discovery that some VTA neurons exhibit multiple vesicular transporters, we have applied ultrastructural and electrophysiological approaches to determine the possible cellular mechanisms by which multiple neurotransmitters are released at the synaptic level. In the process of answering this question, we have revealed ultrastructural architectures suggesting that glutamate and GABA neuronal signaling by VTA neurons can be integrated into a single complex terminal with spatially distinct synaptic release sites for glutamate or GABA (Fig. 1) (Root et al., 2014a). We have also revealed that DA and glutamate neuronal signaling by VTA neurons can be segregated to distinct microdomains within the same axon (Fig 2), allowing for the spatially distinct release of DA or glutamate (Zhang et al., 2015).
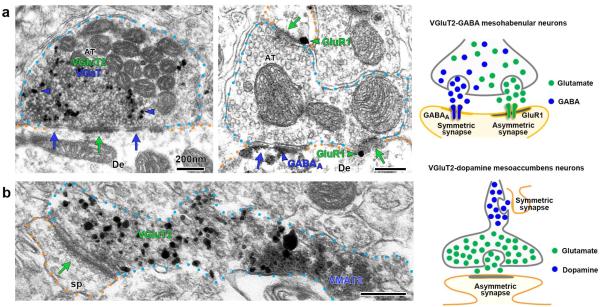
Figure 2. Ultrastructural immunolabeling reveals unpredicted mechanisms of neurotransmission within the mesohabenular and mesoaccumbal pathways.
(a) Lateral habenula micrograph (left panel) showing a single mesohabenular axon terminal containing VGluT2 (scattered dark material detected by immunoperoxidase labeling) and VGaT (gold particles detected by immunogold; blue arrowheads). This single axon terminal forms both an asymmetric synapse (green arrow) and a symmetric synapse (blue arrow) with a common postsynaptic dendrite (De). Postsynaptic to a single axon terminal (middle panel), GluR1 receptors (green arrowhead) are found adjacent to asymmetric synapses (green arrows), while GABAA receptors (blue arrowhead) are found adjacent to symmetric synapses (blue arrow). (b) Nucleus Accumbens micrograph showing a messoaccumbal axon containing both VMAT2 (scattered dark material) and VGluT2 (gold particles). VMAT2 and VGluT2 are segregated within the same axon. Note that the VGluT2 microdomain corresponds to an axon terminal establishing an asymmetric synapse (arrow) with a postsynaptic dendritic spine (sp). All scale bars represent 200 nm.
Multiplexed Signaling by VTA-GluT2 Neurons
Following the discovery of VTA-VGluT2 neurons, further characterization of these neurons demonstrated that they are very diverse in their molecular composition, signaling properties and neuronal connectivity. Whereas many VTA-VGluT2 neurons lack both DAergic and GABAergic markers, there are subpopulations of VTA-VGluT2 neurons that co-express molecules responsible for the synthesis or vesicular transport of either DA or GABA (Li et al., 2013; Root et al., 2014a). Although the distinct targets for VTA-VGluT2 neurons remains to be determined, emerging evidence suggests preferential target sites for specific subsets of VTA-VGluT2 neurons. For instance, by a combination of retrograde tract tracing and in situ hybridization, it has been demonstrated that VTA VGluT2(+)/GAD(+) neurons provide the major mesohabenular input to the LHb (Root et al., 2014a), and by contrast, VTA VGluT2(+)/TH(+) neurons largely target the nAcc shell (Yamaguchi et al., 2011). While the molecular characterization of mesohabenular and mesoaccumbens neurons provides support for multiplexed signaling by some VTA neurons, this characterization does not provide information on the cellular mechanisms by which these neurons release more than one neurotransmitter. As discussed below, recent findings obtained by a combination of cell-type specific anterograde tract tracing and immuno-electron microscopy have demonstrated that the VTA-VGluT2 neurons establish unique synaptic architectures for multiplexed signaling in both the LHb (Root et al., 2014a) and the nAcc (Zhang et al., 2015).
Multiplexed Signaling by Mesohabenular VGluT2 neurons
Recently available viral vectors and transgenic mice have facilitated the elucidation of the cellular mechanisms by which some VTA neurons use two distinct signaling molecules. To determine the synaptic ultrastructural features of mesohabenular axons we have taken advantage of the cell-specific viral tagging of VTA-VGluT2 neurons through the Cre-dependent expression of mCherry tethered to channelrhodopsin (ChR2) under the regulation of the VGluT2 promoter in VGluT2::Cre mice). By applying cell-specific tagging we estimated that more than 70% of mesohabenular axon terminals within the LHb co-express VGluT2 and VGaT (Root et al., 2014a). Moreover, we estimated that within the LHb both VGluT2 and VGaT are present in half of the total population of axon terminals, some of which derive from brain structures others than the VTA (e.g., from the basal ganglia; Shabel et al., 2014). While the presence of VGluT2 and VGaT within the same axon terminal has been established by immuno-electron microscopy, it remains to be determined whether each vesicular transporter is integrated into the membrane of distinct vesicles or in the same vesicular membrane. However, ultrastructural findings of the synaptic composition of individual VGluT2(+)/VGaT(+) axon terminals show that the plasma membrane of single VGluT2(+)/VGaT(+) axon terminals participates in the formation of both asymmetric and symmetric synapses, suggesting that glutamate-signaling is segregated to the asymmetric synapse and GABA-signaling to the symmetric (Figure 2).
In addition to the suggestion that asymmetric synapses participate in excitatory neurotransmission (Peters and Palay, 1996), GluR1-containing AMPA receptors are located in the membrane postsynaptic to the mesohabenular asymmetric synapses, but not to the symmetric synapses (Root et al., 2014a). In contrast, consistent with the suggestion that symmetric synapses participate in inhibitory neurotransmission (Peters and Palay, 1996), GABA-A receptors are located postsynaptically to the mesohabenular symmetric synapses, but not to the asymmetric synapses (Root et al., 2014a). The selective postsynaptic distribution of GluR1 to VGluT2(+)/VGaT(+) mesohabenular terminals making asymmetric synapses and GABA-A to those making symmetric synapses indicates that VGluT2(+)/VGaT(+) terminals release glutamate at the asymmetric synapse, and GABA at the symmetric synapses (Root et al., 2014a). Besides the formation of asymmetric and symmetric synapses by individual VGluT2(+)/VGaT(+) axon terminals, both synapses may target separate postsynaptic dendritic spines or dendritic shafts or share a common postsynaptic dendrite. These ultrastructural findings underlie the multiplexed signaling and potential neuroplastic capacity endowed by dual VGluT2(+)/VGaT(+) axon terminals from the VTA to the LHb.
Multiplexed Signaling by Mesoaccumbens VGluT2-DA neurons
Pioneering electrophysiological in vitro studies demonstrated that dopamine neurons in primary culture have the capability to release glutamate, which lead to the hypothesis that midbrain neurons co-transmit DA and glutamate (Sulzer et al., 1998; Joyce and Rayport 2000; Bourque and Trudeau, 2000). Since then, anatomical studies demonstrated that subsets of TH-positive neurons co-express VGluT2 mRNA throughout the brain (Stornetta et al., 2002; Kawano et al., 2006), including some TH-neurons within the midline nuclei of the VTA in rats (Yamaguchi et al., 2007, 2011) and mice (Yamaguchi et al., 2015). Moreover, findings from optogenetic electrophysiological ex vivo recordings have shown that the VGluT2(+)/TH(+) mesoaccumbens neurons use glutamate as signaling molecule (Stuber et al., 2010; Tecuapetla et al., 2010; Zhang et al., 2015; Mingote et al., 2015), and recent in vitro voltammetry measurements have shown that VGluT2-TH co-expressing neurons that project to nAcc release DA (Zhang et al., 2015).
So far two opposing hypotheses have been proposed to mediate the dual glutamate and DA signaling by VGluT2(+)/TH(+) mesoaccumbens neurons. One of them proposes that glutamate and DA coexist (and are co-released) from the same pool of vesicles (Hnasko et al., 2010, 2012). This hypothesis has been based on the co-immunoprecipitation of VMAT2 and VGluT2 from nAcc preparations (Hnasko et al., 2010). In clear contrast, a recent study has shown lack of VMAT2 and VGluT2 co-immunoprecipitation when ultrastructurally confirmed pure nAcc synaptic vesicles were used (Zhang et al., 2015). These recent findings have led to the hypothesis that dual VGluT2(+)/TH(+) mesoaccumbens neurons contain independent pools of vesicles for the accumulation of either DA or glutamate. Moreover, immuno-electron microscopy findings from intact brain tissue have shown that TH and VGluT2 do not coexist in the same axon terminal in the nAcc of either adult rats (Berube-Carriere et al., 2009; Moss et al., 2011) or mice of any age (Berube-Carriere et al., 2012). These immuno-electron microscopy findings are consistent with nAcc structural studies published over the last 40 years showing that axonal compartments engaged in excitatory signaling do not overlap with axonal compartments engaged in DA signaling (reviewed in Morales and Pickel, 2012). The lack of overlap between DA-vesicles and glutamate-vesicles may result from their segregation into two different sets of axons or segregation into micro-domains within the same axon. Although the possibility of vesicular segregation to different axons has not been discarded, recent immuno-electron microscopy findings indicate that VGluT2-vesicles from VGluT2(+)/TH(+) neurons are located in axon terminals that establish asymmetric synapses, and that axonal segments adjacent to these VGluT2-axonal terminals contain TH, VMAT2 and DAT (Zhang et al., 2015). The segregation between glutamate-vesicles and DA-vesicles within the same axon appears to be highly regulated, as in vivo overexpression of VMAT2, in the rat, does not disrupt the segregation between these two different types of vesicles. Moreover, the vesicular segregation by VGluT2(+)/TH(+) mesoaccumbens neurons is maintained in the nAcc of transgenic mice expressing ChR2 (following their viral mediated expression in VTA neurons under the regulation of either the TH-promoter or VGluT2-promoter; Zhang et al., 2015).
In summary, the characterization of mesoaccumbens and mesohabenular ultrastructural features together with the characterization of their electrophysiological and chemical properties have provided evidence for multiplexed signaling by VTA-VGluT2 neurons. These findings have demonstrated that dual rodent mesoaccumbens VGluT2(+)/TH(+) neurons have adjacent cellular compartments that participate in independent glutamate-signaling and DA-signaling (Zhang et al., 2015). In contrast, the dual rodent mesohabenular VGluT2(+)/GABA(+) neurons concentrate both glutamate-vesicles and GABA-vesicles within a single axon terminal that establishes both excitatory and inhibitory synapses (Root et al., 2014a). Future studies are necessary to determine the molecular and signaling mechanisms involved in the sorting and retention of VGluT2-vesicles, GABA-vesicles (VGaT) and DA-vesicles (VMAT2) to specific microdomains within the same axon. Additional studies are also necessary to determine the extent to which the multiplexed signaling by VTA neurons is affected in brain disorders, such as addiction and depression.
Functional Diversity by VTA Neurons
The functional diversity of VTA neurons has been constantly updated (Unlgess, 2004; Stamatakis et al., 2013; Root et al., 2014b; Mejias-Aponte et al., 2015; Eddine et al., 2015; Kotecki et al., 2015; Beier et al., 2015). As detailed above, the multiplexed neurotransmission of the VTA-VGluT2 neurons is an emerging factor involved in the complexity of VTA function. Based on observations that different combinations of neurotransmitters are multiplexed throughout the brain (Trudeau 2004; Gillespie et al. 2005; Zhou et al., 2005; Gras et al., 2008; Noh et al., 2010; Higley et al., 2011; Tritsch et al. 2012; Hnasko and Edwards, 2012 Münster-Wandowski et al., 2013; Nelson et al., 2014; Root et al., 2014a; Shabel et al., 2014; Qi et al., 2014; Zhang et al., 2015; Fattorini et al., 2015; Saunders et al., 2015), we suggest that multiplexed neurotransmission conveys distinct messages depending on the neurotransmitter content of each circuit, momentary singular or multiplexed signaling, and perhaps even the time scale of neurotransmitter function. Furthermore, we speculate that changes in the influence of one or more of the multiplexed neurotransmitters, by way of either presynaptic of postsynaptic changes, may result from and result in observable changes in behavior. Recent advances in the functional diversity within the VTA neurons targeting the LHb or nAcc will be presented in the following paragraphs.
Functional Diversity by VGluT2 Mesohabenular Neurons
An example of the circuit specific nature of multiplexed neurotransmission is found in the LHb. By combination of optogenetics and electrophysiology, we have shown that activation of the mesohabenular pathway evokes release of GABA and glutamate, and that the co-transmitted GABA is capable of shunting the co-transmitted glutamate-mediated currents (Root et al., 2014a). Therefore, the simultaneous release of glutamate and GABA may be a mechanism by which the glutamatergic excitation within the LHb is autoregulated by the co-transmitted GABA. In vivo recordings of LHb neurons following ChR2 activation of mesohabenular fibers have shown that this activation results in GABA-induced decreases in the firing rates of most recorded LHb neurons, and in glutamate-induced increases in firing rates in fewer neurons. In addition, secondary firing patterns are often observed in which initial increases in firing rates are followed by decreased firing rates or initial decreases in firing rates are followed by increased firing rates. The in vivo recordings of LHb neurons suggest that stimulation on mesohabenular fibers induces predominantly GABAergic neurotransmission. Nevertheless, the observed secondary firing patterns suggest that signaling might also occur over multiple time-scales or that the contribution of each neurotransmitter might be shifted in response to specific stimuli. For instance, rat depression models reduce GABA signaling from the multiplexed glutamate-GABA inputs to LHb from entopeduncular neurons (Shabel et al., 2014).
In addition, findings from combinations of optogenetics and behavioral analysis have shown that mesohabenular stimulation of fibers from different pools of VTA neurons, including multiplexed signaling neurons, promotes different behaviors. For instance, a LHb GABA receptor-mediated reward is evoked by mesohabenular stimulation of fibers expressing ChR2 under the TH-promoter (Stamatakis et al., 2013), likely to include activation of fibers from VGluT2(+)/GAD(+)/TH(+), VGluT2(+)/TH(+)/GAD(−), and VGluT2(−)/GAD(+)/TH(+) mesohabenular neurons. However, a mild reward is evoked by mesohabenular stimulation of fibers expressing ChR2 under the GAD2-promoter (Lammel et al., 2015), likely to include activation of fiber from VGluT2(+)/GAD(+)/TH(+), VGluT2(+)/GAD(+)/TH(−), VGluT2(−)/GAD(+)/TH(−), and VGluT2(−)/GAD(+)/TH(+) mesohabenular neurons. In contrast, a LHb glutamate receptor-mediated conditioned place aversion is evoked by mesohabenular stimulation of fibers expressing ChR2 under the VGluT2-promoter (Root et al., 2014b; Lammel et al., 2015), likely to include activation of fibers from VGluT2(+)/GAD(+)/TH(−), VGluT2(+)/GAD(+)/TH(+), and VGluT2(+)/GAD(−) neurons. These behavioral findings underlie the need for targeted intersectional approaches to dissect the behavioral contributions of each mesohabenular neuronal phenotype.
Multiplexed neurotransmission may affect neuronal regulation over multiple time scales, for instance “prolonged slow-actions” by monoamines (i.e., serotonin or dopamine) and “fast short actions” provided by the concomitant release of glutamate or GABA. This multiple time scale neurotransmission, by neurons endowed with the capacity for multiplexed signaling, may be found in a single DA-glutamate mesoaccumbens axon establishing segregated postsynaptic targets for DA- or glutamate-signaling (Zhang et al., 2015). Although the extent to which these mesoaccumbens DA-glutamate fibers participate in the neurobiology of drugs of abuse remains to be determined, we speculate that these axons may participate in the regulation of neuronal activity in cocaine self-administration. Specifically, electrophysiological recordings have shown that nAcc neurons exhibit rapid phasic firing patterns to related cues and actions to obtain the drug (Peoples et al., 1998; Ghitza et al., 2003, 2004, 2006; Fabbricatore et al., 2009, 2010; Coffey and Barker et al., 2015). The nAcc neurons also exhibit slow-phasic and tonic changes in firing rate that correlate with the pharmacological effects of cocaine, and do not correlate with the rapid phasic firing patterns (Fabbricatore et al., 2010). Furthermore, slow phasic pharmacologic and rapid phasic behavioral firing patterns are similarly processed in downstream accumbal targets (ventral pallidum and lateral preoptic area; Root et al., 2012, 2013; Barker et al., 2014). These dissociable fast and slow signaling patterns in the accumbens are consistent with findings suggesting that glutamate and dopamine each have specific roles in addiction-associated behaviors (Birgner et al., 2010; Alsiö et al., 2011).
Functional Diversity by TH Mesohabenular Neurons
Phenotypic characterizations of VTA-TH neurons have revealed the heterogeneous expression of several transcripts, some of which may be expressed transiently during development or may be induced in the adult brain in response to insults (e.g., drugs, stress, illness). In addition, some of these transcripts may not be translated into detectable protein levels under normal conditions, instead, this translation may depend on VTA circuit activity or be induced as a result of various brain insults (e.g., Bayer and Pickel, 1990, 1991; Garcia-Perez et al., 2014). For instance, we have identified a subset of VTA neurons, in wild type mice, that express TH mRNA, but lack detectable levels of TH-protein in cell bodies, dendrites and axons. Some of these neurons send projections to the LHb (Yamaguchi et al., 2015). In agreement with these findings, revealing TH-mRNA(+)/TH-protein(−) mesohabenular neurons in wild type mice, viral-induced expression of reporter genes (i.e., green-fluorescent-protein under the regulation of the TH-promoter) within the VTA of TH::cre mice has shown expression of fluorescent fibers without detectable TH-protein in the LHb (Stamatakis et al., 2013; Lammel et al., 2015; Stuber et al., 2015). These findings underlie the need to better characterize the VTA cellular composition in wild type mice, and reveal that expression of reporter genes in the mouse under the control of the TH-promoter does not guarantee the selective manipulation or mapping of DA projections.
In contrast to the mouse TH-mRNA(+)/TH-protein(−) mesohabenular neurons, subsets of rat mesohabenular neurons contain detectable levels of TH-protein in the cell bodies, dendrites and axons (Root et al., 2015). However, these rat TH-protein(+) mesohabenular neurons rarely co-express VMAT2-mRNA in their cell bodies or VMAT2-protein in their axon terminals in LHb (Root et al., 2015). The lack of VMAT2 within mesohabenular neurons has also been documented in the mouse (Stamatakis et al., 2013; Lammel et al., 2015). Overall, these findings provide crucial information when considering the functional properties of multi-neurotransmitter neurons, as they demonstrate that specific neuronal subsets have the capacity to synthesize DA but lack the capability to package DA into synaptic vesicles for traditional vesicular release. These finding are intriguing because DA has been detected in LHb homogenates (Phillipson and Pycock, 1982; Root et al., 2015), D2 receptors have been found in a subset of LHb neurons (Aizawa et al., 2012), and exogenous DA evokes currents in LHb neurons, currents that are eliminated by D2 or D4-receptor antagonists (Jhou et al., 2013; Good et al., 2013: Root et al., 2015). However, recordings of LHb neurons from rats treated with toxins for either the elimination of VTA-TH neurons or noradrenergic fibers have demonstrated that noradrenergic habenular afferents specifically activate D4-receptors in the LHb neurons and that VTA TH-expressing neurons are not necessary for this effect (Root et al., 2015). Thus, it seems that the LHb effects on DA-receptors previously ascribed to DA release from mesohabenular fibers may be instead mediated by noradrenergic fibers.
Multiplexed transmission: Future directions and considerations for synaptic plasticity
Our ever-expanding knowledge of multiplexed signaling opens the door to new predictions about synaptic plasticity. For example, though activation of the mesohabenular projection results in glutamate and GABA release, firing patterns of LHb neurons indicate a predominant GABA-induced decrease in firing rate of LHb neurons in rodents (Root et al., 2014a). Drugs of abuse, depression, and stress alter LHb function to favor glutamatergic excitation and demote GABAergic inhibition (Meshul et al., 1998; Li et al., 2011; Shabel et al., 2014), suggesting the potential for mesohabenular plasticity in mediating part of these effects. The ability of “neurotransmitter-switching” depending on circadian and seasonal variations has also been documented (Dulcis et al., 2013; Farajnia et al., 2014), and further investigation is necessary to determine if these factors influence multiplexed signaling.
The postsynaptic signaling dominance by one neurotransmitter may also be used to balance signals from other neurotransmitters and maintain homeostasis. Indeed, it has been shown that the same neurotransmitter may elicit different responses depending on the overall extracellular environment (Laviolette et al., 2002; Twining et al., 2014). For example in the mesohabenular projection, depending on the membrane potential of the postsynaptic LHb neuron, mesohabenular stimulation results in GABA-A receptor or AMPA-receptor currents (Root et al., 2014b). With this in mind, we speculate that drugs of abuse, neurodegenerative diseases, or other circumstances that affect synapses capable of multiplexed neurotransmission may produce aberrant signaling and thus affect cognition and behavior. It is likely that the recent discoveries of unpredicted synaptic arrangements will lead to novel experimental approaches to have a better understanding of how neurotransmission shifts from homeostatic conditions, how certain neurotransmitters become amplified or silenced, and how multiplexed signals might be simultaneously sent and received.
Because many neurotransmitters have multiple postsynaptic and presynaptic effects, multiplexed neurotransmission expands the repertoire of synaptic capabilities of single neurons. In this regard, electrophysiological evidence indicates that DA is capable to affect GABAA-receptors (Tritsch et al., 2012; Kim et al., 2015; Hoerbelt et al., 2015). Thus, when glutamate and DA are multiplexed together—as they are in some mesoaccumbal projections (Zhang et al., 2015)—DA may act to modulate glutamatergic signaling, counter glutamatergic signaling, or might even behave differently depending on the postsynaptic cell (e.g., targeting of D1 receptor neurons or D2 receptor neurons). With this in mind, it is clear that novel technologies and intersectional genetic strategies will be necessary in order to decipher the unique contributions of each component of multiplexed signals (Pupe et al, 2015).
A better understanding of the presynaptic and postsynaptic elements will allow better understanding of how these specialized synapses, described above, manage multiplexed neurotransmission in the presynaptic terminal and are subsequently integrated by the postsynaptic neuron. For example, these elements may work together to facilitate spike-timing dependent mechanisms for plasticity (e.g., Watanabe et al., 2002), as it is known that neuromodulators can affect the temporal window necessary for spike timing dependent activation, or that neuromodulators can cause a switch from long-term potentiation to long term depression (Caporale & Dan 2008; Bissiere et al., 2003). Thus, the spatiotemporal relationship of segregated DAergic and glutamatergic signaling in the nAcc may act to enhance the probability of signal transduction when both transmitters are released within a short time window. A similar mechanism might apply to mesohabenular signaling, although the precise mechanism by which glutamate and GABA might work to facilitate or shunt one another is still unclear. One possibility is that the integration for either a GABAergic or a glutamate response by the post-synaptic cell would depend on the timing of other habenular afferents.
Overall, it is becoming clear that the VTA is far more complex than was initially realized. Indeed, many studies have reported heterogeneous responses of specific VTA neurons (Brischoux et al., 2009; Borgkvist et al., 2011; Margolis et al., 2014; Eddine et al., 2015; Mrejeru et al 2015; Mejias-Aponte et al., 2015), and it would seem likely that this diversity is due (at least in part) to cells that are capable of multiplexed neurotransmission. With this in mind, it is clear that that the discovery of compound cell types has wide-reaching implications for our understanding of VTA circuit functions. Moreover, multiplexed signaling neurons are increasingly identified throughout the brain, suggesting that this unique type of signaling plays important roles in health and disease.
Highlights.
The Ventral Tegmental Area contains neurons capable of multiplexing multiple neurotransmitters.
Novel synaptic arrangements facilitate multiplexed neurotransmission.
Balance shifts in multiplexed neurotransmission may accompany behavioral changes.
Acknowledgements
The Intramural Research Program of the National Institute on Drug Abuse, US National Institutes of Health (IRP/NIDA/NIH) supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: A convergence of theory with fear and reward circuitry. Neurobiology of learning and memory. 2014;108:65–77. doi: 10.1016/j.nlm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. Journal of Comparative Neurology. 2012;520(18):4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- Alfahel-Kakunda A, Silverman WF. Calcium-binding proteins in the substantia nigra and ventral tegmental area during development: correlation with dopaminergic compartmentalization. Developmental brain research. 1997;103(1):9–20. doi: 10.1016/s0165-3806(97)00101-6. [DOI] [PubMed] [Google Scholar]
- Alsiö J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Trudeau LÉ. Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. The Journal of Neuroscience. 2011;31(35):12593–12603. doi: 10.1523/JNEUROSCI.2397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman C, Perlmann T, Wallén Å, Hoffer BJ, Morales M. A selective group of dopaminergic neurons express Nurr1 in the adult mouse brain. Brain research. 1999;851(1):125–132. doi: 10.1016/s0006-8993(99)02149-6. [DOI] [PubMed] [Google Scholar]
- Bai L, Xu H, Collins JF, Ghishan FK. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. Journal of Biological Chemistry. 2001;276(39):36764–36769. doi: 10.1074/jbc.M104578200. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. Journal of Comparative Neurology. 2011;519(6):1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Striano BM, Coffey KC, Root DH, Pawlak AP, Kim OA, West MO. Sensitivity to self-administered cocaine within the lateral preoptic–rostral lateral hypothalamic continuum. Brain Structure and Function. 2014:1–14. doi: 10.1007/s00429-014-0736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Calza L, Pozza M, Le Moal M, Piazza PV. Differential calbindin-immunoreactivity in dopamine neurons projecting to the rat striatal complex. European Journal of Neuroscience. 2000;12(12):4578–4582. [PubMed] [Google Scholar]
- Bayer VE, Pickel VM. Ultrastructural localization of tyrosine hydroxylase in the rat ventral tegmental area: relationship between immunolabeling density and neuronal associations. The Journal of Neuroscience. 1990;10(9):2996–3013. doi: 10.1523/JNEUROSCI.10-09-02996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain research. 1991;559(1):44–55. doi: 10.1016/0006-8993(91)90285-4. [DOI] [PubMed] [Google Scholar]
- Bayer VE, Towle AC, Pickel VM. Ultrastructural localization of neurotensin-like immunoreactivity within dense core vesicles in perikarya, but not terminals, colocalizing tyrosine hydroxylase in the rat ventral tegmental area. Journal of Comparative Neurology. 1991;311(2):179–196. doi: 10.1002/cne.903110202. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. The Journal of neuroscience. 1998;18(21):8648–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Luo L. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162(3):622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl.) 2007;191(2):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinton TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bérubé-Carrière N, Riad M, Dal Bo G, Lévesque D, Trudeau LÉ, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. Journal of Comparative Neurology. 2009;517(6):873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- Bérubé-Carrière N, Guay G, Fortin GM, Kullander K, Olson L, Wallén-Mackenzie Å, Descarries L. Ultrastructural characterization of the mesostriatal dopamine innervation in mice, including two mouse lines of conditional VGLUT2 knockout in dopamine neurons. European Journal of Neuroscience. 2012;35(4):527–538. doi: 10.1111/j.1460-9568.2012.07992.x. [DOI] [PubMed] [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Grevès M, Kullander K. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proceedings of the National Academy of Sciences. 2010;107(1):389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nature neuroscience. 2003;6(6):587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Mrejeru A, Sulzer D. Multiple personalities in the ventral tegmental area. Neuron. 2011;70(5):803–805. doi: 10.1016/j.neuron.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. European Journal of Neuroscience. 2000;12(9):3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proceedings of the National Academy of Sciences. 2009;106(12):4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Tan KR, O’Connor EC, Nikonenko I, Muller D, Lüscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492(7429):452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Falck B, Hillarp NA. Cellular localization of brain monoamines. Acta Physiol Scand. 1962;56(Suppl)(196):1–28. [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38(2):114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Progress in neurobiology. 2010;91(4):313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. The Journal of neuroscience. 1986;6(3):613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. The Journal of neuroscience. 2004;24(4):972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Choi WY, Mingote S, Rayport S. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience. 2009;164(3):1068–1083. doi: 10.1016/j.neuroscience.2009.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey KR, Barker DJ, Gayliard N, Kulik JM, Pawlak AP, Stamos JP, West MO. Electrophysiological evidence of alterations to the nucleus accumbens and dorsolateral striatum during chronic cocaine self-administration. European Journal of Neuroscience. 2015 doi: 10.1111/ejn.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Cholecystokinin-dopamine intearctions. Trends in Pharmacological Sciences. 1991;12:232–236. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe L. Evidence for the existence of monamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of the brain stem neurons. Acta Physiol Scand. 1964;62:1–55. [PubMed] [Google Scholar]
- Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. Journal of neurochemistry. 2004;88(6):1398–1405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. The Journal of Neuroscience. 2010;30(1):218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340(6131):449–453. doi: 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- Eddine R, Valverde S, Tolu S, Dautan D, Hay A, Morel C, Faure P. A concurrent excitation and inhibition of dopaminergic subpopulations in response to nicotine. Scientific reports. 2015;5 doi: 10.1038/srep08184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbricatore AT, Ghitza UE, Prokopenko VF, West MO. Electrophysiological evidence of mediolateral functional dichotomy in the rat accumbens during cocaine self-administration: tonic firing patterns. European Journal of Neuroscience. 2009;30(12):2387–2400. doi: 10.1111/j.1460-9568.2009.07033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbricatore AT, Ghitza UE, Prokopenko VF, West MO. Electrophysiological evidence of mediolateral functional dichotomy in the rat nucleus accumbens during cocaine self-administration II: phasic firing patterns. European Journal of Neuroscience. 2010;31(9):1671–1682. doi: 10.1111/j.1460-9568.2010.07230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J. Comp. Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Farajnia S, Deboer T, Rohling JH, Meijer JH, Michel S. Aging of the suprachiasmatic clock. The Neuroscientist. 2014;20(1):44–55. doi: 10.1177/1073858413498936. [DOI] [PubMed] [Google Scholar]
- Fattorini G, Antonucci F, Menna E, Matteoli M, Conti F. VGLUT1/VGAT co-expression sustains glutamate-gaba co-release and is regulated by activity. Journal of cell science. 2015 doi: 10.1242/jcs.164210. jcs-164210. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu. Rev. Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Foss SM, Li H, Santos MS, Edwards RH, Voglmaier SM. Multiple dileucine-like motifs direct VGLUT1 trafficking. The Journal of Neuroscience. 2013;33(26):10647–10660. doi: 10.1523/JNEUROSCI.5662-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31(2):247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Reimer RJ. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proceedings of the National Academy of Sciences. 2002;99(22):14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. Journal of Comparative Neurology. 2001;435(3):379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- García-Pérez D, López-Bellido R, Rodríguez RE, Laorden ML, Núñez C, Milanés MV. Dysregulation of dopaminergic regulatory mechanisms in the mesolimbic pathway induced by morphine and morphine withdrawal. Brain Structure and Function. 2014:1–19. doi: 10.1007/s00429-014-0761-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Reitböck P, Anichtchik O, Bellucci A, Iovino M, Ballini C, Fineberg E, Spillantini MG. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain. 2010 doi: 10.1093/brain/awq132. awq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Bérod A, Zahm DS, Rostène W. Brain neurotensin, psychostimulants, and stress–emphasis on neuroanatomical substrates. Peptides. 2006;27(10):2364–2384. doi: 10.1016/j.peptides.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Geisler S, Marinelli M, DeGarmo B, Becker ML, Freiman AJ, Beales M, Zahm DS. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33(11):2688–2700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa G, Melis M, Muntoni A, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB 1 receptors. European journal of pharmacology. 1998;341(1):39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. The Journal of neuroscience. 2003;23(19):7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko VF, West MO. Differences between accumbens core and shell neurons exhibiting phasic firing patterns related to drug-seeking behavior during a discriminative-stimulus task. Journal of neurophysiology. 2004;92(3):1608–1614. doi: 10.1152/jn.00268.2004. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Prokopenko VF, West MO, Fabbricatore AT. Higher magnitude accumbal phasic firing changes among core neurons exhibiting tonic firing increases during cocaine self-administration. Neuroscience. 2006;137(3):1075–1085. doi: 10.1016/j.neuroscience.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nature neuroscience. 2005;8(3):332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- Good CH, Wang H, Chen YH, Mejias-Aponte CA, Hoffman AF, Lupica CR. Dopamine D4 receptor excitation of lateral habenula neurons via multiple cellular mechanisms. The Journal of Neuroscience. 2013;33(43):16853–16864. doi: 10.1523/JNEUROSCI.1844-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9(10):3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. The Journal of neuroscience. 2002;22(13):5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Amilhon B, Lepicard ÈM, Poirel O, Vinatier J, Herbin M, El Mestikawy S. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nature neuroscience. 2008;11(3):292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- Grieder TE, Herman MA, Contet C, Tan LA, Vargas-Perez H, Cohen A, George O. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nature neuroscience. 2014 doi: 10.1038/nn.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R, Romo-Parra H, Maqueda J, Vivar C, Ramìrez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. The Journal of neuroscience. 2003;23(13):5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R. The GABAergic phenotype of the “glutamatergic” granule cells of the dentate gyrus. Progress in neurobiology. 2003;71(5):337–358. doi: 10.1016/j.pneurobio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. The dual glutamatergic–GABAergic phenotype of hippocampal granule cells. Trends in neurosciences. 2005;28(6):297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Otsuka M, Morimoto R, Hirota S, Yatsushiro S, Takeda J, Moriyama Y. Differentiation-associated Na+-dependent inorganic phosphate cotransporter (DNPI) is a vesicular glutamate transporter in endocrine glutamatergic systems. Journal of Biological Chemistry. 2001;276(46):43400–43406. doi: 10.1074/jbc.M106244200. [DOI] [PubMed] [Google Scholar]
- Hennigan K, D'Ardenne K, McClure SM. Distinct Midbrain and Habenula Pathways Are Involved in Processing Aversive Events in Humans. The Journal of Neuroscience. 2015;35(1):198–208. doi: 10.1523/JNEUROSCI.0927-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21(22):181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PloS one. 2011;6(4):e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Betensky RA, Raviola E. Corelease of dopamine and GABA by a retinal dopaminergic neuron. The Journal of Neuroscience. 2012;32(38):13281–13291. doi: 10.1523/JNEUROSCI.2213-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65(5):643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter Co-release: mechanism and physiological role. Annual review of physiology. 2012;74:225. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerbelt P, Lindsley TA, Fleck MW. Dopamine Directly Modulates GABAA Receptors. The Journal of Neuroscience. 2015;35(8):3525–3536. doi: 10.1523/JNEUROSCI.4390-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurones. 1980. [DOI] [PubMed]
- Hökfelt T, Everitt BJ, Theodorsson-Norheim E, Goldstein M. Occurrence of neurotensinlike immunoreactivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. Journal of Comparative Neurology. 1984;222(4):543–559. doi: 10.1002/cne.902220407. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Goldstein M. Chemical anatomy of the brain. Science. 1984;225(4668):1326–1334. doi: 10.1126/science.6147896. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Skirboll L, Rehfeld JF, Goldstein M, Markey K, Dann O. A subpopulation of mesencephalic dopamine neurons projecting to limbic areas contains a cholecystokinin-like peptide: evidence from immunohistochemistry combined with retrograde tracing. Neuroscience. 1980;5(12):2093–2124. doi: 10.1016/0306-4522(80)90127-x. [DOI] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. The Journal of neuroscience. 2011;31(32):11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Nolan HE, Luft AR. Topography and collateralization of dopaminergic projections to primary motor cortex in rats. Experimental brain research. 2015;233(5):1365–1375. doi: 10.1007/s00221-015-4211-2. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain research reviews. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KR, Jacobowitz DM. Mapping of the colocalization of calretinin and tyrosine hydroxylase in the rat substantia nigra and ventral tegmental area. Experimental brain research. 1994;99(1):34–42. doi: 10.1007/BF00241410. [DOI] [PubMed] [Google Scholar]
- Jayaraman A, Nishimori T, Dobner P, Uhl GR. Cholecystokinin and neurotensin mRNAs are differentially expressed in subnuclei of the ventral tegmental area. Journal of Comparative Neurology. 1990;296(2):291–302. doi: 10.1002/cne.902960209. [DOI] [PubMed] [Google Scholar]
- Jhou T. Neural mechanisms of freezing and passive aversive behaviors. Journal of Comparative Neurology. 2005;493(1):111–114. doi: 10.1002/cne.20734. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61(5):786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. Journal of Comparative Neurology. 2009b;513(6):566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. The Journal of Neuroscience. 2013;33(17):7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABAA receptor-mediated mechanism. The Journal of neuroscience. 2007;27(26):6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. The Journal of neuroscience. 1992;12(2):483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MP, Rayport S. Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience. 2000;99(3):445–456. doi: 10.1016/s0306-4522(00)00219-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Miller JS. Neurotensin neurons in the ventral tegmental area project to the medial nucleus accumbens. Brain research. 1984;300(1):157–160. doi: 10.1016/0006-8993(84)91351-9. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. Journal of Comparative Neurology. 2009;513(6):597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. γ-Aminobutyric acid cells with cocaine-induced ΔFosB in the ventral tegmental area innervate mesolimbic neurons. Biological psychiatry. 2010;67(1):88–92. doi: 10.1016/j.biopsych.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. Journal of Comparative Neurology. 2006;498(5):581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kim JI, Ganesan S, Luo SX, Wu YW, Park E, Huang EJ, Ding JB. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science. 2015;350(6256):102–106. doi: 10.1126/science.aac4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu JY, Ottersen OP, Hama K. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Experimental brain research. 1987;66(1):191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- Kotecki L, Hearing M, McCall NM, de Velasco EMF, Pravetoni M, Arora D, Weaver CD. GIRK Channels Modulate Opioid-Induced Motor Activity in a Cell Type- and Subunit-Dependent Manner. The Journal of Neuroscience. 2015;35(18):7131–7142. doi: 10.1523/JNEUROSCI.5051-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Konno K, Uchigashima M, Yanagawa Y, Sora I, Minami M, Watanabe M. GABAergic neurons in the ventral tegmental area receive dual GABA/enkephalin-mediated inhibitory inputs from the bed nucleus of the stria terminalis. European Journal of Neuroscience. 2014;39(11):1796–1809. doi: 10.1111/ejn.12503. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Murakami K, Igarashi H, Okada A. The convergence of axon terminals from the mediodorsal thalamic nucleus and ventral tegmental area on pyramidal cells in layer V of the rat prelimbic cortex. European Journal of Neuroscience. 1996;8(7):1340–1349. doi: 10.1111/j.1460-9568.1996.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57(5):760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76:351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Földy C, Wall NR, Beier K, Luo L, Malenka RC. Diversity of Transgenic Mouse Models for Selective Targeting of Midbrain Dopamine Neurons. Neuron. 2015;85(2):429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Seamans JK, Judson Chandler L. Glutamate-Dopamine Cotransmission and Reward Processing in Addiction. Alcoholism: Clinical and Experimental Research. 2006;30(9):1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Lavezzi HN, Zahm DS. The mesopontine rostromedial tegmental nucleus: an integrative modulator of the reward system. Basal ganglia. 2011;1(4):191–200. doi: 10.1016/j.baga.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. The Journal of neuroscience. 2005;25(20):5013–5023. doi: 10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Dopaminergic neurons expressing calbindin in normal and parkinsonian monkeys. Neuroreport. 1991;2(10):601–604. doi: 10.1097/00001756-199110000-00012. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Alexson TO, Van Der Kooy D. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. The Journal of neuroscience. 2002;22(19):8653–8660. doi: 10.1523/JNEUROSCI.22-19-08653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard A, Savard M, Gobeil F, Pierce JP, Pickel VM. The neurokinin-3 (NK3) and the neurokinin-1 (NK1) receptors are differentially targeted to mesocortical and mesolimbic projection neurons and to neuronal nuclei in the rat ventral tegmental area. Synapse. 2009;63(6):484–501. doi: 10.1002/syn.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470(7335):535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Structure and Function. 2013;218(5):1159–1176. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Luo M. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. 2014;81(6):1360–1374. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Borgland SL. Regulation of the mesolimbic dopamine circuit by feeding peptides. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2014.12.046. [DOI] [PubMed] [Google Scholar]
- Liang CL, Sinton CM, German DC. Midbrain dopaminergic neurons in the mouse: co-localization with calbindin-D 28K and calretinin. Neuroscience. 1996;75(2) doi: 10.1016/0306-4522(96)00228-x. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence methods. Acta Physiol. Scand. 1974;412(Suppl):1–48. [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A, Moore RY, Stenevi U. Mesencephalic dopamine neurons projecting to neocortex. Brain Res. 1974;81:325–331. doi: 10.1016/0006-8993(74)90947-0. [DOI] [PubMed] [Google Scholar]
- Lüthi A, Lüscher C. Pathological circuit function underlying addiction and anxiety disorders. Nature neuroscience. 2014;17(12):1635–1643. doi: 10.1038/nn.3849. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. Journal of Comparative Neurology. 2001;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? The Journal of physiology. 2006;577(3):907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. δ-Opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. The Journal of Neuroscience. 2008;28(48):12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area GABAergic neurons. PloS one. 2012;7(7):e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Fujita W, Fields HL. Direct Bidirectional μ-Opioid Control of Midbrain Dopamine Neurons. The Journal of Neuroscience. 2014;34(44):14707–14716. doi: 10.1523/JNEUROSCI.2144-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux M, Mameli M. Cocaine evokes projection-specific synaptic plasticity of lateral habenula neurons. The Journal of Neuroscience. 2012;32(36):12641–12646. doi: 10.1523/JNEUROSCI.2405-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. The Journal of Neuroscience. 2011;31(48):17729–17735. doi: 10.1523/JNEUROSCI.4570-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Ye C, Bonci A, Kiyatkin EA, Morales M. A Subpopulation of Neurochemically-Identified ventral tegmental area Dopamine Neurons Is Excited by Intravenous Cocaine. The Journal of Neuroscience. 2015;35(5):1965–1978. doi: 10.1523/JNEUROSCI.3422-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, Noguchi K, Emre N, Ellison G. Cocaine-induced changes in glutamate and GABA immunolabeling within rat habenula and nucleus accumbens. Synapse. 1998;30(2):211–220. doi: 10.1002/(SICI)1098-2396(199810)30:2<211::AID-SYN11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Mercuri N, Calabresi P, Stanzione P, Bernardi G. Electrical stimulation of mesencephalic cell groups (A9-A10) produces monosynaptic excitatory potentials in rat frontal cortex. Brain research. 1985;338(1):192–195. doi: 10.1016/0006-8993(85)90267-7. [DOI] [PubMed] [Google Scholar]
- El Mestikawy S, Wallén-Mackenzie Å, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nature Reviews Neuroscience. 2011;12(4):204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- Mingote S, Chuhma N, Kusnoor SV, Field B, Deutch AY, Rayport S. Functional Connectome Analysis of Dopamine Neuron Glutamatergic Connections in Forebrain Regions. The Journal of Neuroscience. 2015;35(49):16259–16271. doi: 10.1523/JNEUROSCI.1674-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagu KA. Catechol compounds in rat tissues and in brains of different animals. Nature. 1957;180:244–245. doi: 10.1038/180244a0. [DOI] [PubMed] [Google Scholar]
- Morales M, Pickel VM. Insights to drug addiction derived from ultrastructural views of the mesocorticolimbic system. Annals of the New York Academy of Sciences. 2012;1248(1):71–88. doi: 10.1111/j.1749-6632.2011.06299.x. [DOI] [PubMed] [Google Scholar]
- Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014;282:60–68. doi: 10.1016/j.neuroscience.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J, Ungless MA, Bolam JP. Dopaminergic axons in different divisions of the adult rat striatal complex do not express vesicular glutamate transporters. European Journal of Neuroscience. 2011;33(7):1205–1211. doi: 10.1111/j.1460-9568.2011.07594.x. [DOI] [PubMed] [Google Scholar]
- Mrejeru A, Martí-Prats L, Avegno EM, Harrison NL, Sulzer D. A subset of ventral tegmental area dopamine neurons responds to acute ethanol. Neuroscience. 2015;290:649–658. doi: 10.1016/j.neuroscience.2014.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münster-Wandowski A, Gómez-Lira G, Gutiérrez R. Mixed neurotransmission in the hippocampal mossy fibers. Frontiers in cellular neuroscience. 2013;7 doi: 10.3389/fncel.2013.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, McGeer PL, McGeer EG. Distribution of GABA-T-Intensive neurons in the hat forebrain and midbrain. Journal of Comparative Neurology. 1983;218(2):220–238. doi: 10.1002/cne.902180209. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152(4):1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26(11):2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. An Experimental Study of the fornix system in the rat. J. Comp. Neurol. 1956;104:247–271. doi: 10.1002/cne.901040205. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. Hippocampal projections and related neural pathways to the midbrain in the cat. Brain. 1958;81:319–340. doi: 10.1093/brain/81.3.319. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. Limbic system and hypothalamus: anatomical aspects. Physiol. Rev. 1960;40(suppl. 4):102–104. [PubMed] [Google Scholar]
- Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP, Kreitzer AC. Striatal cholinergic interneurons drive GABA release from dopamine terminals. Neuron. 2014;82(1):63–70. doi: 10.1016/j.neuron.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30(49):16585–16600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nature neuroscience. 2010;13(2):232–238. doi: 10.1038/nn.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends in neurosciences. 2001;24(3):146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Research Reviews. 1987;12(2):117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63(10):895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M, Bonci A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron. 2015;86(5):1145–1157. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Hernan AE. Stimulation of glutamate receptors in the ventral tegmental area is necessary for serotonin-2 receptor-induced increases in mesocortical dopamine release. Neuroscience. 2015;290:159–164. doi: 10.1016/j.neuroscience.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples LL, Gee F, Bibi R, West MO. Phasic firing time locked to cocaine self-infusion and locomotion: dissociable firing patterns of single nucleus accumbens neurons in the rat. The Journal of neuroscience. 1998;18(18):7588–7598. doi: 10.1523/JNEUROSCI.18-18-07588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL. The morphology of synapses. Journal of neurocytology. 1996;25(1):687–700. doi: 10.1007/BF02284835. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Progress in neurobiology. 2004;74(5):301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Phillipson OT, Pycock CJ. Dopamine neurones of the ventral tegmentum project to both medial and lateral habenula. Experimental brain research. 1982;45(1-2):89–94. doi: 10.1007/BF00235766. [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Bonci A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron. 2015;86(5):1145–1157. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Zou J, Drouin-Ouellet J, Kim KYA, Cicchetti F, Awatramani RB. Defining Midbrain Dopaminergic Neuron Diversity by Single-Cell Gene Expression Profiling. Cell reports. 2014;9(3):930–943. doi: 10.1016/j.celrep.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupe S, Wallén-Mackenzie Å. Cre-driven optogenetics in the heterogeneous genetic panorama of the VTA. Trends in neurosciences. 2015 doi: 10.1016/j.tins.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang HL, Wang H, Buendia JDJA, Hoffman AF, Morales M. A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nature communications. 2014;5 doi: 10.1038/ncomms6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayport S. Glutamate is a cotransmitter in ventral midbrain dopamine neurons. Parkinsonism & related disorders. 2001;7(3):261–264. doi: 10.1016/s1353-8020(00)00068-7. [DOI] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Luo M. Habenula “cholinergic” neurons corelease glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69(3):445–452. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Rogers JH. Immunohistochemical markers in rat brain: colocalization of calretinin and calbindin-D28k with tyrosine hydroxylase. Brain research. 1992;587(2):203–210. doi: 10.1016/0006-8993(92)90998-o. [DOI] [PubMed] [Google Scholar]
- Root DH, Fabbricatore AT, Pawlak AP, Barker DJ, Ma S, West MO. Slow phasic and tonic activity of ventral pallidal neurons during cocaine self-administration. Synapse. 2012;66(2):106–127. doi: 10.1002/syn.20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Ma S, Barker DJ, Megehee L, Striano BM, Ralston CM, West MO. Differential roles of ventral pallidum subregions during cocaine self-administration behaviors. Journal of Comparative Neurology. 2013;521(3):558–588. doi: 10.1002/cne.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Zhang S, Wang HL, Hoffman AF, Lupica CR, Morales M. Single rodent mesohabenular axons release glutamate and GABA. Nature neuroscience. 2014a;17(11):1543–1551. doi: 10.1038/nn.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Qi J, Morales M. Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. The Journal of Neuroscience. 2014b;34(42):13906–13910. doi: 10.1523/JNEUROSCI.2029-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Hoffman AF, Good CH, Zhang S, Gigante E, Lupica CR, Morales M. Norepinephrine Activates Dopamine D4 Receptors in the Rat Lateral Habenula. The Journal of Neuroscience. 2015;35(8):3460–3469. doi: 10.1523/JNEUROSCI.4525-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biological psychiatry. 2015;77(10):903–911. doi: 10.1016/j.biopsych.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav. Brain Res. 2002;137(1-2):3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Saunders A, Granger AJ, Sabatini BL. Corelease of acetylcholine and GABA from cholinergic forebrain neurons. eLife. 2015;4:e06412. doi: 10.7554/eLife.06412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Richard JM, Janak PH. Contemporary approaches to neural circuit manipulation and mapping: focus on reward and addiction. Phil. Trans. R. Soc. B. 2015;370(1677):20140210. doi: 10.1098/rstb.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ERE, Brignani S, Adolfs Y, Lemstra S, Demmers J, Vidaki M, Pasterkamp RJ. Subdomain-mediated axon-axon signaling and chemoattraction cooperate to regulate afferent innervation of the lateral habenula. Neuron. 2014;83(2):372–387. doi: 10.1016/j.neuron.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. J. Neurophysiol. 2002;80:1–27. [Google Scholar]
- Seroogy KB, Mehta A, Fallon JH. Neurotensin and cholecystokinin coexistence within neurons of the ventral mesencephalon: projections to forebrain. Experimental brain research. 1987;68(2):277–289. doi: 10.1007/BF00248793. [DOI] [PubMed] [Google Scholar]
- Seroogy K, Schalling M, Brene S, Dagerlind Å, Chai SY, Hökfelt T, Goldstein M. Cholecystokinin and tyrosine hydroxylase messenger RNAs in neurons of rat mesencephalon: peptide/monoamine coexistence studies using in situ hybridization combined with immunocytochemistry. Experimental brain research. 1989;74(1):149–162. doi: 10.1007/BF00248288. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tran T, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. Journal of Comparative Neurology. 1994;342(3):321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Ultrastructural relationships between terminals immunoreactive for enkephalin, GABA, or both transmitters in the rat ventral tegmental area. Brain research. 1995;672(1):261–275. doi: 10.1016/0006-8993(94)01391-t. [DOI] [PubMed] [Google Scholar]
- Seutin V. Dopaminergic neurones: much more than dopamine? British journal of pharmacology. 2005;146(2):167–169. doi: 10.1038/sj.bjp.0706328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Piriz J, Malinow R. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014;345(6203):1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagerberg G, Lindvall O, Bjo A. Origin, course and termination of the mesohabenular dopamine pathway in the rat. Brain research. 1984;307(1):99–108. doi: 10.1016/0006-8993(84)90465-7. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nature neuroscience. 2012;15(8):1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Stuber GD. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80(4):1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. The Journal of neuroscience. 1998;18(19):8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Henriksen SJ, Wilson MC. Transgenic rescue of SNAP-25 restores dopamine-modulated synaptic transmission in the coloboma mutant. Brain research. 1999;847(2):186–195. doi: 10.1016/s0006-8993(99)02023-5. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nature neuroscience. 2013;16(7):966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. Journal of Comparative Neurology. 2002;444(3):191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Striano BM, Barker DJ, Pawlak AP, Root DH, Fabbricatore AT, Coffey KR, West MO. Olfactory tubercle neurons exhibit slow-phasic firing patterns during cocaine self-administration. Synapse. 2014;68(7):321–323. doi: 10.1002/syn.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. The Journal of neuroscience. 2010;30(24):8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Stamatakis AM, Kantak PA. Considerations When Using Cre-Driver Rodent Lines for Studying ventral tegmental area Circuitry. Neuron. 2015;85(2):439–445. doi: 10.1016/j.neuron.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studler JM, Kitabgi P, Tramu G, Herve D, Glowinski J, Tassin JP. Extensive co-localization of neurotensin with dopamine in rat meso-cortico-frontal dopaminergic neurons. Neuropeptides. 1988;11(3):95–100. doi: 10.1016/0143-4179(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. The Journal of neuroscience. 1998;18(12):4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Research Bulletin. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam; Elsevier: 1998. [Google Scholar]
- Takada M, Hattori T. Organization of ventral tegmental area cells projecting to the occipital cortex and forebrain in the rat. Brain research. 1987;418(1):27–33. doi: 10.1016/0006-8993(87)90958-9. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407(6801):189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Tan Y, Williams ES, Zahm DS. Calbindin-D 28kD immunofluorescence in ventral mesencephalic neurons labeled following injections of Fluoro-Gold in nucleus accumbens subterritories: inverse relationship relative to known neurotoxin vulnerabilities. Brain research. 1999;844(1):67–77. doi: 10.1016/s0006-8993(99)01890-9. [DOI] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. Journal of Comparative Neurology. 2014;522(14):3308–3334. doi: 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. The Journal of Neuroscience. 2010;30(20):7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain research. 1996;721(1):140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490(7419):262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LÉ. Glutamate co-transmission as an emerging concept in monoamine neuron function. Journal of Psychiatry and Neuroscience. 2004;29(4):296. [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Gutiérrez R. On cotransmission & neurotransmitter phenotype plasticity. Molecular interventions. 2007;7(3) doi: 10.1124/mi.7.3.5. 138.mun. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Hnasko TS, Wallen-Mackenzie A, Morales M, Rayport S, Sulzer D. The multilingual nature of dopamine neurons. Progress in brain research. 2013;211:141–164. doi: 10.1016/B978-0-444-63425-2.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Wheeler DS, Ebben AL, Jacobsen AJ, Robble MA, Mantsch JR, Wheeler RA. Aversive Stimuli Drive Drug Seeking in a State of Low Dopamine Tone. Biological psychiatry. 2014 doi: 10.1016/j.biopsych.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Gunaydin LA. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Understedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand. 1971;367(Suppl.):1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303(5666):2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends in neurosciences. 2012;35(7):422–430. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain research. 1995;682(1):215–221. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schäfer MKH, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. The Journal of neuroscience. 2002;22(1):142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. Journal of Comparative Neurology. 2008;509(3):302–318. doi: 10.1002/cne.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Qi J, Zhang S, Wang H, Morales M. Rewarding Effects of Optical Stimulation of Ventral Tegmental Area Glutamatergic Neurons. The Journal of Neuroscience. 2015;35(48):15948–15954. doi: 10.1523/JNEUROSCI.3428-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proceedings of the National Academy of Sciences. 2002;99(12):8366–8371. doi: 10.1073/pnas.122210599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Origins of postsynaptic potentials evoked in identified rat neostriatal neurons by stimulation in substantia nigra. Experimental brain research. 1982;45(1-2):157–167. doi: 10.1007/BF00235775. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning, and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. European Journal of Neuroscience. 2007;25(1):106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. The Journal of neuroscience. 2011;31(23):8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Morales M. Glutamate neurons in the substantia nigra compacta and retrorubral field. European Journal of Neuroscience. 2013;38(11):3602–3610. doi: 10.1111/ejn.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Qi J, Wang HL, Zhang S, Morales M. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. European Journal of Neuroscience. 2015 doi: 10.1111/ejn.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM. Increased extracellular dopamine in nucleus accumbens in response to unconditioned and conditioned aversive stimuli: studies using 1 min microdialysis in rats. Journal of neuroscience methods. 2004;138(1):57–63. doi: 10.1016/j.jneumeth.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Yokofujita J, Oda S, Igarashi H, Sato F, Kuroda M. Synaptic characteristics between cortical cells in the rat prefrontal cortex and axon terminals from the ventral tegmental area that utilize different neurotransmitters. International Journal of Neuroscience. 2008;118(10):1443–1459. doi: 10.1080/00207450701870253. [DOI] [PubMed] [Google Scholar]
- Yu Q, Teixeira CM, Mahadevia D, Huang Y, Balsam D, Mann JJ, Ansorge MS. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Molecular psychiatry. 2014;19(6):688–698. doi: 10.1038/mp.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Teixeira CM, Mahadevia D, Huang YY, Balsam D, Mann JJ, Ansorge MS. Optogenetic stimulation of DAergic VTA neurons increases aggression. Molecular psychiatry. 2014b;19(6):635. doi: 10.1038/mp.2014.45. [DOI] [PubMed] [Google Scholar]
- Yu X, Ye Z, Houston CM, Zecharia AY, Ma Y, Zhang Z, Franks NP. Wakefulness Is governed by GABA and histamine cotransmission. Neuron. 2015;87(1):164–178. doi: 10.1016/j.neuron.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Morales M. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nature neuroscience. 2015 doi: 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Salas R, Zhang L, De Biasi M, Dani JA. Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron. 2005;46(1):65–74. doi: 10.1016/j.neuron.2005.02.010. [DOI] [PubMed] [Google Scholar]