Abstract
Targeted therapies and the consequent adoption of “personalized” oncology have achieved notable successes in some cancers; however, significant problems remain with this approach. Many targeted therapies are highly toxic, costs are extremely high, and most patients experience relapse after a few disease-free months. Relapses arise from genetic heterogeneity in tumors, which harbor therapy-resistant immortalized cells that have adopted alternate and compensatory pathways (i.e., pathways that are not reliant upon the same mechanisms as those which have been targeted). To address these limitations, an international task force of 180 scientists was assembled to explore the concept of a low-toxicity “broad-spectrum” therapeutic approach that could simultaneously target many key pathways and mechanisms. Using cancer hallmark phenotypes and the tumor microenvironment to account for the various aspects of relevant cancer biology, interdisciplinary teams reviewed each hallmark area and nominated a wide range of high-priority targets (74 in total) that could be modified to improve patient outcomes. For these targets, corresponding low-toxicity therapeutic approaches were then suggested; many of which were phytochemicals. Proposed actions on each target and all of the approaches were further reviewed for known effects on other hallmark areas and the tumor microenvironment. Potential contrary or procarcinogenic effects were found for 3.9% of the relationships between targets and hallmarks, and mixed evidence of complementary and contrary relationships was found for 7.1%. Approximately 67% of the relationships revealed potentially complementary effects, and the remainder had no known relationship. Among the approaches, 1.1% had contrary, 2.8% had mixed and 62.1% had complementary relationships. These results suggest that a broad-spectrum approach should be feasible from a safety standpoint. This novel approach has potential to help us address disease relapse, which is a substantial and longstanding problem, so a proposed agenda for future research is offered.
Keywords: Multi-targeted, cancer hallmarks, phytochemicals, targeted therapy, integrative medicine
1. Introduction
Cancer is a source of significant and growing mortality worldwide, with an increase to 19.3 million new cancer cases per year projected for 2025. More than half of cancer cases and mortality occur in low- and middle-income countries, and these proportions are expected to increase by 2025 [1]. Current treatments for cancer include surgery, radiotherapy and systemic treatments comprising cytotoxic chemotherapy, hormonal therapy, immunotherapy, and targeted therapies [2]. Cancer continues to stymie clinical treatment efforts, however, and the search for effective therapies continues.
This capstone paper describes the methods and results of a substantial effort by a large international group of biochemical and medical researchers, operating under the name of “The Halifax Project,” sponsored by a non-profit organization, Getting To Know Cancer. It summarizes and draws together material from a series of reviews on the hallmarks of cancer, presented in this special issue of Seminars in Cancer Biology, to present a conceptual framework for a new approach to cancer prevention and therapeutics. This approach involves the targeting of many specific high-priority anticancer mechanisms and pathways within a more comprehensive model of treatment and care. We refer to this as a “broad-spectrum” approach (i.e., an approach aimed at a broad spectrum of important mechanisms and pathways) [3]. The broad-spectrum approach involves combinations of multiple low-toxicity agents that can collectively impact many pathways that are known to be important for genesis and spread of cancer. By making extensive use of chemicals from plants and foods that have already been studied or utilized for cancer prevention and treatment, this approach offers a compelling rationale for addressing the underlying biology of cancer while being efficacious, non-toxic and cost-effective. We come together in the belief that a broad-spectrum approach of this type, in the context of a therapeutic environment including conventional treatment and attentive to optimal health, would provide genuine benefit in clinical outcomes for cancer patients. In this paper we describe the rationale for broad-spectrum therapeutics, detail the methods of the Halifax Project, summarize potential targets and agents related to eleven hallmark features of cancer, propose a research model for the development of broad-spectrum therapies, and call for action to advance this research model.
1.1 Rationale for Broad-Spectrum Approach
Primary motivations for the development of a broad-spectrum approach stem from the distinct limitations that are evident in many current targeted therapies and the personalized medicine paradigm. Molecular target therapies represent a significant advance in the treatment of cancer. They include drugs such as imatinib, an inhibitor of the tyrosine kinase enzyme BCR-ABL, which has made chronic myelogenous leukemia a more manageable disease, and inhibitors of vascular endothelial growth factor receptor (VEGFR), such as sunitinib, sorafenib and bevacizumab, used in renal and colon cancers [2]. Other important treatments based on tumor-specific targets are now in use, including examples such as epidermal growth factor receptor (EGFR) inhibitors (gefitinib, erlotinib) used in lung cancer, and the Her2 inhibitor trastuzumab used in breast cancer. Another approach is the synthetic lethal model [4] exemplified by research on poly ADP ribose polymerase (PARP) inhibition, in which mutational loss of one or more redundant components of a cell survival pathway in tumorigenic cells confers selective sensitivity to drugs that target remaining pathway components.
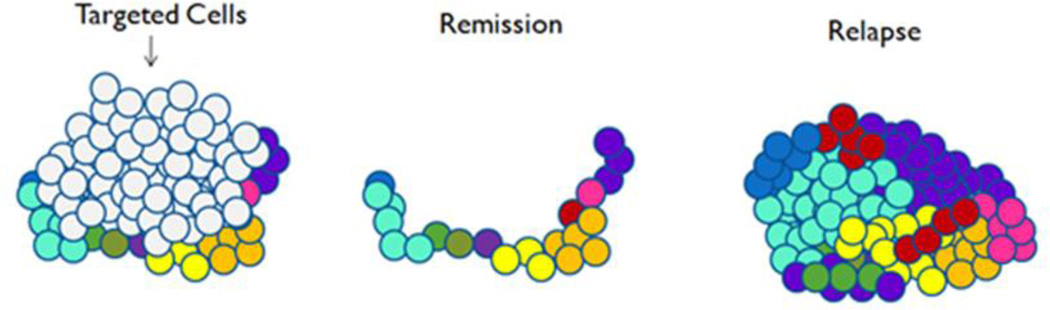
These drugs target cells bearing one, or at most a few mutated gene products or other abnormalities not found on normal cells. In the therapeutic context, the action of the targeted agents can efficiently address malignant cells, without some of the effects on normal cells notorious in cytotoxic chemotherapy. This enables therapeutic responses and remissions. Over time, however, the genetic heterogeneity of tumors increases, engendering resistance to treatment. Resistant cells drive the emergence of increasingly aggressive disease, through clonal expansion and clonal evolution [Figure 1]. Epigenetic modifications, heritable cellular changes not caused by alterations to DNA sequences, but by alterations such as methylation of DNA or modification of the histone protein associated with DNA, may also affect patterns of gene expression and drive cancers [5]. Relapses often occur after only a few months, and tumors reappear, sometimes in exactly the same areas in which they originated [6]. Moreover, targeted agents are not without serious side effects, such as treatment-related mortality with bevacizumab and cardiopulmonary arrest with cetuximab. Meta-analysis of trials of recently approved cancer drugs including targeted therapies versus older drugs showed increased rates of grades 3 and 4 toxicity (OR=1.52), treatment discontinuation (OR=1.33) and toxic deaths (OR = 1.40) [7]. This worsening of adverse effects has gone in large part unacknowledged.
Figure 1.
Diagrammatic representation of removal of susceptible cells by a targeted cancer therapy resulting in disease remission, which leaves genetically heterogeneous resistant cells to proliferate, resulting in relapse.
The efficacy shown to date with targeted therapies, aside from now-established treatments such as bevacizumab and trastuzumab, is nevertheless still limited. Sunitinib, for instance, extends overall survival by 4.6 months in renal cancer, compared with the previous treatment of interferon-α [8]. While statistically significant, this degree of improvement is small comfort to afflicted patients, and challenges the extraordinary monetary investment in drug development as well as costs to the medical system that targeted therapies represent. The MOSCATO 01 trial of molecular triage was able to treat 25 of 111 patients with a variety of advanced cancers using therapies targeted to genomic alterations assessed from tumor biopsies [9]. Of these, 5 patients (20%) experienced partial response and 56% had stable disease. Based on the entire population of 111 patients, this is a partial response of less than 5%, suggesting limited efficacy to date, an outcome also seen in some other studies. [10]. On a more hopeful note however, a combination of pertuzumab with trastuzumab and the chemotherapy agent docetaxel was recently found to extend overall survival among the subset of breast cancer patients whose tumors express Her-2 by 15.7 months [11].
Interestingly, harnessing the body´s immune response against the tumor can also result in impressive durable clinical responses, perhaps because the immune system is a paragon of adaptability and can deal with changes in the mutational landscape of cancer to prevent escape from the therapeutic effect. Immunomodulatory antibodies recently licensed in the United states include ipilimumab as well as nivolumab and pembrolizumab, neutralizing two different inhibitory pathways that block antitumor T cell responses. These agents have achieved some successes in treating late stage cancers refractory to essentially any other treatments [12]. But even with these agents, response rates are still low and predicting who will respond is an unsolved challenge [13,14].
Many of these therapies are somewhat narrowly described as “personalized” because patients’ tumors must be tested for specific mutations to stratify patients to the correct therapy. Viewed in the larger context of individual biological variation, of course, specific mutations drive only the smallest degree of personalization. Truly personalized treatment approaches can be seen to include a much more comprehensive assessment of genetic and even lifestyle factors, such as nutritional, biobehavioral (stress management) strategies, and exercise habits, along with other host variables such as inflammation and immune status. Such an approach to personalizing treatment can be found in the systematic practice of integrative medicine, which played a significant role in the development of this model of broad-spectrum cancer therapy. Some definitions of integrative medicine stress simply the inclusion of complementary and alternative therapies alongside orthodox treatment [15]. A more relevant definition emphasizes a patient-centered, multi-intervention treatment paradigm that addresses the full range of physical, mental, emotional and environmental influences, utilizing an array of disciplines including diet, mind-body and physical activity therapies in addition to conventional therapies and dietary supplements to support optimal health [16], based on laboratory testing that enables comprehensive personalization.
The stratification of patients for these targeted and personalized therapies poses practical challenges. As indicated earlier, over 50% of the increase in cancer incidence by 2025 is projected to occur in the developing world [1]. As industrialization develops in lower-income countries, occupational cancers are expected to increase, potentially aggravating this situation [17]. Cancer treatment in many of these countries is already becoming a social-economic challenge due to the expense and medical infrastructure required [18], and the new generation of treatments may further strain local resources. Currently, the platforms used for testing to personalize regimens include whole exome or whole genome sequencing, whole transcriptome sequencing, and comparative genomic hybridization with still others in development. It is likely that such tests, and related expense, will proliferate in the future. Managing treatment toxicity is also a taxing and complex problem, as these toxicities necessitate additional medical interventions.
The expense of the new targeted therapies is also concerning. Eleven of twelve drugs approved by the US Food and Drug Administration (US FDA) in 2012 were priced above $100,000 US per year per patient – perhaps not surprisingly in view of the accelerating costs of drug development [19]. Clinicians have drawn attention to these high costs: in 2013 more than 100 experts in chronic myeloid leukemia coauthored a paper calling for lower prices and broader access to these drugs [20]. The excessive costs have resulted in drugs not being approved for use by national or regional governments where cost-benefit analyses figure in approval processes [21]. While costs are expected to decrease after expiration of patents on the drugs, the costs for treatment in low- or middle-income countries may continue to be problematic. The potential for unsupportable financial stress on health systems challenges the research community to explore other treatment models that can be more sustainable in the face of the worldwide increase in cancer incidence.
The broad-spectrum approach that we describe here is primarily intended to address the two major issues of therapeutic resistance and cost. It is based on many of the insights of genomic sequencing in cancers. We now know that cancers harbor significant genetic heterogeneity, even within a single patient [6]. Based on this heterogeneity, cancers routinely evolve resistance to treatment through switching from one growth pathway to another [22]. The proposed strategy employs the basic principles of rational drug design, but aims to stem cancer growth by precisely targeting many growth pathways simultaneously. Some effort is now being made in combining targeted agents so that more than one pathway can be affected, but lack of therapeutic success, significant toxicity and costs make this a challenge [23–26].
We see the broad-spectrum approach as one that is complementary to existing therapies, preferably within the context of a genuinely integrative clinical system. Clinical situations in which such an approach might prove useful include (a) as a follow-up maintenance plan to conventional adjuvant treatment; (b) in situations of rare cancers and disease stages for which no accepted treatments exist; (c) for patients who do not tolerate conventional chemotherapy, hormonal therapy or targeted therapies; (d) for patients who experience relapse or progression after targeted treatment; (e) in hospice or palliative care patients where low- or non-invasive strategies are a legitimate and humane option; and (f) in situations in which high-cost agents cannot be obtained. Because of continuous heterogeneity among cancer cells, and their propensity for genomic instability, even a broad-spectrum approach is unlikely to cause complete remission. However, the design of this approach posed a substantial theoretical challenge, for which we chose to use the hallmarks of cancer as a broad organizing framework.
1.2 Hallmarks of cancer as a framework for developing broad-spectrum therapeutics
Douglas Hanahan and Robert A. Weinberg first published their concept of the hallmarks of cancer in 2000 [27]. The hallmarks “constitute an organizing principle that provides a logical framework for understanding the remarkable diversity of neoplastic diseases.” This framework encompasses the biological capabilities that cells acquire during the development of cancers that allow them to become malignancies as we know them. Six hallmarks were proposed in the 2000 publication: sustained proliferative signaling, evading growth suppressors, activating invasion and metastasis, enabling replicative immortality, inducing angiogenesis and resisting cell death. The concept of the hallmarks became widely recognized and influential. In 2011, Hanahan and Weinberg expanded on the initial hallmarks to include other areas of cancer biology that they felt were equally important [28]. They pointed out two enabling characteristics critical to the ability of cells to acquire the six hallmarks, and two new hallmark capabilities. They also singled out the crucial nature of the complex tumor microenvironment in the appearance of the cancer phenotype. The enabling characteristics are genomic instability and tumor-promoting inflammation; the new hallmarks are deregulating cellular energetics and avoiding immune destruction.
The hallmarks framework helps to define domains in which high priority targets can be identified for therapeutic targeting. Hanahan and Weinberg point out that agents are in development that target each of the hallmarks. They also note, however, that in response to targeted therapy, cancers may reduce their reliance on a particular hallmark capability, such as angiogenesis, and instead heighten the activity of another capability, such as invasion and metastasis [29]. This reaction has been clinically verified in the case of glioblastoma [30].
Another model, which was proposed by Vogelstein et al. in 2013 [6], also attempts to describe the mechanisms and pathways that are relevant to many cancers. In this model, “driver” genes that drive cancer growth are distinguished from “passenger” mutations found in cancer cells that impart no growth advantage. Twelve major signaling pathways that drive cancer growth have been elucidated, including signal transducers and activators of transcription (STAT), Notch, DNA damage control and 9 others. These pathways are classified into three cellular processes underlying tumor growth: cell survival, cell fate and genome maintenance. Individual patients with the same cancer can have mutations on different pathways, leading to inter-patient heterogeneity. Yet within each patient there is also substantial heterogeneity, both within each patient’s primary tumor, and among and within metastases, with significance for treatment strategies. For instance, the smallest metastases visible through medical imaging may already have thousands of cells that harbor mutations rendering them resistant to current drugs [31].
Cancer mutations, moreover, are not simply a series of isolated targets. Beneath the surface of the cancer genome is a notably complex cellular signaling network, filled with redundancies. The elucidation of rational therapeutic combinations requires dynamic mechanistic models that reach beyond simple targeting [32]. What propels growth, dissemination and thus ineffective treatment and drug resistance actually appears not to be pathways acting in isolation but interconnected, multidirectional and dynamic networks [33]. Even sorafenib, which inhibits multiple kinases, is susceptible to the rapid development of resistance deriving from crosstalk in pathways such as phosphatidylinositide 3-kinase/protein kinase B (PI3K/Akt) and Janus kinase (JAK)-STAT, hypoxia-induced signaling or the epithelial-to-mesenchymal transition (EMT) [34]. Conventional drug discovery programs are now contemplating systems biology approaches aimed at furthering the network approach to pharmacology. The interdependence of cytokines, chemokines, growth factors, transcription factors, and their resulting proteomes, together with their relevance to cancer prevention and treatment [35], makes systems biology approaches most attractive [36]. This realization makes the significance of a broad-spectrum approach to cancer of even greater importance.
Clinicians as well as researchers recognize the importance of heterogeneity in cancer. A least one clinical center recognizes the significance of this heterogeneity, and intervenes with broad-spectrum approaches to respond to it. In a 2009 book, Life Over Cancer, based on a clinic in operation since 1980, K.I. Block lays out a model of nutraceutical-based targeting of nine “pathways of progression” and six metabolic factors impacting the challenges faced by all cancer patients [3]. The nine growth pathways are proliferation, apoptosis, treatment resistance, immune evasion, angiogenesis, metastasis, cell-to-cell communication, differentiation and immortality. Multiple targeting of these pathways with natural products is used to simultaneously address multiple interconnected growth pathways. Comprehensive molecular profiling maps patients’ growth pathways and provides for relevant natural product intervention. The six metabolic “terrain factors” are oxidation, inflammation, glycemia, blood coagulation, immunity and stress chemistry. Terrain-focused interventions are tailored to patients’ laboratory test results, which are monitored regularly to guide therapeutic modification. Interventions include elimination of maladaptive lifestyle patterns, adjusting exercise habits, improving diet, implementing biobehavioral strategies to diminish adverse consequences of unabated stress/distress, and using natural products and medications that affect specific targets such as C-reactive protein (CRP) [37], interleukin-6 (IL-6), nuclear factor κ-beta (NF-κB) [38], prostaglandin E2 and leukotriene B4 [39] for inflammation. Clinical observations and literature review suggest potential efficacy for this system in breast cancer (including a near-doubling of survival time of breast cancer patients in integrative care) and potentially other cancers [40,41]. Essentially, Block’s clinical model systematically addresses multiple targets and pathways through a specific and selective broad-spectrum approach to treatment. While this system was developed in clinical practice, quite independently from the discussion of hallmarks and enabling characteristics by Hanahan and Weinberg, the conceptual overlap is obvious. That these concepts have already been used in clinical treatment provides powerful support for the viability of a carefully designed broad-spectrum approach.
The model we propose to use to develop a sound framework for a broad-spectrum approach recognizes these broad areas of conceptual overlap and agreement, and can be considered to best align with the hallmarks of cancer framework [27]. Our framework encompasses the molecular and metabolic diversity of malignancy recognized in Hanahan and Weinberg’s hallmarks, Vogelstein’s 12 growth pathways, Block’s pathways of progression and terrain factors, and other emerging research. For the purposes of this project, we treat the 6 hallmarks, 2 enabling characteristics, 2 emerging hallmarks, and the tumor microenvironment equally as hallmarks of malignancy. From a design standpoint, each of these individual areas encompasses an important aspect of cancer’s biology, so each was seen as important to consider for a therapeutic approach aimed at a wide range of high priority targets.
In mid-2012, the framework for this project and approach were shared with Douglas Hanahan. He later independently provided support for this type of approach in a paper, “Rethinking the war on cancer” [42]. Using a military metaphor, he suggests a three-dimensional cancer “battlespace” plan that attacks cancer in a full-scale war rather than individually targeted skirmishes. The first dimension is disruption of cancer’s many capabilities, specifically those figuring in the hallmarks. Rather than just removing one capability, as targeted therapies do, he explains that an ideal approach should target all the hallmark capabilities. The second dimension is defense against cancer’s armed forces, implying specific targeting of the accessory cell types in the tumor microenvironment, such as tumor-promoting inflammatory cells. The third dimension represents the multiple battlefields of cancer: primary tumor, tumor microenvironment, lymph and blood vessels through which tumors disseminate, draining lymph nodes and distant organs. This dimension suggests still more targets.
A rapidly developing sub-discipline in oncology is the application of genetic and immune analysis of tumor tissue and the concomitant use of personalized therapies and prescriptions. These analyses allow better stratification of patients to treatments and clinical decision-making [43]. In the case of breast cancer alone, tests range from Her-2 testing, the basis of trastuzumab treatment to sophisticated suites of tests that analyze dozens of genes. These complex analyses assist in treatment decisions based on correlations with clinical outcomes by predicting treatment response, risk of recurrence and outcome. They suggest the size of the network of genes that affect just one cancer, and emphasize the significance of a broad-spectrum attack. Clinical utility of these tests is still under review [44].
Despite impressive progress in genomic and gene expression profiling, however, it is often impossible to fully characterize the range of immortalized cell variants within any given cancer. The perspectives offered by Hanahan Vogelstein and Block, as well as by the recognition of the network aspects of signaling pathways, however, suggest a larger number of targets may need to be reached. So the 138 driver genes, together with the 12 signaling pathways that comprise them, in addition to the molecular contributors to the hallmarks, and Block’s nine pathways of progression and six terrain factors, help us delineate some of the most significant targets that should be taken into account in development of a broad-spectrum approach.
2. Methods
The effort to develop the concept of broad-spectrum targeting of cancer through a complex combination of agents, emphasizing naturally occurring chemicals, was developed by a non-profit organization, Getting To Know Cancer, and implemented within an initiative called “The Halifax Project.” The aim of the project was to produce a series of reviews of the cancer hallmarks that could collectively assess and prioritize the many target choices that exist, and also identify non-toxic chemicals (primarily from plants or foods) that could safely be combined to produce an optimized broad-spectrum approach that has both prophylactic and therapeutic potential. To that end, it was envisioned that eleven teams of researchers would produce reviews on the ten cancer hallmarks plus the tumor microenvironment, which was treated as a hallmark for the purposes of this project. Each review was to describe the hallmark, its systemic and cellular dysfunctions, and its relationships to other hallmarks. A priority list of relevant therapeutic targets and corresponding approaches suited to those targets was requested, along with a discussion of research needed in the context of goals of the project. Natural compounds were emphasized because of the growing body of literature that supports the low toxicity and interesting potential that many of these substances have demonstrated (i.e., as targeted therapeutics or in cancer prevention), while recognizing the variable effectiveness of these compounds in human trials as well as the undocumented safety or frank toxicity concerns with many natural products [45].
In recognition of the network of signaling pathways involved not only in drug resistance but the interconnection and maintenance of all the hallmarks, the project implemented a cross-validation step in the evaluation of targets and approaches. Because of the diversity of the targets involved in the 11 hallmark areas, it is not unreasonable to suspect that inhibiting or stimulating a target relevant to one hallmark may have an adverse growth effect or clinically adverse effect on a target in another hallmark. For instance, reducing DNA damage is a potential target for counteracting genomic instability. Activation of the immune system can counter DNA damage by eliminating damaged cells. However, activation of the immune system, while reducing overall levels of DNA damage, can contribute to chronic inflammation. [46].
Similar considerations apply to therapeutic approaches. For instance, triptolide, a component of the Chinese herb Tripterygium wilfordii, is known to cause apoptosis in cancer cells [47]. Extracts of the herb have been used in clinical trials for a variety of inflammatory and immune-linked conditions, and have demonstrated both antiinflammatory and immune suppressant activity, raising concern for its effect on immune evasion [48,49].
To address this issue, a specially designated cross-validation team was created within the project to evaluate all selected targets and approaches, i.e., to determine whether the inhibition or activation of targets, and the application of approaches, would have negative effects on other hallmarks. Each potential target-hallmark or approach-hallmark interaction was assessed to determine whether the pair had a complementary interaction (i.e., the interaction of the target or approach with the hallmark facilitated anticancer activity), a contrary interaction (i.e., the interaction of the target or approach with the hallmark had a potential adverse tumor-stimulating or tumor-progression effect), a controversial interaction (i.e., mixed indications of anticancer and tumor-stimulating effects), or no known relationship. A sample cross-validation table for dysregulated metabolism approaches can be accessed as Supplemental Table S1.
It is important to note that the cross-validation team was not given any restrictions for literature selection for this effort, and contributing authors were not restricted to cancer-related research. This approach was taken because it was realized at the outset that this breadth and specificity of knowledge does not yet exist in the literature. As a result, the types and sources of data gathered in this effort varied considerably, although original studies were consistently favored over review articles. Moreover, many studies that were cited in this effort considered only a compound’s ability to instigate or promote an action that mimics a hallmark phenotype in a manner directionally consistent with changes that have been associated with cancer. So while we refer to these as anticancer or tumor-stimulating, the specificity of these activities and their implications for cancer treatment cannot and should not be immediately inferred from this database. In other words, the results from this aspect of the project were only compiled to serve as a starting point for future research, rather than a conclusive guide to therapy.
Targets or approaches that have a substantial number of “contrary” assessments are less attractive for inclusion in the broad-spectrum approach. On the other hand, the use of targets and approaches that appear to have the potential for multiple complementary interactions is consistent with principles of rational drug design, and akin to efforts to design “dirty” drugs (a pharmacological term for drugs with multiple targets – as opposed to single targets -- aimed at multidimensional conditions) [50]. Further evaluation of such “dirty” targets and approaches could be undertaken through more specific application of network pharmacology, for which new tools are currently becoming available [51]. The tabulated results, which appear in the individual reviews, are discussed in a later section of this paper.
The review teams needed for the Halifax Project were formed by first circulating an email to a large number of cancer researchers, seeking expressions of their interest in participation. The email was circulated in July 2012 by Getting To Know Cancer, and scientists were encouraged to submit their details on a dedicated webpage that offered additional project detail. From the pool of 703 cancer scientists who responded to the email, 11 team leaders were selected to each lead a group in producing a review of each hallmark, and an additional leader selected for the cross-validation team. Those leaders were then asked to form their own teams (by drawing from the pool of researchers who expressed interest in the project, and from their own circles of collaborators). Ultimately, 12 teams were formed. Team members were each encouraged to engage a junior researcher as well. This led to fairly large teams but it allowed us to distribute the effort considerably. Team leaders all received project participation guidelines; extensive and ongoing communication from the project leader, Leroy Lowe; copies of the relevant papers of Hanahan and Weinberg; and copies of Life Over Cancer by Block [3] as an example of practical clinical implementation of the broad-spectrum approach. In addition to the 11 teams, two guest editors, Anupam Bishayee and Keith Block, were selected for this special issue of Seminars in Cancer Biology in which the team reviews are published.
The team leaders and other team members who were able to attend the project workshop met in Halifax, Nova Scotia in August 2013 to discuss the project. Drafts of hallmark team papers were submitted in advance, and summary presentations made at the meeting. Other subject matter presentations included presentations on research funding in the natural products area (Jeffrey D. White, Office of Cancer Complementary and Alternative Medicine, National Cancer Institute) and the concept of driver and passenger genes (Bert Vogelstein, Johns Hopkins). Presentations on integrative cancer therapeutics made at the meeting are summarized below (Keith Block, Penny Block, Block Center for Integrative Cancer Treatment). Group discussions were held to facilitate communication among teams and project staff, and to assist teams in exploring the requirements and rationale for selection of targets and approaches.
Each hallmark team contained the following specialists: a lead author with demonstrated expertise in the hallmark area; domain experts who produced the descriptive review; anticancer phytochemical specialists; oncologists; and support researchers. The cross-validation team conducted background literature searches on the submitted targets and compounds from each review team, verifying their activity in relation to the other hallmarks. Results of the cross-validation effort were tabulated and reviewed by the individual teams. Ambiguous results and areas of disagreement were reconciled, and the tables were ultimately incorporated into each hallmark review.
2.1 Selection of targets and approaches
It was assumed from the outset that, in a translational project aimed at the development of a broad-spectrum approach, there would be a practical upper limit to the number of potential targets in any given cancer that could be targeted. So each hallmark team was asked to select and prioritize up to 10 relevant targets for their hallmark area, bearing in mind that each target would serve as a starting point for the identification of a suitable low-toxicity approach that might be used to reach that target. In theory, it was understood that this could lead to as many as 110 targets for the entire project, and since the teams were also asked to select one therapeutic approach for each target, a maximum of 110 potential therapeutic approaches might be selected.
An “approach” was defined in this project as (1) a technique that will cause the body to respond in a manner that will act on the target (e.g., fasting, exercise etc.), or (2) a procedure involving an entity that can act on the target (e.g., phytochemical, dietary modification, synthetic drug, vaccination with peptides, locally administered oncolytic virus etc). Teams were then asked to identify “favored” approaches with patient safety as a top priority (i.e., least likely to cause harm or side effects even in combination with many other approaches). In addition to safety, other practical considerations for choosing favored approaches were suggested as follows:
Efficacy – Greatest potential to achieve the desired action on the intended target across the widest possible range of cancer types
Cost – Less expensive is better, and by no means cost prohibitive
-
Intellectual Property – Free of intellectual property constraints if at all possible.
Approaches that do not have patents, that cannot be patented, and/or those that have patents that are expired are to be given priority over those that have existing patents.
2.1.1 Selection of targets
Extensive discussion took place about the principles of target selection. Certainly targets that are unique to cancer cells and tumor microenvironments, and that are not known to cause side effects when inhibited pharmacologically, would be a primary consideration. Targets induced by viruses or known carcinogens that are of importance in therapy would also be examined. Consideration of the nature of mutations in the cancer genome and the role of epigenetic modification were also discussed.
It is understood that great effort has been made to sequence the cancer genome to identify the most common mutations seen in different cancers. It is also known that different driver mutations may give rise to variant tumor cells, and the number of driver mutations required is limited, with just 2–8 per patient, which could potentially be assessed through whole genome sequencing of individual cancer patients. However, questions arise about treatment, since most of the currently available drugs are not potent enough to target all susceptible cells. Moreover, the toxicity of existing drugs, if administered in combination protocols, is severely limiting, even at the reduced dosages that may be possible when using multiple agents. A strong rationale supports focusing on low toxicity chemistry (e.g., such as that which has been demonstrated by many anticancer and chemopreventive phytochemicals as the foundation for a broad-spectrum approach. A number of phytochemicals enhance absorption of other natural products through such mechanisms as cytochrome P450 modification [52], which could also enhance the possibilities for low-toxicity treatment, i.e., by reducing dosages needed for effective treatment.
Many driver genes are actually tumor suppressor genes, and in these cases, it is the loss of the tumor suppressor gene that allows development of cancer. Drugs cannot target these missing genes. Rather they must target unopposed pathways, such as pathways that are active upstream from the missing suppressor gene. For instance, the tumor suppressor forkhead box 0 (FOX0) normally causes apoptosis. If FOX0 is inactivated in cancer, an unopposed pathway upstream from it is the PI3K/Akt1 signaling pathway, which could alternatively be targeted [53]. The mitogen-activated protein kinase/extracellular-signal regulated kinase/mitogen/extracellular signal-regulated kinase (MEK) pathway, however, can act as a substitute or compensatory pathway to PI3K/Akt1. So, in order to effectively shut down replication, it would seem necessary to address these pathways as well.
Cancer-related signaling pathways, including even those that become driver pathways, may be epigenetically modified prior to their genetic modification in cancer pathogenesis [54]. This suggests an emphasis on chemoprevention or treatment of very early cancers. Targeting may be more straightforward to achieve under these conditions, since it is easier to modulate wildtype pathways pharmacologically than to treat the consequences of the onset of widespread aneuploidy. In this case, the cancer phenotype may well precede the cancer genotype by years or more. Combining knowledge of genetic and epigenetic changes in a particular tumor may result in the targeting of key pathways with fewer agents and reduced cost.
A more general consideration is that both direct and indirect targets and approaches can be considered. Direct targets are those that are familiar to us from targeted therapies – oncogenes, tumor suppressor genes, signaling pathways. Indirect approaches, however, are also potentially useful. For instance, evasion of the immune system is a hallmark of cancer [27], and immunomodulatory targets and approaches are appropriate to support the capacities of immune cells to eliminate tumor cells. Immune regulators are, in a sense, inherently multi-targeted due to the complexity of the responses they induce [55]. However, immunity is frequently compromised in patients under treatment with cytotoxic chemotherapies, as well as in the post-surgical period. Immune system approaches that also support the capacity of patients to tolerate or recover from surgery or toxic therapies indirectly support the health of cancer patients [56]. The potency of the immune system is illustrated by findings that chemotherapy may enhance antitumor immunity if given in the correct sequence, and that cancer refractory to chemotherapy or immune modulation alone may become susceptible to both together [57].
2.1.2 Selection of approaches
The need for low-toxicity agents as constituents suggested that phytochemicals –especially those “pre-screened” in humans owing to their presence in foods or traditional medicines -- should be carefully considered during approach selection. Each hallmark team therefore included cancer researchers who had considerable experience working with phytochemicals. In considering phytochemicals and other low-toxicity agents for inclusion in a broad-spectrum approach, however, several limitations in the literature promptly become clear.
First, the level of evidence for the effects of natural products on particular hallmark targets varies widely. The status of laboratory studies and clinical trials on several well-known phytochemicals, e.g. resveratrol, epigallocatechin gallate (EGCG), curcumin, lycopene and others, was recently reviewed [58]. The pleiotropic nature of the effects of these agents on apoptosis and arrest of cell growth has been emphasized, and their potential use in association with chemotherapy drugs has been acknowledged. Novel strategies based on a strategic combination of phytochemicals with broad-spectrum action together with radiation or chemotherapy agents aimed at overcoming resistance to apoptosis and enhancing sensitivity to treatment are also currently being considered [59,60].
Second, considerable clinical experience with combinations of phytochemicals and other natural agents in treatment of cancer patients exists. Detailed knowledge of the pharmacological effects of combinations of phytochemicals, however, is limited. There is a large literature on herbal combinations used in traditional Chinese medicine in both the laboratory and clinic [61–63], but the quality of older clinical trials is generally low. Additionally, laboratory studies of herbal medicines often use concentrations far higher than are clinically achievable. Supra-physiological concentrations can produce artefactual or irrelevant mechanisms of action or cause toxicity. The limited bioavailability of major phytochemicals makes this especially concerning, although products with improved bioavailability are in development [64]. In general, phytochemical research merits rigorous attention if we hope to gain a more detailed understanding of how these compounds affect the cancer hallmarks. Basic research needs to be followed up with better-designed, statistically powered clinical trials, if we hope to fully realize the therapeutic potential of phytochemicals.
In addition to laboratory studies and clinical trials, approaches may be suggested by epidemiological studies and the observations of integrative medicine, which uses diet and lifestyle therapies to affect medical conditions including cancer. Observational studies of soy consumption, along with corroborating evidence from clinical studies, suggest that dietary consumption of soy foods consistent with levels in the Japanese diet (2–3 servings daily, containing 25–50 mg isoflavones) may be associated with reduced risk of breast cancer incidence and mortality [65]. However, findings from animal studies [66] of negative effects of the soy isoflavone genistein on breast cancer and its treatment suggest some caution and avoidance of simplistic recommendations.
At all levels of investigation, the multi-targeted nature of phytochemicals as well as the integrative therapies is notable. Many isolated phytochemicals and herbals may alter large numbers of targets through multifaceted effects on physiology and metabolism [67–69]. A basic complication of these multi-targeted agents, however, is the lack of mechanistic understanding and scientific acceptance of the roles of synergistic or additive molecules in formulation. Although used by human populations for millennia, there remains a question of how to develop and assess multi-component natural product formulations that are suitable for large-scale production. Genome-wide screening for assessment of targeted effects and experimentation with formulation of some herbs typical of traditional Ayurvedic medicine have recently been attempted in Asian laboratories, and are examples of attempts to better understand effects of multi-component agents [70–72].
3. Hallmarks of cancer
In this section we provide brief summaries of each hallmark review included in this special issue of Seminars in Cancer Biology. Each summary includes the targets and approaches selected in the hallmark review. Tables summarizing the targets and approaches and discussion of the cross-validation results follow. In addition, a summary of the impacts of integrative therapies on cancer-related molecular targets follows the hallmark summary material.
The hallmark summaries are roughly sequenced to capture the acquired capabilities of most cancers (see Figure 2). The section begins with genomic instability, an enabling characteristic, followed by sustained proliferative signaling and evasion of anti-growth signaling, two hallmarks that ensure that proliferation is unabated in cancer cells. These are followed by resistance to apoptosis and replicative immortality, two layers of defense that are believed to be bypassed in all cancers. Then we discuss dysregulated metabolism and tumor-promoting inflammation, which signal an important self-reinforcing evolution in the tumor microenvironment. Sections on angiogenesis and tissue invasion and metastasis speak to disease progression. Finally the tumor microenvironment and immune system evasion summaries relate to the last lines of defense to be defeated in most cancers.
Figure 2.
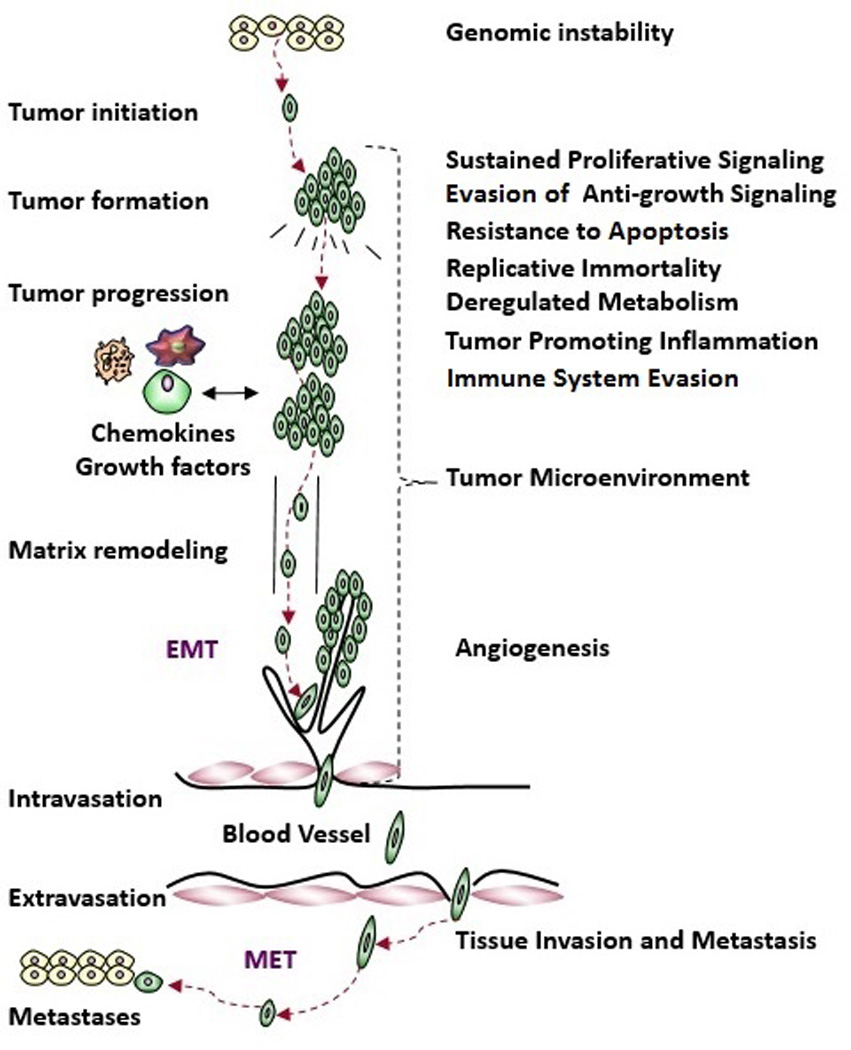
Hallmarks of cancer, sequenced roughly in the order in which these capabilities are acquired by most cancers, as portrayed in the graphical representation of tumor evolution.
3.1 Genomic instability
Genomic instability plays a critical role in cancer initiation and progression. It provides the means by which a cell or subset of cells acquire a selective advantage over neighboring cells, enabling outgrowth and dominance in the tissue microenvironment. In normal cells, the fidelity of the genome is protected at every stage of the cell cycle by checkpoints. In cancer, the presence of aneuploid cells indicates the failure of one or more of these checkpoints. The resulting genomic heterogeneity may offer the cancer “tissue” growth advantages under selective pressures, including hypoxia, immune- and therapy-related challenges. Understanding these checkpoints, and how they are bypassed in cancer cells, may provide opportunities for the development of rational combinatorial or broad-spectrum treatment strategies, including nutraceuticals such as resveratrol [73,74].
A cell, either transformed or normal, must pass through multiple checkpoints during the process of division. These checkpoints are operated by functional complexes of proteins that either enable the cell to pass through the checkpoint (e.g. proto- or oncogenes) or prevent the progression through the cell cycle (i.e. tumor suppressors). The abundance of these proteins, and their functionality, can be modified by genetic changes to their encoding sequences or by non-genetic, or epigenetic, changes that regulate their abundance. Briefly, small changes to the genes that encode proto-oncogenes or tumor suppressors will positively or negatively impact the function of the gene products. These small changes can be induced by environmental and lifestyle factors, such as toxic substances, diet, and smoking, or they can be encoded in the individual at conception. In the case of DNA damage generated by the environment, it is important that the cell repairs the damage effectively. Dysfunction in the molecules that come together to recognize and respond to sites of damage is often associated with human cancer. Thus, an understanding of the genetic or epigenetic status of DNA repair genes, and of the nutraceuticals that may modulate them [75], provides an opportunity to predict, detect, prevent and treat a variety of human cancers.
Growing evidences show that vitamins, minerals, and other dietary factors have profound and protective effects against cancer cells, whether they are grown in the lab, in animals, or studied in human populations. We have identified five targets against genomic instability: (1) prevention of DNA damage; (2) enhancement of DNA repair; (3) targeting deficient DNA repair; (4) impairing centrosome clustering; and, (5) inhibition of telomerase activity. Vitamins D and B, selenium, carotenoids, PARP inhibitors, resveratrol, and isothiocyanates are priority approaches against genomic instability; these approaches may dampen other enabling characteristics of tumor cells, such as replicative immortality, evasion of anti-growth signaling, tumor promoting inflammation, and oncogenic metabolism [73,76–82].
3.2 Sustained proliferative signaling
Proliferation plays an important role in cancer development and progression, as manifested by altered expression and activity of proteins related to the cell cycle [83,84]. Constitutive activation of a large number of signal transduction pathways takes place in cancer; this also stimulates cell growth. Early in tumor development a fibrogenic response is often seen. Along with the development of a hypoxic environment [85,86], this favors the appearance and proliferation of cancer stem cells (CSCs). The survival strategies distinguishing CSCs from normal tissue stem cells involve lack of cellular differentiation and alterations in cell metabolism, such as higher antioxidant levels [83,84]. These alterations take place as cells adapt to the changing microenvironment in affected tissue, prior even to the appearance of tumors. A part of this adaptation embodies epigenetic and genetic alterations in gene expression [6,87] that also confer resistance to many cytotoxic treatments [88,89]. Thus, adaptive resistance is likely acquired early in the pathogenesis of many tumor types.
Once tumors appear, the continued selection of cells with sustained proliferative signaling further promotes tumor heterogeneity. This is accomplished by growth and metastasis, which may be supported by overproduction of appropriate hormones (in hormonally dependent cancers), by promoting angiogenesis, by undergoing EMT, by altering the balance between apoptosis, necrosis and autophagy, and by taking cues from surrounding stromal cells. A number of natural compounds (such as EGCG) have been found to inhibit one or more pathways that contribute to proliferation [90–92]. Many of these compounds are nontoxic at doses that inhibit tumor growth and/or prevent the appearance of tumor. However, one of the keys to their efficacy involves their earliest possible therapeutic application. This is because their efficacy is likely to be the greatest in target tissues prior to the appearance of a tumor where cellular heterogeneity is the least. In addition, many of the steps in carcinogenesis prior to tumor appearance are epigenetic in nature, and are more easily targeted by existing compounds, most of which target wild type molecules. This approach limits adaptive resistance, since early intervention does not have to deal with the issues of aneuploidy, loss of heterozygosity in multiple tumor suppressor genes, and point mutations in oncogenes. The contribution of bioinformatics analyses will be important for identifying signaling pathways and molecular targets that may provide early diagnostic markers and/or critical targets for the development of new drugs or combinations that block tumor formation. Thus, early intervention in pathways and molecules that mediate sustained proliferative signaling will limit adaptive resistance because it targets cells in tissues that have limited genotypic and phenotypic heterogeneity.
Targets selected for sustained proliferative signaling are hypoxia-inducible factor-1 (HIF-1) signaling, NF-κB signaling, PI3K/Akt signaling, wingless-type mouse mammary tumor integration site (Wnt) (β-catenin) signaling, insulin-like growth factor receptor (IGF-1R) signaling, cell cycle [cyclin-dependent kinases (CDKs)/cyclins], androgen receptor signaling, and estrogen receptor signaling. Possible therapeutic approaches include curcumin, genistein and resveratrol.
3.3 Evasion of Anti-growth Signaling
Normal cells must acquire the ability to continuously proliferate in order to transform into malignant phenotypes. However, cells have internal programs (anti-growth signaling) to oppose limitless growth. In order to continue to proliferate, cancer cells must somehow evade many anti-growth signals. In general, anti-growth signaling is mediated by the activation of tumor suppressor genes. The Cancer Genome Atlas has compiled data encompassing all tumor types, which indicates that p53 is the most frequently mutated tumor suppressor gene followed by PTEN, APC, ATM, BRCA2, VHL, RB, CDKN2A, BRCA1 and WT1.
Retinoblastoma protein 1 (RB1) was the first identified tumor suppressor and deletion of this gene is frequently found in cancers [93]. In many cases, the loss of RB is due to defects in upstream signaling molecules such as inactivation of INK4. Loss of p16ink4a results in unopposed activation of CDK4/6, which phosphorylates the RB protein thereby activating E2F-mediated transcription of genes involved in entry into the cell cycle [94].
Another tumor suppressor frequently deleted due to chromosomal loss is p53 [95]. In fact, more than 50% of all tumors have loss of p53 tumor suppressive functions. Recently, mutant p53 has gained renewed attention due to the fact that along with the loss of tumor suppressive functions, mutant p53 gains oncogenic/tumor promoting functions [96].
Epigenetic silencing of tumor suppressor proteins, which includes DNA methylation, histone methylation and acetylation, is another mechanism through which tumor cells evade anti-growth signaling. Many tumor suppressor genes have been found to have promoter hypermethylation in cancers [97]. Finally, anti-growth signaling plays a major role in treatment response and drug development. For example, the patients with human papilloma virus-positive oropharyngeal cancer mostly retain wild-type p53 and have better prognosis and survival.
Although genetic alterations are mostly irreversible, epigenetic repressions are potentially reversible and targets for drug development. At least three histone deacetylase inhibitors, belinostat, vorinostat and romidepsin, are currently approved by the US FDA for cancer treatment. Many natural compounds also target the restoration of tumor suppressors through modifying epigenetic changes [98–102]. Thus, approaches to activate anti-growth signaling will open another chapter for cancer prevention and therapy.
The prioritized targets for anti-growth signaling are RB, p53, phosphatase and tensin homolog (PTEN), Hippo, growth differentiation factor 15 (GDF15), AT-rich interactive domain 1A (ARID1A), Notch, IGF-1R and others. The approaches are inactivation of E2F by down regulation of pRb using CDK inhibitors, activation of p53 through up-regulation of wild-type p53, activation of PTEN to inhibit PI3K-AKT, activation of Hippo pathways by inhibiting Yes-associated protein/transcriptional enhancer activator domain (YAP/TEAD) activity, induction of GDF15 through p53 activation, activation of ARID1A, blocking Notch pathway, and inhibition of IGF-1R to restore tumor suppressor pathways. Suggested phytochemicals for these approaches are EGCG, luteolin, curcumin, genistein, resveratrol, withaferin A, and deguelin. Furthermore, while the evasion of anti-growth signaling is a critical hallmark of cancer, other hallmarks are similarly important and a more integrative approach is necessary to simultaneously target several hallmarks of cancer to combat this deadly disease.
3.4 Resistance to apoptosis
Apoptosis naturally removes aged and unhealthy cells from the body [103]. However, in cancer, cells lose their ability to undergo apoptosis leading to uncontrolled proliferation and multiplication. These malignant cells are often found to overexpress many of the proteins that play important roles in resisting the activation of the apoptotic cascade, and one of the major hallmarks of human cancers is the intrinsic or acquired resistance to apoptosis [104]. Evasion of apoptosis may contribute to tumor development, progression, and also to treatment resistance, since most of the currently available anticancer therapies including chemotherapy, radio- and immunotherapy primarily act by activating death/apoptotic pathways in cancer cells [105]. Hence, a better understanding of the molecular mechanisms underlying tumor resistance to apoptotic cell death is expected to provide the basis for a rational approach to develop molecular targeted therapies.
Apoptosis resistance is multi-factorial and emanates from the interactions of various molecules and signaling pathways at multiple levels. Several mechanisms exist allowing cells to escape programmed cell death. Among them is the overexpression of the anti-apoptotic molecules. B-cell lymphoma-2 (Bcl-2) family proteins play a critical role in the biology of apoptosis resistance. Robust agents against the Bcl-2 homology domain 3 proteins are in development and accelerating toward clinical application. Other cell death mechanisms such as autophagy and necrosis can also be highlighted and strategies against them exist, including the use of natural agent such as EGCG. The role of the chaperone protein heat shock protein 70 (Hsp70) in apoptosis resistance is important, and natural agents may also address this. Various molecular mechanisms support resistance to apoptosis in different disease models such as glioblastoma, multiple myeloma and chronic lymphocytic leukemia. Epigenetic players, particularly the non-coding RNAs/ microRNAs, are also of importance. Novel targets can be pinpointed, such as ecto-nicotinamide dinucleotide disulfide thiol exchanger protein (ENOX) and nuclear export protein chromosomal regional maintenance protein 1(CRM1), along with specific strategies to overcome these important drug resistance promoters. Other targets include inhibition of Mcl-1, activation of tumor autophagy, activation of tumor necrosis, inhibition of Hsp90, inhibition of proteasomes, and inhibition of EGFR and Akt. Approaches to these targets include gossypol, UMI-77, EGCG, triptolide, PXD, selinexor, and inhibitors of EGFR and Akt. Collectively, the knowledge gained through greater understanding of the apoptosis resistance targets and specific strategies is anticipated to bring forward a broad form of therapy that could result in better treatment outcome in patients suffering from therapy-resistant cancers.
3.5 Replicative immortality
Replicative immortality, the ability to undergo continuous self-renewal, is necessary for propagation of normal germ cells, but is not a property of normal somatic cells. When acquired by somatic cells that have sustained genetic damage or instability, replicative immortality allows accumulation of sequential aberrations that confer autonomous growth, invasiveness, and therapeutic resistance [106]. As a result, several mechanisms have evolved to regulate replicative potential as a hedge against malignant progression [107]. Senescence, a viable growth arrest characterized by the inability of affected cells to resume proliferation in the presence of appropriate mitogenic factors, is a specific response to the gradual shortening of chromosomal end structures (telomeres) with each round of cell replication, and a more general response to oncogenic and genotoxic stresses. Senescence often involves convergent interdependent activation of tumor suppressors p53 and p16/pRB [108,109], but can still be induced, albeit with reduced sensitivity, when these suppressors are inactivated. Doses of conventional genotoxic drugs required to achieve cancer cell senescence are often much lower than doses required to achieve outright cell death [110]. Additional targeted therapies may induce senescence specifically in cancer cells by blocking cyclin-dependent kinase mediated inhibition of RB-family proteins [111], or by exploiting cancer cells’ heightened requirements for maintenance of telomere length through the action of the enzyme telomerase [112]. Developing optimized and truly holistic cancer prevention and treatment regimens will likely incorporate strategies that target replicative immortality.
The chief advantage to be gained by the use of senescence-inducing therapeutic regimens is elimination of the tumor’s repopulating ability with reduced collateral damage compared to conventional cytotoxic regimens. There are, however, certain questions and risks associated with this strategy that must be addressed before its clinical adoption. In the case of telomere and telomerase based strategies, replicative senescence may occur more readily in rapidly dividing cancer cells bearing short telomeres than in slowly dividing stem cells with comparatively longer telomeres, but telomere lengths in cancer cells may still be long enough to permit sufficient population doublings for invasion and metastases to occur [112] Moreover, telomere dysfunction promotes the development of chromosomal instability, which in turn can generate mutations that enable cells to become drug resistant and/or activate mechanisms based on alternative lengthening of telomeres for telomere maintenance and/or become more malignant [113]. High priority should therefore be given to further research into the determinants of senescence stability, as the implications of delayed cell cycle re-entry, permanent cytostasis, or eventual clearance may be profoundly different. Lower doses of genotoxic drugs needed to induce senescence may reduce collateral damage to critical normal cells, but allow establishment of dormancy and/or adaptive resistance by cancer cells. The microenvironmental and systemic effects of senescent cells also need further clarification, as factors secreted by senescent cells may promote tumorigenic changes in nearby cells. Conversely, since it is almost impossible to kill all the cells in malignant tumors even using the highest tolerated doses of chemotherapy, combined use of an agent that induces or enhances stable senescence in the cancer cells that manage to retain viability might additively or synergistically increase therapeutic efficacy.
A number of potential targets can be singled out for further research, including telomerase, human telomerase reverse transcriptase (hTERT), mammalian target of rapamycin (mTOR), CDK4/6, CDK 1/2/5/9, Akt and PI3K. Several approaches deserve further research, although the activity of the phytochemicals in particular is still far from clinical utility. These include imetelstat, genistein, perillyl alcohol, palbociclib, dinaciclib, curcumin and EGCG.
3.6 Dysregulated metabolism
Dysregulated metabolism is a hallmark of cancer in which many cancer cells show increased glucose uptake and produce lactate. This characteristic is often called the “Warburg effect” [114], but how and why cancer cells reprogram their metabolic state is not well understood. Recent research has focused on understanding the metabolic changes accompanying oncogenesis [27]. A new model of cancer metabolism positions metabolic rewiring in cancer as a coordinated process to support rapid cellular proliferation by tuning cellular energy production needs towards biosynthetic processes. Indeed, several metabolic shifts associated with cancer can be linked to cellular growth, which serve to support biosynthesis of lipids, proteins, nucleic acids required for tumor formation and survival [115].
In several cases, expression of oncogenes and/or loss of tumor suppressors lead directly to changes in metabolism, by expression, activity, or flux of key metabolic nodes. Several components of glucose and glutamine metabolism have emerged as important regulators of metabolism in cancer. In glucose metabolism, hexokinase 2 (HK2), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) and pyruvate kinase isoform M2 (PKM2) all regulate glycolytic flux. Using a “kitchen sink” analogy for glycolysis, both HK2 and PFKFB3 are regulators of the faucet, and fill up the sink. Conversely, PKM2 regulates the drain. Cancer metabolism turns on the faucet and plugs the drain, which over-spills the glycolytic pathway and provides metabolites used as building blocks for cellular growth. Efforts are underway to identify therapeutic strategies to “turn off the faucet” or “unplug the drain” in glycolysis, limiting cellular growth in cancer. Recent studies have also determined that glutamine is used as a fuel (glutaminolysis) in proliferating cancer cells. Glutamine oxidation can provide carbon and nitrogen for growth, and therefore is an attractive therapeutic target in cancer. Additionally, mutations in genes encoding enzymes directly involved in metabolic pathways have been associated with several types of cancer. Rather than acting as a bystander or facilitator of oncogenesis, aberrant metabolism now has a pro-oncogenic role and has led to the redefinition of some metabolites as ‘oncometabolites’ [116]. Indeed, these oncometabolites are powerful influencers of proliferation, and are also positioned as new therapeutic targets.
In principle, a broad-spectrum approach to target metabolic shifts in cancer is likely to be a promising therapeutic strategy. However, studies using this approach to target dysregulated metabolism in cancer are in their infancy. Lessons could be learned from other strategies to target mitochondria or to target metabolism in order to identify efficacious and safe therapies targeted at cancer metabolism; some drugs targeting metabolism are being re-purposed for their antitumorigenic effects. Several approaches could be mentioned, such as 3-bromopyruvate, 1-(4-pyridinyl)-3-(2-quinolinyl)-2-propen-1-one (PFK-15), 6-[(3-aminophenyl)methyl]-4,6-dihydro-4-methyl-2-(methylsulfinyl)-5H-thieno[2’,3’:4,5]pyrrolo[2,3-d]pyridazin-5-one (TEPP-46), dichloroacetate, hexachlorophene, bis-2-(5-phenylacet-amido-1,2,3-thiadiazol-2-yl)ethyl sulfide (BPTES) and 2,3-Dihydroxy-6-Methyl-7-(phenylMethyl)-4-propyl-1-naphthalenecar-boxylic acid (FX11), but data for these must be regarded as extremely preliminary, and they lack sufficient justification to be included in therapy without further study. Most target proteins or pathways identified as having potential to manipulate cancer metabolism have not been directly tested in the context of other hallmarks. The emerging efficacy of physiological interventions that manipulate cancer outcomes, such as fasting, calorie restriction, or exercise, could influence cancer metabolism and other hallmarks of cancer [117]. Future studies directly testing the ability to manipulate dysregulated metabolism in cancer will be an important and exciting new area of cancer biology that has potential for treating a variety of cancers.
3.7 Tumor-promoting inflammation
Virchow first proposed the role of inflammation in cancer in 1863, while observing the presence of leukocytes in neoplasms, and empirical evidence has since underscored the importance of this linkage [118,119]. The inflammatory milieu promotes a cellular microenvironment that favors the expansion of genomic aberrations and the initiation of carcinogenesis [120]. Chronic inflammation is linked to various phases of tumorigenesis, such as cellular proliferation, transformation, apoptosis evasion, survival, invasion, angiogenesis and metastasis [121–123]. Inflammation is also known to contribute to carcinogenesis through the generation of reactive oxygen species (ROS) and reactive nitrogen species which can damage DNA at the site of the tumor [124]. Free radicals and aldehydes, produced during chronic inflammation, can also induce deleterious gene mutation and post-translational modifications of key cancer-related proteins [125].
In addition, chronic inflammation has an influence on immune system constituents that are directly linked with cancer progression. Under normal conditions, immune cells, including macrophages, granulocytes, mast cells, dendritic cells, innate lymphocytes, and natural killer (NK) cells serve as the front line of defense against pathogens. When tissue disruption occurs, macrophages and mast cells secrete matrix-remodeling proteins, cytokines and chemokines, which activate local stromal cells (e.g., fibroblasts, adipocytes, vascular cells) to recruit circulating leukocytes into damaged tissue (acute inflammation), to eliminate pathogens [126]. However, when these processes are initiated in the tumor microenvironment, they are not resolved, which leads to chronic inflammation of the “damaged” (tumor) tissue. Thus, while acute inflammation normally supports and balances two opposing needs for the repair of damaged tissues (apoptosis and wound healing), chronic inflammation represents a loss of this balance and the resulting confluence of factors has deleterious implications for the immune system [127].
Accordingly, the relationship between tumor-promoting inflammation and cancer is important to consider. Macrophage migration inhibitory factor, cyclooxygenase-2 (COX-2), NF-κB, tumor necrosis factor alpha (TNF-α), inducible nitric oxide synthase (iNOS), Akt, and chemokines are important antiinflammatory targets that might be suitable for a multi-pronged therapeutic approach to inflammation suppression. Additionally, curcumin, resveratrol, EGCG, genistein, lycopene, and anthocyanins are forms of low-cost chemistry with little to no toxicity that could be employed to reach these targets.
Future translational work should make use of promising agents such as these (combined as constituents within a multi-pronged antiinflammatory approach) bearing in mind that some of these targets impact the immune system and can increase the risks associated with infection. Bioavailability challenges are also a concern for a number of these agents but recent advances in delivery systems will help address this issue.
3.8 Angiogenesis
Angiogenesis, the expansion of an existing vasculature, is the main mechanism of blood vessel growth in adults, and is therefore essential for tumor development [128]. Tumor angiogenesis is switched on by changing the balance between angiogenic factors and inhibitors in favor of angiogenesis [129], a process induced by tumor hypoxia as the tumor grows beyond a size of approximately 1 mm3 [128, 130]. At more advanced stages, progressive genomic instability in the tumor leads to mutations in pathways regulating the production of multiple angiogenic factors [131], and stroma cells also become important sources of sustained angiogenic factor production [132]. These collectively result in a stronger and more complex angiogenic factor profile. It is therefore not surprising that targeted neutralization of a single angiogenic factor, which has been the focus for antiangiogenic cancer therapy so far, rarely produces long-term, antitumor effects [132].
Due to the multifactorial nature of tumor angiogenesis this process is likely to be more efficiently treated by targeting multiple aspects of tumor angiogenesis and vascular dysfunction at the same time. Ten of the most important targets for tumor angiogenesis and vascular dysfunction are to inhibit endothelial cell migration/tip cell formation, reduce structural abnormalities of tumor vessels, reduce hypoxia, inhibit lymphangiogenesis, reduce elevated interstitial fluid pressure, reverse poor perfusion, normalize disrupted circadian rhythms, suppress tumor-promoting inflammation, deactivate tumor-promoting fibroblasts and normalize tumor cell metabolism/acidosis.
Currently available non-specific antiangiogenic agents, able to perform some of these tasks, are however quite toxic, which renders them unsuitable for long-term use [131,133,134]. There is an urgent need to identify alternative compounds that could be used in combination over extended periods of time, targeting tumor angiogenesis broadly and thus lowering the risk of resistance. Plant-derived compounds, phytochemicals, are in many cases better tolerated than the synthetic analogues used in cancer therapy today. Furthermore, they often exhibit broader mechanisms of action and sometimes even higher affinity against important cancer targets compared to the synthetic alternatives [135]. Ten phytochemicals that may be effective as approaches to neutralize the 10 identified targets are oleanoic acid, tripterine, silibinin, curcumin, EGCG, kaempferol, melatonin, enterolactone, withaferin A and resveratrol. Further study is needed to determine the optimal use and combination of these phytochemicals in antiangiogenic therapy, focusing on delivery, toxicity and their use in prophylactic regimens.
3.9 Tissue invasion and metastasis
Cancer causes substantial patient morbidity and mortality globally, making it a key health issue. Metastatic dissemination of the disease to distant sites impacts prognosis, with metastatic diseases accounting for a vast percentage of cancer patient mortality [27,136,137]. Cancer cells must overcome particular obstacles in order to successfully disseminate to and establish at a secondary location, progressing through the metastatic cascade. Successful progression through this cascade is linked with numerous established changes in cellular functions leading to the acquisition of an invasive phenotype. This involves loss of cell-cell contact with the main tumor body, invasion, degradation and migration through surrounding tissue and extracellular matrix, secretion of angiogenic/ lymphangiogenic factors and intravasation to the blood/lymph vessel, transport around the body and evasion of the immune system, extravasation at the secondary site and establishment of a secondary tumor [138,139].
Hence, factors influencing these processes such as cell adhesion molecules, proteolytic matrix degrading enzymes, cell motility and factors involved in the process of EMT have all been subject to scientific scrutiny. Additionally, the complex heterogeneity within tumors, together with cellular interactions between tumor cells and other, non-cancerous, cell types have been established to play key roles in metastatic dissemination and add further complexity to this cascade [137, 138]. While advances in the field of cancer research have been made, the process of cancer metastasis and the factors governing cancer spread and establishment at secondary locations are still poorly understood. Current treatment regimes for metastatic disease pose many adverse effects, which can further negatively impact on a subset of patients generally presenting with poorer health conditions. Hence there is a great need to develop new therapeutics that not only target tumor growth and inhibit metastasis but that also have a lower toxicity and reduced inherent side effects. Factors associated with metastasis such disruption of E-cadherin and tight junctions, key signaling pathways, including urokinase-type plasminogen activator, PI3K/AKT, focal adhesion kinase, β-catenin/zinc finger E-box-binding homeobox 1 and transforming growth factor (TGF)-β, together with inactivation of activator protein 1 (AP-1) and suppression of matrix metalloproteinase-9 (MMP-9) activity should be considered as key research priorities.
The need can also be highlighted for new, low toxicity compounds, which interfere with these processes but remain inexpensive alternatives that are readily available and free from intellectual property. Phytochemicals, or natural products, such as those from Agaricus blazei, Albatrellus confluens, Cordyceps militaris, Ganoderma lucidum, Poria cocos and Silybum marianum, together with diet-derived fatty acids gamma-linolenic acid and eicosapentaenoic acid and inhibitory compounds have potential to inhibit these key metastatic events. These potential targets and strategies thus present new therapeutic opportunities to both manage cancer metastasis as well as having holistic effects against many of the hallmarks of cancer.
3.10 Tissue interactions in the tumor microenvironment
Cancer arises in an in vivo tumor microenvironment. This microenvironment is a cause and consequence of tumorigenesis, and consists of cancer cells and host cells that co-evolve dynamically through indirect and direct cellular interactions, producing metabolites and secreting factors that affect cancer progression [140,141]. In turn, this environment regulates the ability of a cancer to grow and survive via multiscale effects on many biological programs including cellular proliferation, growth and metabolism, as well as angiogenesis and hypoxia, innate and adaptive immunity [142]. Specific biological programs could be, based on our most recent understanding, exploited as targets for the prevention and therapy of cancer, including: the inhibition of cholesterol synthesis and metabolites, ROS and hypoxia, macrophage activation and conversion, regulation of dendritic cells, regulation of angiogenesis, fibrosis inhibition, endoglin, and cytokine signaling. These programs emerge as examples of important potential nexuses in the regulation of tumorigenesis and the tumor microenvironment that can be targeted.
Potential targets include metabolic programs that may broadly influence many cell biology programs that impact tumorigenesis and the tumor microenvironment (cholesterol synthesis and metabolites, ROS and hypoxia), inflammation, innate and adaptive immunity-related programs (macrophage conversion, dendritic cell activation, immune signaling), host microenvironment associated cellular programs (fibrosis, angiogenesis), and cytokine-mediated regulatory programs (IL-6, endoglin, and JAK). We have particularly focused on identifying approaches for inhibiting these targets that included natural products that have been suggested to have significant anticancer activity. Some of these molecules may more generally influence tumorigenesis and the microenvironment (berberine), others more specifically target ROS (resveratrol, desoxyrhapontigenin), macrophage conversion (onionin A), indoleamine 2,3-dioxygenase (IDO) regulation of dendritic cells (EGCG), cholesterol synthesis (genistein), fibrosis (naringenin), inflammation and immune signaling (piperine) and JAK signaling (zerumbone). This approach will provide a starting point for examining synergies that might be anticipated in testing certain targets and/or mixtures of natural chemical constituents that may modulate the tumor microenvironment in the treatment and prevention of cancer.
3.11 Immune system evasion
Tumors evade immune attack by several mechanisms including generation of regulatory cells and their secretions, defective antigen presentation, induction of immune suppressive mediators either by cancerous cells themselves or by those in the microenvironment, tolerance, immune deviation and apoptosis.
Current approaches to immune therapy include a) cellular targets, b) molecular targets, c) vaccination therapy, d) therapy by phytochemicals, e) adoptive T cell therapy and f) immunomodulatory antibodies. Of these anticancer agents, the most important are those that are targeted in nature and to lesser extent, those that are non-specific in nature. Targeting specific costimulatory molecules such as cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) [143] or programmed cell death protein (PD1/PD-L1) [144] is considered an important anticancer strategy. Also, anti-PD-1 antibodies are showing enormous therapeutic potential in advanced cancers. Targets that are considered appropriate for broad-spectrum, low-toxicity therapeutics are less specific and include enhancing Th1 responses, enhancing γδ T cells, activation of macrophages, inhibition of Treg lymphocytes, enhancing natural killer cell activity and induction of IL-12.
There are a number of important nonspecific anticancer agents that have been reported, including vaccination therapy, as well as nonspecific bacteria-based therapies [145], and phytochemicals [146–148]. Phytochemicals (the biologically active components of fruits and vegetables) have been shown to exert protective effects against cancer. Examples of potential phytochemical approaches include extracts of Ganoderma lucidum, Trametes versicolor, Astragalus membranaceus, and Lentinus edodes, as well as astaxanthin and the polyphenol resveratrol analogue HS-1793. There is, however, a downside to phytochemical therapy such as their poor absorption by humans and rapid metabolism and excretion. More work is required to assess which phytochemicals block evasion of immune surveillance and also to determine which phytochemicals promote antitumor responses in cancer patients before these can be recognized for therapeutic value in the clinic.
4 Summary of findings on targets and approaches in hallmark reviews
As described above, a cross-validation process was employed to review the proposed actions on each target and all of the approaches for known effects on other hallmark areas and the tumor microenvironment. Anticarcinogenic synergies and confounding/procarcinogenic effects were then compiled and summarized in Tables 1–3. Supplemental table S1, a sample cross-validation table for dysregulated metabolism approaches, was used in construction of Tables 2 and 3. Supplemental tables S2 and S3 contain the aggregated cross-validation tables from each review (with references omitted). More detailed discussion of these interactions can be found in the individual hallmark reviews.
Table 1.
Prioritized targets with summary of information from cross-validation tables.
| Hallmark1,2 | Target (action on target) |
Contrary | Controversial | Complementary | None known |
|---|---|---|---|---|---|
| AP, RI, TPI | Akt (inhbit) | 0 | 0 | 11 | 0 |
| SPS | Androgen receptor signaling (suppress) | 0 | 2 | 8 | 1 |
| TIM | AP-1 (inhibit) | 1 RI | 0 | 7 | 3 |
| EAG | ARID1A (activate) | 1 TIM | 0 | 5 | 5 |
| AP | Bcl-2 (inhibit) | 0 | 1 | 9 | 1 |
| RI | CDK 1/2/5/9 (inhibit) | 1 TME | 0 | 9 | 1 |
| RI | CDK 4/6 (inhibit) | 1 GI | 1 | 8 | 1 |
| SPS | Cell cycle (CDKs/cyclins) (attenuate) | 2 IS, TIM | 0 | 9 | 0 |
| GI | Centrosome clustering (block) | 0 | 0 | 8 | 3 |
| TME | Cholesterol metabolites (inhibit) | 0 | 0 | 7 | 4 |
| TME | Cholesterol synthesis (inhibit) | 0 | 1 | 8 | 2 |
| TPI | COX-2 (inhibit) | 1 AN | 0 | 10 | 0 |
| TPI | CXC chemokine (inhibit) | 0 | 3 | 5 | 3 |
| AN | Disturbed circadian rhythms (normalize) | 0 | 2 | 9 | 0 |
| GI | DNA damage (prevent) | 1 TPI | 3 | 5 | 2 |
| GI | DNA repair (enhance) | 1 TPI | 3 | 5 | 2 |
| EAG, TIM | E-cadherin (restore) | 1 AN | 4 | 4 | 2 |
| EAG | E2F (inactivate) | 1 TME | 0 | 7 | 3 |
| AP | EGFR (inhibit) | 0 | 0 | 10 | 1 |
| AN | Elevated interstitial fluid pressure (reduce) | 0 | 0 | 9 | 2 |
| TME | Endoglin (inhibit) | 0 | 1 | 5 | 5 |
| AN | Endothelial cell migration/tip cell formation (inhibit) | 0 | 0 | 7 | 4 |
| AP | Enox (inhibit) (AP) | 0 | 0 | 5 | 6 |
| SPS | ER signaling (suppress) | 1 TIM | 3 | 7 | 0 |
| EAG | ER stress (induce) | 2 AN, TIM | 1 | 7 | 1 |
| TIM | FAK signaling (inhibit) | 0 | 0 | 9 | 2 |
| TME | Fibrosis (inhibit) | 0 | 0 | 6 | 5 |
| EAG | Growth differentiation factor 15 (induce) | 1 GI | 0 | 5 | 5 |
| SPS | HIF-1 signaling (inhibit) | 0 | 0 | 9 | 2 |
| AP | Hsp90 (inhibit) (AP) | 1 TIM | 0 | 8 | 2 |
| RI | hTERT (inhibit) | 0 | 1 | 8 | 2 |
| AN | Hypoxia (reduce) | 0 | 1 | 10 | 0 |
| TME | IDO (inhibit) | 0 | 1 | 7 | 3 |
| EAG, SPS | IGF-1R (inhibit) | 0 | 0 | 9 | 2 |
| IE | IL-12 (induce) | 1 AP | 0 | 5 | 5 |
| TME | IL-6 (inhibit) | 0 | 3 | 7 | 1 |
| TPI | iNOS (block) | 1 AN | 1 | 6 | 3 |
| TME | JAK (inhibit) | 0 | 0 | 10 | 1 |
| AN | Lymphangiogenesis (impede) | 0 | 1 | 4 | 6 |
| TME | M2 macrophage conversion (inhibit) | 0 | 0 | 7 | 4 |
| IE | Macrophages (activate) | 2 SPS, TIM | 2 | 3 | 4 |
| AP | Mcl-1 (inhibit) | 0 | 0 | 10 | 1 |
| TPI | MIF (block) | 0 | 0 | 9 | 2 |
| TIM | MMP-9 (suppress) | 0 | 1 | 7 | 3 |
| RI | mTOR (inhibit) | 0 | 2 | 8 | 1 |
| SPS, TIM, TPI | NF-KB signaling (inhibit) | 0 | 2 | 8 | 1 |
| IE | NK cell activity (promote) | 0 | 0 | 7 | 4 |
| EAG | NOTCH (block) | 1 AN | 0 | 8 | 2 |
| AP | Nuclear exporter CRM1 (inhibit) | 0 | 0 | 6 | 5 |
| RI | PI3K (inhibit) | 0 | 0 | 11 | 0 |
| EAG, SPS, TIM | PI3K/Akt signaling (inhibit) | 0 | 0 | 11 | 0 |
| AN | Poor perfusion (improve) | 0 | 1 | 7 | 3 |
| AP | Proteasome (inhibit) | 0 | 0 | 10 | 1 |
| TME | ROS (inhibit) | 0 | 2 | 7 | 2 |
| AN | Structural abnormalities of vessel walls (inhibit) | 0 | 0 | 7 | 4 |
| GI | Target deficient DNA repair | 1 TPI | 2 | 5 | 3 |
| GI, RI | Telomerase (inhibit) | 0 | 0 | 10 | 1 |
| TIM | TGF-β (inhibit) | 1 RI | 2 | 7 | 1 |
| IE | Th1 response (promote) | 1 TPI | 0 | 5 | 5 |
| TIM | Tight junctions (promote) | 1 AN | 0 | 6 | 4 |
| TPI | TNF-α (block) | 1 IE | 1 | 8 | 1 |
| IE | Treg lymphocytes (inhibit) | 0 | 1 | 6 | 4 |
| AP | Tumor autophagy (activate) | 1 TPI | 4 | 4 | 2 |
| AN | Tumor cell metabolism/acidosis (normalize) | 0 | 0 | 9 | 2 |
| AP | Tumor necrosis (activate) | 2 AN, TME | 3 | 5 | 1 |
| AN | Tumor-promoting fibro-blasts (deactivate) | 0 | 0 | 9 | 2 |
| AN | Tumor-promoting inflammation (suppress) | 0 | 0 | 7 | 4 |
| TIM | Urokinase plasminogen activator (suppress) | 1 RI | 0 | 7 | 3 |
| TME | VEGF (inhibit) | 0 | 3 | 8 | 0 |
| EAG | Wildtype p53 (upregulate) | 0 | 0 | 10 | 1 |
| SPS | Wnt (B-catenin) (inhibit) | 0 | 3 | 7 | 1 |
| EAG | YAP/TEAD activity (inhibit) | 0 | 0 | 6 | 5 |
| TIM | β-catenin/ZEB1 (inactivate) | 0 | 0 | 7 | 4 |
| IE | γδ T-cell activity (promote) | 2 TPI, AN | 0 | 4 | 5 |
| Totals: | 32 | 62 | 543 | 177 | |
| Percentages: | 3.93% | 7.62% | 66.71% | 21.74% |
For each target, the following items are shown: the hallmark(s) for which it was selected, and the number of other hallmarks with which it has complementary relationships, contrary relationships, no known relationships and controversial relationships. For targets that have contrary relationships, the conflicted hallmark(s) are shown. Totals and percentages of each type of relationship are shown at the end of the table.
AN = Angiogenesis, AP = Resistance to Apoptosis, DM = Dysregulated Metabolism, EAG = Evasion of Anti-Growth Signaling, GI = Genomic Instability, IE = Immune Evasion, RI = Replicative Immortality, SPS = Sustained Proliferative Signaling, TIM = Tissue Invasion and Metastasis, TME = Tumor Microenvironment, TPI = Tumor Promoting Inflammation.
Table 3.
Numbers of targets and therapeutic approaches for each hallmark with the following relationships: complementary relationship, contrary relationship, no known relationship and controversial relationship. Based on cross-validation tables.
| Type of relationship |
Genomic Instability |
Sustained Proliferative Signaling |
Tumor- promoting Inflammation |
Evasion of Anti- growth Signaling |
Resistance to Apoptosis |
Replicative Immortality |
Deregulated Metabolism |
Immune System Evasion |
Angiogenesis | Tissue Invasion and Metastasis |
Tumor Microenvironment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets | |||||||||||
| Complementary | 30 | 52 | 53 | 53 | 62 | 34 | 55 | 44 | 44 | 65 | 61 |
| Contrary | 2 | 1 | 6 | 0 | 1 | 0 | 0 | 2 | 9 | 5 | 3 |
| None known | 52 | 24 | 18 | 20 | 13 | 37 | 23 | 34 | 15 | 7 | 9 |
| Controversial | 1 | 5 | 6 | 7 | 4 | 12 | 5 | 4 | 12 | 3 | 7 |
| Therapeutic Approaches | |||||||||||
| Complementary | 35 | 51 | 44 | 50 | 62 | 37 | 42 | 22 | 40 | 60 | 64 |
| Contrary | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 2 | 1 | 0 |
| None known | 39 | 20 | 26 | 17 | 11 | 37 | 27 | 39 | 23 | 11 | 9 |
| Controversial | 1 | 8 | 5 | 5 | 1 | 1 | 6 | 12 | 7 | 0 | 0 |
Table 2.
Prioritized approaches with summary of information from cross-validation tables.
| Hallmarks1,2 | Approaches | Contrary, Conflicted Hallmarks |
Controversial | Complementary | None Known |
|---|---|---|---|---|---|
| DM | 3-bromopyruvate * | 0 | 0 | 7 | 4 |
| TIM | 5,6-dihydro-4H-pyrrolo[1,2-b]-pyrrazoles * | 0 | 0 | 2 | 9 |
| TPI | Anthocyanins | 0 | 0 | 9 | 2 |
| IE | Astaxanthin | 0 | 0 | 7 | 4 |
| IE | Astragalus membranaceus polysaccharide | 1 AN | 0 | 6 | 4 |
| TME | Berberine | 1 IE | 0 | 9 | 1 |
| DM | BPTES * | 0 | 0 | 5 | 6 |
| GI | Carotenoids | 0 | 1 | 10 | 0 |
| TIM | Cordycepin | 0 | 0 | 8 | 3 |
| AN, EAG, RI, SPS, TME, TPI | Curcumin | 0 | 0 | 11 | 0 |
| EAG | Deguelin | 0 | 0 | 7 | 4 |
| TME | Desoxyrhapontigenin | 0 | 0 | 2 | 9 |
| DM | Dichloroacetate * | 0 | 0 | 7 | 4 |
| RI | Dinacicilib * | 0 | 0 | 6 | 5 |
| AN, AP, EAG, RI, TME, TPI | EGCG | 0 | 0 | 11 | 0 |
| TIM | Eicosapentaenoic acid | 0 | 0 | 8 | 3 |
| AN | Enterolactone | 0 | 0 | 7 | 4 |
| DM | FX11 * | 1 GI | 0 | 2 | 8 |
| TIM | Gamma linolenic acid | 0 | 0 | 7 | 4 |
| TIM | Ganoderic acids | 0 | 0 | 7 | 4 |
| IE | Ganoderma lucidum polysaccharide | 0 | 0 | 9 | 2 |
| EAG, RI, SPS, TME, TPI | Genistein | 0 | 5 | 6 | 0 |
| AP | Gossypol | 0 | 0 | 9 | 2 |
| TIM | Grifolin | 0 | 0 | 6 | 5 |
| DM | GW5074* | 0 | 1 | 3 | 7 |
| DM | Hexachlorophene * | 0 | 0 | 6 | 5 |
| IE | HS-1793 (polyphenol resveratrol analogue)* | 0 | 0 | 5 | 6 |
| RI | Imetelstat* | 0 | 1 | 4 | 6 |
| GI | Isothiocyanate | 0 | 0 | 10 | 1 |
| AN | Kaempferol | 0 | 0 | 7 | 4 |
| IE | Lentinus edodes polysaccharide | 0 | 0 | 8 | 3 |
| EAG | Luteolin | 0 | 0 | 9 | 2 |
| TPI | Lycopene | 0 | 0 | 8 | 3 |
| AN | Melatonin | 0 | 0 | 10 | 1 |
| DM | Metformin* | 0 | 1 | 10 | 0 |
| TME | Naringenin | 0 | 2 | 6 | 3 |
| AN | Oleanoic acid | 0 | 0 | 10 | 1 |
| TME | Onionin A | 0 | 0 | 1 | 10 |
| TIM | Pachymic acid | 0 | 0 | 6 | 5 |
| RI | Palbociclib * | 1 TIM | 0 | 4 | 6 |
| GI | PARP inhibitor* | 0 | 0 | 9 | 2 |
| RI | Perillyl alcohol | 0 | 0 | 10 | 1 |
| TME | Piperine | 1 IE | 0 | 7 | 3 |
| DM | PK15 * | 0 | 0 | 6 | 5 |
| TIM | Polysaccharide (G. lucidum) | 0 | 1 | 8 | 2 |
| AN, DM, EAG, GI, SPS, TME, TPI | Resveratrol | 0 | 2 | 9 | 0 |
| GI | Selenium | 1 TPI | 2 | 6 | 2 |
| AP | Selinexor * | 0 | 0 | 3 | 8 |
| AN, TIM | Silibinin | 0 | 0 | 11 | 0 |
| DM | TEPP-46 * | 0 | 0 | 3 | 8 |
| IE | Trametes versicolor polysaccharide-k | 0 | 0 | 3 | 8 |
| AN | Tripterine | 0 | 0 | 5 | 6 |
| AP | Triptolide | 1 IE | 0 | 9 | 1 |
| AP | UMI-77 * | 0 | 0 | 5 | 6 |
| GI | Vitamin B | 0 | 2 | 3 | 6 |
| GI | Vitamin D | 0 | 0 | 10 | 1 |
| AN, EAG | Withaferin A | 0 | 0 | 9 | 2 |
| TME | Zerumbone | 0 | 0 | 6 | 5 |
| TIM | β-(1–6)-D-glucan (A. blazei) | 0 | 0 | 6 | 5 |
| Totals: | 7 | 18 | 403 | 221 | |
| Percentages: | 1.08% | 2.77% | 62.10% | 34.05% |
For each approach, the following items are shown: the hallmark(s) for which it was selected, and the number of other hallmarks with which it has complementary relationships, contrary relationships, no known relationships and controversial relationships. For approaches that have contrary relationships, the conflicted hallmark(s) are shown. Totals and percentages of each type of relationship are shown at the end of the table. Approaches are natural products except for those noted by asterisks.
AN = Angiogenesis, AP = Resistance to Apoptosis, DM = Dysregulated Metabolism, EAG = Evasion of Anti-Growth Signaling, GI = Genomic Instability, IE = Immune Evasion, RI = Replicative Immortality, SPS = Sustained Proliferative Signaling, TIM = Tissue Invasion and Metastasis, TME = Tumor Microenvironment, TPI = Tumor Promoting Inflammation.
Targeted therapy, synthetic compound or natural product analog/derivative
Table 1 shows an alphabetical listing of prioritized targets from each hallmark review, as well as the number of contrary, controversial, none known and complementary interactions with all other hallmarks. Dysregulated metabolism targets do not appear in the table; too little is known about the targets in this new area of research to reliably assess their interactions with other hallmarks. Of these relationships, 3.98% were contrary, 7.62% were controversial, 21.74% of interaction assessments found no known relationship, and 66.71% were complementary.
Table 2 shows the prioritized therapeutic approaches – the phytochemicals, plant extracts and drugs chosen as modifiers of the priority targets. Of these, 1.08% were contrary, 7.62% were controversial, 34.05% had no known relationships and 62.1% were complementary. Both contrary and controversial interactions indicate potential conflict among the targets and approaches selected for different hallmarks that could result in a broad-spectrum approach with antagonistic, rather than synergistic effects.
The small number of contrary and controversial interactions is encouraging, and suggests that the potential for negative interactions among the selected targets and approach may be limited. However, this may also reflect the common bias in the literature to publish positive antitumor effects. Nearly a third of potential interactions were listed as having no known relationship, suggesting the need for substantially more research in this area. The large number of complementary interactions is also encouraging but may result from indirect or bystander effects.
Table 3, in which the different types of interactions of both targets and approaches are listed for each hallmark, reflects different levels of knowledge regarding hallmarks, as well as varying prevalence of complementary approaches. Genomic instability has the largest number of unknown relationships with the targets and approaches. On the other hand, tumor microenvironment, tissue invasion and metastasis and resistance to apoptosis have the highest number of complementary interactions for both targets and approaches. Small numbers of contrary interactions were found for the different hallmarks for both hallmarks and approaches, but the number of targets for replicative immortality and angiogenesis, reflecting mixed positive and negative interactions, were larger than for other hallmarks.
There are a number of limitations that should be noted in this delineation of cross-hallmark relationships. First, the researchers who assembled these results were not asked to distinguish between direct effects on other hallmark areas and reported effects on other hallmark areas that may have resulted in an indirect or “bystander” effect mediated through a different mechanism. In many cases, but not all, this distinction was made. Therefore it is likely that some of the complementary interactions do not represent a fully independent cross-hallmark relationship, but rather are simply indicative of some sort of downstream effect (e.g., within a signaling cascade or via some other signaling molecule that exerts pleiotropic effects). However, we did not feel that this project needed to investigate the nature of these complementary interactions in detail, especially since the clinical impacts of these interactions would be similar for indirect and direct effects. Instead, our main concern was focused on the possibility that a large number of cross-hallmark relationships might be revealed where actions with procarcinogenic or tumor-promoting potential had been reported. It was more important to identify contrary and controversial cross-hallmark interactions than complementary ones, since targets or approaches that exert procarcinogenic actions would normally need to be more carefully assessed (or avoided altogether) in the development of combination approaches or interventions.
The second limitation of these reports of cross-hallmark relationships is related to data quality. In some instances, the available evidence used to support the indication of a cross-hallmark relationships was robust, consisting of multiple studies involving detailed in vitro and in vivo findings. In other instances, however, the underlying evidence that was used to report the existence of a cross-hallmark relationship was quite weak (e.g., consisting of only a single in vitro study involving a single cell-type). Again, the overarching goal in this project was to create a foundation that would allow us to look systematically across the literature in each of these areas, to help us shape the selection of the targets and approaches in order to comprehensively counter tumor growth pathways. So although we realized that not all of these reports of cross-hallmark relationships represented the same level of evidence, we still wanted to examine available evidence to flag targets and approaches where procarcinogenic actions had been reported.
There was also considerable debate within the task force over the value of tables containing only a simplified indication of a relationship (i.e., + or −) supported by evidence that varied considerably in quality. But since many individual studies and reviews that focus on therapeutic approaches fail to work systematically across the spectrum of incidental actions that might result from combining therapies, it was our opinion that a tabularized framework was the only way to ensure that we had assembled a complete view of cross-hallmark activity.
The types of approaches selected differed among different review teams. While some review teams selected all or mostly phytochemicals or plant extracts, some teams felt that the evidence for these was insufficient, and emphasized other types of molecules, including drugs in development. These may pose more difficulties for translational investigators due to intellectual property, toxicity or other concerns, but may offer advantages in a more clear understanding of their mechanisms. We suggest, however, that the approaches as well as the targets presented in Tables 1 and 2 can be viewed as simply a model for broad-spectrum cancer therapies, rather than as a final list. Some of the recommended approaches are clearly experimental, and further research will likely discover compounds, phytochemical or synthetic, that are not on this list that may be useful in a broad-spectrum approach. The prevalence of interactions where no interactions were found – over 20% for targets and over 30% for approaches – also suggests caution and a need for further research investigating potential cross-hallmark relationships as well as other mechanisms that may lead to toxicities.
Bioavailability of the phytochemicals chosen will also be a concern for future studies. The need for development of better preclinical models for screening compounds and testing rationally designed combinatorial therapies composed of compounds from any source is also obvious, and should clearly be a first step in the development of the broad-spectrum approach.
4.3 Role of integrative therapies in the broad-spectrum approach
Integrative medicine is an approach to health and healing that “makes use of all appropriate therapeutic approaches, healthcare professionals and disciplines to achieve optimal health and healing” [149]. A comprehensive integrative medicine intervention for cancer patients typically includes nutrition education, mind-body medicine and physical activity components, as well as dietary supplements including herbs, nutraceuticals and phytochemicals [3,150]. Such an intervention may contribute uniquely to a broad-spectrum therapeutic approach through its impact on a wide variety of relevant molecular targets and hallmarks. Hallmarks that may be particularly impacted include genomic instability, tumor-promoting inflammation, dysregulated metabolism and immune system evasion. Because of their susceptibility to manipulation by diet, exercise and supplementation, these may be characterized as metabolic hallmarks.
Nutrition has long been the primary focus of research on integrative interventions for cancer. The World Cancer Research Fund and the American Institute for Cancer Research find that diets high in fruits and vegetables substantially reduce risks of several cancers [151]. Cancer prevention diets are also suitable after a cancer diagnosis [152]. For example, colon cancer patients eating a Western diet after diagnosis were at higher risk for recurrence and mortality than those with healthy diets [153]. Breast cancer patients who followed low-fat diets were found to have lost weight and had lower recurrence risks, especially among patients with estrogen receptor-negative cancers [154]. Trials of diets enriched in whole grains, low-glycemic diets, and both low-fat diets and Mediterranean diets enriched in olive oil and almonds reduced levels of inflammation as measured by CRP [155–158]. Low fat diets, weight loss and supplements (anthocyanins and fish oil) have been observed in randomized trials to reduce cytokines and signaling molecules [159–162]. Mind-body interventions have emphasized immune targets, with findings of interventional trials including activation of T cells and lymphokine-activated killer cells and increased natural killer cell activity [163,164]. Exercise interventions have documented effects on survival, IGF-1, natural killer cell activity, and sex hormones [165–168]. While much work remains to be done on integrative interventions, preliminary data suggest that integrative medicine may significantly support a broad-spectrum approach to cancer therapy.
5 Proposed research model
The review process for this project has revealed many potential targets and approaches. The cross-validation activity suggests that only a small number of targets and approaches affect other hallmarks in contrary or controversial ways. Indeed the results suggest that the design of a broad-spectrum approach should in fact be feasible from a safety standpoint. Although considerable research will be needed, disease relapse is a substantial and longstanding problem, so this novel model definitely warrants further investigation.
5.3 In vitro research
An array of in vitro models is available for preliminary study of broad-spectrum formulas. One question is the suitability of receptor-based assays versus cell-based assays. While receptor-based assays may seem more suitable for targeted therapy research, examining the impacts of a putative agent on a molecule such as NF-κB, which is at the intersection of multiple signaling pathways related to inflammation, might be advised. Cultivated cell lines are valuable for preliminary screening of mixtures, but are, in most respects, limited in their predictive ability. Isolated cell lines from clinical samples are an alternative, and use of transformed cancer cells versus non-transformed lines should be discussed. Tissue and organ explants are another useful in vitro model.
Basic research on the properties of the natural product and other approaches selected in the reviews needs to continue. The pharmacology of mixtures and combinations of phytochemicals, bioavailability, dose optimization and synergy are among the areas in which research is needed for many phytochemicals [169,170]. However, multicomponent herbal therapies used in traditional and alternative medicine have not received detailed analysis. Network pharmacology could be a means of exploring these presumed synergisms, and efforts are being made to apply this approach to the complex herbal mixtures used in traditional Chinese medicine [171]. Studies on the pharmacokinetics of herbal extracts and phytochemicals, which often begin at the in vitro level, are also needed [172].
In sum, given the complexity that is immediately suggested when combinations of approaches are possible, we strongly recommend that well-coordinated, multi-faceted programs be pursued initially to ensure that the constituent approaches that are selected are well-characterized using in vitro models, and that delivery methods that are selected for in vivo work receive careful evaluation before animal research is undertaken.
5.4 In vivo research
Multiple in vivo models for further study of broad-spectrum approaches are also available. Two obvious choices are animal tumor models and human tumor xenografts implanted in athymic mice. While human tumor xenografts have the advantage in predicting effects of agents on human cancer cells, animal tumors offer some interesting choices for chemoprevention studies, since several are induced by exposure to various chemicals. The rodent tumors are questionable, however, in their ability to predict human responses to antitumor therapy. Differences in immunity are one consideration, most obviously with athymic mice but also with other animals. Many other differences are known. Rodents and humans, for instance, differ significantly in their blood levels of soy isoflavones after these are administered through a variety of dietary and experimental routes [173]. Isoflavone levels in rodent blood 20 to 150 times those in humans after similar oral intake have been observed, raising questions about the suitability of animals for prediction of phytochemical effects in humans.
Additionally, as shown in different preclinical mouse models, immune and inflammatory responses to cancer differ in young and old individuals, and many cancer treatments are likely to be less effective at older ages. Combination treatment including immunotherapeutic approaches may be most suitable for older animals. Therefore, there is a strong argument for testing and optimizing combination treatments in suitable model systems before attempting to apply them to cancer patients. The US National Cancer Institute Mouse Models of Human Cancer Consortium [174] has tried to provide the scientific community with accurate, reproducible models of human cancers that can be used in translational and preclinical studies. Such improved models could be of great importance for developing combination treatment strategies. Companion animals, such as dogs and cats, which experience several tumors analogous to human cancers, can also act as comparative models for human tumors. [175].
5.5 Clinical trials
Keeping in mind that a broad-spectrum approach may be used not only by itself, but also as adjuvant therapy with conventional agents, there are numerous potential settings for clinical trials, either for proof of principle or therapeutic goals. Preliminary studies could include metabolomic studies to identify metabolites of dietary interventions, or the pharmacokinetics and pharmacodynamics of phytochemical agents. A variety of settings can be contemplated for clinical trials. One period during which a broad-spectrum approach may be particularly appropriate is the perioperative period. Murine data demonstrate that tumor growth accelerates after surgery; there are also numerous anecdotal reports regarding cancer patients in whom rapid growth of metastatic tumors has been noted after surgery [176–181]. Further, there is reasonable human evidence that colon or rectal resection results in significant increases in the plasma levels of numerous proangiogenic proteins after surgery [182–185]. This period is not generally used for chemotherapy administration because of fears of impaired wound healing, but the above findings provide the rationale and motivation for systemically administering selected anticancer agents perioperatively.
Several non-standard chemotherapy agents, including phytochemicals, have been administered perioperatively in small studies. [186–188]. These agents upregulate immune function via nonspecific mechanisms. A Phase I trial assessing the combination of EGCG and silibinin in colorectal cancer is underway, with both agents given orally before and after surgery. [189–191]. Such trials represent an innovative approach to clinical assessment of natural products that can be carried out within a restricted time.
Although clinical trials of phytochemicals and plant extracts in cancer are limited compared to those with conventional chemotherapy, they are by no means lacking. Russo et al. [58] review nearly 50 ongoing and completed trials of phytochemicals and extracts in cancer prevention and therapy, noting that even though clinical research is still limited, preliminary results are promising. Most of the 50 studies took place in the United States, and most included a single phytochemical or single-herb extract. Nearly 3000 controlled trials of Chinese traditional medicine, 90% concerning herbals, were reviewed by Li et al. [192]. Only 16% of traditional medicine trials in this review reported use of adequate methods of randomization, and only a very small percentage reported study blinding, although quality of studies improved through time. Most Chinese herbal formulas contain multiple herbs and are aimed at many targets.
The design and execution of clinical trials of natural chemicals from plants and foods, however, has been challenging worldwide. An herbal products extension of the Consolidated Standards of Reporting Trials (CONSORT) randomized trial reporting guideline has been published to help improve herbal trial reporting [193]. A review of published studies of Panax ginseng, which is common in Chinese formulas but has been studied globally for many conditions, found that only 48% of them reported CONSORT-suggested items, and only 39% reported items from the herbal products extension [194], although these study designs also improved over time.
5.6 Translational considerations
Assuming that translational research work will involve a substantial combination of therapeutic agents such as those proposed in Table 2 as a starting point, a first step would be the selection of specific targets and approaches for preliminary study. To achieve a truly broad-spectrum effect, one strategy might be to use small doses of every approach that lacks significant contrary interferences. While such a mixture might be made up and applied to cell lines, it could be questioned whether the concentrations that could be achieved in the cells would be physiologically relevant, especially given the low bioavailability of many phytochemicals. Most in vitro work on single phytochemicals, however, has actually been conducted at high concentrations that are not achievable in humans. The pharmacokinetics and pharmacodynamics of phytochemicals are complex and many are not yet well known, although progress is being made on some agents [195]. Another method to narrow the number of phytochemicals that need to be in an agent might be to select the phytochemicals that are most widely represented across hallmarks, such as curcumin and resveratrol, and analyze combinations of these agents. Some of the selected approaches, e.g. silibinin, appear to have favorable pharmacokinetics [196]. Other phytochemicals with favorable pharmacokinetics could also be considered for inclusion in a broad-spectrum agent, such as phenethyl isothiocyanate [197]. Research is also urgently needed on the question of the stability of phytochemicals as well as synthetic compounds in mixtures.
Alternative approaches to the question of bioavailability are being explored, especially with the polyphenols. One of the main issues with these compounds, which include quercetin, green tea catechins, curcumin and others, is ensuring that circulating doses of aglycones (one of the active forms of these molecules), are sufficient for activity. After oral supplementation of foodgrade molecules at doses safe for humans (200–500 mg/day), only conjugated forms are found in the bloodstream. As an example, quercetin is not found in the plasma as aglycone or as the parent glycosides: at the doses usually employed in intervention studies, it would be found exclusively as methyl, sulfate or glucuronic acid conjugates [198]. This observation discloses a paradox common to many biologically active phytochemicals: if free aglycones are absent in vivo after a dietary intake or supplementation with high doses, how can we explain the high biological activity of these molecules, largely described in vitro?
Two main hypotheses can be considered. First, conjugated forms of some flavonoids (e.g. quercetin) may be biologically active. Second, after cellular uptake, these metabolites may be de-conjugated, regenerating the free aglycones. To sustain these hypotheses, key issues need to be addressed, such as the efficacy of mechanisms of uptake of polyphenol metabolites and the substrate specificity of each metabolite, which is largely unknown. The use of pure compounds tested in vitro may shed light on these questions. Alternatively, pharmacological doses (2–4 g/day) administered orally [199] may saturate the metabolic pathways of conjugation [200]. Efforts are being made, however, to improve bioavailability of these agents, such as microspheres [201], liposomes [202] and nanoparticles [203]. An additional complication is that individuals may vary in their absorption, distribution, metabolism and elimination of phytochemicals, based in some instances on genetic variability [204], dietary habits [205] and potentially on intestinal microbiota [206].
Considerations of quality control are essential along the spectrum of research from in vitro studies to clinical trials. Good agricultural practice, correct botanical identification and good manufacturing practice are mandatory to prevent adulteration, contamination and toxicity [207]. The example of PC-SPES, a botanical cancer remedy that was found to contain indomethacin, warfarin and synthetic estrogens, leading to its withdrawal from the market in 2002 resulted in greater awareness of the need for a strict approach to quality control [208].
6 Implementation of broad-spectrum research agenda
A variety of practical considerations come into play in translating the proposed research model into a developmental program. These include regulatory considerations, intellectual property, clinical considerations and funding.
6.3 Regulatory considerations
Research on the broad-spectrum model must be undertaken with regulatory constraints in mind. Laws controlling herbal medicines, which would likely apply to the broad-spectrum approach, typically have regulatory paths for herbal or traditional medicine products that differ from those for prescription drugs. Regulations relevant to traditional Chinese herbal medicines, perhaps the closest model for the proposed broad-spectrum approach, are reviewed by Fan et al. [209]. A few examples of national regulations regarding herbal medicines, traditional medicines and natural product drugs follow.
The United States has perhaps the most challenging regulations for drug approval, and regulations for mixtures are particularly complex. Some multicomponent formulas, have nevertheless been tested in clinical trials in the US [210,211], but are still being sold only as dietary supplements, without labeling for use in malignancy. The designation of the Botanical Drugs category may offer opportunities to broad-spectrum agents. A recent court decision declaring natural products unpatentable under US law adds an interesting wrinkle to the regulatory framework [212]. In Canada, development as a high-risk Natural Health Product could be considered [213]. China has a variety of regulatory categories that could be used for multicomponent natural product therapeutics [214]. The relevance of Chinese regulations for multi-targeted drugs has been explored [215]. In the European Union, the Marketing Authorization scheme for conventional drugs would need to be used, rather than the Traditional Herbal Regulation Scheme [216], increasing the challenge for developmental research. In India it is likely that New Chemical Entity approval would be required [217], since use in cancer would likely be considered beyond traditional herbal medicine usage. Japan allows herbal medicines to be registered as prescription or over-the-counter drugs [209]; prescription licensing appears likely for an anticancer therapeutic. A variety of regulations exist in other countries, which are beyond the scope of this paper, and which would need to be explored individually. We expect that working under these strict regulations will be difficult, but we do not see it as impossible.
An additional regulatory consideration is the acceptability of the broad-spectrum approach to institutionally-based ethical review boards needed for clinical research. In institutions located in countries in which multi-component herbal formulas are typical of traditional medicine, ethical approval of such formulas is common, as suggested by the large numbers of clinical studies on traditional Chinese herbal medicine [192] and Japanese Kampo medicine [218]. Trials with multi-component formulas and natural products have been conducted under other regulatory schemes as well. For instance, Phase I and Phase Ib studies of BZL101, an extract of Scutellaria barbata in metastatic breast cancer have been conducted in the United States [219,220]. A 4-herb combination originating in traditional Chinese medicine, PHY906, has been the subject of a Phase I trial as an adjunct to capecitabine in advanced pancreatic cancer, also in the United States [221]. In general, provision of sufficient preclinical and drug formulation information, review of prior clinical studies, and possession of appropriate approvals from national-level agencies will facilitate approval of study protocols.
6.4 Intellectual property
Herbs and natural products in their native forms do not have intellectual property protection, which should help in developing a low-cost, broad-spectrum formulation. Specified extracts and individual phytochemicals may have intellectual property of various types. Researchers could pursue intellectual property protection for specific broad-spectrum therapeutics they develop, as well as licensing to a pharmaceutical company with sufficient resources to support development and testing of the agent. Herbal extracts of some complexity have received patent or trademark status, and have been granted drug approval even in the United States, Examples include a mixture of green tea polyphenols known as Polyphenon E and sold as a patented drug sinecatechins for genital warts [222], and crofelemer, an extract from the South American plant Croton lechleri, approved for HIV-induced diarrhea [223]. The complexities of natural product patenting are beyond the scope of this paper but are covered in depth elsewhere [224].
6.5 Clinical considerations for a multi-component natural product therapeutic
Based on current clinical experience with natural products administered together with conventional drugs, one may anticipate potential concerns with broad-spectrum therapeutics that would be administered jointly with conventional therapies. A primary concern is the interactions between drugs and herbs or phytochemicals, including both pharmacokinetic and pharmacodynamic interactions [225]. This has been of special concern in oncology due to the life-threatening consequences of lowered blood levels of drugs, and the potential for severe side effects when blood levels of a drug are increased or actions of herbal products reinforce those of conventional agents. Antiplatelet activity is common in natural products [226], and may aggravate clinical consequences in patients with thrombocytopenia due to chemotherapy or other drugs [227]. Several other examples of negative interactions are known or suspected. St John’s wort (used for depression), contains the strong cytochrome P450 3A4 inducer hyperforin, which is known to reduce blood levels of many drugs, including irinotecan [228]. Green tea, which is often taken in high doses by cancer patients, has potential interactions with sunitinib [229], with hepatotoxic drugs [230], and with bortezomib. On the other hand, positive interactions have been observed with green tea and erlotinib, a combination now in clinical trials [231]. Curcumin is one of several natural products that act as chemosensitizers and radiosensitizers for several tumors, while protecting normal tissues [232]. The ability of herbs and other natural products to relieve treatment-related side effects should not be overlooked [233,234].
Furthermore, many natural products possess antioxidant activity. The role of oxidation in cancer progression and treatment is controversial [235]. Oxidative stress is increased in late-stage disease [236], which suggests that suppression would be beneficial. Antioxidants may relieve some adverse treatment effects caused by the reactive oxygen species generated by many chemotherapy drugs, but data on this point are not conclusive [237,238]. Randomized trials of antioxidant supplements given with chemotherapy do not find evidence of reduced efficacy, but research with better study design and larger sample size should be conducted [239]. Additionally, some natural antioxidants, including the polyphenols, manifest pro-oxidant properties in cancer cells, due to interactions with metal ions, which contribute to anticancer effects [240]. This pro-oxidant effect has been hypothesized to underlie the broadly multi-targeted actions of polyphenols such as curcumin and EGCG [241]. However, activity of most chemotherapy drugs depends on generation of ROS which should not be abrogated. Additionally, some oxidative metabolites may act as signaling molecules with anticancer activity [242]. Further, intracellular antioxidants may contribute to drug resistance [243]. Our understanding of the interactions of antioxidants and cancer thus continues to develop [244]. Patients are often warned not to supplement with antioxidants during treatment.
6.6 Funding
Development of new clinical agents that could be approved by regulatory agencies is an expensive endeavor. A recent economic model of drug discovery and development in the United States used industry-appropriate assumptions to estimate that the fully capitalized cost of a typical new single-molecule drug developed is now approximately $1.8 billion, 63% of which is attributable to clinical development (Phase I–III studies) [245]. The details of such estimates are beyond the scope of this paper, but the financial challenges are clear. It is our contention that a multi-component broad-spectrum therapeutic approach is needed to complement and balance the current drug discovery paradigm, which focuses on narrowly scoped approaches and singular molecular targets, including targeted therapies, immunotherapy, “one mouse-one patient” avatars that identify personalized therapeutic regimens by implanting patients’ tumors into mice [246] and a variety of other approaches. Such an approach could be expensive to develop, and could face similar costs for trials and approval. However, a broad-spectrum approach could be aimed at wide applicability among many cancer types and subtypes. Thus, initial investment could be more easily recovered than is the case with narrowly-focused target therapies, since it would have utility across a large group of patients. Whether the development of the broad-spectrum approach should be carried forward by governments, for-profit pharmaceutical companies or even non-profit pharmaceutical companies is an open question.
6.7 Importance for low- and middle-income countries
The possibility that a broad-spectrum approach could be developed that is both effective and inexpensive is an important consideration, especially in low- and middle-income countries. One of the cost components of drug development is the cost of target identification and validation. However, in the Halifax Project the strategic list of targets that has been developed has been drawn from the open literature, so individual laboratories or nations that are interested in developing a multi-component therapeutic approach can use this information as a starting point (i.e., as a basis for rationally selecting an array of targets).
7 Summary and conclusions
In spite of the importance of targeted therapies now used in treatment and currently in development, it is clear that most cancers cannot be successfully addressed solely with single-target therapies. The history of cancer treatment has taught us the importance of drug resistance, stemming ultimately from genetic heterogeneity in cancers. Our therapeutic tool kit now includes a large array of cytotoxic chemotherapies, molecular target drugs, immunotherapies and hormonal therapies. A major paradigm in cancer research, in response to the advances in analysis of the cancer genome, is the development of increasingly targeted therapies. Examples illustrating the vigor of research and development in this area are several targeted therapies that have received approval in 2013–2014 by the US FDA, including ceritinib (anaplastic lymphoma kinase inhibitor), ramucirumab (VEGFR2 blocker), ibrutinib (Bruton’s tyrosine kinase inhibitor), trametinib (MEK inhibitor) and dabrafenib (B-Raf inhibitor) [245].
At the same time there is an increasing awareness of a need to develop a therapeutic approach to address the genetic heterogeneity within tumors. Even within this group of newly approved agents, the combination of trametinib and dabrafenib was approved for joint use in 2014, due to the rapid (6–7 months) development of resistance to the sole use of B-Raf inhibitors. The emergence of the concept of multiple hallmarks of cancer [27], the nine pathways of progression [3] the listing of 138 driver genes [6] and the recognition of the importance of network pharmacology [51] all attest to the importance of this issue. A recent review similarly suggests combining antiinflammatory and antioxidant treatment in long-term maintenance therapy of cancer [248]. It is the contention of the Halifax Project that a broad-spectrum approach to cancer prophylaxis and treatment (i.e., simultaneously attacking many targets) is a strategic and promising response to our increasing understanding of the significance of genetic heterogeneity.
Although current drugs have notably increased initial responsiveness to treatment in comparison to traditional approaches to chemotherapy, there remain situations in which a broad-spectrum approach could make real contributions. Some examples include use as follow-up to conventional treatment; for rare cancers; for patients who do not tolerate conventional treatment; for early-stage disease, when aggressive treatment should be avoided; and in hospice and palliative care. If significant interactions with treatments can be avoided, it might even be possible to use such approaches in conjunction with targeted therapies and other treatments.
What are the implications of this broad-spectrum strategy for current clinical practice? First, clinicians should realize that this paper presents a developmental research program, not clinical guidelines. Use of uninformed selections of phytochemical or botanical extracts in poorly-defined clinical situations is unlikely to deliver positive results. Further, as noted above, concerns with interactions of natural products with conventional treatments should be kept in mind. That said, lifestyle therapies appear to affect multiple molecular targets and to improve the health of cancer patients in a variety of ways, and integrative lifestyle modifications should be assessed as a health-promoting foundation for use of broad-spectrum therapeutics [3,150]. Clinical trials are now defining beneficial impacts of natural products [249]. The positive implications of dietary therapies for improvement of the metabolic hallmarks of inflammation, dysregulated metabolism, genomic instability and immune system evasion should be kept in mind [250,251]. Clinicians choosing to use natural product supplements should attend to product quality and be familiar with advances in the formulation of poorly absorbed polyphenols and other phytochemicals [201–203].
The development of the broad-spectrum approach is not without cost. A primary need is further development of preclinical models for testing of combinatorial therapies, including study of the stability, pharmacodynamics and pharmacokinetics of agents comprising multiple phytochemicals and other molecules. While some of the targets and approaches recommended in these reviews are well-known and have been the subject of multiple reviews, others are still only promising leads and may need much better characterization before being adopted as constituents in such an approach. For example, among approaches, curcumin, genistein, resveratrol and EGCG boast a wealth of fundamental research, whereas other approaches such as tripterine, oleanoic acid and withaferin A will require additional basic research. Targets are also in need of more basic research, especially in replicative immortality and in dysregulated metabolism, a field in which studies of relevant targets are just beginning. The approaches analyzed in these areas are similarly only in the most preliminary stages of research. All the hallmarks, however, include targets and approaches that need substantial basic research. Determining how many of the suggested targets should be included in a broad-spectrum approach is also a question that needs substantial research. Supporting these areas of basic research should be an initial goal of funding efforts.
The pharmacology of mixtures of natural products is another area in which basic research is most relevant to the goals of this project. There is certainly a body of research on complex mixtures of natural products [211,215,218,219,221]. A recent study suggested that EGCG lowers the concentration of curcumin needed to reduce proliferation and induce apoptosis in uterine leiomyosarcoma cells [252]. Traditional Chinese medicine formulas have also been subjected to extensive pharmacological testing [253,254]. However, much remains to be done in quantitative optimization of formulas as well as in selection of optimal natural product extracts or phytochemicals. And although this effort emphasized phytochemicals, it is also important and relevant to study defined botanical and food extracts. Standardized black raspberry extract, for instance, has produced positive results in human trials on apoptosis, angiogenesis and several specific targets selected in the project [255]. Aged garlic extract [256] increased immunity in advanced cancer patients, and lyophilized strawberries [257] improved premalignant esophageal lesions. Defined herbal extracts such as PHY 906 and BZL101 mentioned above have demonstrated preliminary clinical antitumor activity [220,221]. Stability and pharmacokinetic properties of complex mixtures are another critical research need, as are proper methods of quality control [258].
The development of complex natural product agents appears ripe for cross-disciplinary approaches as well as attention to the process of translational research. Natural products research, in fact, has long been nurtured most successfully in multidisciplinary and collaborative working groups [259], and the teams that authored the reviews in this special issue were notably interdisciplinary themselves. In view of the challenges as well as the unique opportunities this new concept entails, scientists wishing to take part in the development of broad-spectrum approaches to cancer would do well to commit themselves to a set of new attitudes and skills. Laboratories and grant proposals have achieved success typically based on highly focused exploration of a small intellectual niche. The broad-spectrum approach upends this paradigm. Building linkages with laboratories across campus, or even with the department down the hall, is not always encouraged in academic institutions. But this challenge is not insurmountable, and institutions and granting agencies have successfully mounted efforts that embrace, for instance, natural product development “from the field to the clinic” [260,261]. At the same time, integrative oncology centers globally employ broad-spectrum clinical approaches involving therapies ranging from natural products to meditation in the service of patient needs [262]. There is thus no need to start from absolute zero in building the cross-disciplinary alliances we project will be needed for this effort.
What will be needed is a core group of scientists willing to become advocates for this approach. Advocacy must take place within academic institutions, as institutional silos, perhaps reluctantly, open their doors to collaboration. Institutional review boards and grant offices may need education in the concept of the broad-spectrum approach. Advocacy must take place at higher levels as well. National funding agencies and charitable foundations that currently support cancer research need to heed these recommendations and shift quickly to embrace the rationale for this interdisciplinary team-based approach. Grant review committees may need to confront established interests promoting competing studies with more familiar narrow aims. Creativity in funding initial research efforts will be needed. International agencies interested in addressing the growth of cancer in low to middle income countries might be convinced that broad-spectrum approaches could result in lower-cost and often more culturally acceptable therapeutic tools for these areas.
Now is the time to begin the work of advocating for broad-spectrum therapeutic approaches in cancer. Scientists need to seize the opportunities provided by the unique information provided in this special issue to expand their acquaintance with this model - and perhaps with the scientists themselves who are already involved in this effort. Scientists and clinicians alike should become advocates to their institutions, to funding sources and to the wider public. This dimension of cancer biology and therapy has too much potential to allow it to languish. At the same time, clinical challenges mount, despite the emergence of new targeted therapies. We look forward to seeing concentrated energy and intellect focused on this new approach, and to seeing it yield significant therapeutic benefits in the future.
Supplementary Material
Acknowledgments
Amr Amin was funded by Terry Fox Foundation Grant # TF-13-20 and UAEU Program for Advanced Research (UPAR) # 31S118; Jack Arbiser was funded by NIH AR47901; Alexandra Arreola was funded by NIH NRSA Grant F31CA154080; Alla Arzumanyan was funded by NIH (NIAID) R01: Combination therapies for chronic HBV, liver disease, and cancer (AI076535); Work in the lab of AsfarS. Azmi is supported by NIH R21CA188818 as well as from SkyFoundation Inc. Michigan; Fabian Benencia was supported by NIH Grant R15 CA137499-01; Alan Bilsland was supported by the University of Glasgow, Beatson Oncology Centre Fund, CRUK (www.cancerresearchuk.org) grant C301/A14762; Amancio Carnero was supported by grants to from the Spanish Ministry of Economy and Competitivity, ISCIII (Fis: PI12/00137, RTICC: RD12/0036/0028) co-funded by FEDER from Regional Development European Funds (European Union), Consejeria de Ciencia e Innovacion (CTS-6844 and CTS-1848) and Consejeria de Salud of the Junta de Andalucia (PI-0135-2010 and PI-0306-2012). His work on this project has also been made possible thanks to the Grant PIE13/0004 co-funded by the ISCIII and FEDER funds; Stephanie C. Casey was supported by NIH grant F32CA177139; Mrinmay Chakrabarti was supported by the United Soybean Board; Rupesh Chaturvedi was supported by an NIH NCCAM grant (K01AT007324); Georgia Zhuo Chen was supported by an NIH NCI grant (R33 CA161873-02); Helen Chen acknowledges financial support from the Michael Cuccione Childhood Cancer Foundation Graduate Studentship; Sophie Chen acknowledges financial support from the Ovarian and Prostate Cancer Research Trust, UK; Yi Charlie Chen acknowledges financial support from the West Virginia Higher Education Policy Commission/Division of Science Research, his research was also supported by NIH grants (P20RR016477 and P20GM103434) from the National Institutes of Health awarded to the West Virginia IDeA Network of Biomedical Research Excellence; Maria Rosa Ciriolo was partially supported by the Italian Association for Cancer Research (AIRC) - grant #IG10636, and #15403; Helen M. Coley acknowledges financial support from the GRACE Charity, UK and the Breast Cancer Campaign, UK; Marisa Connell was supported by a Michael Cuccione Childhood Cancer Foundation Postdoctoral Fellowship; Sarah Crawford was supported by a research grant from Connecticut State University; Charlotta Dabrosin acknowledges financial support from the Swedish Research Council and the Swedish Research Society; Giovanna Damia gratefully acknowledges the generous contributions of The Italian Association for Cancer Research (IG14536 to G.D.); Santanu Dasgupta gratefully acknowledges the support of the University of Texas Health Science Centre at Tyler, Elsa U. Pardee Foundation; William K. Decker was supported in part by CPRIT, the Cancer Prevention and research Institute of Texas; Anna Mae E. Diehl was supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the NIH National Institute on Alcohol Abuse and Alcoholism (NIAAA), Gilead and Shire Pharmaceuticals; Q. Ping Dou was partially supported by NIH/NCI (1R01CA20009, 5R01CA127258-05 and R21CA184788), and NIH P30 CA22453 (to Karmanos Cancer Institute); Janice E. Drew was supported by the Scottish Government's Rural and Environment Science and Analytical Services Division; Eyad Elkord thanks the National Research Foundation, United Arab Emirates University and the Terry Fox Foundation for supporting research projects in his lab; Bassel El-Rayes was supported by Novartis Pharmaceutical, Aveo Pharmaceutical, Roche, Bristol Myers Squibb, Bayer Pharmaceutical, Pfizer, and Kyowa Kirin; Mark A. Feitelson was supported by NIH/NIAID grant AI076535; Dean W. Felsher was supported by NIH grants (R01CA170378, U54CA149145, and U54CA143907); Lynnette R Ferguson was financially supported by the Auckland Cancer Society and the Cancer Society of New Zealand; Gary L. Firestone was supported by NIH Public Service grant CA164095 awarded from the National Cancer Institute; Christian Frezza "would like to acknowledge funding from a Medical Research Council CCU-Programme Grant on cancer metabolism, and a unique applicant AICR project grant"; Mark M. Fuster was supported by NIH grant R01-HL107652; Alexandros G. Georgakilas was supported by an EU Marie Curie Reintegration Grant MC-CIG-303514, Greek National funds through the Operational Program ‘Educational and Lifelong Learning of the National Strategic Reference Framework (NSRF)-Research Funding Program THALES (Grant number MIS 379346) and COST Action CM1201 ‘Biomimetic Radical Chemistry’; Michelle F. Green was supported by a Duke University Molecular Cancer Biology T32 Training Grant; Brendan Grue was supported by a National Sciences Engineering and Research Council Undergraduate Student Research Award in Canada; Dorota Halicka was supported by by NIH NCI grant NCIRO1 28704; Petr Heneberg was supported by the Charles University in Prague projects UNCE 204015 and PRVOUK P31/2012, by the Czech Science Foundation projects 15-03834Y and P301/12/1686, by the Czech Health Research Council AZV project 15-32432A, and by the Internal Grant Agency of the Ministry of Health of the Czech Republic project NT13663-3/2012; Matthew D. Hirschey wishes to acknowledge Duke University Institutional Support, the Duke Pepper Older Americans Independence Center (OAIC) Program in Aging Research supported by the National Institute of Aging (P30AG028716-01) and NIH/NCI training grants to Duke University (T32-CA059365-19 and 5T32-CA059365); Lorne J. Hofseth was supported by NIH grants (1R01CA151304, 1R03CA1711326, and 1P01AT003961); Kanya Honoki was supported in part by the grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 24590493); Hsue-Yin Hsu was sup-ported in part by grants from the Ministry of Health and Welfare(CCMP101-RD-031 and CCMP102-RD-112) and Tzu-Chi University(61040055-10) of Taiwan; Lasse D. Jensen was supported by Svenska Sallskapet for Medicinsk Forskning, Gosta Fraenkels Stiftelse, Ak.e Wibergs Stiftelse, Ollie och Elof Ericssons Stiftelse, Linkopings Universitet and the Karolinska Institute, Sweden; Wen G. Jiang wishes to acknowledge the support by Cancer Research Wales, the Albert Hung Foundation, the Fong Family Foundation, and Welsh Government A4B scheme; Lee W. Jones was supported in part by grants from the NIH NCI; W Nicol Keith was supported by the University of Glasgow, Beatson Oncology Centre Fund, CRUK (www.cancerresearchuk.org) grant C301/A14762; Sid P. Kerkar was supported by the NIH Intramural Research Program; Rob J. Kulathinal was supported by the National Science Foundation, and the American Cancer Society; Byoung S. Kwon was supported in part by National Cancer Center (NCC-1310430-2) and National Research Foundation (NRF-2005-0093837); Anne Le was supported by Sol Goldman Pancreatic Cancer Research Fund Grant 80028595, a Lustgarten Fund Grant 90049125 and Grant NIHR21CA169757 (to Anne Le); Michael A. Lea was funded by the The Alma Toorock Memorial for Cancer Research; Ho-Young Lee This work was supported by grants from the National Research Foundation of Korea (NRF), the Ministry of Science, ICT & Future Planning (MSIP), Republic of Korea (Nos. 2011-0017639 and 2011-0030001) and by a NIH grant R01 CA100816; Liang-Tzung Lin was supported in part by a grant from the Ministry of Education of Taiwan (TMUTOP103005-4); Jason W. Locasale acknowledges support from NIH awards (CA168997 and AI110613) and the International Life Sciences Institute; Bal L. Lokeshwar was supported in part by United States’ Public Health Services Grants: NIH R01CA156776 and VA-BLR&D Merit Review Grant No. 5I01-BX001517-02; Valter D. Longo acknowledges support from NIH awards (P01AG034906 and R01AG020642) and from the V Foundation; Costas A. Lyssiotis was funded in part by the Pancreatic Cancer Action Network as a Pathway to Leadership Fellow and through a Dale F. Frey Breakthrough award from the Damon Runyon Cancer Research Foundation; Karen L. MacKenzie wishes to acknowledge the support from the Children's Cancer Institute Australia (affiliated with the University of New South Wales, Australia and the Sydney Children's Hospital Network); Maria Marino was supported by grant from University Roma Tre to M.M. (CLA2013), and by the Italian Association for Cancer Research (AIRC) - grant #Ig15221; Ander Matheu is funded by Carlos III Health Institute (AM: CP10/00539), Basque Foundation for Science (IKERBASQUE) and Marie Curie CIG grant (AM: 2012/712404); Christopher Maxwell was supported by funding from the Canadian Institutes of Health Research, in partnership with the Avon Foundation for Women (OBC-134038) and the Canadian Institutes of Health Research New Investigator Salary Award (MSH-136647); Eoin McDonnell received Duke University Institutional Support; Kapil Mehta was supported by Bayer Healthcare System G4T (Grants4Targets); Gregory A. Michelotti received support from NIH NIDDK, NIH NIAAA, and Shire Pharmaceuticals; Vinayak Muralidhar was supported by the Harvard-MIT Health Sciences and Technology Research Assistantship Award; Elena Niccolai was supported by the Italian Ministry of University and the University of Italy; Virginia R. Parslow gratefully acknowledges the financial support of the Auckland Cancer Society Research Centre (ACSRC); Graham Pawelec was supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) grant number 16SV5536K, and by the European Commission (FP7 259679 “IDEAL”); Peter L. Pedersen was supported by NIH Grant CA-10951; Brad Poore was supported by Sol Goldman Pancreatic Cancer Research Fund Grant 80028595, the Lustgarten Fund Grant 90049125, and Grant NIHR21CA169757 (to Anne Le); Satya Prakash was supported by a Canadian Institutes of Health Research grant (MOP 64308); LizziaRaffaghello was supported by an NIH grant (P01AG034906-01A1)and Cinque per Mille dell’IRPEF–Finanziamento della RicercaSanitaria; Jeffrey C. Rathmell was supported by an NIH grant (R01HL108006); Swapan K. Ray was supported by the United Soybean Board; Domenico Ribatti received funding from the European Union Seventh Framework Programme (FP7/2007– 2013) under grant agreement n°278570; Luigi Ricciardiello was supported by the AIRC Investigator Grants 10216 and 13837, and the European Community’s Seventh Framework Program FP7/2007–2013 under grant agreement 311876; Francis Rodier acknowledges the support of the Canadian Institute for Health Research (FR: MOP114962, MOP125857), Fonds de Recherche Québec Santé (FR: 22624), and the Terry Fox Research Institute (FR: 1030); Gian Luigi Russo contributed to this effort while participating in the Fulbright Research Scholar Program 2013–14; Isidro Sanchez-Garcia is partially supported by FEDER and by MICINN (SAF2012-32810), by NIH grant (R01 CA109335-04A1), by Junta de Castilla y León (BIO/SA06/13) and by the ARIMMORA project (FP7-ENV-2011, European Union Seventh Framework Program). Isidro Sanchez-Garcia's lab is also a member of the EuroSyStem and the DECIDE Network funded by the European Union under the FP7 program; Andrew J. Sanders wishes to acknowledge the support by Cancer Research Wales, the Albert Hung Foundation, the Fong Family Foundation, and Welsh Government A4B scheme; Neeraj K. Saxena was supported by grant funding from NIH NIDDK (K01DK077137, R03DK089130); Dipali Sharma was partially funded by NIH NCI grants (R01CA131294, R21 CA155686), the Avon Foundation and a Breast Cancer Research Foundation grant (90047965); Markus David Siegelin received funding from National Institute of Health, NINDS grant K08NS083732, and the 2013 AACR-National Brain Tumor Society Career Development Award for Translational Brain Tumor Research, Grant Number 13-20-23-SIEG; Neetu Singh was supported by funds from the Department of Science and Technology (SR/FT/LS-063/2008), New Delhi, India; Carl Smythe was supported by Yorkshire Cancer Research and The Wellcome Trust, UK; Carmela Spagnuolo was supported by funding from Project C.I.S.I.A., act n. 191/2009 from the Italian Ministry of Economy and Finance Project CAMPUS-QUARC, within program FESR Campania Region 2007/2013, objectives 2.1, 2.2; Diana M. Stafforini was supported by grants from the National Cancer Institute (5P01CA073992), IDEA Award W81XWH-12-1-0515 from the Department of Defense, and by the Huntsman Cancer Foundation; John Stagg was supported by the Canadian Institutes of Health Research; Pochi R. Subbarayan was supported by the University of Miami Clinical and Translational Science Institute (CTSI) Pilot Research Grant (CTSI-2013-P03) and SEEDS You Choose Awards; Phuoc T. Tran was funded by the DoD (W81XWH-11-1-0272 and W81XWH-13-1-0182), a Kimmel Translational Science Award (SKF-13-021), an ACS Scholar award (122688-RSG-12-196-01-TBG) and the NIH (R01CA166348); Kathryn E. Wellen receives funding from the National Cancer Institute, Pancreatic Cancer Action Network, Pew Charitable Trusts, American Diabetes Association, and Elsa U. Pardee Foundation; Huanjie Yang was partially supported by the Scientific Research Foundation for the Returned Oversea Scholars, State Education Ministry and Scientific and Technological Innovation Project, Harbin (2012RFLXS011); Paul Yaswen was supported by funding from the United States National Institutes of Health (ES019458) and the California Breast Cancer Research Program (17UB-8708); Clement Yedjou was supported by a grant from the National Institutes of Health (Grant # G1200MD007581), through the RCMI-Center for Environmental Health; Xin Yin was supported by NIH/National Heart, Lung, and Blood Institute Training Grant T32HL098062.; Jiyue Zhu was supported by NIH grant R01GM071725;, Massimo Zollo was supported by the EuropeanFP7-TuMIC HEALTH-F2-2008-201662, the Italian Association for Cancer research (AIRC) Grant IG # 11963 and the RegioneCampania L.R:N.5, the European National Funds PON01-02388/12007-2013.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Keith Block is an owner of the Block Center for Integrative Cancer Treatment and of North Shore Nutraceuticals; Charlotte Gyllenhaal is an employee of the Block Center for Integrative Cancer Treatment; Jack Arbiser is the inventor of US Patents involving derivatives of honokiol and NADPH oxidase inhibitors. He has also cofounded ABBY Therapeutics for the development of NADPH oxidase inhibitors; Penny Block is the Executive Director of the Block Center for Integrative Cancer Treatment and President of North Shore Nutraceuticals; Ralph J. DeBerardinis is a member of the scientific advisory boards for Peloton Therapeutics and Agios Pharmaceuticals; Anna Mae E. Diehl has grants from Shire-Research, Metabolon, and Gilead. She is also a consultant for Astrazeneca, Genentech, Japan Tobacco, and the NuSI Foundation; Byoung S. Kwon holds patents for methods regarding anti-CD 137 and adaptive CTL therapeutics; Valter D. Longo has an equity interest in L-Nutra, a company that develops medical food; Kapil Mehta is a scientific advisor to Lifecare Innovations, and holds India Patent 8.765.797, TG2 inhibitors and uses thereof; Michael P. Murphy holds intellectual property in mitochondrial therapies and has ownership shares in a company called Antipodean Pharmaceuticals Inc. which is trying to commercialize some of these compounds; Jeffrey C. Rathmell received indirect compensation from Novartis while working on this project; Luigi Ricciardiello received an unrestricted research grant from SLA Pharma AG, Switzerland.; John Stagg has a sponsored research agreement with Medimmune LLC and is on the scientific advisory board of Surface Oncology; Matthew G. Vander Heiden is a consultant, scientific advisory board member, and owns equity in Agios Pharmaceuticals
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Lyon, France: International Agency for Research on Cancer; [cited 17 July 2014]. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Available from http://globocan.iarc.fr. [Google Scholar]
- 2.Palumbo MO, Kavan P, Miller WH, Jr, Panasci L, Assouline S, Johnson N, et al. Systemic cancer therapy: achievements and challenges that lie ahead. Frontiers in Pharmacology. 2013;4:57. doi: 10.3389/fphar.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block KI. Life Over Cancer. New York: Bantam; 2009. p. 594. 2009. [Google Scholar]
- 4.Kruse V, Rottey S, De Backer O, Van Belle S, Cocquyt V, Denys H. PARP inhibitors in oncology: a new synthetic lethal approach to cancer therapy. Acta Clin Belg. 2011;66(1):2–9. doi: 10.2143/ACB.66.1.2062507. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Hafiz HA, Horwitz KB. Role of epigenetic modifications in luminal breast cancer. Epigenomics. 2015;17:1–16. doi: 10.2217/epi.15.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelstein B, Papadoupoulos N, Velculescu VE, Shou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niraula S, Seruga B, Ocana A, Shao T, Goldstein R, Tannock IF, et al. The price we pay for progress: a meta-analysis of harms of newly approved cancer drugs. J Clin Oncol. 2012;30(24):3012–3019. doi: 10.1200/JCO.2011.40.3824. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollebecque A, Massard C, De Baere T, Auger N, Lacroix L, Koubi-Pick V, et al. Molecular screening for cancer treatment optimization (MOSCATO 01): a prospective molecular triage trial – interim results. J Clin Oncol; Presented at 2013 ASCO Annual Meeting; Chicago IL. 2013. Abstr 2512. [Google Scholar]
- 10.Weiss GJ, Liang WS, Demeure MJ, Kiefer JA, Hostettter G, Izatt T, et al. A pilot study using next-generation sequencing in advanced cancers: feasibility and challenges. PLOS One. 2013;8(10):e76438. doi: 10.1371/journal.pone.0076438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anonymous [Internet] ESMO2014: Final survival analysis from the CLEOPATRA study in patients with HER-2 positive metastatic breast cancer: European Society for Medical Oncology; [Cited 3 October 2014]. Copyright 2014. Available from: http://www.esmo.org/Conferences/ESMO-2014-Congress/News-Articles/Final-Overall-Survival-Analysis-from-the-CLEOPATRA-Study-in-Patients-with-HER2-Positive-Metastatic-Breast-Cancer. [Google Scholar]
- 12.Kefford R, Ribas A, Hamid O, Robert C, Daud A, Wolchok JD, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol. 2014;32(suppl):5s. abstr 3005. [Google Scholar]
- 13.Ribas A, Tumeh PC. The future of cancer therapy: Selecting patients who respond to PD-1/L1 blockade. Clin Cancer Res. 2014;20(19):4982–4984. doi: 10.1158/1078-0432.CCR-14-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rees L, Weil A. Integrated medicine. BMJ. 2001;322(7279):119–120. doi: 10.1136/bmj.322.7279.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block KI, Gyllenhaal C. Chapter 6; Nutritional Interventions in Cancer. In: Abrams D, Weil A, editors. Integrative Oncology. New York: Oxford University Press; 2014. pp. 120–159. [Google Scholar]
- 17.Courtice MN, Lin S, Wang X. An updated review on asbestos and related diseases in China. Int J Occup Environ Health. 2012;18(3):247–253. doi: 10.1179/1077352512Z.00000000021. [DOI] [PubMed] [Google Scholar]
- 18.Iyoke CA, Ugwu GO, Ezugwu EC, Ezugwu FO, Lawani OL, Onyebuchi AK. Challenges associated with the management of gynecological cancers in a tertiary hospital in South East Nigeria. Int J Womens Health. 2014;6:123–130. doi: 10.2147/IJWH.S55797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciociola AA, Cohen LB, Kulkarni P. FDA-Related Matters Committee of the American College of Gastroenterology. How drugs are developed and approved by the FDA: current process and future directions. Am J Gastroenterol. 2014;109(5):620–623. doi: 10.1038/ajg.2013.407. [DOI] [PubMed] [Google Scholar]
- 20.Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439–4442. doi: 10.1182/blood-2013-03-490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaric GS, Sehgal C. The challenge of access to oncology drugs in Canada. Harvard Business Review. Available at: http://hbr.org/product/the-challenge-of-access-to-oncology-drugs-in-canad/an/909E20-PDF-ENG. [Google Scholar]
- 22.Zahreddine H, Borden KLB. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013;4:28. doi: 10.3389/fphar.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringash J, Au HJ, Siu LL, Shapiro JD, Jonker DJ, Zalcberg JR, et al. Quality of life in patients with K-RAS wild-type colorectal cancer: the CO.20 phase 3 randomized trial. Cancer. 2014;120(2):181–189. doi: 10.1002/cncr.28410. [DOI] [PubMed] [Google Scholar]
- 24.Jochems C, Tucker JA, Tsang KY, Madan RA, Dahut WL, Liewehr DJ, et al. A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. Cancer Immunol Immunother. 2014;63(4):407–418. doi: 10.1007/s00262-014-1524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kast RE, Boockvar JA, Brüning A, Cappello F, Chang WW, Cvek B, et al. A conceptually new treatment approach for relapsed glioblastoma: Coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care. Oncotarget. 2013;4:502–530. doi: 10.18632/oncotarget.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhoeff JJ, van Tellingen O, Claes A, Stalpers LJ, van Linde ME, Richel DJ, et al. Concerns about antiangiogenic treatment in patients with glioblastoma multiforme. BMC Cancer. 2009;9:444. doi: 10.1186/1471-2407-9-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komarova NL, Wodarz D. Drug resistance in cancer: principles of emergence and prevention. Proc Natl Acad Sci U S A. 2005;102(27):9714–9719. doi: 10.1073/pnas.0501870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quaranta V, Tyson DR. What lies beneath: looking beyond tumor genetics shows the complexity of signaling networks underlying drug sensitivity. Sci Signal. 2013;6(294):pe32. doi: 10.1126/scisignal.2004715. [DOI] [PubMed] [Google Scholar]
- 33.Ferarrelli LK. Focus issue: networking cancer treatment strategies. Sci Signal. 2013;6(294):eg5. doi: 10.1126/scisignal.2004723. [DOI] [PubMed] [Google Scholar]
- 34.Zhai B, Sun XY. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol. 2013;5(7):345–352. doi: 10.4254/wjh.v5.i7.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401–435. doi: 10.1007/978-3-0348-0837-8_16. [DOI] [PubMed] [Google Scholar]
- 36.Muqbil I, Bao GW, El-Kharraj R, Shah M, Mohammad RM, Sarkar FH, et al. Systems and network pharmacology approaches to cancer stem cells research and therapy. J Stem Cell Res Ther. 2012;(Suppl 7(5)):10413. doi: 10.4172/2157-7633.S7-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley DS, Rasooly R, Jacob RA, Kader AA, Mackey BE. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J Nutr. 2006;136(4):981–986. doi: 10.1093/jn/136.4.981. [DOI] [PubMed] [Google Scholar]
- 38.Peairs AT, Rankin JW. Inflammatory response to a high-fat, low-carbohydrate weight loss diet: effect of antioxidants. Obesity (Silver Spring) 2008;6(7):1573–1578. doi: 10.1038/oby.2008.252. [DOI] [PubMed] [Google Scholar]
- 39.Jolad SD, Lantz RC, Solyom AM, Chen GJ, Bates RB, Timmermann BN. Fresh organically grown ginger (Zingiber officinale): composition and effects on LPS-induced PGE2 production. Phytochemistry. 2004;65(13):1937–1954. doi: 10.1016/j.phytochem.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Block KI, Gyllenhaal C, Tripathy D, Freels S, Mead MN, Block PB, et al. Survival impact of integrative cancer care in advanced metastatic breast cancer. Breast J. 2009;15(4):357–366. doi: 10.1111/j.1524-4741.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 41.Block KI, Gyllenhaal C. Breast Cancer. In: Kohlstadt I, editor. Advancing Medicine with Food and Nutrients. 2nd. Boca Raton, FL: CRC Press, Taylor & Francis Group LLC; 2012. pp. 727–742. [Google Scholar]
- 42.Hanahan D. Rethinking the war on cancer. Lancet. 2014;383(9916):558–563. doi: 10.1016/S0140-6736(13)62226-6. [DOI] [PubMed] [Google Scholar]
- 43.McVeigh TP, Hughes LM, Miller N, Sheehan M, Keane M, Sweeney KJ, et al. The impact of Oncotype DX testing on breast cancer management and chemotherapy prescribing patterns in a tertiary referral centre. Eur J Cancer. 2014;50(16):2763–2770. doi: 10.1016/j.ejca.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marrone M, Stewart A, Dotson WD. Clinical utility of gene-expression profiling in women with early breast cancer: an overview of systematic reviews. Genet Med. 2014 Dec 4; doi: 10.1038/gim.2014.140. Online publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmeiser HH, Nortier JL, Singh R, da Costa GG, Sennesael J, Cassuto-Viguier E, et al. Exceptionally long-term persistence of DNA adducts formed by carcinogenic aristolochic acid I in renal tissue from patients with aristolochic acid nephropathy. Int J Cancer. 2014;135(2):502–507. doi: 10.1002/ijc.28681. [DOI] [PubMed] [Google Scholar]
- 46.Ermolaeva MA, Schumacher B. Systemic DNA damage responses: organismal adaptations to genome instability. Trends Genet. 2014;30(3):95–102. doi: 10.1016/j.tig.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding X, Zhang B, Pei Q, Pan J, Huang S, Yang Y, et al. Triptolide induces apoptotic cell death of human cholangiocarcinoma cells through inhibition of myeloid cell leukemia-1. BMC Cancer. 2014;14(1):271. doi: 10.1186/1471-2407-14-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Triptolide in the treatment of psoriasis and other immunemediated inflammatory diseases. Br J Clin Pharmacol. 2012;74(3):424–436. doi: 10.1111/j.1365-2125.2012.04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv QW, Zhang W, Shi Q, Zheng WJ, Li X, Chen H, et al. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann Rheum Dis. 2015;74(6):1078–1086. doi: 10.1136/annrheumdis-2013-204807. [DOI] [PubMed] [Google Scholar]
- 50.Bastos LF, Coelho MM. Drug repositioning: playing dirty to kill pain. CNS Drugs. 2014;28(1):45–61. doi: 10.1007/s40263-013-0128-0. [DOI] [PubMed] [Google Scholar]
- 51.Hu QN, Deng Z, Tu W, Yang X, Meng ZB, Deng ZX, Liu J. NP: Interactive visual network pharmacology of diseases, targets, and drugs. CPT Pharmacometrics Syst Pharmacol. 2014;3:e105. doi: 10.1038/psp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dudhatra GB, Mody SK, Awale MM, Patel HB, Modi CM, Kumar A, et al. A comprehensive review on pharmacotherapeutics of herbal bioenhancers. Scientific World Journal. 2012;2012:637953. doi: 10.1100/2012/637953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zanella F, Link W, Carnero A. Understanding FOXO, new views on old transcription factors. Curr Cancer Drug Targets. 2010;10(2):135–146. doi: 10.2174/156800910791054158. [DOI] [PubMed] [Google Scholar]
- 54.Feitelson MA, Lian Z, Liu J, Tufan NL, Pan J. Parallel epigenetic and genetic changes in hepatitis B virus associated hepatocellular carcinoma. Cancer Lett. 2006;239(1):10–20. doi: 10.1016/j.canlet.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Vinay DS, Kwon BS. 4-1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep. 2014;47(3):122–129. doi: 10.5483/BMBRep.2014.47.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Miranda Torrinhas RS, Santana R, Garcia T, Cury-Boaventura MF, Sales MM, Curi R, Waitzberg DL. Parenteral fish oil as a pharmacological agent to modulate post-operative immune response: a randomized, double-blind, and controlled clinical trial in patients with gastrointestinal cancer. Clin Nutr. 2013;32(4):503–510. doi: 10.1016/j.clnu.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Ramakrishnan R, Gabrilovich DI. Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol Immunother. 2013;62(3):405–410. doi: 10.1007/s00262-012-1390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russo M, Spagnulo C, Tedesco I, Russo GL. Phytochemicals in cancer prevention and therapy: truth or dare? Toxins. 2010;2:517–551. doi: 10.3390/toxins2040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aravindan S, Natarajan M, Herman TS, Awasthi V, Aravindan N. Molecular basis of 'hypoxic' breast cancer cell radio-sensitization: phytochemicals converge on radiation induced Rel signaling. Radiat Oncol. 2013;8:46. doi: 10.1186/1748-717X-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huq F, Yu JQ, Beale P, Chan C, Arzuman L, Nessa MU, et al. Combinations of platinums and selected phytochemicals as a means of overcoming resistance in ovarian cancer. Anticancer Res. 2014;34(1):541–545. [PubMed] [Google Scholar]
- 61.Chu ES, Sze SC, Cheung HP, Liu Q, Ng TB, Tong Y. An in vitro and in vivo investigation of the antimetastatic effects of a Chinese medicinal decoction, Erxian decoction, on human ovarian cancer models. Integr Cancer Ther. 2013;12(4):336–346. doi: 10.1177/1534735412464519. [DOI] [PubMed] [Google Scholar]
- 62.Liu X, Li Y, Zeng F, Huang Y, Zhou J, Wang Y, et al. Chan-Yu-Bao-Yuan-Tang, the water extract of a Chinese medicine prescription, induces s-phase arrest and mitochondria-mediated apoptosis in human lung adenocarcinoma cells. Integr Cancer Ther. 2012;11(4):337–353. doi: 10.1177/1534735410392579. [DOI] [PubMed] [Google Scholar]
- 63.Wu P, Dugoua JJ, Eyawo O, Mills EJ. Traditional Chinese Medicines in the treatment of hepatocellular cancers: a systematic review and meta-analysis. J Exp Clin Cancer Res. 2009;28:112. doi: 10.1186/1756-9966-28-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanai M, Otsuka Y, Otsuka K, Sato M, Nishimura T, Mori Y, et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol. 2013;71(6):1521–1530. doi: 10.1007/s00280-013-2151-8. [DOI] [PubMed] [Google Scholar]
- 65.Fritz H, Seely D, Flower G, Skidmore B, Fernandes R, Vadeboncoeur S, et al. Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS One. 2013;8(11):e81968. doi: 10.1371/journal.pone.0081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du M, Yang X, Hartman JA, Cooke PS, Doerge DR, Ju YH, et al. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in athymic nude mice. Carcinogenesis. 2012;33(4):895–901. doi: 10.1093/carcin/bgs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hasima N, Aggarwal BB. Cancer-linked targets modulated by curcumin. Int J Biochem Mol Biol. 2012;3(4):328–351. [PMC free article] [PubMed] [Google Scholar]
- 68.McCarty MF, Block KI. Multifocal angiostatic therapy: an update. Integr Cancer Ther. 2005;4(4):301–314. doi: 10.1177/1534735405282475. [DOI] [PubMed] [Google Scholar]
- 69.McCarty MF, Block KI. Toward a core nutraceutical program for cancer management. Integr Cancer Ther. 2006;5(2):150–171. doi: 10.1177/1534735406288443. [DOI] [PubMed] [Google Scholar]
- 70.Subbarayan PR, Sarkar M, Nathanson L, Doshi N, Lokeshwar BL, Ardalan B. in vitro global gene expression analyses support the ethnopharmacological use of Achyranthes aspera. Evid Based Complement Alternat Med. 2013;2013:471739. doi: 10.1155/2013/471739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deocaris CC, Widodo N, Wadhwa R, Kaul SC. Merger of ayurveda and tissue culture-based functional genomics: inspirations from systems biology. J Transl Med. 2008;6:14. doi: 10.1186/1479-5876-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dwivedi V, Anandan EM, Mony RS, Muraleedharan TS, Valiathan MS, Mutsuddi M, et al. In vivo effects of traditional Ayurvedic formulations in Drosophila melanogaster model relate with therapeutic applications. PLoS One. 2012;7(5):e37113. doi: 10.1371/journal.pone.0037113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee SC, Chan JY, Pervaiz S. Spontaneous and 5-fluorouracil-induced centrosome amplification lowers the threshold to resveratrol-evoked apoptosis in colon cancer cells. Cancer Lett. 2010;288:36–41. doi: 10.1016/j.canlet.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 74.Rusin M, Zajkowicz A, Butkiewicz D. Resveratrol induces senescence-like growth inhibition of U-2 OS cells associated with the instability of telomeric DNA and upregulation of BRCA1. Mech Aging Dev. 2009;130:528–537. doi: 10.1016/j.mad.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Ferguson L, Schlothauer R. The potential role of nutritional genomics tools in validating high health foods for cancer control: broccoli as example. Mol Nutr Food Res. 2012;56(1):126–146. doi: 10.1002/mnfr.201100507. [DOI] [PubMed] [Google Scholar]
- 76.Donkena KV, Yuan H, Young CY. Vitamin Bs, one carbon metabolism and prostate cancer. Mini Rev Med Chem. 2010;10:1385–1392. doi: 10.2174/138955710793564106. [DOI] [PubMed] [Google Scholar]
- 77.Hopkins MH, Owen J, Ahearn T, Fedirko V, Flanders WD, Jones DP, et al. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: a randomized, controlled clinical trial. Cancer Prev Res (Phila) 2011;4:1645–1654. doi: 10.1158/1940-6207.CAPR-11-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krishnan AV, Moreno J, Nonn L, Swami S, Peehl DM, Feldman D. Calcitriol as a chemopreventive and therapeutic agent in prostate cancer: role of anti-inflammatory activity. J Bone Miner Res. 2007;22(Suppl 2):V74–V80. doi: 10.1359/jbmr.07s213. [DOI] [PubMed] [Google Scholar]
- 79.Kristal A, Arnold K, Neuhouser M, Goodman P, Platz E, Albanes D, et al. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2010;172:566–577. doi: 10.1093/aje/kwq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharp L, Carsin AE, Cantwell MM, Anderson LA, Murray LJ, Group FS. Intakes of dietary folate and other B vitamins are associated with risks of esophageal adenocarcinoma, Barrett's esophagus, and reflux esophagitis. J Nutr. 2013;143:1966–1973. doi: 10.3945/jn.113.174664. [DOI] [PubMed] [Google Scholar]
- 81.Trejo-Solís C, Pedraza-Chaverrí J, Torres-Ramos M, Jiménez-Farfán D, Cruz Salgado A, et al. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid Based Complement Alternat Med. 2013;2013:705121. doi: 10.1155/2013/705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X-H, Ma J, Smith-Warner S, Lee J, Giovannucci E. Vitamin B6 and colorectal cancer: current evidence and future directions. World J Gastroenterol. 2013;19(7):1005–1010. doi: 10.3748/wjg.v19.i7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vicente-Duenas C, Perez-Caro M, Abollo-Jimenez F, Cobaleda C, Sanchez-Garcia I. Stem-cell driven cancer:"hands-off" regulation of cancer development. Cell Cycle. 2009;8:1314–1318. doi: 10.4161/cc.8.9.8217. [DOI] [PubMed] [Google Scholar]
- 84.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 85.Ji RC. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014;28:6–16. doi: 10.1016/j.canlet.2013.12.001. 346. [DOI] [PubMed] [Google Scholar]
- 86.Luo D, Wang Z, Wu J, Jiang C, Wu J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. Biomed Res Int. 2014;2014:409272. doi: 10.1155/2014/409272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohnishi K, Semi K, Yamada Y. Epigenetic regulation leading to induced pluripotency drives cancer development in vivo. Biochem Biophys Res Commun. 2014;455(1–2):10–15. doi: 10.1016/j.bbrc.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 88.Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell. 2014;54:716–727. doi: 10.1016/j.molcel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Costantini S, Colonna G, Castello G. A holistic approach to study the effects of natural antioxidants on inflammation and liver cancer. Cancer Treat Res. 2014;159:311–323. doi: 10.1007/978-3-642-38007-5_18. [DOI] [PubMed] [Google Scholar]
- 90.Pan MH, Chiou YS, Wang YJ, Ho CT, Lin JK. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011;2:101–110. doi: 10.1039/c0fo00174k. [DOI] [PubMed] [Google Scholar]
- 91.Thakur VS, Gupta K, Gupta S. The chemopreventive and chemotherapeutic potentials of tea polyphenols. Curr Pharm Biotechnol. 2012;13:191–199. doi: 10.2174/138920112798868584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Godbout R, Dryja TP, Squire J, Gallie BL, Phillips RA. Somatic inactivation of genes on chromosome 13 is a common event in retinoblastoma. Nature. 1983;304:451–453. doi: 10.1038/304451a0. [DOI] [PubMed] [Google Scholar]
- 94.Sage J, Straight AF. RB's original. CIN? Genes Dev. 2010;241:329–1333. doi: 10.1101/gad.1948010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trbusek M, Malcikova J. TP53 aberrations in chronic lymphocytic leukemia. Adv Exper Med Biol. 2013;792:109–131. doi: 10.1007/978-1-4614-8051-8_5. [DOI] [PubMed] [Google Scholar]
- 96.Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304–317. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Daniel FI, Cherubini K, Yurgel LS, de Figueiredo MA, Salum FG. The role of epigenetic transcription repression and DNA methyltransferases in cancer. Cancer. 2011;117:677–687. doi: 10.1002/cncr.25482. [DOI] [PubMed] [Google Scholar]
- 98.Liu Z, Xie, Jones W, Pavlovicz RE, Liu S, Yu J. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett. 2009;19:706–709. doi: 10.1016/j.bmcl.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 99.Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga, et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- 100.Nandakumar V, Vaid M, Katiyar SK. (−)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis. 2011;32:537–544. doi: 10.1093/carcin/bgq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Research. 2003;63:7563–7570. [PubMed] [Google Scholar]
- 102.Lee H, Zhang P, Herrmann A, Yang C, Xin H, Wang Z. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc Natl Acad Sci USA. 2012;109:7765–7769. doi: 10.1073/pnas.1205132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Du Toit A. Cell death: balance through a bivalent regulator. Nat Rev Mol Cell Biol. 2013;14:546–547. doi: 10.1038/nrm3637. [DOI] [PubMed] [Google Scholar]
- 104.Morin PJ. Drug resistance and the microenvironment: nature and nurture. Drug Resist Updat. 2003;6:169–172. doi: 10.1016/s1368-7646(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 105.Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46:308–316. doi: 10.1007/s12033-010-9321-2. [DOI] [PubMed] [Google Scholar]
- 106.Chin K, de Solorzano CO, Knowles D, Jones A, Chou W, Rodriguez EG, et al. In situ analyses of genome instability in breast cancer. Nat Genet. 2004;36:984–988. doi: 10.1038/ng1409. [DOI] [PubMed] [Google Scholar]
- 107.Artandi SE, DePinho RA. A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr Opin Genet Dev. 2000;10:39–46. doi: 10.1016/s0959-437x(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 108.Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Nat Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwarze SR, Fu VX, Desotelle JA, Kenowski ML, Jarrard DF. The identification of senescence-specific genes during the induction of senescence in prostate cancer cells. Neoplasia. 2005;7:816–823. doi: 10.1593/neo.05250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- 112.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 113.Hu J, Hwang SS, Liesa M, Gan B, Sahin E, Jaskelioff M, et al. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell. 2012;148:651–663. doi: 10.1016/j.cell.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bensinger SJ, Christofk HR. New aspects of the Warburg effect in cancer cell biology. Semin Cell Dev Biol. 2012;23:352–361. doi: 10.1016/j.semcdb.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 115.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thompson CB. Metabolic enzymes as oncogenes or tumor suppressors. N Engl J Med. 2009;360(8):813–815. doi: 10.1056/NEJMe0810213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30(30):3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- 118.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 119.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 121.Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33:335–351. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 123.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 124.Ohnishi S, Ma N, Thanan R, Pinlaor S, Hammam O, Murata M, et al. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid Med Cell Longev. 2013;2013:387014. doi: 10.1155/2013/387014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nature Reviews Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 126.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khatami M. Inflammation, aging, and cancer: tumoricidal versus tumorigenesis of immunity: a common denominator mapping chronic diseases. Cell Biochem Biophys. 2009;55:55–79. doi: 10.1007/s12013-009-9059-2. [DOI] [PubMed] [Google Scholar]
- 128.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 129.Cao Y. Antiangiogenic cancer therapy. Sem Cancer Biol. 2004;14:139–145. doi: 10.1016/j.semcancer.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 130.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Ann Rev Medicine. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 131.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290(2):H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 132.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang Y, Zhang Y, Cao Z, Ji H, Yang X, Iwamoto H, et al. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc Natl Acad Sci U S A. 2013;110(29):12018–12023. doi: 10.1073/pnas.1301331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chatterjee S, Bhattacharjee B. Use of natural molecules as anti-angiogenic inhibitors for vascular endothelial growth factor receptor. Bioinformation. 2012;8:1249–1254. doi: 10.6026/97320630081249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sporn MB. The war on cancer: a review. Ann N Y Acad Sci. 1997;833:137–146. doi: 10.1111/j.1749-6632.1997.tb48599.x. [DOI] [PubMed] [Google Scholar]
- 138.Guyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 139.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 140.Kenney PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468–3474. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;25:2559–2572. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Casey SC, Li Y, Fan AC, Felsher DW. Oncogene withdrawal engages the immune system to induce sustained cancer regression. J Immunother Cancer. 2014:2–24. doi: 10.1186/2051-1426-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 144.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weber JS, Hua FL, Spears L, Marty V, Kuniyoshi C, Celis E. A phase I trial of an HLA-A1 restricted MAGE-3 epitope peptide with incomplete Freund's adjuvant in patients with resected high-risk melanoma. J Immunother. 1999;22:431–440. doi: 10.1097/00002371-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 146.Patyar S, Joshi R, Byrav DS, Prakash A, Medhi B, Das BK. Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci. 2010;17(1):21. doi: 10.1186/1423-0127-17-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu J, Liu XS, Zhou SF, Wei MQ. Combination of immunotherapy with anerobic bacteria for immunogene therapy of solid tumors. Gene Ther Mol Biol. 2009;13:36–52. [Google Scholar]
- 148.Daviglus ML, Liu K, Pirzada A, Yan LL, Garside DB, Wang R, et al. Relationship of fruit and vegetable consumption in middle-aged men to Medicare expenditures in older age: the Chicago Western Electric Study. J Amer Dietetic Assoc. 2005;105:1735–1744. doi: 10.1016/j.jada.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 149.Academic Consortium for Integrative Medicine. Definition of Integrative Medicine. [updated November 5, 2013, cited November 11, 2013. Available from: http://www.imconsortium.org/about/home.html. [Google Scholar]
- 150.Block KI, Block PB, Gyllenhaal C. Integrative therapies in cancer: modulating a broad spectrum of targets for cancer management. Integr Cancer Ther. 2015;14(2):113–118. doi: 10.1177/1534735414567473. [DOI] [PubMed] [Google Scholar]
- 151.World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 152.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 153.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298(7):754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 154.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 155.Montonen J, Boeing H, Fritsche A, Schleicher E, Joost HG, Schulze MB, et al. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr. 2013;52(1):337–345. doi: 10.1007/s00394-012-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang CY, Noar K, Song X, Lampe JW. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142(2):369–374. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Davis NJ, Crandall JP, Gajavelli S, Berman JW, Tomuta N, Wylie-Rosett J, et al. Differential effects of low-carbohydrate and low-fat diets on inflammation and endothelial function in diabetes. J Diabetes Complications. 2011;25(6):371–376. doi: 10.1016/j.jdiacomp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 158.Urpi-Sarda M, Casas R, Chiva-Blanch G, Romero-Mamani ES, Valderas-Martínez P, Arranz S, Andres-Lacueva C, Llorach R, Medina-Remón A, Lamuela-Raventos RM, Estruch R. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomarkers related to atherosclerosis. Pharmacol Res. 2012;65(6):577–583. doi: 10.1016/j.phrs.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 159.Heymach JV, Shackleford TJ, Tran HT, Yoo SY, Do KA, Wergin M, et al. Effect of low-fat diets on plasma levels of NF-κB-regulated inflammatory cytokines and angiogenic factors in men with prostate cancer. Cancer Prev Res (Phila) 2011;4(10):1590–1598. doi: 10.1158/1940-6207.CAPR-10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pendyala S, Neff LM, Suárez-Fariñas M, Holt PR. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am J Clin Nutr. 2011;93(2):234–242. doi: 10.3945/ajcn.110.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Karlsen A, Retterstøl L, Laake P, Paur I, Bøhn SK, Sandvik L, et al. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. 2007;137(8):1951–1954. doi: 10.1093/jn/137.8.1951. [DOI] [PubMed] [Google Scholar]
- 162.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25(8):1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eremin O, Walker MB, Simpson E, Heys SD, Ah-See AK, Hutcheon AW, et al. Immuno-modulatory effects of relaxation training and guided imagery in women with locally advanced breast cancer undergoing multimodality therapy: a randomised controlled trial. Breast. 2009;18(1):17–25. doi: 10.1016/j.breast.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 164.Lutgendorf SK, Mullen-Houser E, Russell D, Degeest K, Jacobson G, Hart L, et al. Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach. Brain Behav Immun. 2010;24(8):1231–1240. doi: 10.1016/j.bbi.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 166.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: metaanalysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kruijsen-Jaarsma M, Révész D, Bierings MB, Buffart LM, Takken T. Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev. 2013;19:120–143. [PubMed] [Google Scholar]
- 168.Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28(9):1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jiang J1, Eliaz I, Sliva D. Synergistic and additive effects of modified citrus pectin with two polybotanical compounds, in the suppression of invasive behavior of human breast and prostate cancer cells. Integr Cancer Ther. 2013;12(2):145–152. doi: 10.1177/1534735412442369. [DOI] [PubMed] [Google Scholar]
- 170.Bishayee A, Thoppil RJ, Waghray A, Kruse JA, Novotny NA, Darvesh AS. Dietary phytochemicals in the chemoprevention and treatment of hepatocellular carcinoma: in vivo evidence, molecular targets, and clinical relevance. Curr Cancer Drug Targets. 2012;12(9):1191–1232. [PubMed] [Google Scholar]
- 171.Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2):110–120. doi: 10.1016/S1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- 172.Darvesh AS, Bishayee A. Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr Cancer. 2013;65(3):329–344. doi: 10.1080/01635581.2013.767367. [DOI] [PubMed] [Google Scholar]
- 173.Setchell KD, Brown NM, Zhao X, Lindley SL, Heubi JE, King EC, et al. Soy isoflavone phase II metabolism differs between rodents and humans: implications for the effect on breast cancer risk. Am J Clin Nutr. 2011 Nov;94(5):1284–1294. doi: 10.3945/ajcn.111.019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marks C. Mouse models of human cancer consortium (MMHCC) from NCI. Dis Model Mech. 2009;2(3–4):111. doi: 10.1242/dmm.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009;6(10):e1000161. doi: 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goshima H, Saji S, Furuta T, Taneumura H, Takao H, Kida H, Takahashi H. Experimental study on preventive effects of lung metatastases using LAK cells induced from various lymphocytes--special references to enhancement of lung metastasis after laparotomy stress. J Jap Surg Soc. 1989;90:1245–1250. [PubMed] [Google Scholar]
- 177.Allendorf JDF, Bessler M, Kayton ML, Oesterling SD, Treat MR, Nowygrod R, Whelan RL. Increased tumor establishment and growth after laparotomy vs laparoscopy in a murine model. Arch Surg. 1995;130:649–653. doi: 10.1001/archsurg.1995.01430060087016. [DOI] [PubMed] [Google Scholar]
- 178.Eggermont AM, Steller EP, Marquet RL, Jeekel J, Sugarbaker PH. Local regional promotion of tumor growth after abdominal surgery is dominant over immunotherapy with interleukin-2 and lymphokine activated killer cells. Cancer Detect Prev. 1988;12:421–429. [PubMed] [Google Scholar]
- 179.Peeters CF, de Waal RM, Wobbes T, Westphal JR, Ruers TJ. Outgrowth of human liver metastases after resection of the primary colorectal tumor: a shift in the balance between apoptosis and proliferation. Int J Cancer. 2006;119:1249–1253. doi: 10.1002/ijc.21928. [DOI] [PubMed] [Google Scholar]
- 180.Lange PH, Hekmat K, Bosl G, et al. Accelerated growth of testicular cancer after cytoreductive surgery. Cancer. 1980;45:1498–1506. doi: 10.1002/1097-0142(19800315)45:6<1498::aid-cncr2820450633>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 181.Crawford SE, Flores-Stadler EM, Huang L, Tan XD, Ranalli M, Mu Y, Gonzalez-Crussi F. Rapid growth of cutaneous metastases after surgical resection of thrombospondin-secreting small blue round cell tumor of childhood. Hum Pathol. 1998;29(10):1039–1044. doi: 10.1016/s0046-8177(98)90410-5. [DOI] [PubMed] [Google Scholar]
- 182.Shantha Kumara HM, Cabot JC, Yan X, Herath SA, Luchtefeld M, Kalady MF, et al. Minimally invasive colon resection is associated with a persistent increase in plasma PlGF levels following cancer resection. Surg Endosc. 2011;25(7):2153–2158. doi: 10.1007/s00464-010-1514-z. [DOI] [PubMed] [Google Scholar]
- 183.Shantha Kumara HM, Tohme ST, Herath SA, Yan X, Senagore AJ, Nasar A, et al. Plasma soluble vascular adhesion molecule-1 levels are persistently elevated during the first month after colorectal cancer resection. Surg Endosc. 2012;26(6):1759–1764. doi: 10.1007/s00464-011-2112-4. [DOI] [PubMed] [Google Scholar]
- 184.Kumara HM, Shantha, Feingold D, Kalady M, Dujovny N, Senagore A, Hyman N, et al. Colorectal resection is associated with persistent proangiogenic plasma protein changes: postoperative plasma stimulates in vitro endothelial cell growth, migration, and invasion. Ann Surg. 2009;249(6):973–977. doi: 10.1097/SLA.0b013e3181a6cd72. [DOI] [PubMed] [Google Scholar]
- 185.Shantha Kumara HM, Kirchoff D, Naffouje S, Grieco M, Herath SA, Dujovny N, et al. Plasma from the second and third weeks after open colorectal resection for cancer stimulates in vitro endothelial cell growth, migration, and invasion. Surg Endosc. 2012;26(3):790–795. doi: 10.1007/s00464-011-1953-1. [DOI] [PubMed] [Google Scholar]
- 186.Kim IY, Yan X, Tohme S, Ahmed A, Cordon-Cardo C, Shantha Kumara HM, et al. CpG ODN, Toll Like Receptor (TLR)-9 agonist, inhibits metastatic colon adenocarcinoma in a murine hepatic tumor model. J Surg Res. 2012;174(2):284–290. doi: 10.1016/j.jss.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 187.Carter JJ, Feingold DL, Oh A, Kirman I, Wildbrett P, Stapleton G, et al. Perioperative immunomodulation with Flt3 kinase ligand or a whole tumor cell vaccine is associated with a reduction in lung metastasis formation after laparotomy in mice. Surg Innov. 2006;13(1):41–47. doi: 10.1177/155335060601300107. [DOI] [PubMed] [Google Scholar]
- 188.Wildbrett P1, Oh A, Carter JJ, Schuster H, Bessler M, Jaboci CA, Whelan RL. Increased rates of pulmonary metastases following sham laparotomy compared to CO2 pneumoperitoneum and the inhibition of this effect with perioperative immunomodulation. Surgical Endosc. 2002;16(8):1162–1170. doi: 10.1007/s00464-001-8158-y. [DOI] [PubMed] [Google Scholar]
- 189.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol Epigallocatechin-3-gallate. Cancer Res. 2006;66(5):2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 190.Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008;269(2):352. doi: 10.1016/j.canlet.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yan X, Gardner TR, Grieco M, Herath SA, Jang JH, Kirchoff D, et al. Perioperative Polyphenon E- and siliphos-inhibited colorectal tumor growth and metastases without impairment of gastric or abdominal wound healing in mouse models. Surg Endosc. Surg Endosc. 2012;26(7):1856–1864. doi: 10.1007/s00464-011-2114-2. [DOI] [PubMed] [Google Scholar]
- 192.Li X, Yang G, Li X, Zhang Y, Yang J, Chang J, Sun X, et al. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in Chinese. PLoS One. 2013;8(4):e60338. doi: 10.1371/journal.pone.0060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C, et al. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144(5):364–367. doi: 10.7326/0003-4819-144-5-200603070-00013. [DOI] [PubMed] [Google Scholar]
- 194.Shergis JL, Zhang AL, Zhou W, Xue CC. Quality and risk of bias in Panax ginseng randomized controlled trials: a review. Am J Chin Med. 2013;41(2):231–252. doi: 10.1142/S0192415X13500171. [DOI] [PubMed] [Google Scholar]
- 195.Gescher A, Steward WP, Brown K. Resveratrol in the management of human cancer: how strong is the clinical evidence? Ann N Y Acad Sci. 2013;1290:12–20. doi: 10.1111/nyas.12205. [DOI] [PubMed] [Google Scholar]
- 196.Zhu HJ, Brinda BJ, Chavin KD, Bernstein HJ, Patrick KS, Markowitz JS. An assessment of pharmacokinetics and antioxidant activity of free silymarin flavonolignans in healthy volunteers a dose escalation study. Drug Metab Dispos. 2013;419(9):1679–1685. doi: 10.1124/dmd.113.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Morris ME, Dave RA. Pharmacokinetics and pharmacodynamics of phenethyl isothiocyanate: implications in breast cancer prevention. AAPS J. 2014;16(4):705–713. doi: 10.1208/s12248-014-9610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Russo GL, Russo M, Spagnuolo C, Tedesco I, Bilotto S, Iannitti R, Palumbo R. Quercetin: a pleiotropic kinase inhibitor against cancer. Cancer Treat Res. 2014;159:185–205. doi: 10.1007/978-3-642-38007-5_11. [DOI] [PubMed] [Google Scholar]
- 199.Lamson DW, Brignall MS. Antioxidants and cancer, part 3: quercetin. Altern Med Rev. 2000 Jun;5(3):196–208. [PubMed] [Google Scholar]
- 200.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2001;(8S Suppl):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 201.Pereira AG, Fajardo AR, Nocchi S, Nakamura CV, Rubira AF, Muniz EC. Starch-based microspheres for sustained-release of curcumin: preparation and cytotoxic effect on tumor cells. Carbohydr Polym. 2013;98(1):711–720. doi: 10.1016/j.carbpol.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 202.Ranjan AP, Mukerjee A, Helson L, Gupta R, Vishwanatha JK. Efficacy of liposomal curcumin in a human pancreatic tumor xenograft model: inhibition of tumor growth and angiogenesis. Anticancer Res. 2013;33(9):3603–3609. [PubMed] [Google Scholar]
- 203.Shehzad A, Ul-Islam M, Wahid F, Lee YS. Multifunctional polymeric nanocurcumin for cancer therapy. J Nanosci Nanotechnol. 2014;14(1):803–814. doi: 10.1166/jnn.2014.9103. [DOI] [PubMed] [Google Scholar]
- 204.Chen Y, Kuehl GE, Bigler J, Rimorin CF, Schwarz Y, Shen DD, et al. UGT1A6 polymorphism and salicylic acid glucuronidation following aspirin. Pharmacogenet Genomics. 2007;17(8):571–579. doi: 10.1097/01.fpc.0000236339.79916.07. [DOI] [PubMed] [Google Scholar]
- 205.Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr Rev. 2014 Jul;72(7):429–452. doi: 10.1111/nure.12114. [DOI] [PubMed] [Google Scholar]
- 206.Hanhineva K, Aura AM, Rogachev I, Matero S, Skov T, Aharoni A, et al. In vitro microbiotic fermentation causes an extensive metabolite turnover of rye bran phytochemicals. PLoS One. 2012;7(6):e39322. doi: 10.1371/journal.pone.0039322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.van Breemen RB, Fong HH, Farnsworth NR. Ensuring the safety of botanical dietary supplements. Am J Clin Nutr. 2008;87(2):509S–513S. doi: 10.1093/ajcn/87.2.509S. [DOI] [PubMed] [Google Scholar]
- 208.Sovak M, Seligson AL, Konas M, Hajduch M, Dolezal M, Machala M, Nagourney R. Herbal composition PC-SPES for management of prostate cancer: identification of active principles. J Natl Cancer Inst. 2002;94(17):1275–1281. doi: 10.1093/jnci/94.17.1275. [DOI] [PubMed] [Google Scholar]
- 209.Fan TP, Deal G, Koo HL, Rees D, Sun H, Chen S, et al. Future development of global regulations of Chinese herbal products. J Ethnopharmacol. 2012;140:568–586. doi: 10.1016/j.jep.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 210.Huang EC, Zhao Y, Chen G, Baek SJ, McEntee MF, Minkin S, et al. Zyflamend, a polyherbal mixture, down regulates class I and class II histone deacetylases and increases p21 levels in castrate-resistant prostate cancer cells. BMC Complement Altern Med. 2014;14:68. doi: 10.1186/1472-6882-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Capodice JL, Gorroochurn P, Cammack AS, Eric G, McKiernan JM, Benson MC, et al. Zyflamend in men with high-grade prostatic intraepithelial neoplasia: results of a phase I clinical trial. J Soc Integr Oncol. 2009;7(2):43–51. [PubMed] [Google Scholar]
- 212.Wong AY, Chan AW. Myriad and its implications for patent protection of isolated natural products in the United States. Chin Med. 2014;9:17. doi: 10.1186/1749-8546-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Health Canada. [Internet] Pathway for licensing Natural Health Products making Modern Health Claims. Ottawa (ON): Health Canada; No copyright. [updated 27 December 2012; cited 13 April 2014]. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodnatur/legislation/docs/modern-eng.php. [Google Scholar]
- 214.World Health Organization. Report of a WHO global survey. Geneva, Switzerland: World Health Organization; 2005. National policy on traditional medicine and regulation of herbal medicines. [Google Scholar]
- 215.Wang Y, Fan X, Qu H, Gao X, Cheng Y. Strategies and techniques for multi-component drug design from medicinal herbs and traditional Chinese medicine. Curr Top Med Chem. 2012;12(12):1356–1362. doi: 10.2174/156802612801319034. [DOI] [PubMed] [Google Scholar]
- 216.Medicines and Healthcare Products Regulatory Agency. [Internet] London: The Agency; C 2014. [cited 13 April 2014]. Permitted Indications under the Directive on Traditional Herbal Medicinal Products. Available from: http://www.mhra.gov.uk/home/groups/es-herbal/documents/websiteresources/con009363.pdf. [Google Scholar]
- 217.Sachan V, Kohli Y, Gautam R. Regulatory issues for herbal products – a review. [Internet] India: Greater Noida, U.P.; 2010. [cited July 8 2014]. Available from: http://www.scribd.com/doc/26680241/REGULATORY-ISSUES-FOR-HERBAL-PRODUCTS-A-REVIEW. [Google Scholar]
- 218.Gao JJ, Song PP, Qi FH, Kokudo N, Qu XJ, Tang W. Evidence-based research on traditional Japanese medicine, Kampo, in treatment of gastrointestinal cancer in Japan. Drug Discov Ther. 2012;6(1):1–8. [PubMed] [Google Scholar]
- 219.Rugo H, Stivelman E, Perez A, Vogel C, Franco S, Tan Chiu E, Melisko M, et al. Phase I trial and antitumor effects of BZL101 for patients with metastatic breast cancer. Breast Cancer Res Treat. 2007;(10591):17–28. doi: 10.1007/s10549-006-9430-6. [DOI] [PubMed] [Google Scholar]
- 220.Perez AT, Arun B, Tripathy D, Tagliaferri MA, Shaw HS, Kimmick GG, Cohen I, et al. A phase IB dose escalation trial of Scutellaria barbata (BZL 101) for patients with metastatic breast cancer. Breast Cancer Res Treat. 2010;120(1):111–118. doi: 10.1007/s10549-009-0678-5. [DOI] [PubMed] [Google Scholar]
- 221.Saif MW, Li J, Lamb L, Kaley K, Elligers K, Jiang Z, Bussom S, et al. First-in-human phase II trial of the botanical formulation PHY906 with capecitabine as second-line therapy in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2014;73(2):373–380. doi: 10.1007/s00280-013-2359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Meltzer SM, Monk BJ, Tewari KS. Green tea catechins for treatment of external genital warts. Am J Obstet Gynecol. 2009;200(3):233.e1–233.e7. doi: 10.1016/j.ajog.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 223.Tradtrantip L, Namkung W, Verkman AS. Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol. 2010;77(1):69–78. doi: 10.1124/mol.109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Krattiger A, Mahoney RT, Nelsen L, Thomson JA, Bennet AB, Satyanarayana K, et al., editors. Oxford, UK: Center for the Management of Intellectual Property, and Davis, US: Public Intellectual Property Resources for Agriculture; 2007. Intellectual Property Management in Health and Agricultural Innovation: A Handbook of Best Practices. [Google Scholar]
- 225.He SM, Yang AK, Li XT, Du YM, Zhou SF. Effects of herbal products on the metabolism and transport of anticancer agents. Expert Opin Drug Metab Toxicol. 2010;6(10):1195–1213. doi: 10.1517/17425255.2010.510132. [DOI] [PubMed] [Google Scholar]
- 226.Fuentes E, Palomo I. Relationship between platelet PPARs, cAMP Levels, and P-selectin expression: antiplatelet activity of natural products. Evid Based Complement Alternat Med. 2013;2013:861786. doi: 10.1155/2013/861786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mousa SA. Antithrombotic effects of naturally derived products on coagulation and platelet function. Methods Mol Biol. 2010;663:229–240. doi: 10.1007/978-1-60761-803-4_9. [DOI] [PubMed] [Google Scholar]
- 228.Madabushi R, Frank B, Drewelow B, Derendorf H, Butterweck V. Hyperforin in St. John's wort drug interactions. Eur J Clin Pharmacol. 2006;62(3):225–233. doi: 10.1007/s00228-006-0096-0. [DOI] [PubMed] [Google Scholar]
- 229.Ge J, Tan BX, Chen Y, Yang L, Peng XC, Li HZ, et al. Interaction of green tea polyphenol epigallocatechin-3-gallate with sunitinib: potential risk of diminished sunitinib bioavailability. J Mol Med (Berl) 2011;89(6):595–602. doi: 10.1007/s00109-011-0737-3. [DOI] [PubMed] [Google Scholar]
- 230.Lu Y, Sun J, Petrova K, Yang X, Greenhaw J, Salminen WF, et al. Metabolomics evaluation of the effects of green tea extract on acetaminophen-induced hepatotoxicity in mice. Food Chem Toxicol. 2013;62:707–721. doi: 10.1016/j.fct.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 231.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27(16):2712–2725. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62(7):919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 233.Rao S, Dinkar C, Vaishnav LK, Rao P, Rai MP, Fayad R, et al. The Indian spice turmeric delays and mitigates radiation-induced oral mucositis in patients undergoing treatment for head and neck cancer: an investigational study. Integr Cancer Ther. 2013;13(3):201–210. doi: 10.1177/1534735413503549. [DOI] [PubMed] [Google Scholar]
- 234.Barton DL, Liu H, Dakhil SR, Linquist B, Sloan JA, Nichols CR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst. 2013;105(16):1230–1238. doi: 10.1093/jnci/djt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Watson J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013;3(1):120144. doi: 10.1098/rsob.120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Panis C, Victorino VJ, Herrera AC, Freitas LF, De Rossi T, Campos FC, et al. Differential oxidative status and immune characterization of the early and advanced stages of human breast cancer. Breast Cancer Res Treat. 2012;133(3):881–888. doi: 10.1007/s10549-011-1851-1. [DOI] [PubMed] [Google Scholar]
- 237.Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials. Int J Cancer. 2008;123(6):1227–1239. doi: 10.1002/ijc.23754. [DOI] [PubMed] [Google Scholar]
- 238.Panis C, Herrera AC, Victorino VJ, Campos FC, Freitas LF, De Rossi T, et al. Oxidative stress and hematological profiles of advanced breast cancer patients subjected to paclitaxel or doxorubicin chemotherapy. Breast Cancer Res Treat. 2012;133(1):89–97. doi: 10.1007/s10549-011-1693-x. [DOI] [PubMed] [Google Scholar]
- 239.Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev. 2007;33(5):407–418. doi: 10.1016/j.ctrv.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 240.Ullah MF, Ahmad A, Khan HY, Zubair H, Sarkar FH, Hadi SM. The prooxidant action of dietary antioxidants leading to cellular DNA breakage and anticancer effects: implications for chemotherapeutic action against cancer. Cell Biochem Biophys. 2013;67(2):431–438. doi: 10.1007/s12013-011-9303-4. [DOI] [PubMed] [Google Scholar]
- 241.Azmi AS, Sarkar FH, Hadi SM. Pro-oxidant activity of dietary chemopreventive agents: an under-appreciated anti-cancer property. F1000Research. 2013;2:135. doi: 10.12688/f1000research.2-135.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Manello F, Tonti GA, Pagliarini S, Benedetti S, Canestrari F, Zhu W, et al. The 8-epimer of prostaglandin f(2 alpha), a marker of lipid peroxidation and oxidative stress, is decreased in the nipple aspirate fluid of women with breast cancer. Int J Cancer. 2007;120(9):1971–1976. doi: 10.1002/ijc.22522. [DOI] [PubMed] [Google Scholar]
- 243.Backos DS, Franklin CC, Reignan P. The role of glutathione in brain tumor drug resistance. Biochem Pharmacol. 2012;83:1005–1012. doi: 10.1016/j.bcp.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 244.Mencalha A, Victorino VJ, Cecchini R, Panis C. Mapping oxidative damage in breast cancer: understanding the basic to reach the clinics. Anticancer Res. 2014;34(3):1127–1140. [PubMed] [Google Scholar]
- 245.Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9(3):203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 246.Malaney P, Nicosia SV, Davé V. One mouse, one patient paradigm: New avatars of personalized cancer therapy. Cancer Lett. 2014;344(1):1–12. doi: 10.1016/j.canlet.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.U.S. Food and Drug Administration [Internet] Drug Approvals and Databases. Washington: The Administration; [updated April 30, 2014, cited May 6, 2014]. [about 2 screens]. Available from: http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm. [Google Scholar]
- 248.Crawford S. Anti-inflammatory/antioxidant use in long-term maintenance cancer therapy: a new therapeutic approach to disease progression and recurrence. Ther Adv Med Oncol. 2014;6(2):52068. doi: 10.1177/1758834014521111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Golombick T, Diamond TH, Manoharan A, Ramakrishna R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: a randomized, double-blind placebo-controlled crossover 4g study and an open-label 8g extension study. Am J Hematol. 2012;87(5):455–460. doi: 10.1002/ajh.23159. [DOI] [PubMed] [Google Scholar]
- 250.Shivappa N, Hébert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. 2015;113(4):665–671. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women’s Health Initiative. Cancer Causes Control. 2014;26(3):399–408. doi: 10.1007/s10552-014-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kondo A, Takeda T, Li B, Tsuiji K, Kitamura M, Wong TF, Yaegashi N. Epigallocatechin-3-gallate potentiates curcumin's ability to suppress uterine leiomyosarcoma cell growth and induce apoptosis. Int J Clin Oncol. 2013;18(3):380–388. doi: 10.1007/s10147-012-0387-7. [DOI] [PubMed] [Google Scholar]
- 253.Xiong F, Jiang M, Huang Z, Chen M, Chen K, Zhou J, et al. A novel herbal formula induces cell cycle arrest and apoptosis in association with suppressing the PI3K/AKT pathway in human lung cancer A549 cells. Integr Cancer Ther. 2014;13(2):152–160. doi: 10.1177/1534735413503544. [DOI] [PubMed] [Google Scholar]
- 254.Wang P, Chen Z, Meng ZQ, Luo JM, Lin JH, Zhou ZH, et al. Ski acts as therapeutic target of qingyihuaji formula in the treatment of SW1990 pancreatic cancer. Integr Cancer Ther. 2010;9(1):50–58. doi: 10.1177/1534735409359179. [DOI] [PubMed] [Google Scholar]
- 255.Stoner GD. Foodstuffs for preventing cancer; the preclinical and clinical development of berries. Cancer Prev Res (Phila) 2009;(293):187–194. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, et al. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J Nutr. 2006;136(3 Suppl):816S–820S. doi: 10.1093/jn/136.3.816S. [DOI] [PubMed] [Google Scholar]
- 257.Chen T, Yan F, Qian J, Guo M, Zhang H, Tang X, et al. Randomized phase II trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev Res (Phila) 2012;5(1):41–50. doi: 10.1158/1940-6207.CAPR-11-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Xu JD, Mao Q, Shen H, Zhu LY, Li SL, Yan R. Ultra-high performance liquid chromatography coupled with photo-diode array and quadrupole/time-of-flight mass spectrometry based chemical profiling approach to evaluate the influence of preparation methods on the holistic quality of Qiong-Yu-Gao, a traditional complex herbal medicine. J Chromatogr A. 2013;1304:154–168. doi: 10.1016/j.chroma.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 259.Kinghorn AD, Carcache de Blanco EJ, Chai HB, Orjala J, Farnsworth NR, Soejarto DD, et al. Discovery of anticancer agents of diverse natural origin. Pure Appl Chem. 2009;81(6):1051–1063. doi: 10.1351/PAC-CON-08-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Farnsworth NR, Mahady GB. Research highlights from the UIC/NIH Center for Botanical Dietary Supplements Research for Women's Health: Black cohosh from the field to the clinic. Pharm Biol. 2009;47(8):755–760. doi: 10.1080/13880200902988637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Weaver CM, Barnes S, Wyss JM, Kim H, Morré DM, Morré DJ, et al. Research Highlights from the Purdue-UAB Botanicals Research Center for Age Related Diseases. Pharm Biol. 2009;47(8):768–773. doi: 10.1080/13880200902988603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cramer H, Cohen L, Dobos G, Witt CM. Integrative oncology: best of both worlds-theoretical, practical, and research issues. Evid Based Complement Alternat Med. 2013;2013:383142. doi: 10.1155/2013/383142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.