Abstract
The negative efficacy outcomes of double-blinded, randomized, placebo-controlled Phase III human clinical trials with selenomethionine (SeMet) and SeMet-rich selenized-yeast (Se-yeast) for prostate cancer prevention and Se-yeast for prevention of non-small cell lung cancer (NSCLC) in North America lead to rejection of SeMet/Se-yeast for cancer prevention in Se-adequate populations. We identify two major lessons from the outcomes of these trials: 1) The antioxidant hypothesis was tested in wrong subjects or patient populations. 2) The selection of Se agents was not supported by cell culture and preclinical animal efficacy data as is common in drug development. We propose that next-generation forms of Se (next-gen Se), such as methylselenol precursors, offer biologically appropriate approaches for cancer chemoprevention but these are faced with formidable challenges. Solid mechanism-based preclinical efficacy assessments and comprehensive safety studies with next-gen Se will be essential to re-vitalize the idea of cancer chemoprevention with Se in the post-SELECT era. We advocate smaller mechanism-driven Phase I/II trials with these next-gen Se to guide and justify future decisions for definitive Phase III chemoprevention efficacy trials.
1. INTRODUCTION AND SCOPE OF REVIEW
Early clinical studies suggested that supplementation of selenium (Se) in the form of selenized yeast (Se-yeast), which contains mostly selenomethionine (SeMet), might decrease the risk of cancers in China (1,2) and US (3). The impressive initial “positive” efficacy of Se-yeast in the Nutritional Prevention of Cancer Trial (NPCT, also known as the “Clark Trial” after the late Dr. Larry Clark, its principal investigator) in the US to reduce risk of cancers of the prostate (Table 1), lung and colon and all cancer-mortality (3–6) motivated initiation of a number of Phase III trials in North America against prostate and lung cancer (7–11), the first and largest of which being the Selenium and vitamin E Cancer Prevention Trial [SELECT] (7,8). All four published trials with cancer incidence as primary endpoint showed no efficacy for SeMet or Se-yeast (Table 1). These trials (7–11) all followed the gold standard of evidence-based medicine, i.e., they were of a double-blinded, randomized, placebo-controlled design, and owing to the rigor of these well-executed trials, the null efficacy outcomes are considered conclusive. Follow-up analyses of SELECT have even shown slight enhancement of prostate cancer (PCa) risk associated with α-tocopheryl acetate form of vitamin E (12) and in a subgroup of men on SeMet that had high baseline Se status (13). These outcomes had serious adverse impacts on the credibility and reputation of the field of cancer chemoprevention research which had suffered already from the adverse outcome of the Alpha-Tocopherol Beta-Carotene (ATBC) trial showing enhancement of risk of lung and prostate cancer in male smokers given beta-carotene supplementation (14). Potter boldly entitled his 2014 review article “The failure of cancer chemoprevention” (15). Because these Se trials conclusively invalidated SeMet and Se-yeast for cancer prevention use in populations with adequate Se intake/status, many have cast grave doubts about the merit of Se for cancer chemoprevention.
Table 1.
Cancer Intervention Outcomes of Published Clinical Trials with SeMet/Se-yeast in North America
| Trial | Se form & Dose µg Se/day |
Baseline Se ng/ml |
Post-Rx Se* ng/ml (max) |
Outcome** parameter |
RR*** Rx/Placebo |
1o Ref |
|---|---|---|---|---|---|---|
| Clark NPCT | Se-yeast, 200 | 113 (median) | 190 | PCa (2nd) | 0.37 (p=0.002) | (3) |
| Clark NPCT (follow-up) |
1 tertile, <106 | PCa (2nd) | 0.14 | (5) | ||
| 2 tertile, 107–123 | PCa (2nd) | 0.33 | ||||
| 3 tertile, >123 | PCa (2nd) | 1.14 | ||||
| SELECT | SeMet, 200 | 136 (median) | 251(max283) | PCa (1o) | 1.04 (p>0.15) | (8) |
| SWOG9917 | SeMet, 200 | 137 (median) | 261 (max305) | PCa (1o) | 0.97 (p=0.73) | (9) |
| ECOG NBT | Se-Yeast, 200 | 126 (median) | Not available | PCa (1o) | 0.94 (p=0.18) | (10) |
| Se-Yeast, 400 | Not available | “ “ | 0.90 (p=0.17) | |||
| ECOG5597 | Se-Yeast, 200 | Not available | Lung Ca (1o) | 1.25 (p=0.294) | (11) | |
| Lung Ca |
Highest median/mean reported for that study
Outcome parameter: planned primary endpoint (1o) vs. added secondary endpoint (2nd) of the respective trial.
The Negative Biopsy Trial (NBT)-US, New Zealand
95% Confidence intervals were not presented in most of these studies.
We do not share such pessimism. Given the well documented metabolic and biochemical differences between SeMet and other forms of Se (16,17) (Fig. 1), we have argued that the failure of SeMet in SELECT should not and cannot be taken to indicate that next-generation forms of Se (next-gen Se) are ineffective for cancer prevention (18). Many have opined on possible reasons for failure to demonstrate SeMet efficacy as those offered by authors of the original paper reporting the outcome of SELECT (8) and in subsequent reviews, including baseline Se status of subjects, dosage and chemical forms of Se, and stage of cancer development targeted (19–21). We recently elaborated on lessons learned for two major causes of the failure of SELECT and other trials with Se (22): 1) The use of non-Se-deficient human subjects for testing the Se anti-oxidant hypothesis, and 2) an incorrect choice of Se form for cancer chemoprevention in a Se-adequate population.
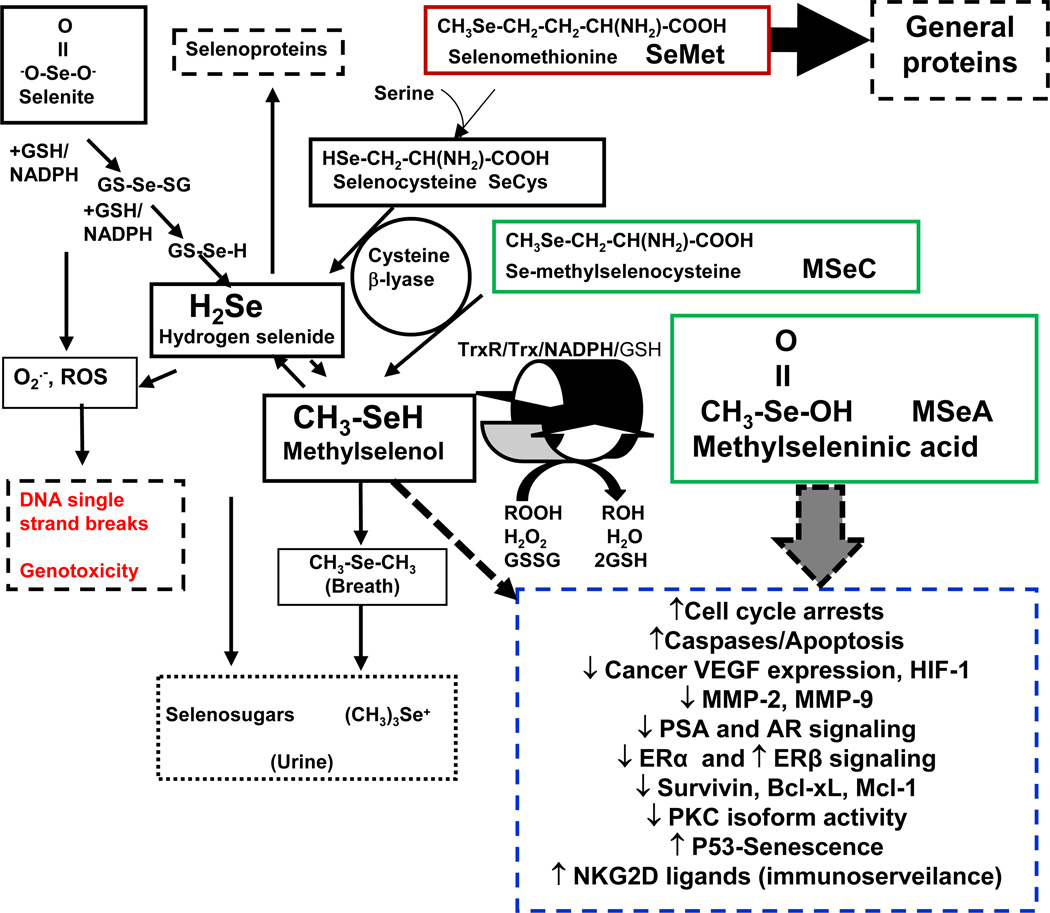
Fig. 1. Structures and possible metabolic pathways for methylseleninic acid (MSeA), Se-methylselenocysteine (MSeC), selenoamino acids and inorganic selenite in a chemoprevention context.
For selenoamino acids, tissue cysteine β-lyases release hydrogen selenide and methylselenol from selenocysteine and methylselenocysteine, respectively. SeMet leads to massive tissue accumulation of Se due to its non-specific incorporation into general proteins in place of Met. Selenite can cause DNA damage, likely through reactive oxygen species. Methylselenol pool may be selectively enriched by precursor compounds or functional foods such as Se-garlic, bypassing the hydrogen selenide pool. Depending on the entry point to a likely MSeA-MSeH redox cycle, the cellular effect and molecular targets of a next-gen methyl Se will be the integrative balance of MSeA, MSeH and their redox intermediary metabolites. Reported cellular effects and molecular targets of MSeA-MSeH are summarized.
In this article, we summarize the outcomes of these trials and further expand on these two major lessons. We will discuss recent progress on next-gen Se forms that can be considered for future translational cancer chemoprevention studies. We posit that it would be a mistake to abandon research on next-gen Se because of the failure of SeMet or Se-yeast to prevent prostate and lung cancer in Se sufficient human populations. In addition, we highlight some novel synthetic Se compounds in the R&D pipeline.
2. LESSONS FROM MAJOR CLINICAL TRIALS THAT FAILED TO VALIDATE CANCER PREVENTIVE ACTIVITIES OF SEMET OR SE-YEAST
Summary of human clinical trial outcomes
Table 1 summarizes the primary efficacy outcomes from Clark’s NPCT (3–5) to the latest published trials. As evident from the summarized data, the NPCT (3,4) indicated that risk of PCa was reduced in a baseline Se-specific manner in subjects in the lower 2 tertiles of serum Se (<106 ng Se/ml and 107–123 ng Se/ml) but there was no risk reduction for the highest tertile (>123 ng Se/ml). Lung and colon cancer risks were also reduced by Se-yeast supplementation. Limitations of this study were not only that PCa was a secondary endpoint not planned in the original design, but also the small number of PCa cases, among others. Subsequent trials (7–11) were all designed with either PCa or lung Ca as the primary endpoint and adequately powered, but did not find efficacy against PCa and lung cancer. The baseline Se levels at enrollment for these newer trials were higher than in NPCT. In SELECT, further analyses of the effects of SeMet compared with those of vitamin E (dl-α-tocopheryl acetate) supplementation stratified by baseline Se status (toe nail Se levels) showed that SeMet did not benefit men with low Se status but increased the risk of high-grade PCa among men with high baseline Se status (13). Vitamin E supplement increased the risk of PCa among men with low Se status in this analysis (13). The study authors cautioned that men should avoid Se (SeMet) or vitamin E (dl-α-tocopheryl acetate) supplementation at doses that exceed recommended dietary intakes. Christensen recently discussed the baseline Se and intervention efficacy issues in considerable depth (21), and we also articulated two major lessons from these trials (22). In the following sections we offer an updated and integrated discussion of these points.
Lesson 1: The antioxidant hypothesis was tested in wrong subjects/patient populations
The best studied biochemical activity of Se is its function as an integral part of Se-dependent glutathione peroxidases (SeGPX), which are selenoprotein antioxidant enzymes. It has been estimated that achieving 1 µM or 80 ng/mL is the upper limit for a maximal SeGPX response to supplemental Se in healthy adults (23) (Fig. 2), which is more than sufficiently provided by the National Research Council’s recommended daily allowance of 55 µg Se for adults (24). A more recent study in Se-deficient subjects in China with SeMet supplementation provided a similar estimate of Se intake needed to support maximal plasma levels of selenoprotein P (SEPP1), another selenoprotein (25). Since SeGPX3 and SEPP1 make up virtually all the selenoproteins in the blood, the authors measured plasma levels of these two proteins after a 40-week placebo-controlled, double-blinded SeMet supplementation trial in 98 healthy Chinese subjects with baseline daily dietary Se intake of 14 µg. Fourteen subjects each were assigned randomly to a daily dose of 0, 21, 35, 55, 79, 102, and 125 µg Se as SeMet. Plasma SEPP1 concentration reached a maximum at the 35-µg supplement dose, which amounted to 49 µg total Se ingestion/day. Plasma GPX3 activity reached a plateau by 21 µg supplemental Se, i.e., 35 µg total Se ingestion/day. However, the total plasma Se levels continued to increase dose-dependently without plateauing. Because of its function as Se carrier, SEPP1 was considered the better plasma biomarker for assessing optimal expression of all selenoproteins (25). On the basis of the Se intake needed for SEPP1 optimization with adjustments for body weight and individual variation, the authors extrapolated 75 µg Se/day as the SeMet dose for US residents required to ensure full expression of selenoproteins (25).
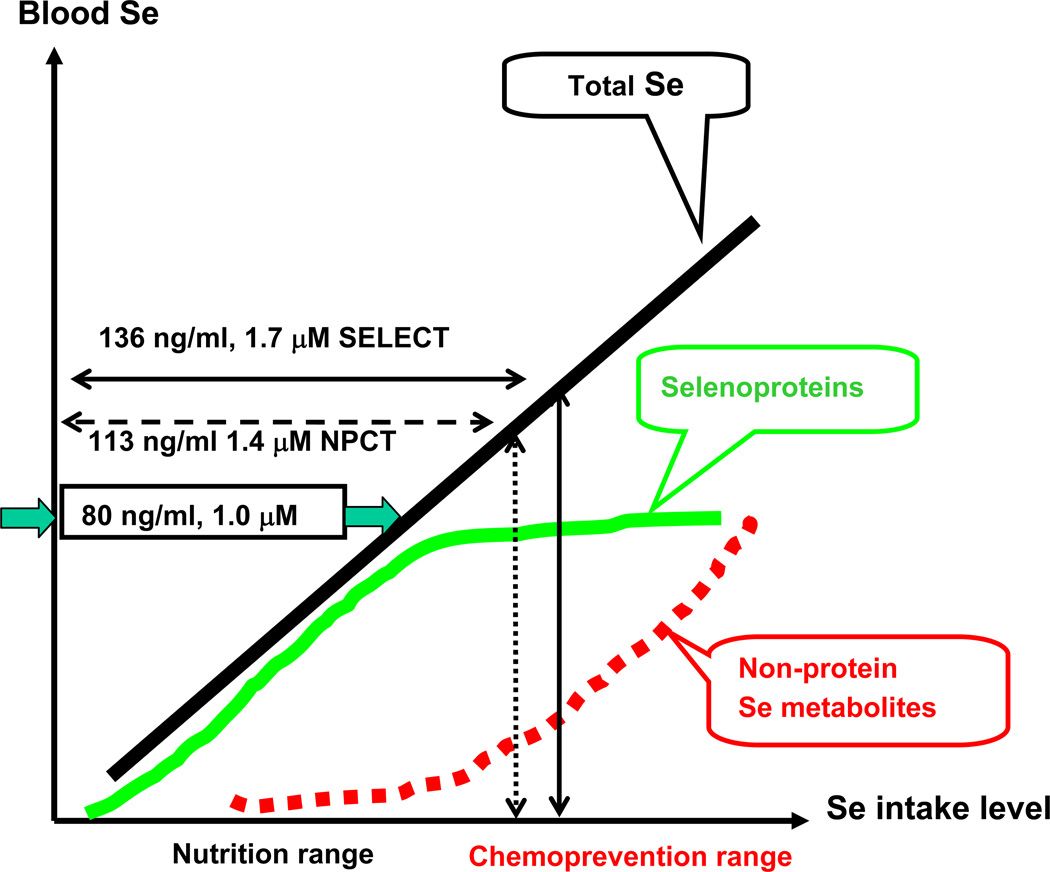
Fig. 2. Schematic relationship of different pools of Se in blood circulation as a function of Se intake level in the nutritional range and in chemoprevention range.
Mean or medium baseline plasma Se at enrollment for NPCT vs. SELECT was marked in reference to plasma Se threshold value to support full selenoprotein activity.
According to the Third National Health and Nutrition Examination Survey (NHANES III), the mean Se intake in the US of individuals of all ages in 1999–2000 was 103 µg (26), nearly twice the NRC daily allowance of 55 µg for adults (24). The mean serum Se concentration in US adults was 1.58 µM and the median was 1.56 µM based on more than 18,000 samples in NHANES III (27,28). In the NPCT study the placebo group had a similar baseline plasma Se level of 113 ng Se/ml (1.4 µM) (Table 1), and the plasma Se level in the supplemented group was increased by some 67% to 190 ng/mL or 2.4 µM (3,5). Only 1.5% of all subjects had Se levels lower than 80 ng/mL (5). In spite of the increase of total Se level in the plasma, the plasma GPX3 activity of selected subjects before and after Se supplementation was not increased in NCPT (29).
In SELECT, the median baseline plasma Se level was 136 ng/ml (1.72 µM), higher than that in the NPCT, and SeMet supplementation increased median levels to 223, 232, 228 and 251 ng/ml in four subsequent years (2.82–3.17 µM) (8) (Table 1). In the SWOG9917 trial in men with high grade prostatic intraepithelial neoplasia (HG-PIN), the baseline median plasma/serum Se level was 135 to 138 ng/mL in the placebo and Se supplemented group, respectively (9) (Table 1). In the NBT study with Se-yeast in men with elevated PSA but negative biopsies, the mean baseline Se values were 125, 127, and 127 ng/ml, respectively for the placebo, 200-µg and 400-µg cohort (10). In the report of the lung cancer trial with Se-yeast, the baseline Se values were not presented, but only fewer than 1% of subjects were rated below the normal range (11). In all these Se trials, the baseline Se status was therefore more than nutritionally adequate. Based on analyses of baseline Se in these trials and work by Combs (30) and Richie (31), Christensen re-emphasized the notion of a threshold dose as suggested by the PCa outcome in the lowest tertile group in the NPCT, a dose below which SeMet/Se-yeast might be beneficial as a supplement to improve Se anti-cancer function (21). However, subjects with such low Se status are rare in the US. In other words, Se status in most US residents is more than adequate from a nutritional perspective. Thus, the hypothesis of cancer prevention by dietary Se can only be tested in populations with marginal to deficient Se intake.
Lesson 2. The dose levels and selection of Se agents was not supported by cell culture and animal efficacy data
Dose levels in cell culture and human studies
In cell culture studies, SeMet exposure levels of 2 orders of magnitude higher than circulating total Se level are needed to show modest growth inhibitory effects in cancer cell lines. For example, SeMet exposure inhibited the growth of A549 lung cancer cells with an IC50 of 65 µM and of HT29 colon cancer cells with an IC50 of 130 µM (32). In PCa cells, 100–500 µM SeMet were needed to induce growth suppression and apoptosis (33). Two additional studies with colon cancer cell lines reported cell cycle arrest with SeMet levels above 100 µM (34,35). Whether colonic luminal SeMet concentrations can reach such high levels following dietary exposure should be evaluated to assess the relevance of these observed effects.
For non-alimentary tract cancers, the only likely route of exposure to SeMet as dietary supplement is through absorption and delivery via the blood stream. It is very unlikely that such extreme high SeMet levels are achievable through oral SeMet supplements. Indeed, the highest mean Se level resulting from supplementation of SeMet or Se-yeast in the reported trials was 305 ng Se/mL (3.9 µM) (Table 1). In a Phase I dose escalation study, 4,800 and 7,200 µg SeMet/dose (twice per day for 7 days) resulted in serum Se levels of 15 µM and 20 µM on day 8, respectively (36). The 7,200 µg SeMet dose, twice per day regimen represented a Se intake that was 29 fold higher than that used in the SELECT and other PCa trials (Table 1), but the resulting blood level was lower than that needed in cell culture experiments to reduce growth.
Combs and coworkers (30) evaluated the relationship of serum levels of selenoproteins (GPX3, SEPP1) and plasma Se in a cohort of healthy, Se-replete men (n = 106) and women (n = 155) from North Dakota at baseline in a SeMet supplementation study. Plasma Se was 142 ng/ml, with GPX3 and serum-derived SEPP1 calculated to comprise 20% and 34%, respectively, of the total plasma Se. The balance, non-specific components (calculated from difference between total Se minus Se in GPX3 and SEPP1 pools) accounted for virtually all of the inter-individual variation in total plasma Se. After a year-long supplementation of these healthy American adults with SeMet at doses of 50, 100 or 200 µg Se/day vs. placebo (37), GPX3 activity or SEPP1 concentrations were not increased, but the Se contents of plasma, urine and buccal cells were increased dose-dependently and each of these plateaued after 9 months and were linearly related to the “effective” Se dose defined as µg/day per kg0·75 (metabolic body weight). The authors concluded that the most responsive Se-biomarkers in this Se-sufficient cohort were those related to body Se pools, i.e., plasma, buccal cell and urinary Se concentrations. As the plasma selenoprotein pools were saturated without supplementation, the percentage represented by the non-specific pool increased with increasing doses of supplementation as illustrated in Figure 2.
Richie et al. directly compared SeMet and Se-yeast supplement in men and measured serum Se forms(31). Less than 1 ng/mL SeMet was observed in the circulation of either SeMet- or Se-yeast-supplemented subjects. Non-specific incorporation of SeMet into general proteins is the most probable fate of the supplemented SeMet. Therefore, it is likely that SeMet in vivo will be in nM range and cannot elicit cellular responses observed in the µM range of exposure in cell culture models.
Animal models showed no efficacy of SeMet or α-tocopheryl acetate
Two general types of animal models had been used for efficacy assessment: primary carcinogenesis induced by chemical-hormonal carcinogenesis in immune-competent rodents and “take” and growth of primary and metastatic PCa xenografts in immunocompromised mice or allografts in syngeneic mice. For the former category, a couple of studies conducted in the late 1990’s before SELECT did not find any efficacy of SeMet and α-tocopheryl acetate alone or in combination or of Se-yeast on a prostate carcinogenesis model in rats. These negative results were initially communicated in meeting proceedings and the full results were only published after SELECT results had been published (38) (39). In one study, the potential of SeMet and α-tocopheryl acetate was examined to modulate PCa development in testosterone plus estradiol-treated Noble (NBL/Crl) rats, a model that involves sex hormone-induced oxidative stress and prostatic inflammation (39). One week following the implantation with hormone-filled Silastic implants, rats were fed diets containing SeMet (1.5 or 3.0 mg/kg) or dl-α-tocopheryl acetate (2,000 or 4,000 mg/kg), in a natural ingredient diet. The development of prostate carcinomas was not affected by dietary treatment with either agent. In the other study (38), McCormick et al. stimulated prostate epithelial cell proliferation by a sequential regimen of cyproterone acetate followed by testosterone propionate in Wistar-Unilever (WU) rats which subsequently received a single i.v. injection of the methylating carcinogen N-methyl-N-nitrosourea (MNU) followed by chronic androgen stimulation via subcutaneous implantation of testosterone-containing Silastic implants. From 1 week post-MNU, carcinogen-treated rats were fed either a control basal diet or a diet supplemented with SeMet (3 or 1.5 mg/kg diet), dl-α-tocopheryl acetate (vitamin E, 4,000 or 2,000 mg/kg diet), SeMet + vitamin E (3 + 2,000 mg/kg diet or 3 + 500 mg/kg diet), or Se-yeast (Se levels at 9 or 3 mg/kg diet). At termination of experiment 13 months post-MNU, PCa incidence as verified by histopathology was not statistically significant different in any group receiving dietary supplementation with Se or Se-yeast alone or Se and vitamin E. In the group receiving only vitamin E, slightly but significantly more animals developed carcinomas in the dorsolateral or anterior prostate, similar to what occurred in SELECT. Together, both studies showed that SeMet and/or α-tocopheryl acetate supplementation did not prevent PCa in rats fed diets with nutritionally adequate levels of Se and vitamin E. Importantly, in hindsight, the results of these animal studies were predictive of the outcome of SELECT.
In a recently published study (40), we examined modulation of testosterone plus 17β-estradiol-induced prostatic oxidative stress, dysplasia, and inflammation by SeMet at 1.5 or 3.0 mg Se/kg in NIH-07 diet in Noble (NBL)/Crl rats treated with these hormones via slow-release Silastic implants for 16 wk. Hormone treatment increased immunohistochemical staining for 8-hydroxydeoxyguanosine (8-OHdG) in the prostatic sites of hormone-induced preneoplasia (P < 0.05), but SeMet did not attenuate 8-OHdG staining and dysplasia in the lateral prostate. Glutathione-peroxidase (SeGPXs) activity (P < 0.05) and mRNA expression were induced by testosterone plus 17β-estradiol (P < 0.0001) but not changed by SeMet. SeMet did not cause significant responses in expression and activity of SeGPX and manganese superoxide dismutase (MnSOD), except for a reduction of MnSOD protein expression in the lateral prostate (P < 0.01). We noted significant (P < 0.01) opposite apoptosis/cell proliferation balance responses to SeMet to testosterone plus 17β-estradiol in the lateral and dorsal prostate, which may help to account for why these hormones induce lesions selectively in the lateral lobe of NBL rats.
Xenograft studies conducted before or since SELECT was initiated did not support any in vivo anti-cancer activity of SeMet (41,42). In a study with orthotopic PC3 xenograft tumors in the prostates of 6-week-old male nude mice fed a Se adequate diet (0.07 mg/kg), supplementation with different Se forms at two different concentrations (0.3 and 3 mg/L) in drinking water, SeMet, Se-Yeast, or MSeC did not retard the growth of primary prostatic tumors and the development of retroperitoneal lymph node metastases, yet surprisingly sodium selenate did (41). We evaluated the growth inhibitory effects of SeMet and selenite in comparison to the next-gen Se forms MSeA and MSeC (See Fig. 1 for structure and metabolism) on DU145 and PC-3 human PCa xenografts in athymic nude mice (42). Each Se was given by a daily single oral dose regimen starting the day after subcutaneous inoculation of cancer cells. Serum, liver, and tumor Se content confirmed supplementation status. SeMet did not have any inhibitory effect in spite of an order of magnitude higher Se retention in liver and tumors of the SeMet-treated mice than in those from mice treated with selenite or MSeA and MSeC in either model. MSeA and MSeC each exerted a dose-dependent inhibition of DU145 xenograft growth. Selenite treatment increased DNA single-strand breaks in peripheral lymphocytes, whereas the other Se forms did not. In the PC-3 xenograft model, only MSeA was growth inhibitory at a dose of 3 mg/kg body weight. These data demonstrated superior in vivo growth inhibitory efficacy of MSeA over SeMet and selenite, against two human PCa xenograft models without the genotoxic effects of selenite.
Recently, Yan and DeMars compared the effects of dietary supplementation with SeMet vs. MSeA on metastasis of murine Lewis lung carcinoma in syngenic male C57BL/6 mice (43). Mice were fed AIN93G control diet or that diet supplemented with MSeA or SeMet at 2.5 mg Se/kg for 4 weeks at which time they were given a intramuscular or subcutaneous injection of the tumor cells. Dietary MSeA reduced lung metastasis yield two weeks after intramuscular injection or two weeks after surgical removal of subcutaneous tumors. However, SeMet did not have any inhibitory effect.
In summary, the data from cell culture studies and the animal models do not support cancer preventive activity of SeMet or SeMet-rich Se-yeast, even with doses much higher than those tested in multiple clinical trials.
3. NEXT-GEN SE COMPOUNDS IN CANCER CHEMOPREVENTION: METHYLSELENOL-MSEA REDOX AND POTENTIAL MECHANISMS OF ACTION
Non-selenoprotein Se metabolites and the mono-methyl Se pool hypothesis
As discussed in preceding section, Se deficiency is not a health concern in the US (See schematic in Fig. 2). Thus, most animal models and cell culture studies since the mid-1980s have dealt with supra-nutritional or potentially therapeutic levels of Se. Cell culture studies have by necessity used cancerous cells as the cell targets due to lack of appropriate precancerous cell culture models. Most animal models have shown cancer chemopreventive activity of Se intake that is 20–50 times greater than the rodent nutritional requirement (17). Based on a large body of data from these studies, Ip and Ganther as well as Combs (17,44) articulated that cancer chemoprevention by Se in the nutritionally adequate subjects is independent of the antioxidant activity of plasma or tissue SeGPX. This paradigm was based on the observation that the dietary level of Se (2 ppm [mg/kg] or greater as selenite or other Se forms) needed to achieve a significant cancer preventive activity in rodent animal models far exceeded that required (i.e., 0.1 ppm) to support maximal SeGPX (SeGPX3) expression in the blood or the target tissues from which experimental cancers arise. This view has been extended to the other selenoproteins identified subsequently in the past 2 decades, including phospholipid glutathione peroxidase (Ph-SeGPX, also known as GPX4), selenoprotein P (SEPP1 also known as Sel-P), selenoprotein W (Sel-W), and thyroixine de-iodinases (TDI) and thioredoxin reductases (TrxR) (16,45,46). Studies with suppression of selenoproteins in genetically modified mice (increased prostate and colon cancer risk when SeGPXs is decreased in the presence of adequate dietary Se) and with TrxR1 knockdown cells (decreased lung cancer cell growth when TrxR1 is knocked down) indicate likely contradicting roles of several of these proteins as regulators of cancer risk in the nutritional range of Se intake (47). Pertinent to the role of selenoprotein in mouse prostatic epithelium, Luchman et al (48) created mice with a prostate-specific deletion of Trsp, a gene that encodes a transfer RNA (Sec tRNA) required for the insertion of selenocysteine residues into all selenoproteins during their translation. By 6 weeks of age, these Trsp-deficient mice exhibited widespread PIN lesions in all prostatic lobes, which then progressed to high-grade dysplasia and microinvasive carcinoma by 24 weeks with increased lipid peroxidation markers in Trsp-deficient epithelial cells. This novel model of prostate neoplasia is consistent with the idea that SeGPXs and possibly other selenoproteins are tumor suppressors in the murine prostate. Dietary Se deficiency thus conceivably increases cancer risk in these mice and correction of this deficiency should lower cancer risk (anti-oxidant hypothesis).
Excess Se beyond the need for selenoprotein synthesis (i.e., hydrogen selenide is co-translationally incorporated into SeCys-containing selenoproteins) is methylated to methylselenol, which is further methylated and excreted as dimethylselenide (volatile and excreted via the breath) and trimethylselenonium (excreted in urine) or converted into selenosugars (See Fig. 1). SeMet is predominantly incorporated into general proteins in place of Met (non-specific substitution) or metabolized to SeCys through a trans-selenation pathway similar to the transulfuration pathway in Met-cysteine cycle. The efficiency of the latter pathway will be dependent on the metabolic capacity of the cell types and organs.
Ip and Ganther originated the active metabolite hypothesis based on data from mammary chemical carcinogenesis models (17,49) which identified as the likely active Se form a mono-methylated Se species, presumably methylselenol. They proposed that the chemopreventive efficacy of a given Se compound depends on the rate of its metabolic conversion to this active Se form(s). Circumstantial evidence in support of this hypothesis was obtained by comparing the cancer chemopreventive efficacy of forms of Se that fed into different Se metabolite pools, with precursors of methylselenol displaying greater preventive efficacy than those for hydrogen selenide or dimethylselenide in the chemically induced rodent mammary carcinogenesis model (50,51). Their subsequent work showed that the alkylselenol and allylselenol precursor compounds were more active against mammary carcinogenesis than methylselenol precursors on an equal molar basis of dietary Se intake (52,53). However, these structure-activity studies have not been extended beyond the mammary carcinogenesis model for assessing the general applicability of this hypothesis for other organ sites.
Cell culture studies by us and others have shown that next-gen Se compounds that are putative precursors of methylselenol induce numerous cellular, biochemical and gene expression responses that are distinct from those induced by the forms of Se that enter the hydrogen selenide pool (16,17,22,45,46). These major cellular and biochemical effects are schematically summarized in Figure 1 and detailed in earlier reviews (16,22,45).
Sodium selenite and sodium selenide are examples of genotoxic forms of Se that feed into the hydrogen selenide (H2Se) pool. Selenite rapidly (within a few hours of Se exposure) induce DNA single strand breaks (SSBs), S phase or G2/M cell cycle arrest, and subsequent cell death by apoptosis and necrosis (16). Sodium selenide and SeCys recapitulate the DNA SSB and apoptosis inducing effects of selenite in mouse mammary carcinoma cells (54). An MnSOD mimetic compound, copper dipropylsalicylate, blocked DNA SSBs and apoptosis, indicating that ROS, but not selenite per se triggered these events (55). More studies have provided further support for ROS (superoxide generation) as intermediates for activating p53 by Serine phosphorylation in apoptosis induction by selenite in LNCaP PCa cells (56,57). We have further shown that selenite at a daily oral dose of 3 mg/kg body weight to xenograft tumor bearing nude mice increased DNA SSBs in peripheral lymphocytes, whereas the same dose of MSeA or MSeC did not have this effect (42). Further studies in animal models and in humans will be necessary to confirm the in vivo genotoxicity of selenite.
Methylselenol precursors
Putative methylselenol precursors such as methylselenocyanate (MSeCN) and MSeC have been shown to induce apoptosis of mammary tumor epithelial cells and leukemia cells without the induction of DNA SSBs (54,58,59). Furthermore, methyl Se-induced cancer cell apoptosis was caspase-dependent, whereas caspase involvement in selenite-induced cell death appeared to vary with the p53 status. Methyl Se compounds or methylselenol generated enzymatically led to G1 arrest (58–63) or G2 arrest (64) in many cancer cells. Inhibitory effects on cyclin dependent kinases (63,65) and protein kinase C (66) have been attributed to the methyl Se pool. With regards to genotoxicity, a daily oral dose of 3 mg/kg body weight of MSeA and MSeC significantly suppressed DU145 human PCa xenograft growth without increasing DNA SSBs in the peripheral lymphocytes of the host mice, whereas the same dosage of selenite caused increased DNA SSBs and was ineffective for suppressing xenograft growth (42).
In addition to these cellular effects, MSeA exerts a rapid inhibitory effect on the expression of key molecules involved in angiogenesis regulation. For example, sub-apoptotic doses of MSeA inhibited the expression and secretion of the angiogenic factor VEGF in several cancer cell lines (67). MSeA also inhibited the expression of matrix metalloproteinase (MMP)-2 in the vascular endothelial cells (67,68). These effects in combination with a potent inhibitory effect on cell cycle progression of vascular endothelial cells (61,62) indicate a key inhibitor function of MSeA on angiogenic switch regulation in early neoplastic lesions and tumors (16). Furthermore, MSeA and methylselenol released by methioninase from SeMet inhibited expression of the androgen receptor and its signaling to regulate prostate specific antigen (PSA) expression (69–71) as well as PSA protein stability (69) in PCa cells. MSeA has been also shown to inhibit estrogen receptor signaling in breast and endometrial cancer cells (72–75) and is a novel suppressor of aromatase expression in human ovarian tumor cells (76). MSeA can also potentiate apoptosis signaling induced by chemotherapeutic drugs or biologics in various cancer cell types through inhibition of survival molecules such as survivin, Bcl-XL (77), and Mcl-1(78,79).
Methylselenol-MSeA redox cycle through thioredoxin reductase
Gromer and Gross (80) examined Ganther’s hypothesis (49) that methylselenol and MSeA might exert their effects by inhibition of the selenoenzyme thioredoxin reductase (TrxR) via irreversible formation of a diselenide bridge. However, they showed that MSeA did not act as an inhibitor of mammalian TrxR but was an excellent substrate for reduction by this enzyme. Nascent methylselenol efficiently reduced both H2O2 and glutathione disulfide (GSSG). They also found that MSeA was a poor substrate for human glutathione reductase, which is not a selenoprotein, and that the catalytic SeCys residue of mammalian TrxR was essential for MSeA reduction to methylselenol.
Gopalakrishna’s group showed (81) that MSeA, but not methylselenol, inactivated specific protein kinase C (PKC) isoforms, especially those involved in tumor progression. They showed that MSeA inactivated pure PKC enzyme activity, which could be reversed by the TrxR system or thiol agents, but methylselenol did not. In DU145 and LNCaP human PCa cell lines under serum-starved conditions, MSeA (at 5 µM) decreased PKC activity within 5 to 15 minutes. The extent of PKC inactivation in these cell lines was observed to be less than that of the pure PKC enzyme, possibly due to TrxR-mediated MSeA-MSeH redox cycling to remove MSeA from the site of action. The increase in PKC inactivation, in particular PKCepsilon isoform, was associated with increased apoptosis and cell arrest. As an illustration of selectivity, a 10-times higher concentration of MSeA was required to inactivate protein kinase A. The PKC inactivation was further enhanced when MSeA was converted from methylselenol by PKC-bound phospholipid peroxides within close proximity to PKC thioclusters. Nanomolar Se concentrations were needed for oxidation of the catalytic unit of PKC by the MSeA-methylselenol redox cycle, highlighting the specificity of MSeA in inactivating PKC.
The same group subsequently showed that MSeA at submicromolar concentrations prevented the transformation of prostate epithelial cells but micromolar levels were required to inhibit cell growth, invasion and induce apoptosis in PCa cells (82). Over-expression of PKCepsilon attenuated MSeA inhibition of epithelial cell transformation and PCa cell apoptosis. In addition, increased TrxR expression caused resistance to MSeA treatment and inhibition of TrxR increased the sensitivity of cancer cells to MSeA (82). These studies suggest that both PKCepsilon and TrxR can negate the anti-cancer efficacy of MSeA. Therefore, methylselenol, MSeA, and their redox cycling intermediates, especially in the localized protein microenvironment of thioclusters, may provide specific targeting niches to negatively regulate enzymatic activities involved in cancer promotion or growth. In retrospect, many of the reported activities that we and others attributed to the “methylselenol pool” likely represent the summation of actions of the dynamic MSeA-MSeH redox cycling (See Figure 1).
Recent findings that MSeA induced senescence of normal primary lung fibroblasts suggest a possible mechanism to increase a barrier to early cancer lesion progression (83). In this study, normal primary fibroblasts and PC3 PCa and HCT116 colon cancer cells were treated with low micromolar concentrations of MSeA for 48 h, followed by a recovery of 1–7 days. Cellular senescence, as evidenced by senescence-associated beta-galactosidase staining and lack of 5-bromo-2-deoxyuridine incorporation, was observed in normal fibroblastic cells, but not in cancer cells. In addition, the ataxia telangiectasia mutated (ATM) DNA damage response protein was rapidly activated by MSeA and its kinase activity was required for the induced senescence response. Subsequently depletion of p53 was demonstrated to attenuate senescence, disrupt the MSeA-induced cell cycle arrest, and increased genome instability (84). Pretreatment with KU55933, an ATM kinase inhibitor, or NU7026, an inhibitor of DNA-dependent protein kinase, desensitized MSeA cytotoxicity in normal fibroblasts but not in fibroblasts with knocked down p53. These results suggest that ATM-p53 DNA damage response is critical for senescence induction by MSeA in normal cells. In particular relevance to PCa chemoprevention, we have obtained preliminary evidence that MSeA treatment of Pten-K/O mice super-activated p53 and senescence in vivo in the early prostate lesions (see later in this review).
Pertinent to the MSeA-MSeH hypothesis, MSeA, MSeC and dimethylselenide (DMDSe) were observed to stimulate the cell surface expression of ligands for the lymphocyte receptor NKG2D in Jurkat T cells, specifically inducing the expression of MICA/B major histocompatibility complex class I related chain genes, which are up-regulated in stressed cells for immune system recognition (85). MSeA and DMDSe induced a maximal MICA/B response at 5 µM, whereas selenite, selenate, SeMet, selenocysteine, and hydrogen selenide had no effect on the cell surface expression of MICA/B at the protein and mRNA level. These data suggest that next-gen methyl Se could improve NKG2D-based cancer immune surveillance and prevention. Taken together, cell culture studies suggest that next-gen Se affects multiple cellular processes related not only to pre/cancerous epithelial cells but also to their microenvironments including endothelial cells and immune cells conducive for chemopreventive action.
4. EFFICACY STUDIES OF NEXT-GEN SE TO INHIBIT PROSTATE AND OTHER PRECLINICAL CANCER MODELS
Whereas both MSeC and MSeA have been shown to inhibit chemically-induced mammary carcinogenesis in the 1990’s (51,86), their anti-cancer efficacy in prostate or other non-mammary organs has only recently been tested by us and others. The lack of interest in further studying these next-gen Se in mammary carcinogenesis models was due, in major part, to the NPCT outcome of 2 breast cancer cases in placebo group vs. 6 cases in Se-yeast group of the very few women enrolled in that trial (3).
Xenograft models
We have shown that orally-administered MSeA and MSeC dose-dependently (1 and 3 mg Se/kg) inhibited the growth of DU145 human PCa xenografts in athymic nude mice, whereas selenite and SeMet did not (42). MSeA was more active than MSeC against PC-3 xenograft growth. Measurement of tissue Se content showed that SeMet treatment led to 9.1-fold more liver Se retention and approximately 3.6 times higher tumor Se levels than mice treated with an equal dose of methyl-Se, despite no potency of SeMet to inhibit DU145 or PC-3 xenograft growth. The observed massive tissue Se accumulation supports non-specific incorporation of SeMet into proteins. The lack of anti-cancer efficacy of SeMet in the presence of elevated circulating and tissue Se accumulation agreed well with earlier work with SeMet in conventional rodent models (51,87). Oral bolus administration of MSeA activity against breast cancer xenograft growth has also been reported (88).
Allograft models
Lindshield and coworkers (89) evaluated the effects of dietary lycopene (250 mg/kg diet), MSeC (1 mg/kg diet), and γ-tocopherol (200 mg/kg diet) alone and in combination on the growth of androgen-dependent Dunning R3327-H rat prostate adenocarcinomas in male, Copenhagen rats. AIN-93G diets containing these micronutrients were fed for 4 to 6 weeks prior to subcutaneous tumor implantation. After 18 weeks, MSeC consumption significantly (P = 0.003) decreased final tumor area and tumor weight, but lycopene and γ-tocopherol did not alter these parameters. Tumor growth inhibition by MSeC was not associated with affecting circulating male hormone level; none of these agents consumed alone or in combination altered serum testosterone or dihydrotestosterone concentrations.
Metastasis models
Yan and DeMars (43) showed that AIN93G diet supplemented with MSeA at 2.5 mg Se/kg (ppm) significantly reduced pulmonary metastatic yield compared with controls, but SeMet did not have such an effect. Supplementation with MSeA also decreased plasma concentrations of urokinase-type plasminogen activator and plasminogen activator inhibitor-1. Furthermore, MSeA reduced plasma concentrations of VEGF, bFGF, and PDGF. SeMet did not affect any of the aforementioned measurements. Thus, inhibition of tumor cell invasion and angiogenesis may be a mechanism by which MSeA inhibited tumor metastasis.
Primary prostate carcinogenesis in transgenic/knockout mice models
We used the transgenic adenocarcinoma mouse prostate (TRAMP) model to test the efficacy of MSeA and MSeC against prostate carcinogenesis and to characterize potential mechanisms (18). Sexually mature male TRAMP mice of C57B/6 background (8 weeks old) were given a daily oral dose of MSeA or MSeC at 3 mg Se/kg body weight and were euthanized at either 18 or 26 weeks of age. By 18 weeks of age, the dorsolateral prostate weights for the MSeA- and MSeC-treated groups were lower than for the water control (P < 0.01) and at 26 weeks genitourinary weight (reflecting tumor load) was much lower. The efficacy was accompanied by delayed histologic lesion development, increased apoptosis, and decreased proliferation without changes of T-antigen expression in the dorsolateral prostate of Se-treated mice. In another experiment, MSeA given to TRAMP mice from 10 or 16 weeks of age increased their survival to 50 weeks of age and delayed the time of death due to neuroendocrine carcinomas and other lesions in the prostate and seminal vesicle hypertrophy. Wild-type mice receiving MSeA from 10 weeks did not exhibit decreased body weights or genitourinary weight compared with the control mice and serum alanine aminotransferase (a sign of hepatic toxicity) was not increased. Therefore, these Se compounds selectively inhibit prostate carcinogenesis without toxicity or effects on the normal prostate.
Christensen and coworkers studied the interaction of MSeC with isoflavones in TRAMP mice (90). They did not observe efficacy of MSeC (at 3ppm Se in diet) against TRAMP tumor readouts (urogenital tract weight, lesion severity). They pointed out several differences from the studies done by our group. These include route/dose of MSeC delivery (15 µg Se per mouse throughout a day by diet vs. 75 µg Se per mouse as daily bolus dose) and mouse genetic background (C57/B/FVB hybrid vs. C57B background). Additionally, the small number of mice per group and inability to separate NE-Ca lineage from epithelial lesions limited their statistical power (90).
In a more recent study, we documented the inhibition of prostate specific Pten-knockout (KO)-driven carcinogenesis by MSeA in association with a super-activation of p53/p21-senescence axis as a preventive barrier to prostate tumorigenesis in vivo (Wang et al, submitted to Cancer Prevention Research, 2015). We utilized probasin-Cre (Pb-Cre) transgenic mice expressing Cre to delete Pten in prostate epithelium to investigate whether oral administration of MSeA inhibited AKT-driven prostate lesion severity and progression, relevant to human prostate cancer etiology. We treated Pten-KO and age-matched wild type male mice from 8 weeks of age with water or MSeA at a dose of 3 mg Se/kg body weight five times a week for 4 or 25 weeks. After 4 weeks, MSeA treatment significantly increased expression of P53 and P21Cip1 proteins and senescence-associated-β-galactosidase staining, and reduced the Ki-67 cell proliferation index in Pten KO prostate epithelium. After 25 weeks, MSeA administration significantly suppressed development of HG-PIN, reduced tumor weight, and prevented emergence of invasive carcinoma. Mechanistically, the long-term MSeA treatment not only sustained P53-mediated senescence, but also markedly reduced AKT phosphorylation and androgen receptor abundance in the Pten KO prostate. Importantly, these cellular and molecular changes were not observed in the prostate of wild type littermates treated with MSeA. Since P53 signaling is likely to be intact in HG-PIN compared to advanced PCa, the selective super-activation of P53-mediated senescence by MSeA in these mouse studies and the results of the aforementioned cell culture studies in normal fibroblasts indicating senescence induction by MSeA (83) (84), suggest a new mechanistic paradigm of cancer chemoprevention by strengthening a barrier to cancer progression through induction of irreversible senescence with additional suppression of oncogenic androgen receptor and AKT signaling. A comparison of the efficacy of MSeA and MSeC with SeMet remains to be determined regarding the specificity of effects via the p53-p21-senescence pathway.
5. “MOLECULAR TARGETS” OF NEXT-GEN SE IN CANCER MODELS
We also examined the impact of acute high dose Se treatments (i.e., daily single oral gavage of 2 mg Se per kilogram of body weight for 3 days) of female Sprague-Dawley rats bearing 1-methyl-1-nitrosourea-induced mammary carcinomas to increase the probability of detecting in vivo apoptosis and the associated gene/protein changes in the cancerous epithelial cells (91). Whereas control carcinomas doubled in volume in 3 days, MSeC and selenite treatments caused regression of approximately half of the carcinomas, accompanied by a 3- to 4-fold increase of morphologically detectable apoptosis and approximately 40% inhibition of 5-bromo-2’-deoxyuridine incorporation index of the cancerous epithelial cells. The mRNA levels of growth arrest-DNA damage inducible 34 (gadd34), gadd45, and gadd153 genes were not higher in the Se-treated carcinomas than in the gavage or diet restriction control groups contrary to expectation (59). The gadd34 and gadd153 proteins in the Se-treated carcinomas were localized in the nonepithelial stromal cells and not induced in the epithelial cancer cells. On the other hand, both Se forms decreased the expression of cyclin D1 and increased levels of P27Kip1 and c-Jun NH2-terminal kinase activation in a majority of the mammary carcinomas. The lack of induction of gadd genes in vivo by MSeA was confirmed in a human PCa xenograft model in athymic nude mice. Collectively, these experiments showed the induction of cancer epithelial cell apoptosis and inhibition of cell proliferation by Se in vivo through the potential involvement of cyclin D1, P27Kip1, and c-Jun NH2-terminal kinase pathways, but cast doubt on the three gadd genes as mediators of Se action in vivo.
Proteomic profiling
We have applied iTRAQ (isobaric tag for relative and absolute quantization [iTRAQ, Applied Biosystems])-proteomic approaches to profile protein changes of the TRAMP dorsolateral prostate and to characterize their modulation by MSeA and MSeC to identify potential molecular targets of Se (92). The expression of 75 proteins was significantly different between TRAMP and wild-type mice. MSeA mainly affected proteins related to prostate functional differentiation, androgen receptor signaling, protein (mis)folding, and endoplasmic reticulum-stress responses (e.g., GRP78), whereas MSeC affected proteins involved in phase II detoxification (e.g., GSTM1) or cytoprotection, and in stromal cells. Although MSeA and MSeC were about equally efficacious against tumor development in the TRAMP model, their distinct affected protein profiles suggest biological differences in their molecular targets.
We also analyzed the proteome signatures of prostate from mice treated with MSeA, MSeC, SeMet and selenite for hypothesis generation concerning their potential in vivo molecular targets and cancer risk modification (93). Nude mice bearing subcutaneous PC-3 xenografts were treated daily with each Se form (3 mg Se/kg) orally for 45 days. This analysis indicated that 72 proteins were significantly modulated by one or more Se forms. MSeA and MSeC each induced separate sets of tumor suppressor proteins and suppressed different onco-proteins. A similar spectrum of proteins was induced by selenite and MSeC which were related to energy metabolism (e.g., fatty-acid synthase), whereas those induced by SeMet included vimentin and heat-shock protein-70. While proteome changes induced by MSeA were associated with PCa risk reduction, desirable risk-reducing signatures induced by MSeC (e.g., inducing GSTM1 and increasing GPX-3) were counterbalanced by risk-promoting patterns shared with selenite and SeMet (e.g., inducing fatty acid synthase, a known metabolic oncoprotein for PCa). The balance of oncogenic vs. suppressor protein patterns in the prostate may impact the direction of PCa risk modification by a given Se form.
While methylselenol has been assumed an in vivo active anti-cancer metabolite pool (16,17), the proteomic data suggest unique potential molecular targets for each of these diverse chemoprevention-active Se forms with surprisingly little “protein targets” overlap between MSeA and MSeC. Together, the proteomic data suggest MSeA and MSeC are not interchangeable and point to a possible adverse PCa risk profile for MSeC through onco-proteins such as fatty acid synthase. These data, when considered in the framework of dynamic MSeA-MSeH redox cycling, would be reasonable depending on the entry point of the next-gen Se to fuel the redox cycle. Whether such findings are relevant for the mammary gland and other organ sites should be examined to assess their generalizability. Pre-IND standard toxicology and toxico-omic investigations of the tissues/organs exposed to these two Se forms in non-rodent mammalian species such as dog, pig, or primate may help to predict and inform the potential adverse impacts of each next-gen Se form for human translational consideration.
6. SPECIATION OF SE METABOLITES
Although extremely challenging technologically, the identification and quantitation of Se species in tissues and bodily fluids can be important to understanding their anti-cancer functions and hypothesis testing. In general, characterization of these chemicals in Se-enriched plants and microorganisms is less thorny due to their higher abundance than for animal specimens. The dominant chemical forms and their abundance in Se-enriched yeast, garlic, broccoli, carrot, onion, as well as unfortified grains, vegetables, meats and seafood have been documented (94). Currently, techniques such as high performance liquid chromatography (HPLC) and gas chromatography (GC) coupled with different mass spectrometry (MS) are routinely used for Se speciation and quantitation. Following earlier methods developed by Gromer and Gross for trapping methylselenol (80), procedures for trapping of volatile Se compounds in nitric acid or hydrogen peroxide have been developed and evaluated for the analysis of volatile metabolites such as dimethylselenide (DMSe) and dimethyl diselenide (95). Inductively coupled plasma mass spectrometry (ICP-MS) is the most sensitive and robust detection method for elemental selenium available to date. Selenium has 5 naturally occurring stable isotopes: 74Se, 76Se, 77Se, 78Se, and 80Se. 82Se has a very long half-life and, for practical purposes, can be considered to be stable. Their molar fractions in naturally occurring Se are 0.9%, 9.4%, 7.6%, 23.8%, 49.6% and 8.7%, respectively (96). These Se isotopes can be detected and quantitated by ICP-MS, but since ICP-MS provides only elemental information about Se compounds, it is effective only when the retention times of samples in analysis with ICP-MS coupled with HPLC/GC can be matched with those of certified Se standards. On the other hand, electrospray ionization, atmospheric pressure chemical ionization, and matrix-assisted laser desorption ionization-tandem mass spectrometry have been used for the identification of unknown Se metabolites, but these methods are limited by their detection sensitivity. HPLC/GC coupled with elemental and molecular MS can provide complementary data to increase accuracy of Se speciation (97,98).
Using stable isotopic tracing, Suzuki et al. pioneered analysis of the bioavailability and metabolism of MSeA by comparing the uptake, distribution, and metabolism of MSeA with selenite, selenate, and methylselenonic acid (99). They depleted the rats of endogenous Se with a single stable isotope (the most abundant stable isotope, 80Se as selenite) and then fed such rats a Se deficient diet (less than 0.02 µg/g) and Se-free distilled water for 10 days. Then four isotopic pure 82Se-selenite, 78Se-selenate, 77Se-MSeA and 76Se-methylselenonic acid simultaneously were administered by gavage, each at the dose of 25 µg Se/kg body weight. Analysis of serum, urine, and organs over various time points indicated that the overall availability of Se was, in decreasing order of magnitude, selenate (6+ valent) > selenite (4+) = MSeA (4+) > methylselenonic acid (6+). All 4 Se compounds, especially selenate, were efficiently transformed into selenosugar (in liver and urine), cellular glutathione peroxidase (cGPx, in liver, kidney, lung, and pancreas), extracellular glutathione peroxidase (eGPx, plasma SeGpx3) and selenoprotein P (in serum). The 6+valent Se (selenate and methylselenonic acid) were detected in serum, lung, pancreas, and urine in their intact forms, whereas 4+valent Se forms (MSeA and selenite) were not detected in the intact forms at all. Trimethylselenonium (TMSe) was detected in the lung, kidney, and urine from rats administered MSeA or methylselenonic acid, but not selenite and selenate. Overall, these data are consistent with MSeA producing MSeH, which in turn either is methylated to TMSe or is demethylated to selenide for the synthesis of selenosugar and selenoproteins (99).
Subsequently, the same group (100) compared the tissue distribution of Se by orally administering a mixture of 76Se-MSeC, 82Se-MSeA and 82Se-selenite (10 µg Se/kg each) to rats that had been depleted of natural abundance Se as described above (99). Three hours after administration, the Se in MSeC was taken up more efficiently by most organs, especially the pancreas and duodenum, than Se in MSeA and selenite, which delivered similar amounts of Se to most organs except kidney, liver, and spleen. In these three organs, these compounds were retained to the same extent as after MSeC administration. HPLC-ICP-MS detected the intact form of MSeC, but not MSeA and selenite in the liver supernatant, consistent with the previous report (99). The metabolic fates of these Se compounds in individuals adequate in Se are unknown.
Using similar approaches, availability and metabolism of SeMet and MSeC were studied by Ohta et al. using MSeA as a reference methylselenol source (101). A mixture of 77Se-SeMet, 76Se-MSeC, and 82Se-MSeA (each at 25 µg Se/kg) was orally administered to rats and urine and exhaled gas were analyzed by HPLC-ICP-MS and GC-ICP-MS, respectively. The proportion of 77Se-TMSe was much less than that of 76Se- and 82Se-TMSe in urine, suggesting that 77Se-SeMet was less efficiently metabolized to TMSe. In exhaled gas, there was significantly less DMSe originating from 77Se-SeMet than 76Se-DMSe and 82Se-DMSe originating from 76Se-MSeC and 82Se-MSeA, respectively. Since DMSe and TMSe are generally accepted as the metabolites of MSeH, the results suggested that both MSeA and MSeC were converted to MSeH more efficiently than SeMet. The dosages of Se compounds used in these studies (10–25µg Se/kg) were much less than those used in efficacy studies reported by us (3 mg Se/kg) and others. Taken together, these pioneering studies documented the different pharmacokinetic behaviors of next-gen Se compounds MSeA and MSeC compared to SeMet and selenite. These data were consistent with both MSeA and MSeC being able to generate MSeH in vivo, and indicated that MSeC could deliver more Se to target organ(s), partially in its parent form, than MSeA.
7. TRANSLATIONAL INVESTIGATIONS INVOLVING NEXT-GEN SE
Pre-IND toxicology for MSeC
Preclinical toxicology studies of MSeC, in both short and long term experiments, have been conducted by the National Cancer Institute (NCI) through its Division of Cancer Prevention Rapid Access to Preventive Intervention Development (RAPID) Program (102) and enabled IND approval for MSeC. In short term experiments, CD rats were treated by daily gavage at doses of 0, 0.5, 1.0, or 2.0 mg Se/kg/day (0, 3, 6, or 12 mg/m2/day), and beagle dogs at doses of 0, 0.15, 0.3, or 0.6 mg Se/kg/day (0, 3, 6, or 12 mg/m2/day) for 28 days. In rats, MSeC induced dose-related hepatomegaly in both sexes. Blood analyses and biochemistry routines showed mild anemia, thrombocytopenia, and elevated liver enzymes in high dose females only. Microscopic examination revealed hepatocellular degeneration (in high dose males and in females of all dose groups); arrested spermatogenesis (in high dose males); and atrophy of corpora lutea (in middle and high dose females). In dogs, MSeC induced mild anemia in middle and high dose males, and in high dose females. Microscopic lesions in dogs in the liver consisted of peliosis and vacuolar degeneration in high dose males and midzonal necrosis in males in all dose groups. Based on liver pathology seen in female rats in all dose groups, the no observed adverse effect level (NOAEL) for MSeC in rats was estimated to be less than 0.5 mg Se/kg/day. In dogs, the NOAEL for MSeC was estimated to be less than 0.15 mg Se/kg/day based on alterations in hematology parameters and liver morphology in male dogs in all dose groups. Taking the NOAEL of dogs as reference (~ 0.1 mg Se/kg), an equivalent value extrapolated allometrically (by factor of 2) to humans for a 70 kg person is 3,500 µg Se/day. We are not aware of similar studies for MSeA that would need to be done to obtain an IND.
Human pharmacokinetics study with MSeC
The first human single-dose pharmacokinetic study of MSeC was conducted by Marshall and colleagues (103). They randomized healthy male volunteers in double-blinded fashion to receive either a single oral dose of MSeC at one of three different concentrations or placebo. In the first step, five subjects received 400 µg of Se and one received placebo; in the second step, five subjects received 800 µg of Se and one received placebo; in the third step, five subjects received 1,200 µg of Se and one received placebo. The results show that the most distinct concentration curve is for the 1,200 µg dose, with an apparent dose-response pattern for lower doses over that of placebo. For the three MSeC dose cohorts, tmax were similar, ranging between 3 and 5 hours. The mean Se Cmax increased from 10 for placebo to 22.8, 30.75, and 63.2 ng/mL (~0.8 µM) in the low, medium, and high dose subjects, respectively. Similar studies would be conducted once IND status has been granted for MSeA and other next-gen Se.
8. NOVEL SYNTHETIC SE COMPOUNDS IN R&D PIPELINE
Since the early 1990s, el-Bayoumi and colleagues have generated synthetic aromatic selenocyanate compounds and evaluated their chemopreventive efficacy in many preclinical carcinogenesis models with clear efficacy (104). Their exemplary compound was 1,4-phenylenebis-(methylene)selenocyanate (p-XSC) and the NCI-Chemoprevention Branch supported plans for its further preclinical development (105). In spite of favorable subchronic dietary exposure safety profiles in rat models (106), NCI contract-supported toxicological studies in dogs showed unacceptable liver damage, abolishing further human translational consideration of this class of compounds
More recently, Sharma, Amin and colleagues have developed novel Se compounds through substitution of sulfur with Se in existing drugs/compounds of a number of anti-cancer functional drug categories with improved anti-cancer actions against a number of organ sites including melanoma and lung cancer (107–110). Additional novel drug approaches include synthesis of an isosteric Se analog of the PPARbeta/delta agonist GW501516 (111) and rational incorporation of Se into temozolomide that elicited superior in vitro antitumor activity associated with both apoptotic and autophagic cell death (112). Such agents are in early stage development and merit rigorous evaluations. Other investigators have attempted to conjugate redox-active Se to monoclonal antibodies in hope of enhancing targeted cancer delivery for greater therapeutic benefit, but the success of this approach is not yet clear (113).
9. CONCLUSIONS AND FUTURE DIRECTIONS
The outcomes of the four published randomized trials with SeMet or Se-yeast for prevention of prostate and lung cancer in Northern America have had a negative impact on the field of Se cancer research. We elaborated on two factors that, in our opinion, were the major reasons for the failure of these studies to detect preventive efficacy of SeMet or Se-yeast, namely inappropriate subject/patient populations selected for testing the anti-oxidant hypothesis and forms of Se chosen for human clinical trials that were ineffective in cell culture and animal models. Mechanistic studies have indicated that both Se forms and dosages are critical factors for chemoprevention efficacy, depending on their entry into two distinct Se metabolite pools. In cell culture models, the MSeA-MSeH redox pair exert many desirable attributes of cancer chemoprevention, including targeting of key signaling pathways, angiogenic switch regulators, immune surveillance, and invasion and metastasis molecules as well as sex hormone signaling in gender-specific cancers. Accumulating data support MSeC and MSeA as more promising candidates than SeMet for future clinical investigations of cancer chemopreventive efficacy. Proteomic profiling has uncovered more differences than similarities for the proteome affected by distinct next-gen Se forms. Based on lessons learned from the past Se clinical trials, it will be essential and necessary that efficacy validation and chronic safety evaluations (including diabetes risk of SeMet exposure) as well as the molecular targets and cellular pathways affected be thoroughly investigated in relevant preclinical animal models with respect to each of these effective next-gen Se to provide scientific rationale for future translational studies in humans. Mechanism-driven smaller Phase I/II trials should and must be carried out with next-gen Se agents that are preclinically proven safe and efficacious to guide decisions about future definitive Phase III chemoprevention efficacy trials.
Acknowledgments
We regret the inability to cite many worthy papers due to space limitations. This work was supported by US National Institutes of Health National Cancer Institute grant (R01 CA172169 to Lü, Deng and Bosland)
References
- 1.Yu SY, Zhu YJ, Li WG. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol Trace Elem Res. 1997;56:117–124. doi: 10.1007/BF02778987. [DOI] [PubMed] [Google Scholar]
- 2.Yu SY, Li WG, Zhu YJ, Yu WP, Hou C. Chemoprevention trial of human hepatitis with selenium supplementation in China. Biol Trace Elem Res. 1989;20:15–22. doi: 10.1007/BF02919094. [DOI] [PubMed] [Google Scholar]
- 3.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 4.Clark LC, Dalkin B, Krongrad A, Combs GF, Jr, Turnbull BW, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81:730–734. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 5.Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr, Slate EH, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 6.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 7.Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM, Jr, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 8.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall JR, Tangen CM, Sakr WA, Wood DP, Jr, Berry DL, et al. Phase III Trial of Selenium to Prevent Prostate Cancer in Men with High-grade Prostatic Intraepithelial Neoplasia: SWOG S9917. Cancer Prev Res (Phila) 2011;4:1761–1769. doi: 10.1158/1940-6207.CAPR-10-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Algotar AM, Stratton MS, Ahmann FR, Ranger-Moore J, Nagle RB, et al. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate. 2013;73:328–335. doi: 10.1002/pros.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karp DD, Lee SJ, Keller SM, Wright GS, Aisner S, et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: ECOG 5597. J Clin Oncol. 2013;31:4179–4187. doi: 10.1200/JCO.2013.49.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J Natl Cancer Inst. 2014;106:djt456. doi: 10.1093/jnci/djt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 15.Potter JD. The failure of cancer chemoprevention. Carcinogenesis. 2014;35:974–982. doi: 10.1093/carcin/bgu063. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Jiang C. Selenium and cancer chemoprevention: hypotheses integrating the actions of selenoproteins and selenium metabolites in epithelial and non-epithelial target cells. Antioxid Redox Signal. 2005;7:1715–1727. doi: 10.1089/ars.2005.7.1715. [DOI] [PubMed] [Google Scholar]
- 17.Ip C. Lessons from basic research in selenium and cancer prevention. J Nutr. 1998;128:1845–1854. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Bonorden MJ, Li GX, Lee HJ, Hu H, et al. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev Res (Phila) 2009;2:484–495. doi: 10.1158/1940-6207.CAPR-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Bayoumy K. The negative results of the SELECT study do not necessarily discredit the selenium-cancer prevention hypothesis. Nutr Cancer. 2009;61:285–286. doi: 10.1080/01635580902892829. [DOI] [PubMed] [Google Scholar]
- 20.Hatfield DL, Gladyshev VN. The Outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol Interv. 2009;9:18–21. doi: 10.1124/mi.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen MJ. Selenium and prostate cancer prevention: what next-if anything? Cancer Prev Res (Phila) 2014;7:781–785. doi: 10.1158/1940-6207.CAPR-14-0197. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Jiang C, Zhang J. Cancer Prevention with Selenium: costly lessons and difficult but bright future prospects. In: Kong A-NT, editor. Inflammation, Oxidative Stress and Cancer. CRC Press Taylor Francis; 2014. pp. 477–494. [Google Scholar]
- 23.Neve J. Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity. J Trace Elem Med Biol. 1995;9:65–73. doi: 10.1016/S0946-672X(11)80013-1. [DOI] [PubMed] [Google Scholar]
- 24.Food, Nutrition Board IoM, Selenium. Dietary references intakes for vitamin C, vitamin E, selenium and carotenoids. Washington DC: National Academy Press; 2000. pp. 284–324. [Google Scholar]
- 25.Xia Y, Hill KE, Li P, Xu J, Zhou D, et al. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am J Clin Nutr. 2010;92:525–531. doi: 10.3945/ajcn.2010.29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ervin RB, Wang CY, Wright JD, Kennedy-Stephenson J. Dietary intake of selected minerals for the United States population: 1999–2000. Adv Data. 2004:1–5. [PubMed] [Google Scholar]
- 27.Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003;52:2346–2352. doi: 10.2337/diabetes.52.9.2346. [DOI] [PubMed] [Google Scholar]
- 28.Niskar AS, Paschal DC, Kieszak SM, Flegal KM, Bowman B, et al. Serum selenium levels in the US population: Third National Health and Nutrition Examination Survey, 1988–1994. Biol Trace Elem Res. 2003;91:1–10. doi: 10.1385/BTER:91:1:1. [DOI] [PubMed] [Google Scholar]
- 29.Combs GF, Jr, Clark LC, Turnbull BW. An analysis of cancer prevention by selenium. Biofactors. 2001;14:153–159. doi: 10.1002/biof.5520140120. [DOI] [PubMed] [Google Scholar]
- 30.Combs GF, Jr, Watts JC, Jackson MI, Johnson LK, Zeng H, et al. Determinants of selenium status in healthy adults. Nutr J. 2011;10:75. doi: 10.1186/1475-2891-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richie JP, Jr, Das A, Calcagnotto AM, Sinha R, Neidig W, et al. Comparative effects of two different forms of selenium on oxidative stress biomarkers in healthy men: a randomized clinical trial. Cancer Prev Res (Phila) 2014;7:796–804. doi: 10.1158/1940-6207.CAPR-14-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redman C, Xu MJ, Peng YM, Scott JA, Payne C, et al. Involvement of polyamines in selenomethionine induced apoptosis and mitotic alterations in human tumor cells. Carcinogenesis. 1997;18:1195–1202. doi: 10.1093/carcin/18.6.1195. [DOI] [PubMed] [Google Scholar]
- 33.Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer Epidemiol Biomarkers Prev. 2000;9:1171–1182. [PubMed] [Google Scholar]
- 34.Chigbrow M, Nelson M. Inhibition of mitotic cyclin B and cdc2 kinase activity by selenomethionine in synchronized colon cancer cells. Anticancer Drugs. 2001;12:43–50. doi: 10.1097/00001813-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Goel A, Fuerst F, Hotchkiss E, Boland CR. Selenomethionine induces p53 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cancer Biol Ther. 2006;5:529–535. doi: 10.4161/cbt.5.5.2654. [DOI] [PubMed] [Google Scholar]
- 36.Fakih MG, Pendyala L, Smith PF, Creaven PJ, Reid ME, et al. A phase I and pharmacokinetic study of fixed-dose selenomethionine and irinotecan in solid tumors. Clin Cancer Res. 2006;12:1237–1244. doi: 10.1158/1078-0432.CCR-05-2004. [DOI] [PubMed] [Google Scholar]
- 37.Combs GF, Jr, Jackson MI, Watts JC, Johnson LK, Zeng H, et al. Differential responses to selenomethionine supplementation by sex and genotype in healthy adults. Br J Nutr. 2012;107:1514–1525. doi: 10.1017/S0007114511004715. [DOI] [PubMed] [Google Scholar]
- 38.McCormick DL, Rao KV, Johnson WD, Bosland MC, Lubet RA, et al. Null activity of selenium and vitamin e as cancer chemopreventive agents in the rat prostate. Cancer Prev Res (Phila) 2010;3:381–392. doi: 10.1158/1940-6207.CAPR-09-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozten N, Horton L, Lasano S, Bosland MC. Selenomethionine and alpha-tocopherol do not inhibit prostate carcinogenesis in the testosterone plus estradiol-treated NBL rat model. Cancer Prev Res (Phila) 2010;3:371–380. doi: 10.1158/1940-6207.CAPR-09-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozten N, Schlicht M, Diamond AM, Bosland MC. L-selenomethionine does not protect against testosterone plus 17beta-estradiol-induced oxidative stress and preneoplastic lesions in the prostate of NBL rats. Nutr Cancer. 2014;66:825–834. doi: 10.1080/01635581.2014.904907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corcoran NM, Najdovska M, Costello AJ. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J Urol. 2004;171:907–910. doi: 10.1097/01.ju.0000092859.16817.8e. [DOI] [PubMed] [Google Scholar]
- 42.Li GX, Lee HJ, Wang Z, Hu H, Liao JD, et al. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis. 2008;29:1005–1012. doi: 10.1093/carcin/bgn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan L, Demars LC. Dietary supplementation with methylseleninic acid, but not selenomethionine, reduces spontaneous metastasis of Lewis lung carcinoma in mice. Int J Cancer. 2011 doi: 10.1002/ijc.27355. [DOI] [PubMed] [Google Scholar]
- 44.Combs GF, Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998;79:179–192. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 45.Lu J, Hu H, Jiang C. Regulation of signaling pathways by selenium in cancer. In: YJD Surh Z, Cadenas E, Packer L, editors. Dietary Modulation of Cell Signaling pathways (Book) CRC Press; 2009. p. 42. [Google Scholar]
- 46.Ip C, Dong Y, Ganther HE. New concepts in selenium chemoprevention. Cancer Metastasis Rev. 2002;21:281–289. doi: 10.1023/a:1021263027659. [DOI] [PubMed] [Google Scholar]
- 47.Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN. Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci. 2014;39:112–120. doi: 10.1016/j.tibs.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luchman HA, Villemaire ML, Bismar TA, Carlson BA, Jirik FR. Prostate epithelium-specific deletion of the selenocysteine tRNA gene Trsp leads to early onset intraepithelial neoplasia. Am J Pathol. 2014;184:871–877. doi: 10.1016/j.ajpath.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999;20:1657–1666. doi: 10.1093/carcin/20.9.1657. [DOI] [PubMed] [Google Scholar]
- 50.Ip C, Ganther HE. Activity of methylated forms of selenium in cancer prevention. Cancer Res. 1990;50:1206–1211. [PubMed] [Google Scholar]
- 51.Ip C, Hayes C, Budnick RM, Ganther HE. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Res. 1991;51:595–600. [PubMed] [Google Scholar]
- 52.Ip C, Vadhanavikit S, Ganther H. Cancer chemoprevention by aliphatic selenocyanates: effect of chain length on inhibition of mammary tumors and DMBA adducts. Carcinogenesis. 1995;16:35–38. [PubMed] [Google Scholar]
- 53.Ip C, Zhu Z, Thompson HJ, Lisk D, Ganther HE. Chemoprevention of mammary cancer with Se-allylselenocysteine and other selenoamino acids in the rat. Anticancer Res. 1999;19:2875–2880. [PubMed] [Google Scholar]
- 54.Lu J, Jiang C, Kaeck M, Ganther H, Vadhanavikit S, et al. Dissociation of the genotoxic and growth inhibitory effects of selenium. Biochem Pharmacol. 1995;50:213–219. doi: 10.1016/0006-2952(95)00119-k. [DOI] [PubMed] [Google Scholar]
- 55.Lu J. Apoptosis and angiogenesis in cancer prevention by selenium. Adv Exp Med Biol. 2001;492:131–145. doi: 10.1007/978-1-4615-1283-7_11. [DOI] [PubMed] [Google Scholar]
- 56.Zhao R, Xiang N, Domann FE, Zhong W. Expression of p53 enhances selenite-induced superoxide production and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:2296–2304. doi: 10.1158/0008-5472.CAN-05-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu H, Jiang C, Schuster T, Li GX, Daniel PT, et al. Inorganic selenium sensitizes prostate cancer cells to TRAIL-induced apoptosis through superoxide/p53/Bax-mediated activation of mitochondrial pathway. Mol Cancer Ther. 2006;5:1873–1882. doi: 10.1158/1535-7163.MCT-06-0063. [DOI] [PubMed] [Google Scholar]
- 58.Lu J, Pei H, Ip C, Lisk DJ, Ganther H, et al. Effect on an aqueous extract of selenium-enriched garlic on in vitro markers and in vivo efficacy in cancer prevention. Carcinogenesis. 1996;17:1903–1907. doi: 10.1093/carcin/17.9.1903. [DOI] [PubMed] [Google Scholar]
- 59.Kaeck M, Lu J, Strange R, Ip C, Ganther HE, et al. Differential induction of growth arrest inducible genes by selenium compounds. Biochem Pharmacol. 1997;53:921–926. doi: 10.1016/s0006-2952(97)00103-2. [DOI] [PubMed] [Google Scholar]
- 60.Jiang C, Wang Z, Ganther H, Lu J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol Cancer Ther. 2002;1:1059–1066. [PubMed] [Google Scholar]
- 61.Wang Z, Jiang C, Ganther H, Lu J. Antimitogenic and proapoptotic activities of methylseleninic acid in vascular endothelial cells and associated effects on PI3K-AKT, ERK, JNK and p38 MAPK signaling. Cancer Res. 2001;61:7171–7178. [PubMed] [Google Scholar]
- 62.Wang Z, Jiang C, Lu J. Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Mol Carcinog. 2002;34:113–120. doi: 10.1002/mc.10056. [DOI] [PubMed] [Google Scholar]
- 63.Zhu Z, Jiang W, Ganther HE, Thompson HJ. Mechanisms of cell cycle arrest by methylseleninic acid. Cancer Res. 2002;62:156–164. [PubMed] [Google Scholar]
- 64.Wang L, Hu H, Wang Z, Xiong H, Cheng Y, et al. Methylseleninic acid suppresses pancreatic cancer growth involving multiple pathways. Nutr Cancer. 2014;66:295–307. doi: 10.1080/01635581.2014.868911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sinha R, Medina D. Inhibition of cdk2 kinase activity by methylselenocysteine in synchronized mouse mammary epithelial tumor cells. Carcinogenesis. 1997;18:1541–1547. doi: 10.1093/carcin/18.8.1541. [DOI] [PubMed] [Google Scholar]
- 66.Sinha R, Kiley SC, Lu JX, Thompson HJ, Moraes R, et al. Effects of methylselenocysteine on PKC activity, cdk2 phosphorylation and gadd gene expression in synchronized mouse mammary epithelial tumor cells. Cancer Lett. 1999;146:135–145. doi: 10.1016/s0304-3835(99)00250-5. [DOI] [PubMed] [Google Scholar]
- 67.Jiang C, Ganther H, Lu J. Monomethyl selenium--specific inhibition of MMP-2 and VEGF expression: implications for angiogenic switch regulation. Mol Carcinog. 2000;29:236–250. doi: 10.1002/1098-2744(200012)29:4<236::aid-mc1006>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 68.Jiang C, Jiang W, Ip C, Ganther H, Lu J. Selenium-induced inhibition of angiogenesis in mammary cancer at chemopreventive levels of intake. Mol Carcinog. 1999;26:213–225. doi: 10.1002/(sici)1098-2744(199912)26:4<213::aid-mc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 69.Cho SD, Jiang C, Malewicz B, Dong Y, Young CY, et al. Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Mol Cancer Ther. 2004;3:605–611. [PubMed] [Google Scholar]
- 70.Dong Y, Lee SO, Zhang H, Marshall J, Gao AC, et al. Prostate specific antigen expression is down-regulated by selenium through disruption of androgen receptor signaling. Cancer Res. 2004;64:19–22. doi: 10.1158/0008-5472.can-03-2789. [DOI] [PubMed] [Google Scholar]
- 71.Zhao H, Whitfield ML, Xu T, Botstein D, Brooks JD. Diverse effects of methylseleninic acid on the transcriptional program of human prostate cancer cells. Mol Biol Cell. 2004;15:506–519. doi: 10.1091/mbc.E03-07-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SO, Nadiminty N, Wu XX, Lou W, Dong Y, et al. Selenium disrupts estrogen signaling by altering estrogen receptor expression and ligand binding in human breast cancer cells. Cancer Res. 2005;65:3487–3492. doi: 10.1158/0008-5472.CAN-04-3267. [DOI] [PubMed] [Google Scholar]
- 73.Shah YM, Kaul A, Dong Y, Ip C, Rowan BG. Attenuation of estrogen receptor alpha (ERalpha) signaling by selenium in breast cancer cells via downregulation of ERalpha gene expression. Breast Cancer Res Treat. 2005;92:239–250. doi: 10.1007/s10549-005-3203-5. [DOI] [PubMed] [Google Scholar]
- 74.Shah YM, Al-Dhaheri M, Dong Y, Ip C, Jones FE, et al. Selenium disrupts estrogen receptor (alpha) signaling and potentiates tamoxifen antagonism in endometrial cancer cells and tamoxifen-resistant breast cancer cells. Mol Cancer Ther. 2005;4:1239–1249. doi: 10.1158/1535-7163.MCT-05-0046. [DOI] [PubMed] [Google Scholar]
- 75.Cai L, Mu LN, Lu H, Lu QY, You NC, et al. Dietary selenium intake and genetic polymorphisms of the GSTP1 and p53 genes on the risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:294–300. doi: 10.1158/1055-9965.EPI-05-0680. [DOI] [PubMed] [Google Scholar]
- 76.Gao R, Zhao L, Liu X, Rowan BG, Wabitsch M, et al. Methylseleninic acid is a novel suppressor of aromatase expression. J Endocrinol. 2012;212:199–205. doi: 10.1530/JOE-11-0363. [DOI] [PubMed] [Google Scholar]
- 77.Hu H, Li GX, Wang L, Watts J, Combs GF, Jr, et al. Methylseleninic acid enhances taxane drug efficacy against human prostate cancer and down-regulates antiapoptotic proteins Bcl-XL and survivin. Clin Cancer Res. 2008;14:1150–1158. doi: 10.1158/1078-0432.CCR-07-4037. [DOI] [PubMed] [Google Scholar]
- 78.Guo X, Yin S, Dong Y, Fan L, Ye M, et al. Enhanced apoptotic effects by the combination of curcumin and methylseleninic acid: potential role of Mcl-1 and FAK. Mol Carcinog. 2013;52:879–889. doi: 10.1002/mc.21933. [DOI] [PubMed] [Google Scholar]
- 79.Yin S, Dong Y, Li J, Fan L, Wang L, et al. Methylseleninic acid potentiates multiple types of cancer cells to ABT-737-induced apoptosis by targeting Mcl-1 and Bad. Apoptosis. 2012;17:388–399. doi: 10.1007/s10495-011-0687-9. [DOI] [PubMed] [Google Scholar]
- 80.Gromer S, Gross JH. Methylseleninate is a substrate rather than an inhibitor of mammalian thioredoxin reductase. Implications for the antitumor effects of selenium. J Biol Chem. 2002;277:9701–9706. doi: 10.1074/jbc.M109234200. [DOI] [PubMed] [Google Scholar]
- 81.Gundimeda U, Schiffman JE, Chhabra D, Wong J, Wu A, et al. Locally generated methylseleninic acid induces specific inactivation of protein kinase C isoenzymes: relevance to selenium-induced apoptosis in prostate cancer cells. J Biol Chem. 2008;283:34519–34531. doi: 10.1074/jbc.M807007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gundimeda U, Schiffman JE, Gottlieb SN, Roth BI, Gopalakrishna R. Negation of the cancer-preventive actions of selenium by over-expression of protein kinase Cepsilon and selenoprotein thioredoxin reductase. Carcinogenesis. 2009;30:1553–1561. doi: 10.1093/carcin/bgp164. [DOI] [PubMed] [Google Scholar]
- 83.Wu M, Kang MM, Schoene NW, Cheng WH. Selenium compounds activate early barriers of tumorigenesis. J Biol Chem. 2010;285:12055–12062. doi: 10.1074/jbc.M109.088781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu M, Wu RT, Wang TT, Cheng WH. Role for p53 in selenium-induced senescence. J Agric Food Chem. 2011;59:11882–11887. doi: 10.1021/jf203012a. [DOI] [PubMed] [Google Scholar]
- 85.Hagemann-Jensen M, Uhlenbrock F, Kehlet S, Andresen L, Gabel-Jensen C, et al. The selenium metabolite methylselenol regulates the expression of ligands that trigger immune activation through the lymphocyte receptor NKG2D. J Biol Chem. 2014;289:31576–31590. doi: 10.1074/jbc.M114.591537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- 87.Ip C, Hayes C. Tissue selenium levels in selenium-supplemented rats and their relevance in mammary cancer protection. Carcinogenesis. 1989;10:921–925. doi: 10.1093/carcin/10.5.921. [DOI] [PubMed] [Google Scholar]
- 88.Qi Y, Fu X, Xiong Z, Zhang H, Hill SM, et al. Methylseleninic acid enhances paclitaxel efficacy for the treatment of triple-negative breast cancer. PLoS One. 2012;7:e31539. doi: 10.1371/journal.pone.0031539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lindshield BL, Ford NA, Canene-Adams K, Diamond AM, Wallig MA, et al. Selenium, but not lycopene or vitamin E, decreases growth of transplantable dunning R3327-H rat prostate tumors. PLoS One. 2010;5:e10423. doi: 10.1371/journal.pone.0010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Christensen MJ, Quiner TE, Nakken HL, Lephart ED, Eggett DL, et al. Combination effects of dietary soy and methylselenocysteine in a mouse model of prostate cancer. Prostate. 2013;73:986–995. doi: 10.1002/pros.22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang W, Jiang C, Pei H, Wang L, Zhang J, et al. In vivo molecular mediators of cancer growth suppression and apoptosis by selenium in mammary and prostate models: lack of involvement of gadd genes. Mol Cancer Ther. 2009;8:682–691. doi: 10.1158/1535-7163.MCT-08-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Wang L, Anderson LB, Witthuhn B, Xu Y, et al. Proteomic profiling of potential molecular targets of methyl-selenium compounds in the transgenic adenocarcinoma of mouse prostate model. Cancer Prev Res (Phila) 2010;3:994–1006. doi: 10.1158/1940-6207.CAPR-09-0261. [DOI] [PubMed] [Google Scholar]
- 93.Zhang J, Wang L, Li G, Anderson LB, Xu Y, et al. Mouse prostate proteomes are differentially altered by supranutritional intake of four selenium compounds. Nutr Cancer. 2011;63:778–789. doi: 10.1080/01635581.2011.563029. [DOI] [PubMed] [Google Scholar]
- 94.Fairweather-Tait SJ, Collings R, Hurst R. Selenium bioavailability: current knowledge and future research requirements. Am J Clin Nutr. 2010;91:1484S–1491S. doi: 10.3945/ajcn.2010.28674J. [DOI] [PubMed] [Google Scholar]
- 95.Winkel L, Feldmann J, Meharg AA. Quantitative and qualitative trapping of volatile methylated selenium species entrained through nitric acid. Environmental science & technology. 2010;44:382–387. doi: 10.1021/es902345m. [DOI] [PubMed] [Google Scholar]
- 96.De Laeter JR, Bohlke JK, De Bievre P, Hidaka H, Peiser HS, et al. Atomic weights of the elements: Review 2000 - (IUPAC technical report) Pure and Applied Chemistry. 2003;75:683–800. [Google Scholar]
- 97.Ogra Y, Anan Y. Selenometabolomics: Identification of selenometabolites and specification of their biological significance by complementary use of elemental and molecular mass spectrometry. Journal of Analytical Atomic Spectrometry. 2009;24:1477–1488. [Google Scholar]
- 98.Pedrero Z, Madrid Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: a review. Analytica chimica acta. 2009;634:135–152. doi: 10.1016/j.aca.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki KT, Ohta Y, Suzuki N. Availability and metabolism of (77)Se-methylseleninic acid compared simultaneously with those of three related selenocompounds. Toxicology and Applied Pharmacology. 2006;217:51–62. doi: 10.1016/j.taap.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Suzuki KT, Tsuji Y, Ohta Y, Suzuki N. Preferential organ distribution of methylselenol source Se-methylselenocysteine relative to methylseleninic acid. Toxicology and Applied Pharmacology. 2008;227:76–83. doi: 10.1016/j.taap.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 101.Ohta Y, Kobayashi Y, Konishi S, Hirano S. Speciation analysis of selenium metabolites in urine and breath by HPLC- and GC-inductively coupled plasma-MS after administration of selenomethionine and methylselenocysteine to rats. Chemical research in toxicology. 2009;22:1795–1801. doi: 10.1021/tx900202m. [DOI] [PubMed] [Google Scholar]
- 102.Johnson WD, Morrissey RL, Kapetanovic I, Crowell JA, McCormick DL. Subchronic oral toxicity studies of Se-methylselenocysteine, an organoselenium compound for breast cancer prevention. Food Chem Toxicol. 2008;46:1068–1078. doi: 10.1016/j.fct.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marshall JR, Ip C, Romano K, Fetterly G, Fakih M, et al. Methyl selenocysteine: single-dose pharmacokinetics in men. Cancer Prev Res (Phila) 2011;4:1938–1944. doi: 10.1158/1940-6207.CAPR-10-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.el-Bayoumy K, Upadhyaya P, Chae YH, Sohn OS, Rao CV, et al. Chemoprevention of cancer by organoselenium compounds. J Cell Biochem Suppl. 1995;22:92–100. doi: 10.1002/jcb.240590812. [DOI] [PubMed] [Google Scholar]
- 105.Committee NCI. Clinical development plan: 1,4-phenylenebis-(methylene)selenocyanate (p-XSC). NCI-DCPC Chemoprevention Branch and Agent Development Committee Report. Journal of Cellular Biochemistry. 1996;26S:8. [PubMed] [Google Scholar]
- 106.Conaway CC, Upadhyaya P, Meschter CL, Kurtzke C, Marcus LA, et al. Subchronic toxicity of benzyl selenocyanate and 1,4-phenylenebis(methylene)selenocyanate in F344 rats. Fundam Appl Toxicol. 1992;19:563–574. doi: 10.1016/0272-0590(92)90095-y. [DOI] [PubMed] [Google Scholar]
- 107.Sharma AK, Amin S. Post SELECT: selenium on trial. Future Med Chem. 2013;5:163–174. doi: 10.4155/fmc.12.203. [DOI] [PubMed] [Google Scholar]
- 108.Sharma AK, Sharma A, Desai D, Madhunapantula SV, Huh SJ, et al. Synthesis and anticancer activity comparison of phenylalkyl isoselenocyanates with corresponding naturally occurring and synthetic isothiocyanates. J Med Chem. 2008;51:7820–7826. doi: 10.1021/jm800993r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma A, Sharma AK, Madhunapantula SV, Desai D, Huh SJ, et al. Targeting Akt3 signaling in malignant melanoma using isoselenocyanates. Clin Cancer Res. 2009;15:1674–1685. doi: 10.1158/1078-0432.CCR-08-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crampsie MA, Pandey MK, Desai D, Spallholz J, Amin S, et al. Phenylalkyl isoselenocyanates vs phenylalkyl isothiocyanates: thiol reactivity and its implications. Chem Biol Interact. 2012;200:28–37. doi: 10.1016/j.cbi.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharma AK, Sk UH, He P, Peters JM, Amin S. Synthesis of isosteric selenium analog of the PPARbeta/delta agonist GW501516 and comparison of biological activity. Bioorg Med Chem Lett. 2010;20:4050–4052. doi: 10.1016/j.bmcl.2010.05.094. [DOI] [PubMed] [Google Scholar]
- 112.Cheng Y, Sk UH, Zhang Y, Ren X, Zhang L, et al. Rational incorporation of selenium into temozolomide elicits superior antitumor activity associated with both apoptotic and autophagic cell death. PLoS One. 2012;7:e35104. doi: 10.1371/journal.pone.0035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Misra S, Boylan M, Selvam A, Spallholz JE, Bjornstedt M. Redox-active selenium compounds--from toxicity and cell death to cancer treatment. Nutrients. 2015;7:3536–3556. doi: 10.3390/nu7053536. [DOI] [PMC free article] [PubMed] [Google Scholar]