Abstract
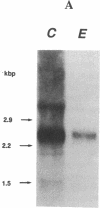
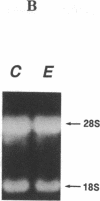
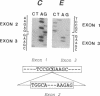
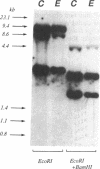
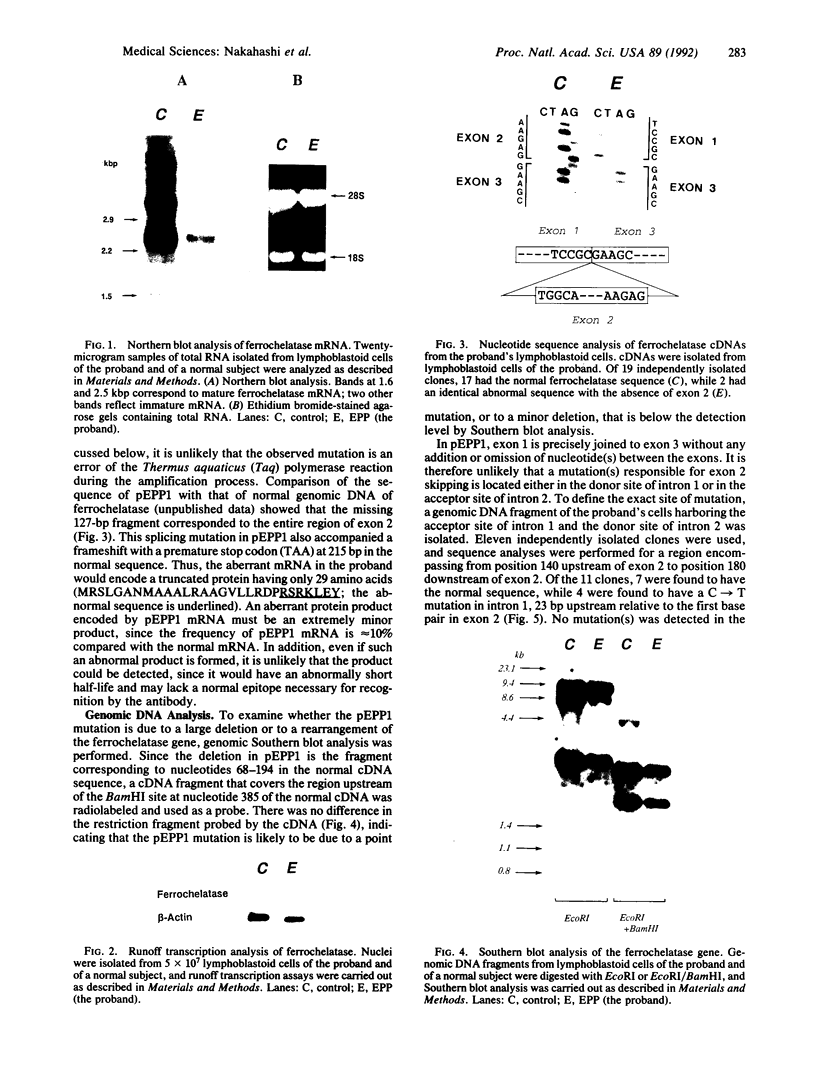
The molecular basis of an inherited defect of ferrochelatase in a patient with erythropoietic protoporphyria (EPP) was investigated. Ferrochelatase is the terminal enzyme in the heme biosynthetic pathway and catalyzes the insertion of ferrous iron into protoporphyrin IX to form heme. In Epstein-Barr virus-transformed lymphoblastoid cells from a proband with EPP, enzyme activity, an immunochemically quantifiable protein, and mRNA content of ferrochelatase were about one-half the normal level. In contrast, the rate of transcription of ferrochelatase mRNA in the proband's cells was normal, suggesting that decreased ferrochelatase mRNA is due to an unstable transcript. cDNA clones encoding ferrochelatase in the proband, isolated by amplification using the polymerase chain reaction, were found to be classified either into those encoding the normal protein or into those encoding an abnormal protein that lacked exon 2 of the ferrochelatase gene, indicating that the proband is heterozygous for the ferrochelatase defect. Genomic DNA analysis revealed that the abnormal allele had a point mutation, C----T, near the acceptor site of intron 1. This point mutation appears to be responsible for the post-transcriptional splicing abnormality resulting in an aberrant transcript of ferrochelatase in this patient.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adema G. J., van Hulst K. L., Baas P. D. Uridine branch acceptor is a cis-acting element involved in regulation of the alternative processing of calcitonin/CGRP-l pre-mRNA. Nucleic Acids Res. 1990 Sep 25;18(18):5365–5373. doi: 10.1093/nar/18.18.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga S. J., Benz E. J., Jr Nonsense mutations in the human beta-globin gene affect mRNA metabolism. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2056–2060. doi: 10.1073/pnas.85.7.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer J. R., Bonkowsky H. L., Ebert P. S., Mahoney M. J. Inheritance in protoporphyria. Comparison of haem synthetase activity in skin fibroblasts with clinical features. Lancet. 1976 Jul 31;2(7979):226–228. doi: 10.1016/s0140-6736(76)91027-8. [DOI] [PubMed] [Google Scholar]
- Bloomer J. R., Brenner D. A., Mahoney M. J. Study of factors causing excess protoporphyrin accumulation in cultured skin fibroblasts from patients with protoporphyria. J Clin Invest. 1977 Dec;60(6):1354–1361. doi: 10.1172/JCI108895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Bloomer J. R., Ebert P. S., Mahoney M. J. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975 Nov;56(5):1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley S. S., Tanaka M., Everett M. A. Diminished erythroid ferrochelatase activity in protoporphyria. J Lab Clin Med. 1975 Jul;86(1):126–131. [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- DeLeo V. A., Poh-Fitzpatrick M., Mathews-Roth M., Harber L. C. Erythropoietic protoporphyria. 10 years experience. Am J Med. 1976 Jan;60(1):8–22. doi: 10.1016/0002-9343(76)90528-3. [DOI] [PubMed] [Google Scholar]
- Deybach J. C., de Verneuil H., Boulechfar S., Grandchamp B., Nordmann Y. Point mutations in the uroporphyrinogen III synthase gene in congenital erythropoietic porphyria (Günther's disease). Blood. 1990 May 1;75(9):1763–1765. [PubMed] [Google Scholar]
- Deybach J. C., de Verneuil H., Phung N., Nordmann Y., Puissant A., Boffety B. Congenital erythropoietic porphyria (Günther's disease): enzymatic studies on two cases of late onset. J Lab Clin Med. 1981 Apr;97(4):551–558. [PubMed] [Google Scholar]
- Eperon L. P., Graham I. R., Griffiths A. D., Eperon I. C. Effects of RNA secondary structure on alternative splicing of pre-mRNA: is folding limited to a region behind the transcribing RNA polymerase? Cell. 1988 Jul 29;54(3):393–401. doi: 10.1016/0092-8674(88)90202-4. [DOI] [PubMed] [Google Scholar]
- Estes P. A., Cooke N. E., Liebhaber S. A. A difference in the splicing patterns of the closely related normal and variant human growth hormone gene transcripts is determined by a minimal sequence divergence between two potential splice-acceptor sites. J Biol Chem. 1990 Nov 15;265(32):19863–19870. [PubMed] [Google Scholar]
- Fu X. D., Katz R. A., Skalka A. M., Maniatis T. The role of branchpoint and 3'-exon sequences in the control of balanced splicing of avian retrovirus RNA. Genes Dev. 1991 Feb;5(2):211–220. doi: 10.1101/gad.5.2.211. [DOI] [PubMed] [Google Scholar]
- Fujita H., Sassa S. The rapid and decremental change in haem oxygenase mRNA during erythroid differentiation of murine erythroleukaemia cells. Br J Haematol. 1989 Dec;73(4):557–560. doi: 10.1111/j.1365-2141.1989.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Kauppinen R., Mignotte V., Peltonen L., Mustajoki P., Roméo P. H., Goossens M., Nordmann Y. Molecular analysis of acute intermittent porphyria in a Finnish family with normal erythrocyte porphobilinogen deaminase. Eur J Clin Invest. 1989 Oct;19(5):415–418. doi: 10.1111/j.1365-2362.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., de Rooij F., Beaumont C., Wilson P., Deybach J. C., Nordmann Y. A point mutation G----A in exon 12 of the porphobilinogen deaminase gene results in exon skipping and is responsible for acute intermittent porphyria. Nucleic Acids Res. 1989 Aug 25;17(16):6637–6649. doi: 10.1093/nar/17.16.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries R. K., Ley T. J., Anagnou N. P., Baur A. W., Nienhuis A. W. Beta O-39 thalassemia gene: a premature termination codon causes beta-mRNA deficiency without affecting cytoplasmic beta-mRNA stability. Blood. 1984 Jul;64(1):23–32. [PubMed] [Google Scholar]
- Ishida N., Fujita H., Noguchi T., Doss M., Kappas A., Sassa S. Message amplification phenotyping of an inherited delta-aminolevulinate dehydratase deficiency in a family with acute hepatic porphyria. Biochem Biophys Res Commun. 1990 Oct 15;172(1):237–242. doi: 10.1016/s0006-291x(05)80199-8. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Taylor S. I. A nonsense mutation causing decreased levels of insulin receptor mRNA: detection by a simplified technique for direct sequencing of genomic DNA amplified by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jan;87(2):658–662. doi: 10.1073/pnas.87.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreivi J. P., Zerivitz K., Akusjärvi G. Sequences involved in the control of adenovirus L1 alternative RNA splicing. Nucleic Acids Res. 1991 May 11;19(9):2379–2386. doi: 10.1093/nar/19.9.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H. C., Nasim F. H., Grabowski P. J. Control of alternative splicing by the differential binding of U1 small nuclear ribonucleoprotein particle. Science. 1991 Mar 1;251(4997):1045–1050. doi: 10.1126/science.1825520. [DOI] [PubMed] [Google Scholar]
- Lamola A. A., Piomelli S., Poh-Fitzpatrick M. G., Yamane T., Harber L. C. Erythropoietic protoporphyria and lead intoxication: the molecular basis for difference in cutaneous photosensitivity. II. Different binding of erythrocyte protoporphyrin to hemoglobin. J Clin Invest. 1975 Dec;56(6):1528–1535. doi: 10.1172/JCI108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGNUS I. A., JARRETT A., PRANKERD T. A., RIMINGTON C. Erythropoietic protoporphyria. A new porphyria syndrome with solar urticaria due to protoporphyrinaemia. Lancet. 1961 Aug 26;2(7200):448–451. doi: 10.1016/s0140-6736(61)92427-8. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M. M., Drouin G. L., Duffy L. Isolation of human ferrochelatase. Arch Dermatol. 1987 Apr;123(4):429–430. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahashi Y., Taketani S., Okuda M., Inoue K., Tokunaga R. Molecular cloning and sequence analysis of cDNA encoding human ferrochelatase. Biochem Biophys Res Commun. 1990 Dec 14;173(2):748–755. doi: 10.1016/s0006-291x(05)80099-3. [DOI] [PubMed] [Google Scholar]
- Sassa S., Fujita H., Doss M., Hassoun A., Verstraeten L., Mercelis R., Kappas A. Hereditary hepatic porphyria due to homozygous delta-aminolevulinic acid dehydratase deficiency: studies in lymphocytes and erythrocytes. Eur J Clin Invest. 1991 Apr;21(2):244–248. doi: 10.1111/j.1365-2362.1991.tb01817.x. [DOI] [PubMed] [Google Scholar]
- Sassa S., Zalar G. L., Poh-Fitzpatrick M. B., Anderson K. E., Kappas A. Studies in porphyria: functional evidence for a partial deficiency of ferrochelatase activity in mitogen-stimulated lymphocytes from patients with erythropoietic protoporphyria. J Clin Invest. 1982 Apr;69(4):809–815. doi: 10.1172/JCI110520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S., Tokunaga R. Purification and substrate specificity of bovine liver-ferrochelatase. Eur J Biochem. 1982 Oct;127(3):443–447. doi: 10.1111/j.1432-1033.1982.tb06892.x. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A., Inoue K., Sakamoto H., Shimura Y. A secondary structure at the 3' splice site affects the in vitro splicing reaction of mouse immunoglobulin mu chain pre-mRNAs. Nucleic Acids Res. 1989 Oct 25;17(20):8159–8169. doi: 10.1093/nar/17.20.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went L. N., Klasen E. C. Genetic aspects of erythropoietic protoporphyria. Ann Hum Genet. 1984 May;48(Pt 2):105–117. doi: 10.1111/j.1469-1809.1984.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Wu J., Manley J. L. Mammalian pre-mRNA branch site selection by U2 snRNP involves base pairing. Genes Dev. 1989 Oct;3(10):1553–1561. doi: 10.1101/gad.3.10.1553. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Grandchamp B., Romeo P. H., Raich N., Beaumont C., Goossens M., Nicolas H., Nordmann Y. Molecular analysis of uroporphyrinogen decarboxylase deficiency in a family with two cases of hepatoerythropoietic porphyria. J Clin Invest. 1986 Feb;77(2):431–435. doi: 10.1172/JCI112321. [DOI] [PMC free article] [PubMed] [Google Scholar]