Abstract
Objective To evaluate the bioavailability and safety of a novel vaginal capsule containing solubilized bioidentical 17β-estradiol for vulvar and vaginal atrophy and compare its pharmacokinetics with that of an approved vaginal estradiol tablet in healthy postmenopausal women.
Methods Two randomized, single-dose, two-way cross-over, relative bioavailability trials compared the pharmacokinetics of a solubilized vaginal estradiol softgel capsule (TX-004HR, test) with that of a vaginal estradiol tablet (Vagifem®, reference) in postmenopausal women (aged 40–65 years) at 10-μg and 25-μg doses. In each study, women were randomly assigned to receive a single dose of the test capsule or reference tablet, followed by a single dose of the alternate drug after a 14-day washout.
Results Thirty-five women completed the 10-μg study and 36 completed the 25-μg study. Significantly lower systemic levels of estradiol, estrone, and estrone sulfate at both doses of the test product were observed compared with equivalent doses of the reference product, with lower AUC0-24 and C max and earlier t max. No adverse events were reported in either trial.
Conclusion TX-004HR, a novel estradiol vaginal softgel capsule, exhibited significantly lower systemic exposure than equivalent doses of an approved vaginal estradiol tablet at both 10-μg and 25-μg doses. Both doses of each product were safe and well-tolerated.
KEYWORDS: Bioavailability, estradiol, menopause, pharmacokinetics, vaginal estrogen therapy, vulvar and vaginal atrophy
Introduction
Vulvar and vaginal atrophy (VVA), a component of the genitourinary syndrome of menopause (GSM), is a common condition, with symptoms occurring in up to 50% of postmenopausal women1 , 2. Complaints include vaginal dryness, vulvar and vaginal itching and irritation, and pain with sexual activity3. Approximately 40% of affected women use over-the-counter (OTC), herbal, or prescription products to treat their symptoms3. For symptomatic women who do not respond to non-hormonal interventions, local low-dose estrogen therapy is preferred when vaginal symptoms associated with VVA are the only complaint4 , 5.
Publication of the data from the Women’s Health Initiative (WHI) in 20026 resulted in decreased prescribing of systemic menopausal hormone therapy (HT)7–9. By 2013, the REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs (REVIVE) survey reported that only 7% of women with VVA were being treated with prescription therapy alone3. Despite proven minimal systemic exposure10, the boxed warning for estrogen class labeling is required for low-dose vaginal estrogen products and may impact clinicians’ and women’s treatment decisions11. Surveys of postmenopausal women with VVA indicate that they are concerned about the safety of current treatments2 , 3 and are dissatisfied with the efficacy of available products and how they are administered3. Thus, there is a need for a new, safe and convenient treatment option that results in lower systemic concentration of estradiol and its metabolites.
Solubilized 17β-estradiol (TX-004HR) in a new softgel capsule (VagiCap™, TherapeuticsMD, Inc., Boca Raton, FL, USA) has been developed for vaginal administration for the treatment of moderate to severe dyspareunia associated with VVA. The novel capsule was designed to provide local efficacy (i.e. estrogenic effect on the vaginal tissue) without increasing systemic exposure of estradiol. In addition, it was designed for convenience; it is easily inserted without the need for an applicator and is not as messy as creams.
TX-004HR is anticipated to provide a treatment option for women with VVA with early onset of action, low systemic estrogen exposure, and ease of use, resulting in better compliance than with currently available products. The objectives of the two studies reported here were to demonstrate the pharmacokinetic and safety profiles of TX-004HR at doses of 10 μg and 25 μg in healthy postmenopausal women, and to compare its pharmacokinetic parameters with those resulting from the same doses of an approved solid vaginal tablet of estradiol.
Methods
Study design
Two randomized, two-treatment, two-period, two-sequence, single-dose, open-label, two-way cross-over, relative bioavailability studies were conducted to obtain data on the pharmacokinetic properties and safety of TX-004HR, an investigational vaginal softgel capsule that contains solubilized 17β-estradiol (test), compared with an estradiol vaginal tablet (reference; Vagifem®; Novo Nordisk, Plainsboro, NJ, USA) approved by the FDA to treat menopause-related atrophic vaginitis12. One study compared doses of 10 μg and the other compared doses of 25 μg for each product in healthy postmenopausal women.
Eligible subjects were randomly assigned to receive a single dose of both the test product and the reference product, sequentially, during two study periods in one of two possible sequences (TR or RT; T = test, R = reference) according to a computer-generated randomization schedule. To minimize bias, the bioanalytical analyst was blinded to the randomization code. Each study period was separated by a 14-day washout, and both studies lasted 17 days.
For each study period, women were admitted to the clinical facility at least 11 h before dosing (with an overnight fast of at least 10 h) and remained in the facility for at least 24 h after dosing. At admission, participants were screened for recent drug or alcohol use and for pregnancy. A trained female investigator administered either the TX-004HR capsule (1–2 inches inside the vaginal canal) or the reference tablet (using the manufacturer’s vaginal applicator and directions for insertion), after which women remained supine for 4 h. Participants received standard meals at scheduled times and were instructed to refrain from strenuous exercise. Subjects were monitored for safety before, during, and at the end of the study with frequent clinical examinations, vital sign assessments, laboratory values, and well-being questionnaires.
Thirteen 10-ml blood samples were collected during each study period via an indwelling intravenous cannula. Sampling occurred 1 h, 0.5 h, and immediately before dosing; and then at 1, 2, 4, 6, 8, 10, 12, 14, 18, and 24 h after dosing. An 8-ml blood sample was collected at the conclusion of the study for a post-study safety assessment (at 24 h after the second study period). Pharmacokinetic samples were centrifuged (4000 rpm for 10 min at 4 °C) within 30 min of collection, and the separated plasma was transferred into polypropylene tubes. Aliquots for both studies were stored at −30 °C until analyses.
Study participants
Both pharmacokinetic studies recruited postmenopausal women aged 40–65 years with a body mass index (BMI) between 18.50 and 29.99 kg/m2. Women were eligible to participate if they were generally healthy and postmenopausal, as defined by plasma estradiol concentration <50 pg/ml, follicle stimulating hormone level >40 IU/l, and cessation of vaginal bleeding for ≥12 months or 6 weeks post-bilateral oophorectomy with or without hysterectomy. Candidates were required to discontinue prescription drugs 14 days before the study.
The studies excluded women who were pregnant or breast-feeding, had any allergy or hypersensitivity to estradiol and related drugs, were current smokers, or had a substance abuse history, undiagnosed abnormal vaginal bleeding, history of breast cancer or thromboembolic disorders, or any current or prior medical condition that might compromise pharmacokinetic outcomes or represent a safety risk. Participants were excluded from using vaginal medications including vaginal hormone products within the past week, estrogen-containing lotions/gels within the past 4 weeks, transdermal or oral HT within the past 8 weeks, progestational implants, estrogen or estrogen/progestational injectables within the past 3 months, or estrogen pellets/progestational injectables within the past 6 months.
An independent ethics committee approved the protocol for both studies. Participants provided written informed consent before any study-related activities. The trials were conducted in accordance with Good Clinical Practices, US federal regulations, and the Declaration of Helsinki.
Study endpoints
The primary pharmacokinetic endpoints in both studies were area under the concentration–time curve from zero to 24 h (AUC0–24) and maximum concentration (C max) for estradiol, estrone, and estrone sulfate (baseline adjusted and unadjusted). Time to maximum concentration (t max) was a secondary endpoint. The studies were designed to evaluate the relative bioavailability of two separate doses (10 μg and 25 μg) of the test product (TX-004HR) compared with that of the reference product (Vagifem). Safety endpoints included adverse events, hematologic and biochemical findings from the screening (pre-study) laboratory results, and a post-study laboratory assessment.
Analytical methods
Concentrations of estradiol, estrone, and estrone sulfate in human plasma were ascertained using a validated liquid chromatography–tandem mass spectrometry method in both studies. The lower limits of quantification (LLOQ) for the 10-μg study were 1.00 pg/ml for estradiol, 2.5 pg/ml for estrone, and 10 ng/dl for estrone sulfate. In the 25-μg study, the LLOQ were 2.00 pg/ml for estradiol, 9.91 pg/ml for estrone, and 20.08 pg/ml for estrone sulfate. The analyses for the 10-μg study were performed at Esoterix Endocrinology (Calabasas, CA, USA) and for the 25-μg product at Micro Therapy Research Labs Private Ltd. (Chennai, India). All concentration values below the LLOQ were set to zero for the analyses.
Estimates of the pharmacokinetic parameters were performed for estradiol, estrone and estrone sulfate for the 10-μg study at Biostudy Solutions, LLC (Wilmington, NC, USA) using non-compartmental modeling in WinNonlin software version 5.2 (Pharsight Corporation, USA), and at Micro Therapy Research Labs Private Ltd. (Chennai, India) for the 25-μg study using WinNonlin software version 5.3 to perform non-compartmental modeling.
Statistical analyses
In each trial, a sample size of 36 subjects was considered sufficient for evaluating relative bioavailability between the test and reference formulations, taking into account the possibility of study dropouts.
Statistical analyses were performed with SAS version 9.1.3 (10-μg study) and SAS version 9.2 (25-μg study; SAS Institute, Cary, NC, USA). Participants who completed both study periods were included in the statistical and pharmacokinetic analyses. Women who had negative concentrations of a hormone at all sample time points after baseline correction were excluded from statistical evaluation.
Descriptive statistics were calculated for baseline-adjusted and unadjusted estradiol, estrone, and estrone sulfate for C max, AUC0–24, and t max for both the test and reference products at each study dose. Test and reference products were compared using analysis of variance (ANOVA) for AUC0–24 and C max (ln-transformed) for estradiol, estrone, and estrone sulfate concentrations. The models included terms for sequence, subjects-within-sequence, period, and treatment effects. ANOVA was performed in the 10-μg study using general linear models (PROC GLM); in the 25-μg study, linear mixed models (PROC MIXED) were used. The threshold for statistical significance was p < 0.05. Test-to-reference ratios and 90% confidence intervals (CIs) for the geometric means of C max and AUC0–24 were used to compare the bioavailability of the test and reference products. Analyses were performed on baseline-adjusted and unadjusted data. Results for baseline-adjusted data are reported.
Results
Participant disposition and baseline characteristics
In the 10-μg study, 85 healthy postmenopausal women were screened and 36 were enrolled; 35 participants completed both periods of the study (one participant failed to report for check-in for period 2). Mean age was 50.4 years and mean BMI was 25.4 kg/m2 for those who completed the study. In the 25-μg study, the investigators screened 61 healthy postmenopausal women and 36 were enrolled; mean age was 49.9 years and mean BMI was 25.6 kg/m2. All 36 women completed the study. Table 1 summarizes the subject characteristics.
Table 1.
Demographic characteristics of the subjects who completed the studies.
|
10-μg Study (n = 35) |
25-μg Study (n = 36) |
|||
|---|---|---|---|---|
| Parameter | Mean ± SD | Range | Mean ± SD | Range |
| Age (years) | 50.40 ± 4.96 | 42.00–63.00 | 49.86 ± 3.83 | 43.00–58.00 |
| Height (cm) | 150.14 ± 5.13 | 140.00–161.00 | 149.69 ± 5.42 | 135.00–162.00 |
| Weight (kg) | 57.26 ± 8.59 | 43.00–73.00 | 57.47 ± 6.83 | 50.00–75.00 |
| Body mass index (kg/m2) | 25.36 ± 3.32 | 18.73–29.90 | 25.61 ± 2.24 | 21.64–29.90 |
SD, standard deviation
In the 10-μg study, some women had negative concentrations of hormones after baseline correction and therefore were excluded from analyses. For this reason, one subject was excluded from estradiol calculations, two subjects were excluded from estrone calculations, and 11 subjects were excluded from estrone sulfate calculations. Resultant participant numbers were 34 for estradiol, 33 for estrone, and 24 for estrone sulfate. In the 25-μg study, one subject was excluded from estrone calculations and one subject was excluded from estrone sulfate calculations, both for the test product. All 36 women were included for the estradiol calculations, but there were 35 participants in both the estrone and estrone sulfate calculations.
Pharmacokinetic results
Plasma concentrations of estradiol, estrone, and estrone sulfate were determined for all women who completed the 10-μg or 25-μg studies.
Estradiol
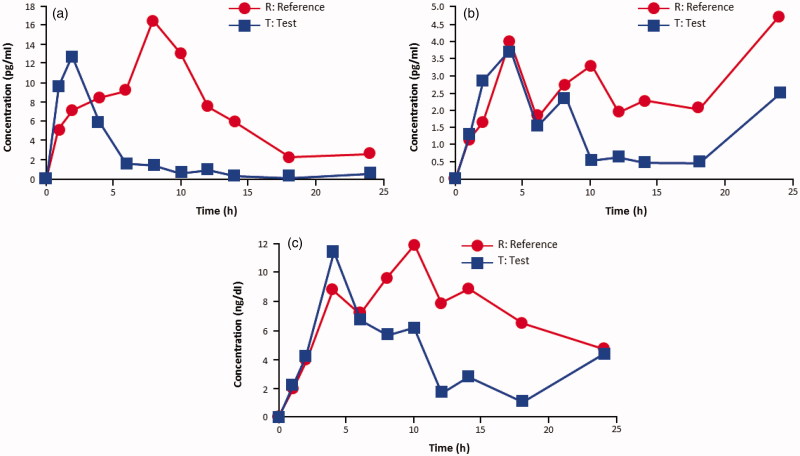
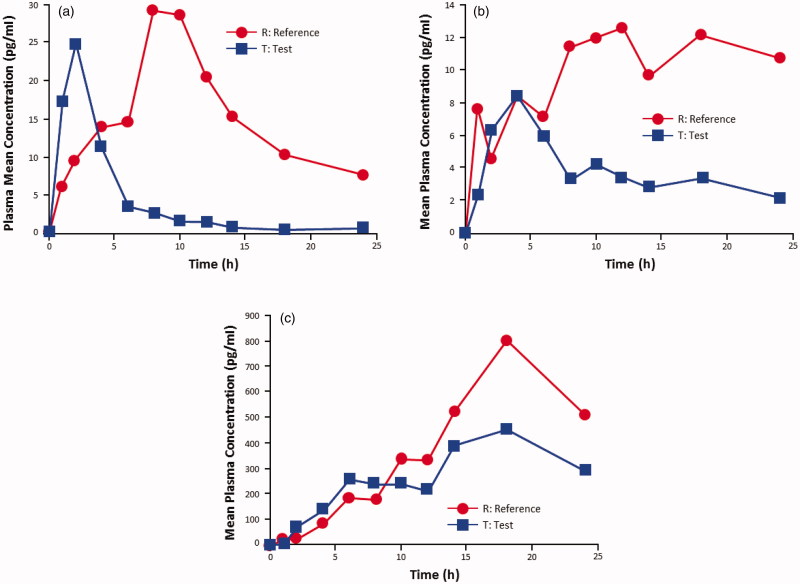
With the 10-μg dose, the maximum serum concentration of estradiol (C max) was significantly lower for the test product (baseline-adjusted geometric mean 14.38 pg/ml) compared with the reference drug (20.38 pg/ml; p = 0.0194; Table 2). With the 25-μg dose, estradiol C max was also significantly lower for the test product (23.08 pg/ml) compared with the reference product (42.70 pg/ml; p < 0.0001; Table 2). The AUC0-24 for estradiol was significantly lower with both doses of the test product versus the reference drug (p < 0.0001 for both comparisons; Table 2). Descriptive analysis showed that the test product had an earlier t max of approximately 2 h for estradiol at both doses vs. approximately 9 h for the reference product at 10 μg and approximately 11 h at 25 μg (Table 2).
Table 2.
Mean estrogen values in pharmacokinetic trials (geometric mean; baseline adjusted), and ANOVA results for comparisons between test a and reference b products. p Values indicate statistical significance at <0.05 between test and reference products.
|
AUC0-24 (pg·h/ml) |
Cmax (pg/ml) |
tmax (h) |
||||||
|---|---|---|---|---|---|---|---|---|
| Dose (μg) | Test | Reference | p | Test | Reference | p | Test | Reference |
| Estradiol | ||||||||
| 10 | 49.62 | 132.92 | <0.0001 | 14.38 | 20.38 | 0.0194 | 1.75 | 9.28 |
| 25 | 89.21 | 292.1 | <0.0001 | 23.08 | 42.70 | <0.0001 | 1.85 | 11.18 |
| Estrone | ||||||||
| 10 | 24.24 | 48.24 | 0.0002 | 5.15 | 6.98 | 0.0127 | 5.87 | 9.07 |
| 25 | 50.22 | 165.5 | <0.0001 | 10.69 | 23.58 | 0.0002 | 5.14 | 11.48 |
|
Estrone sulfate |
||||||||
| 10 | 66.6 (ng·h/dl) | 121.6 (ng·h/dl) | 0.0091 | 12.2 (ng/dl) | 16.9 (ng/dl) | 0.0366 | 5.5 | 8.8 |
| 25 | 4290 | 7330 | 0.0031 | 497.6 | 730.6 | 0.0042 | 11.75 | 15.87 |
a, Test product: TX-004HR; TherapeuticsMD, Inc., Boca Raton, FL, USA; b, reference product: Vagifem®; Novo Nordisk, Plainsboro, NJ, USA.
In both studies, the test product resulted in a lower, more modest systemic estradiol exposure than the reference product (Table 3; Figures 1a and 2a). The baseline-adjusted test-to-reference ratios (expressed as percentages) for estradiol C max were 72% and 54% in the 10-μg and the 25-μg studies, respectively (Table 3). The test-to reference ratios for baseline-adjusted estradiol AUC0–24 were 38% and 31% in the 10-μg and the 25-μg studies, respectively (Table 3).
Table 3.
Statistical results of test product versus reference product for estrogens (baseline-adjusted).
|
10-μg dose
a
|
25-μg dose
b
|
|||||
|---|---|---|---|---|---|---|
| Analyte/parameter | Intra-subject CV% | Test-to-reference ratio c (×100) | 90% CI | Intra-subject CV% | Test-to-reference ratio c (×100) | 90% CI |
| Estradiol | ||||||
| AUC0-24 (pg·h/ml) | 70.64 | 37.95 | 29.21–49.31 | 70.4 | 30.5 | 23.7–39.3 |
| Cmax (pg/ml) | 60.68 | 71.54 | 56.82–90.08 | 54.0 | 54.1 | 44.2–66.1 |
| Estrone | ||||||
| AUC0-24 (pg·h/ml) | 73.66 | 50.51 | 38.37–66.50 | 157 | 31.0 | 19.8–48.4 |
| Cmax (pg/ml) | 47.59 | 74.50 | 61.69–89.97 | 99.6 | 45.8 | 33.0–63.6 |
| Estrone sulfate | ||||||
| AUC0–24 (10 μg: ng.h/dl; 25 μg: pg.h/ml) | 73.87 | 57.87 | 41.68–80.35 | 82.6 | 57.8 | 43.2–77.3 |
| Cmax (10 μg: ng/dl; 25 μg: pg/ml) | 48.02 | 74.55 | 59.43–93.51 | 58.8 | 67.1 | 53.8–83.6 |
CV, coefficient of variation; CI, confidence interval
a, estradiol, n = 34; estrone, n = 33; estrone sulfate, n = 24; b, estradiol, n = 36; estrone and estrone sulfate, n = 35; c, test product: TX-004HR; TherapeuticsMD, Inc., Boca Raton, FL, USA; reference product: Vagifem®; Novo Nordisk, Plainsboro, NJ, USA. All test–reference ratios are statistically significant by ANOVA (α = 0.05)
Figure 1.
Linear plot of baseline-corrected mean plasma concentration versus time for (a) estradiol (n = 34), (b) estrone (n = 33), and (c) estrone sulfate (n = 24) after treatment with the test and reference preparations, each at 10 μg. Test product: TX-004HR; TherapeuticsMD, Inc., Boca Raton, FL. Reference product: Vagifem®; Novo Nordisk, Plainsboro, NJ.
Figure 2.
Linear plot of baseline-corrected mean plasma concentration versus time for (a) estradiol (n = 36), (b) estrone (n = 36), and (c) estrone sulfate (n = 36) after treatment with the test and reference preparations, each at 25 μg. Test product: TX-004HR; TherapeuticsMD, Inc., Boca Raton, FL. Reference product: Vagifem®; Novo Nordisk, Plainsboro, NJ.
Estrone and estrone sulfate
Estrone and estrone sulfate maximum serum concentrations (C max) were significantly lower for the test product compared with the reference drug at both the 10-μg and the 25-μg doses (p < 0.05 for all comparisons; Table 2). The AUC0-24 for estrone and estrone sulfate was also significantly lower with both doses of the test product versus the reference drug (p < 0.01 for all comparisons; Table 2).
In both studies, estrone and estrone sulfate exposures were lower with the test product than with the reference product (Table 3; Figures 1b, 1c, 2b, 2c). Test-to-reference ratios for baseline-adjusted estrone C max and AUC0–24 for the10-μg dose were 75% and 51%, respectively. For the 25-μg dose, test-to-reference ratios for baseline-adjusted estrone C max and AUC0–24 were 46% and 31%, respectively. Test-to-reference ratios for baseline-adjusted C max and AUC0–24 for estrone sulfate were 75% and 58%, respectively, for the 10-μg dose; and 67% and 58%, respectively, for the 25-μg dose.
Safety results
In the 10-μg study, 35 subjects were exposed to both the test and reference drugs, and in the 25-μg study, all 36 women were exposed. No adverse events were reported in either trial with either the test or reference drug. None of the clinical laboratory parameters in the post-study assessments of either trial were found to be significantly different from baseline parameters.
Discussion
These studies evaluated the bioavailability and safety of single 10-μg and 25-μg doses of a novel estradiol vaginal softgel capsule, TX-004HR, compared with equivalent doses of an approved vaginal estradiol tablet in healthy postmenopausal women. The extent of systemic estradiol, estrone, and estrone sulfate exposure with TX-004HR at both the 10-μg dose and the 25-μg dose was significantly lower than that of the reference product at the same doses. The reference product is the product with the lowest systemic exposure of estradiol available on the US market13. Both the test and reference study medications were safe and well tolerated in all study subjects at the 10-μg and 25-μg doses.
Serum estradiol levels did not rise above the normal reference level for postmenopausal women (i.e. approximately 20 pg/ml10) with 10 μg of TX-004HR, and were close to that level with 25 μg of TX-004HR. Furthermore, levels of estradiol, estrone, and estrone sulfate were significantly lower than those found with the reference product. With TX-004HR, both doses had a more rapid systemic absorption profile, and a faster return to baseline serum estradiol levels (within 6–10 h for 10 μg and 10–14 h for 25 μg) than the reference product, which took approximately 18 h to return to baseline in the 10-μg study and did not return to baseline within the 24-h monitoring period in the 25-μg study.
Low-dose, vaginal estrogen therapy is intended to treat symptoms locally while avoiding the risks with systemic absorption5. However, systemic levels higher than the reference range for postmenopausal women have been reported in pharmacokinetic studies of some low-dose vaginal estradiol treatments14 , 15. Eugster-Hausmann and colleagues reported a relatively rapid rise in serum estradiol levels within 8 h of administration of a 25-μg dose of the reference drug used in this study, which decreased within hours, but remained above the postmenopausal reference levels for 2 weeks14. With the 10-μg dose in the same study, serum estradiol levels rose to approximately 24 pg/ml within 8 h of administration, then remained below 10 pg/ml for the remainder of the study14. A Cochrane Review comparing vaginal estrogen products (creams, rings, and tablets) reported that conjugated equine estrogen creams had a significantly higher incidence of the adverse effects of uterine bleeding and breast pain, which may be indicators of systemic estrogen exposure, compared with vaginal estradiol tablets16. Another review of vaginal estrogen products found that peak serum estradiol levels were consistently higher with creams than with tablets10. While the doses of estradiol in the test and reference products compared in the present studies were similar, the pharmacokinetic results suggest that TX-004HR may have a better safety profile with less overall systemic exposure than other compounds.
There is currently a need for new vaginal therapy options for treating menopausal VVA3. As previously indicated, the REVIVE survey reported that only 7% of women with symptoms of VVA received prescription therapy alone3. Additionally, 23–42% of the symptomatic women who were using any VVA-specific therapy (OTC moisturizers/lubricants or prescription products) were dissatisfied with their current treatments3. The population of women who are at risk for VVA is expected to increase over the next two decades17, and untreated symptoms of VVA tend to persist and worsen with age18. The International Menopause Society recommends that VVA treatment be started early to prevent irreversible changes and that treatment be continued to maintain benefits, indicating that women will need to find treatment options that are more acceptable for long-term use4. If approved, TX-004HR could provide an alternative treatment option for women with VVA that is safe, effective, and easy to use. Women surveyed in a pilot study of TX-004HR reported of ease of use and overall satisfaction with the product19.
Data in the current study show that TX-004HR achieves significantly lower systemic drug exposure at both 10-μg and 25-μg doses than an FDA-approved low-dose alternative, and is safe and well-tolerated. If approved, this new vaginal softgel capsule could offer postmenopausal women another option for treating VVA, with less systemic exposure and easier administration than comparable, currently available, vaginal estradiol products.
Acknowledgements
The authors would like to acknowledge Jolene Mason, PhD and Laura Ninger, ELS of Precise Publications, LLC for their assistance in the writing of this manuscript.
Conflict of interest
Dr Pickar was formerly an employee of Wyeth Research; has received consultant fees from Wyeth/Pfizer, Besins Healthcare, Shionogi Inc., Metagenics, Radius Health, Inc, and TherapeuticsMD; and has stock options with TherapeuticsMD. Dr Bernick, Dr Mirkin, and Ms Amadio are employees of TherapeuticsMD.
Source of funding
TherapeuticsMD sponsored the study and funded the medical writing support provided by Jolene Mason, PhD and Laura Ninger, ELS of Precise Publications, LLC.
References
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6:2133–42. doi: 10.1111/j.1743-6109.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- Simon JA, Kokot-Kierepa M, Goldstein J, Nappi RE. Vaginal health in the United States: results from the Vaginal Health: Insights, Views & Attitudes survey. Menopause. 2013;20:1043–8. doi: 10.1097/GME.0b013e318287342d. [DOI] [PubMed] [Google Scholar]
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10:1790–9. doi: 10.1111/jsm.12190. [DOI] [PubMed] [Google Scholar]
- De Villiers TJ, Pines A, Panay N.et alUpdated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric 201316316–37. [DOI] [PubMed] [Google Scholar]
- Management of symptomatic vulvovaginal atrophy 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888–902. doi: 10.1097/GME.0b013e3182a122c2. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: results from the National Health and Nutrition Examination Survey, 1999–2010. Obstet Gynecol. 2012;120:595–603. doi: 10.1097/AOG.0b013e318265df42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkellner AR, Denison SE, Eldridge SL, Lenzi LL, Chen W, Bowlin SJ. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women's Health Initiative. Menopause. 2012;19:616–21. doi: 10.1097/gme.0b013e31824bb039. [DOI] [PubMed] [Google Scholar]
- Jewett PI, Gangnon RE, Trentham-Dietz A, Sprague BL. Trends of postmenopausal estrogen plus progestin prevalence in the United States between 1970 and 2010. Obstet Gynecol. 2014;124:727–33. doi: 10.1097/AOG.0000000000000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric. 2015;18:121–34. doi: 10.3109/13697137.2014.947254. [DOI] [PubMed] [Google Scholar]
- Manson JE, Goldstein SR, Kagan R.et alWhy the product labeling for low-dose vaginal estrogen should be changed. Menopause 201421911–16. [DOI] [PubMed] [Google Scholar]
- Vagifem® Prescribing Information . 2003. [Google Scholar]
- Weisberg E, Ayton R, Darling G.et alEndometrial and vaginal effects of low-dose estradiol delivered by vaginal ring or vaginal tablet. Climacteric 2005883–92. [DOI] [PubMed] [Google Scholar]
- Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10 μg 17β-estradiol vaginal tablets. Climacteric. 2010;13:219–27. doi: 10.3109/13697137.2010.483297. [DOI] [PubMed] [Google Scholar]
- Labrie F, Cusan L, Gomez JL.et alEffect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause 20091630–6. [DOI] [PubMed] [Google Scholar]
- Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD001500.pub2. [DOI] [PubMed] [Google Scholar]
- Howden LM, Meyer JA. http://www.census.gov/prod/cen2010/doc/sf1.pdf [Google Scholar]
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351–8. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- Kingsberg S, Amadio J, Graham S, Bernick B, Mirkin S. 2015. http://www.isswshmeeting.org/index.php?option=com_content&view=article&id=126&abstract_id=56 [Google Scholar]