Abstract
Cell proliferation, metabolism, migration and survival are coordinated through the tight control of two target of rapamycin (TOR) kinase complexes: TORC1 and TORC2. Here, we show that a novel phosphorylation of fission yeast Gad8 (AGC kinase) on the evolutionarily conserved threonine 6 (Thr6) prevents the physical association between Gad8 and TORC2. Accordingly, this block to protein interactions by Gad8 Thr6 phosphorylation decreases TORC2-controlled activation of Gad8. Likewise, phosphorylation of Gad8 Thr6, possibly by PKC, prevents the association of Gad8 with TORC2 thereby increasing TORC2 activity, because it reduces Gad8-mediated feedback inhibition of TORC2. Consistently, the introduction of a Gad8 T6D mutant, that mimics phosphorylation, increased TORC2 activity. Increased PKCPck2 expression prevented Gad8–TORC2 binding and so reduced the TORC2-mediated phosphorylation of Gad8 serine 546 that activates Gad8. Interestingly, independent of the Ser546 phosphorylation status, Gad8 Thr6 phosphorylation is important for remodelling the actin cytoskeleton and survival upon potassium ion and heat stresses. In contrast, Ser546 phosphorylation is required for the control of G1 arrest, mating, cell length at division and vascular size. Finally, these findings reveal a novel mode of TORC2 activation that is essential for cell survival following stress.
Keywords: TORC2, Gad8, PKC, SGK1, Schizosaccharomyces pombe
1. Introduction
Target of rapamycin (TOR) is a highly conserved protein kinase that coordinates cell growth and proliferation with changes in the cellular environment and stress [1]. In all organisms, TOR forms two structurally and functionally distinct multi-protein complexes, TORC1 and TORC2. These complexes are defined by unique interacting components that are highly conserved across species; in mammalian cells, regulatory-associated protein of mTOR (Raptor) defines TORC1, whereas the TORC2 complex uniquely incorporates rapamycin-insensitive companion of mTOR (Rictor) and Sin1 [2–4]. Mip1 and Ste20 represent the fission yeast homologues of Raptor and Rictor, respectively [5]. The unique TORC1 and TORC2 interacting proteins are thought to provide substrate specificity for each individual TOR complex [6].
Fission yeast contains two TOR kinases; Tor2 is the main TORC1 kinase, whereas the majority of TORC2 incorporates the non-essential Tor1 kinase [7–9]. In general, because TORC1 is sensitive to rapamycin [10], this has accelerated the understanding of the TORC1 signalling. TORC1 is essential for anabolic growth and proliferation [11]. In contrast, TORC2 regulation and signalling are less well understood. TORC2 has been shown to control metabolism, the cytoskeleton and cell survival after exposure to stress [10].
The fission yeast Gad8 protein is a member of the AGC subfamily of protein kinases (reviewed in [12]) and is regulated by TORC2. Phosphorylation of the T-loop of Gad8 by PDK1Ksg1 activates the kinase; however, full Gad8 activation requires additional TORC2-dependent phosphorylation within its carboxy-terminus [13,14]. Environmentally induced enhancement of PDK1Ksg1 activity boosts Gad8 activity [14] to promote a conserved feedback loop that downregulates TORC2 activity, because phosphorylation of the TORC2 ATP binding site by Gad8 reduces TORC2 activity [15]. Importantly, simultaneous Gad8 activation by TORC2 and Gad8 feedback inhibition of TORC2 would constitute a futile cycle as the two modifications negate one another. It is therefore highly likely that additional mechanisms provide tighter regulation of this negative feedback loop. We have previously shown that Gad8 ser546 phosphorylation is very dynamic as it is dephosphorylated within minutes of TORC2 inhibition. In contrast, dephosphorylation of the TORC2 ATP binding site, to increase TORC2 activity, occurs with slower kinetics [15].
We now show that phosphorylation of fission yeast Gad8 on the conserved residue Thr6 reduces the physical interaction between Gad8 and TORC2 to decrease both Gad8-mediated inhibition of TORC2 along with TORC2-controlled activation of Gad8. Thr6 represents a novel PKC consensus phosphorylation site that is conserved in a subset of AGC kinases, including mammalian SGK1/2 and the Saccharomyces cerevisiae kinases Ypk1 and Sch9. Our findings reveal a novel mode of TORC2 activation that is important for actin remodelling and survival following either potassium ion or heat stress. Finally, we show that Gad8 Ser546 phosphorylation is required for the control of G1 arrest, mating, cell length at division and vascular size, but not for remodelling of the actin cytoskeleton and survival upon potassium ion and heat stresses.
2. Results
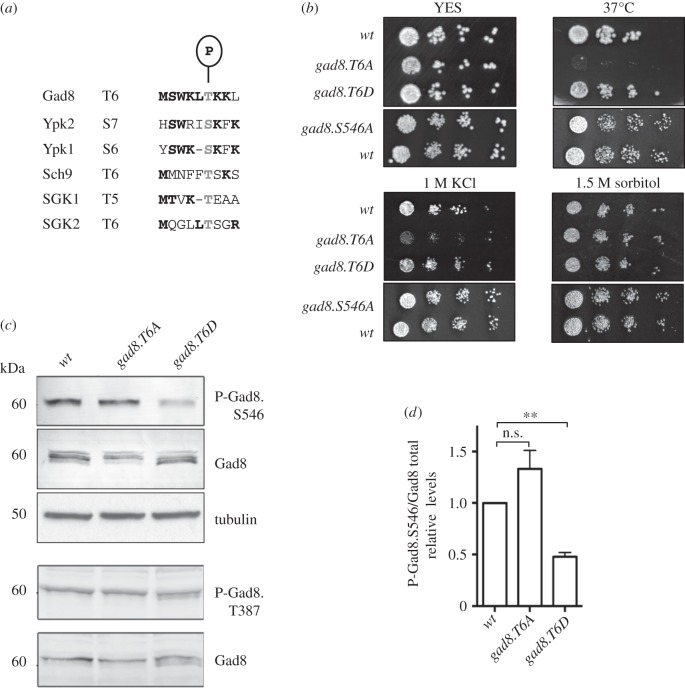
2.1. The evolutionarily conserved Thr6 of Gad8 is phosphorylated
We have previously shown that environmentally induced enhancement of PDK1Ksg1 activity boosts Gad8 activity to promote a conserved feedback loop that downregulates TORC2 activity, because phosphorylation of the TORC2 ATP binding site by Gad8 reduces kinase activity [15]. In addition, we found that Gad8 is heavily phosphorylated [15]. To gain further insights into the functional consequences of Gad8 phosphorylation, and its role in Gad8 control of TORC2 activity, Gad8 was immunoprecipitated from wild-type fission yeast cells, and sites of Gad8 phosphorylation were identified via tandem mass spectrometry. Two well-characterized sites of phosphorylation were identified, the PDK1Ksg1 controlled T-loop residue Thr387 and the TORC2-controlled Ser546 [14] (data not shown) alongside a novel phosphorylation event at threonine 6 (figure 1a and electronic supplementary material, figure S1A). Thr6 is conserved in the homologous kinases in S. cerevisiae (Ypk1p, Ypk2p and Sch9p) and humans (SGK1 and SGK2; figure 1a), highlighting the potential for a novel, conserved, mode of regulation that exploits phosphorylation of Thr6 within this family of AGC kinases. Gad8 is also an AKT homologue; however, the amino-terminal Thr6 is not conserved in AKT.
Figure 1.
Gad8 Thr6 is phosphorylated. (a) Gad8 Thr6 is evolutionarily conserved. (b) Stress response of gad8 mutants. (c) Gad8 protein and Thr387 and Ser546 phosphorylation levels in T6A and T6D mutants. (d) Relative Gad8 Ser546 phosphorylation levels in T6A and T6D mutants.
2.2. Gad8 Thr6 regulates cell survival following heat and potassium ion stress
The TORC2 Gad8 signalling pathway is required for tolerance of a variety of stresses [14,16–18]. To assess the significance of phosphorylation on Schizosaccharomyces pombe Gad8 Thr6 in the response to environmental stress, it was mutated to either alanine (T6A), to block signalling, or aspartic acid (T6D), to mimic constitutive phosphorylation. Interestingly, the gad8.T6A mutant was specifically sensitive to growth at increased temperature and was slow growing upon exposure to KCl (figure 1b). Neither gad8.T6A nor gad8.T6D altered tolerance to other stresses including: hydrogen peroxide, CaCl2 and sorbitol-induced osmotic stress (electronic supplementary material, figure S1B). Because Gad8 activity is required for cell growth in response to all these stresses [14,16], our initial data suggested that Thr6 phosphorylation moderately attenuated Gad8 function, rather than completely blocking its kinase activity.
2.3. Gad8 Thr6 phosphorylation affects Gad8 S546 phosphorylation
We next assessed the impact of Gad8 Thr6 phosphorylation on Gad8 protein levels and phosphorylation. Gad8 protein levels were slightly reduced in gad8.T6A mutant cells (figure 1c and electronic supplementary material, figure S1C,D); however, Gad8 protein stability and turnover were unaffected by the phosphorylation status of Thr6 (electronic supplementary material, figure 1SE,F). As mentioned previously, PDK1Ksg1 phosphorylation of the T-loop of Gad8, at position Thr387, is essential for kinase activity, whereas additional TORC2-dependent phosphorylation at the carboxy-terminal further enhances kinase activity. The TORC2-controlled phosphorylation of Gad8 Ser546 is equivalent to the mTORC2-controlled phosphorylation of AKT on the hydrophobic motif (Ser473), which activates AKT in mammals [13,19]. The relative level of Ser546 phosphorylation was reduced in the gad8.T6D mutant (figure 1c,d). In contrast, the PDK1Ksg1-dependent phosphorylation at Thr387 was unaffected by Thr6 phosphorylation status (figure 1c), suggesting that the impact of Thr6 phosphorylation on additional Gad8 phosphorylation events is specific to the TORC2-dependent regulation of Gad8.
TORC2 deletion mutants (tor1.Δ, ste20.Δ and sin1.Δ) are all sensitive to increased temperature and potassium levels [14,16,17]. As mentioned above, the sensitivity to either heat or potassium stress was unaffected by the gad8.T6D mutation (figure 1b), yet TORC2-controlled Ser546 phosphorylation is reduced in the gad8.T6D mutant (figure 1c,d). Exposing the gad8.S546A mutant to heat and potassium stress established that Gad8 Ser546 phosphorylation is not critical for cell survival following heat or potassium stress, because the gad8.S546A mutant showed no growth defect compared with wild-type cells when exposed to these stresses (figure 1b and table 1). In contrast, gad8.T6A mutant cells with normal levels of Ser546 phosphorylation were sensitive to heat and potassium stress (figure 1b and table 1). The mechanistic basis for enhanced sensitivity to heat or potassium stress of the gad8.T6A mutant is currently unclear.
Table 1.
Summary of the effect of Gad8 Thr6, S546 and kinase dead mutants on Gad8 function.
| phenotype | gad8.T6A | gad8.T6D | gad8.S546A | gad8.KD* |
|---|---|---|---|---|
| Gad8.S546 phosphorylation | unaffected | reduced | n.a. | increased (KD: kinase dead mutant) |
| Tor1 activity | unaffected | increased | increased | increased |
| the T6 phenotype is S546-dependent and similar to Gad8.KD (Gad8 activity is likely to determine phenotype) | ||||
| cell length at division | reduced | increased | increased | increased |
| mating efficiency | unaffected | reduced | reduced | reduced |
| G1 arrest | delayed | small delay | delayed | delayed |
| the T6 phenotype is S546-dependent but opposite to Gad8.KD. (TORC2 activity is likely to determine phenotype, but sensitive to lack of Gad8 activity) | ||||
| vacuolar size | reduced | increased | increased | reduced |
| the T6 phenotype is S546 independent but similar to Gad8.KD (T6-dependent phenotype but sensitive to lack of Gad8 and increased TORC2 activities) | ||||
| NETO | unaffected | reduced | unaffected | reduced |
| 37° stress | sensitive | unaffected | unaffected | sensitive |
| KCl stress | slow growth | unaffected | unaffected | sensitive |
| phenotype is T6 independent | ||||
| osmotic stress | unaffected | unaffected | unaffected | sensitive |
| oxidative stress | unaffected | unaffected | unaffected | sensitive |
| CaCl2 stress | unaffected | unaffected | unaffected | sensitive |
| nutrient stress | unaffected | unaffected | slight sensitivity | sensitive |
2.4. Thr6 affects cell and vacuolar size through its impact upon Ser546 phosphorylation
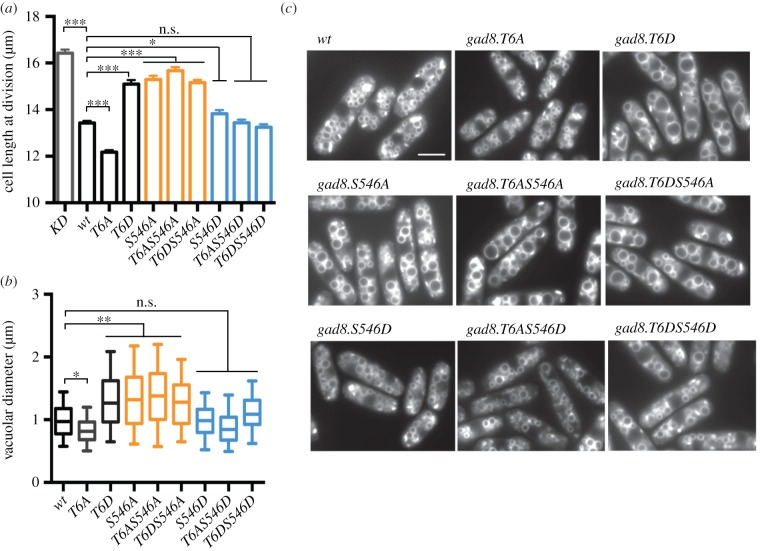
Cell size at division is increased in gad8 deficient mutants. Cell size at division is also increased in the TORC2 deficient deletion mutants tor1.Δ, ste20.Δ and sin1.Δ, indicating that the G2/M transition is delayed when either Gad8 or TORC2 function is compromised [16,18]. Although the molecular mechanisms are currently unclear, the delay to mitotic commitment that underlies the increase in size at division of gad8 deficient mutants suggests that Gad8 plays a key role in regulating the timing of the G2/M transition [16,18]. To assess the impact of Thr6 phosphorylation upon cell size control, we measured cell size at division of Thr6 mutants. The cell size at division of gad8.T6D mimicked the kinase dead gad8.KD and the TORC2 deficient deletion mutants tor1.Δ, ste20.Δ and sin1.Δ in being increased (figure 2a and table 1) [16,18]. Conversely, cell size at division was reduced in gad8.T6A (figure 2a). The gad8.S546A mutation phenocopied the increased cell length at division of gad8.T6D (figure 2a and table 1). Therefore, the increase in size at division of gad8.T6D mutants may stem from their reduction in Ser546 phosphorylation (figure 1c). To test this possibility, the cell size of different combinations of Thr6 and Ser546 double mutants were measured (figure 2a). Importantly, the gad8.T6A-S546A double mutant resembled gad8.S546A, as both appeared elongated. Together, these data suggest that the cell size at division of Gad8 Thr6 mutants is indeed dictated by the status of TORC2-mediated phosphorylation of serine 546 on Gad8.
Figure 2.
Gad8 Thr6 regulates cell and vacuolar size through its effect on Ser546 phosphorylation. (a) Cell length at division of indicated gad8 mutants. (b) Vacuolar size of indicated gad8 mutants. (c) FM4-64 staining of vacuoles in indicated gad8 mutants.
The nutritional environment also determines cell size at division of fission yeast [20,21]. Gad8 is essential for the advancement of mitosis that leads to cell division at a reduced cell length following nutrient stress [18]. However, Thr6 phosphorylation does not appear to be required for this nutrient stress-induced advancement of mitosis and cell division (electronic supplementary material, figure S2a).
The TORC2 mutants tor1, ste20, sin1 and gad8 mutants are all required for vacuolar (yeast lysosomes) integrity and size [19]. Consequently, the vacuoles of cells lacking gad8.Δ are small and fragmented [19]. We therefore assessed the impact of Thr6 phosphorylation status upon vacuolar size. Vacuoles were enlarged in gad8.T6D (figure 2b,c and table 1) and shrunken in gad8.T6A mutants. The gad8.S546A mutant also induced an increase in vacuole size. Examination of double mutants, that combined mutations at Ser546 with Thr6 mutants, established that vacuolar size is also determined by the phosphorylation status of Ser546 (figure 2b,c).
The ability to form autophagosomes and so survive nitrogen or glucose starvation might be influenced by altered vacuolar function. However, all gad8 Thr6 mutants survived nitrogen starvation. Autophagy in fission yeast is therefore unlikely to be affected by Thr6 phosphorylation status (electronic supplementary material, figure S2b). In summary, the Thr6 phosphorylation-dependent impact on cell length and vacuole size appears to be mediated via TORC2-controlled Ser546 phosphorylation of Gad8.
2.5. Gad8 Thr6 phosphorylation is important for sexual differentiation
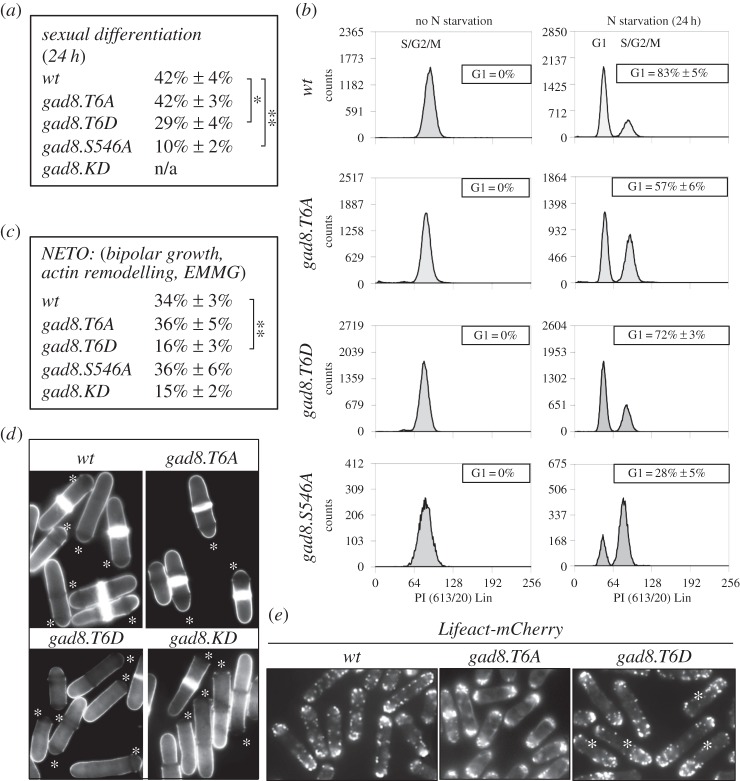
Gad8 was initially identified in fission yeast as a G1 arrest deficient (gad) mutant. Consequently, gad8.KD mutants are unable to undergo sexual differentiation and mating and are therefore sterile [14]. Flow cytometric analysis of DNA content in wild-type fission yeast cultures, grown in a good nitrogen source, does not reveal a G1 population [22]. However, 24 h of nitrogen starvation induces G1 arrest in 80% of wild-type cells and, when mixed with cells of the opposite mating type, 40% of wild-type cells undergo sexual differentiation and mating (figure 3a,b). Both G1 arrest and sexual differentiation were reduced by 75% in the gad8.S546A mutant (figure 3a,b). In the gad8.T6A mutant, the level of G1 arrests was reduced by 25% (figure 3b). Nevertheless, the mating efficiency of gad8.T6A mutants was similar to wild-type cells. Therefore, once arrested in G1, gad8.T6A mutants mate efficiently (figure 3a). In contrast, sexual differentiation and mating were reduced in gad8.T6D mutant even though 70% of cells arrested in G1 (figure 3a,b). Thus, Gad8 regulates G1 arrest and sexual differentiation via independent mechanisms yet regulation through phosphorylation of Thr6 is important for both functions (table 1).
Figure 3.
Gad8 Thr6 regulates sexual differentiation and F-actin re-modelling. (a) Sexual differentiation efficiency assay. (b) The efficiency of G1 arrest in gad8 mutants when starved for nitrogen. The data shown are from a single representative experiment out of three repeats. (c) The frequency of NETO of indicated gad8 mutants. (d) Calcofluor staining of indicated gad8 mutants, the asterisk marks the new cell ends. (e) Lifeact-mCherry is used to visualize F-actin in the gad8 thr6 Lifeact-mCherry double mutants, the asterisk marks long mono-polar cells in the gad8.T6D mutants.
2.6. Gad8 Thr6 phosphorylation is important for remodelling of the F-actin cytoskeleton
Following the induction of sexual differentiation, the cell must change the positioning of the growth zone (projection tip) to initiate the growth at new sites that will support growth towards a mating partner. This switch in actin organization is determined by a signalling cascade that is triggered by a gradient of sex pheromones [23–26]. Consequently, the actin cytoskeleton is continuously reorganized during courtship before being set in a new polarized pattern typical of conjugating cells [25]. It has already been established that S. cerevisiae Ypk1p and human SGK1 regulate actin organization [27,28]. Because the reduction in the mating efficiency of the gad8.T6D mutant cannot be explained by a defect in the competence to arrest cell cycle progression in G1 (figure 3b), we explored the possibility that it may arise from inefficient reorganization of the F-actin cytoskeleton by monitoring F-actin remodelling in Thr6 mutants.
Fission yeast cells are rod shaped and grow by tip extension. A ‘newborn’ fission yeast cell grows in a mono-polar fashion from the old cell end. Mid-way through G2, the actin cytoskeleton and the growth machinery is activated at the new cell end (new end take off, NETO) to initiate a phase of bipolar growth that persists until division [29]. Therefore, NETO can be used as a proxy for the ability to reorganize actin in fission yeast. Calcofluor can be used to visualize NETO, as the growing ends of fission yeast cells stain more intensely with this fluorescent dye. The number of cells undergoing NETO (bright at both cell ends) in the gad8.KD kinase dead and gad8.T6D mutants was reduced, whereas both gad8.T6A and gad8.S546A cells were NETO proficient (figure 3c and table; figure 3d, asterisks indicate the new cell ends). We next used Lifeact-mCherry to visualize F-actin [30]. The presence of long cells in the gad8.T6D Lifeact-mCherry double mutant, which had F-actin localized at the old end only, confirmed that gad8.T6D cells are NETO deficient (figure 3e). In summary, the reduced mating efficiency of gad8.T6D cells correlated with a reduction in the ability to reorganize the F-actin cytoskeleton.
2.7. Gad8 Thr6 phosphorylation specifically reduces TORC2-controlled activation of Gad8 and Gad8-mediated feedback inhibition of TORC2
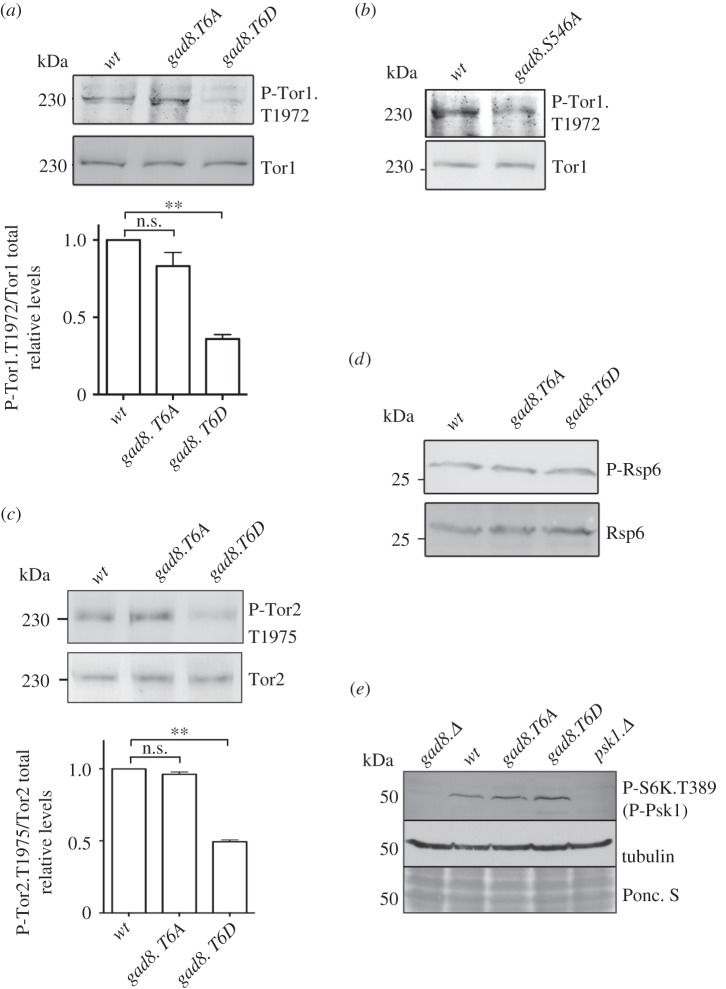
Our phenotypic analyses suggest that Gad8 Thr6 phosphorylation is required for the execution of only a subset of Gad8 functions. Gad8 Thr6 phosphorylation is important for cell survival upon heat and potassium stresses and F-actin reorganization in mating. For the reorganization of the F-actin cytoskeleton, this requirement is independent of the Ser546 phosphorylation status. To gain further insights into the mechanistic basis for this specificity of Thr6 on Gad8 function, we assessed Gad8 activity and the ability of the Thr6 mutant cells to regulate known Gad8 substrates. We began by developing an in vitro assay to monitor Gad8 kinase activity. We have previously reported direct phosphorylation of Tor1.T1972 as part of TORC2 by Gad8 [15]. Thus, a small fragment of Tor1 fused to GST was expressed in, and isolated from, Escherichia coli for use as in vitro substrate. Phospho-specific Tor1.T1972 antibodies measured Gad8 activity towards T1972 on this recombinant Tor1 substrate. A twofold serial dilution of immunoprecipitated wild-type Gad8 established that our in vitro assays monitored kinase activity within a linear range (electronic supplementary material, figure S3A). Thr6 phosphorylation status did not affect Gad8 in vitro kinase activity towards the Tor1–GST fusion protein (electronic supplementary material, figure S3B).
We next assessed Gad8 in vivo kinase activity. The Gad8-dependent in vivo phosphorylation of Tor1.T1972 was reduced in the gad8.T6D and gad8.S546A mutants (figure 4a,b). In contrast, the Tor1.T1972 phosphorylation status was not altered by the gad8.T6A mutation (figure 4a). Fission yeast has two TOR kinases [31]; the Gad8-dependent phosphorylation of Tor1 Thr1972 is conserved for Tor2 (the main kinase of TORC1) [7–9] as phosphorylation of Tor2 Thr1975 also is Gad8 dependent [15]. Tor2.T1975 phosphorylation resembled Tor1.T1972 phosphorylation in also being reduced by the gad8.T6D mutation (figure 4c). Interestingly, the Thr6 mutants had no impact on two additional Gad8-dependent phosphorylation events in vivo (figure 4d,e). The S. pombe ribosome protein 6 (Rps6) is a homologue of the S6 protein that is phosphorylated by mammalian S6 kinase [32]. We have previously shown that phosphorylation of Rps6 is regulated by Gad8 [33]. Mutation of Thr6 had no impact upon Gad8-controlled Rsp6 phosphorylation (figure 4d). In fission yeast, Rsp6 phosphorylation is also regulated by TORC1 and the Psk1 kinase; furthermore, the activation of Psk1 is TORC1 dependent [34]. Interestingly, we found that this TORC1-dependent phosphorylation of Psk1 also required Gad8 (figure 4e). This dependency for Gad8 in regulation of Psk1 activation may provide an explanation for the role of Gad8 and TORC2 in S6 phosphorylation. However, importantly, the Gad8-dependent Psk1 phosphorylation was unaffected by the phosphorylation status of Thr6.
Figure 4.
Gad8 Thr6 phosphorylation reduces Gad8-controlled phosphorylation of Tor1 and Tor2. (a) Gad8 phosphorylation of the Gad8-specific substrate Tor1.T1972 in T6A and T6D mutants, along with quantification of relative Tor1.T1972 phosphorylation levels. (b) Gad8 phosphorylation of the Gad8-specific substrate Tor1.T1972 in the S546A mutant. (c) Gad8 phosphorylation of the Gad8-specific substrate Tor2.T1975 in T6A and T6D mutants, along with quantification of relative Tor2.T1975 phosphorylation levels. (d) Gad8-dependent phosphorylation of Rsp6 in T6A and T6D mutants. (e) Gad8 phosphorylation of the Gad8-dependent substrate Psk1 in T6A and T6D mutants.
Together, our data suggest that Thr6 phosphorylation has no impact on in vitro kinase activity and no impact on two in vivo phosphorylation sites (on Rsp6 and Psk1) regulated by Gad8. However, Thr6 affects the reciprocal regulation of TORC2 and Gad8 activities in vivo, because both Gad8.S546 (figure 1c) and Tor1.T1972 (figure 4a) phosphorylation are reduced in gad8.T6D mutants.
2.8. Thr6 phosphorylation prevents physical association between Gad8 and TORC2
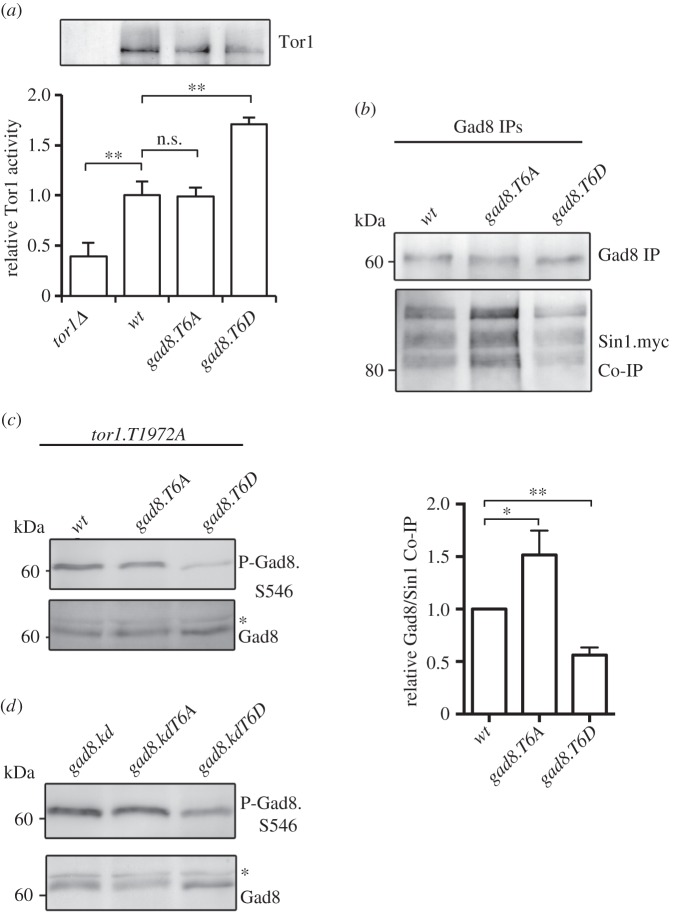
Because phosphorylation of Tor1.T1972 by Gad8 is inhibitory for TORC2 functions [15], the reduction in Tor1.T1972 phosphorylation in the gad8.T6D mutant (figure 4a) is likely to elevate TORC2 activity. To test this possibility, we used established assays to measure in vitro Tor1 kinase activity in the Thr6 mutants [15,35]. As anticipated by the reduction in T1972 phosphorylation, in vitro Tor1 activity increased in the gad8.T6D mutant (figure 5a), yet was unaffected in the gad8.T6A mutant. This gad8.T6D-dependent increase in in vitro TORC2 activity is in stark contrast to the observed reduction in TORC2-dependent Gad8.S546 phosphorylation (figure 1c).
Figure 5.
Gad8 Thr6 phosphorylation reduces TORC2 and Gad8 interactions. (a) Tor1 in vitro kinase assays. Immunoprecipitated Tor1 from the gad8.T6D mutant has a higher Tor1 kinase activity than wild-type Gad8. (b) Gad8 was immunoprecipitated from T6A and T6D mutant and Sin1 (TORC2) co-immunoprecipitation measured. (c) TORC2 phosphorylation of the Gad8 Ser546 in hyperactive tor1.T1972A and T6A and T6D double mutants. (d) TORC2 phosphorylation of the Gad8 Ser546 in gad8.KD kinase dead and T6A and T6D double mutants. (b,c) Asterisk indicates a background band.
To gain more insight into this conundrum, we next considered the possibility that Thr6 phosphorylation may decrease the reciprocal Gad8–TORC2 regulation by compromising the physical interaction between Gad8 and TORC2. We therefore assessed the interaction between Gad8 and the TORC2-specific component Sin1 [5]. An increase in the levels of TORC2-Sin1 co-precipitating with Gad8.T6A mutant protein, and a converse reduction in the association in a Gad8.T6D precipitation, suggested that Thr6 phosphorylation status does indeed regulate the interaction between Gad8 and TORC2-Sin1 (figure 5b).
To further validate the role of Thr6 in regulating Gad8's interaction with TORC2, we generated a tor1.T1972A gad8.T6D double mutant. We have previously established that tor1.T1972A mutants have increased TORC2 activity, because Tor1.T1972A cannot be inhibited by Gad8. Consequently, tor1.T1972A mutants have increased Gad8.S546 phosphorylation [15]. Consistent with the reduced binding of gad8.T6D to TORC2-Sin1 (figure 5b), Gad8.S546 phosphorylation was still reduced in the tor1.T1972A gad8.T6D double mutant (figure 5c) despite the increase in kinase activity of Tor1.T1972A mutant proteins. We next took advantage of the catalytically inactivating gad8.KD kinase dead mutant, in which TORC2 activity also is increased, because the loss of Gad8 activity blocks its ability to inhibit TORC2 [15]. Introducing this kinase dead mutation alongside the T6D in a gad8.KD-T6D double mutant also failed to rescue the reduced Gad8.S546 phosphorylation of gad8.T6D mutants (figure 5d). Together, these data suggest that elevated TORC2 activity cannot rescue the lower levels of Ser546 phosphorylation in the gad8.T6D mutant.
In summary, the gad8.T6D mutation reduced the association between Gad8 and TORC2-Sin1. This reduced protein interaction can explain the specific inability of gad8.T6D to regulate T1972 phosphorylation of Tor1 (TORC2) (figure 4a) and the inability of TORC2 to regulate Gad8.S546 in the gad8.T6D mutant (figure 1c).
2.9. Thr6 lies within a protein kinase C consensus site
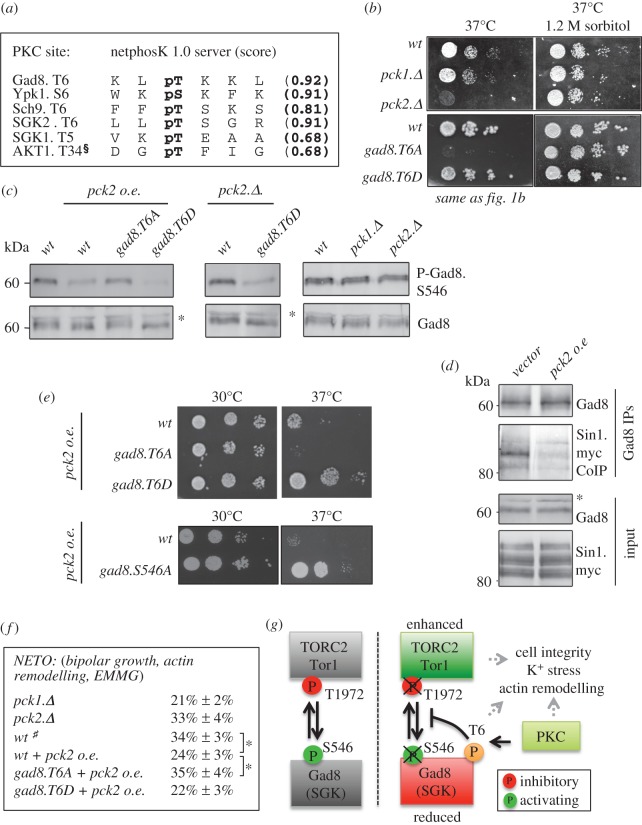
Together, our data suggest that the kinase that phosphorylates Thr6 regulates a subset of TORC2- and Gad8-dependent activities. To identify the candidate Thr6 kinase, we submitted the Gad8 sequence to the NetPhosK 1.0 server [36]. Thr6 of Gad8 along with the sites found in the Gad8 homologues, YPK1, Sch9 and SGK kinases, all conformed to the consensus for phosphorylation by protein kinase C (PKC; figure 6a). In mammalian cells, the Gad8 homologue AKT has been shown to be phosphorylated by PKC on Thr34 (figure 6a) [37,38]. AKT.T34 is found within the membrane binding PH domain of the kinase (electronic supplementary material, figure S4A). Thr34 phosphorylation prevents the recruitment of AKT to membranes [37]. Importantly, none of the amino terminal PKC consensus sites in Gad8, Ypk1 and Sch9 lie within the respective membrane binding regions (electronic supplementary material, figure S4A). The mammalian homologues, the SGKs, do not have a predicted membrane-binding domain (electronic supplementary material, figure S4A). Interestingly, the Thr6-regulated processes (potassium sensitivity, actin cytoskeleton and heat stress that activates the cell wall integrity pathway) are all regulated by the fission yeast PKC homologues PKCPck1 and PKCPck2 [39,40].
Figure 6.
High levels of PKC reduce Gad8 binding to TORC2-Sin1. (a) Gad8.Thr6 resembles a protein kinase C phosphorylation motif that is conserved in other AGC kinases. §Has previously been identified by Powell et al. [37]. (b) Heat stress sensitivity of pck2.Δ and gad8.T6A mutants is rescued by osmotic stabilizer. (c) Pck2 overexpression in T6A and T6D mutants. Pck2 reduces Gad8.S546 phosphorylation through Thr6. (d) Pck2 overexpression reduces Gad8 and Sin1 (TORC2) co-immunoprecipitation. (e) Stress response of gad8 T6A, T6D and S546A mutants following Pck2 overexpression. (f) Measurement of actin remodelling (NETO, new end take off) in T6A and T6D mutants. Hash symbol denotes identical to figure 3c. (g) Schematic suggesting that PKC-dependent regulation of Gad8 Thr6 blocks Gad8 binding to TORC2 and therefore reduces Gad8-dependent TORC2 inhibition and TORC2 activation of Gad8. (c,d) Asterisk indicates a background band.
2.10. PKCPck2 reduces Gad8 S546 phosphorylation
In fission yeast, several PKCPck1 and PKCPck2 functions are redundant. However, only pck2.Δ mutants are sensitive to heat stress (figure 6b). The rescue of this heat sensitivity by the addition of sorbitol to buffer against osmotic shock indicates that the temperature sensitivity of pck2.Δ deletion cells arises from cell lysis at the elevated temperature (figure 6b). Sorbitol rescued the heat stress sensitivity (figures 1b and 6b) and cell lysis at high temperatures of gad8.T6A mutant cells as well (electronic supplementary material, figure S4B). While the gad8.T6D mutant was not sensitive to heat stress (figure 1c), a gad8.T6D pck2.Δ double mutant was (electronic supplementary material, figure S4C), implying that PKCPck2 has multiple targets that are important for cell integrity at high temperatures. One prediction of increased phosphorylation of Gad8 Thr6 by PKC would be a decrease in Gad8 Ser546 phosphorylation, whereas complete loss of PKC would be predicted to have very limited impact, because the gad8.T6A mutant has no impact on Gad8 S546 phosphorylation (figure 1c). PKCPck2 was ectopically expressed from the nmt42 promoter [41]. Increased PKCPck2 expression in wild-type cells, but not in the gad8.T6A mutant, reduced Ser546 phosphorylation, whereas deletion of either pck1.Δ or pck2.Δ had no impact on Ser546 phosphorylation levels (figure 6c). Importantly, this PKCPck2 expression rescued the temperature sensitivity of pck2.Δ mutant (electronic supplementary material, figure S4D). PKCPck2 is known to regulate the Pmk1 MAPK kinase required for cell integrity [42] and Pmk1 has been linked to TORC2 regulation [43]. However, expression of PKCPck2 in a pmk1.Δ still reduced Gad8 Ser546 phosphorylation (electronic supplementary material, figure S4E). Together, our data suggest that PKCPck2 influences Gad8 Ser546 phosphorylation status as an indirect consequence of its phosphorylation of Gad8 Thr6.
2.11. PKCPck2 prevents Gad8–TORC2 binding
A second prediction arising from PKCPck2-dependent regulation of Thr6 is that elevation of Pck2 levels will reduce Gad8 binding to TORC2-Sin1. Gad8 was therefore immunoprecipitated from cells expressing PKCPck2 ectopically (pkc2 oe) and the amount of Sin1 co-immunoprecipitating with Gad8 was determined by Western blotting. In a phenocopy of gad8.T6D mutant cells, PKCPck2 expression reduced the amount of Sin1 binding to Gad8 (figure 6d). Furthermore, in line with the prediction of PKCPck2-dependent regulation of Thr6 phosphorylation, PKCPck2 failed to rescue the heat sensitivity of gad8.T6A cells (figure 6e). In contrast, growth of the gad8.T6D and gad8.S546A mutants following heat stress was enhanced by PKCPck2 expression (figure 6e). Interestingly, the gad8.T6D and gad8.S546A mutants both have reduced inhibitory Tor1.T1972 (TORC2) phosphorylation (figure 4a,b). Therefore, elevated PKCPck2 expression in combination with elevated TORC2 activity may be stimulating growth during heat stress.
It is well established that protein kinase C regulates actin organization in both yeast and mammals [44,45]. PKCpck2+ ectopic expression led to a 30% reduction in the frequency at which cells executed NETO (re-arrangement of the F-actin cytoskeleton; figure 6f). Importantly, this PKCPck2 expression had no impact on the ability of gad8.T6A cells to execute NETO (figure 6f). Thus, the PKCPck2-dependent decline in the ability of cells to initiate the actin re-modelling that underpins growth at the new cell tip is also Gad8 Thr6-dependent.
3. Discussion
Gad8 Thr6 constitutes a novel PKC consensus phosphorylation site conserved in a subset of AGC kinases, including SGK1/2 in mammals and Ypk1 and Sch9 in S. cerevisiae. Gad8 Thr6 phosphorylation reduces Gad8–TORC2 binding. Thus, Gad8 Thr6 phosphorylation enhances TORC2 activity (figure 5a) because it blocks the ability of Gad8 to bind and place the inhibitory phosphate at position T1972 of Tor1 (figure 4a) that downregulates TORC2 activity [15]. Reduced Gad8–TORC2 binding in the gad8.T6D mutant also diminishes TORC2 phosphorylation of Gad8 S546 (summarized in figure 6g).
The impact of PKC control of Thr6 phosphorylation is highlighted by the gad8.T6A mutant. Because Gad8 and TORC2-Sin1 binding is increased in this mutant, at first it seems surprising that Gad8-controlled Tor1 T1972 phosphorylation and TORC2-controlled Gad8 S546 phosphorylation are not significantly increased. However, the explanation probably lies in the opposing nature of the TORC2 and Gad8 regulation. In this model, the absence of Thr6 regulation in the gad8.T6A mutant probably promotes a futile regulatory cycle. More specifically, reduced TORC2 activity (due to increased Tor1 T1972 phosphorylation) will reduce Gad8 activity and thereby reduce Gad8-controlled inhibition of TORC2. Consequently, the impact of either of the two phosphorylation events is abolished, to result in unchanged ‘steady-state’ activities of both kinases. Therefore, the PKC control of Thr6 phosphorylation can disrupts this futile regulatory cycle and thus activates TORC2 by blocking Gad8-mediated feedback inhibition (figure 6g).
The impact of Gad8 Thr6, S546 and kinase dead mutants are summarized in table 1. We find that Thr6 phosphorylation alters cell and vacuolar size, G1 arrest and mating as a result of the reduction in activating TORC2-controlled Gad8.S546 phosphorylation, which stems from the decline in TORC2 and Gad8 association. In contrast, Gad8 Thr6 regulation acts independently of Gad8 S546 phosphorylation in actin remodelling (NETO), cell survival following heat stress (cell integrity) and potassium stress (table 1). At present, it is unclear why some Gad8-dependent phenotypes are Thr6-dependent but Ser546 independent. As mentioned previously, phosphorylation of the T-loop of Gad8 by PDK1Ksg1 activates the kinase, whereas additional TORC2-dependent phosphorylation, within its carboxy-terminus, further activates kinase activity [13,14]. All of the Thr6-dependent phenotypes are sensitive to lack of Gad8 kinase activity (table 1), suggesting that at least some level of Gad8 activity is required. However, the gad8.T6A mutant, which is sensitive to potassium and heat stress, does not appear to have altered Gad8 or TORC2 activity. Therefore, the gad8.T6A-dependent phenotypes might be a result of reduced binding of Gad8 to an as yet unidentified substrate.
Finally, the Thr6-dependent phenotypes, survival following heat stress (cell integrity), potassium stress and actin remodelling, are all functions that have previously been ascribed to the human Gad8 homologues SGK1/2 [28,46,47] in which Thr6 is conserved (figure 6a). Importantly, mTORC2 and human PKC are also well known regulators of actin remodelling, crucial for cell migration [10,28,45]. Therefore, the Thr6-dependent regulation of the actin cytoskeleton is likely to be conserved in mammalian cells. In addition, hyperglycaemia (high blood sugar level) has been shown to activate PKC [48]. Furthermore, elevated PKC and mTORC2 activities, along with decreased SGK1 activity, have recently been associated with insulin resistance in mammalian cells [49–52]. Interestingly, the novel mode of AGC kinase and TORC2 regulation that we present here predicts that PKC-induced phosphorylation of SGK1 Thr5 would promote mTORC2 activity and reduce SGK1 activity. Therefore, PKC and elevated SGK1 Thr5 phosphorylation may contribute to insulin resistance through control of mTORC2 and SGK1 activities. Together, our data suggest that environmentally induced PKC can indirectly boost TORC2 activity by preventing AGC kinase feedback inhibition of TORC2 (figure 6g).
4. Material and methods
4.1. Strains
Strains used in this study are listed in the electronic supplementary material, table S1. Unless otherwise specified, cells were cultured at 28°C in Edinburgh minimal media [21] using 20 mM l-glutamic acid (EMMG) as a nitrogen source. Cells were grown exponentially for 48 h before being harvested at early exponential phase of 1.5 × 106 cells ml−1.
To assay sexual differentiation, 4 × 106 cells were mixed with equal numbers of cells of the opposite mating types and the mixture spotted onto sporulating agar before incubation at 30°C. Mating efficiency was determined at the indicated time by 2 × zygotes/[cells + (2 × zygotes)]. Nitrogen starvation was applied as follows. Cells were cultured at 28°C in minimal sporulating liquid media (MSL) [53] to a density of 1.5 × 106 cells ml−1 and filtered into MSL minus nitrogen source.
For stress sensitivity spot test assays, cells were grown in yeast extract media (YES) to a cell density of 1.5 × 106 cells ml−1. Ten-fold dilution series starting with 5 × 104 cells were spotted on media indicated.
4.2. Cell length and division ratio measurement
Cells were grown at 28°C in EMMG to 1.5 × 106 cells ml−1 and filtered into media using 20 mM l-proline as nitrogen source (EMMP). Cells were harvested at indicated time point, fixed with 3% formaldehyde, washed with PBS and stained by calcofluor. Images of cells were obtained using a CoolSNAP HQ2 CCD camera and processed with ImageJ. The ratio of cells undergoing NETO was estimated from calcofluor-stained cells (growing cell tips stain brighter with calcofluor). Cell length at division was measured (in each analysis, more than 200 cells were measured/counted).
4.3. Vacuolar staining
In order to analyse vacuolar morphology, cells were grown to a density of 1.8 × 106 cells ml−1 before FM4-64 dye 5 mg ml−1 was added to the culture in the ratio 1 : 10 000 (dye : culture, v/v) and cells were incubated for a further 30 min before images of the living cells were captured with a CoolSNAP HQ2 CCD camera and processed with ImageJ. For each culture, at least 500 vacuoles were measured.
4.4. Molecular manipulations and generation of single point mutations
Standard site-directed mutagenesis was used to generate gad8 point mutations, and the mutated gad8 allele was introduced into cells through transformation. The recombinant gene was then used to replace the ura4+ gene in the gad8::ura4+. The resulting strains were back-crossed, and prototroph progeny was selected. The presence of the mutation was verified by PCR. Thus, all gad8 point mutations used in this study are single point mutants integrated into the gad8 locus, and they are all prototrophic strains.
For generation of the Tor1–GST fusion substrate in the Gad8 kinase assay, Tor1 was amplified using the following primers, 5′-CTCGGATCCATCTCGCATTTCCATCACACTTTCGAAG-3′ and 5′-ACGCGTCGACAAGTCTCCAATTAATCAAAGGGTCATAG-3′, and cloned into pET-41a(+) vector. Tor1–GST was expressed in E. coli BL-21 cells. Pck2 was amplified by PCR from genomic wt DNA, sequenced and cloned into pRep42 plasmid to facilitate Pck2 expression from the nmt42 promoter [41].
4.5. Western blotting
TCA precipitation protocol was followed for total protein extracts [54]. The following dilutions of antibodies were used in this study. 1/100 anti-Tor1, 1/100 anti-P-Tor1.T1972, 1/100 anti-P-Tor2.S1975, 1/1000 anti-P-Gad8.S546, 1/100 anti-P-Gad8.T387, 1/100 anti-Gad8 antibodies, 1/2000 phospho-(Ser/Thr) Akt substrate (PAS) antibody (Cell Signalling) and 1/1000 S6 antibody (Abcam). Anti-p-Tor1.T1972, anti-P-Gad8.S546 and anti-P-Gad8.T378 were all generated by Eurogentec. Alkaline phosphatase coupled secondary antibodies were used for all blots followed by direct detection with NBT/BCIP (VWR) substrates on PVDF membranes.
4.6. Large-scale Gad8 immunoprecipitation for mass spectrometry analysis
A 20 l of fission yeast culture at 3 × 106 cells ml−1 were harvested and re-suspended in 20% TCA. Cells were disrupted using a Spex6870 freezer mill in liquid nitrogen. After washing with 0.1% TCA, the sample was re-suspended in sample buffer (80 mM Tris pH 7.5, 5 mM DTT, 5 mM EDTA) plus 2% SDS with 3 min boiling. About 4.5 volumes of sample buffer plus 1% triton X-100 were added to the supernatant. The mix was centrifuged at 10 000g for 5 min. IP buffer (0.5% Doc, 50 mM NaF, 0.2 mM Na3VO4, 20 mM Na-β-glycerophosphate, 1 mM PMSF and protease inhibitors) was added to the supernatant. The Gad8 kinase was immunoprecipitated on protein G dynal beads for 30 min at 4°C. The beads were then washed six times with sample buffer plus inhibitors, then heated to 80°C for 10 min prior to electrophoreses.
4.7. Gad8 for phospho-site mapping
Large-scale Gad8 immunoprecipitations were run on a Nupage Bis–Tris 4–12% SDS–PAGE gel (Invitrogen), The Gad8 Coomassie-stained band was excised and digested with either 20 ng sequencing-grade trypsin (Sigma-Aldrich), 400 ng LysN (Associates of Cape Cod) or 350 ng Elastase (Calbiochem) in 100 µl 40 mM ammonium bicarbonate, 9% (v/v) acetonitrile at 37°C for 18 h. The peptides were separated using a Nano-Acquity UPLC system (Waters) using a Waters NanoAcquity BEH C18 column (75 µm ID, 1.7 µm, 25 cm) with a gradient of 1–25% (v/v) of acetonitrile, 0.1% formic acid over 30 min at a flow rate of 400 nl min−1. The LTQ-Orbitrap XL mass spectrometer was operated in parallel data-dependent mode where the MS survey scan was performed at a nominal resolution of 60 000 (at m/z 400) resolution in the Orbitrap analyser between m/z range of 400–2000. The top six precursors were selected for CID in the LTQ at normalized collision energy of 35% using multi-stage activation at m/z 98.0, 49.0 and 32.7 Da.
4.8. Gad8 in vitro kinase assay
Tor1–GST was purified as follows. BL-21 cell expression of Tor1–GST was disrupted with lysis buffer (50 mM Tris pH 8.0, 100 mM NaCl, 10 mM EGTA, 10 mM EDTA, 0.1% Triton, 1 mM DTT, 1 mM PMSF, protease inhibitor cocktail). The supernatant was incubated with glutathione sepharose for 2 h at 4°C. The sepharose was washed 10× with lysis buffer and Tor1–GST was eluted with elution buffer (as lysis buffer but using 50 mM Tris pH 9.6 plus 6 mg ml−1 glutathione). The in vitro kinase assay was performed as previously described [14]. Briefly, Gad8 was immunoprecipitated in IP buffer (50 mM Tris pH 7.6, 150 mM KCl, 5 mM EDTA, 1 mM EDTA, 10% glycerol, 0.2% NP40, 20 mM β-glycerophosphate, 0.1 mM Na3VO4, 15 mM PNPP, 1 mM PMSF and protease inhibitors) and resuspended in kinase assay buffer (20 mM HEPES pH 7.5, 10 mM MgCl2, 1 mM DTT, 20 mM β-glycerophosphate, 0.1 mM Na3VO4 and 15 mM PNPP). It was mixed with 3 µg of Tor1–GST and 25 µM of ATP, incubated at 32°C for 30 min. The reaction was stopped by 5 min boiling with 2× loading buffer.
4.9. Tor1 in vitro kinase assay
Schizosaccharomyces pombe cells were harvested from early exponential phase cultures of 1.5 × 106 cells ml−1. Cells were resuspended in IP buffer (50 mM Tris pH 7.6, 150 mM KCl, 5 mM EDTA, 1 mM EDTA, 10% glycerol, 0.2% NP40, 20 mM β-glycerophosphate, 0.1 mM Na3VO4, 15 mM PNPP, 1 mM PMSF and protease inhibitors). Following cell lysis, Tor1 was immunoprecipitated on protein G Dynal beads and used directly in in vitro Tor1 kinase assays. The kinase assays were performed using the K-LISA mTOR activity kit that includes substrate according to Ikai et al. [35].
4.10. Statistics
Asterisks represent statistical significance (p < 0.05) as determined by Student's t-test.
Supplementary Material
Acknowledgements
We thank M. Balasubramanian and K. Shiozaki for strains, and Iain Hagan for stimulating discussions and comments on the manuscript.
Competing interests
We have no competing interests.
Funding
A Cancer Research UK Senior Fellowship (C10888/A11178), Manchester and Flinders Universities supported this work.
References
- 1.Laplante M, Sabatini DM. 2012. mTOR signaling in growth control and disease. Cell 149, 274–293. (doi:10.1016/j.cell.2012.03.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175. (doi:10.1016/S0092-8674(02)00808-5) [DOI] [PubMed] [Google Scholar]
- 3.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. 2002. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457–468. (doi:10.1016/S1097-2765(02)00636-6) [DOI] [PubMed] [Google Scholar]
- 4.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302. (doi:10.1016/j.cub.2004.06.054) [DOI] [PubMed] [Google Scholar]
- 5.Otsubo Y, Yamamato M. 2008. TOR signaling in fission yeast. Crit. Rev. Biochem. Mol. Biol. 43, 277–283. (doi:10.1080/10409230802254911) [DOI] [PubMed] [Google Scholar]
- 6.Buel G, Blenis J. 2016. Seeing mTORC1 specificity. Science 352, 2 (doi:10.1126/science.aad9696) [DOI] [PubMed] [Google Scholar]
- 7.Matsuo T, Otsubo Y, Urano J, Tamanoi F, Yamamoto M. 2007. Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol. Cell Biol. 27, 3154–3164. (doi:10.1128/MCB.01039-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez B, Moreno S. 2006. Fission yeast Tor2 promotes cell growth and represses cell differentiation. J. Cell Sci. 119, 4475–4485. (doi:10.1242/jcs.03241) [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Hatanaka M, Nagao K, Nakaseko Y, Kanoh J, Kokubu A, Ebe M, Yanagida, M. 2007. Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells 12, 1357–1370. (doi:10.1111/j.1365-2443.2007.01141.x) [DOI] [PubMed] [Google Scholar]
- 10.Cybulski N, Hall MN. 2009. TOR complex 2: a signaling pathway of its own. Trends Biochem. Sci. 34, 620–627. (doi:10.1016/j.tibs.2009.09.004) [DOI] [PubMed] [Google Scholar]
- 11.Bar-Peled L, Sabatini DM. 2014. Regulation of mTORC1 by amino acids. Trends Cell Biol. 24, 400–406. (doi:10.1016/j.tcb.2014.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearce LR, Komander D, Alessi DR. 2010. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11, 9–22. (doi:10.1038/nrm2822) [DOI] [PubMed] [Google Scholar]
- 13.Jacinto E, Lorberg A. 2008. TOR regulation of AGC kinases in yeast and mammals. Biochem. J. 410, 19–37. (doi:10.1042/BJ20071518) [DOI] [PubMed] [Google Scholar]
- 14.Matsuo T, Kubo Y, Watanabe Y, Yamamoto M. 2003. Schizosaccharomyces pombe AGC family kinase Gad8p forms a conserved signaling module with TOR and PDK1-like kinases. EMBO J. 22, 3073–3083. (doi:10.1093/emboj/cdg298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halova L, Du W, Kirkham S, Smith DL, Petersen J. 2013. Phosphorylation of the TOR ATP binding domain by AGC kinase constitutes a novel mode of TOR inhibition. J. Cell Biol. 203, 595–604. (doi:10.1083/jcb.201305103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda K, Morigasaki S, Tatebe H, Tamanoi F, Shiozaki K. 2008. Fission yeast TOR complex 2 activates the AGC-family Gad8 kinase essential for stress resistance and cell cycle control. Cell Cycle 7, 358–364. (doi:10.4161/cc.7.3.5245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisman R, Choder M. 2001. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J. Biol. Chem. 276, 7027–7032. (doi:10.1074/jbc.M010446200) [DOI] [PubMed] [Google Scholar]
- 18.Hartmuth S, Petersen J. 2009. Fission yeast Tor1 functions as part of TORC1 to control mitotic entry through the stress MAPK pathway following nutrient stress. J. Cell Sci. 122, 1737–1746. (doi:10.1242/jcs.049387) [DOI] [PubMed] [Google Scholar]
- 19.Tatebe H, Morigasaki S, Murayama S, Zeng CT, Shiozaki K. 2010. Rab-family GTPase regulates TOR complex 2 signaling in fission yeast. Curr. Biol. 20, 1975–1982. (doi:10.1016/j.cub.2010.10.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen J, Nurse P. 2007. TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat. Cell Biol. 9, 1263–1272. (doi:10.1038/ncb1646) [DOI] [PubMed] [Google Scholar]
- 21.Fantes P, Nurse P. 1977. Control of cell size at division in fission yeast by growth-modulated size control over nuclear division. Exp. Cell Res. 107, 377–386. (doi:10.1016/0014-4827(77)90359-7) [DOI] [PubMed] [Google Scholar]
- 22.Knutsen JH, Rein ID, Rothe C, Stokke T, Grallert B, Boye E. 2011. Cell-cycle analysis of fission yeast cells by flow cytometry. PLoS ONE 6, e17175 (doi:10.1371/journal.pone.0017175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen J, Weilguny D, Egel R, Nielsen O. 1995. Characterization of fus1 of Schizosaccharomyces pombe: a developmentally controlled function needed for conjugation. Mol. Cell Biol. 15, 3697–3707. (doi:10.1128/MCB.15.7.3697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen J, Nielsen O, Egel R, Hagan IM. 1998. FH3, a domain found in formins, targets the fission yeast formin Fus1 to the projection tip during conjugation. J. Cell Biol. 141, 1217–1228. (doi:10.1083/jcb.141.5.1217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen J, Nielsen O, Egel R, Hagan IM. 1998. F-actin distribution and function during sexual differentiation in Schizosaccharomyces pombe. J. Cell Sci. 111, 867–876. [DOI] [PubMed] [Google Scholar]
- 26.Bendezu FO, Martin SG. 2013. Cdc42 explores the cell periphery for mate selection in fission yeast. Curr. Biol. 23, 42–47. (doi:10.1016/j.cub.2012.10.042) [DOI] [PubMed] [Google Scholar]
- 27.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. 2004. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128. (doi:10.1038/ncb1183) [DOI] [PubMed] [Google Scholar]
- 28.Schmid E, Gu S, Yang W, Munzer P, Schaller M, Lang F, Stournaras C, Shumilina E. 2013. Serum- and glucocorticoid-inducible kinase SGK1 regulates reorganization of actin cytoskeleton in mast cells upon degranulation. Am. J. Physiol. Cell Physiol. 304, C49–C55. (doi:10.1152/ajpcell.00179.2012) [DOI] [PubMed] [Google Scholar]
- 29.Mitchison JM, Nurse P. 1985. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 75, 357–376. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, et al. 2012. Nonmedially assembled F-actin cables incorporate into the actomyosin ring in fission yeast. J. Cell Biol. 199, 831–847. (doi:10.1083/jcb.201209044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisman R, Finkelstein S, Choder M. 2001. Rapamycin blocks sexual development in fission yeast through inhibition of the cellular function of an FKBP12 homolog. J. Biol. Chem. 276, 24 736–24 742. (doi:10.1074/jbc.M102090200) [DOI] [PubMed] [Google Scholar]
- 32.Nakashima A, Sato T, Tamanoi F. 2010. Fission yeast TORC1 regulates phosphorylation of ribosomal S6 proteins in response to nutrients and its activity is inhibited by rapamycin. J. Cell Sci. 123, 777–786. (doi:10.1242/jcs.060319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du W, Hálová L, Kirkham S, Atkin J, Petersen J. 2012. TORC2 and the AGC kinase Gad8 regulate phosphorylation of the ribosomal protein S6 in fission yeast. Biol. Open 1, 884–888. (doi:10.1242/bio.20122022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakashima A, Otsubo Y, Yamashita A, Sato T, Yamamoto M, Tamanoi F. 2012. Psk1, an AGC kinase family member in fission yeast, is directly phosphorylated and controlled by TORC1 and functions as S6 kinase. J. Cell Sci. 125, 5840–5849. (doi:10.1242/jcs.111146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikai N, Nakazawa N, Hayashi T, Yanagida M. 2011. The reverse, but coordinated, roles of Tor2 (TORC1) and Tor1 (TORC2) kinases for growth, cell cycle and separase-mediated mitosis in Schizosaccharomyces pombe. Open Biol. 1, 110007 (doi:10.1098/rsob.110007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. 2004. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4, 1633–1649. (doi:10.1002/pmic.200300771) [DOI] [PubMed] [Google Scholar]
- 37.Powell DJ, Hajduch E, Kular G, Hundal HS. 2003. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Mol. Cell Biol. 23, 7794–7808. (doi:10.1128/MCB.23.21.7794-7808.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weyrich P, Neuscheler D, Melzer M, Hennige AM, Haring HU, Lammers R. 2007. The Par6alpha/aPKC complex regulates Akt1 activity by phosphorylating Thr34 in the PH-domain. Mol. Cell. Endocrinol. 268, 30–36. (doi:10.1016/j.mce.2007.01.011) [DOI] [PubMed] [Google Scholar]
- 39.Arellano M, Valdivieso MH, Calonge TM, Coll PM, Duran A, Perez P. 1999. Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J. Cell Sci. 112, 3569–3578. [DOI] [PubMed] [Google Scholar]
- 40.Kobori H, Toda T, Yaguchi H, Toya M, Yanagida M, Osumi M. 1994. Fission yeast protein-kinase-c gene homologs are required for protoplast regeneration—a functional-link between cell-wall formation and cell-shape control. J. Cell Sci. 107, 1131–1136. [DOI] [PubMed] [Google Scholar]
- 41.Maundrell K. 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123, 127–130. (doi:10.1016/0378-1119(93)90551-D) [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Mir L, Soto T, Franco A, Madrid M, Viana RA, Vicente J, Gacto M, Perez P, Cansado J. 2014. Rho1 GTPase and PKC ortholog Pck1 are upstream activators of the cell integrity MAPK pathway in fission yeast. PLoS ONE 9, e88020 (doi:10.1371/journal.pone.0088020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen A, Kupiec M, Weisman R. 2014. Glucose activates TORC2-Gad8 protein via positive regulation of the cAMP/cAMP-dependent protein kinase A (PKA) pathway and negative regulation of the Pmk1 protein-mitogen-activated protein kinase pathway. J. Biol. Chem. 289, 21 727–21 737. (doi:10.1074/jbc.M114.573824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helliwell SB, Schmidt A, Ohya Y, Hall MN. 1998. The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol. 8, 1211–1214. (doi:10.1016/S0960-9822(07)00511-8) [DOI] [PubMed] [Google Scholar]
- 45.Larsson C. 2006. Protein kinase C and the regulation of the actin cytoskeleton. Cell. Signal. 18, 276–284. (doi:10.1016/j.cellsig.2005.07.010) [DOI] [PubMed] [Google Scholar]
- 46.Lang F, Henke G, Embark HM, Waldegger S, Palmada M, Bohmer C, Vallon V. 2003. Regulation of channels by the serum and glucocorticoid-inducible kinase—implications for transport, excitability and cell proliferation. Cell. Physiol. Biochem. 13, 41–50. (doi:10.1159/000070248) [DOI] [PubMed] [Google Scholar]
- 47.Lang F, Stournaras C. 2013. Serum and glucocorticoid inducible kinase, metabolic syndrome, inflammation, and tumor growth. Hormones 12, 160–171. (doi:10.14310/horm.2002.1401) [DOI] [PubMed] [Google Scholar]
- 48.Geraldes P, King GL. 2010. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 106, 1319–1331. (doi:10.1161/CIRCRESAHA.110.217117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao X, et al. 2013. Exercise protects against diet-induced insulin resistance through downregulation of protein kinase Cbeta in mice. PLoS ONE 8, e81364 (doi:10.1371/journal.pone.0081364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang W, Bansode R, Mehta M, Mehta KD. 2009. Loss of protein kinase Cbeta function protects mice against diet-induced obesity and development of hepatic steatosis and insulin resistance. Hepatology 49, 1525–1536. (doi:10.1002/hep.22815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu H, et al. 2014. Hepatic serum- and glucocorticoid-regulated protein kinase 1 (SGK1) regulates insulin sensitivity in mice via extracellular-signal-regulated kinase 1/2 (ERK1/2). Biochem. J. 464, 281–289. (doi:10.1042/BJ20141005) [DOI] [PubMed] [Google Scholar]
- 52.Destefano MA, Jacinto E. 2013. Regulation of insulin receptor substrate-1 by mTORC2 (mammalian target of rapamycin complex 2). Biochem. Soc. Trans. 41, 896–901. (doi:10.1042/BST20130018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egel R, Willer M, Kjaerulff S, Davey J, Nielsen O. 1994. Assessment of pheromone production and response in fission yeast by a halo test of induced sporulation. Yeast 10, 1347–1354. (doi:10.1002/yea.320101012) [DOI] [PubMed] [Google Scholar]
- 54.Caspari T, Dahlen M, Kanter-Smoler G, Lindsay HD, Hofmann K, Papadimitriou K, Sunnerhagen P, Carr AM. 2000. Characterization of Schizosaccharomyces pombe Hus1: a PCNA-related protein that associates with Rad1 and Rad9. Mol. Cell Biol. 20, 1254–1262. (doi:10.1128/MCB.20.4.1254-1262.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.