Abstract
Chlorine is a commonly used, reactive compound to which humans can be exposed via accidental or intentional release resulting in acute lung injury. Formulations of rolipram (a phosphodiesterase inhibitor), triptolide (a natural plant product with anti-inflammatory properties), and budesonide (a corticosteroid), either neat or in conjunction with poly(lactic:glycolic acid) (PLGA), were developed for treatment of chlorine-induced acute lung injury by intramuscular injection. Formulations were produced by spray-drying, which generated generally spherical microparticles that were suitable for intramuscular injection. Multiple parameters were varied to produce formulations with a wide range of in vitro release kinetics. Testing of selected formulations in chlorine-exposed mice demonstrated efficacy against key aspects of acute lung injury. The results show the feasibility of developing microencapsulated formulations that could be used to treat chlorine-induced acute lung injury by intramuscular injection, which represents a preferred route of administration in a mass casualty situation.
Keywords: acute lung injury, pulmonary edema, airway hyperreactivity, microencapsulation
Introduction
Chlorine is a gaseous chemical that is used in multiple types of industrial applications, including chemical syntheses, production of plastics, water treatment, and bleaching operations (Evans, 2005). Chlorine is one of the top ten chemicals produced by mass in the U.S., and much of it is transported from production sites to end use locations. Chlorine gas is highly reactive and produces respiratory toxicity when inhaled (White and Martin, 2010). Human exposure to chlorine can occur through industrial and household accidents. Large accidental releases of chlorine have resulted from train derailments, and these have led to human casualties (Joyner and Durel, 1962; Weill et al., 1969; Jones et al., 1986; Van Sickle et al., 2009). Chlorine is also considered a chemical threat agent, having been used as a chemical weapon in World War I and more recently in the Iraq War. Because chlorine is easily acquired and deployed, there are concerns that it could be used in a terrorist attack on the US populace. The U.S. Department of Homeland Security has estimated that a large-scale chlorine release in an urban area could produce as many as 100,000 hospitalizations for respiratory injuries (Homeland). Because casualties on this scale would likely overwhelm local health care capabilities, there is interest in developing medical countermeasures that could be used to treat acute lung injury induced by chlorine gas inhalation.
Chlorine damages cells lining the respiratory tract, and inhalation of chlorine at high concentrations can produce acute lung injury characterized by epithelial-endothelial barrier disruption, pulmonary edema, pneumonitis, and airway obstruction. Clinical symptoms include dyspnea, cough, hypoxemia, and bilateral infiltrates on chest X-ray (Van Sickle et al., 2009). Treatment for chlorine-induced lung injury involves primarily supportive care such as oxygen administration and mechanical ventilation. Although a variety of drugs, including ß-adrenergic agonists, corticosteroids, and sodium bicarbonate, have been used off-label (Van Sickle et al., 2009), there are currently no FDA-approved medical countermeasures for chlorine-induced acute lung injury.
We are developing treatments that could be administered after chlorine exposure to ameliorate lung injury. We have shown previously that rolipram (a type 4 phosphodiesterase inhibitor), triptolide (a natural plant diterpenoid), and budesonide (a corticosteroid) inhibit various aspects of acute lung injury in mice exposed to chlorine gas. Rolipram inhibited pulmonary edema and airway hyperreactivity induced by chlorine inhalation (Chang et al., 2012). Triptolide inhibited chlorine-induced inflammation (Hoyle et al., 2010), and budesonide inhibited chlorine-induced inflammation and pulmonary edema (Chen et al., 2013). We previously administered rolipram, triptolide, and budesonide by different routes in initial experiments to establish efficacy of treatments. In the present study, we developed and tested formulations to optimize intramuscular delivery of the compounds of interest for treating chlorine-induced acute lung injury. The intramuscular route is attractive for administering countermeasures because it potentially allows for rapid treatment of large numbers of casualties by first responders. We developed a variety of formulations for each active compound and conducted in vitro and in vivo assessments in an effort to produce optimized countermeasures.
Materials and Methods
Materials
Rolipram and triptolide were obtained from Tocris (Ellisville, MO), and budesonide was obtained from Medisca (Plattsburgh, NY). Poly(lactic:glycolic acid) (PLGA) was purchased from Lakeshore Biomaterials (Birmingham, AL), and polyethylene glycol 3350 was purchased from Spectrum Chemical (New Brunswick, NJ).
Production of spray-dried formulations
The active pharmaceutical ingredients, in addition to PLGA and polyethylene glycol (PEG) when present, were dissolved in methylene chloride or ethanol. These solutions were spray dried using a Pro-C-epT 4M8 spray drier (Pro-C-epT, Zelzate, Belgium) with bifluid nozzle to produce microparticles. Payload analysis for the active compounds was performed by dissolution of formulations in DMSO and analysis by HPLC. Average deviation between actual and target payloads was 4.5%. The designations for the formulations refer to the target composition of the dried microparticles.
Scanning electron microscopy
Scanning electron microscopy was conducted using a model EVO 50 (Carl Zeiss, GmbH) environmental instrument operated at 20 keV. Both quadrant backscatter and secondary electron (Everhart-Thornley) detectors were used for this study. Samples were sputtered with AuPd prior to imaging in high-vacuum mode.
In vitro release assay
Release of active compounds from formulations into phosphate buffered saline at 37 °C over time was measured. Compounds were measured by HPLC using C18 columns with UV detection. For rolipram, the mobile phase was 40% acetonitrile in water with detection at 280 nm. For triptolide, the mobile phase was 40% acetonitrile in water with detection at 215 nm. For budesonide, the mobile phase was 70% methanol in water with detection at 240 nm.
Particle sizing
Particle sizing was determined using a Malvern Mastersizer with Hydro 2000S accessory. Approximately 200 mg of dry sample was suspended in 10 ml of a 0.1% (w/w) Tween 80 in water solution and mixed using a bench top vortex mixer. The suspension was added drop-wise to the sample chamber of the instrument until the proper obscuration was attained (6-20%). The sample was mixed to allow flow through the imaging cell with an impeller at 2800 rpm and sonication at 50% of maximum. A general purpose mathematical model that assumed spherical particles and a refractive index of 1.460 were used to calculate particle size distribution. An average of three sample measurements (5000 captures per measurement) was reported for each sample and plotted as a function of volume or number percent versus particle size.
Chlorine exposure and treatments
All animal experiments were approved by the University of Louisville Institutional Animal Care and Use Committee and were performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). Male FVB/NJ mice were purchased from The Jackson Laboratory at 8 weeks of age and housed for 1-2 weeks after receipt prior to chlorine exposure. Mice were housed under specific pathogen-free conditions with 3-5 mice per cage and were randomly assigned to treatment groups before chlorine exposure. Mice were exposed to a target dose of 240 ppm-h (240 ppm for 1 h) chlorine by whole body exposure as previously described (Chang et al., 2012). Actual doses averaged 237 ± 5 ppm-h (mean ± SD). Mice received 0.1 mg/kg buprenorphine subcutaneously for analgesia b.i.d. starting immediately after exposure until they were euthanized. Mice were treated with formulations containing rolipram, triptolide, or budesonide by intramuscular injection. Formulations were suspended in Dulbecco's phosphate buffered saline without calcium or magnesium containing 0.1% Tween 80, and 20 μl per mouse of each suspension was injected intramuscularly. As controls, placebo injections of the vehicle alone or PLGA microparticles without the active pharmaceutical component were performed. For analysis of pulmonary edema 6 h after exposure, mice received a single injection of rolipram 1 h after the end of the chlorine exposure. For analysis of airway hyperreactivity 1 day after exposure, mice received a total of three doses of rolipram: one dose 1 h after exposure, a second dose 10-12 h after exposure, and a third dose the following day 2 h before pulmonary function measurements were made. For analysis of neutrophils in lavage fluid 48 h after exposure or in tissue sections 6 h after exposure, mice were given a single treatment of triptolide formulations 1 h after chlorine exposure. Budesonide formulations were given b.i.d. for 2 days starting 1 h after chlorine exposure prior to analysis of lavage fluid neutrophils 48 h after exposure. In all cases, the labeling in the figures represents the amount of active compound administered in each dose.
Analysis of lung injury
Pulmonary edema was measured by analyzing extravascular lung water (Chang et al., 2012). Airway hyperreactivity was assessed by measuring respiratory system resistance at baseline and in response to increasing doses of methacholine as described (Chang et al., 2012). Lung lavage and analysis of the recovered cells was performed as described (Tian et al., 2008). Analysis of neutrophils in tissue sections from lungs collected 6 h after chlorine exposure was performed by immunostaining for the neutrophil marker Ly-6G as described (Hoyle et al., 2010).
Pharmacokinetic analysis
Male FVB/NJ mice (8 weeks old) were purchased from The Jackson Laboratory and used 1-2 weeks after receipt. Mice were injected intramuscularly with 360 μg of spray-dried neat rolipram formulation or 300 μg of spray-dried neat budesonide formulation. At various times after drug injection, blood was collected for preparation of plasma, following which the animals were euthanized for collection of lung tissue. Plasma and lung homogenates made in phosphate buffered saline were spiked with internal standard (dexamethasone) and extracted with methyl tert-butyl ether. Samples for LC-MS/MS analysis were prepared by drying the organic phase and dissolving the material in 50% methanol. HPLC separation was performed on a C8 column with gradient elution from 90% solution A (10 mM formic acid in water) 10% solution B (10 mM formic acid in methanol) to 10% solution A 90% solution B.
Data analysis
In vitro release data are shown as means of duplicate samples. Pharmacokinetic data are shown as means of 2-3 replicates for which rolipram or budesonide was detected. Other data are presented as group means ± standard error of the mean (SEM). Effects of treatment on airway reactivity to methacholine were analyzed by repeated measures analysis of variance (ANOVA). Effects of exposure condition/treatment on extravascular lung water and lung inflammation were analyzed using one-way ANOVA with Bonferroni's Multiple Comparison Test. Data were transformed before analysis if necessary to produce normally distributed data. Differences were considered to be statistically significant at the p<0.05 level. Pharmacokinetic parameters were calculated using WinNonlin Professional (version 5.2.1, Pharsight Corporation, Mountain View, CA).
Results
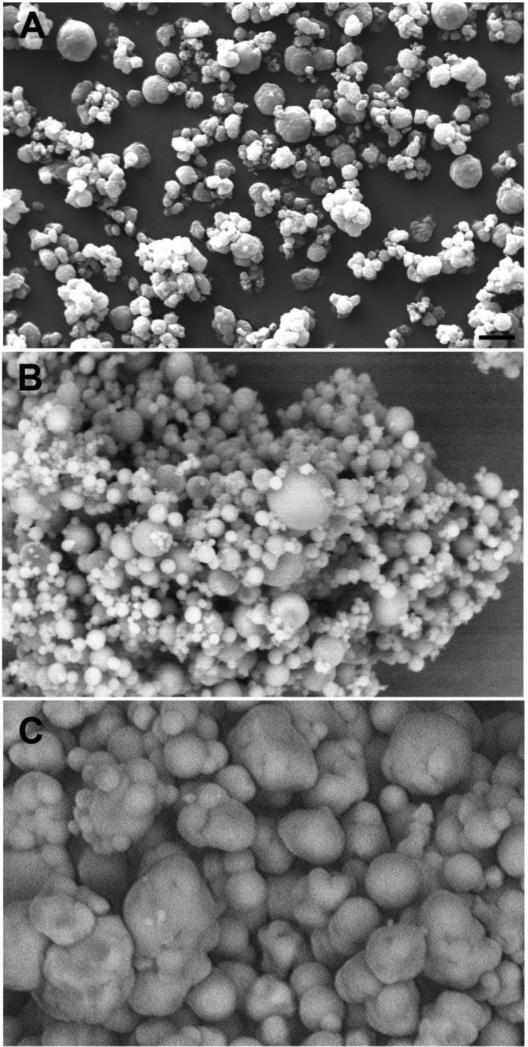
Formulations for intramuscular injection of rolipram, triptolide, and budesonide were developed. A list of formulations with selected properties is shown in Table S1. Parameters were investigated for producing intramuscular injectable formulations suitable for treating effects of lung injury that developed 6 to 48 h after chlorine exposure, including inflammation, impaired pulmonary function, and pulmonary edema. Goals were to develop formulations that could be suspended in small volumes for intramuscular injection and that would rapidly release the active compounds. Formulations were produced by spray drying solutions of the active compounds made in organic solvents. Formulations comprising both the neat compounds and the drugs encapsulated in PLGA microparticles were produced. PLGA formulations typically allow for an initial rapid “burst” release followed by a longer period of controlled release (Allison, 2008). This type of release kinetics may be beneficial for treating chlorine-induced effects in which lung injury develops rapidly but also continues to progress during the first day after exposure. The compounds of interest with or without PLGA were dissolved in an organic solvent and spray-dried to produce microparticles. The size and shape of the particles were assessed by scanning electron microscopy, and the ability of the particles to release the active component was evaluated by in vitro release assays. The effects of the percentage of active compound and nozzle size for spray drying were investigated. Figure 1 shows scanning electron micrographs of selected rolipram microparticles produced by spray drying. The spray dried formulation of neat rolipram contained particles that were generally spherical in shape but had irregular surfaces (Fig. 1A). Rolipram-PLGA particles were generally spherical, and, as expected, the larger nozzle size appeared to produce larger particles (Fig. 1B and 1C).
Figure 1. Scanning electron micrographs of rolipram formulations.
Rolipram formulations were produced by spray drying and evaluated by scanning electron microscopy. A) Neat rolipram spray-dried from methylene chloride solution, 0.4 mm nozzle. B) 20% rolipram/80% PLGA spray-dried from methylene chloride, 0.4 mm nozzle. C) 40% rolipram/60% PLGA spray-dried from methylene chloride, 0.6 mm nozzle. Scale bar in panel A represents 10 μm for all panels.
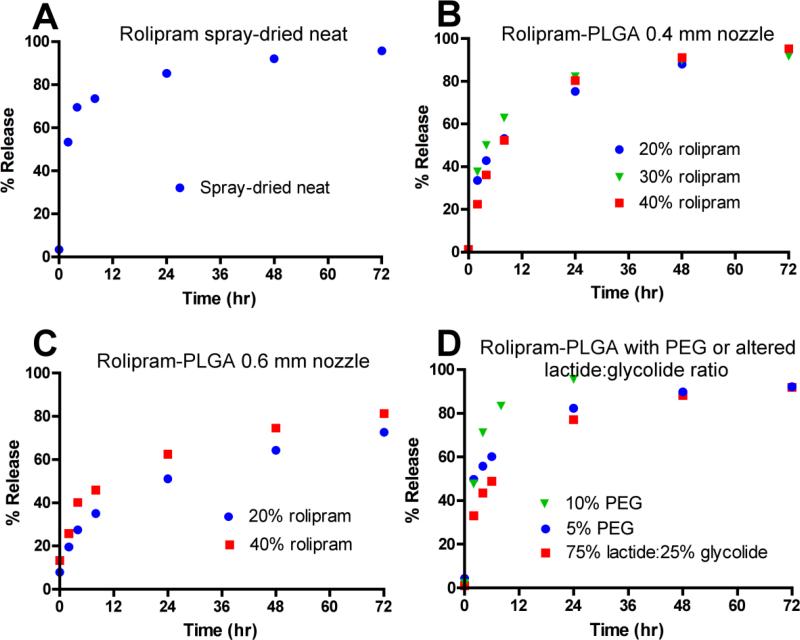
Spray-dried neat rolipram particles rapidly released the active compound into solution as judged by in vitro release assay, with 70% released by 4 h (Fig. 2A). The rate of release slowed after this, with 85% being released by 24 hr. Rolipram-PLGA particles generated by spray-drying using the 0.4 mm nozzle exhibited an initial release (36-50% by 4 h) that was slower than that of the spray-dried neat particles, but a 24 hr release (75-82%) that was similar (Fig. 2B). Thus these rolipram-PLGA particles released more rolipram during the 4-24 h time segment than did the spray-dried neat rolipram. Rolipram-PLGA particles loaded with different amounts of rolipram (20%, 30%, and 40%) had similar release kinetics except for a slightly slower initial release from the 40% rolipram/60% PLGA formulation (Fig. 2B). Rolipram-PLGA particles made using the 0.6 mm nozzle had even slower release kinetics, with 27-40% release at 4 h and 51-62% at 24 h (Fig. 2C). Most of the PLGA formulations were made using a 50% lactide:50% glycolide ratio. One formulation was tested in which this ratio was altered to determine the effects on release kinetics. Increasing the lactide:glycolide ratio to 75%:25% did not produce a major difference in release kinetics (compare 75% lactide:25% glycolide in Fig. 2D with 40% rolipram in Fig. 2B). Addition of PEG into rolipram-PLGA particles dose-dependently increased the initial rate of release [at 4 h 56% release for 40% rolipram/55% PLGA/5% PEG and 71% release for 40% rolipram/50% PLGA/10% PEG (Fig. 2D) compared with 36% release for 40% rolipram/60% PLGA (Fig. 2B)]. The rolipram-PLGA formulation containing 10% PEG had the highest release at 24 h of all the formulations tested (96%, Fig. 2D).
Figure 2. In vitro release kinetics for rolipram formulations.
Rolipram formulations produced by spray drying were evaluated using in vitro release assays. A) Neat rolipram, 0.4 mm nozzle. B) Rolipram/PLGA formulations containing 20%, 30%, and 40% rolipram, 0.4 mm nozzle. C) Rolipram/PLGA formulations containing 20% and 40% rolipram, 0.6 mm nozzle. D) Rolipram/PLGA formulations containing 40% rolipram with altered lactide:glycolide ratio or addition of PEG, 0.4 mm nozzle.
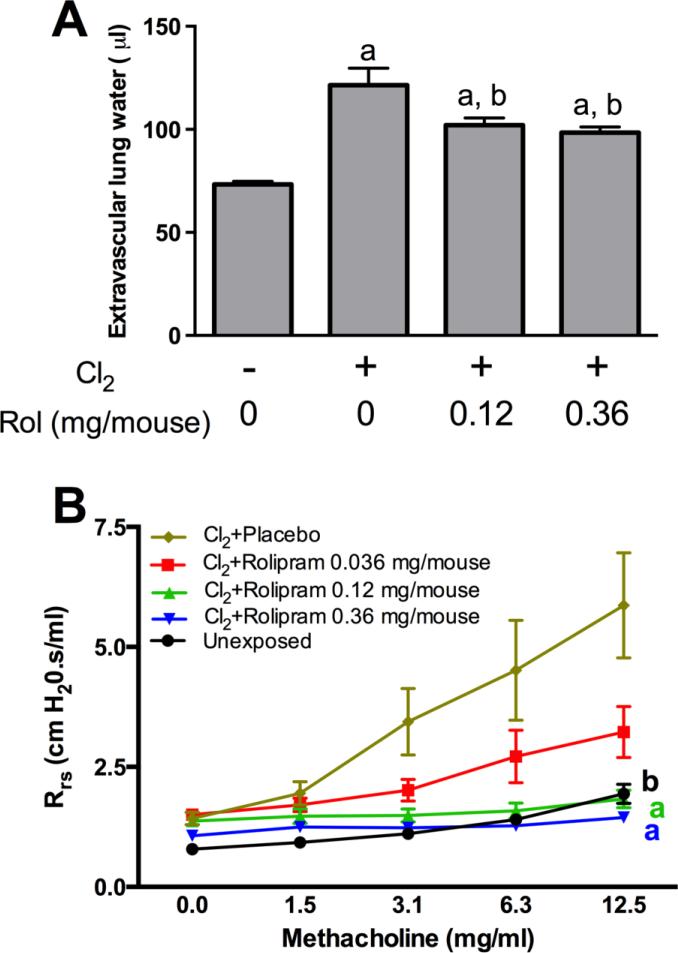
Selected rolipram formulations were tested for the ability to inhibit key aspects of lung injury induced by chlorine inhalation. FVB/NJ mice were exposed to chlorine, injected with rolipram formulations inramuscularly starting 1 h after exposure, and evaluated for pulmonary edema 6 h after exposure and airway reactivity to methacholine 1 day after exposure. One formulation, 40% rolipram/60% PLGA, produced a significant inhibition of chlorine-induced pulmonary edema as measured by extravascular lung water (Fig. 3A). The other formulations that were tested in vivo, spray-dried neat rolipram and 40% rolipram/55% PLGA/5% PEG, did not significantly inhibit chlorine-induced pulmonary edema (Table 1). Spray-dried neat rolipram inhibited chlorine-induced airway hyperreactivity in a dose-dependent manner (Fig. 3B). The other two formulations that were tested also inhibited chlorine-induced airway hyperreactivity up to 100% (Table 1). The formulation with 40% rolipram/50% PLGA/10% PEG, which had the fastest release kinetics, could not be tested in vivo because it formed sticky aggregates that could not be injected intramuscularly.
Figure 3. Inhibition of chlorine-induced pulmonary edema and airway hyperreactivity by rolipram formulations.
A) Pulmonary edema. FVB/NJ mice were exposed to chlorine and treated with 40% rolipram/60% PLGA or placebo beads intramuscularly 1 h after the end of the chlorine exposure. Pulmonary edema was assessed by measurement of extravascular lung water in left lungs collected 6 hr after chlorine exposure. a, p<0.001 vs. no chlorine; b, p<0.05 vs. chlorine, no rolipram. n=9-12 mice/group. B) Airway reactivity. FVB/NJ mice were exposed to chlorine and treated with spray dried neat rolipram or vehicle intramuscularly 1 hr after chlorine exposure, 10-12 h after exposure, and 2 h before pulmonary function testing on the day after exposure. Respiratory system resistance was measured at baseline and following inhalation of increasing doses of methacholine. a, dose-response curves p<0.01 vs. Cl2+Placebo. b, dose-response curve p<0.05 vs. Cl2+Placebo. n=6-8 mice/group.
Table 1.
Summary of results for rolipram formulations that were tested in vivo.
| Formulation1 | In vitro release at 4 h | Maximum inhibition of airway hyperreactivity | Maximum inhibition of pulmonary edema |
|---|---|---|---|
| 100% rolipram | 70% | 100% | Not significant |
| 40% rolipram/60% PLGA | 36% | 100% | 48% |
| 40% rolipram/55% PLGA/5% PEG | 56% | 100% | Not significant |
All generated by spray drying of methylene chlorine solutions using 0.4 mm nozzle; PLGA formulations contain 50:50 ratio of lactide:glycolide.
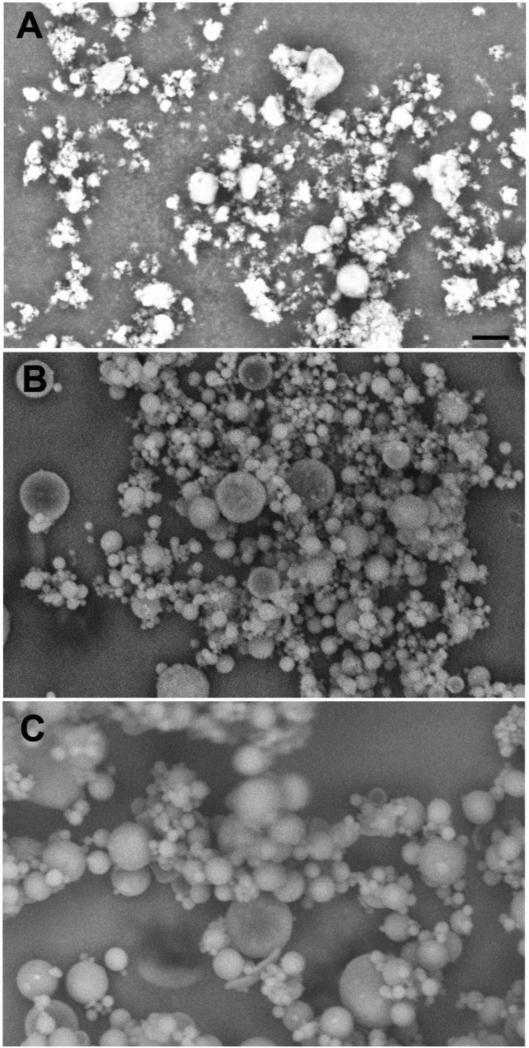
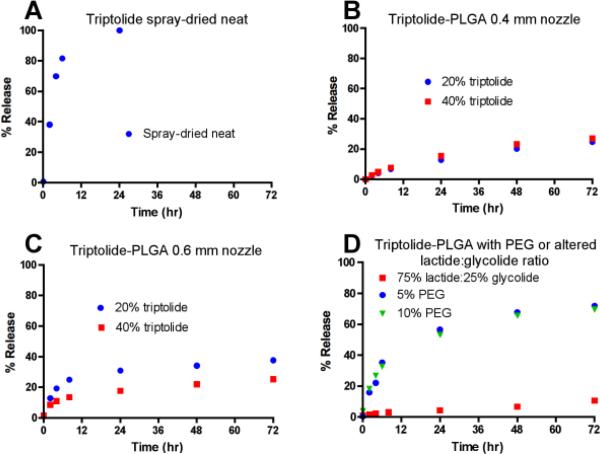
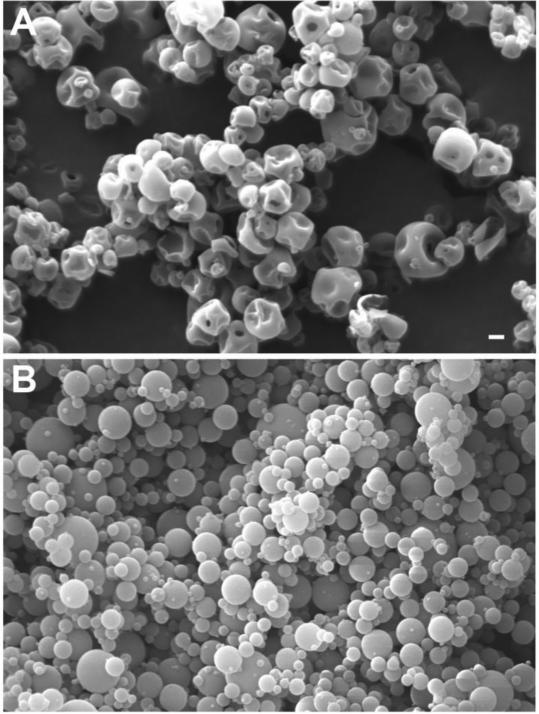
Formulations for intramuscular injection were produced for triptolide, which is a natural plant product with potent anti-inflammatory activity (Hoyle et al., 2010). Figure 4 shows scanning electron micrograph images for selected triptolide formulations. A formulation of neat spray-dried triptolide had large amounts of sub-micron particles (Figure 4A). Triptolide-PLGA formulations had larger, spherical particles (Fig. 4B and 4C), with the 0.6 mm nozzle producing the largest particles (Fig. 4C). Neat spray-dried triptolide produced the fastest release of any of the triptolide formulations as measured by in vitro release assays (70% by 4 h and 100% by 24 h; Fig. 5 A). Triptolide-PLGA formulations produced with either the 0.4 mm (Fig. 5B) or 0.6 mm (Fig. 5C) nozzle had slower release kinetics, with less than 40% release by 24 h. Increasing the lactide:glycolide ratio resulted in even slower release (Fig. 5D). Addition of 5 or 10% PEG to the triptolide-PLGA formulations increased release at 24 hr to approximately 60% (Fig. 5D).
Figure 4. Scanning electron micrographs of triptolide formulations.
Triptolide formulations were produced by spray drying and evaluated by scanning electron microscopy. A) Neat triptolide spray-dried from methylene chloride solution, 0.4 mm nozzle. B) 40% triptolide/60% PLGA spray-dried from methylene chloride, 0.4 mm nozzle. C) 40% triptolide/60% PLGA spray-dried from methylene chloride, 0.6 mm nozzle. Scale bar in panel A represents 10 μm for all panels.
Figure 5. In vitro release kinetics for triptolide formulations.
Triptolide formulations produced by spray drying were evaluated using in vitro release assays. A) Neat triptolide, 0.4 mm nozzle. B) Triptolide/PLGA formulations containing 20% and 40% triptolide, 0.4 mm nozzle. C) Triptolide/PLGA formulations containing 20% and 40% triptolide, 0.6 mm nozzle. D) Triptolide/PLGA formulations containing 40% triptolide with altered lactide:glycolide ratio or addition of PEG, 0.4 mm nozzle.
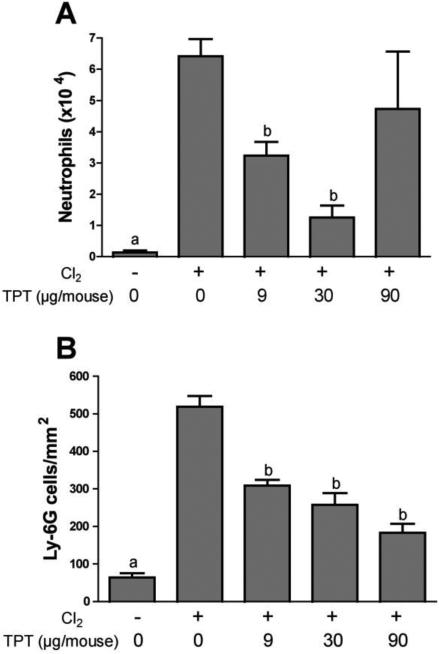
Selected triptolide formulations were tested in vivo by assessing inhibition of neutrophil influx after chlorine exposure. Neutrophils were measured in lavage fluid collected 48 h after chlorine exposure because we have shown that lavage fluid neutrophils are consistently and significantly elevated at this time (Tian et al., 2008; Hoyle et al., 2010). Using this assay, the formulation consisting of 40% triptolide/55% PLGA/5% PEG was shown to significantly inhibit neutrophil influx up to 82% at the intermediate dose tested (30 μg/mouse; Fig. 6A). A higher dose of triptolide (90 μg/mouse) resulted in a higher level of inflammation, suggesting potential toxicity of triptolide. Similar results for 40% triptolide/60% PLGA formulations generated from both the 0.4 mm and 0.6 mm nozzles were obtained but with a somewhat smaller maximal inhibition of lavage fluid neutrophils (Table 2). Neat spray-dried triptolide, which had the fastest in vitro release kinetics, did not result in any significant inhibition of chlorine-induced neutrophil influx (Table 2). In addition, this formulation produced clear toxic effects, as all the mice treated with the highest dose (90 μg/mouse) died. Anti-inflammatory effects of selected triptolide formulations were confirmed by a second assay in which early influx of neutrophils was detected in lung tissue sections by immunostaining 6 h after chlorine exposure (Hoyle et al., 2010). Figure 6B shows significant inhibition of neutrophil influx into lung tissue by all three doses of 40% triptolide/60% PLGA that were tested.
Figure 6. Inhibition of chlorine-induced lung inflammation by triptolide formulations.
A) Lavage fluid neutrophils. FVB/NJ mice were exposed to chlorine and treated with 40% triptolide/55% PLGA/5% PEG or placebo intramuscularly 1 h after the end of the chlorine exposure. Neutrophils were enumerated in lung lavage fluid collected 48 h after chlorine exposure. a, p<0.05 vs. other groups; b, p<0.05 vs. chlorine, no triptolide. n=6-9 mice/group. B) Lung tissue neutrophils. FVB/NJ mice were exposed to chlorine and treated with 40% triptolide/60% PLGA or placebo particles intramuscularly 1 h after chlorine exposure. Immunostaining was performed for the neutrophil marker Ly-6G in sections of lungs collected 6 h after exposure, and labeled cells were counted. a, p<0.05 vs. other groups; b, p<0.001 vs. chlorine, no triptolide. n=7-8 mice/group.
Table 2.
Summary of results for triptolide formulations that were tested in vivo.
| Formulation1 | Nozzle size (mm) | In vitro release at 4 h | Maximum inhibition of lavage fluid neutrophils |
|---|---|---|---|
| 100% triptolide | 0.4 | 70% | Not significant, toxic2 |
| 40% triptolide/60% PLGA | 0.4 | 5% | 49% |
| 40% triptolide/60% PLGA | 0.6 | 11% | 65% |
| 40% triptolide/55% PLGA/5% PEG | 0.4 | 22% | 82% |
All generated by spray drying of methylene chloride solutions using indicated nozzle size; PLGA formulations contain 50:50 ratio of lactide:glycolide.
9/9 mice receiving the highest triptolide dose (90 μg/mouse) died prior to the planned study end point.
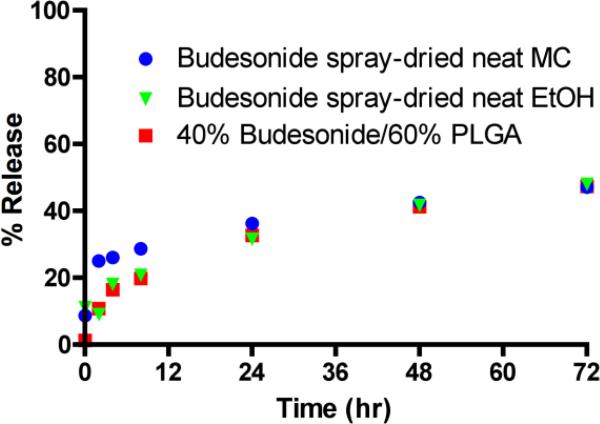
Budesonide formulations were produced by spray drying solutions made in methylene chloride or ethanol, and the solvents used produced different particle shapes. Spray drying of budesonide dissolved in methylene chloride resulted in hollow, dimpled spheres, whereas the ethanol solution produced solid spherical particles (Figure 7). The latter formulation appeared to have the smallest particle size of any of the materials that were imaged. The spray-dried neat budesonide formulations, in addition to a 40% budesonide/60% PLGA formulation, had slow initial release rates (16-26% at 4 h), and none of the formulations released more than 50% of the active compound by 72 h in the in vitro release assays (Fig. 8). Although budesonide release appeared inefficient, at least as measured in vitro, the spray-dried neat formulation produced from ethanol solution was highly effective in preventing neutrophil influx, as it inhibited the number of neutrophils in lavage fluid by almost 90% (Fig. 9). Similar results were also obtained for the other spray-dried neat formulation and the budesonide-PLGA formulation (Table 3).
Figure 7. Scanning electron micrographs of budesonide formulations.
Budesonide formulations were produced by spray drying and evaluated by scanning electron microscopy. A) Neat budesonide spray-dried from methylene chloride solution, 0.4 mm nozzle. B) Neat budesonide spray-dried from ethanol solution, 0.4 mm nozzle. Scale bar in panel A represents 1 μm for both panels.
Figure 8. In vitro release kinetics for budesonide formulations.
Budesonide formulations produced by spray drying were evaluated using in vitro release assays.
Figure 9. Inhibition of chlorine-induced lung inflammation by budesonide formulation.
FVB/NJ mice were exposed to chlorine and treated with placebo or spray-dried neat (ethanol) budesonide formulation intramuscularly b.i.d. for 2 days starting 1 h after the end of the chlorine exposure. Neutrophils were enumerated in lung lavage fluid collected 48 h after chlorine exposure. a, p<0.001 vs. other groups. n=6-9 mice/group.
Table 3.
Summary of results for budesonide formulations that were tested in vivo.
| Formulation1 | In vitro release at 4 h | Maximum inhibition of lavage fluid neutrophils |
|---|---|---|
| 100% budesonide (methylene chloride) | 26% | 91% |
| 100% budesonide (ethanol) | 18% | 88% |
| 40% budesonide/60% PLGA (methylene chloride) | 16% | 87% |
All generated by spray drying of solutions in the indicated solvent using 0.4 mm nozzle; PLGA formulation contains 50:50 ratio of lactide:glycolide.
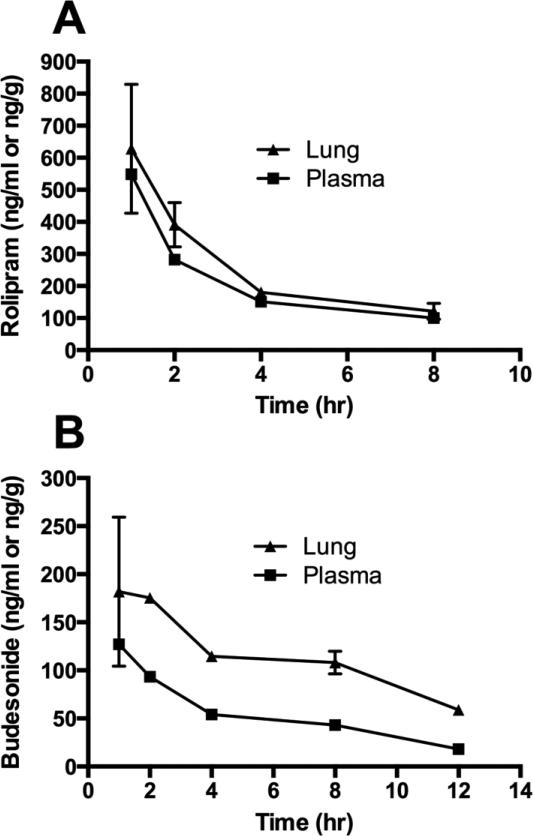
Pharmacokinetic analysis in mice was performed for one rolipram formulation and one budesonide formulation (Fig. 10). The spray-dried neat rolipram formulation and the spray-dried neat budesonide (ethanol) formulation were injected intramuscularly, and levels of the active compounds were measured in plasma and lung. For the rolipram formulation, levels were similar between plasma and lung (Fig. 10A). Peak levels in plasma and lung measured 1 h after injection were 550 ng/ml and 630 ng/g, respectively. The t1/2 for rolipram was 4.2 h in plasma and 3.8 h in lung. Total exposure based on the area under the curve was 2,240 h-ng/ml for plasma and 2,670 h-ng/ml for lung for an injected dose of 360 μg. For the budesonide formulation, the levels in lung were higher than in plasma (Fig. 10B). Peak levels in plasma and lung measured 1 hr after injection were 130 ng/ml and 180 ng/g, respectively. The t1/2 for budesonide was not calculated owing to insufficient measurable data points in the terminal elimination phase. Total exposure for budesonide was 860 h-ng/ml for plasma and 2,060 h-ng/ml for lung for an injected dose of 300 μg.
Figure 10. Pharmacokinetic analysis of rolipram and budesonide formulations in mice.
Mice were injected with formulations i.m., and plasma and lung tissue were collected at various times after injection for measurement of rolipram (A) or budesonide (B). n=2-3 mice/group.
Discussion
Because of the potential for accidental or intentional release of chlorine resulting in a large number of exposed individuals, countermeasure strategies for treating casualties quickly and efficiently are needed. Intramuscular injection represents an attractive route of administration for countermeasures to treat chlorine-induced lung injury, as large numbers of patients could be treated quickly by first responders. One challenge for delivery by this route is the development of preparations having suitably high drug concentrations and low volumes for intramuscular injection. In the present study, countermeasures were injected as suspensions of microparticles containing the active pharmaceutical ingredients alone or in conjunction with PLGA. Microparticles of rolipram, triptolide, and budesonide produced by spray drying with or without PLGA could be easily suspended and injected in small volumes. Although in some cases the addition of PEG as a disintegrant produced material that did not form free-flowing suspensions, the spray-drying process was generally successful in producing formulations suitable for intramuscular injection.
PLGA is a biocompatible and biodegradable polymer that has been used extensively in formulating drugs for i.m. injection (Makadia and Siegel, 2011). Multiple products formulated with PLGA for intramuscular administration have been approved by the FDA for use in the U.S., including Leupron Depot®, Vivitrol®, Sandostatin® LAR Depot, Risperdal Consta®, Trelstar®, and Signifor® LAR. Incorporation of compounds into PLGA microparticles allows for controlled drug release for periods up to weeks or months (Mazzei et al., 1989; Su et al., 2009). For our purposes, some controlled release was desirable to extend the therapeutic window for the drugs of interest, all of which have short systemic half-lives (Ryrfeldt et al., 1982; Krause and Kuhne, 1988; Shao et al., 2007). However a relatively short period of release, on the order of hours to a day, was considered appropriate for countermeasure treatment; to achieve this we explored the use of smaller microparticles as well as those that contained the active pharmaceutical ingredients in the absence of polymer. Encapsulation of drugs into PLGA microparticles can be achieved by multiple methods, including spray drying, phase separation, and solvent evaporation/emulsion processes (Makadia and Siegel, 2011). In pilot studies, we explored solvent evaporation methods for formulating PLGA microparticles. These showed inefficient loading and tendency for particle agglomeration (not shown), leading to adoption of the spray drying techniques which were superior in both these respects.
After injection of microparticle formulations, systemic delivery is achieved through release of active compound from the microparticles. For particles containing neat compounds, this is based on the dissolution of the drug in the surrounding fluid, which is dependent on the solubility of the compound and the size of the particles. Release of active compounds from PLGA particles is more complex with multiple processes coming into play, including burst release of compound adsorbed to the surface of particles, leaching of compound through pores in the particles, and slower release accompanying the breakdown of the PLGA polymer (Makadia and Siegel, 2011). Altering parameters involved in production of the particles can thus in theory be used to achieve a large variety of release properties. This was shown in practice with our spray-dried formulations of rolipram, triptolide, and budesonide, which exhibited a wide range of in vitro release kinetics. Spray drying with a larger nozzle increased particle size as expected and produced slower release kinetics for rolipram, but this was not evident for triptolide formulations. Incorporation of PLGA into rolipram and triptolide formulations resulted in generally slower release kinetics, and this was particularly apparent for triptolide. By contrast, in vitro release kinetics for budesonide formulations did not differ appreciably with or without PLGA. Addition of PEG to PLGA particles has been used to accelerate drug release kinetics (Cleek et al., 1997). Addition of 10% PEG to rolipram/PLGA formulations resulted in a small increase in the rate of rolipram release. For triptolide/PLGA formulations, from which release in the absence of PEG was inefficient, addition of either 5% or 10% PEG caused a pronounced increase in the rate of release.
When inhaled, chlorine reacts with airway lining fluid components and cells lining the respiratory tract and rapidly triggers a cascade of events leading to lung injury (Squadrito et al., 2010). Because of the rapid initiation of lung injury, treatment with countermeasures that quickly deliver efficacious drug levels is desirable. However, because chlorine-induced lung injury can progress for many hours after exposure, there is also a need to balance fast initial release with maintaining systemic drug concentrations at effective levels for longer periods of time. Therefore it was difficult to predict a priori based on in vitro release profiles which formulations would most effectively inhibit lung injury parameters. The three rolipram formulations that were tested in vivo all showed similar effects completely inhibiting chlorine-induced airway hyperreactivity. These formulations differed in their ability to inhibit pulmonary edema, as only one of the three, that containing 40% rolipram/60% PLGA, significantly reduced extravascular lung water. The 40% rolipram/60% PLGA formulation had slower initial in vitro release compared with the spray-dried neat and 40% rolipram/55% PLGA/5% PEG formulations that did not inhibit pulmonary edema. If these release properties are replicated in vivo, the 40% rolipram/60% PLGA formulation may provide a more sustained release of rolipram during a critical period after chlorine exposure during which pulmonary edema develops. This idea was consistent with the pharmacokinetic results for the spray-dried neat rolipram formulation, which produced a rapid but short-lived spike in plasma and lung rolipram levels.
Phosphodiesterase inhibitors including rolipram raise the intracellular levels of the second messenger cyclic AMP, which can have multiple beneficial effects in the lung following injury (Hoyle, 2010). Rolipram has been shown to inhibit lung injury in animals exposed to bleomycin (Pan et al., 2009), hyperoxia (Mehats et al., 2008), and Streptococcus pneumoniae (Tavares et al., 2015). For chlorine injury, we previously showed in mice inhibition by rolipram of pulmonary edema and airway hyperreactivity, which for the lung function measurements involved three rolipram treatments between chlorine exposure and assessment 24 hr after exposure (Chang et al., 2012). By contrast, rolipram was reported to have no significant effect on airway reactivity when administered as a single 10 mg/kg intraperitoneal dose 1 hr after exposure (Wigenstam et al., 2015). These findings add additional support to the importance of maintaining rolipram levels for an extended time beyond the initial postexposure period. The rolipram formulations administered intramuscularly in the current study exerted near maximal effects at 0.12 mg/mouse (approximately 4 mg/kg), and this was generally similar in potency to the systemic rolipram treatments we examined previously (Chang et al., 2012). For a possible future use in humans, an expected human dose based on allometric scaling would be 0.3 mg/kg (Freireich et al., 1966; U.S. Department of Health and Human Services, 2005). Rolipram has been used clinically as an antidepressant at 0.5 mg/dose (i.e. 0.008 mg/kg for a 60 kg person); an anticipated rolipram dose for treating chlorine injury in humans would therefore be considerably larger than has been used previously.
Triptolide is a natural plant product derived from Tripterygium wilfordii that has potent anti-inflammatory and immunosuppressive properties. Triptolide treatment has been shown to inhibit lung inflammation and injury in multiple lung disease models, including lipopolysaccharide injury (Wei and Huang, 2014), pulmonary fibrosis induced by radiation (Yang et al., 2015) or bleomycin (Krishna et al., 2001), and allergic inflammation and airway remodeling (Chen et al., 2011; Chen et al., 2015). In the present study, three of the triptolide formulations that were tested in vivo produced significant inhibition of chlorine-induced inflammation. These three formulations had relatively slow in vitro release profiles, and within this group, the anti-inflammatory effect appeared to be proportional to the extent of initial release. These results were in contrast to those with spray-dried neat triptolide, which showed rapid in vitro release but produced toxic effects in vivo. We and others have shown previously the inibition of chlorine-induced lung inflammation by triptolide administered intraperitoneally, with maximal effects observed around 1 mg/kg (Hoyle et al., 2010; Wigenstam et al., 2015). Here we observed anti-inflammatory effects at a triptolide dose of 30 μg/mouse (approx. equivalent to 1 mg/kg), but a higher dose (90 μg/mouse, approx. 3 mg/kg) produced toxic effects when admininstered intramuscularly as a formulation with a higher rate of release. The results suggest that formulations with controlled release properties will be beneficial for triptolide treatment to avoid toxic levels of this compound. A dose equivalent to 1 mg/kg in mice would be 0.08 mg/kg in humans. Extracts of Tripterygium wilfordii have been used at doses of 1 mg/kg/day in clinical trials for treatment of kidney diseases, but the triptolide content in the extracts has not been reported (Wu et al., 2013; Chen et al., 2014).
Corticosteroids may be beneficial for treating chlorine-induced lung injury because they inhibit inflammation, promote alveolar fluid clearance, and stimulate surfactant production (Ballard, 1989; Smoak and Cidlowski, 2004; Guney et al., 2007). Corticosteroids, including dexamethasone, budesonide, mometasone, bethamethasone, and terbutaline, have been shown to ameliorate aspects of lung injury following chlorine inhalation in animal models (Demnati et al., 1998; Wang et al., 2002; Wang et al., 2004; Wang et al., 2005; Chen et al., 2013; Jonasson et al., 2013; Wigenstam et al., 2015). Budesonide inhibited chlorine-induced lung inflammation, hypoxemia, and edema (Wang et al., 2002; Wang et al., 2004; Wang et al., 2005; Chen et al., 2013). In the present study, all three budesonide formulations that were produced showed good anti-inflammatory effects in the in vivo chlorine model. In vitro, these formulations showed slow initial release and a maximum release of less than 50%. In vivo pharmacokinetic assay for the spray-dried neat formulation showed that the maximal budesonide concentration in plasma and lung was achieved within 1 hr after injection, suggesting that in vivo release for this formulation may be more efficient than what was observed in vitro. Our previous results injecting budesonide solution intraperitoneally showed that administration of a dose of 10 mg/kg budesonide was required to achieve a maximal (approximately 90%) inhibition of neutrophil influx into the lung (Chen et al., 2013). In the present study, this level of inhibition was achieved using doses of 30 μg/mouse (approximately 1 mg/kg) of the budesonide formulations. The spray-dried formulations for intramuscular injection therefore represent a significant improvement in potency for producing the desired anti-inflammatory effects. An equivalent human dose would be 0.08 mg/kg. Budesonide products for human use generally involve topical or local rather than systemic administration, but an oral budesonide product for treatment of ulcerative colitis (Uceris®) is taken as a dose of 9 mg (0.15 mg/kg for a 60 kg person). Therefore the effective dose in mice for the budesonide formulations we developed is within a generally equivalent range of dosing that has been shown to be safe in humans.
Intramuscular injection represents a desirable route of delivery for countermeasures in chemical disaster situations, as treatments can be administered rapidly in the field to large numbers of casualties. We developed spray dried formulations that were compatible with intramuscular injection for rolipram, triptolide, and budesonide. We were able to achieve a large range of in vitro release properties by altering multiple parameters in the production of the formulations. Treatment of mice with selected formulations produced significant inhibition of various aspects of chlorine-induced lung injury, including airway hyperreactivity, pulmonary edema, and inflammation. In the case of budesonide formulations, an increase in the potency of this drug against chlorine-induced lung inflammation was achieved. The results indicate that spray-dried countermeasure formulations represent viable treatment strategies for chlorine-induced lung injury.
Supplementary Material
Highlights.
Chlorine causes lung injury when inhaled and is considered a chemical threat agent.
Countermeasures for treatment of chlorine-induced acute lung injury are needed.
Formulations containing rolipram, triptolide, or budesonide were produced.
Formulations with a wide range of release properties were developed.
Countermeasure formulations inhibited chlorine-induced lung injury in mice.
Acknowledgments
Funding Source
This work was supported by the National Institutes of Health CounterACT Program through the Office of the Director and the National Institute of Environmental Health Sciences (Award U01 ES015673 to G.W.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison SD. Analysis of initial burst in PLGA microparticles. Expert Opin. Drug Deliv. 2008;5:615–628. doi: 10.1517/17425247.5.6.615. [DOI] [PubMed] [Google Scholar]
- Ballard PL. Hormonal regulation of pulmonary surfactant. Endocr. Rev. 1989;10:165–181. doi: 10.1210/edrv-10-2-165. [DOI] [PubMed] [Google Scholar]
- Chang W, Chen J, Schlueter CF, Rando RJ, Pathak YV, Hoyle GW. Inhibition of chlorine-induced lung injury by the type 4 phosphodiesterase inhibitor rolipram. Toxicol. Appl. Pharmacol. 2012;263:251–258. doi: 10.1016/j.taap.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Ma Y, Wang X, Yu S, Li L, Dai B, Mao Z, Liu H, Liu S, Mei C. Triptolide-containing formulation in patients with autosomal dominant polycystic kidney disease and proteinuria: an uncontrolled trial. Am. J. Kidney Dis. 2014;63:1070–1072. doi: 10.1053/j.ajkd.2014.01.418. [DOI] [PubMed] [Google Scholar]
- Chen J, Mo Y, Schlueter CF, Hoyle GW. Inhibition of chlorine-induced pulmonary inflammation and edema by mometasone and budesonide. Toxicol. Appl. Pharmacol. 2013;272:408–413. doi: 10.1016/j.taap.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lv Z, Jiang S. The effects of triptolide on airway remodelling and transforming growth factor-beta(1)/Smad signalling pathway in ovalbumin-sensitized mice. Immunol. 2011;132:376–384. doi: 10.1111/j.1365-2567.2010.03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lv Z, Zhang W, Huang L, Lin X, Shi J, Zhang W, Liang R, Jiang S. Triptolide suppresses airway goblet cell hyperplasia and Muc5ac expression via NF-kappaB in a murine model of asthma. Mol. Immunol. 2015;64:99–105. doi: 10.1016/j.molimm.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Cleek RL, Ting KC, Eskin SG, Mikos AG. Microparticles of poly(DL-lactic-co-glycolic acid)/poly(ethylene glycol) blends for controlled drug delivery. J. Control. Release. 1997;48:259–268. [Google Scholar]
- Demnati R, Fraser R, Martin JG, Plaa G, Malo JL. Effects of dexamethasone on functional and pathological changes in rat bronchi caused by high acute exposure to chlorine. Toxicol. Sci. 1998;45:242–246. doi: 10.1006/toxs.1998.2532. [DOI] [PubMed] [Google Scholar]
- Evans RB. Chlorine: state of the art. Lung. 2005;183:151–167. doi: 10.1007/s00408-004-2530-3. [DOI] [PubMed] [Google Scholar]
- Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- Guney S, Schuler A, Ott A, Hoschele S, Zugel S, Baloglu E, Bartsch P, Mairbaurl H. Dexamethasone prevents transport inhibition by hypoxia in rat lung and alveolar epithelial cells by stimulating activity and expression of Na+-K+-ATPase and epithelial Na+ channels. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L1332–1338. doi: 10.1152/ajplung.00338.2006. [DOI] [PubMed] [Google Scholar]
- Homeland HSC. The Homeland Security Council, Planning Scenarios: Executive Summaries. 2004 [Google Scholar]
- Hoyle GW. Mitigation of chlorine lung injury by increasing cyclic AMP levels. Proc. Am. Thorac. Soc. 2010;7:284–289. doi: 10.1513/pats.201001-002SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle GW, Hoyle CI, Chen J, Chang W, Williams RW, Rando RJ. Identification of triptolide, a natural diterpenoid compound, as an inhibitor of lung inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;298:L830–L836. doi: 10.1152/ajplung.00014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, Commission of Life Sciences, National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Jonasson S, Wigenstam E, Koch B, Bucht A. Early treatment of chlorine-induced airway hyperresponsiveness and inflammation with corticosteroids. Toxicol. Appl. Pharmacol. 2013 doi: 10.1016/j.taap.2013.04.037. [DOI] [PubMed] [Google Scholar]
- Jones RN, Hughes JM, Glindmeyer H, Weill H. Lung function after acute chlorine exposure. Am. Rev. Resp. Dis. 1986;134:1190–1195. doi: 10.1164/arrd.1986.134.6.1190. [DOI] [PubMed] [Google Scholar]
- Joyner RE, Durel EG. Accidental liquid chlorine spill in a rural community. J. Occup. Med. 1962;4:152–154. [PubMed] [Google Scholar]
- Krause W, Kuhne G. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica. 1988;18:561–571. doi: 10.3109/00498258809041693. [DOI] [PubMed] [Google Scholar]
- Krishna G, Liu K, Shigemitsu H, Gao M, Raffin TA, Rosen GD. PG490-88, a derivative of triptolide, blocks bleomycin-induced lung fibrosis. Am. J. Pathol. 2001;158:997–1004. doi: 10.1016/S0002-9440(10)64046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzei T, Mini E, Eandi M, Reali EF, Fioretto L, Bartoletti R, Rizzo M, Calabro G, Periti P. Pharmacokinetics, endocrine and antitumour effects of leuprolide depot (TAP-144-SR) in advanced prostatic cancer: a dose-response evaluation. Drugs Exp. Clin. Res. 1989;15:373–387. [PubMed] [Google Scholar]
- Mehats C, Franco-Montoya ML, Boucherat O, Lopez E, Schmitz T, Zana E, Evain-Brion D, Bourbon J, Delacourt C, Jarreau PH. Effects of phosphodiesterase 4 inhibition on alveolarization and hyperoxia toxicity in newborn rats. PLoS One. 2008;3:e3445. doi: 10.1371/journal.pone.0003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JB, Hou YH, Zhang GJ. Rolipram attenuates bleomycin A5-induced pulmonary fibrosis in rats. Respirology. 2009;14:975–982. doi: 10.1111/j.1440-1843.2009.01606.x. [DOI] [PubMed] [Google Scholar]
- Ryrfeldt A, Andersson P, Edsbacker S, Tonnesson M, Davies D, Pauwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur. J. Respir. Dis. Suppl. 1982;122:86–95. [PubMed] [Google Scholar]
- Shao F, Wang G, Xie H, Zhu X, Sun J, A, J. Pharmacokinetic study of triptolide, a constituent of immunosuppressive chinese herb medicine, in rats. Biol. Pharm. Bull. 2007;30:702–707. doi: 10.1248/bpb.30.702. [DOI] [PubMed] [Google Scholar]
- Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech. Ageing Dev. 2004;125:697–706. doi: 10.1016/j.mad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Squadrito GL, Postlethwait EM, Matalon S. Elucidating Mechanisms of Chlorine Toxicity: Reaction Kinetics, Thermodynamics, and Physiological Implications. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299:L289–L300. doi: 10.1152/ajplung.00077.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Sun F, Shi Y, Jiang C, Meng Q, Teng L, Li Y. Effects of formulation parameters on encapsulation efficiency and release behavior of risperidone poly(D,L-lactide-co-glycolide) microsphere. Chem. Pharm. Bull. (Tokyo) 2009;57:1251–1256. doi: 10.1248/cpb.57.1251. [DOI] [PubMed] [Google Scholar]
- Tavares LP, Garcia CC, Vago JP, Queiroz-Junior CM, Galvao I, David BA, Rachid MA, Silva PM, Russo RC, Teixeira MM, Sousa LP. Inhibition of PDE4 During Pneumococcal Pneumonia Reduces Inflammation and Lung Injury in Mice. Am. J. Respir. Cell Mol. Biol. 2015 doi: 10.1165/rcmb.2015-0083OC. [DOI] [PubMed] [Google Scholar]
- Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhal. Toxicol. 2008;20:783–793. doi: 10.1080/08958370802007841. [DOI] [PubMed] [Google Scholar]
- U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research Guidance For Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005 http://www.fda.gov/downloads/Drugs/.../Guidances/UCM078932.pdf.
- Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL. Acute health effects after exposure to chlorine gas released after a train derailment. Am. J. Emerg. Med. 2009;27:1–7. doi: 10.1016/j.ajem.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Winskog C, Edston E, Walther SM. Inhaled and intravenous corticosteroids both attenuate chlorine gas-induced lung injury in pigs. Acta Anaesthesiol. Scand. 2005;49:183–190. doi: 10.1111/j.1399-6576.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang L, Walther SM. Inhaled budesonide in experimental chlorine gas lung injury: influence of time interval between injury and treatment. Intensive Care Med. 2002;28:352–357. doi: 10.1007/s00134-001-1175-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang L, Walther SM. Administration of aerosolized terbutaline and budesonide reduces chlorine gas-induced acute lung injury. J. Trauma. 2004;56:850–862. doi: 10.1097/01.ta.0000078689.45384.8b. [DOI] [PubMed] [Google Scholar]
- Wei D, Huang Z. Anti-inflammatory effects of triptolide in LPS-induced acute lung injury in mice. Inflammation. 2014;37:1307–1316. doi: 10.1007/s10753-014-9858-5. [DOI] [PubMed] [Google Scholar]
- Weill H, George R, Schwarz M, Ziskind M. Late evaluation of pulmonary function after acute exposure to chlorine gas. Am. Rev. Resp. Dis. 1969;99:374–379. [PubMed] [Google Scholar]
- White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc. Am. Thorac. Soc. 2010;7:257–263. doi: 10.1513/pats.201001-008SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigenstam E, Koch B, Bucht A, Jonasson S. N-acetyl cysteine improves the effects of corticosteroids in a mouse model of chlorine-induced acute lung injury. Toxicol. 2015;328:40–47. doi: 10.1016/j.tox.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Wu L, Mao J, Jin X, Fu H, Shen H, Wang J, Liu A, Shu Q, Du L. Efficacy of triptolide for children with moderately severe Henoch-Schonlein purpura nephritis presenting with nephrotic range proteinuria: a prospective and controlled study in China. Biomed. Res. Int. 2013;2013:292865. doi: 10.1155/2013/292865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhang M, Chen C, Cao Y, Tian Y, Guo Y, Zhang B, Wang X, Yin L, Zhang Z, O'Dell W, Okunieff P, Zhang L. Triptolide Mitigates Radiation-Induced Pulmonary Fibrosis. Radiat. Res. 2015;184:509–517. doi: 10.1667/RR13831.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.