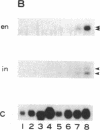
Abstract
Genetic analysis in Drosophila has shown that engrailed (en) plays an important role in segmentation and neurogenesis. A closely related gene, invected (in), is coexpressed with en in the posterior developmental compartments where en is known to specify cell state. We report here the isolation of two en-like cDNAs from the middle silk glands of Bombyx mori larvae. Sequence analysis revealed that they are the counterparts of Drosophila en and in. Four highly conserved domains, including the homeodomain, were identified in these En and In proteins from Bombyx and Drosophila. In addition, two en-specific and one in-specific domains could also be found. These structurally homologous genes might share a similar role in Bombyx development. They were found to be coexpressed in the middle silk gland but not in the posterior silk gland during the fourth molt/fifth intermolt period. We speculate that these Bombyx en-like genes might be involved in the compartmentalization of the silk gland.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brivanlou A. H., Harland R. M. Expression of an engrailed-related protein is induced in the anterior neural ectoderm of early Xenopus embryos. Development. 1989 Jul;106(3):611–617. doi: 10.1242/dev.106.3.611. [DOI] [PubMed] [Google Scholar]
- Campbell G. L., Caveney S. Engrailed gene expression in the abdominal segment of Oncopeltus: gradients and cell states in the insect segment. Development. 1989 Aug;106(4):727–737. doi: 10.1242/dev.106.4.727. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coleman K. G., Poole S. J., Weir M. P., Soeller W. C., Kornberg T. The invected gene of Drosophila: sequence analysis and expression studies reveal a close kinship to the engrailed gene. Genes Dev. 1987 Mar;1(1):19–28. doi: 10.1101/gad.1.1.19. [DOI] [PubMed] [Google Scholar]
- Davidson D., Graham E., Sime C., Hill R. A gene with sequence similarity to Drosophila engrailed is expressed during the development of the neural tube and vertebrae in the mouse. Development. 1988 Oct;104(2):305–316. doi: 10.1242/dev.104.2.305. [DOI] [PubMed] [Google Scholar]
- Davis C. A., Holmyard D. P., Millen K. J., Joyner A. L. Examining pattern formation in mouse, chicken and frog embryos with an En-specific antiserum. Development. 1991 Feb;111(2):287–298. doi: 10.1242/dev.111.2.287. [DOI] [PubMed] [Google Scholar]
- Davis C. A., Joyner A. L. Expression patterns of the homeo box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev. 1988 Dec;2(12B):1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- Davis C. A., Noble-Topham S. E., Rossant J., Joyner A. L. Expression of the homeo box-containing gene En-2 delineates a specific region of the developing mouse brain. Genes Dev. 1988 Mar;2(3):361–371. doi: 10.1101/gad.2.3.361. [DOI] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The sequence specificity of homeodomain-DNA interaction. Cell. 1988 Sep 23;54(7):1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolecki G. J., Humphreys T. An engrailed class homeo box gene in sea urchins. Gene. 1988 Apr 15;64(1):21–31. doi: 10.1016/0378-1119(88)90477-5. [DOI] [PubMed] [Google Scholar]
- Fjose A., Eiken H. G., Njølstad P. R., Molven A., Hordvik I. A zebrafish engrailed-like homeobox sequence expressed during embryogenesis. FEBS Lett. 1988 Apr 25;231(2):355–360. doi: 10.1016/0014-5793(88)80849-4. [DOI] [PubMed] [Google Scholar]
- Fjose A., McGinnis W. J., Gehring W. J. Isolation of a homoeo box-containing gene from the engrailed region of Drosophila and the spatial distribution of its transcripts. Nature. 1985 Jan 24;313(6000):284–289. doi: 10.1038/313284a0. [DOI] [PubMed] [Google Scholar]
- Gardner C. A., Darnell D. K., Poole S. J., Ordahl C. P., Barald K. F. Expression of an engrailed-like gene during development of the early embryonic chick nervous system. J Neurosci Res. 1988 Oct-Dec;21(2-4):426–437. doi: 10.1002/jnr.490210234. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Poole S. J., Kornberg T. B. The Drosophila engrailed protein is phosphorylated by a serine-specific protein kinase. Nucleic Acids Res. 1988 Jul 25;16(14A):6637–6647. doi: 10.1093/nar/16.14.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama C., Ali Z., Kornberg T. B. Region-specific recombination and expression are directed by portions of the Drosophila engrailed promoter. Genes Dev. 1990 Jul;4(7):1079–1093. doi: 10.1101/gad.4.7.1079. [DOI] [PubMed] [Google Scholar]
- Han K., Levine M. S., Manley J. L. Synergistic activation and repression of transcription by Drosophila homeobox proteins. Cell. 1989 Feb 24;56(4):573–583. doi: 10.1016/0092-8674(89)90580-1. [DOI] [PubMed] [Google Scholar]
- Hatta K., Schilling T. F., BreMiller R. A., Kimmel C. B. Specification of jaw muscle identity in zebrafish: correlation with engrailed-homeoprotein expression. Science. 1990 Nov 9;250(4982):802–805. doi: 10.1126/science.1978412. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., de la Torre J. R., Holt C., Harland R. M. Cephalic expression and molecular characterization of Xenopus En-2. Development. 1991 Mar;111(3):715–724. doi: 10.1242/dev.111.3.715. [DOI] [PubMed] [Google Scholar]
- Hui C. C., Matsuno K., Suzuki Y. Fibroin gene promoter contains a cluster of homeodomain binding sites that interact with three silk gland factors. J Mol Biol. 1990 Jun 20;213(4):651–670. doi: 10.1016/S0022-2836(05)80253-0. [DOI] [PubMed] [Google Scholar]
- Hui C. C., Suzuki Y., Kikuchi Y., Mizuno S. Homeodomain binding sites in the 5' flanking region of the Bombyx mori silk fibroin light-chain gene. J Mol Biol. 1990 Jun 5;213(3):395–398. doi: 10.1016/S0022-2836(05)80201-3. [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., O'Farrell P. H. Activation and repression of transcription by homoeodomain-containing proteins that bind a common site. Nature. 1988 Dec 22;336(6201):744–749. doi: 10.1038/336744a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A. L., Herrup K., Auerbach B. A., Davis C. A., Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science. 1991 Mar 8;251(4998):1239–1243. doi: 10.1126/science.1672471. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Martin G. R. En-1 and En-2, two mouse genes with sequence homology to the Drosophila engrailed gene: expression during embryogenesis. Genes Dev. 1987 Mar;1(1):29–38. doi: 10.1101/gad.1.1.29. [DOI] [PubMed] [Google Scholar]
- Kassis J. A., Poole S. J., Wright D. K., O'Farrell P. H. Sequence conservation in the protein coding and intron regions of the engrailed transcription unit. EMBO J. 1986 Dec 20;5(13):3583–3589. doi: 10.1002/j.1460-2075.1986.tb04686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P. On the role of the engrailed+ gene in the internal organs of Drosophila. EMBO J. 1984 Dec 1;3(12):2839–2844. doi: 10.1002/j.1460-2075.1984.tb02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Morata G. Compartments in the wing of Drosophila: a study of the engrailed gene. Dev Biol. 1976 Jun;50(2):321–337. doi: 10.1016/0012-1606(76)90155-x. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Struhl G. Further studies of the engrailed phenotype in Drosophila. EMBO J. 1982;1(7):827–833. doi: 10.1002/j.1460-2075.1982.tb01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. H. Simple method for constructing phylogenetic trees from distance matrices. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1085–1089. doi: 10.1073/pnas.78.2.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C., Willard H. F., Rommens J. M., Joyner A. L. Chromosomal localization of the human homeo box-containing genes, EN1 and EN2. Genomics. 1989 Feb;4(2):206–209. doi: 10.1016/0888-7543(89)90301-7. [DOI] [PubMed] [Google Scholar]
- Maekawa H., Suzuki Y. Repeated turn-off and turn-on of fibroin gene transcription during silk gland development of Bombyx mori. Dev Biol. 1980 Aug;78(2):394–406. doi: 10.1016/0012-1606(80)90343-7. [DOI] [PubMed] [Google Scholar]
- Martinez S., Alvarado-Mallart R. M. Expression of the homeobox Chick-en gene in chick/quail chimeras with inverted mes-metencephalic grafts. Dev Biol. 1990 Jun;139(2):432–436. doi: 10.1016/0012-1606(90)90312-7. [DOI] [PubMed] [Google Scholar]
- Ohkuma Y., Horikoshi M., Roeder R. G., Desplan C. Binding site-dependent direct activation and repression of in vitro transcription by Drosophila homeodomain proteins. Cell. 1990 May 4;61(3):475–484. doi: 10.1016/0092-8674(90)90529-n. [DOI] [PubMed] [Google Scholar]
- Patel N. H., Kornberg T. B., Goodman C. S. Expression of engrailed during segmentation in grasshopper and crayfish. Development. 1989 Oct;107(2):201–212. doi: 10.1242/dev.107.2.201. [DOI] [PubMed] [Google Scholar]
- Patel N. H., Martin-Blanco E., Coleman K. G., Poole S. J., Ellis M. C., Kornberg T. B., Goodman C. S. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989 Sep 8;58(5):955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Walldorf U., Fleig R., Gehring W. J. Comparison of homeobox-containing genes of the honeybee and Drosophila. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9971–9975. doi: 10.1073/pnas.86.24.9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblat D. A., Price D. J., Wedeen C. J. Segmentation in leech development. Development. 1988;104 (Suppl):161–168. doi: 10.1242/dev.104.Supplement.161. [DOI] [PubMed] [Google Scholar]