Abstract
Paired receptors on NK cells recognize similar ligands with varied strength of binding ability and perform different functions. The CD300 molecules are emerging as novel immune regulators in health and disease due to their interaction with their lipid-nature ligands. Particularly, the paired receptors CD300c and CD300a have been shown to elicit activating and inhibitory capabilities, respectively. In the current study, we seek to investigate the expression and function of CD300c on human NK cells. We demonstrate that IL-2 and IL-15 treatment significantly induce CD300c expression exclusively on CD56bright NK cells. CD300c up-regulation requires STAT5 and its expression is inhibited by IL-4. Consistently, IL-2 secreted from activated CD4+ T cells specifically induces the expression of CD300c on CD56bright NK cells. Crosslinking CD300c with a specific antibody enhances the proficiency of CD56bright NK cells to degranulate and induce chemokine and cytokine secretion. We also show the differential binding of CD300a and CD300c to their ligands phosphatidylethanolamine (PE) and phosphatidylserine (PS) and their differential ability to affect CD56bright NK cell functions. Our results provide an insight into the novel set of paired receptors CD300a and CD300c that are distinctively expressed on CD56bright NK cells with varied effector functions.
Natural Killer (NK) cells are known for their pivotal role in the innate immune system; displaying natural cytotoxicity against tumor-transformed and virus-infected cells, as well as secreting immune-regulatory cytokines1,2,3. Their function is regulated by a multitude of both activating and inhibitory receptors4,5. Complex interactions of different cellular targets with ligands for both types of receptors determine NK cell inhibition (tolerance) or activation (missing self and stress-induced self). In addition, cytokines such as IL-12, IL-15, IL-18 and IL-1β secreted from monocytes, macrophages and dendritic cells (DC) are primary signals that activate NK cells6,7,8,9. In recent years, the importance of NK cell-mediated regulation of adaptive immune responses has also been explored in various scenarios, such as in NK-DC cross talk, the interaction with antigen presenting cells and also through the effect that they have in modulating T and B cell responses7,10,11,12,13,14. Moreover, it has been shown that stimulatory signals like IL-2 from the adaptive immune system (antigen-specific T cells) activate the CD56bright NK cell subset in secondary lymphoid organs and is able to modulate its effector functions15,16.
Human NK cells are phenotypically characterized by the expression of CD56 and lack of CD3 on their cell surface. Examining the surface density of CD56 expression, NK cells are divided into two distinct subsets, CD56bright and CD56dim. In the periphery, approximately 90% of human NK cells are CD56dim expressing high levels of CD16 (FcγRIII) and are predominantly cytotoxic in function. In contrast, only 5–10% of NK cells are CD56bright and CD16dim/neg with a predilection for secreting pro-inflammatory cytokines17,18,19,20. Similar to their varied differences in functions, these two subsets express a different array of receptors on their surface, which include activating and inhibitory receptors, adhesion molecules and chemokine receptors21,22,23. Some of these variations determine the homing of NK cells to different lymphoid tissues. For example, CD56bright NK cells home to the secondary lymphoid organs, where they comprise roughly 90% of the NK cell population15. Furthermore, CD56bright and CD56dim cells differ in their response to IL-2 for proliferation. CD56bright cells constitutively express high levels of both high and intermediate-affinity IL-2 receptors on their surface, which allow them to proliferate even under low concentrations of IL-224,25,26. Similar to IL-2, IL-15 also binds with high affinity to the hetero-trimeric receptor complexes, which consist of IL-2/15Rβ (CD122), the common chain (γc or CD132), and IL-15Rα9,15,27. The γc is the main component that transduces the signal via Janus tyrosine-kinase (JAK)-3 to phosphorylate further downstream signaling molecules like signal transducer and activator of transcription (STAT) molecules. This signaling is specific to each receptor complex. In this case, IL-2 and IL-15 mainly activate STAT5 to induce cellular functions such as activation, proliferation and also regulate the receptor repertoire of NK cells27,28.
The human CD300 family of receptors is a group of eight type-I membrane glycoproteins that harbor a single IgV-like extracellular domain and regulate a diverse array of immune processes. This family is clustered on chromosome 17. Seven members (CD300 a-h) are expressed on leukocytes29,30. The eighth member, CD300g, is found only on endothelial cells31. The human activating receptors, CD300b, CD300c, CD300d, CD300e and CD300h associate with different adaptor molecules such as FcεRIγ chain, DNAX-activating protein (DAP)-12 or DAP10 through their charged residues in the trans-membrane domain. In contrast, the human inhibitory receptors, CD300a and CD300f, elicit inhibitory signals via their immuno-receptor tyrosine-based inhibitory motifs (ITIMs) in the cytoplasmic tail29. The ligands for this family of receptor are mostly of lipid nature, including phosphatidylserine (PS) and phosphatidylethanolamine (PE), two amino-phospholipids that are expressed on the outer leaflet of the plasma membrane of dead and activated cells32,33. The highly homologous members CD300a and CD300c are considered as paired receptors with inhibitory and activating roles, respectively34. Although transcripts encoding these receptors were reported in cells from both myeloid and lymphoid lineages, the receptor expression of CD300a and CD300c are indistinguishable on the cell surface due to the lack of specific antibodies. However, recently we have shown that a specific antibody clone, TX45, recognizes CD300c but not CD300a35.
Previously, the role of CD300a on NK cells has been investigated to elicit signals to inhibit target cell killing by NK cells36,37. However, the expression and function of CD300c on NK cells has yet to be elucidated. Here, we show that expression of CD300c is induced uniquely on CD56bright NK cells upon treatment with either IL-2 or IL-15. In addition, we demonstrate that its expression is inhibited in the presence of IL-4 and regulated by the transcription factor STAT5. We also show that cross-linking of CD300c with a specific monoclonal antibody enhances effector functions (degranulation and cytokine secretion) of CD56bright NK cells. Finally, we demonstrate the differential binding of CD300a and CD300c to their ligands PE and PS, and reveal the distinct response of CD56bright NK cells after interacting with these ligands. Our results provide an insight into a novel set of paired receptors - CD300a and CD300c - that are expressed on CD56bright NK cells.
Results
CD300c is exclusively expressed on CD56bright NK cells
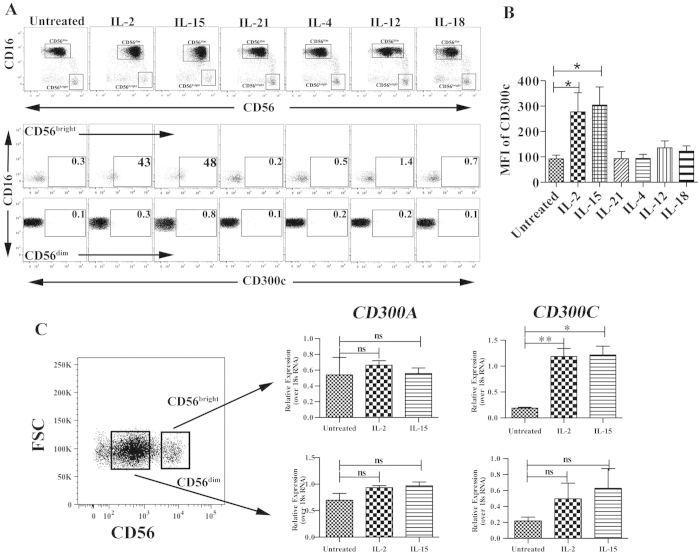
Recently, we demonstrated that CD300c is expressed on human monocytes using the specific monoclonal antibody clone TX4535. To assess the expression of CD300c on NK cells, we used the same antibody and investigated whether cytokine stimulation influences the expression of CD300c. We stimulated with a wide variety of cytokines that activate NK cells and confirmed that IL-2 and IL-15 significantly up-regulate CD300c expression solely on the CD56bright (CD56high CD16neg) subset. In contrast, no expression was detected on CD56dim (CD56low CD16pos) subset of NK cells. When NK cells were treated with IL-4, IL-12, IL-18 and IL-21, we observed that CD300c expression was not changed on either of the NK cell subsets (Fig. 1A,B). Moreover, within the CD56bright NK cell subset a small population of CD56high CD16dim NK cells also expressed CD300c upon IL-2 and IL-15 treatment (Supplementary Figure). Since CD300c expression is dependent on IL-2, we tested its expression on NK cells after a prolonged treatment of IL-2 for 7 days mimicking a phenomenon of lymphokine-activated killer (LAK) cells. We show that CD300c expression is maintained only on CD56bright subsets but not on CD56dim subset (Supplementary Figure). To further confirm that the expression of CD300c is transcriptionally regulated, we sorted the two NK cell subsets and analyzed the respective mRNA levels after treatment with IL-2 and IL-15. Previously, we and others have demonstrated that CD300a is expressed on the cell surface of all NK cell subsets35,36, therefore we chose CD300a as a control. We confirmed that transcripts encoding CD300c were significantly up-regulated in the CD56bright NK cells upon cytokine stimulation but not on CD56dim NK cells. On the contrary, IL-2 and IL-15 stimulation did not have any impact in the transcript levels encoding CD300a (Fig. 1C). These results indicate that expression of the paired receptors CD300a and CD300c are differentially regulated on CD56bright NK cells.
Figure 1. CD300c is exclusively expressed on CD56bright NK cells.
(A) The gating strategy of NK cells is shown to distinguish the CD56bright and CD56dim populations (upper panel). The dot plots in the bottom panel indicate the percentage of CD300c+ NK cells upon various cytokine stimuli for 40 hours. (B) The bar graph shows significant up-regulation of CD300c expression on CD56bright subsets that are stimulated with IL-2 and IL-15. The data are from independent experiments from 4–6 donors. (C) The specific mRNA transcripts of CD300A and CD300C were determined by RT-PCR analysis on the sorted populations of different subsets of unstimulated, IL-2 and IL-15 stimulated NK cells. The y-axis denotes the normalized expression over 18sRNA and the corresponding ΔΔCt values are plotted. The data is from four healthy donors. The error bars indicate the average ± SEM.
Expression of CD300c is regulated by STAT5 and IL-4
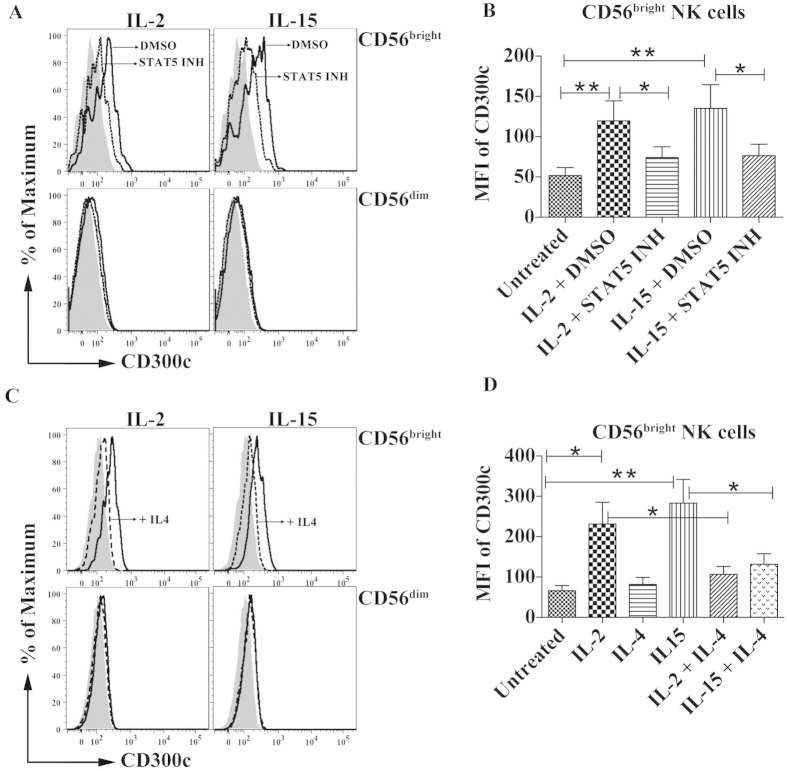
One of the major mechanisms that are triggered by IL-2 and IL-15 is the activation of STAT527. To determine the role of the latter, we treated NK cells with an inhibitor, N′-((4-Oxo-4H-chromen-3-yl)methylene)nicotinohydrazide, that blocks the biological function of STAT5 in the presence of either IL-2 or IL-15, and we observed that the expression of CD300c was significantly reduced on the CD56bright NK cell subset (Fig. 2A,B). In T cells, it was demonstrated that IL-4 inhibited IL-2-triggered STAT5 activation through a mechanism that involves down-regulation of the kinase activity of JAK1 and JAK338. Therefore, we studied the role of IL-4 in regulating IL-2 and IL-15 mediated expression of CD300c. Inclusion of IL-4 into the culture in conjunction with either IL-2 or IL-15 significantly decreased CD300c expression on CD56bright NK cells when compared with IL-2 or IL-15 treated cells (Fig. 2C,D). Therefore, our results indicate that CD300c expression on CD56bright NK cells is dependent on STAT5 activation and negatively regulated by IL-4.
Figure 2. Expression of CD300c is regulated by STAT5 and IL-4.
(A) Purified NK cells were stimulated with either IL-2 or IL-15 in the presence or absence of STAT5 inhibitor. Representative histograms show the expression of CD300c on different NK cell subsets that are either untreated (shaded), IL-2 or IL-15 plus vehicle DMSO as represented (thick line) and IL-2 or IL-15 plus STAT5 inhibitor (dotted line). (B) Bar graph represents the MFI of CD300c expression on CD56bright NK cells with indicated treatments. (C) Representative histograms show the expression of CD300c on different NK cell subsets that are either untreated (shaded), IL-2 or IL-15 as represented (thick line) and IL-2 or IL-15 with IL-4 (dotted line). (D) Bar graph represents the MFI of CD300c expression on CD56bright NK cells with indicated treatments. The data are from independent experiments from 4 donors. The error bars indicate the average ± SEM.
T cell-derived IL-2 induces the expression of CD300c on CD56bright NK cells
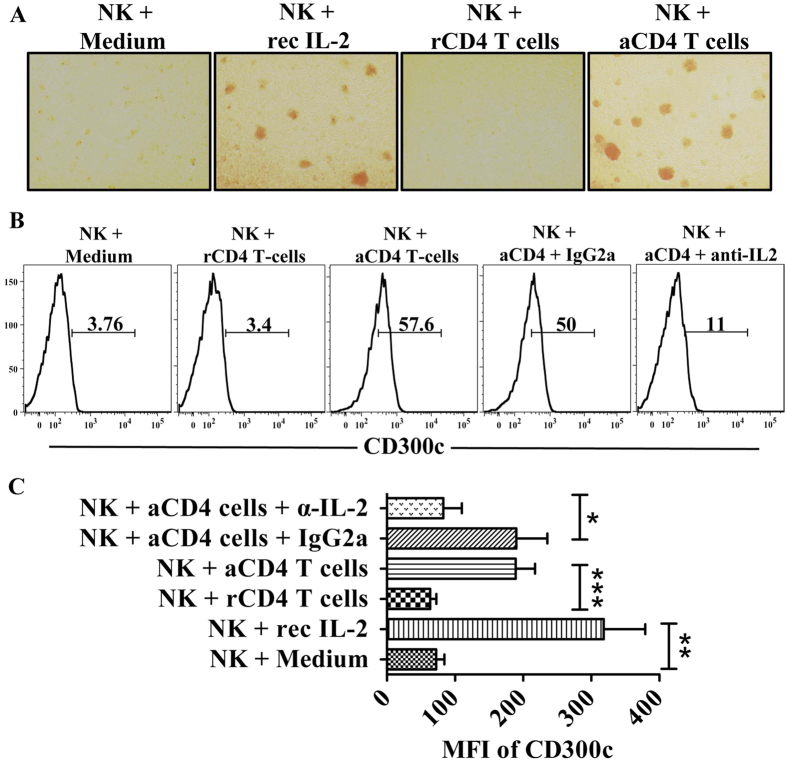
Many studies have shown that CD56bright NK cells localize in the secondary lymphoid organs and play an important role in the ongoing adaptive immune responses9. During these responses, APC-activated T cells secrete IL-2 and eventually interact with the high affinity IL-2 receptor present of CD56bright cells enhancing the NK and T cell cross talk15. We thus examined the expression of CD300c on CD56bright NK cells in a transwell assay with CD4+ T cells as their counterpart. In our experiments, we purified autologous naïve CD4+ T cells and NK cells from the same donor. Expanded and activated CD4+ T cells were obtained using anti-CD3/CD28 beads. Similar to the results observed with recombinant IL-2, the morphology of the NK cells is more clustered when cultured in the presence of activated CD4+ T cells suggesting that NK cells are more activated. This phenomenon is not observed when NK cells were incubated with either medium alone or resting CD4+ T cells (Fig. 3A). Then, we determined the expression of CD300c on CD56bright NK cells when cultured with resting and activated CD4+ T cells. As expected, CD300c expression is enhanced and this effect was inhibited only when a specific antibody was used to block the biological activity of IL-2. The isotype control did not show any effect. On the other hand, medium alone and resting CD4+ T cells did not induce any change in CD300c expression (Fig. 3B,C). Thus, the IL-2 secreted from activated CD4+ T cells specifically upregulates CD300c expression on CD56bright NK cells.
Figure 3. T cell-derived IL-2 induces the expression of CD300c on CD56bright NK cells.
(A) NK cells form clusters when cultured either in the presence of recombinant IL-2 (rIL-2) or activated CD4+ T cells in a trans-well assay. (B) The histograms represent the percentage of CD300c+ CD56bright NK cells cultured under several conditions in the transwell assay as described in Materials and Methods. (C) Bar graph represents the MFI of CD300c expression on CD56bright NK cells cultured under the indicated conditions in the transwell assay. The data are from independent experiments from 4 donors. The error bars indicate the average ± SEM. rCD4 T-cells: resting CD4+ T cells; aCD4 T-cells: activated CD4+ T cells; α-IL-2: neutralizing anti-IL-2 mAb; rec IL-2: recombinant IL-2.
Crosslinking of CD300c induces CD56bright NK cells to degranulate and secrete cytokines
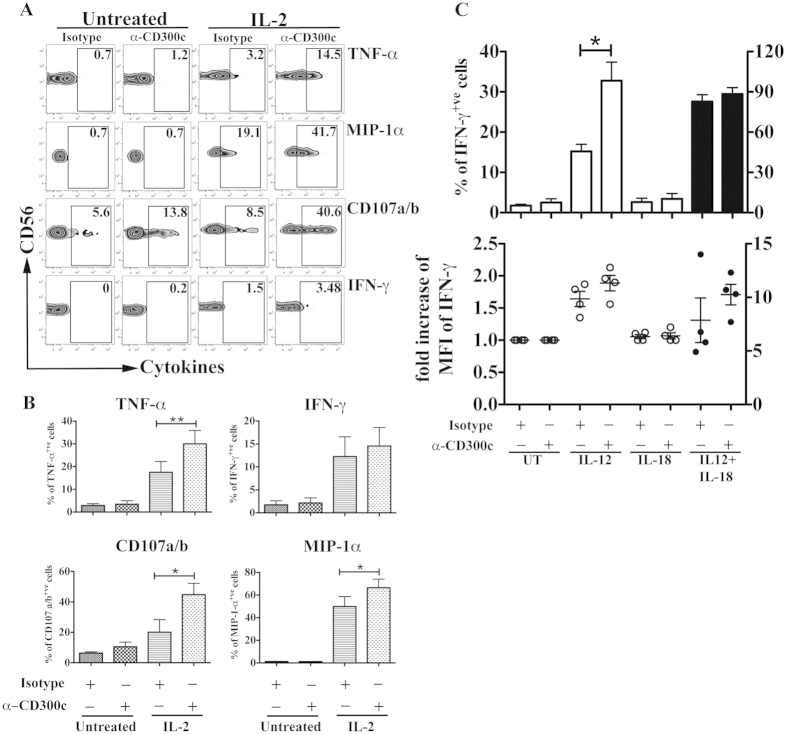
To determine whether the expression of CD300c on CD56bright NK cells possesses a functional role, CD300c was crosslinked with the specific monoclonal antibody clone TX4535, and assessed for the ability of NK cells to degranulate and produce cytokines. Crosslinking of IL-2 pre-activated NK cells with TX45 significantly increased NK cell degranulation, as measured by the expression of CD107a/b and induction of the pro-inflammatory cytokine TNF-α and chemokine MIP-1α (Fig. 4A,B), when compared with only IL-2 pre-activated NK cells. On the other hand, cross-linking of CD300c in IL-2 non-treated NK cells did not show any significant effects.
Figure 4. Crosslinking of CD300c induces CD56bright NK cells to degranulate and cytokine secretion.
Purified NK cells were both left untreated or pre-treated with IL-2 and cross-linked with isotype or anti-CD300c (clone TX45) to assess NK cell functions. (A) The zebra plots represent the production of various cytokines and degranulation markers (CD107a/b) from CD56bright NK cells, gated as in Fig. 1 (gated as CD56bright CD16neg). (B) Bar graphs represent the percentage of CD56bright NK cells producing cytokines and expressing CD107a/b after different stimulation conditions. The data are from independent experiments from at least 4 donors. (C) Purified NK cells were both left untreated or pre-treated with IL-2 and crosslinked with isotype or anti-CD300c (clone TX45), and in the absence or presence of IL-12 and IL-18. The upper panel shows the percentage of IFN-γ+ CD56bright NK cells. The lower panel represents the fold increase in the MFI IFN-γ+ CD56bright NK cells. The Y axis on the left correspond to the white bars and the white symbols, while the Y axis on the right is for the black bars and the black symbols. The data are from independent experiments from 4 donors. The error bars indicate the average ± SEM.
Although we observed a small percentage of IFN-γ producing cells after IL-2 pre-activation, the effect of CD300c mediated signaling of IFN-γ secretion in IL-2 activated CD56bright NK cells was not clear and pronounced. Because IL-12 and IL-18 are important monokines for IFN-γ production by NK cells39, we hypothesized that crosslinking of CD300c may synergize with IL-12 and/or IL-18 in inducing IFN-γ production by IL-2 pre-activated CD56bright NK cells. Therefore, in a different set of experiments, purified NK cells were treated with IL-2 to up-regulate the expression of CD300c and then crosslinked with anti-CD300c monoclonal antibodies in the presence or absence of IL-12 and IL-18. While IL-12 alone can induce IFN-γ secretion, we found a significant increase when NK cells were simultaneously crosslinked with anti-CD300c antibody (Fig. 4C-top). These effects were not observed when cells were crosslinked with anti-CD300c monoclonal antibodies in the presence of IL-18. When NK cells were stimulated with both monokines, there was no significant effect of CD300c mediated-signals on the number of IFN-γ secreting cells, but we observed an enhanced secretion of IFN-γ per cell basis as shown by an increase in the median fluorescence intensity (MFI) of IFN-γ producing cells (Fig. 4C-bottom). Thus, the activating receptor CD300c has a co-stimulatory role for IFN-γ production by CD56bright NK cells.
Differential binding of paired receptors CD300a and CD300c to PE and PS
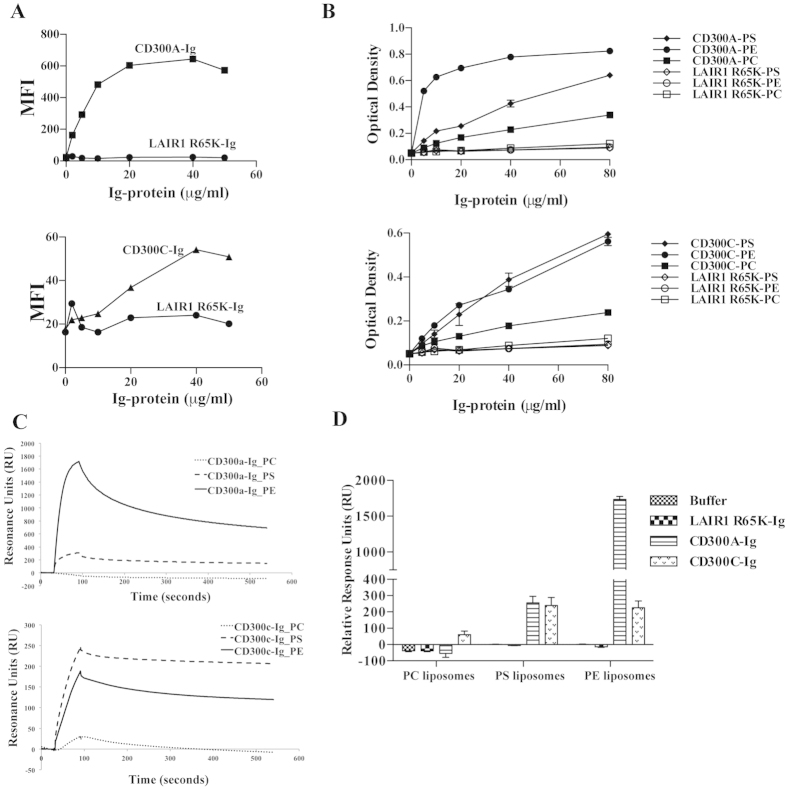
In our previous publication we have demonstrated that CD300a interacts with PE and PS exposed on the outer leaflet of the plasma membrane of dead cells32. Since the extracellular domain of CD300c is highly homologous to CD300a we hypothesized that CD300c might have a similar binding pattern to these two amino-phospholipids. Therefore, in this report we examined the ability of CD300c to bind PS and PE using multiple assays. For this analysis, we generated a chimeric protein consisting of CD300c extracellular domain and the Fc fragment of human IgG2. Consistent with our reported results32, we observed that the fusion protein CD300a-Ig binds to dead cells in increasing concentrations and the binding is saturated at 20 μg/ml; while the negative control did not show any binding (Fig. 5A-top). Similarly, CD300c-Ig also binds to dead cells in a concentration-dependent manner but to a lesser extent compared to CD300a-Ig (Fig. 5A-bottom). Since the plasma membrane asymmetry on the dead cell surface exposes predominantly the amino-phospholipids PS and PE40, we next assessed the ability of these two receptors to bind each amino-phospholipid separately. We tested the binding of fusion proteins to pure lipids in a concentration dependent manner. While CD300c-Ig binds to both PS and PE at the same level, CD300a-Ig predominantly binds to PE (Fig. 5B). The negative control protein LAIR R65K-Ig did not show any binding to any of the lipids. The relevant binding of fusion proteins to the negative control lipid phosphatidylcholine (PC) is minimal or absent. Furthermore, to validate the differential binding of CD300a-Ig and CD300c-Ig to PS and PE, we performed Surface Plasmon Resonance (SPR) experiments by immobilizing PC, PS and PE liposomes on L1 chip. Consistent with the results above, we observed that CD300a-Ig predominantly binds to PE liposomes, while CD300c-Ig binds to PS and PE liposomes to a similar extent. Interestingly, this is similar to the exhibited binding of CD300a-Ig to PS (Fig. 5C,D). Altogether, these results indicate that the paired receptors CD300a and CD300c have different binding characteristics to PS and PE.
Figure 5.
(A) Binding of the purified fusion proteins CD300a-Ig (top graph) and CD300c-Ig (bottom graph) to dead Jurkat cells. Increasing concentrations of fluorochrome labeled CD300a-Ig and CD300c-Ig proteins were incubated with dead Jurkat cells and then acquired in a flow cytometer. LAIR1 R65K-Ig fusion protein served as negative control. Graphs represent the binding (MFI) of the fusion proteins to dead cells. The data are representative of two independent experiments. (B) ELISA assay shows binding of increasing concentrations of CD300a-Ig (top graph) and CD300c-Ig (bottom graph) fusion proteins to pure lipids that are coated on plates. LAIR1 R65K-Ig fusion protein served as negative control. The data is a representative of 2 independent experiments. (C) Binding of CD300-Ig fusion proteins to liposomes. Liposomes of specified compositions were prepared and coupled to a L1 biosensor. The binding of CD300a-Ig (top) and CD300c-Ig (bottom) was analyzed by allowing the proteins to pass through the L1 sensor. The curves represent the binding of fusion proteins to liposome coated L1 chips. (D) The binding (RU) for plateau values is shown in the bar graph and the error bars represent the average ± SEM. LAIR1 R65K-Ig fusion protein served as negative control. Results shown are from 3 independent experiments.
PS and PE differentially regulate the function of CD56bright NK cells
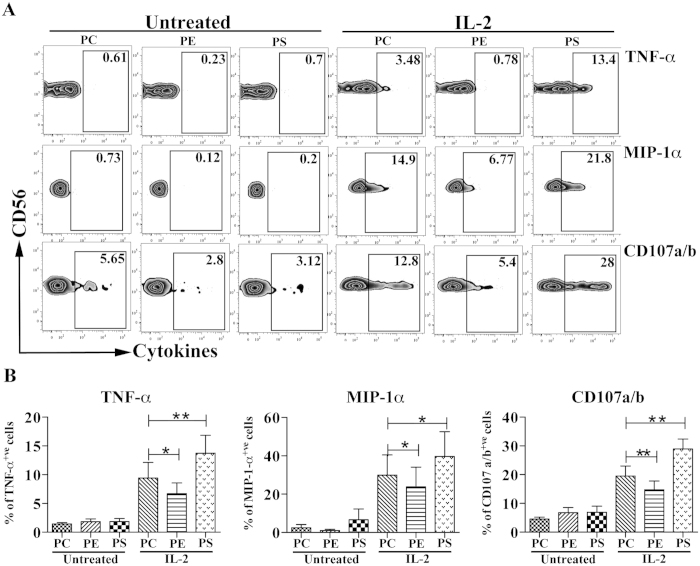
Next, our focus was to understand the functional consequences of NK cells in response to the interaction with the lipids PC, PS and PE. NK cells were purified and were either untreated or pre-treated with recombinant IL-2. As expected, in this set of experiments, we observed a small number of TNF-α+ and MIP-1α+, as well as degranulating (CD107a/b) IL-2 pre-activated CD56bright NK cells when they were cultured on plates coated with the negative control lipid PC, whereas the IL-2 non-stimulated cells did not show any effector function. Interestingly, IL-2 pre-stimulated NK cells on plates coated with PE showed a significant decrease of cytokine production and degranulation, indicating that PE is an inhibitory ligand (Fig. 6A,B). On the other hand, PS stimulation of IL-2 pre-activated NK cells significantly enhanced the number of cytokine producing and degranulating NK cells, indicating that PS acts as an activating ligand (Fig. 6A,B). These results suggest that the interaction of PE with CD300a is predominant over the interaction with CD300c, which produces an inhibitory signal as a result. In addition, crosslinking of CD300c by PS on IL-2 pre-activated NK cells overrides the crosslinking of CD300a, resulting in a positive signal.
Figure 6. Purified NK cells were both left untreated or pre-treated with IL-2 and cross-linked with pure lipids coated on a plate as mentioned in Materials and Methods.
(A) The zebra plots represent the secretion of various cytokines and degranulation markers (CD107a/b) from CD56bright NK cells, gated as in Fig. 1 (gated as CD56bright CD16neg) stimulated under the indicated conditions. (B) Bar graphs represent the percentage of TNF-α, MIP-1α and CD107a/b positive CD56bright NK cells. The data are from independent experiments from 4 donors. The error bars indicate the average ± SEM.
Discussion
NK cell responses are balanced by a multitude of activating and inhibitory receptors5. The receptors expression and function is regulated by various cytokine stimuli5. In this study, we investigated the expression pattern of CD300c, an activating receptor that belongs to the CD300 family, which recognizes lipids as their ligands. Signaling through IL-2 and IL-15 induced the expression of CD300c on CD56bright NK cells but not on the CD56dim population in a mechanism that involves STAT5. Interestingly, IL-4, a potent regulator of IL-2 mediated activation of NK cells41, inhibits IL-2/IL-15 dependent expression of CD300c on CD56bright NK cells, which is consistent with the ability of IL-4 to inhibit IL-2 triggered STAT5 activation on human T cells38.
In humans, the CD56bright NK cell subset has been shown to play an important role in adaptive immunity15. CD56bright NK cells, being the most abundant NK cell population in the secondary lymphoid organs, have the capacity to produce abundant cytokines and are thus considered to possess a regulatory role during adaptive immune responses (9, 12, 20). Throughout NK cell development in the secondary lymph nodes, a CD34+ precursor population has been identified and shown to differentiate into CD56bright NK cells upon stimulation with either IL-2 or IL-15. Further, these cells reside in the T cell enriched regions of the lymph nodes16. Since antigen-activated T cells secrete IL-2, we postulate that the neighboring CD56bright NK cells up-regulate CD300c expression on their surface as a consequence of T cell activation. The data from the transwell assays validate the role of activated CD4+ T cells derived IL-2 in the induction of CD300c on CD56bright NK cells.
It has previously been demonstrated that crosslinking of CD300c activates human monocytes to induce pro-inflammatory responses35,42. In this report, we show that an antibody against CD300c can induce cytokine secretion and degranulation only in IL-2 pre-activated CD56bright NK cells. The induction of TNF-α, MIP1-α and degranulation (CD107a/b) by IL-2 pre-activated CD56bright NK cells were strongly enhanced after crosslinking of CD300c. On the other hand, IFN-γ induction was minimally increased after CD300c cross-linking on IL-2 pre-activated CD56bright NK cells. However, CD300c mediated signals synergized with IL-12 and IL-18 to produce high amounts of IFN-γ on IL-2 pre-activated cells. Recently, it has been shown that the crosstalk between NK cells and other myeloid cells, especially dendritic cells and macrophages, mutually benefit by reciprocal activation. Cytokines derived from myeloid cells, such as IL-12, IL-15 and IL-18, activate NK cells. Post activation, NK cells secrete pro-inflammatory cytokines like TNF-α that initiate DC maturation and IFN-γ that activates inflammatory macrophages7,11. In this particular study, we envisage that the unique expression and function of CD300c on CD56bright NK cells might play an important co-stimulatory role in the secondary lymphoid organs in the presence of endogenous T-cell derived IL-2 and cytokines (IL-12, IL-15 and IL-18) derived from antigen presenting cells (APCs).
Among the myriad of activating and inhibitory receptors on NK cells, the paired ones possess highly homologous sequences in the extracellular domain and have opposing functions in immune regulation43. Some of the best-described examples are the killer cell immunoglobulin-like receptors (KIR) family and C-type lectin receptor family that recognize MHC Class I molecules44. Our data also demonstrated recognition of same ligands by the paired receptors CD300a and CD300c, although with different affinities. PE and PS are the lipids that are exposed on the outer leaflet when the cell membrane is compromised40. In line with our previous reports, our current studies demonstrated binding of CD300a to dead cells and the corresponding amino-phospholipids PE and PS. A similar phenomenon was observed for the activating receptor CD300c. The intriguing feature is that the strength of binding between the two receptors to dead cells and PE is very different. CD300a exhibited a stronger binding to dead cells and to PE than CD300c. On the other hand, both CD300a and CD300c exhibited similar binding to PS. Our observaions are also supported and published by Takahashi et al.42. Therefore, it is acceptable to conclude that the ligand interactions to the activating receptor CD300c are weaker than to its inhibitory counterpart CD300a. The above phenomena are consistent with other paired receptors belonging to different families. For example, KIR2DL1 (KIR family) and CD94/NKG2A (C-type lectin family) have higher binding affinity to HLA-CLys80 and HLA-E, respectively, than their activating counter parts KIR2DS1 and CD94/NKG2C43.
Our studies described the ability of PS to induce cytokine production and degranulation by CD56bright NK cells. Considering that PS is not only exposed on apoptotic cells, but also on activated T cells, B cells and monocytes45,46,47, it might engage CD300c on CD56bright NK cells. Predominantly CD56bright NK cell subset has the ability to lyse autologous activated T cells compared to CD56dim NK cell subset48,49. Moreover, it has been shown that tonsilar CD56bright NK cells can influence T cell polarization during primary immune responses by secretion of IFN-γ50. Therefore, it is highly persuasive that CD300c on CD56bright NK cells may facilitate the cross talk between these NK cells and other activated leukocytes. In the past decade, there has also been emerging evidence that CD56bright NK cells restrain T cell responses during various autoimmune diseases51. The prognoses of autoimmune diseases is influenced by an activated immune system, such as activated T cells in multiple sclerosis (MS)52 and polyclonal activated B cells in systemic lupus erythematous (SLE)53. For example, treatment of MS patients with Dacluzimab, an anti-CD25 humanized monoclonal antibody, selectively increases the subset of CD56bright NK cells and is responsible for the cytotoxicity of activated T cells54. Others have shown that killing of activated T cells by IL-2 activated NK cells involves the NKG2D, LFA-1 and TRAIL receptors48,49. Similarly, it may be very possible that CD300c has also an important role, along with other receptors, in the killing of activated T cells.
Contrary to PS activating function, we observed that PE induced a negative response in degranulation and cytokine response of IL-2 pre-activated CD56bright NK cells. Our previous studies32 and current data demonstrate that CD300a strongly binds to PE and the binding to PS is weaker, while CD300c binds similarly PE and PS. On the other hand, CD300a binds PE stronger than CD300c, while both receptors similarly bind PS (Fig. 5). While it was expected that interaction with PE would deliver a negative signal, we did not know what to expect when IL-2 (or IL-15) pre-activated CD56bright NK cells interacted with PS. Interestingly, cells de-granulated and produced cytokines in response to PS, indicating that in this scenario the positive signal (CD300c-mediated) overrides the negative signal (CD300a-mediated). It is important to note that the NK cells used in our assays are always pre-activated with IL-2 (or IL-15) to induce CD300c expression on the CD56bright subset. We therefore do not know whether in the absence of pre-activation that interaction with PS would deliver an inhibitory signal. Unfortunately, the experiments cannot be performed with freshly isolated CD56bright NK cells since they do not express (or express very low levels) of CD300c on their cell surface. However, to avoid the co-stimulatory effect of IL-2, cells were rested for 24 hours after cytokine treatment, which may suggest that CD56bright NK cells expressing CD300c do not need to be in a highly activated state to produce cytokines and degranulate after encountering PS.
The finding that two phospholipids, PS and PE, are metabolically related40, but differentially regulate the functions of CD56bright NK cells through their interaction with CD300a and CD300c clearly indicate that further studies are necessary to perform in-depth characterization of the immune-regulatory roles of these paired receptors on NK cells. CD300a and CD300c on CD56bright NK cells, with their opposing functions, will modulate the threshold for cell activation after interacting with PE and PS expressing dead and activated cells. Consequently, this might play an important role in maintaining the homeostasis of secondary lymphoid organs during the cross-talk between activated T cells and CD56bright NK cells.
Methods
Primary Cells
Leukapheresis blood packs from healthy donors were obtained under an institutional review board-approved protocol at the National Institutes of Health. All donors provided written informed consent. All the cell isolation methods were carried out in accordance with the approved laboratory guidelines. Peripheral blood mononuclear cells (PBMCs) were obtained by ficoll density centrifugation. NK cells were purified from PBMCs using a negative selection method. Naïve CD4+ T cells were isolated from PBMCs using a double purification step involving the removal of CD25+ regulatory cells and then using a negative selection method for the purification of naïve CD4+ T cells. All the kits were purchased from Stem Cell Technologies and isolated using RoboSep. Cells were cultured in Iscove´s Modified Dulbecco´s Medium (IMDM) supplemented with 10% fetal-bovine serum, L-glutamine, sodium pyruvate and non-essential amino acids.
Antibodies and Reagents
Fluorochrome-labeled antibodies used for flow cytometric analyses were obtained from the following vendors: anti-CD3 (UCHT1), anti-CD16 (eBioCB16), anti-CD56 (CMSSB), anti-CD107a (eBioH4A3), anti-CD107b (eBioH4B4), anti-CD300c (TX45), anti-TNFα (MAb11), anti-MIP1α (PFFM3) and anti-CD69 (FN50) were from eBiosciences and anti-IFN-γ (B27) was from BD Biosciences. Purified antibodies anti-human IL-2 (AB12-3G4), mouse IgG2a κ isotype (eBM2a), mouse IgG1 κ (MOPC-21) and anti-CD300c (TX45) were obtained from eBiosciences. For the real time PCR experiments, primers for CD300A, CD300C and 18sRNA genes were purchased from SA Biosciences. STAT5 inhibitor N′-((4-Oxo-4H-chromen-3-yl)methylene)nicotinohydrazide was purchased from Calbiochem/EMD Millipore. Recombinant IL-2 was obtained from National Cancer Institute, Frederick, MD. Cytokines IL-12, IL-15 and IL-4 are from R&D Systems, IL-18 is from Medical and Biological Laboratories (MBL) and IL-21 is from Peprotech. Phospholipids 1-palmitoyl-2-oleoyl (PO) phosphatidylserine (POPS) (PS), phosphatidylethanolamine (POPE) (PE) and phosphatidylcholine (POPC) (PC) were purchased from Avanti Polar Lipids.
NK cell Stimulation
Purified NK cells were stimulated with cytokines IL-2 (100–200 U/ml), IL-15 (10 ng/ml), IL-4 (50 ng/ml), IL-12 (10 ng/ml), IL-18 (100 ng/ml) and IL-21 (50 ng/ml) for 40 hours. In the inhibition assays, vehicle DMSO (Sigma) and STAT5 inhibitor were used at 250 nM concentrations during NK cell stimulation. Post incubation, all the respective samples were stained for multiple surface markers and acquired in either LSR-II or Fortessa X-20 (BD Biosciences). Lymphocytes were electronically gated based on forward and side scatter parameters, and NK cells were further gated based on the expression of CD3, CD56 and CD16 (CD3−CD56+CD16+/−). Flow cytometric data were analyzed using FlowJo software (Tree Star).
Transwell Assays
Purified NK cells were cultured in the inner compartment and the naïve CD4+ T cells were in the outer compartment in a Transwell 24-well plate. The CD4+ T cells were activated with human T-activator CD3/CD28 Dyna beads (Life Technologies). As a control NK cells were also incubated with resting CD4+ T cells in the trans-well system. When indicated, the isotype control IgG2a κ and anti-human IL-2 (eBiosciences) were added at a concentration of 10 μg/ml to the activated T cells compartment. After 2 days of incubation, the NK cells in the inner compartment were observed in a bright field microscope for the cluster formation and later the cells were harvested and analyzed for CD300c surface expression. Recombinant IL-2 (100–200 U/ml) was used as a positive control.
Functional Experiments
Freshly isolated NK cells were stimulated with recombinant IL-2 for 40 hours, washed thoroughly and starved from IL-2 for 24 hours. For antibody cross-linking experiments, both untreated and IL-2 pre-stimulated NK cells were cross-linked with two different monoclonal antibodies; isotype control (MOPC-21) and anti-CD300c (TX45) plated on a 24-well plate at 10 μg/ml concentrations. For lipid stimulation experiments, the lipids PC, PS and PE were diluted in 100% methanol and air-dried. Later the IL-2 starved NK cells were incubated with the lipid-coated plates. After overnight incubation, the NK cells were stained with appropriate surface receptors and intracellular cytokines. The anti-CD107a/b monoclonal antibodies and monensin (Golgi-stop, a protein transport inhibitor) were added during the culture for the last six hours before harvesting. Subsequently, the cells were acquired in a flow cytometer (BD LSR-II) and analyzed by using FlowJo software (Tree Star).
Binding Assays
The Ig fusion proteins were constructed, purified and fluorescently labeled as previously described55. The cell binding assay protocol was followed as mentioned in our previous report32. Briefly, UV-treated Jurkat cells were incubated with the Ig fusion proteins in the presence of binding buffer (10 mM HEPES pH 7.4, 140 mM NaCl, 2.5 mM CaCl2 and 2 mM MgCl2) for 45 minutes on ice and washed twice with wash buffer (PBS containing 1% of FCS) and re-suspended in PBS for flow cytometric analysis. Cells were also stained with Annexin V-APC (eBiosciences) and 7AAD (Beckmann Coulter) to differentiate between dead and live cells. ELISA binding studies were performed as previously described32. Briefly, purified lipids PC, PS and PE were diluted in 100% methanol and air-dried. After subsequent washes and blocking, the wells were incubated with different human Ig fusion proteins, washed and incubated with anti-human Ig (Fcγ specific) antibody coupled to horseradish peroxidase HRP (Jackson Immuno Research) for one hour at RT. The peroxidase activity was analyzed by using TMB substrate (ImmunoPure TMB Substrate Kit-Pierce) and the absorbance was measured at 450 nm in a spectrophotometer. The Surface Plasmon Resonance (SPR) analysis protocol was followed as described earlier and performed in Biacore T20032. Briefly, the liposomes composed of various lipids PC, PS and PE were captured on the L1 sensor chip and the human-Ig fusion proteins were injected and carried in flow to study their binding to liposomes. BIACORE T200 Control Software and BIAevaluation Software were used to analyze the SPR experiments.
Statistical analysis
Data were analyzed using GraphPad Prism software. The data were plotted as bar graphs representing the average ± standard error of the mean (SEM). Pair wise comparisons were examined by a paired Student’s t-test. NS: not significant; *P < 0.05, **P < 0.01 ***P < 0.001.
Additional Information
How to cite this article: Dimitrova, M. et al. CD300c is uniquely expressed on CD56bright Natural Killer Cells and differs from CD300a upon ligand recognition. Sci. Rep. 6, 23942; doi: 10.1038/srep23942 (2016).
Supplementary Material
Acknowledgments
We thank Dr. John E. Coligan for allowing us to use their blood bank services at the National Institute of Health. This work was funded by the Intramural Program of the Food and Drug Administration and Project PI13/00889, integrated into the “Plan Estatal de I+D+I 2013-2016” and funded by the “ISCIII-Subdirección de Evaluación y Fomento de la Investigación-Fondo Europeo de Desarrollo Regional (FEDER)”.
Footnotes
Author Contributions V.R.S. and M.D. performed the experiments; V.R.S., O.Z. and F.B. analyzed results; V.R.S. designed the research; V.R.S. and F.B. wrote the paper. All the authors reviewed the manuscript.
References
- Becknell B. & Caligiuri M. A. Natural killer cells in innate immunity and cancer. J Immunother 31, 685–692, doi: 10.1097/CJI.0b013e318182de23 (2008). [DOI] [PubMed] [Google Scholar]
- Caligiuri M. A. Human natural killer cells. Blood 112, 461–469, doi: 10.1182/blood-2007-09-077438 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol 47, 187–376 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Nunes J. A. & Vely F. Natural killer cell signaling pathways. Science 306, 1517–1519, doi: 10.1126/science.1103478 (2004). [DOI] [PubMed] [Google Scholar]
- Vivier E., Tomasello E., Baratin M., Walzer T. & Ugolini S. Functions of natural killer cells. Nat Immunol 9, 503–510, doi: 10.1038/ni1582 (2008). [DOI] [PubMed] [Google Scholar]
- Aste-Amezaga M., D’Andrea A., Kubin M. & Trinchieri G. Cooperation of natural killer cell stimulatory factor/interleukin-12 with other stimuli in the induction of cytokines and cytotoxic cell-associated molecules in human T and NK cells. Cell Immunol 156, 480–492, doi: 10.1006/cimm.1994.1192 (1994). [DOI] [PubMed] [Google Scholar]
- Cooper M. A., Fehniger T. A., Fuchs A., Colonna M. & Caligiuri M. A. NK cell and DC interactions. Trends Immunol 25, 47–52 (2004). [DOI] [PubMed] [Google Scholar]
- Cooper M. A. et al. Interleukin-1beta costimulates interferon-gamma production by human natural killer cells. Eur J Immunol 31, 792–801 (2001). [DOI] [PubMed] [Google Scholar]
- Cooper M. A. et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 97, 3146–3151 (2001). [DOI] [PubMed] [Google Scholar]
- Carbone E. et al. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol 29, 4022–4029, doi: (1999). [DOI] [PubMed] [Google Scholar]
- Ferlazzo G. et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA 101, 16606–16611, doi: 10.1073/pnas.0407522101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlazzo G. et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol 172, 1455–1462 (2004). [DOI] [PubMed] [Google Scholar]
- Gerosa F. et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 195, 327–333 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. M., Scalzo A. A., Yokoyama W. M., Smyth M. J. & Degli-Esposti M. A. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol 4, 175–181, doi: 10.1038/ni880 (2003). [DOI] [PubMed] [Google Scholar]
- Fehniger T. A. et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 101, 3052–3057, doi: 10.1182/blood-2002-09-2876 (2003). [DOI] [PubMed] [Google Scholar]
- Freud A. G. et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 22, 295–304, doi: 10.1016/j.immuni.2005.01.013 (2005). [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R. & Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol 136, 4480–4486 (1986). [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Cwirla S. & Phillips J. H. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol 143, 3183–3191 (1989). [PubMed] [Google Scholar]
- Ellis T. M. & Fisher R. I. Functional heterogeneity of Leu 19“bright”+ and Leu 19“dim”+ lymphokine-activated killer cells. J Immunol 142, 2949–2954 (1989). [PubMed] [Google Scholar]
- Cooper M. A., Fehniger T. A. & Caligiuri M. A. The biology of human natural killer-cell subsets. Trends Immunol 22, 633–640 (2001). [DOI] [PubMed] [Google Scholar]
- Andre P. et al. Modification of P-selectin glycoprotein ligand-1 with a natural killer cell-restricted sulfated lactosamine creates an alternate ligand for L-selectin. Proc Natl Acad Sci USA 97, 3400–3405, doi: 10.1073/pnas.040569797 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. J. et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol 166, 6477–6482 (2001). [DOI] [PubMed] [Google Scholar]
- Frey M. et al. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol 161, 400–408 (1998). [PubMed] [Google Scholar]
- Baume D. M. et al. Differential responses to interleukin 2 define functionally distinct subsets of human natural killer cells. Eur J Immunol 22, 1–6, doi: 10.1002/eji.1830220102 (1992). [DOI] [PubMed] [Google Scholar]
- Caligiuri M. A. et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest 91, 123–132, doi: 10.1172/JCI116161 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri M. A. et al. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med 171, 1509–1526 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meazza R., Azzarone B., Orengo A. M. & Ferrini S. Role of common-gamma chain cytokines in NK cell development and function: perspectives for immunotherapy. J Biomed Biotechnol 2011, 861920, doi: 10.1155/2011/861920 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman Y., Spolski R. & Leonard W. J. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol 9, 480–490, doi: 10.1038/nri2580 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego F. The CD300 molecules: an emerging family of regulators of the immune system. Blood 121, 1951–1960, doi: 10.1182/blood-2012-09-435057 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizuma K., Tahara-Hanaoka S., Noguchi E. & Shibuya A. Identification and characterization of CD300H, a new member of the human CD300 immunoreceptor family. J Biol Chem, doi: 10.1074/jbc.M115.643361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu H. et al. CD300 antigen like family member G: A novel Ig receptor like protein exclusively expressed on capillary endothelium. Biochem Biophys Res Commun 348, 183–191, doi: 10.1016/j.bbrc.2006.07.047 (2006). [DOI] [PubMed] [Google Scholar]
- Simhadri V. R. et al. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood 119, 2799–2809, doi: 10.1182/blood-2011-08-372425 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. P., O’Driscoll M. & Litman G. W. Specific lipid recognition is a general feature of CD300 and TREM molecules. Immunogenetics 64, 39–47, doi: 10.1007/s00251-011-0562-4 (2012). [DOI] [PubMed] [Google Scholar]
- Zenarruzabeitia O., Vitalle J., Eguizabal C., Simhadri V. R. & Borrego F. The Biology and Disease Relevance of CD300a, an Inhibitory Receptor for Phosphatidylserine and Phosphatidylethanolamine. J Immunol 194, 5053–5060, doi: 10.4049/jimmunol.1500304 (2015). [DOI] [PubMed] [Google Scholar]
- Simhadri V. R., Mariano J. L., Gil-Krzewska A., Zhou Q. & Borrego F. CD300c is an activating receptor expressed on human monocytes. J Innate Immun 5, 389–400, doi: 10.1159/000350523 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankry D. et al. Expression and function of CD300 in NK cells. J Immunol 185, 2877–2886, doi: 10.4049/jimmunol.0903347 (2010). [DOI] [PubMed] [Google Scholar]
- Lankry D., Rovis T. L., Jonjic S. & Mandelboim O. The interaction between CD300a and phosphatidylserine inhibits tumor cell killing by NK cells. Eur J Immunol 43, 2151–2161, doi: 10.1002/eji.201343433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A., Sengupta T. K., Ruiz D. C., Yang E. & Ivashkiv L. B. IL-4 selectively inhibits IL-2-triggered Stat5 activation, but not proliferation, in human T cells. J Immunol 162, 1261–1269 (1999). [PubMed] [Google Scholar]
- Agaugue S., Marcenaro E., Ferranti B., Moretta L. & Moretta A. Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood 112, 1776–1783, doi: 10.1182/blood-2008-02-135871 (2008). [DOI] [PubMed] [Google Scholar]
- Vance J. E. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res 49, 1377–1387, doi: 10.1194/jlr.R700020-JLR200 (2008). [DOI] [PubMed] [Google Scholar]
- Nagler A., Lanier L. L. & Phillips J. H. The effects of IL-4 on human natural killer cells. A potent regulator of IL-2 activation and proliferation. J Immunol 141, 2349–2351 (1988). [PubMed] [Google Scholar]
- Takahashi M. et al. Human CD300C delivers an Fc receptor-gamma-dependent activating signal in mast cells and monocytes and differs from CD300A in ligand recognition. J Biol Chem 288, 7662–7675, doi: 10.1074/jbc.M112.434746 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanietsky N. & Mandelboim O. Paired NK cell receptors controlling NK cytotoxicity. FEBS Lett 584, 4895–4900, doi: 10.1016/j.febslet.2010.08.047 (2010). [DOI] [PubMed] [Google Scholar]
- Lopez-Botet M. et al. Paired inhibitory and triggering NK cell receptors for HLA class I molecules. Hum Immunol 61, 7–17 (2000). [DOI] [PubMed] [Google Scholar]
- Appelt U. et al. Viable, apoptotic and necrotic monocytes expose phosphatidylserine: cooperative binding of the ligand Annexin V to dying but not viable cells and implications for PS-dependent clearance. Cell Death Differ 12, 194–196, doi: 10.1038/sj.cdd.4401527 (2005). [DOI] [PubMed] [Google Scholar]
- Choi S. C. et al. Cutting edge: mouse CD300f (CMRF-35-like molecule-1) recognizes outer membrane-exposed phosphatidylserine and can promote phagocytosis. J Immunol 187, 3483–3487, doi: 10.4049/jimmunol.1101549 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S. R., Mancini M., Rosen A. & Schlissel M. S. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol 164, 1322–1332 (2000). [DOI] [PubMed] [Google Scholar]
- Cerboni C. et al. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood 110, 606–615, doi: 10.1182/blood-2006-10-052720 (2007). [DOI] [PubMed] [Google Scholar]
- Nielsen N., Odum N., Urso B., Lanier L. L. & Spee P. Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS One 7, e31959, doi: 10.1371/journal.pone.0031959 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi B., Bougras G., Muller W. A., Ferlazzo G. & Munz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol 36, 2394–2400, doi: 10.1002/eji.200636290 (2006). [DOI] [PubMed] [Google Scholar]
- Schleinitz N., Vely F., Harle J. R. & Vivier E. Natural killer cells in human autoimmune diseases. Immunology 131, 451–458, doi: 10.1111/j.1365-2567.2010.03360.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B. et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA 103, 5941–5946, doi: 10.1073/pnas.0601335103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A. & Isenberg D. A. Systemic lupus erythematosus. N Engl J Med 358, 929–939, doi: 10.1056/NEJMra071297 (2008). [DOI] [PubMed] [Google Scholar]
- Bielekova B. et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol 66, 483–489, doi: 10.1001/archneurol.2009.50 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. et al. A single residue, arginine 65, is critical for the functional interaction of leukocyte-associated inhibitory receptor-1 with collagens. J Immunol 182, 5446–5452, doi: 10.4049/jimmunol.0804052 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.