Abstract
Executive function is a critical behavioral trait rarely studied in relation to potential neurotoxicants. Prenatal exposure to polybrominated diphenyl ethers (PBDEs) and perfluoroalkyl substances (PFASs) has been associated with adverse neurodevelopment, but there is limited research on executive function. Data from 256 mother-child pairs in the Health Outcomes and Measures of the Environment Study, a prospective birth cohort (2003-2006, Cincinnati, OH), was used to examine maternal serum PBDEs and PFASs and executive function in children ages 5 and 8 years. Maternal serum PBDEs and PFASs were measured at 16±3 weeks gestation. Executive function was assessed with the parent-rated Behavior Rating Inventory of Executive Function (BRIEF), which yields composite measures: behavioral regulation index, metacognition index, and global executive composite. Higher BRIEF scores indicate executive function impairments. Linear mixed models and generalized estimating equations were used to estimate covariate-adjusted associations between PBDEs and PFASs and executive function. A 10-fold increase in BDE-153 was associated with poorer behavior regulation (β=3.23, 95% CI 0.60, 5.86). Higher odds of having a score ≥60 in behavior regulation (OR=3.92, 95% CI 1.76, 8.73) or global executive functioning (OR=2.34, 95% CI 1.05, 5.23) was observed with increased BDE-153. Each ln-unit increase in perfluorooctane sulfonate (PFOS) was associated with poorer behavior regulation (β=3.14, 95% CI 0.68, 5.61), metacognition (β=3.10, 95% CI 0.62, 5.58), and global executive functioning (β=3.38, 95% CI 0.86, 5.90). However, no association was observed between perfluorooctanoate (PFOA) and executive function. Prenatal exposures to BDE-153 and PFOS may be associated with executive function deficits in school-age children.
Keywords: Polybrominated diphenyl ethers (PBDEs), perfluoroalkyl substances (PFASs), executive function, neurodevelopment, prenatal, exposure
1. Introduction
Beginning in the 1970s, PBDEs were used as synthetic flame retardants in a number of consumer products, including polyurethane foams, electronics, and some textiles. Since then, PBDEs have been detected throughout the environment and in humans (Darnerud et al., 2001). Dietary intake and dust ingestion are the primary sources of human exposure. Animal models have demonstrated that PBDEs are endocrine disruptors and neurotoxicants (Costa and Giordano, 2007). Several epidemiologic studies have suggested neurotoxic effects of prenatal exposure to PBDEs. Increased attention deficit/hyperactivity disorder (ADHD) related behaviors and decrements in cognitive function have been reported among children with higher exposures to prenatal PBDEs (Chen et al., 2014; Eskenazi et al., 2013; Herbstman et al., 2010; Roze et al., 2009). However, null associations have been reported between prenatal and perinatal PBDEs and neurodevelopment in a birth cohort in Menorca, Spain (Gascon et al., 2011) and among mother-child pairs in Taiwan (Chao et al., 2011; Shy et al., 2011), although the reported concentrations of PBDEs in Europe and Taiwan were much lower than in the US.
PFASs are used as surfactants and surface treatments in firefighting foams, personal care products, cleaning products, upholstery, and non-stick cookware (Kissa, 2001). Prenatal exposure to PFASs reduces thyroid hormone concentrations in animal studies and may be neurotoxic (Johansson et al., 2008; Lau et al., 2003; Luebker et al., 2005). However, human studies have yielded inconsistent results with regard to neurodevelopment. No association was reported between prenatal perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) and neurologic development in the Danish National Birth Cohort (DNBC) (Fei et al., 2008; Fei and Olsen, 2011) or between perinatal PFOS and PFOA exposure and neuropsychological development in the Norwegian Human Milk Study (HUMIS) (Forns et al., 2015). In contrast, increases in Full Scale IQ (FSIQ) and decreases in ADHD behaviors were reported with prenatal PFOA exposure (Stein et al., 2013). In addition, a recent study reported prenatal perfluoroundecanoate (PFUnDA) and perfluorononanoate (PFNA) were associated with decrements in FSIQin children (Wang et al., 2015).
Although the relation between prenatal PBDEs and PFASs and several components of neurodevelopment have been explored, there is limited research on their relation with executive function in children. Executive function encompasses higher order neurocognitive processes, including cognitive flexibility, goal planning, and information processing. Deficits in executive functioning can hinder an individual’s ability to formulate goals, effectively perform, and focus. Identifying and providing intervention to children with executive function deficits at an early age is imperative as individuals who have undergone substantial cognitive loss can still be independent and functional if executive function is intact (Lezak et al., 2004). Environmental chemical exposure may disrupt normal neurodevelopment, particularly during brain development when rapid structural and functional changes occur (Viberg et al., 2003). Previous studies have reported poorer executive function among children exposed to maternal tobacco smoking during pregnancy and lead exposure during childhood (Canfield et al., 2004; Piper and Corbett, 2012; Roy et al., 2009).
Only one study has examined prenatal PBDEs and its relation with executive function. Sagiv et al (2015) recently reported poorer executive function in children aged 9 and 12 years with measured concentrations of prenatal ∑PBDEs (BDE-47, -99, -100, and -153). While there are no studies on prenatal PFASs and executive function, Stein et al (2014) examined childhood PFOA concentrations at 2-8 years of age and executive function at 6-12 years. Compared to the lowest quartile of PFOA concentrations, boys in the highest quartile had better executive functioning, while girls had poorer executive functioning based on mother reports. Given that PBDEs and PFASs are environmentally persistent and PBDEs are an order of magnitude higher in the US compared with Europe or Asia, their potential neurotoxicity could imply considerable impact on children’s neurodevelopment at the population level. In this study, we examined the relation between prenatal exposure to PBDEs or PFASs and executive function in children at ages 5 and 8 years.
2. Materials and methods
2.1. Study participants and design
Our sample consisted of participants enrolled between March 2003 and February 2006 in the Health Outcomes and Measures of the Environment (HOME) Study, an ongoing prospective birth cohort in the Greater Cincinnati area (Ohio, USA). To be enrolled in the study, women had to meet the following eligibility criteria: 1) ≥18 years of age; 2) 16±3 weeks of gestation; 3) residing in a home built prior to 1978 (a criterion relating to a goal of the larger HOME Study examining lead exposures); 4) intending to continue prenatal care and deliver at any of the collaborating obstetric practices and hospitals; 5) HIV negative; and 6) not receiving seizure, thyroid, or chemotherapy/radiation medications. Of the 468 enrolled women, 65 dropped out prior to delivery, 3 delivered stillborn infants, and 10 delivered twins. From the 390 women who remained to deliver live singleton infants, we restricted our study to 256 mother-child pairs who had concentrations of PBDEs (n=246) or PFASs (n=242) measured at enrollment and at least one executive function assessment at age 5 (n=201) or 8 years (n=222). The study protocol was approved by the Institutional Review Boards at the Cincinnati Children’s Hospital Medical Center and the Centers for Disease Control and Prevention (CDC).
2.2. Prenatal PBDE measurements
Maternal serum samples were collected at 16±3 weeks of gestation and stored at −80°C until analysis. Two grams of serum were used for measurements of PBDEs, with samples assigned to 24 sample batches (Jones et al., 2012; Sjodin et al., 2004). Every batch included three quality control and three method blank samples comprised of bovine serum (Gibco Inc., Grand Island, NY) diluted 1:40 with water, to reduce any target analytes in the blank serum to a level less than one order of magnitude lower than the limit of detection (LOD). No PBDEs were detected in the bovine serum prior to dilution. Measurements of PBDEs (BDE-17, -28, -47, -66, -85, -99, -100, -153, -154, and -183) were made using gas chromatography/isotope dilution-high resolution mass spectrometry using DFS instruments (ThermoFisher, Bremen, Germany) (Sjodin et al., 2004). Analytical data were corrected by subtracting the median blank value. The LODs were determined as the higher of 3 times the standard deviation (SD) of the method blanks analyzed or as instrumental LOD defined as the injected amount known to produce a signal/noise ratio >10. The LODs were 0.2-2.0 ng/g lipid (BDE-17, -28, -66, -85, -100, -153, -154, and -183), 0.3-8.2 ng/g lipid (BDE-47), and 0.2-5.7 ng/g lipid (BDE-99). We analyzed data of congeners with detection frequencies >80% (BDEs 28, 47, 99, 100, and 153) and total PBDEs (∑PBDEs), sum of BDE-28, -47, -99, -100, and -153, resulting in a total of 246 samples of PBDEs. Total serum lipids were determined based on measurements of triglycerides and total cholesterol using standard enzymatic methods (Phillips et al., 1989). Concentrations of PBDEs were expressed on a serum lipid basis (ng/g lipid).
2.3. Prenatal PFAS measurements
Maternal serum samples collected at 16±3 weeks of gestation were also used to measure PFASs with additional subjects collected at 26 weeks of gestation (21 of 242 participants) and within 24 hours of parturition (56 of 242 participants). PFOA, PFOS, perfluorohexane sulfonate (PFHxS), PFNA, and perfluorodecanoate (PFDeA) were quantified using on-line solid-phase extraction coupled to high-performance liquid chromatography-tandem mass spectrometry (Kato et al., 2011) on a Symbiosis on-line SPE system (Spark Holland, Plainsboro, NJ) coupled with an API 4000 mass spectrometer (Applied Biosystems, Foster City, CA). Isotope-labeled internal standards for quantification included: 13C2-PFOA, 13C4-PFOS, 18O2-PFHxS, 13C5-PFNA, and 13C2-PFDeA. Calibration standards were spiked into calf serum to account for potential matrix effects (Kato et al., 2014). The LODs were 0.082 ng/mL (PFNA), 0.1 ng/mL (PFOA, PFHxS, PFDeA), and 0.2 ng/mL (PFOS). Each analytic batch included reagent blanks and low-concentration and high-concentration quality control (QC) materials, prepared from a calf serum pool. The coefficients of variation of repeated measurements of the QC materials were ~6% (Kato et al., 2014). Concentrations were averaged among women who had >1 sample to obtain a measure of prenatal PFASs for PFOA, PFOS, PFHxS, PFNA, and PFDeA (in ng/mL), resulting in a total of 242 women with either an averaged concentration of prenatal PFASs or a measurement at 16 or 26 weeks of gestation. These PFASs were detected in >95% of maternal serum samples.
2.4. Executive function assessment
Executive function was assessed at ages 5 and 8 years using the Behavior Rating Inventory of Executive Function (BRIEF), a valid and reliable parent-reported questionnaire (Gioia et al., 2000a; Gioia et al., 2000b; Skogerbo et al., 2012). A parent who had extensive contact over the past 6 months with the child, which was predominantly the mother in the HOME Study, completed the BRIEF, which takes approximately 10-15 minutes to complete. HOME Study staff provided the questionnaire to the parents with minimal instructions, to be completed on their own. It yields the subscales: inhibit (impulse control); shift (ability to transition); emotional control (emotional response management); working memory (information retention for task completion); plan/organize (planning goals and systematically executing steps); initiate (independently starting tasks and generating problem-solving strategies); organization of materials (orderliness); and monitor (self-observation of routines). Combining the BRIEF subscales generates composite measures, including behavioral regulation index (inhibit + shift + emotional control), metacognition index (working memory + plan/organize + initiate + organization of materials + monitor), and global executive composite (derived from all 8 scales). A trained psychologist scored the BRIEF to obtain raw scores and then converted them to standardized T-scores based on sex-specific norms for the age ranges provided in the BRIEF manual, which were 5-7 and 8-10 years. The scoring algorithm was implemented using SAS (SAS Institute Inc., Cary, NC). Assessments of BRIEF composites at age 5 and 8 years were significantly correlated with each other (rs=0.59-0.62, p<0.001). A total of 201 and 222 BRIEF assessments were completed at age 5 and 8 years, respectively. Normalized BRIEF T-scores had a mean of 50±10, with higher scores indicating impairments in executive function (see Supplemental material, Table S1). BRIEF scores 1.5 SDs above the mean (≥65) are considered clinically significant (Gioia et al., 2000a). However, due to our modest sample size, we defined BRIEF scores 1 SD above the mean (≥60) “at risk” of a clinically relevant executive function problem.
2.5. Statistical analyses
Maternal serum concentrations of PBDEs and PFASs <LOD were replaced with the LOD/√2 (Hornung and Reed, 1990). The concentrations of PBDEs and PFASs were not normally distributed and were log10- and ln-transformed (due to a lower range of concentrations), respectively. We used linear mixed models to estimate β coefficients and 95% confidence intervals (CIs) for individual BDE congeners, ∑PBDEs, and the five individual PFASs in relation to BRIEF composite measures while accounting for repeated outcome measurements. Interaction terms between PBDEs or PFASs (continuous) and child age (categorical) were not statistically significant for any of the BRIEF composite measure models. Thus, overall estimates for both 5 and 8 years are provided rather than age-specific estimates (see Supplemental Material, Table S2). To determine whether effect modification by child sex or maternal education was present, we also tested interaction terms between continuous concentrations of PBDEs or PFASs and child sex or maternal education, with p<0.20 considered significant.
The relation between prenatal PBDEs or PFASs and being “at risk” of a BRIEF score with clinical relevance (≥60) was examined using generalized linear models to obtain odds ratios (ORs) and 95% CIs. Generalized estimating equations were used to account for repeated outcome measures. We also examined BRIEF scores considered clinically significant (≥65) in a sensitivity analysis. For PFASs, we additionally examined the associations only using concentrations at 16±3 weeks to avoid potential problems using averaged prenatal concentrations. The associations between tertiles of PBDEs and PFASs and BRIEF summary measures were assessed for linear trend using the median value in each tertile as a continuous variable in the regression models (Greenland, 1995). We additionally estimated the regression coefficients of PBDEs with additional adjustment for each PFAS (in separate models), and vice versa, to determine whether estimates varied with mutual adjustment. We also adjusted by parity to see if our conclusions changed.
Covariates included in final regression models were based on results of bivariate analyses examining the relationship with executive function (p<0.20). Final models included the following covariates: maternal age at enrollment, race, education, family income, maternal depression (assessed by Beck Depression Inventory II at enrollment) (Beck et al., 1996), marital status, child sex, Home Observation for Measurement of the Environment inventory score measured at the 1-year home visit (Caldwell and Bradley, 1984), maternal serum cotinine (ng/mL, continuous) at enrollment, and maternal IQ (assessed by Wechsler Abbreviated Scale of Intelligence, continuous) (Wechsler, 1999). Maternal serum polychlorinated biphenyl (∑PCB) concentrations (PCB congeners-28, 74, 99, 105, 118, 146, 153, 156, 170, 180, 183, 187, 194, 199, and 206), dichlorodiphenyldichloroethylene (DDE), dichlorodiphenyltrichloroethane (DDT), and maternal blood lead concentrations were considered as potential confounders, but they were not associated with PBDEs or PFASs and global executive functioning at 8 years. Maternal urinary concentrations of dialkyl phosphate (DAP) metabolites [nmol/g Cr, log10-transformed, continuous], a marker of organophosphate exposure, at 16±3 weeks of gestation were additionally included in the models as a sensitivity analysis.
3. Results
3.1. Participant characteristics
Maternal serum concentrations of BDE-47, -99, and PFOS were comparable to pregnant women in the National Health and Nutrition Examination Survey (NHANES) 2003-2004 (Table 1). However, HOME Study women had higher PFOA and lower BDE-153 concentrations compared to NHANES pregnant women (Woodruff et al., 2011). Positive correlations were observed between BDE congeners (rs=0.49-0.92, p<0.01), and among PFASs (rs=0.19-0.80, p<0.01) (see Supplemental material, Table S3). Women included in the analysis were similar to those excluded due to missing information on PBDEs, PFASs, or child executive function assessments (n=134), except those included were more likely to be married/living with a partner and had a slightly lower IQ score (105.8 vs 109.4).
Table 1.
Maternal concentrations of polybrominated diphenyl ether congenersa (ng/g lipid) and perfluoroalkyl substances b (ng/mL), HOME Study
Odds ratios and 95% confidence intervals of having BRIEF scores ≥60 at ages 5 and/or 8 years by 10-fold increases in maternal serum concentrations of PBDEs (ng/g lipid) or 1-unit increase in ln-PFASs (ng/mL). Models adjusted for maternal age, race, education, income, maternal serum cotinine, maternal depression, Home Observation for Measurement of the Environment score, maternal IQ, marital status, and child sex.
| n | Min | <LOD (%) |
Percentile | Max | GM (GSD) |
NHANESe GM (GSD) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 95th | |||||||
| PBDEs | ||||||||||
| ∑PBDEsc,d | 226 | 5.9 | - | 21.6 | 36.7 | 78.0 | 215.6 | 2046.9 | 41.3 (2.6) | -f |
| BDE-17 | 226 | <LOD | (97.4) | 0.3 | 0.3 | 0.4 | 1.0 | 4.0 | 0.4 (1.6) | - |
| BDE-28d | 226 | <LOD | (18.6) | 0.7 | 1.1 | 1.9 | 4.8 | 31.4 | 1.2 (2.3) | - |
| BDE-47d | 246 | 3.0 | - | 11.1 | 19.7 | 35.6 | 103 | 1290 | 21.5 (2.6) | 23.9 (2.2) |
| BDE-66 | 226 | <LOD | (97.8) | 0.3 | 0.3 | 0.4 | 1.1 | 2.6 | 0.4 (1.6) | - |
| BDE-85 | 226 | <LOD | (49.6) | 0.4 | 0.6 | 1.0 | 3.5 | 38.7 | 0.6 (2.3) | - |
| BDE-99d | 240 | 0.6 | - | 2.7 | 4.5 | 8.5 | 33.0 | 465 | 5.0 (2.7) | 5.5 (0.8) |
| BDE-100d | 226 | <LOD | (1.3) | 2.2 | 3.7 | 8.2 | 26.1 | 172 | 4.2 (2.8) | 6.1 (0.9) |
| BDE-153d | 226 | <LOD | (1.3) | 2.5 | 4.7 | 9.2 | 47.6 | 152 | 5.5 (2.9) | 9.9 (3.0) |
| BDE-154 | 226 | <LOD | (55.3) | 0.3 | 0.5 | 1.0 | 3.0 | 28.7 | 0.6 (2.3) | - |
| BDE-183 | 226 | <LOD | (76.1) | 0.3 | 0.4 | 0.6 | 1.1 | 9.3 | 0.4 (1.7) | - |
| PFASs | ||||||||||
| PFOAd | 242 | 0.5 | - | 3.6 | 5.4 | 7.5 | 12.0 | 24.5 | 5.3 (1.7) | 2.4 (0.2) |
| PFOSd | 242 | 0.4 | - | 8.8 | 13.2 | 17.8 | 29.4 | 57.2 | 12.6 (1.8) | 12.3 (1.0) |
| PFHxSd | 242 | <LOD | (0.8) | 0.9 | 1.5 | 2.4 | 5.0 | 32.5 | 1.4 (2.2) | - |
| PFNAd | 241 | 0.1 | - | 0.7 | 0.9 | 1.1 | 1.9 | 2.9 | 0.9 (1.5) | - |
| PFDeAd | 225 | 0.1 | - | 0.1 | 0.2 | 0.3 | 0.5 | 1.3 | 0.2 (1.6) | - |
Abbreviations: GM, geometric mean; GSD, geometric standard deviation; Max, maximum; Min, minimum.
LODs: PFOA, PFHxS, and PFDeA (0.1 ng/mL); PFOS (0.2 ng/mL); PFNA (0.082 ng/mL); BDE-17, BDE-28, BDE-66, BDE-85, BDE-100, BDE-153, BDE-154, and BDE-183 (0.2-2.0 ng/g lipid); BDE-47 (0.3-8.2 ng/g lipid); BDE-99 (0.2-5.7 ng/g lipid).
Measured at 16±3 weeks of gestation
Average of three measurements (16 and 26 weeks gestation and within 24 hours of parturition)
Includes congeners with detection frequencies >80% (BDEs 28, 47, 99, 100, and 153)
Included in statistical analyses
Serum concentrations in pregnant women in NHANES 2003-200437
Not available
Mothers who were non-White, less educated, of lower income, not married or living alone, moderately/severely depressed, and who had a Home Observation for Measurement of the Environment score <40 had significantly higher concentrations of ∑PBDEs (Table 2). Poorer global executive composite scores at age 8 years were also observed among children of these women. Women who were non-Hispanic white, of higher income, and who were minimally/mildly depressed had significantly higher PFOA and PFOS concentrations. While maternal IQ was inversely correlated with ∑PBDEs and global executive composite scores at age 8 years, a positive correlation was observed with PFOS (Table 3). Maternal serum cotinine levels were positively correlated with ∑PBDEs and global executive composite scores, and negatively correlated with PFOS.
Table 2.
Maternal serum concentrations of ∑PBDEs (ng/g lipid) and select PFASs (ng/mL) and BRIEF global executive composite scores at age 8 years by maternal and child characteristics, HOME Studya
| Maternal and Child Characteristics | ∑PBDEs | PFOA | PFOS | GEC | |||
|---|---|---|---|---|---|---|---|
| n b | GM (GSD) | n b | GM (GSD) | GM (GSD) | n | Mean (SD) | |
| Maternal age, years | |||||||
| <25 | 55 | 47.7 (2.1) | 61 | 5.6 (1.9) | 12.0 (2.1) | 59 | 50.5 (10.5) |
| 25-34 | 139 | 42.1 (2.7) | 147 | 5.1 (1.7) | 12.8 (1.6) | 131 | 47.1 (10.2) |
| ≥35 | 32 | 30.0 (2.5) | 34 | 5.6 (1.8) | 13.3 (1.7) | 32 | 48.7 (11.1) |
| Race/ethnicityb,c,d,e | |||||||
| Non-Hispanic White | 139 | 35.1 (2.6) | 146 | 5.7 (1.7) | 14.0 (1.6) | 134 | 46.5 (9.8) |
| Non-Hispanic Black and Others | 87 | 53.8 (2.4) | 96 | 4.7 (1.8) | 10.8 (1.9) | 88 | 50.9 (10.9) |
| Educationb,d,e | |||||||
| High school or less | 60 | 53.2 (2.2) | 64 | 5.0 (1.8) | 10.6 (1.9) | 61 | 50.0 (9.9) |
| Some college/2 yr degree | 59 | 43.4 (2.2) | 62 | 5.1 (1.7) | 12.6 (1.9) | 57 | 50.9 (11.2) |
| Bachelor’s | 67 | 34.4 (2.8) | 73 | 5.5 (1.7) | 13.6 (1.6) | 65 | 45.9 (9.5) |
| Graduate or professional | 40 | 35.9 (3.0) | 43 | 5.6 (1.7) | 14.7 (1.5) | 39 | 45.5 (10.6) |
| Family Incomeb,c,d,e | |||||||
| <$40,000 | 91 | 56.0 (2.4) | 99 | 4.8 (1.8) | 11.1 (1.9) | 92 | 50.9 (10.1) |
| $40,000-$79,999 | 77 | 38.3 (2.7) | 80 | 5.3 (1.6) | 12.6 (1.5) | 72 | 46.6 (10.8) |
| ≥$80,000 | 58 | 28.4 (2.4) | 63 | 6.0 (1.7) | 15.6 (1.7) | 58 | 46.1 (9.8) |
| Maternal Depressionb,c,d,e | |||||||
| Minimal/mild | 206 | 39.5 (2.5) | 219 | 5.5 (1.7) | 13.3 (1.7) | 200 | 47.6 (10.5) |
| Moderate/severe | 19 | 69.0 (2.9) | 21 | 3.9 (1.8) | 7.8 (2.2) | 20 | 55.1 (7.8) |
| Home Observation for Measurement of the Environment scoreb,d,e |
|||||||
| ≥40 | 133 | 35.0 (2.6) | 144 | 5.5 (1.7) | 13.8 (1.6) | 129 | 46.2 (10.4) |
| 35-39 | 40 | 54.2 (2.5) | 43 | 5.3 (1.6) | 11.9 (1.8) | 42 | 51.6 (10.6) |
| <35 | 39 | 53.1 (2.3) | 39 | 4.8 (1.9) | 10.5 (2.1) | 35 | 50.8 (8.5) |
| Marital statusb,e | |||||||
| Married/living with partner | 170 | 37.4 (2.7) | 182 | 5.3 (1.7) | 13.0 (1.7) | 165 | 46.9 (10.0) |
| Not married, living alone | 56 | 55.9 (2.1) | 60 | 5.3 (1.7) | 11.6 (1.8) | 57 | 52.1 (10.8) |
| Child Sex | |||||||
| Male | 100 | 37.8 (2.5) | 111 | 5.1 (1.7) | 12.3 (1.7) | 99 | 47.9 (11.2) |
| Female | 126 | 44.4 (2.6) | 131 | 5.4 (1.8) | 12.9 (1.8) | 123 | 48.5 (9.9) |
| Breastfeeding duration, months | |||||||
| <1 | 92 | 35.9 (2.5) | 104 | 4.9 (1.7) | 12.4 (1.7) | 90 | 47.1 (10.3) |
| 1-5 | 58 | 39.1 (2.7) | 61 | 5.5 (1.9) | 12.4 (2.0) | 51 | 50.1 (10.9) |
| ≥6 | 67 | 51.4 (2.5) | 75 | 5.7 (1.6) | 13.2 (1.6) | 68 | 49.3 (10.3) |
Abbreviations: GM, geometric mean; GSD, geometric standard deviation; GEC, Global Executive Composite; SD, standard deviation.
Frequencies may not add to the total number of participants because of missing values.
Includes individuals with a GEC score at 5 or 8 years
p < 0.05 for: b ∑PBDEs;
PFOA;
PFOS;
GEC (two-sided p values using ANOVA or t-test)
Table 3.
Maternal serum concentrations of ∑PBDEs (ng/g lipid) and select PFASs (ng/mL) and global executive composite scores at age 8 years by maternal characteristics, HOME Study
| Maternal characteristics (GM±GSD)a | ∑PBDEs | PFOA | PFOS | GEC | ||||
|---|---|---|---|---|---|---|---|---|
| n | Pearson r | n | Pearson r | n | Pearson r | n | Pearson r | |
| Maternal IQc,e,f | ||||||||
| (Mean±SD 105.9±15.4) | 213 | −0.19 | 229 | 0.01 | 229 | 0.14 | 209 | −0.17 |
| Maternal cotininec,e,f | ||||||||
| (0.06±19.9 ng/mL) | 224 | 0.29 | 240 | −0.01 | 240 | −0.16 | 220 | 0.21 |
| Maternal Serum PCBsb | ||||||||
| (44.7±1.8 ng/g lipid) | 225 | −0.07 | 217 | 0.03 | 217 | 0.13 | 197 | −0.03 |
| Maternal Blood Lead Levelsf | ||||||||
| (0.65±1.5 μg/dL) | 226 | 0.10 | 242 | −0.02 | 242 | −0.03 | 222 | 0.14 |
| Maternal Urinary DAPc,f | ||||||||
| (75.4±4.8 nmol/g Cr) | 222 | −0.19 | 239 | −0.02 | 239 | 0.09 | 218 | −0.15 |
| Maternal Serum p,p’-DDTc | ||||||||
| (2.6±1.7 ng/g lipid) | 226 | 0.14 | 218 | −0.02 | 218 | −0.01 | 198 | 0.09 |
| Maternal Serum p,p’-DDE | ||||||||
| (75.9±1.9 ng/g lipid) | 226 | 0.12 | 232 | 0.05 | 232 | 0.11 | 215 | −0.06 |
Abbreviations: GM, geometric mean; GSM, geometric standard deviation; GEC, Global Executive Composite; SD, standard deviation.
Measured at baseline
Sum of congeners with detection frequencies >75% (congeners-28, 74, 99, 105, 118, 146, 153, 156, 170, 180, 183, 187, 194, 199, and 206)
p < 0.05 for: ∑PBDEs;
PFOA;
PFOS;
GEC
3.2. Maternal concentrations of PBDEs and PFASs and executive function
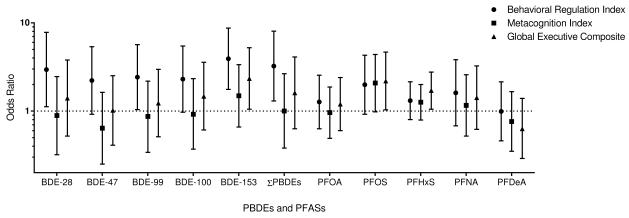
Poorer (higher) scores in behavior regulation, metacognition, and global executive functioning were observed with a 10-fold increase in ∑PBDEs, although associations were not statistically significant (Table 4). Maternal serum BDE-153 was significantly associated with poorer behavior regulation index scores in children ages 5 and 8 years (β=3.23, 95% CI 0.60, 5.86). Higher odds of having a behavior regulation index score ≥60 was observed across all BDE congeners and ∑PBDEs (Figure 1); statistically significant associations were noted with BDE-28 (OR=2.95, 95% CI 1.12, 7.82), BDE-99 (OR=2.42, 95% CI 1.04, 5.66), BDE-153 (OR=3.92, 95% CI 1.76, 8.73), and ∑PBDEs (OR=3.23, 95% CI 1.30, 8.05). A 10-fold increase in BDE-153 was also associated with higher odds of having a global executive composite score ≥60 (OR=2.34, 95% CI 1.05, 5.23). Significant ORs were observed between BDE-153 and several BRIEF subscales, including inhibit, emotional control, initiate, and organization of materials (Supplemental material, Table S4). BDE-47, -99, -100, and ∑PBDEs were also significantly associated with the emotional control subscale.
Table 4.
Estimated score differences and 95% confidence intervals in executive function scores at ages 5 and/or 8 years by increasesa in maternal serum concentrations of polybrominated diphenyl ethers (ng/g lipid) and perfluoroalkyl substances (ng/mL), HOME Studyb
| n | Behavioral Regulation Indexc |
Metacognition Indexd |
Global Executive Functione |
|
|---|---|---|---|---|
| PBDEs | ||||
| BDE-28 | 205 | 2.48 (−0.80, 5.75) | 1.91 (−1.35, 5.16) | 2.21 (−1.13, 5.55) |
| BDE-47 | 223 | 1.39 (−1.59, 4.37) | 0.49 (−2.53, 3.51) | 0.82 (−2.24, 3.89) |
| BDE-99 | 217 | 2.35 (−0.57, 5.27) | 1.31 (−1.59, 4.22) | 1.78 (−1.20, 4.76) |
| BDE-100 | 205 | 1.80 (−1.07, 4.66) | 1.02 (−1.83, 3.87) | 1.37 (−1.55, 4.29) |
| BDE-153 | 205 | 3.23 (0.60, 5.86) | 1.69 (−0.93, 4.31) | 2.46 (−0.23, 5.15) |
| ∑PBDEs | 205 | 2.77 (−0.31, 5.85) | 1.68 (−1.37, 4.74) | 2.21 (−0.93, 5.35) |
| PFASs | ||||
| PFOA | 219 | 1.11 (−1.22, 3.44) | 0.58 (−1.77, 2.93) | 1.06 (−1.33, 3.45) |
| PFOS | 218 | 3.14 (0.68, 5.61) | 3.10 (0.62, 5.58) | 3.38 (0.86, 5.90) |
| PFHxS | 219 | 1.19 (−0.54, 2.91) | 1.31 (−0.43, 3.04) | 1.36 (−0.41, 3.12) |
| PFNA | 218 | 2.57 (−0.26, 5.40) | 1.37 (−1.49, 4.23) | 2.01 (−0.89, 4.92) |
| PFDeA | 204 | −0.70 (−3.31, 1.92) | −1.24 (−3.87, 1.39) | −1.13 (−3.79, 1.54) |
PBDEs were log10-transformed; PFASs were ln-transformed
Adjusted by maternal age, race, education, income, maternal serum cotinine, maternal depression, Home Observation for Measurement of the Environment score, maternal IQ, marital status, and child sex.
Behavioral Regulation Index: Inhibit + Shift + Emotional Control
Metacognition Index: Initiate + Working Memory + Plan/Organize + Organization of Materials + Monitor
Global Executive Composite: all 8 scales
Figure 1.
Prenatal PFOA concentrations were not associated with behavior regulation (β=1.11, 95% CI -1.22, 3.44), metacognition (β=0.58, 95% CI -1.77, 2.93), or global executive functioning (β=1.06, 95% CI -1.33, 3.45) in school-age children (Table 4). Maternal serum PFOS concentrations were associated with poorer behavior regulation, metacognition, and global executive functioning, with approximately a 3 point increase in all summary measures with a 1 ln-unit increase in PFOS concentrations. Significant ORs for global executive composite scores ≥60 were observed for PFOS (OR=2.19, 95% CI 1.03, 4.66) and PFHxS (OR=1.71, 95% CI 1.05, 2.77) (Figure 1). Higher odds of behavior regulation index (OR=1.99, 95% CI 0.92, 4.30) and metacognition scores ≥60 (OR=2.08, 95% CI 0.98, 4.38) were also observed with 1-unit increase in ln-PFOS, although they were borderline significant. Statistically significant positive associations were observed between maternal PFOS and several BRIEF subscales ≥60, including inhibit, working memory, plan/organize, and monitor (Supplemental material, Table S4).
A significant linear trend was observed between tertiles of BDE-153 and behavior regulation index scores ≥60 (ptrend=0.002), and the trend between PFOS and behavior regulation index or global executive composite scores was marginally significant (ptrend=0.06) (Supplemental material, Table S5).
3.3. Child sex and maternal education differences
While we did not observe differences in BRIEF composite scores by child sex, we noted statistically significant effect measure modifications by child sex between BDE-153 and behavior regulation index (pinteraction=0.064) and global executive function (pinteraction=0.142) (Supplemental material, Table S6). Increases in BDE-153 concentrations were associated with poorer behavior regulation index scores in male children (β=5.81, 95% CI 2.03, 9.58), but not in females (β=0.91, 95% CI -2.68, 4.49). Poorer global executive functioning was observed with a 10-fold increase in BDE-153 in male children (β=4.57, 95% CI 0.70, 8.44), but not in females (β=0.59, 95% CI -3.09, 4.26). Effect measure modification by child sex was also noted between PFOS and metacognition (pinteraction=0.060) and global executive function (pinteraction=0.130). However, poorer metacognition and global executive function scores were observed among female children with a unit increase in ln-PFOS concentrations (β=5.16, 95% CI 1.89, 8.43 and β=5.07, 95% CI 1.74, 8.39), but not in males (β=0.57, 95% CI -3.05, 4.19 and β=1.32, 95% CI -2.35, 4.98). Statistically significant effect measure modification was also observed by maternal education between several BDE congeners and behavior regulation index and global executive function. A 10-fold increase in prenatal PBDEs was associated with poorer behavior regulation index and global executive composite scores in children with mothers with a high school education or less, while null associations were observed in children with mothers with a higher level of education (results not shown).
3.4. Sensitivity analyses
Similar results were observed when we examined PBDEs and BRIEF scores ≥65; BDE-153 was significantly associated with every BRIEF summary measure, and higher odds of having a behavior regulation index score ≥65 was noted with all BDE congeners (results not shown). For PFASs, we found higher odds of having a score ≥65 between maternal PFOS concentrations and metacognition score, and between PFHxS concentrations and behavior regulation index and metacognition. No differences in executive function findings were observed between averaged concentrations of PFASs and concentrations measured at 16±3 weeks of gestation. Associations between prenatal PFOS and BRIEF composite measures remained even after adjustment by BDE congeners (results not shown). However, the relation between BDE-153 and behavior regulation index was slightly attenuated after adjustment for PFOS (β=2.40, 95% CI -0.32, 5.12, p=0.08). Additionally adjusting by maternal urinary DAP concentrations did not change the overall conclusions (results not shown).
4. Discussion
In the present study, we found higher odds of being at risk for behavioral regulation problems with increasing concentrations of several BDE congeners and their sum. Maternal serum BDE-153, PFOS, and PFHxS concentrations were also associated with being at risk for global executive functioning problems. However, no association was noted between maternal serum concentrations of PFOA and executive function. We observed a 3 point increase in behavior regulation index with a 10-fold increase in BDE-153, and a 3 point increase across all BRIEF summary measures with a 1-unit increase in ln-PFOS. Previously, global executive composite scores were reported to increase by 5 points in children 5-18 years who had mothers who smoked during pregnancy (Piper and Corbett, 2012).
Mechanisms by which PBDEs may exert neurotoxic effects include apoptotic neuronal death through oxidative stress, disruption of signal transduction, and reduction in cell migration and differentiation into neurons and oligodendrocytes (Costa and Giordano, 2007; Dingemans et al., 2011; Schreiber et al., 2010). Associations observed with BDE-153 may be due to its higher ability to bioaccumulate and bind to brain tissue than BDE- 47, -99, and -100. Staskal et al (2006) administered a 1 mg/kg intravenous dose of BDE-47, -99, -100, or -153 to mice and found significantly higher concentrations of BDE-153 than other congeners in the brain tissue; BDE-153 concentrations in the brain were four times higher than in blood. Further, metabolism and excretion rates were lower for BDE-153. In another study, BDE-153 uptake by rat neurons correlated with altered translocation of protein kinase C, which is associated with neuronal development (Kodavanti et al., 2005). PBDEs have been observed to cause hyperactivity in rats exposed to low doses during gestational development (Kuriyama et al., 2005). In addition, learning and memory impairments and hyperactivity have also been reported among mice perinatally exposed to PBDEs (Branchi et al., 2002; Branchi et al., 2005; Koenig et al., 2012). PFASs may have direct effects on the central nervous system. They have been reported to increase levels of calcium/calmodulin-dependent protein kinase II, growth-associated protein-43, and synaptophysin, proteins which may influence neuronal growth and synaptogenesis; PFOS has also been observed to affect the calcium ion channel by increasing Ca2+/calmodulin-dependent protein kinase IIα expression and subsequently neuroendocrine function in animal models (Austin et al., 2003; Johansson et al., 2009; Liu et al., 2010). Compared to PFOA, PFOS has a stronger effect in vitro in inhibiting DNA synthesis and increasing lipid peroxidation in undifferentiated PC12 cells, and interfering with differentiation by promoting cholinergic rather than dopaminergic neurotransmitter phenotypes (Slotkin et al., 2008). In addition, delayed learning was reported in female mice of dams who were exposed to both PFOS and restraint stress (Fuentes et al., 2007). Developmental neurotoxicity may also be mediated by PBDEs’ and PFASs’ effects on thyroid hormones, which are essential for proper brain development (Williams, 2008). Several epidemiologic studies have reported that prenatal concentrations of PBDEs and PFASs disrupt thyroid hormones (Abdelouahab et al., 2013; Chevrier et al., 2010; Stapleton et al., 2011; Vuong et al., 2015; Wang et al., 2014; Webster et al., 2014). We previously observed a significant association between prenatal PBDEs and higher maternal serum thyroxine (T4) and triiodothyronine (T3) in the HOME Study (Vuong et al., 2015). However, lower levels of maternal T4 during gestation have been associated with cognitive deficits (Haddow et al., 1999; Henrichs et al., 2013; Julvez et al., 2013). Recently, a non-monotonic relationship was reported in the Generation R Study, with low and high maternal T4concentrations associated with lower IQ in school-aged children (Korevaar et al., 2015). Maternal hyperthyroidism has been linked with childhood ADHD (Andersen et al., 2014). Certain ADHD phenotypes may stem from deficits in certain executive function domains (Willcutt et al., 2005).
Several studies have reported that prenatal PBDEs are associated with adverse neurodevelopment. In a cohort of women who were pregnant on September 11, 2001 and subsequently delivered in hospitals within a two mile radius of the World Trade Center, cord serum concentrations of BDE-47, -100, and -153 were inversely associated with FSIQ scores in children 4 years (Herbstman et al., 2010). Significant decrements in FSIQ scores were additionally observed among children at age 7 years in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort with prenatal PBDEs and at age 5 years in the HOME Study (Chen et al., 2014; Eskenazi et al., 2013). In addition, cognitive deficits were reported with BDE-209 exposure in breast milk among infants 8-12 months of age in southern Taiwan (Chao et al., 2011). Conversely, no association was reported between prenatal BDE-47 and cognitive function in children 4 years of age in the Menorca birth cohort (Gascon et al., 2011). Further, two Taiwanese studies reported null findings between ∑14PBDEs in breast milk and PBDEs in cord blood and social-emotional scales (Chao et al., 2011; Shy et al., 2011). However, Eskenazi et al (2013) reported poorer attention and higher ADHD behaviors with increased maternal exposure to ∑PBDEs. Maternal serum concentrations of BDE-47 were associated with more ADHD behaviors and hyperactivity in HOME Study children (Chen et al., 2014). Sagiv et al. (2015) examined the relation between prenatal PBDEs and executive function in school-aged children (Sagiv et al., 2015). Adverse associations between measured prenatal ∑PBDEs (BDE-47, -99, -100, and -153) and BRIEF metacognition (β=3.3, 95% CI 0.4, 6.3) and global executive function (β=3.1, 95% CI 0.2, 6.04) were reported in children 9 years in the CHAMACOS Study (Sagiv et al., 2015). Adverse BRIEF composite scores were also reported with metacognition (β=2.7, 95% CI 0.2, 5.1) and global executive function (β=2.6, 95% CI 0.1, 5.1) among children 12 years with higher measured prenatal concentrations of ∑PBDEs. In addition, poorer working memory was observed with a 10-fold increase in measured ∑PBDEs (β=3.5, 95% CI 1.0, 6.0) and overall (measured and estimated) ∑PBDEs (β=2.5, 95% CI 0.5, 4.4). Our study found a 10-fold increase in BDE-153 concentrations was associated with a 3-point increase in behavior regulation, which aligns with findings from the CHAMACOS Study. However, we did not observe significant deficits in metacognition or global executive function.
Studies of prenatal PFASs and neurodevelopment are inconsistent. In the DNBC, no association was found between PFOA or PFOS on developmental motor and mental milestones at 6 and 18 months of age or behavioral disorders at 7 years (Fei et al., 2008; Fei and Olsen, 2011). Null associations were also observed between perinatal PFOS and PFOA measured in breast milk and neuropsychological development in children at 6, 12, and 24 months in the HUMIS study (Forns et al., 2015). However, decrements in IQ were also noted in children ages 5 and 8 years with increased prenatal PFUnDA and PFNA concentrations, respectively (Wang et al., 2015). Among children 6-12 years in the C8 Health Project, protective associations were reported between estimated in utero PFOA and FSIQ and ADHD behavior (Stein et al., 2013). Executive function was also examined in children 6-12 years of age in the C8 Health Project (Stein et al., 2014). Childhood PFOA concentrations measured at 2-8 years was not associated with deficits in executive function. However, sex-specific results indicated better executive function in boys in the highest quartile compared to the lowest quartile of PFOA concentrations, and poorer executive function in girls. Inconsistent results between studies may be due to differences in sample size, varying neurodevelopmental outcomes, and child age at assessment. Differing concentrations of PFASs between cohorts may also influence results. For instance, due to contaminated drinking water, median PFOA concentrations among women in the C8 Health Project was 43.7 ng/ml (interquartile range [IQR] 11.7-110.8 ng/mL) (Stein et al., 2013), much higher than in the HOME Study (5.4 ng/mL, [3.6-7.5]) or the DNBC (5.4 ng/mL, [4.0-7.1]) (Fei et al., 2008; Fei and Olsen, 2011). Varying postnatal exposures may also contribute to the inconsistent findings between studies.
We observed poorer scores in behavioral regulation among males with increased BDE-153 exposure, and poorer metacognition scores among females with increased PFOS exposure. Previous studies have reported conflicting results regarding effect modification by child sex with PBDEs or PFASs and neurodevelopment. In the CHAMACOS Study, no sex differences were reported for prenatal PBDEs and BRIEF assessed executive function (Sagiv et al., 2015). Further, no sex differences were found between maternal serum BDE-47 and child FSIQ in the HOME Study (Chen et al., 2014), and a previous study of in utero PFOA exposure reported that child sex did not modify the Numerical Operations score in the C8 Health Project (Stein et al., 2013). However, sex specific differences were reported in the C8 Health Project between childhood PFOA and executive function, with better performance in boys and poorer performance in girls (Stein et al., 2014). Inconsistent results between studies may be due to differences in neurodevelopmental measures, specific PBDEs and PFASs, and study populations. Our participants were similar with regard to maternal age at delivery as the Taiwan Maternal and Infant Cohort Study, HUMIS Study, DNBC, New York cohort, and the Menorca cohort (Fei et al., 2008; Fei and Olsen, 2011; Forns et al., 2015; Gascon et al., 2011; Herbstman et al., 2010; Wang et al., 2015). However, maternal age at delivery in HOME Study mothers was younger than mothers in the C8 Health Project and somewhat older than mothers in the CHAMACOS Study (Eskenazi et al., 2013; Sagiv et al., 2015; Stein et al., 2013; Stein et al., 2014). Participants in the CHAMACOS Study also consisted of Mexican Americans, while the HOME Study was primarily comprised of non-Hispanic white and black women. While the mechanism underlying such differences is unclear, future studies should examine child sex as a potential modifier.
Our study had several notable strengths, including its longitudinal study design and use of repeated measurements of executive function at ages 5 and 8 years. In addition, executive function was assessed by the BRIEF, which has high test-retest reliability across clinical scales (Gioia et al., 2000b). Numerous possible confounders were taken into account, including sociodemographics, maternal IQ, and a nurturing home environment. Further, sensitivity analyses additionally adjusting by parity did not change our overall conclusions.
Our findings are subject to several limitations, including loss to follow-up and outcome misclassification. A total of 126 (32%) HOME Study children did not have any BRIEF assessment completed. However, attrition was nondifferential as children lost to follow-up were similar with regard to socioeconomic status, prenatal concentrations of PBDEs and PFASs, and early developmental assessments. Outcome misclassification is also a concern, because the BRIEF was only completed by one parent. BRIEF assessments are based on an individual’s perspective and previous observations, which may differ between parents. In addition, we do not have a teacher completed BRIEF, which would have been less biased than the parental assessed BRIEF. Further, some higher-brominated compounds, particularly BDE-209, were not measured. Deca-BDE was still used in the US at the time of the age 5 and 8 follow-ups; however, most studies of the general population have reported low levels of BDE-209 near 5 ng/g lipid (USEPA, 2010). Nevertheless, BDE-209 may be a potential neurotoxicant as several animal models have reported altered cognitive and motor function with gestational and postnatal exposure to BDE-209 (Costa and Giordano, 2011). Further, a significant inverse association was reported between BDE-209 in breast milk and cognitive development in infants 8-12 months of age (Chao et al., 2011). Lastly, postnatal exposures of PBDEs and PFASs were not examined.
5. Conclusions
These findings suggest that concentrations of maternal serum PBDEs and PFASs during pregnancy may be associated with poorer executive function in school-age children. Several BDE congeners, in particular BDE-153, were significantly associated with worse behavior regulation among children 5 and 8 years. Prenatal exposure to PFOS and PFHxS was associated with poorer global executive functioning. Given that the persistence of PBDEs and PFASs has resulted in detectable serum concentrations worldwide, the observed deficits in executive function may have a large impact at the population level. Further research is needed to replicate these findings and to elucidate the mechanisms involved in the potential neurotoxicity of PBDEs and PFASs.
Supplementary Material
Highlights.
Prenatal BDE-153 and PFOS was associated with poorer executive function in children
PBDEs were adversely associated with behavior regulation
BDE-47, -99, -100, -153, and ∑PBDEs were associated with poorer emotional control
PFOS was associated with both behavior regulation and metacognition impairment
No association was observed between PFOA and executive function in children
Acknowledgments
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS P01 ES11261, R01 ES014575, R01 ES020349, and R00 ES020346; EPA P01 R829389). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIEHS or the Centers for Disease Control and Prevention (CDC). We acknowledge the CDC laboratory staff who participated in the analysis of the samples for environmental chemicals.
Abbreviations
- ADHD
Attention deficit/hyperactivity disorder
- BRIEF
Behavior Rating Inventory of Executive Function
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- DNBC
Danish National Birth Cohort
- FSIQ
Full Scale IQ
- GM
Geometric mean
- HOME
Health Outcomes and Measures of the Environment
- LOD
Limit of detection
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds ratio
- PBDEs
Polybrominated diphenyl ethers
- PCBs
Polychlorinated biphenyls
- PFASs
Perfluoroalkyl substances
- PFDeA
Perfluorodecanoate
- PFHxS
Perfluorohexane sulfonate
- PFNA
Perfluorononanoate
- PFOA
Perfluorooctanoate
- PFOS
Perfluorooctane sulfonate
- PFUnDA
Perfluoroundecanoate
- QC
Quality control
- SD
Standard deviation
- T4
Thyroxine
- T3
Triiodothyronine
Footnotes
Competing Financial Interests
The authors declare they have no competing financial interests.
Disclosure
Dr. Lanphear has served as an expert witness and as a consultant to the California Attorney General’s Office, but has not personally received compensation for these services. Dr. Lanphear has also served as a paid consultant on a U.S. Environmental Protection Agency research study and to the California Department of Toxic Substance Control. Dr. Braun was financially compensated for conducting a reanalysis of the international pooled study of lead exposure for the plaintiffs in a public nuisance case. None of these activities are directly related to the present study.
The study protocol was approved by the Institutional Review Boards at the Cincinnati Children’s Hospital Medical Center and the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelouahab N, et al. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol. 2013;178:701–13. doi: 10.1093/aje/kwt141. [DOI] [PubMed] [Google Scholar]
- Andersen S, et al. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG. 2014 doi: 10.1111/1471-0528.12681. [DOI] [PubMed] [Google Scholar]
- Austin ME, et al. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Perspect. 2003;111:1485–9. doi: 10.1289/ehp.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, et al. Beck Depression Inventory. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Branchi I, et al. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioural development. Neurotoxicology. 2002;23:375–84. doi: 10.1016/s0161-813x(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Branchi I, et al. Early developmental exposure to BDE 99 or Aroclor 1254 affects neurobehavioural profile: interference from the administration route. Neurotoxicology. 2005;26:183–92. doi: 10.1016/j.neuro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home Observation for Measurement of the Environment. University of Arkansas; Little Rock, AR: 1984. [Google Scholar]
- Canfield RL, et al. Impaired neuropsychological functioning in lead-exposed children. Dev Neuropsychol. 2004;26:513–40. doi: 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- Chao HR, et al. Levels of breast milk PBDEs from southern Taiwan and their potential impact on neurodevelopment. Pediatr Res. 2011;70:596–600. doi: 10.1203/PDR.0b013e3182320b9b. [DOI] [PubMed] [Google Scholar]
- Chen A, et al. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: The HOME Study. Environ Health Perspect. 2014;122:856–62. doi: 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, et al. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect. 2010;118:1444–9. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–67. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Is decabromodiphenyl ether (BDE-209) a developmental neurotoxicant? Neurotoxicology. 2011;32:9–24. doi: 10.1016/j.neuro.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO, et al. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(Suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, et al. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119:900–7. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121:257–62. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, et al. Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy. Environ Health Perspect. 2008;116:1391–5. doi: 10.1289/ehp.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, Olsen J. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ Health Perspect. 2011;119:573–8. doi: 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, et al. Perfluoroalkyl substances measured in breast milk and child neuropsychological development in a Norwegian birth cohort study. Environ Int. 2015;83:176–82. doi: 10.1016/j.envint.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Fuentes S, et al. Influence of maternal restraint stress on the long-lasting effects induced by prenatal exposure to perfluorooctane sulfonate (PFOS) in mice. Toxicol Lett. 2007;171:162–70. doi: 10.1016/j.toxlet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Gascon M, et al. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ Int. 2011;37:605–11. doi: 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gioia GA, et al. Behavior Rating Inventory of Executive Function. Psychological Assessment Resources; Odessa, FL: 2000a. [Google Scholar]
- Gioia GA, et al. TEST REVIEW Behavior Rating Inventory of Executive Function. Child Neuropsychology. 2000b;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Greenland S. Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology. 1995;6:450–4. doi: 10.1097/00001648-199507000-00025. [DOI] [PubMed] [Google Scholar]
- Haddow JE, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Henrichs J, et al. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf) 2013;79:152–62. doi: 10.1111/cen.12227. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–9. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- Johansson N, et al. Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol Sci. 2009;108:412–8. doi: 10.1093/toxsci/kfp029. [DOI] [PubMed] [Google Scholar]
- Johansson N, et al. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29:160–9. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Jones R, et al. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Compd. 2012;74:97–98. [Google Scholar]
- Julvez J, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology. 2013;24:150–7. doi: 10.1097/EDE.0b013e318276ccd3. [DOI] [PubMed] [Google Scholar]
- Kato K, et al. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A. 2011;1218:2133–7. doi: 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- Kato K, et al. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003-2006. Environ Sci Technol. 2014;48:9600–8. doi: 10.1021/es501811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissa E. Fluorinated surfactants and repellents. Marcel Dekker, Inc.; New York, NY: 2001. [Google Scholar]
- Kodavanti PR, et al. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol Sci. 2005;88:181–92. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Koenig CM, et al. Maternal transfer of BDE-47 to offspring and neurobehavioral development in C57BL/6J mice. Neurotoxicol Teratol. 2012;34:571–80. doi: 10.1016/j.ntt.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korevaar TI, et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 2015 doi: 10.1016/S2213-8587(15)00327-7. [DOI] [PubMed] [Google Scholar]
- Kuriyama SN, et al. Developmental exposure to low dose PBDE 99: effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect. 2005;113:149–54. doi: 10.1289/ehp.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci. 2003;74:382–92. doi: 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- Lezak MD, et al. Neuropsychological assessment. Oxford University Press; New York: 2004. [Google Scholar]
- Liu X, et al. Effects of subchronic perfluorooctane sulfonate exposure of rats on calcium-dependent signaling molecules in the brain tissue. Arch Toxicol. 2010;84:471–9. doi: 10.1007/s00204-010-0517-9. [DOI] [PubMed] [Google Scholar]
- Luebker DJ, et al. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: dose-response, and biochemical and pharamacokinetic parameters. Toxicology. 2005;215:149–69. doi: 10.1016/j.tox.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Phillips DL, et al. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Corbett SM. Executive function profile in the offspring of women that smoked during pregnancy. Nicotine Tob Res. 2012;14:191–9. doi: 10.1093/ntr/ntr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, et al. Lead exposure and behavior among young children in Chennai, India. Environ Health Perspect. 2009;117:1607–11. doi: 10.1289/ehp.0900625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E, et al. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–8. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, et al. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9-12 years of age. Neurotoxicol Teratol. 2015 doi: 10.1016/j.ntt.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber T, et al. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ Health Perspect. 2010;118:572–8. doi: 10.1289/ehp.0901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy CG, et al. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers. Bull Environ Contam Toxicol. 2011;87:643–8. doi: 10.1007/s00128-011-0422-9. [DOI] [PubMed] [Google Scholar]
- Sjodin A, et al. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem. 2004;76:1921–7. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- Skogerbo A, et al. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on executive function in 5-year-old children. BJOG. 2012;119:1201–10. doi: 10.1111/j.1471-0528.2012.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, et al. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect. 2008;116:716–22. doi: 10.1289/ehp.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, et al. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119:1454–9. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskal DF, et al. Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicol Sci. 2006;94:28–37. doi: 10.1093/toxsci/kfl091. [DOI] [PubMed] [Google Scholar]
- Stein CR, et al. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology. 2013;24:590–9. doi: 10.1097/EDE.0b013e3182944432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, et al. Perfluorooctanoate exposure in a highly exposed community and parent and teacher reports of behaviour in 6-12-year-old children. Paediatr Perinat Epidemiol. 2014;28:146–56. doi: 10.1111/ppe.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA . An exposure assessment of polybrominated diphenyl ethers. Washington, DC: 2010. [Google Scholar]
- Viberg H, et al. Neurobehavioral derangements in adult mice receiving decabrominated diphenyl ether (PBDE 209) during a defined period of neonatal brain development. Toxicol Sci. 2003;76:112–20. doi: 10.1093/toxsci/kfg210. [DOI] [PubMed] [Google Scholar]
- Vuong AM, et al. Maternal polybrominated diphenyl Ether (PBDE) exposure and thyroid hormones in maternal and cord sera: The HOME Study, Cincinnati, USA. Environ Health Perspect. 2015;123:1079–85. doi: 10.1289/ehp.1408996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Prenatal exposure to perfluroalkyl substances and children's IQ: The Taiwan maternal and infant cohort study. Int J Hyg Environ Health. 2015;218:639–44. doi: 10.1016/j.ijheh.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect. 2014;122:529–34. doi: 10.1289/ehp.1306925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, et al. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population-based cohort study. Environ Res. 2014;133:338–47. doi: 10.1016/j.envres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Willcutt EG, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–46. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–94. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, et al. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011;119:878–85. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.