Abstract
Proximity assays are immunohistochemical tools that utilise two or more DNA-tagged aptamers or antibodies binding in close proximity to the same protein or protein complex. Amplification by PCR or isothermal methods and hybridisation of a labelled probe to its DNA target generates a signal that enables sensitive and robust detection of proteins, protein modifications or protein–protein interactions. Assays can be carried out in homogeneous or solid phase formats and in situ assays can visualise single protein molecules or complexes with high spatial accuracy. These properties highlight the potential of proximity assays in research, diagnostic, pharmacological and many other applications that require sensitive, specific and accurate assessments of protein expression.
Keywords: Immunoassays, Immuno-PCR, Proximity ligation assay, Proximity extension assay, In situ assays
1. Introduction
The remarkable advances made over the last fifty years or so in all areas of the life sciences, medicine, diagnostics, forensics and biotechnology are inconceivable without the contributions from two key technologies: the polymerase chain reaction (PCR) for the detection of nucleic acids and antibody-based methods for the detection of proteins.
-
•
PCR is typified by its exquisite sensitivity and simplicity of use, for example the ease with which specific primers can be synthesised and modified. These properties have led to the widespread use of PCR and its complement, reverse transcription (RT)-PCR, for the analysis of mutations, SNPs and DNA methylation, the analysis of gene expression, as well as a pervasive presence in diagnostic assays aimed at identifying pathogens [1]. The introduction of real-time quantitative PCR (qPCR) [2], [3], [4], which uses fluorescence to detect PCR amplicons provided a simple and reproducible method for the detection of nucleic acids and, crucially, affords the very large dynamic range required for accurate quantification of mRNA.
-
•
Antibodies are characterised by their diversity, specificity and ability to bind to target epitopes in complex biological samples such as serum and whole cell lysates. They are used in a wide range of immunoassays, e.g. the enzyme-linked immunosorbent assay (ELISA) [5], which measure signals emanating from the affinity interactions of antibodies with their target molecules. Antibodies are also an essential component of flow cytometry, which allows the analysis of the expression of cell surface and intracellular molecules, characterisation and definition of different cell types in heterogeneous cell populations, assessment of the purity of isolated subpopulations, and analysis of cell size and volume. This has enabled the detailed study of cellular protein expression, location, modification and interaction [6], the discovery of protein biomarkers in serum and plasma for diagnostic applications such as early detection and monitoring of disease [7] and the rapid and specific detection of pathogen-specific proteins [8] together with the emergence of antibody-derived drug-conjugate molecules as promising next generation therapeutics [9].
The ever-increasing availability of new antibodies continues to expand the potential of the immunohistochemical repertoire. At the same time, there has been a continuous stream of improvements and novel developments of nucleic-acid detection methods, including the emergence of isothermal amplification methods such as rolling circle amplification (RCA) [10]. The combination of these technologies, leading first to the development of immuno-PCR (iPCR) and, more recently proximity ligation (PLA) and extension (PEA) assays, couples the detection specificity of the antibody with the amplification power of PCR or RCA. This arsenal is beginning to provide researchers with a powerful tool for the detection and quantification of cellular, pathogen and GMO-specific proteins as well as diagnostic biomarkers [11]. This emergence of proximity assays into the main stream of proteomic research is reflected in the number of papers citing the technology, which have increased fourfold between 2010 and 2014 from 41 to 156, with 55 papers already published in 2015.
2. Immuno-PCR
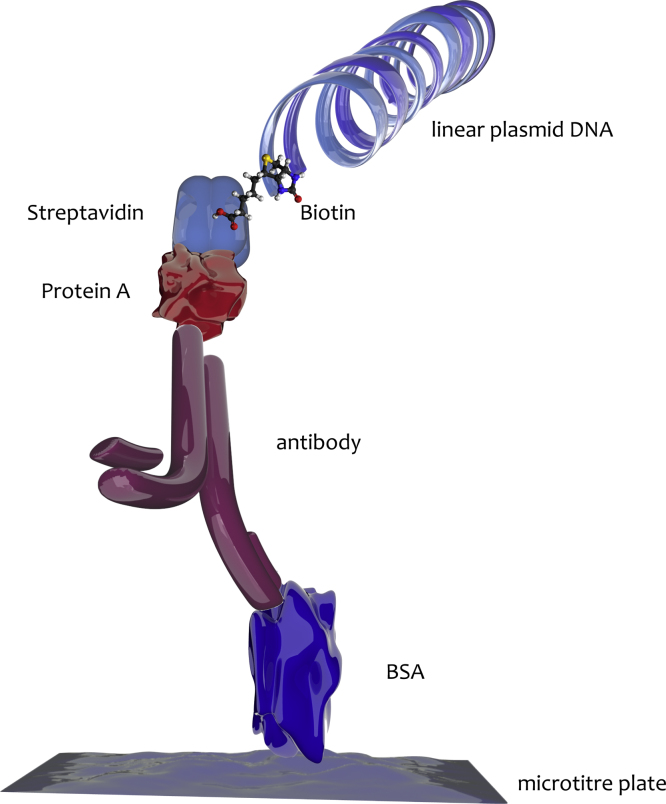
The original iPCR, which was first described in 1992 [12], involved amplification of a biotinylated, linear plasmid DNA linked to antigen/monoclonal antibody complexes immobilised on microtiterplate wells through a streptavidin-protein A chimera (Fig. 1). This modification significantly enhanced the sensitivity of an equivalent ELISA, permitting the detection of as few as several hundred targets by means of ethidium bromide-stained agarose gel electrophoresis. Additional changes created a more universal iPCR by substituting the fusion protein with commercially available biotinylated secondary antibodies, thus circumventing the variability and lack of specificity associated with the use of protein A [13]. Although assay throughput and sensitivity was increased further when readout by gel electrophoresis was replaced with fluorogenic PCR-ELISA [14], iPCR still required time-intensive and laborious post-PCR analysis. This was addressed by using qPCR to detect antigen/antibody complexes, which simplified iPCR by reducing the number of handling steps and, crucially, increased the dynamic range of the assay [15], [16]. Eventually, the most advantageous assay format was identified as consisting of a sandwich assemblage: a capture antibody is adsorbed directly to the surface of a PCR plate well, sample and detection antibody, which is coupled to a DNA-label, are premixed and transferred to the PCR plate [17]. At the time, the marker DNA was covalently coupled to the antibody, but since the covalent conjugation of oligonucleotides to antibodies can be difficult and time consuming, this has now been largely replaced by a combination of biotinylated antibodies and streptavidin-linked oligonucleotides. Today, iPCR in its various manifestations has become a robust method that provides the specificity and sensitivity required e.g. for assessing the success of novel drug design [18] or measuring the pharmacokinetics [19] and toxicokinetics [20] of drug metabolism. It has also been used for the detection of protein biomarkers of cancers [21], [22], [23], [24], [25] and viral infectious agents [26], [27]. Chimera Biotec (http://www.chimera-biotec.com) is the best-known provider of iPCR-based assays and assay development services with numerous applications targeting many kinds of macromolecular analyte.
Fig. 1.
The original iPCR made use of a recombinant streptavidin-protein A chimera with bispecific affinity for DNA and antibodies to link linear plasmid DNA to an antibody specific for bovine serum albumin (BSA), which was immobilised on the surface of microtitre wells. Binding of the antibody to BSA resulted in a specific antigen–antibody–DNA conjugate that was detected by agarose gel electrophoresis after PCR amplification with plasmid-specific primers.
3. Proximity ligation assays
Arguably the main drawbacks of iPCR are its non-homogeneous nature, which requires extensive washing steps to ensure minimal background signal. Proximity assays address this issue and the first of these, PLA, was first demonstrated in 2002 [28]. At first, PLA made use of two DNA aptamers [29], which bind their targets with affinities and specificities that are comparable to those of monoclonal antibodies [30] and can be designed so that they only require a single epitope on a protein surface [31]. However, difficulties with aptamer design and the availability of a vast pool of commercial antibodies has resulted in antibody-based PLAs becoming the most popular way of implementing this assay [32]. Today, the most common method uses two antibodies, with the requirement for a dual binding event making a false positive result less likely and thus reducing background noise.
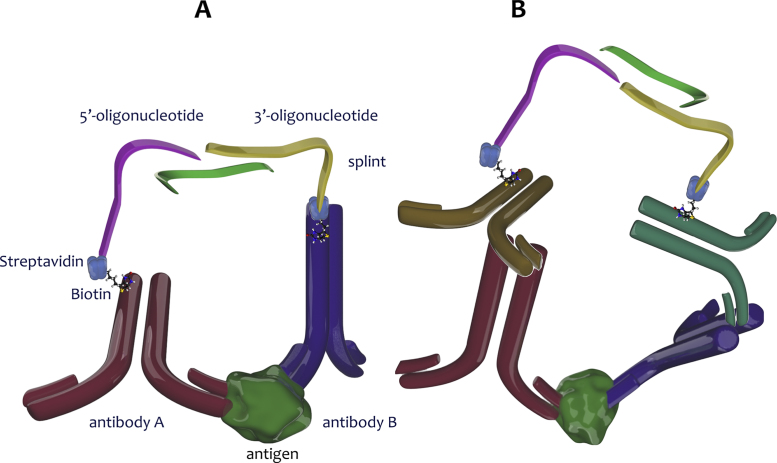
PLA probes are assembled either through noncovalent attachment of biotinylated oligonucleotides to streptavidin and subsequent interaction of that complex with biotinylated antibodies [33] or, more commonly today, through generation of oligonucleotides covalently attached to streptavidin at either their 5′- or 3′-ends, allowing them to interact directly with biotinylated antibodies. PLA can use either monoclonal or polyclonal antibodies, as well as a combination of the two. There are two alternative approaches for detecting the antibody/antigen interaction: one uses direct primary antibody conjugation (Fig. 2A), the other indirect scheme uses a secondary antibody linked to DNA for detection (Fig. 2B). It is also possible to conjugate oligonucleotides directly to Fab fragments, which improves the dimensional detection limit of PLA [34]. At its simplest, a single biotinylated monoclonal antibody can be divided into two groups for conjugation with a 5′- or 3′-oligonucleotide, respectively (Fig. 3A). This design is applicable to antigen targets assembled in multimeric formats such as protein homodimers and repeated motifs expressed on the surfaces of virions. The subsequent workflow is both simple and rapid and following a 60 min annealing of antibodies, antigen and splint in a microtitre plate (Fig. 3B), a brief 10 min ligation (Fig. 3C) generates amplifiable DNA templates (Fig. 3D) that can be detected using a number of different readout formats. Fig. 3E shows results obtained using a standard qPCR thermal cycler (CFX Connect, Biorad, Hercules, CA, USA) or a digital PCR (Constellation, Formulatrix, Bedford, MA, USA) setup. In addition, flow cytometry [35], loop-mediated isothermal amplification [36] or DNA sequencing [37], [38] have also been employed. The indirect form of PLA follows the same principle, except that unmodified primary antibodies are detected with secondary antibodies that are conjugated to the DNA strands.
Fig. 2.
Direct and indirect PLA. (A) Biotinylated antibodies bind pairwise to adjacent epitopes on target proteins. This brings the two streptavidin-oligonucleotide tails, one coupled through its 5′-end, the other through its 3′-end, into close proximity. The connector oligonucleotide (splint) hybridises to both oligonucleotides, resulting in adjacent free 3′-OH and 5′-phosphate moieties. The gap is ligated and the resulting continuous DNA strand can be amplified and detected. (B) The indirect form of PLA follows the same principle, except that unmodified primary antibodies are detected with secondary antibodies that are conjugated to the DNA strands.
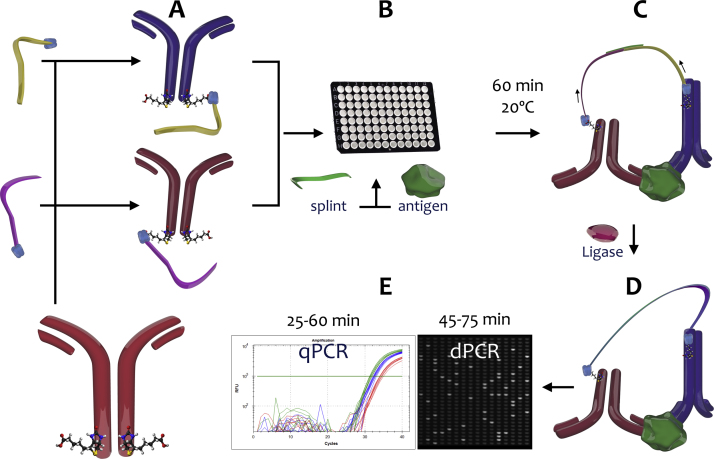
Fig. 3.
PLA workflow. (A) Oligonucleotides synthesised with a streptavidin molecule at their 5′- or 3′-ends are combined with biotinylated antibodies to form proximity probes. (B) Probes, sample and splint are combined and both probes bind simultaneously to their epitopes on the target antigen, if present, and the 5′- and 3′-oligonucleotides are joined by the splint. (C) DNA ligase connects the gap and thus joins the two oligonucleotides. (D) This generates a full length DNA amplicon that can be amplified and detected by several methods. (E) Detection by qPCR or dPCR is shown.
The intra-assay coefficients of variation (CVs) with qPCR as readout range from less than 10% to greater than 30% [39]. This has been improved by the development of a digital PLA (dPLA) based on amplified single molecule detection, which shows significant improvements in precision and detection sensitivity over qPCR readout [40]. The dPLA workflow is the same as that for standard PLA up to and including the ligation step. Instead of PCR amplification, two oligonucleotides complementary to either end of the single stranded oligonucleotides connecting the two antibodies are added, generating two restriction sites. Following digestion, released DNA strands are circularised via a second DNA ligation reaction and the circularised reporter DNA molecules are amplified, in this case by RCA. Amplification products form random coils, which after are detected by hybridisation with fluorescence dye labelled probes and counted using a dedicated microfluidic detection instrument.
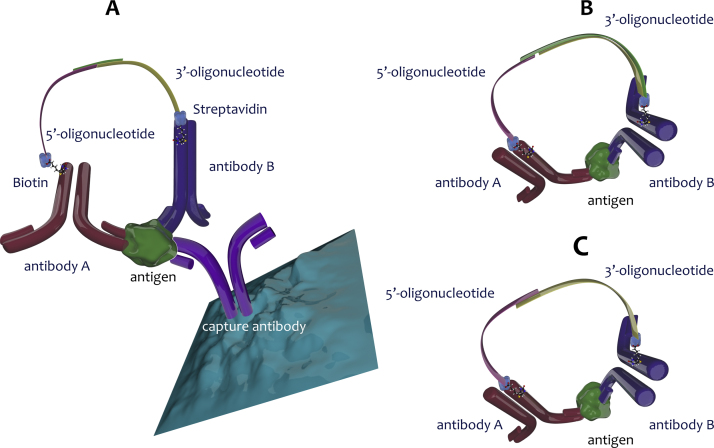
PLAs can be carried out in solution as a homogeneous assay, which has the advantage of minimising operator intervention, obviating the requirement for washes and hence facilitating maximum speed. Like immuno-PCR, PLA can also be configured in a solid phase format using an immobilised capture antibody, with two proximity probes detecting captured target molecules [41], an approach that may be more suitable for detecting protein directly from biofluids such as blood or faeces (Fig. 4A). Configuring the assay through the binding of three independent affinity reagents to the same target molecule can further enhance specificity of signal generation [42]. In this triple antibody specific proximity ligation assay, the third proximity ligation probe replaces the connector oligonucleotide as a ligation template. Short blocking oligonucleotides prevent ligation in the absence of the target molecule and considerably minimise background noise.
Fig. 4.
A. Solid phase PLA. (A) Capture antibody immobilised in a microtitre well binds target antigen, with unbound particles and other sample components removed by washes. Proximity probes are then added to the well and a PLA is carried out. (B) First generation PEA. The 3′-oligonucleotide is double stranded with a 3′-overhang. In the presence of target antigen, the probe oligonucleotides can hybridise to each other, leading to the extension of one oligonucleotide into a DNA template that can be detected and quantified. (C) Second generation PEA. Single stranded oligonucleotides hybridise directly to each other, with one becoming extended by a DNA polymerase to generate an amplifiable DNA template.
Another proximity-mediated detection system, in situ PLA, can detect and visualise target proteins and protein complexes expressed by fixed cells and on tissue slide sections [43], [44]. In situ PLA uses RCA to amplify ligation products, which are generated through the ligation of two connector oligonucleotides to the two oligonucleotides conjugated to the antibodies. This results in the formation of a circular, single stranded DNA molecule, with one of the antibody-conjugated DNA molecules serving as a primer for the RCA. Following the addition of a DNA polymerase, a long DNA product is formed that remains covalently attached to one of the PLA probes. Consequently, a concatameric repeat of the same sequence aggregates in submicron spots, generating discrete, localised signals after hybridisation of fluorescence-labelled oligonucleotide probes complementary to the RCA product. Signals can be detected and digitally counted using a standard fluorescence microscope [45], [46], [47], [48], [49]. The reaction is so efficient that care must be taken when using RCA in a quantitative manner due to the possibility of non-linear saturation of the RCA signal [50].
Multiplex PLAs have been developed, where the ligation of numerous PLA probes linked to different combinations of 5′- and 3′-oligonucleotides results in unique sequences that serve as primer sites for target-specific amplification and quantification by qPCR [51]. This has been extended so that four 24-plex panels profiling 74 putative biomarkers can be analysed with high sensitivity, yet using very low levels of sample [52]. Kits for carrying out homogeneous PLAs are commercially available from Life Technologies, Carlsbad, CA, USA, those for in situ PLA from Olink Bioscience, Uppsala, Sweden or Sigma–Aldrich, St. Louis, Mo, USA.
4. Proximity extension assays
PEA is an alternative to PLA and was developed because proximity probes joined by a DNA ligase suffer from recovery loss in complex biological fluids [53]. The main difference between the two is that in a PEA the ligation event is replaced by a DNA polymerisation step. In its original design, one of the PEA probes consisted of a double stranded-oligonucleotide attached to the antibody at its 3′-end, with a nine nucleotide or so 3′-overhang at its 5′-end. This overhang was complementary to the 3′-end of the oligonucleotide bound to the other antibody partner (Fig. 4B). Following incubation of the proximity probes with a sample containing antigen recognised by the probes, the overhanging 3′-end could hybridise to the 5′-oligonucleotide and, following the addition of a DNA polymerase, the free 3′-OH was extended in the 5′–3′ direction towards the attachment site of the 5′-oligonucleotide. This generated a full-length amplicon and hybridisation site for the upstream primer and thus allows for the amplification and detection of the target antigen by PCR. This arrangement has now been replaced by a modification, where each of the two single-stranded oligonucleotides contain a complementary site for pair-wise annealing with the other oligonucleotide, allowing extension by a DNA polymerase [54]. This obviates the requirement for a double-stranded oligonucleotide with a 3′-overhang (Fig. 4C). Furthermore, judicious choice of DNA polymerase allows minimisation of background noise and so improves the sensitivity of the assay [54]. PEA is amenable to multiplexing [54], [55] and features the same advantages of PLA, including very low sample consumption, high sensitivity and specificity detection in a homogeneous reaction [7]. For maximum sensitivity, PEA requires the use of DNA polymerases with intact 3′–5′ exonuclease as they reduce background noise by degrading non-proximal DNA strands. Kits for carrying out PEAs are commercially available from Olink Bioscience, Uppsala, Sweden.
5. Applications
The ultra-low sample requirement of homogeneous PLA and the ability of solid phase PLA to investigate larger sample volumes has made this technology a useful tool for a wide range of proteomic studies [56]. PCR-based assays only detect nucleic acids and cannot reliably provide information about the relationship between RNA and cellular protein levels, the extent of post-translational modifications or protein/protein interactions. This is important, since transcriptome and proteome are in a constant state of dynamic flux involving the transcription or degradation of RNAs and the translation, modification, interaction and turnover of proteins. This results in a complex, variable and sample-dependent [57] relationship between mRNA levels and protein expression, making extrapolation of changes in RNA levels to those of proteins difficult. Indeed, somewhere between 30 and 70% of the variability in protein levels can be explained by concordance to mRNA levels [58], [59] and translation efficiency turns out to be the best predictor of protein levels [60]. This challenge is not confined to nuclear genes, as mitochondrial proteins can also show significant upregulation in the absence of any change in mRNA levels [61]. Furthermore, correlations are very much gene-dependent and are substantially stronger for genes involved in the maintenance of cellular processes and structural properties compared with regulatory genes [60].
PLA encourages an integrated approach for measuring relative changes in miRNA, mRNA and protein expression from the same starting sample and on a single analytical platform [62]. This makes it a useful method for the validation of potential biomarkers for clinical diagnostic needs, for example by simplifying the analysis of cellular protein/protein interactions [63], [64], screening for inhibitors of such interactions and posttranslational modifications [65], [66] or the interaction of cellular and viral proteins [67]. PLA is finding increased use for cancer biomarker profiling, where its multiplexing capabilities [51], [68] allow the simultaneous analysis of tens of proteins at a time [69] and make possible the parallel visualisation of multiple protein complexes in tumour cells [70], [71]. It provides an ultrasensitive assay for the detection of PSA [72] and has been used for the in situ quantification of EGFR receptor dimerisation and activation [63]. PLA has identified functional differences between different mutations, which may help with the development of mutation-specific targeted therapies [73]. Specifically, its ability to identify mutant EGFR dimer configurations that can evade blockade by anti-EGFR treatment may permit a more accurate patient selection for EGFR-targeted treatment in glioblastoma multiforme, the most common primary brain tumour [74]. In breast cancer, it has been used to demonstrate that elevated levels of HER2:HER2 and HER2:HER3 [75] as well as protein kinase 6:HER2 complexes [66] are significantly associated with differences in overall patient survival. Furthermore, specific interactions between Smad proteins and AP-1 transcription factors activate a programme of TGFβ-induced breast cancer cell invasion [76], with Akt activation identified as an important driver of progression [77]. Interestingly, measuring activated forms of Akt by in situ PLA does not correlate with phosphorylated Akt or the Akt isoforms as measured by IHC, suggesting that the isoform-specific PLA assays are providing additional clinically relevant information. PLA is showing similar promise for the molecular dissection of a number of other cancers, including lung [78], [79], colorectal [80] and prostate [81] cancers. There are fewer publications describing the use of PEA, but this assay has been used to identify five plasma protein biomarkers associated with colorectal cancer, with three of them additionally found to be discriminators of early-stage cancer [55].
Pathogen detection is another application for proximity assays, suggested by the original proof of concept [41]. PLA has been used for bacterial [82], [83] and viral [84], [85] pathogen detection, identification of bacterial spores [86] as well as analyses of mechanisms underlying viral pathogenicity [87] and may have a role in the rapid identification of pathogens in environmental samples for biosecurity applications [88]. Proximity assays may be of particular interest for the development of assays targeting infectious fungal diseases, bloodstream and nosocomial infections, which are associated with high rates of morbidity and mortality. Early treatment is the foundation for successful disease management, yet its diagnosis is challenging due to the limitations of the methods currently used. Since pathogens are most effectively eliminated in the earliest stages of infection, there is an urgent need for early detection of infectious microbes. Current immunodiagnostic methods, e.g. ELISAs and lateral flow devices (LFD) lack the sensitivity for earliest possible diagnosis. PCR is more sensitive, but the detection of microbial DNA does not definitively prove the presence of a viable microorganism causing a given infection. Furthermore, there are contamination issues with PCR-based assays, not least because PCR reagents are produced in bacteria and fungi and may contain microbial DNA impurities. Proximity assays targeting proteins expressed only during active growth would provide functional information and allow earlier, clinically more relevant detection than ELISA or LFD but without the disadvantages associated with PCR-based detection [89].
6. Conclusions
Proximity assays expand the range of DNA amplification applications to include the sensitive, accurate and robust identification of proteins through the amplification of a surrogate DNA template after antibody binding [62]. This provides a variety of novel approaches for the direct detection of proteins, with the homogenous format of PLA allowing specific detection and quantification from unfractionated cell lysates and biofluids while in situ PLA provides a powerful means of localising proteins and interrogating protein/protein interactions. Today, most proximity assays make use of the extensive collection of commercially available antibodies, although advances in the selection and production of aptamers are likely to see their use increase in future [90], [91]. This is going to increase the attractiveness of proximity assays for use as tools for next generation pathology surveillance [92], live cell imaging [93], unravelling the details of cellular migration characteristic of embryogenesis and cancer metastasis [94] as well as protein biomarker validation [95] essential for the implementation of personalised medicine [96]. Furthermore, there is an obvious application of proximity assays for the detection of pathogens where the detection of nucleic acids by itself does not indicate viability or ability to invade [89]. Finally, at this early stage it is also important to consider benchmarks for assay standardisation, reproducibility and transparency of reporting [11] analogous to the MIQE guidelines [97]. In conclusion, proximity assays provide an integrated approach to the measurement of changes in gene and protein expression, protein modification and interactions from the same starting sample and readouts on a single analytical platform.
References
- 1.Bustin S.A. IUL Press; La Jolla, CA: 2004. A–Z of quantitative PCR. [Google Scholar]
- 2.Higuchi R., Fockler C., Dollinger G., Watson R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology (NY) 1993;11:1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- 3.Gibson U.E., Heid C.A., Williams P.M. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 4.Heid C.A., Stevens J., Livak K.J., Williams P.M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 5.Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 6.Uhlen M., Bjorling E., Agaton C., Szigyarto C.A., Amini B., Andersen E. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 2005;4:1920–1932. doi: 10.1074/mcp.M500279-MCP200. [DOI] [PubMed] [Google Scholar]
- 7.Solier C., Langen H. Antibody-based proteomics and biomarker research – current status and limitations. Proteomics. 2014;14:774–783. doi: 10.1002/pmic.201300334. [DOI] [PubMed] [Google Scholar]
- 8.Skottrup P.D., Nicolaisen M., Justesen A.F. Towards on-site pathogen detection using antibody-based sensors. Biosens Bioelectron. 2008;24:339–348. doi: 10.1016/j.bios.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 9.Li J., Zhu Z. Research and development of next generation of antibody-based therapeutics. Acta Pharmacol Sin. 2010;31:1198–1207. doi: 10.1038/aps.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lizardi P.M., Huang X., Zhu Z., Bray-Ward P., Thomas D.C., Ward D.C. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat Genet. 1998;19:225–232. doi: 10.1038/898. [DOI] [PubMed] [Google Scholar]
- 11.Hansen M.C., Nederby L., Henriksen M.O., Hansen M., Nyvold C.G. Sensitive ligand-based protein quantification using immuno-PCR: a critical review of single-probe and proximity ligation assays. Biotechniques. 2014;56:217–228. doi: 10.2144/000114164. [DOI] [PubMed] [Google Scholar]
- 12.Sano T., Smith C.L., Cantor C.R. Immuno-PCR: very sensitive antigen detection by means of specific antibody–DNA conjugates. Science. 1992;258:120–122. doi: 10.1126/science.1439758. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H., Fisher R.J., Papas T.S. Universal immuno-PCR for ultra-sensitive target protein detection. Nucleic Acids Res. 1993;21:6038–6039. doi: 10.1093/nar/21.25.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niemeyer C.M., Adler M., Blohm D. Fluorometric polymerase chain reaction (PCR) enzyme-linked immunosorbent assay for quantification of immuno-PCR products in microplates. Anal Biochem. 1997;246:140–145. doi: 10.1006/abio.1996.9989. [DOI] [PubMed] [Google Scholar]
- 15.Sims P.W., Vasser M., Wong W.L., Williams P.M., Meng Y.G. Immunopolymerase chain reaction using real-time polymerase chain reaction for detection. Anal Biochem. 2000;281:230–232. doi: 10.1006/abio.2000.4578. [DOI] [PubMed] [Google Scholar]
- 16.Adler M., Wacker R., Niemeyer C.M. A real-time immuno-PCR assay for routine ultrasensitive quantification of proteins. Biochem Biophys Res Commun. 2003;308:240–250. doi: 10.1016/s0006-291x(03)01364-0. [DOI] [PubMed] [Google Scholar]
- 17.Lind K., Kubista M. Development and evaluation of three real-time immuno-PCR assemblages for quantification of PSA. J Immunol Methods. 2005;304:107–116. doi: 10.1016/j.jim.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Sergeeva A., Kolonin M.G., Molldrem J.J., Pasqualini R., Arap W. Display technologies: application for the discovery of drug and gene delivery agents. Adv Drug Deliv Rev. 2006;58:1622–1654. doi: 10.1016/j.addr.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber R., Eisenbraun J., Miletzki B., Adler M., Scheer R., Klein R. Pharmacokinetics of natural mistletoe lectins after subcutaneous injection. Eur J Clin Pharmacol. 2010;66:889–897. doi: 10.1007/s00228-010-0830-5. [DOI] [PubMed] [Google Scholar]
- 20.He X., McMahon S., Henderson T.D., Griffey S.M., Cheng L.W. Ricin toxicokinetics and its sensitive detection in mouse sera or feces using immuno-PCR. PLoS ONE. 2010;5:e12858. doi: 10.1371/journal.pone.0012858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasai N., Kobayashi K., Shioya S., Yoshikawa Y., Yotsumoto F., Miyamoto S. Soluble heparin-binding EGF-like growth factor (HB-EGF) detected by newly developed immuno-PCR method is a clear-cut serological biomarker for ovarian cancer. Am J Transl Res. 2012;4:415–421. [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S., Cho H., Nam E.J., Kim S.W., Kim Y.T., Park Y.W. Autoantibodies against stress-induced phosphoprotein-1 as a novel biomarker candidate for ovarian cancer. Genes Chromosomes Cancer. 2010;49:585–595. doi: 10.1002/gcc.20769. [DOI] [PubMed] [Google Scholar]
- 23.Lee G., Cheung A.P., Li B., Ge B., Chow P.M. Molecular and immuno-characteristics of immunoglobulin-like glycoproteins in cancer cell-expressed biomarker, CA215. Immunol Invest. 2012;41:429–446. doi: 10.3109/08820139.2012.661007. [DOI] [PubMed] [Google Scholar]
- 24.Patani N., Jiang W.G., Mokbel K. Brain-derived neurotrophic factor expression predicts adverse pathological & clinical outcomes in human breast cancer. Cancer Cell Int. 2011;11:23. doi: 10.1186/1475-2867-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Ren J., Pan K., Ma J., Li J., Shen L. Detection of gastric carcinoma-associated MG7-Ag by serum immuno-PCR assay in a high-risk Chinese population, with implication for screening. Int J Cancer. 2010;126:469–473. doi: 10.1002/ijc.24739. [DOI] [PubMed] [Google Scholar]
- 26.Barletta J.M., Edelman D.C., Constantine N.T. Lowering the detection limits of HIV-1 viral load using real-time immuno-PCR for HIV-1 p24 antigen. Am J Clin Pathol. 2004;122:20–27. doi: 10.1309/529T-2WDN-EB6X-8VUN. [DOI] [PubMed] [Google Scholar]
- 27.Barletta J., Bartolome A., Constantine N.T. Immunomagnetic quantitative immuno-PCR for detection of less than one HIV-1 virion. J Virol Methods. 2009;157:122–132. doi: 10.1016/j.jviromet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Fredriksson S., Gullberg M., Jarvius J., Olsson C., Pietras K., Gustafsdottir S.M. Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol. 2002;20:473–477. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- 29.Famulok M., Mayer G., Blind M. Nucleic acid aptamers-from selection in vitro to applications in vivo. Acc Chem Res. 2000;33:591–599. doi: 10.1021/ar960167q. [DOI] [PubMed] [Google Scholar]
- 30.Pai S., Roberts A., Ellington A.D. Aptamer amplification: divide and signal. Expert Opin Med Diagn. 2008;2:1333–1346. doi: 10.1517/17530050802562016. [DOI] [PubMed] [Google Scholar]
- 31.Yang L., Ellington A.D. Real-time PCR detection of protein analytes with conformation-switching aptamers. Anal Biochem. 2008;380:164–173. doi: 10.1016/j.ab.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gullberg M., Gustafsdottir S.M., Schallmeiner E., Jarvius J., Bjarnegard M., Betsholtz C. Cytokine detection by antibody-based proximity ligation. Proc Natl Acad Sci U S A. 2004;101:8420–8424. doi: 10.1073/pnas.0400552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darmanis S., Kahler A., Spangberg L., Kamali-Moghaddam M., Landegren U., Schallmeiner E. Self-assembly of proximity probes for flexible and modular proximity ligation assays. Biotechniques. 2007;43:443–444. doi: 10.2144/000112551. 446, 448 passim. [DOI] [PubMed] [Google Scholar]
- 34.Klasener K., Maity P.C., Hobeika E., Yang J., Reth M. B cell activation involves nanoscale receptor reorganizations and inside-out signaling by Syk. Elife. 2014;3:e02069. doi: 10.7554/eLife.02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leuchowius K.J., Weibrecht I., Landegren U., Gedda L., Soderberg O. Flow cytometric in situ proximity ligation analyses of protein interactions and post-translational modification of the epidermal growth factor receptor family. Cytometry A. 2009;75:833–839. doi: 10.1002/cyto.a.20771. [DOI] [PubMed] [Google Scholar]
- 36.Jiang X., Cheng S., Chen W., Wang L., Shi F., Zhu C. Comparison of oligonucleotide-labeled antibody probe assays for prostate-specific antigen detection. Anal Biochem. 2012;424:1–7. doi: 10.1016/j.ab.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Nong R.Y., Wu D., Yan J., Hammond M., Gu G.J., Kamali-Moghaddam M. Solid-phase proximity ligation assays for individual or parallel protein analyses with readout via real-time PCR or sequencing. Nat Protoc. 2013;8:1234–1248. doi: 10.1038/nprot.2013.070. [DOI] [PubMed] [Google Scholar]
- 38.Ebai T., Kamali-Moghaddam M., Landegren U. Parallel protein detection by solid-phase proximity ligation assay with real-time PCR or sequencing. Curr Protoc Mol Biol. 2015;109 doi: 10.1002/0471142727.mb2010s109. 20.10.1–20.10.25. [DOI] [PubMed] [Google Scholar]
- 39.Darmanis S., Nong R.Y., Hammond M., Gu J., Alderborn A., Vanelid J. Sensitive plasma protein analysis by microparticle-based proximity ligation assays. Mol Cell Proteomics. 2010;9:327–335. doi: 10.1074/mcp.M900248-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ke R., Nong R.Y., Fredriksson S., Landegren U., Nilsson M. Improving precision of proximity ligation assay by amplified single molecule detection. PLOS ONE. 2013;8:e69813. doi: 10.1371/journal.pone.0069813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gustafsdottir S.M., Nordengrahn A., Fredriksson S., Wallgren P., Rivera E., Schallmeiner E. Detection of individual microbial pathogens by proximity ligation. Clin Chem. 2006;52:1152–1160. doi: 10.1373/clinchem.2005.065847. [DOI] [PubMed] [Google Scholar]
- 42.Schallmeiner E., Oksanen E., Ericsson O., Spangberg L., Eriksson S., Stenman U.H. Sensitive protein detection via triple-binder proximity ligation assays. Nat Methods. 2007;4:135–137. doi: 10.1038/nmeth974. [DOI] [PubMed] [Google Scholar]
- 43.Soderberg O., Gullberg M., Jarvius M., Ridderstrale K., Leuchowius K.J., Jarvius J. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 44.Koos B., Andersson L., Clausson C.M., Grannas K., Klaesson A., Cane G. Analysis of protein interactions in situ by proximity ligation assays. Curr Top Microbiol Immunol. 2014;377:111–126. doi: 10.1007/82_2013_334. [DOI] [PubMed] [Google Scholar]
- 45.Jarvius M., Paulsson J., Weibrecht I., Leuchowius K.J., Andersson A.C., Wahlby C. In situ detection of phosphorylated platelet-derived growth factor receptor beta using a generalized proximity ligation method. Mol Cell Proteomics. 2007;6:1500–1509. doi: 10.1074/mcp.M700166-MCP200. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y., Gu J., Hagner-McWhirter A., Sathiyanarayanan P., Gullberg M., Soderberg O. Western blotting via proximity ligation for high performance protein analysis. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.O111.011031. O111.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung J., Lifland A.W., Zurla C., Alonas E.J., Santangelo P.J. Quantifying RNA–protein interactions in situ using modified-MTRIPs and proximity ligation. Nucleic Acids Res. 2013;41:e12. doi: 10.1093/nar/gks837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gobet N., Ketterer S., Meier M. Design and validation of DNA libraries for multiplexing proximity ligation assays. PLOS ONE. 2014;9:e112629. doi: 10.1371/journal.pone.0112629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu B., Zhang B., Chen G., Yang H., Tang D. Proximity ligation assay with three-way junction-induced rolling circle amplification for ultrasensitive electronic monitoring of concanavalin A. Anal Chem. 2014;86:7773–7781. doi: 10.1021/ac501690v. [DOI] [PubMed] [Google Scholar]
- 50.Mocanu M.M., Varadi T., Szollosi J., Nagy P. Comparative analysis of fluorescence resonance energy transfer (FRET) and proximity ligation assay (PLA) Proteomics. 2011;11:2063–2070. doi: 10.1002/pmic.201100028. [DOI] [PubMed] [Google Scholar]
- 51.Fredriksson S., Dixon W., Ji H., Koong A.C., Mindrinos M., Davis R.W. Multiplexed protein detection by proximity ligation for cancer biomarker validation. Nat Methods. 2007;4:327–329. doi: 10.1038/nmeth1020. [DOI] [PubMed] [Google Scholar]
- 52.Lundberg M., Thorsen S.B., Assarsson E., Villablanca A., Tran B., Gee N. Multiplexed homogeneous proximity ligation assays for high-throughput protein biomarker research in serological material. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.004978. M110.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundberg M., Eriksson A., Tran B., Assarsson E., Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39:e102. doi: 10.1093/nar/gkr424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Assarsson E., Lundberg M., Holmquist G., Bjorkesten J., Thorsen S.B., Ekman D. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLOS ONE. 2014;9:e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorsen S.B., Lundberg M., Villablanca A., Christensen S.L., Belling K.C., Nielsen B.S. Detection of serological biomarkers by proximity extension assay for detection of colorectal neoplasias in symptomatic individuals. J Transl Med. 2013;11:253. doi: 10.1186/1479-5876-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soderberg O., Leuchowius K.J., Kamali-Moghaddam M., Jarvius M., Gustafsdottir S., Schallmeiner E. Proximity ligation: a specific and versatile tool for the proteomic era. Genet Eng (N Y) 2007;28:85–93. doi: 10.1007/978-0-387-34504-8_5. [DOI] [PubMed] [Google Scholar]
- 57.Guo Y., Xiao P., Lei S., Deng F., Xiao G.G., Liu Y. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai) 2008;40:426–436. doi: 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 58.Gry M., Rimini R., Stromberg S., Asplund A., Ponten F., Uhlen M. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics. 2009;10:365. doi: 10.1186/1471-2164-10-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shankavaram U.T., Reinhold W.C., Nishizuka S., Major S., Morita D., Chary K.K. Transcript and protein expression profiles of the NCI-60 cancer cell panel: an integromic microarray study. Mol Cancer Ther. 2007;6:820–832. doi: 10.1158/1535-7163.MCT-06-0650. [DOI] [PubMed] [Google Scholar]
- 60.Schwanhausser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 61.Margineantu D.H., Emerson C.B., Diaz D., Hockenbery D.M. Hsp90 inhibition decreases mitochondrial protein turnover. PLoS ONE. 2007;2:e1066. doi: 10.1371/journal.pone.0001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swartzman E., Shannon M., Lieu P., Chen S.M., Mooney C., Wei E. Expanding applications of protein analysis using proximity ligation and qPCR. Methods. 2010;50:S23–S26. doi: 10.1016/j.ymeth.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 63.Gajadhar A., Guha A. A proximity ligation assay using transiently transfected, epitope-tagged proteins: application for in situ detection of dimerized receptor tyrosine kinases. Biotechniques. 2010;48:145–152. doi: 10.2144/000113354. [DOI] [PubMed] [Google Scholar]
- 64.Zieba A., Wahlby C., Hjelm F., Jordan L., Berg J., Landegren U. Bright-field microscopy visualization of proteins and protein complexes by in situ proximity ligation with peroxidase detection. Clin Chem. 2010;56:99–110. doi: 10.1373/clinchem.2009.134452. [DOI] [PubMed] [Google Scholar]
- 65.Leuchowius K.J., Jarvius M., Wickstrom M., Rickardson L., Landegren U., Larsson R. High content screening for inhibitors of protein interactions and post-translational modifications in primary cells by proximity ligation. Mol Cell Proteomics. 2010;9:178–183. doi: 10.1074/mcp.M900331-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aubele M., Spears M., Ludyga N., Braselmann H., Feuchtinger A., Taylor K.J. In situ quantification of HER2-protein tyrosine kinase 6 (PTK6) protein–protein complexes in paraffin sections from breast cancer tissues. Br J Cancer. 2010;103:663–667. doi: 10.1038/sj.bjc.6605836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y.J., Chen Y.H., Chow L.P., Tsai Y.H., Chen P.H., Huang C.Y. Heat shock protein 72 is associated with the hepatitis C virus replicase complex and enhances viral RNA replication. J Biol Chem. 2010;285:28183–28190. doi: 10.1074/jbc.M110.118323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fredriksson S., Horecka J., Brustugun O.T., Schlingemann J., Koong A.C., Tibshirani R. Multiplexed proximity ligation assays to profile putative plasma biomarkers relevant to pancreatic and ovarian cancer. Clin Chem. 2008;54:582–589. doi: 10.1373/clinchem.2007.093195. [DOI] [PubMed] [Google Scholar]
- 69.Darmanis S., Nong R.Y., Vanelid J., Siegbahn A., Ericsson O., Fredriksson S. ProteinSeq: high-performance proteomic analyses by proximity ligation and next generation sequencing. PLoS ONE. 2011;6:e25583. doi: 10.1371/journal.pone.0025583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leuchowius K.J., Clausson C.M., Grannas K., Erbilgin Y., Botling J., Zieba A. Parallel visualization of multiple protein complexes in individual cells in tumor tissue. Mol Cell Proteomics. 2013;12:1563–1571. doi: 10.1074/mcp.O112.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moreira J.M., Thorsen S.B., Brunner N., Stenvang J. Proximity probing assays for simultaneous visualization of protein complexes in situ. Expert Rev Proteomics. 2013;10:219–221. doi: 10.1586/epr.13.22. [DOI] [PubMed] [Google Scholar]
- 72.Zhu L., Koistinen H., Wu P., Narvanen A., Schallmeiner E., Fredriksson S. A sensitive proximity ligation assay for active PSA. Biol Chem. 2006;387:769–772. doi: 10.1515/BC.2006.096. [DOI] [PubMed] [Google Scholar]
- 73.Bunse L., Schumacher T., Sahm F., Pusch S., Oezen I., Rauschenbach K. Proximity ligation assay evaluates IDH1R132H presentation in gliomas. J Clin Invest. 2015;125:1–14. doi: 10.1172/JCI77780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gajadhar A.S., Bogdanovic E., Munoz D.M., Guha A. In situ analysis of mutant EGFRs prevalent in glioblastoma multiforme reveals aberrant dimerization, activation, and differential response to anti-EGFR targeted therapy. Mol Cancer Res. 2012;10:428–440. doi: 10.1158/1541-7786.MCR-11-0531. [DOI] [PubMed] [Google Scholar]
- 75.Spears M., Taylor K.J., Munro A.F., Cunningham C.A., Mallon E.A., Twelves C.J. In situ detection of HER2:HER2 and HER2:HER3 protein–protein interactions demonstrates prognostic significance in early breast cancer. Breast Cancer Res Treat. 2012;132:463–470. doi: 10.1007/s10549-011-1606-z. [DOI] [PubMed] [Google Scholar]
- 76.Sundqvist A., Zieba A., Vasilaki E., Herrera Hidalgo C., Soderberg O., Koinuma D. Specific interactions between Smad proteins and AP-1 components determine TGFbeta-induced breast cancer cell invasion. Oncogene. 2013;32:3606–3615. doi: 10.1038/onc.2012.370. [DOI] [PubMed] [Google Scholar]
- 77.Spears M., Cunningham C.A., Taylor K.J., Mallon E.A., Thomas J.S., Kerr G.R. Proximity ligation assays for isoform-specific Akt activation in breast cancer identify activated Akt1 as a driver of progression. J Pathol. 2012;227:481–489. doi: 10.1002/path.4022. [DOI] [PubMed] [Google Scholar]
- 78.Wu H., Shang L.Q., Chen R.L., Yang S.M., Wang S.L., Wang J. Significance of Trask protein interactions in brain metastatic cohorts of lung cancers. Tumour Biol. 2015 doi: 10.1007/s13277-015-3053-7. [DOI] [PubMed] [Google Scholar]
- 79.Smith M.A., Hall R., Fisher K., Haake S.M., Khalil F., Schabath M.B. Annotation of human cancers with EGFR signaling-associated protein complexes using proximity ligation assays. Sci Signal. 2015;8:ra4. doi: 10.1126/scisignal.2005906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang M.A., Kim M.S., Kim J.Y., Shin Y.J., Song J.Y., Jeong J.H. A novel pyrido-thieno-pyrimidine derivative activates p53 through induction of phosphorylation and acetylation in colorectal cancer cells. Int J Oncol. 2015;46:342–350. doi: 10.3892/ijo.2014.2720. [DOI] [PubMed] [Google Scholar]
- 81.Mehraein-Ghomi F., Kegel S.J., Church D.R., Schmidt J.S., Reuter Q.R., Saphner E.L. Targeting androgen receptor and JunD interaction for prevention of prostate cancer progression. Prostate. 2014;74:792–803. doi: 10.1002/pros.22800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu C., Deng X., Shi F. Rapid detection of Brucella abortus by a novel proximity ligation-based loop-mediated isothermal amplification method. J Rapid Methods Autom Microbiol. 2009;17:154–163. [Google Scholar]
- 83.Leslie D.C., Sohrabi A., Ikonomi P., McKee M.L., Landers J.P. Size-based separations as an important discriminator in development of proximity ligation assays for protein or organism detection. Electrophoresis. 2010;31:1615–1622. doi: 10.1002/elps.201000008. [DOI] [PubMed] [Google Scholar]
- 84.Nordengrahn A., Gustafsdottir S.M., Ebert K., Reid S.M., King D.P., Ferris N.P. Evaluation of a novel proximity ligation assay for the sensitive and rapid detection of foot-and-mouth disease virus. Vet Microbiol. 2008;127:227–236. doi: 10.1016/j.vetmic.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 85.Van Wesenbeeck L., Meeuws H., De Wolf H., Stuyver L. Evaluation of a proximity extension assay for the detection of H1 2009 pandemic influenza viruses. J Virol Methods. 2013;193:77–84. doi: 10.1016/j.jviromet.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 86.Pai S., Ellington A.D., Levy M. Proximity ligation assays with peptide conjugate ‘burrs’ for the sensitive detection of spores. Nucleic Acids Res. 2005;33:e162. doi: 10.1093/nar/gni150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inaba J., Kim B.M., Shimura H., Masuta C. Virus-induced necrosis is a consequence of direct protein–protein interaction between a viral RNA-silencing suppressor and a host catalase. Plant Physiol. 2011;156:2026–2036. doi: 10.1104/pp.111.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goransson J., Ke R., Nong R.Y., Howell W.M., Karman A., Grawe J. Rapid identification of bio-molecules applied for detection of biosecurity agents using rolling circle amplification. PLOS ONE. 2012;7:e31068. doi: 10.1371/journal.pone.0031068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson G., Ferrini A., Dolan S.K., Nolan T., Agrawal S., Doyle S. Biomarkers for invasive aspergillosis: the challenges continue. Biomark Med. 2014;8:429–451. doi: 10.2217/bmm.13.129. [DOI] [PubMed] [Google Scholar]
- 90.Fodey T., Leonard P., O’Mahony J., O’Kennedy R., Danaher M. Developments in the production of biological and synthetic binders for immunoassay and sensor-based detection of small molecules. Trends Anal Chem. 2011;30:254–269. [Google Scholar]
- 91.Kong H.Y., Byun J. Nucleic acid aptamers: new methods for selection, stabilization, and application in biomedical science. Biomol Ther (Seoul) 2013;21:423–434. doi: 10.4062/biomolther.2013.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koos B., Kamali-Moghaddam M., David L., Sobrinho-Simoes M., Dimberg A., Nilsson M. Next-generation pathology-surveillance of tumor microecology. J Mol Biol. 2015;5:2013–2022. doi: 10.1016/j.jmb.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 93.Salipalli S., Singh P.K., Borlak J. Recent advances in live cell imaging of hepatoma cells. BMC Cell Biol. 2014;15:26. doi: 10.1186/1471-2121-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chan J.S., Teo Z., Sng M.K., Tan N.S. Probing for protein–protein interactions during cell migration: limitations and challenges. Histol Histopathol. 2014;29:965–976. doi: 10.14670/HH-29.965. [DOI] [PubMed] [Google Scholar]
- 95.Blokzijl A., Nong R., Darmanis S., Hertz E., Landegren U., Kamali-Moghaddam M. Protein biomarker validation via proximity ligation assays. Biochim Biophys Acta. 2014;1844:933–939. doi: 10.1016/j.bbapap.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 96.Blokzijl A., Friedman M., Ponten F., Landegren U. Profiling protein expression and interactions: proximity ligation as a tool for personalized medicine. J Intern Med. 2010;268:232–245. doi: 10.1111/j.1365-2796.2010.02256.x. [DOI] [PubMed] [Google Scholar]
- 97.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]