Abstract
Posttraumatic stress disorder (PTSD) has been described as the only neuropsychiatric disorder with a known cause, yet effective behavioral and pharmacotherapies remain elusive for many afflicted individuals. PTSD is characterized by heightened noradrenergic signaling, as well as a resistance to extinction learning. Research aimed at promoting more effective treatment of PTSD has focused on memory erasure (disrupting reconsolidation) and/or enhancing extinction retention through pharmacological manipulations. Propranolol, a β-adrenoceptor antagonist, has received considerable attention for its therapeutic potential in PTSD, although its impact on patients is not always effective. In this review, we briefly examine the consequences of β-noradrenergic manipulations on both reconsolidation and extinction learning in rodents and in humans. We suggest that propranolol is effective as a fear-reducing agent when paired with behavioral therapy soon after trauma when psychological stress is high, possibly preventing or dampening the later development of PTSD. In individuals who have already suffered from PTSD for a significant period of time, propranolol may be less effective at disrupting reconsolidation of strong fear memories. Also, when PTSD has already developed, chronic treatment with propranolol may be more effective than acute intervention, given that individuals with PTSD tend to experience long-term, elevated noradrenergic hyperarousal.
Keywords: fear, extinction, reconsolidation, propranolol, PTSD, consolidation, norepinephrine
1. Introduction
Posttraumatic stress disorder (PTSD) affects approximately 8% of the United States general population in their lifetime (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995; Kessler et al., 2005), and is characterized by heightened arousal and a resistance to extinction learning (Liberzon & Sripada, 2008; Pitman et al., 2012; Rauch, Shin, & Phelps, 2006; Shin & Handwerger, 2009; VanElzakker, Dahlgren, Davis, Dubois, & Shin, 2014). While the pathophysiology of PTSD is poorly understood, dysregulated signaling of the stress-related neurotransmitter norepinephrine (NE) has been identified as a key biomarker underlying PTSD symptomatology (Geracioti et al., 2001; Kosten, Mason, Giller, Ostroff, & Harkness, 1987; Southwick et al., 1997, 1999; Yehuda, Southwick, Giller, Ma, & Mason, 1992). However, the only FDA approved treatments for PTSD are the selective serotonin reuptake inhibitors, sertraline (Zoloft) and paroxetine (Paxil), which have limited efficacy (Tawa & Murphy, 2013). Nonetheless, pharmacotherapies that either dampen NE transmission, such as the α1-adrenoceptor antagonist prazosin, the α2 agonist clonidine, and the non-selective β antagonist propranolol, or enhance NE transmission such as the α2 antagonist yohimbine, have shown some success in diminishing the exaggerated fear responding associated with PTSD (Belkin & Schwartz, 2015; Morris & Bouton, 2007; Powers, Smits, Otto, Sanders, & Emmelkamp, 2009; Raskind et al., 2003; Strawn & Geracioti, 2008; Tawa & Murphy, 2013; Taylor, Freeman, & Cates, 2008; Wangelin, Powers, Smits, & Tuerk, 2013). Yohimbine, as well as the non-selective β agonist isoproterenol, can enhance extinction learning (Cain, Blouin, & Barad, 2004; Do-Monte et al., 2010; Morris & Bouton, 2007; Powers et al., 2009), as well as memory consolidation or reconsolidation (Dębiec, Bush, & LeDoux, 2011; Gazarini, Stern, Carobrez, & Bertoglio, 2013). For these reasons, there has been a resurgence of interest in using noradrenergic drugs as adjuncts to cognitive-behavioral therapies for PTSD.
In this regard, animal studies of inhibitory avoidance, and Pavlovian fear conditioning studies in both animals and humans have provided insight into the neurobiological underpinnings of aversive learning and memory that contribute to the development and expression of PTSD (Bowers & Ressler, 2015; Fanselow & Poulos, 2005; LeDoux, 2000; Maren, 2001; Maren, Phan, & Liberzon, 2013; Myers & Davis, 2007; Roozendaal, McEwen, & Chattarji, 2009). Here we primarily focus on reviewing Pavlovian fear conditioning studies because the interpretation of drug studies using an inhibitory avoidance design may be less clear, since the accuracy and specificity of learning are difficult to parse. In particular, post-training drug manipulations resulting in “better memory” (i.e., a longer latency to enter the aversive chamber) may reflect a more accurate recall of the initial training experience. However, it is possible that this apparent memory enhancement actually reflects reduced accuracy of memory for the training context (Atucha & Roozendaal, 2015). This problem can be bypassed through the use of multiple contexts, which are frequently used in studies of Pavlovian fear extinction, where this literature may better model PTSD relevant processes.
Initially, many studies of Pavlovian fear conditioning focused on NE and memory consolidation, the process through which a temporary short-term memory is stabilized into a persistent long-term memory, a procedure that in part involves protein synthesis (Johansen, Cain, Ostroff, & LeDoux, 2011). NE plays a crucial role in memory consolidation and propranolol can impair consolidation in both animal models and human subjects (Berlau & McGaugh, 2006; Cahill, Pham, & Setlow, 2000; Introini-Collison & Baratti, 1986; Lonergan, Olivera-Figueroa, Pitman, & Brunet, 2013; McGaugh, 2000; Roozendaal, Okuda, Van der Zee, & McGaugh, 2006; Wilson, Pham, & Sullivan, 1994). The molecular mechanisms by which propranolol (and NE itself) affects aversive learning and memory processes are only beginning to be elucidated, but they may include the MAPK and JAK/STAT3 pathways, among others. Johansen, LeDoux and colleagues have suggested that postsynaptic β-adrenergic signaling in the lateral nucleus of the amygdala interacts with the MAPK pathway to modulate acquisition and consolidation of fear memories (Johansen et al., 2011). Another group found that infusion of the inflammatory cytokine IL-6 into the basolateral amygdala modulates fear extinction learning through the JAK/STAT3 pathway (Hao et al., 2014), and other studies have linked NE (and propranolol) with IL-6 signaling (Norris & Benveniste, 1993).
There is growing interest in the role of NE in memory reconsolidation as well as extinction learning. As described below in Figures 1 and 2, studies of reconsolidation and extinction both typically begin with fear acquisition (i.e., conditioning), comprising the pairing of a neutral conditioned stimulus (CS) with an aversive unconditioned stimulus (US), such as a mild footshock. Many reconsolidation studies use a single CS-US pairing, and the following day animals are presented with one CS-alone trial to “reactivate” the fear memory. In contrast, for studies of extinction learning, conditioning typically consists of multiple (3–5) CS-US pairings, perhaps yielding a stronger association. As such, fear extinction requires many CS-alone presentations to acquire a new CS-no US memory. While fear-related measures, such as freezing in rodents and autonomic skin conductance in humans, are similarly used to quantify both reconsolidation and extinction, differences between these two paradigms in the acquisition phase in particular need to be considered when comparing the effects of drug manipulations.
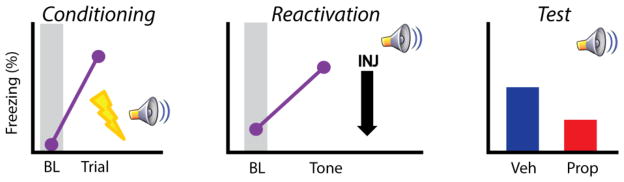
Figure 1.
Schematic representation showing the effect of post-reactivation propranolol on reconsolidation. In rodents, conditioning typically consists of a single CS-US pairing (left). The next day animals receive 1 CS reactivation trial, immediately followed by propranolol or vehicle administration (middle). When tested at later time points in the absence of drug, propranolol treated animals show reduced fear responding (right). Abbreviations: baseline period (BL), injection (INJ), vehicle (Veh), propranolol (Prop).
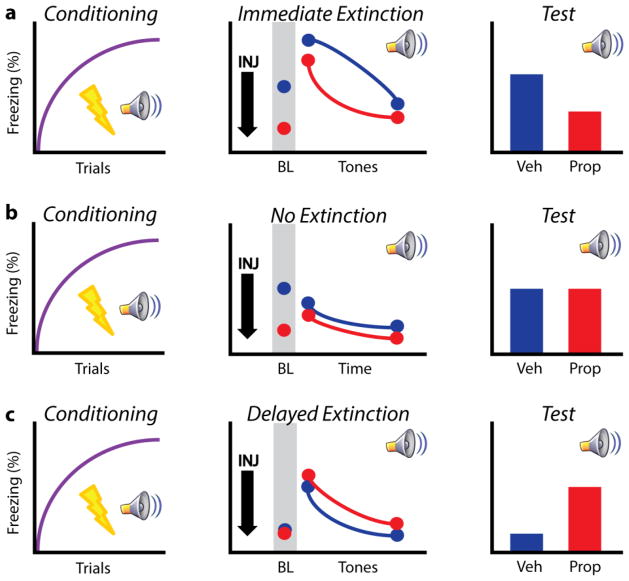
Figure 2.
Schematic data showing the bi-directional effect of propranolol treatment on extinction learning at different time points. a) Animals are typically conditioned with several CS-US pairings (left). In an immediate extinction procedure, animals received either vehicle or propranolol 30 min prior to extinction training (and immediately after conditioning). Propranolol treatment reduced elevated baseline levels of freezing and attenuated fear throughout the session (middle). When tested for extinction recall later (in a drug free state), prior propranolol treatment significantly reduced the spontaneous recovery of fear, ameliorating the immediate extinction deficit (right). b) Similar to animals undergoing immediate extinction procedures, “no-extinction” controls received vehicle or propranolol immediately following conditioning, but were exposed to the extinction context in the absence of CS presentation. Propranolol reduced elevated levels of baseline freezing, although no difference was observed between groups throughout the session (middle). Upon test, animals froze at similarly high levels (right). c) In delayed extinction, animals were conditioned using the same protocol as in a and b. However, animals were returned to their home cage following conditioning and not injected. The following day, rats received vehicle or propranolol administration 30 min prior to an extinction session. No differences were observed at baseline or during tone presentation (middle). When tested for extinction recall (in a drug free state), prior propranolol treatment impaired retrieval relative to vehicle controls (right). Abbreviations as in Figure 1 legend. Data are adapted from Fitzgerald et al. (2015).
Psychotherapies, some of which are thought to mimic aspects of extinction learning, are frequently used to counteract PTSD, although behavioral therapy alone is not always effective (Bryant, 2002; Mayou, Ehlers, & Hobbs, 2000; Rose, Brewin, Andrews, & Kirk, 1999). Because individuals with PTSD display exaggerated fear responses, clinicians and scientists have attempted to inhibit pathological fear with pharmaceuticals via two distinct mechanisms: 1) blocking memory reconsolidation after reactivating traumatic memories or 2) enhancing long-term extinction learning associated with exposure therapy. When a consolidated memory is retrieved it is thought to enter a labile state which may be subject to manipulation and possibly erasure (Alberini & LeDoux, 2013). It has been shown that inhibiting protein synthesis immediately after a brief memory reactivation is sufficient to attenuate conditional fear in rodents (Nader, Schafe, & LeDoux, 2000; Rudy, Biedenkapp, Moineau, & Bolding, 2006).
Propranolol, a commonly prescribed ‘beta-blocker’ that can cross the blood-brain barrier, has received considerable attention for its noted effects on reconsolidation blockade (Brunet et al., 2008; Debiec & LeDoux, 2004; Soeter & Kindt, 2012). One possibility is that propranolol acts indirectly to inhibit protein synthesis, thereby disrupting reconsolidation and erasing the fear memory. An alternative but not mutually exclusive possibility is that propranolol, which has known anxiolytic effects (Brantigan, Brantigan, & Joseph, 1982), may help reduce the psychological stress associated with encountering a feared stimulus upon extinction training, helping to restore an optimal level of NE signaling to promote extinction learning. Here we review the existing literature comparing the efficacy of propranolol in reconsolidation versus its effects on extinction learning, both in rodents and in humans.
2. Does propranolol prevent reconsolidation and partially erase fear memories?
Individuals who suffer from PTSD often exhibit heightened fear responses. This may reflect hyperconditioning, a resistance to extinction learning, or a combination of the two (Milad et al., 2009; Pitman, 1988; Pitman et al., 2012). In the laboratory setting, Pavlovian fear conditioning is widely used in both rodents and humans to investigate emotional learning and memory. Animals are typically trained by pairing a neutral CS with an aversive US, such as a mild footshock. With one or more pairings, animals learn to exhibit conditioned fear responses (CR) such as freezing (i.e., immobility) or potentiated acoustic startle, which are accompanied by autonomic changes such as increased respiration and heart rate (Davis, 1992; LeDoux, 2000; Maren, 2001). Rodent fear conditioning studies typically use freezing as their principal behavioral measure of fear, although it cannot be assumed with certainty that freezing always provides a reliable readout of the subjective state of fear in animals.
When a consolidated fear memory is retrieved or reactivated it is believed to enter a labile state and may be reconsolidated in a protein synthesis dependent manner (Nader et al., 2000). This idea has far reaching implications for the treatment of trauma-related disorders such as PTSD, insofar as PTSD is characterized by recurring thoughts or images of the traumatic event. It follows then, that one may be able to disrupt the reconsolidation of a fearful memory when it is retrieved and this would partially abolish that fear memory (Maren, 2011). It is unlikely that even very effective disruption of reconsolidation could fully erase a fear memory, as some degree of declarative knowledge of the memory would probably remain intact, both in human subjects and in rodents. Nonetheless, there is high interest in gaining a greater understanding of the neurobiology of reconsolidation and pharmacological means of its disruption as a treatment for PTSD.
2.1 Rodents
In a potentially major breakthrough, Debiec and LeDoux (2004) found that systemic administration of propranolol successfully blocked reconsolidation in rodents. In this study, rats were fear conditioned and the following day received one CS “reactivation” trial followed by propranolol. This single injection was sufficient to reduce conditioned responding to the CS when tested at several time points beyond drug (see Figure 1 for schematic data) (Debiec & LeDoux, 2004). In a follow up study, it was shown that β-adrenoceptor stimulation enhanced fear memory reconsolidation (making that memory resistant to extinction) and that this can indeed be blocked by propranolol (Dębiec et al., 2011). This effect has been replicated in the rodent literature, further demonstrating that propranolol reduces fear when delivered after a brief memory reactivation (Abrari, Rashidy-Pour, Semnanian, & Fathollahi, 2008; Muravieva & Alberini, 2010). In addition to cued fear memories, propranolol effectively blocks reconsolidation of context fear in rats (Schneider et al., 2014). Collectively, these data indicate that propranolol administration immediately after brief memory retrieval dampens fear responding long term and may do so by interfering with reconsolidation.
The mechanism by which propranolol attenuates reconsolidation is unknown; however, many studies have proposed that it indirectly disrupts protein synthesis, thought to be a necessary component of reconsolidation (Nader et al., 2000). Given that PTSD is a chronic disorder and trauma reminders occur frequently, it is possible that remote fear memories are less sensitive to disruption due to overconsolidation processes (Pitman & Delahanty, 2005). Interestingly, it has been shown that recent, but not remote, fear memories are susceptible to disruption via protein synthesis inhibitors (Milekic & Alberini, 2002; Suzuki et al., 2004; Taherian et al., 2014). Moreover, others have shown that post-reactivation propranolol has no effect on reconsolidation in rats (Pitman et al., 2011). The reasons for these discrepancies are unclear, but it is possible that the weak conditioning parameters (1 CS-US pairing) used in the majority of these reconsolidation studies results in weak fear memories, making them more susceptible to erasure and interference. There is some evidence, however, to suggest that strong fear memories are also susceptible to manipulation (Duvarci, Nader, & LeDoux, 2008; Taherian et al., 2014). Additionally, the initial reactivation tests are typically given 24 hrs post-conditioning in these studies, making them fairly recent memories as opposed to more remote ones that would be associated with chronic PTSD. Overall, propranolol may be mildly effective at disrupting reconsolidation, but this is likely limited by the age and strength of the memory.
2.2 Humans
Due to the debilitating nature of stress and trauma-related disorders such as PTSD, there is high interest in both behavioral and pharmacotherapies aimed at alleviating symptoms or preventing these disorders from developing at all. Considerable effort has been placed on erasing the original fear memory by disrupting reconsolidation after memory retrieval in humans. Indeed, many studies have shown that propranolol appears to disrupt reconsolidation processes, thereby dampening fear responding in healthy volunteers as well as individuals with PTSD (Brunet et al., 2008, 2011, 2014; Kindt, Soeter, & Vervliet, 2009; Lonergan et al., 2013; Poundja, Sanche, Tremblay, & Brunet, 2012; Schwabe, Nader, Wolf, Beaudry, & Pruessner, 2012; Soeter & Kindt, 2011, 2012). Typically, the experimental designs in these studies consisted of one or more memory reactivation trials with or without propranolol. Fear memories were then tested at a later time point, in a drug free state. Propranolol treatment attenuated physiological fear measures (Brunet et al., 2008, 2011, 2014; Kindt et al., 2009; Soeter & Kindt, 2011, 2012), and both men and women with chronic PTSD reported a better quality of life (Poundja et al., 2012). Collectively, these data appear to strongly suggest that propranolol effectively blocks the reconsolidation of fear memories in both healthy volunteers and individuals with PTSD.
It is, however, somewhat surprising that a single, acute dose of propranolol (or any drug for that matter) would permanently erase, or even strongly weaken, a longstanding fear memory. Many studies have argued that targeting reconsolidation may be unlikely to work given that only weak and recently formed memories are most susceptible to erasure (Milekic & Alberini, 2002; Parsons & Ressler, 2013; Suzuki et al., 2004) and that memories do not necessarily undergo reconsolidation upon reactivation, unless there is new information to be encoded (Sevenster, Beckers, & Kindt, 2012). This suggests that there may be certain “boundary conditions” influencing reconsolidation. For example, Nader and colleagues have suggested that reconsolidation may only occur when certain conditions are met, including that strong, recent memories resist reconsolidation but that extinction learning is not sufficient to block reconsolidation (Duvarci, Mamou, & Nader, 2006; Wang, de Oliveira Alvares, & Nader, 2009). Moreover, individuals with PTSD may also exhibit enhanced conditioning, possibly making these fear memories even more difficult to target (Pitman, 1988). These strongly encoded fear memories also undergo frequent reactivation which may result in overconsolidation, further limiting the idea that acute propranolol could abolish them (Pitman & Delahanty, 2005).
Additionally, given the nature of the reconsolidation procedures used in many of the human studies, it is unclear whether propranolol is blocking reconsolidation, enhancing extinction learning, or some combination of the two. For example, Poundja and colleagues (2012) examined subjects with chronic PTSD. Individuals were asked to actively recall a traumatic event while under the influence of propranolol. This process was repeated once a week for six weeks. While the authors claim that the observed decrement in fear responding reflected disruption of reconsolidation and a weakened fear memory, an alternative interpretation is that these recall sessions trigger extinction-like processes which may be enhanced by repeated propranolol administration (Pitman et al., 2002). A similar confound arises in the studies by Brunet and colleagues (2008, 2011, 2014). Because patients received multiple drug-reactivation sessions, it is difficult to parse the outcomes, although this does not preclude the observation that propranolol appears to be an effective fear-reducing agent in some situations. Additional issues arise in the interpretation of these results because the effects are not always replicable, even when carried out by the same laboratory in some cases (Spring et al., 2015; Tollenaar, Elzinga, Spinhoven, & Everaerd, 2009a, 2009b; Wood et al., 2015), bringing into question whether propranolol modulates reconsolidation. In summary, compelling evidence to support the notion that acute or even subchronic propranolol treatment effectively blocks reconsolidation in human subjects is lacking and many of the experimental designs make it difficult to dissociate effects on extinction versus reconsolidation processes.
3. Does propranolol enhance extinction learning and augment exposure therapy?
Conditional fear can be extinguished with repeated presentation of the CS in the absence of the US, ultimately resulting in a reduction of CRs. Extinction, however, is highly susceptible to various forms of relapse as indexed by increased conditioning responding with the passage of time (spontaneous recovery), the presentation of an unsignaled US (reinstatement), upon encountering the CS in a non-extinguished context (renewal), (Bouton, 2000; Goode & Maren, 2014; Hermans, Craske, Mineka, & Lovibond, 2006; Maren et al., 2013; Vervliet, Craske, & Hermans, 2013), and relapse can be promoted by psychological stress (Maren & Holmes, 2015). Extinction learning is also sensitive to the timing relative to trauma, and it is typically less effective when given soon after the traumatic experience (Chang, Berke, & Maren, 2010; Fitzgerald, Giustino, Seemann, & Maren, 2015; Kim, Jo, Kim, Kim, & Choi, 2010; Maren, 2014; Maren & Chang, 2006). The notion that stress and the timing of extinction learning relative to trauma can disrupt its long-term efficacy calls into question the benefit of early therapeutic interventions such as psychological debriefing, which is often used to reduce the impact, or even prevent the development, of PTSD. A number of studies have shown that these early intervention strategies are ineffective when used alone (Bryant, 2002; Mayou et al., 2000; Rose et al., 1999), but may be improved by pharmacological adjuncts (de Kleine, Rothbaum, & van Minnen, 2013).
3.1 Rodents
Despite a vast literature on extinction and its relevance to PTSD treatment, no existing drugs stand out for their ability to broadly reduce conditional fear. Propranolol has been studied extensively, but with mixed results, leading many to believe it is an ineffective therapeutic option (Raskind et al., 2003; Strawn & Geracioti, 2008; Tawa & Murphy, 2013). In addition, many studies in rodents have shown that systemic propranolol prior to delayed extinction impairs long-term retention (Cain et al., 2004; Do-Monte et al., 2010; Fitzgerald et al., 2015). One possibility is that, similar to extinction learning itself, the efficacy of propranolol as a fear-reducing agent is sensitive to the timing of administration. This may reflect differences in basal levels of NE at the onset of extinction (high in “immediate” extinction (i.e., when extinction is carried out minutes to several hours after fear conditioning, and relatively low in delayed extinction). In line with this hypothesis, it is well established that elevated NE signaling impairs prefrontal function (Arnsten, 2009) and that the prelimbic (PL) and infralimbic (IL) subdivisions of the medial prefrontal cortex (mPFC) crucially regulate fear and its extinction (Giustino & Maren, 2015; Milad & Quirk, 2012; Quirk & Beer, 2006; Riga et al., 2014; Sotres-Bayon & Quirk, 2010).
We have recently shown that footshock stress rapidly and persistently alters mPFC single-unit activity for up to an hour after conditioning (a time frame that corresponds with the immediate extinction deficit) (Fitzgerald et al., 2015). Systemic propranolol administered prior to conditioning acted to normalize mPFC firing in the aftermath of trauma, blunting both increases and decreases in firing rate in separate populations of neurons. Thus, propranolol delivered immediately after conditioning may stabilize mPFC function and promote successful extinction. Indeed, propranolol effectively enhances extinction learning under conditions where it normally fails; that is, when extinction trials are delivered soon after conditioning, when psychological stress is high (Figure 2a). Systemic propranolol administered immediately after conditioning (and 30 min prior to immediate extinction training) produced marked effects on extinction learning. Propranolol treatment attenuated the normally high levels of baseline fear (prior to CS presentation) associated with immediate extinction while reducing fear throughout extinction training (Figure 2a). When animals were tested for extinction retrieval 48 hrs later, prior propranolol treatment reduced the spontaneous recovery of fear (Figure 2a). It is possible that propranolol treatment immediately post conditioning resulted in a weaker fear memory, by interfering with consolidation processes. However, animals that received post-conditioning propranolol and underwent a “no-extinction” protocol (i.e., no CS presentations) showed similar levels of spontaneous recovery to vehicle controls on test (Figure 2b). Interestingly, propranolol still effectively reduced baseline freezing levels in this no-extinction procedure, but had no long-term effect on fear memory, suggesting that propranolol treatment indeed facilitated extinction learning as opposed to dampening the strength of the CS-US association (Figure 2b) (Fitzgerald et al., 2015).
As noted above, propranolol given prior to delayed extinction may have detrimental effects on learning (Cain et al., 2004; Do-Monte et al., 2010; Fitzgerald et al., 2015). To explore this bi-directional effect of timing, in the same study described above (Fitzgerald et al., 2015), a separate group of animals received propranolol 30 min prior to delayed extinction (24 hrs post conditioning) (Figure 2c). Whereas this treatment had no within session effect (while on drug), it actually impaired retrieval when tested the following day (Figure 2c). These data suggest that the timing of propranolol administration relative to trauma is a key factor in its therapeutic efficacy. Importantly, propranolol administration alone would not be expected to dampen long-term fear responding as propranolol had no long-term effect in the no-extinction animals. In summary, propranolol appears to effectively enhance immediate extinction while impairing delayed extinction.
Rodent microdialysis studies have linked footshock stress, which is typically the stressor used in animal fear conditioning, with amygdalar (Galvez, Mesches, & McGaugh, 1996), hippocampal (Hajós-Korcsok et al., 2003), and medial prefrontal cortical (Ishizuka et al., 2000) release of NE, bolstering the hypothesis put forth by Fitzgerald et al. (2015) that recent footshock exposure during immediate extinction is characterized by elevated NE signaling. Less appears to be known about endogenous NE signaling during fear extinction or reconsolidation, where many studies of these two phenomena have focused on manipulating such signaling with exogenous noradrenergic drugs. Two older studies found that forebrain depletion of NE (with little effect on dopamine) using the drug 6-hydroxydopamine produced resistance to extinction learning (Mason & Fibiger, 1979; Mason, Roberts, & Fibiger, 1979). More recently, Berlau and McGaugh (2006) showed that infusion of NE into right hemisphere amygdala enhanced extinction, and others have shown that β-adrenoceptor activation in infralimbic cortex is necessary for extinction (Mueller, Porter, & Quirk, 2008). These four studies, which are probably more relevant to delayed than immediate extinction, collectively suggest that enhancing endogenous NE signaling promotes delayed extinction whereas depleting NE impairs such extinction, consistent with the Fitzgerald et al. (2015) propranolol data.
3.2 Humans
In humans, psychological debriefing can be given soon after trauma in an attempt to reduce or prevent PTSD development. The timing of this behavioral therapy mirrors that of immediate extinction procedures in rodents, where the latter approach often yields unsuccessful long-term fear reduction, as described above. It is possible that propranolol may effectively enhance the long-term efficacy of debriefing in humans. Interestingly, several studies have reported that propranolol treatment soon after trauma produced no difference from untreated controls (McGhee et al., 2009; Nugent et al., 2010; Stein, Kerridge, Dimsdale, & Hoyt, 2007). Importantly, these studies suggest that propranolol alone may be ineffective, but did not address the efficacy of propranolol as an adjunct to behavioral therapies aimed at reducing fear.
Similar to its use in rodents, it is possible that propranolol is most effective when coupled with behavioral therapies soon after trauma in humans, and is less effective or even worsens the outcome at delayed time points. In support of this idea, Bos and colleagues (2012) showed that propranolol delivered prior to extinction training had no effect on physiological response properties (startle reflex and skin conductance), but impaired extinction learning based on levels of US expectancy in healthy humans (Bos, Beckers, & Kindt, 2012). In addition, it has been shown that propranolol delivered either right before or right after extinction training failed to line with our data and other studies demonstrating that propranolol administration impairs delayed extinction in rodents (Cain et al., 2004; Do-Monte et al., 2010; Fitzgerald et al., 2015). It remains possible that propranolol would be most effective soon after trauma when coupled with behavioral therapies, acting to reduce psychological stress and enhance learning. In a pilot study, Pitman and colleagues (2002) have shown that propranolol administered four times daily for 10 consecutive days (with the first dose coming within 6 hrs of trauma exposure), coupled with behavioral counseling, significantly reduced long-term fear; this suggests that propranolol can be used to prevent PTSD development. Moreover, propranolol given three times daily for 1 week immediately following trauma reduced the development of PTSD symptomatology (Vaiva et al., 2003). These data also lend support to the idea that chronic propranolol treatment, starting soon after trauma, might effectively reduce PTSD symptomatology long-term, although there is little empirical evidence on this topic (Ostrowski & Delahanty, 2014).
4. Conclusions
There remains an unmet need regarding effective treatment for PTSD, although various behavioral interventions, pharmacotherapies, or their combination have shown some promise (Bukalo, Pinard, & Holmes, 2014; Fitzgerald, Seemann, & Maren, 2014; Ostrowski & Delahanty, 2014). An ambiguous view on propranolol has emerged in the literature, given discrepant findings in both rodents and humans and a debate on the memory processes propranolol is most effective at modulating (reconsolidation versus extinction). Here we propose that propranolol may be most effective as a long-term fear-reducing agent when administered soon after trauma and coupled with extinction training. We suggest that propranolol effectively dampens elevated NE signaling under high psychological stress, thereby promoting successful extinction learning. This can also explain why propranolol may be ineffective at modulating or even hinder delayed extinction in rodents and humans. The fact that propranolol facilitated immediate extinction and impaired delayed extinction indicates that it may be most useful under conditions in which stress is high (e.g., at the onset of immediate extinction) and NE levels are likely elevated beyond what is optimal for learning (Fitzgerald et al., 2015). In contrast, stress is relatively low at the onset of delayed extinction and the fear-inducing stimulus might elicit an “optimal” amount of NE release to facilitate learning; this signaling via β-adrenoceptors may be reduced to a suboptimal level by acute propranolol. This line of reasoning may also suggest that if too high a dose of propranolol is administered before immediate extinction, it could also impair learning by reducing NE signaling below an optimal level. In addition, long-term administration of propranolol may effectively reduce tonically elevated NE signaling for individuals with chronic PTSD (i.e., those who would no longer benefit from acute propranolol paired with exposure therapy).
We also suggest here that propranolol is less likely to disrupt reconsolidation, especially in individuals with chronic PTSD in which fear memories are neither recent nor weak, which was not the case in many of the animal studies that showed propranolol blocks reconsolidation. The human literature on propranolol and reconsolidation may actually strengthen the argument that propranolol facilitates extinction learning under stress given the multiple reactivation experimental designs described above, rather than this drug solely dampening or eliminating the fear memory. Overall, the mixed findings on propranolol and reconsolidation make it difficult to support the notion that propranolol might permanently abolish fear memories, although it may dampen them when certain boundary conditions for reconsolidation are met (Wang et al. 2009, Duvarci et al. 2006).
Highlights.
Propranolol coupled with extinction soon after trauma reduces long-term fear
Chronic propranolol may block tonically elevated levels of NE associated with PTSD
It is unlikely that acute propranolol erases strong fear memories in PTSD
Acknowledgments
Funding
Supported by a grant from the National Institutes of Health (R01MH065961) and a McKnight Memory and Cognitive Disorders Award to SM.
Footnotes
Conflicts of Interest
None declared.
Author Contributions
TFG, PJF and SM wrote and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: dependence upon training intensity. Neurobiology of Learning and Memory. 2008;89(2):178–184. doi: 10.1016/j.nlm.2007.07.005. http://doi.org/10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Alberini CM, LeDoux JE. Memory reconsolidation. Current Biology. 2013;23(17):R746–R750. doi: 10.1016/j.cub.2013.06.046. http://doi.org/10.1016/j.cub.2013.06.046. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. http://doi.org/10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atucha E, Roozendaal B. The inhibitory avoidance discrimination task to investigate accuracy of memory. Frontiers in Behavioral Neuroscience. 2015;9 doi: 10.3389/fnbeh.2015.00060. http://doi.org/10.3389/fnbeh.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin M, Schwartz T. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs in Context. 2015;4:1–5. doi: 10.7573/dic.212286. http://doi.org/10.7573/dic.212286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiology of Learning and Memory. 2006;86(2):123–132. doi: 10.1016/j.nlm.2005.12.008. http://doi.org/10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bos MGN, Beckers T, Kindt M. The effects of noradrenergic blockade on extinction in humans. Biological Psychology. 2012;89(3):598–605. doi: 10.1016/j.biopsycho.2012.01.007. http://doi.org/10.1016/j.biopsycho.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Bouton ME. A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychology. 2000;19(1 Suppl):57–63. doi: 10.1037/0278-6133.19.suppl1.57. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Ressler KJ. An Overview of Translationally Informed Treatments for Posttraumatic Stress Disorder: Animal Models of Pavlovian Fear Conditioning to Human Clinical Trials. Biological Psychiatry. 2015;78(5):E15–27. doi: 10.1016/j.biopsych.2015.06.008. http://doi.org/10.1016/j.biopsych.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. The American Journal of Medicine. 1982;72(1):88–94. doi: 10.1016/0002-9343(82)90592-7. [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. Journal of Psychiatric Research. 2008;42(6):503–506. doi: 10.1016/j.jpsychires.2007.05.006. http://doi.org/10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Brunet A, Poundja J, Tremblay J, Bui E, Thomas E, Orr SP, Pitman RK. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. Journal of Clinical Psychopharmacology. 2011;31(4):547–550. doi: 10.1097/JCP.0b013e318222f360. http://doi.org/10.1097/JCP.0b013e318222f360. [DOI] [PubMed] [Google Scholar]
- Brunet A, Thomas É, Saumier D, Ashbaugh AR, Azzoug A, Pitman RK, Tremblay J. Trauma reactivation plus propranolol is associated with durably low physiological responding during subsequent script-driven traumatic imagery. Canadian Journal of Psychiatry. 2014;59(4):228–232. doi: 10.1177/070674371405900408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA. Early Interventions Following Psychological Trauma. CNS Spectrums. 2002;7(09):650–654. doi: 10.1017/s1092852900022136. http://doi.org/10.1017/S1092852900022136. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Pinard CR, Holmes A. Mechanisms to medicines: elucidating neural and molecular substrates of fear extinction to identify novel treatments for anxiety disorders. British Journal of Pharmacology. 2014;171(20):4690–4718. doi: 10.1111/bph.12779. http://doi.org/10.1111/bph.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Pham CA, Setlow B. Impaired memory consolidation in rats produced with beta-adrenergic blockade. Neurobiology of Learning and Memory. 2000;74(3):259–266. doi: 10.1006/nlme.1999.3950. http://doi.org/10.1006/nlme.1999.3950. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learning & Memory (Cold Spring Harbor, NY) 2004;11(2):179–187. doi: 10.1101/lm.71504. http://doi.org/10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PloS One. 2010;5(8):e11971. doi: 10.1371/journal.pone.0011971. http://doi.org/10.1371/journal.pone.0011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. http://doi.org/10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dębiec J, Bush DEA, LeDoux JE. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats--a possible mechanism for the persistence of traumatic memories in PTSD. Depression and Anxiety. 2011;28(3):186–193. doi: 10.1002/da.20803. http://doi.org/10.1002/da.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129(2):267–272. doi: 10.1016/j.neuroscience.2004.08.018. http://doi.org/10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- de Kleine RA, Rothbaum BO, van Minnen A. Pharmacological enhancement of exposure-based treatment in PTSD: a qualitative review. European Journal of Psychotraumatology. 2013;4 doi: 10.3402/ejpt.v4i0.21626. http://doi.org/10.3402/ejpt.v4i0.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte FHM, Kincheski GC, Pavesi E, Sordi R, Assreuy J, Carobrez AP. Role of beta-adrenergic receptors in the ventromedial prefrontal cortex during contextual fear extinction in rats. Neurobiology of Learning and Memory. 2010;94(3):318–328. doi: 10.1016/j.nlm.2010.07.004. http://doi.org/10.1016/j.nlm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Mamou CB, Nader K. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. The European Journal of Neuroscience. 2006;24(1):249–260. doi: 10.1111/j.1460-9568.2006.04907.x. http://doi.org/10.1111/j.1460-9568.2006.04907.x. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. De novo mRNA synthesis is required for both consolidation and reconsolidation of fear memories in the amygdala. Learning & Memory. 2008;15(10):747–755. doi: 10.1101/lm.1027208. http://doi.org/10.1101/lm.1027208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. http://doi.org/10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Giustino TF, Seemann JR, Maren S. Noradrenergic blockade stabilizes prefrontal activity and enables fear extinction under stress. Proceedings of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1500682112. 201500682. http://doi.org/10.1073/pnas.1500682112. [DOI] [PMC free article] [PubMed]
- Fitzgerald PJ, Seemann JR, Maren S. Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Research Bulletin. 2014;105:46–60. doi: 10.1016/j.brainresbull.2013.12.007. http://doi.org/10.1016/j.brainresbull.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiology of Learning and Memory. 1996;66(3):253–257. doi: 10.1006/nlme.1996.0067. http://doi.org/10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Gazarini L, Stern CAJ, Carobrez AP, Bertoglio LJ. Enhanced noradrenergic activity potentiates fear memory consolidation and reconsolidation by differentially recruiting α1- and β-adrenergic receptors. Learning & Memory (Cold Spring Harbor, NY) 2013;20(4):210–219. doi: 10.1101/lm.030007.112. http://doi.org/10.1101/lm.030007.112. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. The American Journal of Psychiatry. 2001;158(8):1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Frontiers in Behavioral Neuroscience. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. http://doi.org/10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Maren S. Animal models of fear relapse. ILAR Journal/National Research Council, Institute of Laboratory Animal Resources. 2014;55(2):246–258. doi: 10.1093/ilar/ilu008. http://doi.org/10.1093/ilar/ilu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós-Korcsok E, Robinson DD, Yu JH, Fitch CS, Walker E, Merchant KM. Rapid habituation of hippocampal serotonin and norepinephrine release and anxiety-related behaviors, but not plasma corticosterone levels, to repeated footshock stress in rats. Pharmacology, Biochemistry, and Behavior. 2003;74(3):609–616. doi: 10.1016/s0091-3057(02)01047-x. [DOI] [PubMed] [Google Scholar]
- Hao Y, Jing H, Bi Q, Zhang J, Qin L, Yang P. Intra-amygdala microinfusion of IL-6 impairs the auditory fear conditioning of rats via JAK/STAT activation. Behavioural Brain Research. 2014;275:88–95. doi: 10.1016/j.bbr.2014.08.052. http://doi.org/10.1016/j.bbr.2014.08.052. [DOI] [PubMed] [Google Scholar]
- Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biological Psychiatry. 2006;60(4):361–368. doi: 10.1016/j.biopsych.2005.10.006. http://doi.org/10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Baratti CM. Opioid peptidergic systems modulate the activity of beta-adrenergic mechanisms during memory consolidation processes. Behavioral and Neural Biology. 1986;46(2):227–241. doi: 10.1016/s0163-1047(86)90710-7. [DOI] [PubMed] [Google Scholar]
- Ishizuka Y, Ishida Y, Jin Q, Kato K, Kunitake T, Mitsuyama Y, Kannan H. Differential profiles of nitric oxide and norepinephrine releases in the paraventricular nucleus region in response to mild footshock in rats. Brain Research. 2000;862(1–2):17–25. doi: 10.1016/s0006-8993(00)02061-8. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147(3):509–524. doi: 10.1016/j.cell.2011.10.009. http://doi.org/10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. http://doi.org/10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kim SC, Jo YS, Kim IH, Kim H, Choi JS. Lack of Medial Prefrontal Cortex Activation Underlies the Immediate Extinction Deficit. The Journal of Neuroscience. 2010;30(3):832–837. doi: 10.1523/JNEUROSCI.4145-09.2010. http://doi.org/10.1523/JNEUROSCI.4145-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nature Neuroscience. 2009;12(3):256–258. doi: 10.1038/nn.2271. http://doi.org/10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Mason JW, Giller EL, Ostroff RB, Harkness L. Sustained urinary norepinephrine and epinephrine elevation in post-traumatic stress disorder. Psychoneuroendocrinology. 1987;12(1):13–20. doi: 10.1016/0306-4530(87)90017-5. http://doi.org/10.1016/0306-4530(87)90017-5. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. http://doi.org/10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Progress in Brain Research. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. http://doi.org/10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lonergan MH, Olivera-Figueroa LA, Pitman RK, Brunet A. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: a meta-analysis. Journal of Psychiatry & Neuroscience: JPN. 2013;38(4):222–231. doi: 10.1503/jpn.120111. http://doi.org/10.1503/jpn.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. http://doi.org/10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70(5):830–845. doi: 10.1016/j.neuron.2011.04.023. http://doi.org/10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Nature and causes of the immediate extinction deficit: a brief review. Neurobiology of Learning and Memory. 2014;113:19–24. doi: 10.1016/j.nlm.2013.10.012. http://doi.org/10.1016/j.nlm.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Chang C. Recent fear is resistant to extinction. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(47):18020–18025. doi: 10.1073/pnas.0608398103. http://doi.org/10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holmes A. Stress and Fear Extinction. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.180. http://doi.org/10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews. Neuroscience. 2013;14(6):417–428. doi: 10.1038/nrn3492. http://doi.org/10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason ST, Fibiger H. Noradrenaline, fear and extinction. Brain Research. 1979;165(1):47–56. doi: 10.1016/0006-8993(79)90043-x. [DOI] [PubMed] [Google Scholar]
- Mason ST, Roberts DC, Fibiger HC. Interaction of brain noradrenaline and the pituitary-adrenal axis in learning and extinction. Pharmacology, Biochemistry, and Behavior. 1979;10(1):11–16. doi: 10.1016/0091-3057(79)90161-8. [DOI] [PubMed] [Google Scholar]
- Mayou RA, Ehlers A, Hobbs M. Psychological debriefing for road traffic accident victims. The British Journal of Psychiatry. 2000;176(6):589–593. doi: 10.1192/bjp.176.6.589. http://doi.org/10.1192/bjp.176.6.589. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a Century of Consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. http://doi.org/10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGhee LL, Maani CV, Garza TH, Desocio PA, Gaylord KM, Black IH. The effect of propranolol on posttraumatic stress disorder in burned service members. Journal of Burn Care & Research. 2009;30(1):92–97. doi: 10.1097/BCR.0b013e3181921f51. http://doi.org/10.1097/BCR.0b013e3181921f51. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. http://doi.org/10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear Extinction as a Model for Translational Neuroscience: Ten Years of Progress. Annual Review of Psychology. 2012;63(1):129–151. doi: 10.1146/annurev.psych.121208.131631. http://doi.org/10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36(3):521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behavioral Neuroscience. 2007;121(3):501–514. doi: 10.1037/0735-7044.121.3.501. http://doi.org/10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. The Journal of Neuroscience. 2008;28(2):369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. http://doi.org/10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learning & Memory (Cold Spring Harbor, NY) 2010;17(6):306–313. doi: 10.1101/lm.1794710. http://doi.org/10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12(2):120–150. doi: 10.1038/sj.mp.4001939. http://doi.org/10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–726. doi: 10.1038/35021052. http://doi.org/10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Norris JG, Benveniste EN. Interleukin-6 production by astrocytes: induction by the neurotransmitter norepinephrine. Journal of Neuroimmunology. 1993;45(1–2):137–145. doi: 10.1016/0165-5728(93)90174-w. [DOI] [PubMed] [Google Scholar]
- Nugent NR, Christopher NC, Crow JP, Browne L, Ostrowski S, Delahanty DL. The efficacy of early propranolol administration at reducing PTSD symptoms in pediatric injury patients: a pilot study. Journal of Traumatic Stress. 2010;23(2):282–287. doi: 10.1002/jts.20517. http://doi.org/10.1002/jts.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Milad MR, Metzger LJ, Lasko NB, Gilbertson MW, Pitman RK. Effects of beta blockade, PTSD diagnosis, and explicit threat on the extinction and retention of an aversively conditioned response. Biological Psychology. 2006;73(3):262–271. doi: 10.1016/j.biopsycho.2006.05.001. http://doi.org/10.1016/j.biopsycho.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Ostrowski SA, Delahanty DL. Prospects for the pharmacological prevention of post-traumatic stress in vulnerable individuals. CNS Drugs. 2014;28(3):195–203. doi: 10.1007/s40263-014-0145-7. http://doi.org/10.1007/s40263-014-0145-7. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nature Neuroscience. 2013;16(2):146–153. doi: 10.1038/nn.3296. http://doi.org/10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK. Post-traumatic stress disorder, conditioning, and network theory. Psychiatric Annals. 1988;18(3):182–189. [Google Scholar]
- Pitman RK, Delahanty DL. Conceptually driven pharmacologic approaches to acute trauma. CNS Spectrums. 2005;10(2):99–106. doi: 10.1017/s109285290001943x. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Milad MR, Igoe SA, Vangel MG, Orr SP, Tsareva A, Nader K. Systemic mifepristone blocks reconsolidation of cue-conditioned fear; propranolol prevents this effect. Behavioral Neuroscience. 2011;125(4):632–638. doi: 10.1037/a0024364. http://doi.org/10.1037/a0024364. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Liberzon I. Biological studies of post-traumatic stress disorder. Nature Reviews. Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. http://doi.org/10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological Psychiatry. 2002;51(2):189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Poundja J, Sanche S, Tremblay J, Brunet A. Trauma reactivation under the influence of propranolol: an examination of clinical predictors. European Journal of Psychotraumatology. 2012;3 doi: 10.3402/ejpt.v3i0.15470. http://doi.org/10.3402/ejpt.v3i0.15470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Smits JAJ, Otto MW, Sanders C, Emmelkamp PMG. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: a randomized placebo controlled trial of yohimbine augmentation. Journal of Anxiety Disorders. 2009;23(3):350–356. doi: 10.1016/j.janxdis.2009.01.001. http://doi.org/10.1016/j.janxdis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. http://doi.org/10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. The American Journal of Psychiatry. 2003;160(2):371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. http://doi.org/10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC. Optogenetic dissection of medial prefrontal cortex circuitry. Frontiers in Systems Neuroscience. 2014;8 doi: 10.3389/fnsys.2014.00230. http://doi.org/10.3389/fnsys.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10(6):423–433. doi: 10.1038/nrn2651. http://doi.org/10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(17):6741–6746. doi: 10.1073/pnas.0601874103. http://doi.org/10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Brewin CR, Andrews B, Kirk M. A randomized controlled trial of individual psychological debriefing for victims of violent crime. Psychological Medicine. 1999;29(4):793–799. doi: 10.1017/s0033291799008624. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Biedenkapp JC, Moineau J, Bolding K. Anisomycin and the reconsolidation hypothesis. Learning & Memory. 2006;13(1):1–3. doi: 10.1101/lm.157806. http://doi.org/10.1101/lm.157806. [DOI] [PubMed] [Google Scholar]
- Schneider AM, Simson PE, Daimon CM, Mrozewski J, Vogt NM, Keefe J, Kirby LG. Stress-dependent opioid and adrenergic modulation of newly retrieved fear memory. Neurobiology of Learning and Memory. 2014;109:1–6. doi: 10.1016/j.nlm.2013.11.013. http://doi.org/10.1016/j.nlm.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Wolf OT, Beaudry T, Pruessner JC. Neural signature of reconsolidation impairments by propranolol in humans. Biological Psychiatry. 2012;71(4):380–386. doi: 10.1016/j.biopsych.2011.10.028. http://doi.org/10.1016/j.biopsych.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiology of Learning and Memory. 2012;97(3):338–345. doi: 10.1016/j.nlm.2012.01.009. http://doi.org/10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Shin LM, Handwerger K. Is posttraumatic stress disorder a stress-induced fear circuitry disorder? Journal of Traumatic Stress. 2009;22(5):409–415. doi: 10.1002/jts.20442. http://doi.org/10.1002/jts.20442. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Noradrenergic enhancement of associative fear memory in humans. Neurobiology of Learning and Memory. 2011;96(2):263–271. doi: 10.1016/j.nlm.2011.05.003. http://doi.org/10.1016/j.nlm.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Stimulation of the Noradrenergic System during Memory Formation Impairs Extinction Learning but not the Disruption of Reconsolidation. Neuropsychopharmacology. 2012;37(5):1204–1215. doi: 10.1038/npp.2011.307. http://doi.org/10.1038/npp.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current Opinion in Neurobiology. 2010;20(2):231–235. doi: 10.1016/j.conb.2010.02.005. http://doi.org/10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological Psychiatry. 1999;46(9):1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA, Nicolaou AL, Nagy LM, Charney DS. Noradrenergic and serotonergic function in posttraumatic stress disorder. Archives of General Psychiatry. 1997;54(8):749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- Spring JD, Wood NE, Mueller-Pfeiffer C, Milad MR, Pitman RK, Orr SP. Prereactivation propranolol fails to reduce skin conductance reactivity to prepared fear-conditioned stimuli. Psychophysiology. 2015;52(3):407–415. doi: 10.1111/psyp.12326. http://doi.org/10.1111/psyp.12326. [DOI] [PubMed] [Google Scholar]
- Stein MB, Kerridge C, Dimsdale JE, Hoyt DB. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. Journal of Traumatic Stress. 2007;20(6):923–932. doi: 10.1002/jts.20270. http://doi.org/10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Geracioti TD. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depression and Anxiety. 2008;25(3):260–271. doi: 10.1002/da.20292. http://doi.org/10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory Reconsolidation and Extinction Have Distinct Temporal and Biochemical Signatures. The Journal of Neuroscience. 2004;24(20):4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. http://doi.org/10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taherian F, Vafaei AA, Vaezi GH, Eskandarian S, Kashef A, Rashidy-Pour A. Propranolol-induced Impairment of Contextual Fear Memory Reconsolidation in Rats: A similar Effect on Weak and Strong Recent and Remote Memories. Basic and Clinical Neuroscience. 2014;5(3):231–239. [PMC free article] [PubMed] [Google Scholar]
- Tawa J, Murphy S. Psychopharmacological treatment for military posttraumatic stress disorder: An integrative review. Journal of the American Association of Nurse Practitioners. 2013;25(8):419–423. doi: 10.1111/1745-7599.12016. http://doi.org/10.1111/1745-7599.12016. [DOI] [PubMed] [Google Scholar]
- Taylor HR, Freeman MK, Cates ME. Prazosin for treatment of nightmares related to posttraumatic stress disorder. American Journal of Health-System Pharmacy. 2008;65(8):716–722. doi: 10.2146/ajhp070124. http://doi.org/10.2146/ajhp070124. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Immediate and prolonged effects of cortisol, but not propranolol, on memory retrieval in healthy young men. Neurobiology of Learning and Memory. 2009a;91(1):23–31. doi: 10.1016/j.nlm.2008.08.002. http://doi.org/10.1016/j.nlm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Psychophysiological responding to emotional memories in healthy young men after cortisol and propranolol administration. Psychopharmacology. 2009b;203(4):793–803. doi: 10.1007/s00213-008-1427-x. http://doi.org/10.1007/s00213-008-1427-x. [DOI] [PubMed] [Google Scholar]
- Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, Marmar CR. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biological Psychiatry. 2003;54(9):947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Kathryn Dahlgren M, Caroline Davis F, Dubois S, Shin LM. From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiology of Learning and Memory. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. http://doi.org/10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: state of the art. Annual Review of Clinical Psychology. 2013;9:215–248. doi: 10.1146/annurev-clinpsy-050212-185542. http://doi.org/10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Wangelin BC, Powers MB, Smits JAJ, Tuerk PW. Enhancing exposure therapy for PTSD with yohimbine HCL: Protocol for a double-blind, randomized controlled study implementing subjective and objective measures of treatment outcome. Contemporary Clinical Trials. 2013;36(2):319–326. doi: 10.1016/j.cct.2013.08.003. http://doi.org/10.1016/j.cct.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Wang S-H, de Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nature Neuroscience. 2009;12(7):905–912. doi: 10.1038/nn.2350. http://doi.org/10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Pham TC, Sullivan RM. Norepinephrine and posttraining memory consolidation in neonatal rats. Behavioral Neuroscience. 1994;108(6):1053–1058. doi: 10.1037//0735-7044.108.6.1053. [DOI] [PubMed] [Google Scholar]
- Wood NE, Rosasco ML, Suris AM, Spring JD, Marin MF, Lasko NB, Pitman RK. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psychiatry Research. 2015;225(1–2):31–39. doi: 10.1016/j.psychres.2014.09.005. http://doi.org/10.1016/j.psychres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick S, Giller EL, Ma X, Mason JW. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. The Journal of Nervous and Mental Disease. 1992;180(5):321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]