Abstract
The progressive nature of osteoarthritis is manifested by the dynamic increase of degenerated articular cartilage, which is one of the major characteristics of this debilitating disease. As articular chondrocytes become exposed to inflammatory stress they enter a pro-catabolic state, which leads to the secretion and activation of a plethora of proteases. In aim to detect the disease before massive areas of cartilage are destroyed, various protein and non-protein biomarkers have been examined in bodily fluids and correlated with disease severity. This review will discuss the widely research extracellular degraded products as well as products generated by affected cellular pathways upon increased protease activity. While extracellular components could be more abundant, cleaved cellular proteins are less abundant and are suggested to possess a significant effect on cell metabolism and cartilage secretome. Subtle changes in cell secretome could potentially act as indicators of the chondrocyte metabolic and biological state. Therefore, it is envisioned that combined biomarkers composed of both cell and extracellular-degraded secretome could provide a valuable platform for testing drug efficacy to halt disease progression at its early stages.
Keywords: Biomarkers, chondrocytes, osteoarthritis, proteome, proteolytic enzymes
Introduction
Osteoarthritis (OA) is a progressive age-related disease, wherein cartilage extracellular matrix (ECM) is degraded in response to mechanical joint destabilization and long-term low-grade inflammatory exposure (Burrage et al., 2006; Goldring & Goldring, 2006; Iannone & Lapadula, 2003). As a result of progressive articular cartilage destruction, joint space narrows, possibly causing ankyolisis of the joint, severe pain and restricted movement. The diagnosis of OA often occurs by assessing joint space on an X-ray radiograph by an orthopedic specialist. However, since the disease is latently progressed, pain and radiographic evidence is manifested only at its late stages, when a significant amount of cartilage has been damaged, at which point the patient is referred for joint replacement surgery and physical therapy. In fact, joint replacement surgeries are becoming extremely common and present a significant socio-economic burden (Litwic et al., 2013). It is expected that the burden of OA will increasingly rise in developed countries, especially given that extended life expectancy and increased obesity and BMI – two risk factors of OA – within these population (Litwic et al., 2013).
The field of OA research has been facing two major challenges in the past decade: (1) identifying and developing quantifiable and measurable diagnostic biomarkers and (2) developing efficacious disease modifying osteoarthritis drug (DMOAD) to hinder cartilage degeneration at its early stages. Ideally, diagnostic and prognostic biomarkers would support the development of DMOAD to reverse or hinder OA development. Despite that currently DMOADs are unavailable; it appears that developing bioassays for early detection of OA would be a good starting point, given that such measures could also provide a platform for examining drug efficacy.
A diagnostic biomarker will only indicate the pathology exists, whereas a prognostic biomarker will provide an indication of the pathophysiological severity and likelihood of progressing (Abramson & Krasnokutsky, 2006; Karsdal et al., 2011). In such a case wherein a given biomarker is both prognostic and diagnostic, it would be expected that its generation is tightly linked to the disease pathogenesis, making it also a potential drug target. To this end, the disease mechanism must be fully understood, before a candidate biomarker is attempted to be detected in bodily fluids.
Much of the basic research in the field of OA clearly shows that chondrocytes, residing within articular surfaces, undergo significant stress related to inflammatory, metabolic and mechanical stresses (Abella et al., 2014; Blanco et al., 1998; Brady et al., 2014; Burrage et al., 2006; Goldring & Goldring, 2006; Iannone & Lapadula, 2003). This leads to a pronounced activation of matrix-degrading enzymes targeted at the extracellular milieu, but also at some intracellular targets. In the field of OA, much of the focus has been towards understanding the mechanisms by which ECM is degraded and the resulting degradation products as potential diagnostic and prognostic biomarkers. Fewer reports have examined the cellular proteins affected by enhanced proteolytic activity within the cell, albeit their potential link to disease development. This concise review will highlight both cellular and extracellular targets and discuss the pathways attributes of cellular protease activity. Despite OA being considered as a joint disease involving cartilage, bone, tendon and synovium, this review will focus mostly on chondrocytes and the process leading to cartilage destruction by cartilaginous matrix degrading enzymes.
Stimulators of catabolism in cartilage
Several stimulators of cartilage degradation via catabolic enzymatic activity and expression have been extensively demonstrated with regard to OA research. The major stimulators described in the literature are cytokines possessing a pro-inflammatory effect. One such cytokine is tumor necrosis factor alpha (TNFα) which binds toll-like receptor (TLR)-2, -4 and has been implicated to enhance matrix metalloproteinase (MMP)-3, -13 and cyclooxygenase (COX)-2 via nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) dependent mechanism, indicating that its effect is not only pro-catabolic but also pro-apoptotic (Kim et al., 2006; Sakai et al., 2001). Albeit the pro-catabolic impact of TNFα in articular cartilage, it is likely to be more pronounce in rheumatoid arthritis (RA) or inflammatory arthritis, as its levels in the synovial fluid are markedly higher in RA compared to OA (Manicourt et al., 2000).
An additional cytokine involved in OA pathogenesis is Interleukin 1 beta (IL1β) which elicits increased levels of MMP-1-13, A Disintegrin And Metalloproteinase with Thrombospondin Motifs (ADAMTS)-4, 5 and 9 via mitogen-activated protein kinases (MAPK) p38 signaling pathway (Demircan et al., 2005; Mengshol et al., 2000, 2001). Similar findings were also reported for IL-17 and oncostatin M (OSM), a member of the IL6 family (El Mabrouk et al., 2007; Sylvester et al., 2004). Interestingly, many of the proinflammatory cytokines exacerbate a chemokine-dependent reaction that not only induces expression of matrix degrading enzymes but inhibit ECM synthesis in articular chondrocytes (Borzi et al., 2000; Yuan et al., 2001).
Increased matrix degradation results in breakdown products which may possess new functional attributes. Breakdown oligosaccharides derived from hyaluronic acid (HA) have been shown to promote ADAMTS-4, -5 expression through their capacity to interact with CD44 receptors upon chondrocytes (Ariyoshi et al., 2012). Another example for the influence of matrix breakdown fragments in the exacerbation of pro-catabolic enzymes is the capacity of fibronectin fragments to bind cell-surface integrins and enhanced MMP and reactive oxygen species (ROS) in chondrocytes by regulating NFκB and MAPK activity (Del Carlo et al., 2007).
The depletion of cartilaginous matrix is often accompanied with enhanced chondrocytes apoptosis and cellular caspase levels. For example, caspase 3 and 9 have been enhanced in superficial zone of articular cartilage and in clustered chondrocytes undergoing apoptosis and correlated with OA severity grade (Matsuo et al., 2001). It was also suggested that structural changes in the extracellular framework of chondrocytes elicits a pro-apoptotic effect by initiating FAS signaling (Kim et al., 2001), possibly through the generation of new breakdown products.
Although a plethora of extrinsic biochemical factors (Figure 1A) deriving from degraded matrix of signaling molecules may perpetuate OA pathogenesis, it is clear that mechanical stimuli and its magnitude could also facilitate changes in ECM synthesis. Several elegant studies by the Kandel group show that short-term cyclic mechanical compression enhances matrix remodeling and synthesis in chondrocytes via MAPK/AP-1 pathway (De Croos et al., 2006; Spiteri et al., 2010). Furthermore, Li et al. showed that the extent of loading and combination with pro-inflammatory stimuli could be extremely detrimental to the state of cartilage (Li et al., 2013). In fact, an eight day moderate loading regimen in the presence of pro-inflammatory stimuli was not considered to result in a pro-catabolic or pro-apoptotic affect in bovine articular cartilage (Li et al., 2013). It is possible that variations in-vitro explant load systems may stem from differences in responsiveness of the various cartilage zones (Raizman et al., 2009) or the limitations in reproducing the exact biomechanical profiles and alterations of the joint which are often caused by lifelong changes in BMI, joint misuse and age-related joint laxity.
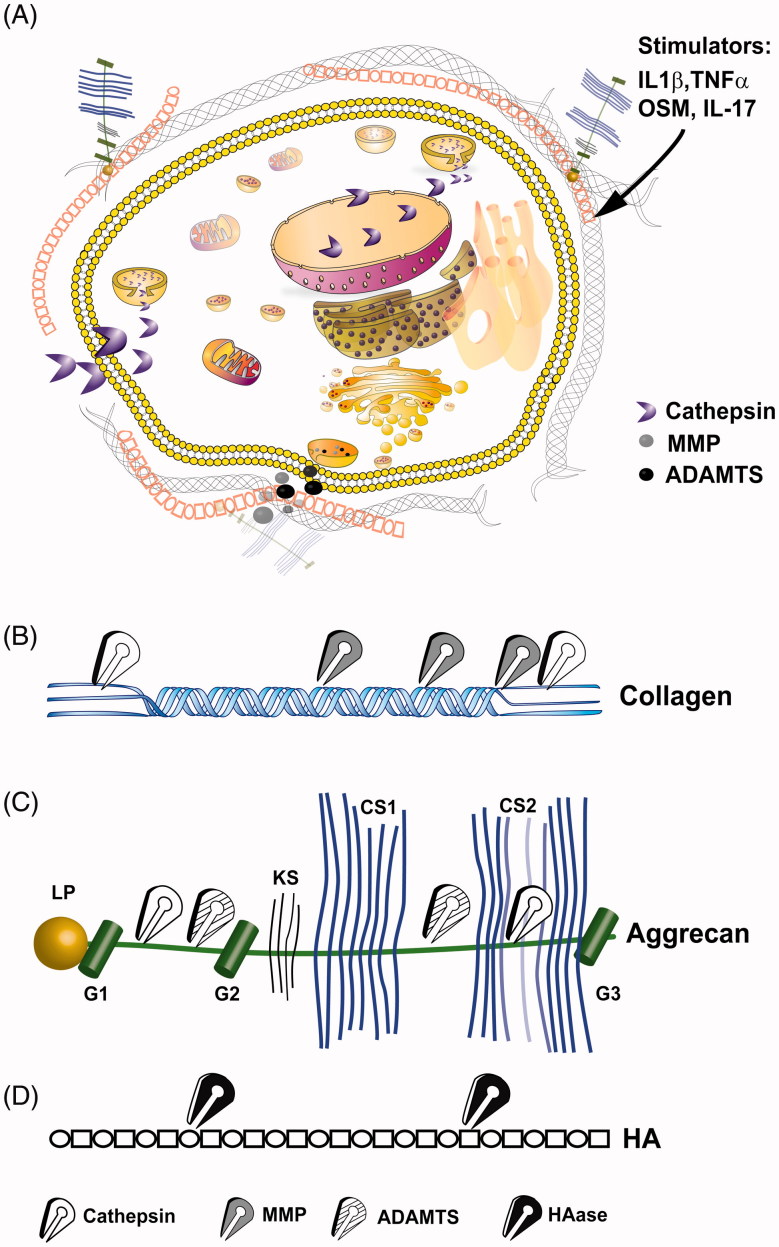
Figure 1.
Intracellular and extracellular proteolytic targeting of articular cartilage. (A) Illustration of an articular chondrocyte embedded in extracellular matrix composed of HA, aggrecan and collagen type II fibrils. Upon pro-catabolic stress, proteolytic enzymes as MMPs and ADAMTS are released to the extracellular space via exocytotic vesicles, while lysosomal cathepsins are also found throughout the cellular compartments, due to leaky lysosomes. One cellular protein target of cathepsins is SIRT1 which is cleaved (i.e. 75SIRT1) and exported from the nucleus under proinflamtory conditions, as also reported for Bid (Cirman et al., 2004; Dvir-Ginzberg et al., 2011). (B) Collagen type II cleavage by cathepsins (white scissors), MMP (grey scissors). MMPs target the collagen type II triple helix, generating Helix-II, C2C, Coll2-1NO2/Coll2-1 and C1,C2 neo-peptides (Dejica et al., 2012; Rousseau & Delmas, 2007). Cathepsins are capable of cleaving the collagen type II N-telopeptide (i.e. generating C2K, PIINP and PIIANP) and C-telopeptide (i.e. generating CTX-II neopeptide fragments) (Dejica et al., 2012; Rousseau & Delmas, 2007). (C) ADAMTS (striped scissors) mediated cleavage of aggrecan between the G1/G2 domains and several regions between the G2 and G3 which are glycosylated with keratin sulfate (KS) and chondroitin sulfate (CS) (Fosang et al., 2008). Cathepsin (B and D; blue scissors) additionally cleave G1/G2 domains and targets chondroitin sulfate attachment regions (Handley et al., 2001; Mort et al., 1998). (D) Linear hyaluronic acid is cleaved by hyaluronidase (HAse; black scissors) at the β(1,4) site between N-acetyl-d-glucosamine and d-gluroniuc acid leading to smaller and random fragments which reduce the overall molecular weight of the HA mesh within articular cartilage.
In essence, many signaling molecules, some of which indicated in Figure 1(A), are capable of facilitating cross talk with cartilage and driving pro-catabolic responses which are often accompanied with progressive chondrocytes apoptosis. Before reaching the state of apoptosis, it appears that one factor leads to chondrocyte susceptibility perpetuated by another, causing a “snowball” effect that ends inevitably in extensive cell death. Interestingly, cartilage tissue is not permissive for clearance of apoptotic cell debris, a fact that may increase the instances of secondary necrosis in apoptotic chondrocytes. This may possibly lead to a unique secretome profile of chondrocyte-derived molecules that may otherwise not permeate into the synovial fluid or blood. Hence, not only fragments of cartilaginous matrix and signaling molecules but also chondrocyte secretome derived from the inner chondrocyte proteome may predict cartilage state and possibly OA severity.
Proteases and their ECM targets
The primary constituent of cartilage is collagen type II which provides the tissue with tensile strength and is targeted for proteolytic degradation by MMPs, namely MMP-1,-8,-13 and -14 (Burrage et al., 2006; Fosang & Beier, 2011; Troeberg & Nagase, 2012), which are zinc-dependent endopeptidases. In fact, murine MMP13 nulls are resistant to OA, indicating it is a primary protease in the pathogenesis of the disease (Little et al., 2009). This is supported by the numerous reports indicating that MMP13 is enhanced during proinflammatory stimulation of articular chondrocytes (El Mabrouk et al., 2007; Kim et al., 2006; Mengshol et al., 2000, 2001; Sakai et al., 2001; Sylvester et al., 2004). Collagenase activity of MMPs target the collagen type II triple helix, generating Helix-II, C2C, Coll2-1NO2/Coll2-1 and C1,C2 neo-peptides, wherein C1 is derived from the collagen type I triple helix (Dejica et al., 2012; Rousseau & Delmas, 2007). Additional to MMPs, cathepsins are capable of cleaving the collagen type II N-telopeptide (i.e. generating C2K, PIINP and PIIANP) and C-telopeptide (i.e. generating CTX-II neopeptide fragments) (Dejica et al., 2012; Rousseau & Delmas, 2007). Peptides derived from collagen type II C-telopeptide (CPII) and their levels compared to N-propeptide fragments (PIINP and PIIANP) which are correlative to matrix synthesis, may provide an overall indication regarding cartilaginous matrix homeostasis and its potential disruption (Karsdal et al., 2011; Rousseau & Delmas, 2007). It is therefore not surprising that these fragments are found in blood and urine of OA patients correlating with the burden of disease and used as a prognostic measure for OA (Abramson & Krasnokutsky, 2006). Nitration of collagen type II derived triple helix (Coll2-1NO2, a.a. 108–116) has also been examined and compared to Coll2-1, which is the un-nitrated form (Deberg et al., 2005a,b). Sera examination of Coll2-1NO2 and Coll2-1 show it is enhanced in sera of OA and RA (Deberg et al., 2005a), while a one-year examination of these fragments in urine samples from 75 patients with primary knee OA revealed that they are predictive markers for radiographic OA progression (Charni et al., 2005). As an additional triple helix derivate, Helix-II (a.a. 622–632) showed great promise as a biomarker capable of distinguishing amongst healthy, knee OA or RA (Charni et al., 2005). More recently, Eyre and Weis reported an error in the core peptide sequence against which the Helix-II antibodies were generated, indicating that the clinical data require some re-evaluation (Eyre & Weis, 2009). Figure 1(A) illustrates the gross regions of collagen type II cleavage, generated fragments and responsible proteolytic enzymes.
Aggrecanase (ADAMTS), especially ADAMTS-4 and -5 have been implicated to target the proteoglycan aggrecan between the unglycosylated G1 and G2 domains and several regions between the G2 and G3 which are glycosylated with keratin sulfate (KS) and chondroitin sulfate (CS) (Fosang et al., 2008). Mice bearing inactive ADAMTS-5 were more resistant to inflammatory arthritis (Stanton et al., 2005), while double knockouts for ADAMTS-4 and -5 developed lower severities of OA (Majumdar et al., 2007). Furthermore, aggrecan cleavage at the G1/G2 interglobular domain was previously attributed to cathepsin B and D, the last also targets chondroitin sulfate attachment regions (Handley et al., 2001; Mort et al., 1998). Figure 1(C) illustrates aggrecan regions targeted by cathepsins and ADAMTS. The cleavage of aggrecan generates an ARGS neopeptide which is generated from the G1/G2 cleavage site was found increased in synovial fluid with OA severity (Germaschewski et al., 2014). The examination of keratin sulfate (KS) and chondroitin sulfate (CS) using antibodies against both epitopes show that they are elevated in OA cartilage and synovial fluid, their detection was significantly lower in serum, hence significantly restricting their use as an OA biomarker (Garvican et al., 2010).
Hyaluronidase (HAase) is a protease that targets the linear Hyaluronic acid at the β(1,4) site between N-acetyl-d-glucosamine and d-Gluroniuc acid (Figure 1D). Nagava et al. examined the presence of HAase in SF and serum of RA and OA patients, and also found it is correlated with synovitis and RA rather than OA (Nagaya et al., 1999). No reports regarding the detection of HA polymer fragments have been attempted, possibly due to its high variation and potency in numerous tissues other than cartilage. Hyaluronan is often intra-articularly injected to relive OA symptoms, hence posing an additional restriction in attempting to detect its cleaved polymers.
Different from the previously indicated catabolic enzymes involved in cartilage breakdown, lysosomal cysteine proteases, particularly cathepsin K, have also been shown to contribute to cartilage ECM degradation (Dejica et al., 2012; Panwar et al., 2013). However, cathepsins are generally more potent and active in acidic pHs, whereas activities of MMPs and ADAMTS are less influenced by pH. Cathepsin potency in acidic pHs enables efficient turnover of cellular proteins confined to the lysosomes into peptide or amino acids. It has been previously established that OA cartilage and synovial fluid possess acidic pH (Konttinen et al., 2002), which may favor cathepsin catabolic activity. Albeit many reports regarding the involvement of cathepsin K in OA (Konttinen et al., 2002; Salminen-Mankonen et al., 2007), its involvement in enhanced bone resorption facilitated by osteoclast activity in osteoporosis suggests it may not be an ideal candidate biomarker for OA, especially that both conditions are prevalent during progressive ages. On the other hand, cathepsin S and B are enhanced under pro-inflammatory stress and remain relatively active at pH 6–7 supporting that they may target collagen type II and possibly other ECM constituents of cartilage (Baici et al., 1995; Caglic et al., 2013; Dvir-Ginzberg et al., 2011, Salminen-Mankonen et al., 2007). Figure 2(A) shows primary OA-derived chondrocytes exposed to cytokine treatment with enhanced levels of cathepsin B in cell cellular compartments. It has been well established that cathepsins may permeate through the lysosome during inflammatory conditions and thus modulate various cellular and extracellular targets (Dvir-Ginzberg et al., 2011; Guicciardi et al., 2000) (Figure 1A). Solau-Gervais et al. examined the activity of cathepsin B, L, dipeptidyl peptidase and calpain in synovial tissues, and found cathepsin and calpain activities were augmented in RA and OA patients compared to controls (Solau-Gervais et al., 2007). RA exhibited more enzymatic activity of these proteases than OA (Solau-Gervais et al., 2007). These data support the relationship of cathepsin activity, expression and synovial inflammation which is in line with numerous basic studies (Appleton et al., 2007; Lambert et al., 2014). It may be therefore possible to categorize inflammatory state of OA based on the type, activity and level of cathepsins, which may be therapeutically targeted, using personalized medicine.
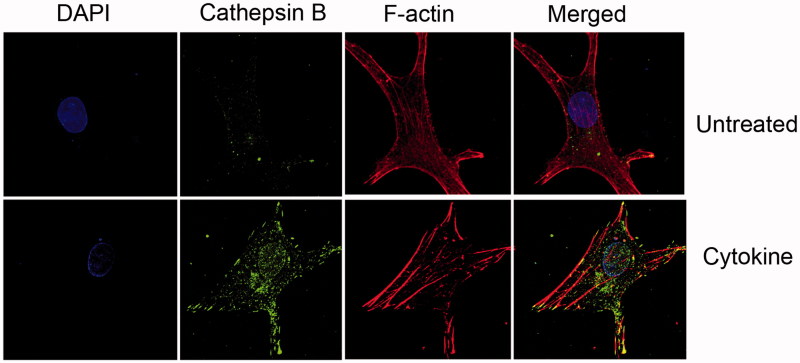
Figure 2.
Increased cellular cathepsin during cytokine treatment of human chondrocytes. OA human-derived chondrocytes were obtained following an informed consent from individuals undergoing total knee replacement (Hadassah Institutional approval obtained). Chondrocytes were isolated and cultured, treated (Cytokine: 50 ng/ml TNFα+2 ng/ml IL1β, 24 h) and stained as previously described (Dvir-Ginzberg et al., 2011; Oppenheimer et al., 2012). Cells stained with DAPI (i.e. nuclei in blue fluorescence), cathepsin B (i.e. active cathepsin B in green florescence), Phalloidine (i.e. F-actin filaments in red florescence), × 600 magnification. The images clearly show that cytokine treatment renders increased cathepsin B levels throughout the cell compartment, as well as the nucleus. The micrographs are representative images of data previously described by Oppenheimer et al., Arthritis and Rheumatology (Oppenheimer et al., 2012).
It should be noted that many of the above-indicated proteases have natural inhibitors. For example, tissue inhibitor of metalloproteinases (TIMPs) inhibits MMP/ADAMTS activity, while cystatins are potent inhibitors of many cathepsins. Overall, these matrix degrading enzymes are necessary for normal ECM turnover, their augmented activity and levels disrupt the normal homeostasis. It is the net effect matrix degrading enzyme expression and levels versus their inhibitors which will determine the state of joint cartilage, therefore more efforts in detecting enzyme activity levels could provide good indicators of disease severity.
Protease cellular targets and affected pathways
While MMP/ADAMTS are often generated and target extracellular components, it is less expected that they would directly affect cellular signaling circuits. One example for the impact of proteases on cell regulation is syndecan 4, which is a transmembrane heparan sulfate proteoglycan capable of associating augmenting ADAMTS-5 activation in collagen type X-expressing chondrocytes (Echtermeyer et al., 2009). Syndecan 4 ectodomain shedding by protease activity (Fitzgerald et al., 2000) could rescue OA phenotype as observed in Syndican null mice (Echtermeyer et al., 2009).
Within the cell, caspases cysteine proteases are involved in inducing cell apoptosis under extrinsic and intrinsic stress conditions. Given that chronic low-grade inflammation is involved in OA pathogenesis, it is not surprising that caspases are active in OA articular chondrocytes (Blanco et al., 1998). The increase in cell apoptosis is accompanies with pro-catabolic joint destruction that appears with OA. The canonical caspase pathway is a mitochondrial-dependent signaling pathways which is induced by an extrinsic death signal, often caused by ligand binding to the death receptor, as TNFα/TLR or FASL/FAS. As part of this process, cytochrome C, which is loosely associated with the inner mitochondrial membrane, is released through mitochondrial pores that are increasingly formed in the outer mitochondrial membrane. The increase of cellular ROS during pro-death signaling also aids in the release of cytochrome C from its mitochondrial anchor cardiolipin, enabling the increase levels of soluble cytochrome C in the cell cytoplasm. Increasing ROS levels additionally enhance lysosomal permeability and cause the dispersity of lysosomal proteases like cathepsins throughout the cells (Figures 1(A) and 2A). Cellular cathepsins are known to cleave BH3 interacting-domain death agonist (Bid) to form truncated Bid (tBid) which binds BCL-2-like protein 4 (Bax) and their multimerization to form pores through which cytochrome C escapes the mitochondria (Cirman et al., 2004). Another example for cathepsin activity within the cell is the capacity of cathepsin L to cleave the N-terminal domain of histone 3 during mouse embryogenesis, thereby possibly effecting the gene regulation during ESC differentiation (Duncan et al., 2008).
With regard to articular chondrocytes, our group identified enhanced cathepsin B levels throughout the cell upon cytokine stimulation results in C-terminal truncation of the protein deacetylase SIRT1, hence inducing its nuclear export and influencing numerous cellular pathways (Dvir-Ginzberg et al., 2011; Oppenheimer et al., 2012). These data were also supported by in-vivo observations, showing Sirt1 wild-type (WT) 9-month-old strains presenting the 75SIRT1 cleaved variant in articular chondrocytes compared to haploinsufficient mice (Gabay et al., 2012). More interestingly, 9-month-old WT mice exhibited reduced OA severity and chondrocyte apoptosis compared with age-matched haploinsufficient mice, indicating 75SIRT1 could rise from stressed chondrocytes primed to apoptose, making it a potential biomarker candidate for predicting OA susceptibility. More recent studies showed that high-fat diet (HFD) fed mice and adipose express a caspase 1 N-terminally cleaved variant of SIRT1 (Chalkiadaki & Guarente, 2012). These observations suggest that the inflammasome are activated in adipose tissue, and thus induce N-terminal truncation of SIRT1. The inflammasome is a multi-protein oligomer composed of Nod-like receptors (NLRs), apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase 1. Upon assembly, pro-caspase 1 becomes active and cleaved pro-IL1β, pro-IL-18 perpetuating a pro-inflammatory response and activating pyroptosis process, which is a type of programmed cell death induced by pathogens (Franchi et al., 2009). Therefore, it is not surprising that the activation and assembly of inflammasome has been linked to exposure to microbial molecules or insoluble crystals such as urate crystals and fibrilar amyloid-β, hence associating this mechanism with Gout, Atherosclerosis, Alzheimer's and Diabetes (Tschopp & Schroder, 2010). The engulfment of crystalline or other particulate structures have been reported to cause lysosomal rupture and cathepsin B release prior to inflammasome assembly. Notably, cathepsin B has been suggested to cleave and activate the NLR family and facilitate inflammasome formation (Franchi et al., 2009), hence it appears that both inflammasome and apoptosome pathways are affected by cathepsin activity within the cytoplasm.
Summery and discussion
In recent years, a substantial degree of effort has been invested in identifying biomarkers for OA predisposition and severity. These biomarkers and/or advanced imaging techniques not only identify the exact stage of joint destruction and OA severity but also pose an attractive setting to test drug efficacy and prognosis. Hence, the identification of novel means to detect OA susceptibility is paramount for the discovery of novel drug targets as well as validating their efficacy. It is notable that the past decade of research yielded various markers based on matrix degradation products and enzymes. The most remarkable and consistent of those is the collagen type II degradation fragments, CTXII which have been identified in serum and urine of OA patients (Abramson & Krasnokutsky, 2006). Nonetheless, biomarkers which are responsive to the biological state of cartilage and chondrocytes are less successfully identified. Given that cartilage is continuously remodeled, it would be difficult to assess subtle changes of collagen type II fragments and correlate them with remission of disease. It is therefore envisioned that an ideal biomarker or combination thereof would be significantly altered during disease progression and remission. For this reason, biomarkers derived from key signaling circuits linked to chondrocyte biology may answer this unmet limitation. Moreover, the process of enhanced ECM proteolysis is attractive given the abundance of cartilaginous fragments and their relative ease of detection, however, detection of proteolytically processed cellular components may be difficult, especially given the restricted amount of articular chondrocytes compared to other tissues. Nonetheless if this is achieved, such cellular biomarkers could be of paramount importance in delivering a biological beacon of cartilage nature. Cathepsins and caspases are the two major enzymes partaking in cellular processes. While caspases are activated in cell stress and death, cathepsins are primarily lysosomal and participate in protein turnover. Under stress, cathepsins participate in cleavage of extracellular aggrecan and collagen type II (Handley et al., 2001; Mort et al., 1998), as well as cellular SIRT1, Histone 3, Bid and indirectly IL1β/18 via inflammasome activation (Cirman et al., 2004; Duncan et al., 2008; Dvir-Ginzberg et al., 2011; Franchi et al., 2009; Tschopp & Schroder, 2010).
More specifically, the cleavage of SIRT1, an important regulator of cartilage homeostasis and survival (Dvir-Ginzberg & Steinmeyer, 2013; Dvir-Ginzberg et al., 2008; Gabay et al., 2013; Oppenheimer et al., 2014), could be a good indicator of cartilage stress especially if it is present in synovial secretome and blood. An interesting study recently published showed that SIRT1 (full-length) is reduced in Alzheimer susceptible patients, using an ELISA based method, showing an overall reduction of serum Sirt1 with age and susceptibility to Alzheimer's (Kumar et al., 2013). Given that SIRT1 reduction may be an age-dependent process, analyzing tissue-specific SIRT1-derived proteolytic variants could make a more distinctive observation regarding particular pathologies, as OA.
Summarizing the efforts in the next years should target cell-derived proteolytic products which are more likely linked to the biological state of the cells and may enable, based on their levels and timing of detection, the application of disease modifying agents as a therapeutic intervention. This not only answers an unmet need in the biomarker arena of OA, but also opens a new venue of personalized care for OA susceptible individuals based on the disease stage and severity.
Declaration of interest
Authors declare no conflict of interest. M. D.-G. is a partner in the D-BOARD Consortium, Novel Diagnostics and Biomarkers for Early Identification of Chronic Inflammatory Joint Diseases. The research leading to these results has received full funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 305815.
References
- Abella V, Scotece M, Conde J, et al. Adipokines, metabolic syndrome and rheumatic diseases. J Immunol Res. 2014;2014 doi: 10.1155/2014/343746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson S, Krasnokutsky S. Biomarkers in osteoarthritis. Bull NYU Hosp Jt Dis. 2006;64:77–81. [PubMed] [Google Scholar]
- Appleton CT, Pitelka V, Henry J, Beier F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 2007;56:1854–68. doi: 10.1002/art.22711. [DOI] [PubMed] [Google Scholar]
- Ariyoshi W, Takahashi N, Hida D, et al. Mechanisms involved in enhancement of the expression and function of aggrecanases by hyaluronan oligosaccharides. Arthritis Rheum. 2012;64:187–97. doi: 10.1002/art.33329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baici A, Horler D, Lang A, et al. Cathepsin B in osteoarthritis: zonal variation of enzyme activity in human femoral head cartilage. Ann Rheum Dis. 1995;54:281–8. doi: 10.1136/ard.54.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco FJ, Guitian R, Vazquez-Martul E, et al. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–9. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Borzi RM, Mazzetti I, Cattini L, et al. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 2000;43:1734–41. doi: 10.1002/1529-0131(200008)43:8<1734::AID-ANR9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Brady MA, Waldman SD, Ethier CR. The application of multiple biophysical cues to engineer functional neocartilage for treatment of osteoarthritis (part I: cellular response) Tissue Eng Part B Rev 2014 doi: 10.1089/ten.TEB.2013.0757. . [Epub ahead of print]. PMID: 24919456. [DOI] [PubMed] [Google Scholar]
- Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–43. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- Caglic D, Repnik U, Jedeszko C, et al. The proinflammatory cytokines interleukin-1alpha and tumor necrosis factor alpha promote the expression and secretion of proteolytically active cathepsin S from human chondrocytes. Biol Chem. 2013;394:307–16. doi: 10.1515/hsz-2012-0283. [DOI] [PubMed] [Google Scholar]
- Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16:180–8. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charni N, Juillet F, Garnero P. Urinary type II collagen helical peptide (HELIX-II) as a new biochemical marker of cartilage degradation in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2005;52:1081–90. doi: 10.1002/art.20930. [DOI] [PubMed] [Google Scholar]
- Cirman T, Oresic K, Mazovec GD, et al. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of bid by multiple papain-like lysosomal cathepsins. J Biol Chem. 2004;279:3578–87. doi: 10.1074/jbc.M308347200. [DOI] [PubMed] [Google Scholar]
- De Croos JN, Dhaliwal SS, Grynpas MD, et al. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biol. 2006;25:323–31. doi: 10.1016/j.matbio.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Deberg M, Labasse A, Christgau S. New serum biochemical markers (Coll 2-1 and Coll 2-1 NO2) for studying oxidative-related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2005a;13:258–65. doi: 10.1016/j.joca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Deberg MA, Labasse AH, Collette J, et al. One-year increase of Coll 2-1, a new marker of type II collagen degradation, in urine is highly predictive of radiological OA progression. Osteoarthritis Cartilage. 2005b;13:1059–65. doi: 10.1016/j.joca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Dejica VM, Mort JS, Laverty S, et al. Increased type II collagen cleavage by cathepsin K and collagenase activities with aging and osteoarthritis in human articular cartilage. Arthritis Res Ther. 2012;14:R113. doi: 10.1186/ar3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Carlo M, Schwartz D, Erickson EA, Loeser RF. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic Biol Med. 2007;42:1350–8. doi: 10.1016/j.freeradbiomed.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demircan K, Hirohata S, Nishida K, et al. ADAMTS-9 is synergistically induced by interleukin-1beta and tumor necrosis factor alpha in OUMS-27 chondrosarcoma cells and in human chondrocytes. Arthritis Rheum. 2005;52:1451–60. doi: 10.1002/art.21010. [DOI] [PubMed] [Google Scholar]
- Duncan EM, Muratore-Schroeder TL, Cook RG, et al. Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell. 2008;135:284–94. doi: 10.1016/j.cell.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J Biol Chem. 2008;283:36300–10. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir-Ginzberg M, Gagarina V, Lee EJ, et al. Tumor necrosis factor alpha-mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis Rheum. 2011;63:2363–73. doi: 10.1002/art.30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir-Ginzberg M, Steinmeyer J. Towards elucidating the role of SirT1 in osteoarthritis. Front Biosci (Landmark Ed) 2013;18:343–55. doi: 10.2741/4105. [DOI] [PubMed] [Google Scholar]
- Echtermeyer F, Bertrand J, Dreier R, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–6. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- El Mabrouk M, Sylvester J, Zafarullah M. Signaling pathways implicated in oncostatin M-induced aggrecanase-1 and matrix metalloproteinase-13 expression in human articular chondrocytes. Biochim Biophys Acta. 2007;1773:309–20. doi: 10.1016/j.bbamcr.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Weis MA. The Helix-II epitope: a cautionary tale from a cartilage biomarker based on an invalid collagen sequence. Osteoarthritis Cartilage. 2009;17:423–6. doi: 10.1016/j.joca.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, Wang Z, Park PW, et al. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–24. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang AJ, Beier F. Emerging frontiers in cartilage and chondrocyte biology. Best Pract Res Clin Rheumatol. 2011;25:751–66. doi: 10.1016/j.berh.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Fosang AJ, Rogerson FM, East CJ, Stanton H. ADAMTS-5: the story so far. Eur Cell Mater. 2008;15:11–26. doi: 10.22203/ecm.v015a02. [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay O, Oppenhiemer H, Meir H, et al. Increased apoptotic chondrocytes in articular cartilage from adult heterozygous SirT1 mice. Ann Rheum Dis. 2012;71:613–16. doi: 10.1136/ard.2011.200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay O, Zaal KJ, Sanchez C, et al. Sirt1-deficient mice exhibit an altered cartilage phenotype. Joint Bone Spine. 2013;80:613–20. doi: 10.1016/j.jbspin.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvican ER, Vaughan-Thomas A, Clegg PD, Innes JF. Biomarkers of cartilage turnover. Part 2: Non-collagenous markers. Vet J. 2010;185:43–9. doi: 10.1016/j.tvjl.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Germaschewski FM, Matheny CJ, Larkin J, et al. Quantitation OF ARGS aggrecan fragments in synovial fluid, serum and urine from osteoarthritis patients. Osteoarthritis Cartilage. 2014;22:690–7. doi: 10.1016/j.joca.2014.02.930. [DOI] [PubMed] [Google Scholar]
- Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:376–8. [PubMed] [Google Scholar]
- Guicciardi ME, Deussing J, Miyoshi H, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–37. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley CJ, Mok MT, Ilic MZ, et al. Cathepsin D cleaves aggrecan at unique sites within the interglobular domain and chondroitin sulfate attachment regions that are also cleaved when cartilage is maintained at acid pH. Matrix Biol. 2001;20:543–53. doi: 10.1016/s0945-053x(01)00168-8. [DOI] [PubMed] [Google Scholar]
- Iannone F, Lapadula G. The pathophysiology of osteoarthritis. Aging Clin Exp Res. 2003;15:364–72. doi: 10.1007/BF03327357. [DOI] [PubMed] [Google Scholar]
- Karsdal MA, Woodworth T, Henriksen K, et al. Biochemical markers of ongoing joint damage in rheumatoid arthritis--current and future applications, limitations and opportunities. Arthritis Res Ther. 2011;13:215. doi: 10.1186/ar3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, Cho ML, Choi HY, et al. The catabolic pathway mediated by toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54:2152–63. doi: 10.1002/art.21951. [DOI] [PubMed] [Google Scholar]
- Kim HA, Suh DI, Song YW. Relationship between chondrocyte apoptosis and matrix depletion in human articular cartilage. J Rheumatol. 2001;28:2038–45. [PubMed] [Google Scholar]
- Konttinen YT, Mandelin J, Li TF, et al. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 2002;46:953–60. doi: 10.1002/art.10185. [DOI] [PubMed] [Google Scholar]
- Kumar R, Chaterjee P, Sharma PK, et al. Sirtuin1: a promising serum protein marker for early detection of Alzheimer's disease. PLoS One. 2013;8:e61560. doi: 10.1371/journal.pone.0061560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Dubuc JE, Montell E, et al. Gene expression pattern of cells from inflamed and normal areas of osteoarthritis synovial membrane. Arthritis Rheumatol. 2014;66:960–8. doi: 10.1002/art.38315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Frank EH, Wang Y, et al. Moderate dynamic compression inhibits pro-catabolic response of cartilage to mechanical injury, tumor necrosis factor-alpha and interleukin-6, but accentuates degradation above a strain threshold. Osteoarthritis Cartilage. 2013;21:1933–41. doi: 10.1016/j.joca.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little CB, Barai A, Burkhardt D, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–33. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar MK, Askew R, Schelling S, et al. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56:3670–4. doi: 10.1002/art.23027. [DOI] [PubMed] [Google Scholar]
- Manicourt DH, Poilvache P, Van Egeren A, et al. Synovial fluid levels of tumor necrosis factor alpha and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheum. 2000;43:281–8. doi: 10.1002/1529-0131(200002)43:2<281::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Nishida K, Yoshida A, et al. Expression of caspase-3 and -9 relevant to cartilage destruction and chondrocyte apoptosis in human osteoarthritic cartilage. Acta Med Okayama. 2001;55:333–40. doi: 10.18926/AMO/32000. [DOI] [PubMed] [Google Scholar]
- Mengshol JA, Vincenti MP, Brinckerhoff CE. IL-1 induces collagenase-3 (MMP-13) promoter activity in stably transfected chondrocytic cells: requirement for Runx-2 and activation by p38 MAPK and JNK pathways. Nucleic Acids Res. 2001;29:4361–72. doi: 10.1093/nar/29.21.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengshol JA, Vincenti MP, Coon CI, et al. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–11. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Mort JS, Magny MC, Lee ER. Cathepsin B: an alternative protease for the generation of an aggrecan ‘metalloproteinase’ cleavage neoepitope. Biochem J. 1998;335:491–4. doi: 10.1042/bj3350491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya H, Ymagata T, Ymagata S, et al. Examination of synovial fluid and serum hyaluronidase activity as a joint marker in rheumatoid arthritis and osteoarthritis patients (by zymography) Ann Rheum Dis. 1999;58:186–8. doi: 10.1136/ard.58.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer H, Gabay O, Meir H, et al. 75-kd sirtuin 1 blocks tumor necrosis factor alpha-mediated apoptosis in human osteoarthritic chondrocytes. Arthritis Rheum. 2012;64:718–28. doi: 10.1002/art.33407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer H, Kumar A, Meir H, et al. Set7/9 impacts COL2A1 expression through binding and repression of SirT1 histone deacetylation. J Bone Miner Res. 2014;29:348–60. doi: 10.1002/jbmr.2052. [DOI] [PubMed] [Google Scholar]
- Panwar P, Du X, Sharma V, et al. Effects of cysteine proteases on the structural and mechanical properties of collagen fibers. J Biol Chem. 2013;288:5940–50. doi: 10.1074/jbc.M112.419689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizman I, De Croos JN, St-Pierre JP, et al. Articular cartilage subpopulations respond differently to cyclic compression in vitro. Tissue Eng A. 2009;15:3789–98. doi: 10.1089/ten.TEA.2008.0530. [DOI] [PubMed] [Google Scholar]
- Rousseau JC, Delmas PD. Biological markers in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3:346–56. doi: 10.1038/ncprheum0508. [DOI] [PubMed] [Google Scholar]
- Sakai T, Kambe F, Mitsuyama H, et al. Tumor necrosis factor alpha induces expression of genes for matrix degradation in human chondrocyte-like HCS-2/8 cells through activation of NF-kappaB: abrogation of the tumor necrosis factor alpha effect by proteasome inhibitors. J Bone Miner Res. 2001;16:1272–80. doi: 10.1359/jbmr.2001.16.7.1272. [DOI] [PubMed] [Google Scholar]
- Salminen-Mankonen HJ, Morko J, Vuorio E. Role of cathepsin K in normal joints and in the development of arthritis. Curr Drug Targets. 2007;8:315–23. doi: 10.2174/138945007779940188. [DOI] [PubMed] [Google Scholar]
- Solau-Gervais E, Zerimech F, Lemaire R, et al. Cysteine and serine proteases of synovial tissue in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 2007;36:373–7. doi: 10.1080/03009740701340172. [DOI] [PubMed] [Google Scholar]
- Spiteri C, Raizman I, Pilliar RM, Kandel RA. Matrix accumulation by articular chondrocytes during mechanical stimulation is influenced by integrin-mediated cell spreading. J Biomed Mater Res A. 2010;94:122–9. doi: 10.1002/jbm.a.32706. [DOI] [PubMed] [Google Scholar]
- Stanton H, Rogerson FM, East CJ, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–52. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Sylvester J, Liacini A, Li WQ, Zafarullah M. Interleukin-17 signal transduction pathways implicated in inducing matrix metalloproteinase-3, -13 and aggrecanase-1 genes in articular chondrocytes. Cell Signal. 2004;16:469–76. doi: 10.1016/j.cellsig.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824:133–45. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–15. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Yuan GH, Masuko-Hongo K, Sakata M, et al. The role of C-C chemokines and their receptors in osteoarthritis. Arthritis Rheum. 2001;44:1056–70. doi: 10.1002/1529-0131(200105)44:5<1056::AID-ANR186>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]