Significance
Acute lung injury, including impaired alveolar fluid clearance, is a life-threatening complication of severe respiratory virus infection, and effective treatment is lacking. Understanding the mechanism of this complication may suggest novel therapies. Here, we found that, in vitro, influenza A/H5N1 infection impaired alveolar fluid clearance more than did seasonal virus, mimicking its greater severity in patients. We demonstrated that this impairment is mediated by the release of soluble factors from infected cells, leading to down-regulation of alveolar sodium and chloride transporters. Mesenchymal stromal cells prevented or reduced this effect in vitro and in vivo in A/H5N1-infected mice. These cells provide a potentially effective treatment for acute lung injury in severe influenza.
Keywords: influenza, avian, acute lung injury, mesenchymal stromal cells, alveolar fluid clearance
Abstract
Influenza can cause acute lung injury. Because immune responses often play a role, antivirals may not ensure a successful outcome. To identify pathogenic mechanisms and potential adjunctive therapeutic options, we compared the extent to which avian influenza A/H5N1 virus and seasonal influenza A/H1N1 virus impair alveolar fluid clearance and protein permeability in an in vitro model of acute lung injury, defined the role of virus-induced soluble mediators in these injury effects, and demonstrated that the effects are prevented or reduced by bone marrow-derived multipotent mesenchymal stromal cells. We verified the in vivo relevance of these findings in mice experimentally infected with influenza A/H5N1. We found that, in vitro, the alveolar epithelium’s protein permeability and fluid clearance were dysregulated by soluble immune mediators released upon infection with avian (A/Hong Kong/483/97, H5N1) but not seasonal (A/Hong Kong/54/98, H1N1) influenza virus. The reduced alveolar fluid transport associated with down-regulation of sodium and chloride transporters was prevented or reduced by coculture with mesenchymal stromal cells. In vivo, treatment of aged H5N1-infected mice with mesenchymal stromal cells increased their likelihood of survival. We conclude that mesenchymal stromal cells significantly reduce the impairment of alveolar fluid clearance induced by A/H5N1 infection in vitro and prevent or reduce A/H5N1-associated acute lung injury in vivo. This potential adjunctive therapy for severe influenza-induced lung disease warrants rapid clinical investigation.
Acute lung injury is a continuum of clinical and radiographic changes, terminating at its most severe, with acute respiratory distress syndrome. Infection with highly pathogenic avian influenza (HPAI) viruses of the H5N1 and more recent H7N9 subtypes often leads to acute lung injury whereas seasonal influenza viruses and the 2009 pandemic H1N1 influenza viruses do so more rarely. The underlying mechanisms of influenza-related acute lung injury remain unclear, and effective therapies are lacking. Viruses that are highly pathogenic to humans (e.g., H5N1 viruses) may differ intrinsically from the less pathogenic (LP) (e.g., seasonal H1N1) viruses in their replication competence, cell tropism, and/or cytokine dysregulation (1, 2). Early treatment of H5N1 disease with the antiinfluenza drug oseltamivir is helpful but does not ensure a favorable outcome (3). Thus, effective adjunctive therapies that do not compromise beneficial host defenses are needed (4).
H5N1 (5) and H7N9 (6) influenza viruses target alveolar epithelial cells, which form the crucial gas exchange interface in the lung. These cells also help to maintain intraalveolar and intravascular fluid homeostasis by vectorial transport of sodium, chloride, and water from the apical to the basolateral surface of the alveolar epithelium [alveolar fluid clearance (AFC)]. Impaired AFC and increased alveolar protein permeability (APP) contribute to acute lung injury (7). Therapies that normalize alveolar fluid clearance are likely to be free of off-target effects, unlike immunomodulation, that may promote virus replication.
Human bone marrow-derived multipotent mesenchymal stromal cells (MSCs) have applications in multiple clinical disorders, including sepsis, myocardial infarction, diabetes, and acute renal failure (8). Allogeneic MSC therapy has beneficial preclinical effects on endotoxin-, bacteria-, and ventilator-induced acute lung injury (9) via MSC secretion of the soluble paracrine growth factors angiopoietin-1 (Ang1) and keratinocyte growth factor (KGF) (9, 10). MSCs can also transfer mitochondria and microvesicles that modulate immunity and epithelial response to injury (11). Current clinical trials are testing MSCs as a therapy for sepsis and acute respiratory distress syndrome (12). However, little is known about the impact of MSCs on acute respiratory viral infections, including influenza, with the exception of a study in which MSCs failed to reduce influenza-induced lung injury in mice (13). Here, we showed that influenza A/H5N1 virus infection dysregulates AFC and APP in vitro by inducing infected cells to release soluble mediators that down-regulate alveolar sodium and chloride transporters. When we cocultured alveolar epithelium with MSCs, these injury mechanisms were prevented or reduced. We then treated mice infected with influenza A/H5N1 with MSCs and demonstrated a clinically significant reduction in lung pathology and increased survival in association with a modulation of these pathogenic mechanisms in vivo.
Results
HPAI H5N1 Virus Infection Reduces AFC and Enhances APP in Human Alveolar Epithelial Cells.
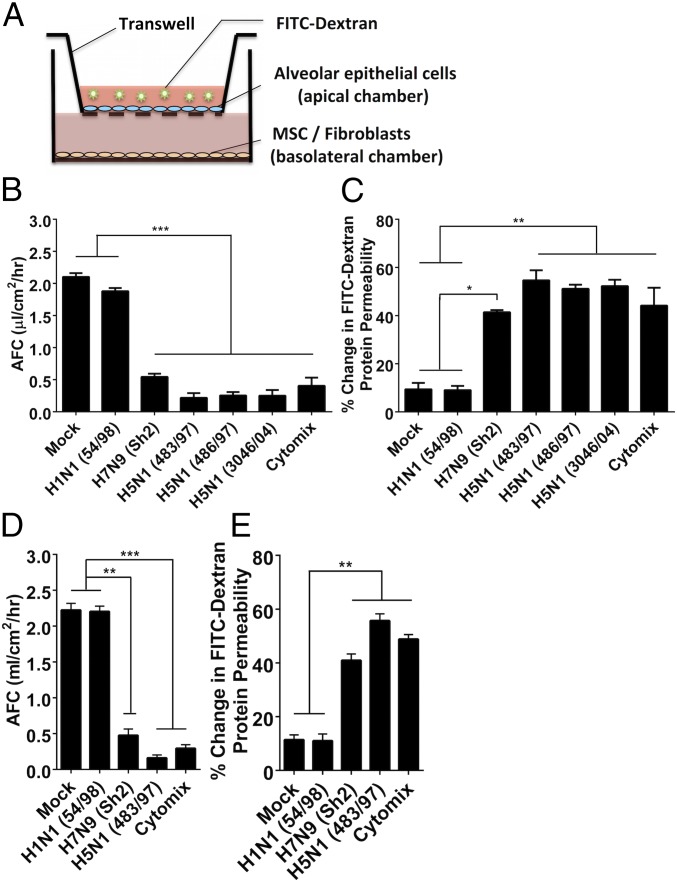
Monolayers of primary human alveolar epithelial cells demonstrating tight junctions were grown on apical chambers of transwell culture inserts in which the basolateral chamber can be used for coculture with MSCs, or with fibroblasts or PBS as controls (Fig. 1A). Influenza H5N1, H7N9, and human seasonal H1N1 influenza viruses replicated comparably in these cells (Fig. S1). Under the experimental conditions used, ZO-1 tight junction staining of the alveolar epithelial was not affected in virus-infected cells, and the transepithelial resistance (>1,000 ohms) was maintained for up to 48 h postinfection. Although H1N1 54/98 virus minimally affected AFC and APP, the H5N1 viruses (483/97, 486/97, and 3046/04) and H7N9 virus (Sh2) (complete virus names are listed in Materials and Methods) caused much greater reduction of AFC (all P < 0.0001) (Fig. 1B) and greater increase in APP (P = 0.008, 0.007, 0.007, and 0.032, respectively) (Fig. 1C) than did mock infection at 24 h postinfection (p.i.). A mixture of proinflammatory cytokines [Cytomix (IL-1β, TNF-α, and IFN-γ)] served as positive control. The other human seasonal influenza viruses, H1N1 (OK/447), H3N2 (1174/99), and H3N2 (OK/370), affected AFC and APP similarly to H1N1 54/98 (Fig. S2). Virus-free conditioned medium from virus-infected alveolar epithelial cells (after virus filtration) similarly affected AFC (Fig. 1D) and APP (Fig. 1E). Therefore, the observed effects were caused by soluble mediators released from H5N1 or H7N9 virus-infected, but not H1N1 virus-infected, alveolar epithelial cells, rather than direct viral cytopathic effects.
Fig. 1.
Highly pathogenic influenza viruses significantly reduce human alveolar fluid clearance (AFC) and significantly increase human alveolar protein permeability (APP). (A) Schematic diagram of the in vitro lung injury model. FITC-dextran, fluorescein isothiocyanate-dextran; MSC, mesenchymal stromal cells. (B and C) Infection with HPAI H5N1 (A/HongKong/483/97, A/HongKong/486/97, and A/HongKong/3046 /04) and H7N9 (A/Shanghai/2/2013) viruses (MOI = 0.1) significantly (B) reduced the AFC and (C) increased the APP of human alveolar epithelial cells compared with infection with LP influenza H1N1 virus (A/HongKong/54/98). Cytomix containing proinflammatory cytokines (IL-1β, TNF-α, and IFN-γ) was used as an injury-inducing positive control. Data are the mean ± SE from three experiments. *P < 0.05; **P < 0.01; ***P < 0.005. (D and E) The effect of conditioned medium (virus-free supernatant) from cells infected with H5N1 and H7N9 influenza viruses affected the AFC and APP of alveolar epithelial cells comparably with the live influenza viruses and (D) reduced the AFC and (E) increased the APP significantly more than supernatant from cells infected with H1N1 virus. Data are the mean ± SE from three experiments. **P < 0.01; ***P < 0.005.
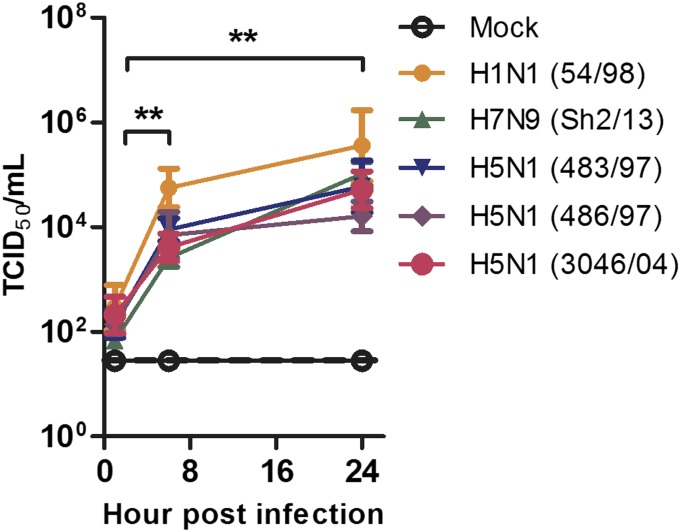
Fig. S1.
In vitro replication kinetics of HPAI H5N1 (A/HongKong/483/97, A/Hong Kong/486/97, A/HongKong/3046/04) influenza viruses, HPAI H7N9 (A/Shanghai/2/2013) influenza virus, and an LP influenza virus (A/HongKong/54/98) in human alveolar epithelial cells. All viruses replicated productively, and a statistically significant increase in titers was detected at 8 h and 24 h p.i. Data are the mean ± SE from three experiments. **P < 0.01.
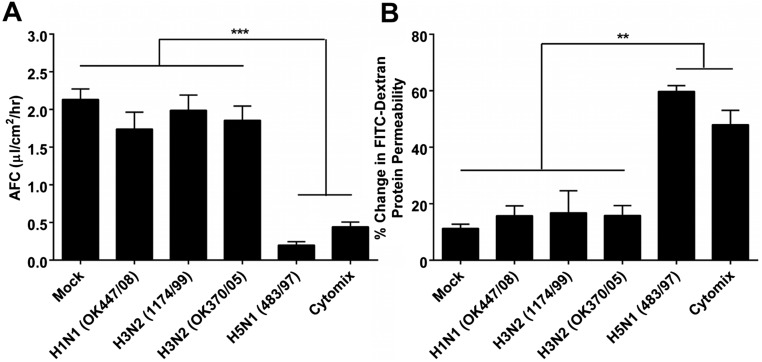
Fig. S2.
Highly pathogenic influenza H5N1 virus significantly reduces human alveolar fluid clearance (AFC) and significantly increases human alveolar protein permeability (APP) compared with low pathogenic seasonal influenza viruses. (A and B) HPAI H5N1 (A/HongKong/483/97) virus infection significantly (A) reduced the AFC and (B) increased the APP of human alveolar epithelial cells compared with infection with the LP influenza viruses A/Oklahoma/447/08 (H1N1), A/HongKong/1174/99 (H3N2), and A/Oklahoma/370/05 (H3N2).
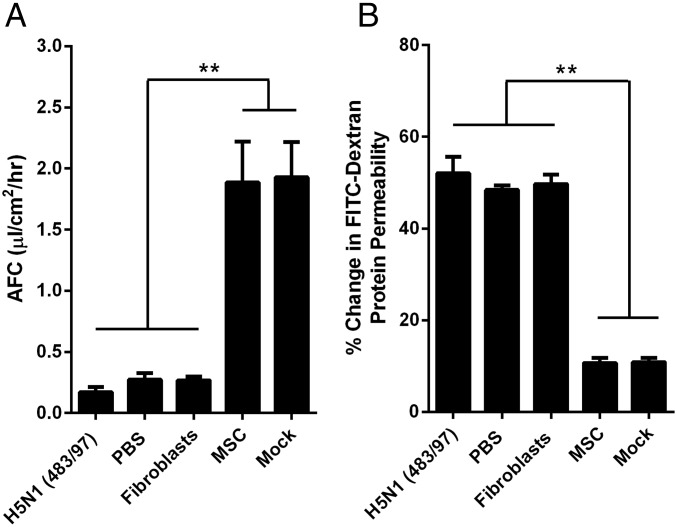
Coculture with MSCs Reduces the Adverse Effect of H5N1 Infection on AFC, APP, and Proinflammatory Cytokine Responses in Human Alveolar Epithelial Cells.
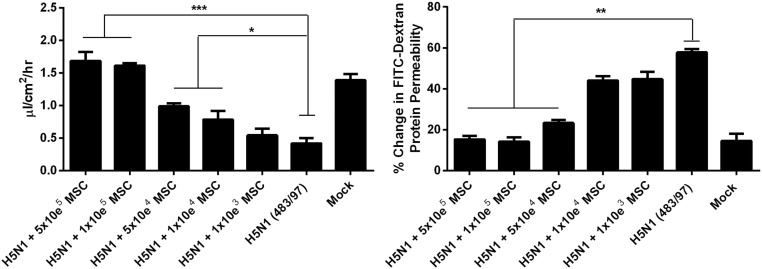
When H5N1 483/97-infected alveolar epithelial cells were cocultured (100,000 cells per well) with MSCs, the viral effects on AFC and APP were significantly moderated (Fig. 2), showing levels similar to those levels in mock-infected cells. Coculture with human lung fibroblasts or PBS had no effect (Fig. 2). MSCs have a dose-dependent effect on H5N1-impaired AFC and APP. However, even with 10,000 cells, MSCs still mediate a significant decrease in the impairment of AFC and reduction of the increased APP, respectively (Fig. S3).
Fig. 2.
MSCs prevent or reduce H5N1 virus impairment of alveolar epithelial AFC and APP. Alveolar epithelial cell monolayers on the apical chamber of transwell culture inserts were infected with H5N1 (A/HongKong/483/97) influenza virus (MOI = 0.1). Cells cocultured with MSCs in the basolateral chamber showed a significantly greater increase in net AFC (A) and decrease in APP (B) across the transwell inserts than did untreated infected cells, mock-cocultured cells, or cells cocultured with fibroblasts. Data represent the mean ± SE from three experiments. **P < 0.01.
Fig. S3.
The effect of MSCs on H5N1-impaired AFC (Left) and APP (Right) is dependent on the number of MSC used for coculture. Alveolar epithelial cell monolayers on the apical chamber of transwell culture inserts were infected with H5N1 (A/HongKong/483/97) influenza virus (MOI = 0.1). Influenza virus-infected cells cocultured with different cell numbers of MSCs in the basolateral chamber. Data represent the mean ± SE from three experiments, *P < 0.05; **P < 0.01; ***P < 0.005.
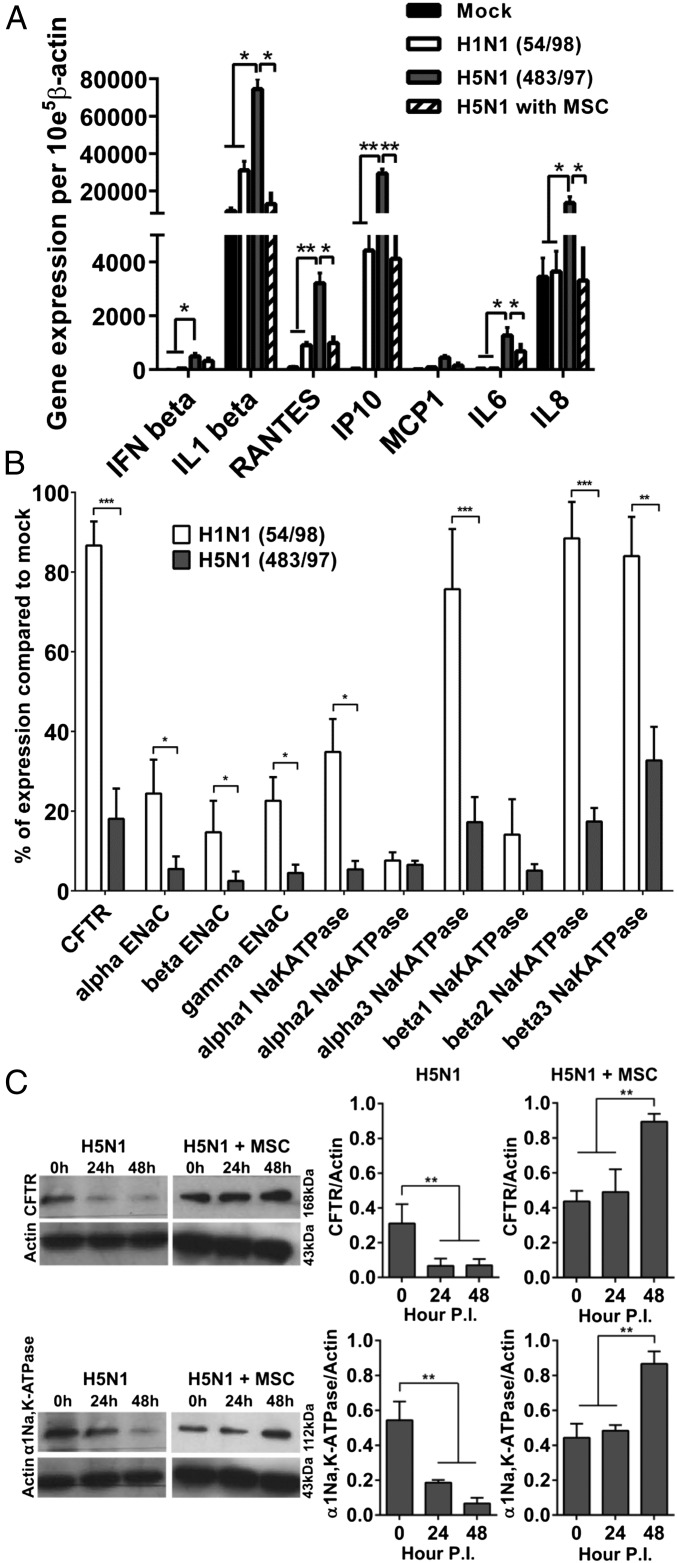
The mRNA expression of the major proinflammatory cytokines and chemokines IFN-β, interleukin (IL)-1β, RANTES, IP10, IL6, and IL8 was significantly greater in alveolar epithelial cells infected with H5N1 483/97 virus than in the cells infected with human influenza H1N1 54/98 (P = 0.041, 0.035, 0.006, 0.003, 0.031, and 0.038, respectively) or mock-infected (P = 0.033, 0.042, 0.004, 0.003, 0.024, and 0.034, respectively). Coculture of H5N1 virus-infected alveolar epithelial cells with MSCs significantly reduced IL-1β (P = 0.035), RANTES (P = 0.024), IL6 (P = 0.022), IL8 (P = 0.031), and IP10 (P = 0.006) mRNA levels compared with the cells without MSC coculture (Fig. 3A).
Fig. 3.
H5N1 influenza virus infection of alveolar epithelium induces greater cytokine and chemokine expression and lower sodium and chloride transporter expression than does H1N1 virus infection whereas coculture with MSCs prevents or reduces the H5N1 effects. See Fig. 2 for experimental details. (A) Alveolar epithelial cells infected with HPAI H5N1 virus showed greater gene expression of proinflammatory cytokines (IL1-β; IL-6; IL-8) and chemokines (RANTES; IP-10) than did cells infected with H1N1 virus whereas coculture with MSCs reduced expression of cytokines and chemokines. (B) H5N1 virus infection down-regulated expression of the major sodium and chloride transporters (with the exception of α2-Na,K-ATPase and β1-Na,K-ATPase) to a greater extent than infection with H1N1 virus. Data are percent expression compared with that in mock-infected cells. (C) Western blot analysis showed reduced expression of CFTR and α1-Na,K-ATPase protein in H5N1 virus-infected alveolar epithelial cells at 24 h p.i. and further reduction of α1-Na,K-ATPase at 48 h p.i. With MSC coculture, expression of CFTR and α1-Na,K-ATPase was increased at 24 h p.i. and increased more substantially at 48 h p.i. Shown is mean ± SE protein expression relative to β-actin expression in three experiments. *P < 0.05; **P < 0.01; ***P < 0.005.
Coculture with MSCs Prevents Down-Regulation of Sodium and Chloride Transporter Proteins in H5N1 Virus-Infected Alveolar Epithelial Cells.
Major sodium and chloride transporter mRNA levels were decreased by H1N1 54/98 infection and decreased even more by H5N1 483/97 infection (Fig. 3B). These effects were confirmed in the protein expression of cystic fibrosis transmembrane conductance regulator (CFTR) (3.2 times less protein expression at both 24 h and 48 h p.i with H5N1, both P = 0.006) and α1Na,K-ATPase (3.1 times and 7.1 times less protein expression at 24 h and 48 h p.i. with H5N1, P = 0.008 and 0.006, respectively) (Fig. 3C). These H5N1-induced effects were prevented by coculture with MSCs at 48 h p.i, and expression of the transporter proteins CFTR and α1Na,K-ATPase was significantly enhanced (P < 0.009 and 0.007, respectively) (Fig. 3C).
MSCs Cocultured with H5N1-Infected Alveolar Epithelial Cells Secrete Ang1 and KGF.
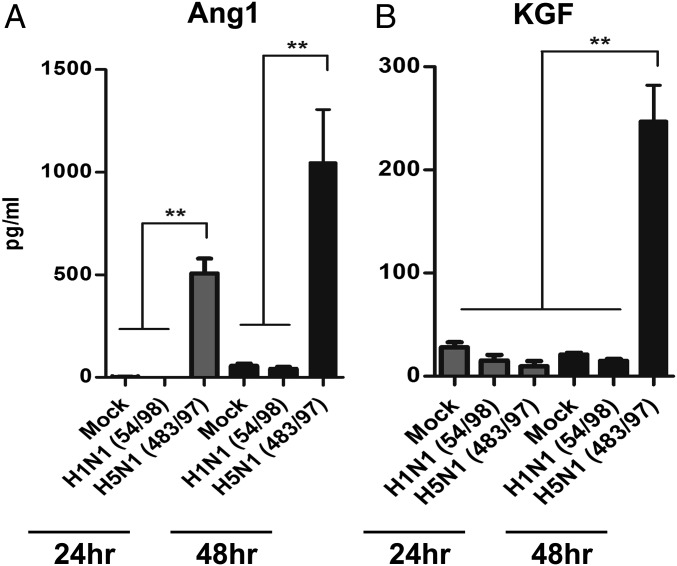
Ang1 and KGF are paracrine soluble factors associated with the beneficial effects of MSCs in preclinical models (10). After coculture of MSCs with alveolar epithelial cells infected with H5N1 virus, Ang1 in MSC supernatants increased by a factor of 500 at 24 h p.i. and a factor of 1,015 at 48 h p.i., compared with levels in mock cocultures (both P = 0.006) (Fig. 4A). Similarly, KGF secretion by MSCs cocultured with H5N1 virus-infected alveolar epithelial cells was greater than that in mock cocultures by a factor of 253 (P = 0.005) at 48 h p.i. (Fig. 4B). These effects were not observed in MSCs cocultured with H1N1 virus-infected alveolar epithelial cells.
Fig. 4.
Growth factor secretion by mesenchymal stromal cells is enhanced by coculture with H5N1 influenza virus-infected alveolar epithelial cells and contributes to reduction of AFC and APP impairment. Secretion of (A) Ang1 and (B) KGF by MSCs was increased significantly more by coculture with alveolar epithelium infected with H5N1 (A/HongKong/483/97) influenza virus vs. H1N1 (A/HongKong/54/98) virus, as measured by ELISA. Mock, mock infection. Data are the mean ± SE from three experiments. **P < 0.01.
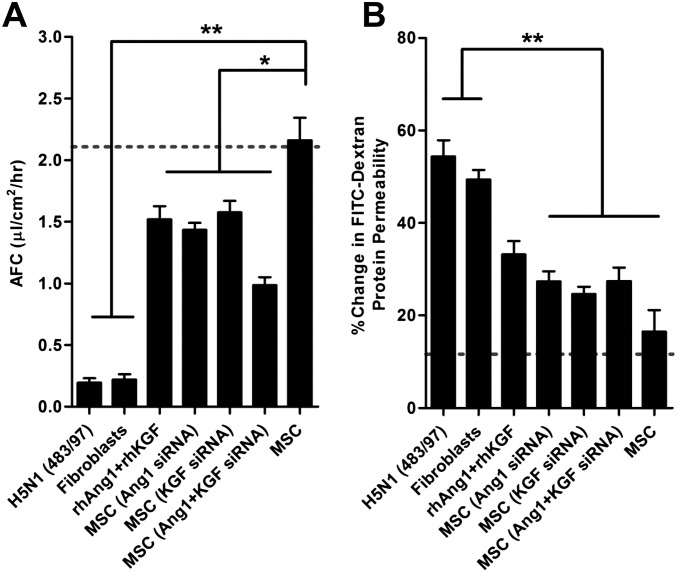
To assess the effect of Ang1 and KGF on the AFC and APP of H5N1 virus-infected alveolar epithelial cells, we selectively knocked down Ang1 and/or KGF protein in MSCs by using Ang1 and KGF siRNAs, separately or together. We cocultured H5N1 483/97-infected human alveolar epithelial cells on the transwell membrane with MSCs transfected with the siRNA or with scrambled siRNA as control on the transwell basolateral chamber. Pretreatment with Ang1 siRNA or KGF siRNA reduced the MSC-mediated increase of AFC by 35% and 30%, respectively, compared with that of mock or MSCs with scrambled siRNA-transfected (P = 0.036 and 0.042, respectively) (Fig. S4A). Similarly, MSC pretreatment with Ang1 siRNA or KGF siRNA reduced the MSC-mediated decrease of APP by 44% and 39%, respectively, compared with that of mock or MSC with scrambled siRNA-transfected (P = 0.008 and 0.007, respectively) (Fig. S4B). Further, siRNA knock-down of both Ang1 and KGF reduced the ability of MSCs to restore AFC and APP by 58% and 46%, respectively (P = 0.027 and 0.007, respectively) (Fig. S4). Conversely, addition of 100 ng/mL recombinant human (rh) Ang1 and KGF alone to H5N1 virus-infected alveolar epithelial cells in the absence of MSCs reduced the virus-mediated decrease of AFC by ∼60% (Fig. S4A) and the increase of APP by ∼56% (Fig. S4B). Therefore, MSC mediated the reduction of AFC and APP impairment in part via secreted Ang1 and KGF. However, these two factors together did not reduce H5N1-mediated lung injury to the extent achieved by MSC coculture, suggesting that other soluble factors or other mechanisms play a synergistic role.
Fig. S4.
Growth factor secretion by mesenchymal stromal cells contributes to reduction of AFC and APP impairment. (A and B) Ang1 and KGF mRNA was knocked down in MSCs by using 300 nM Ang1 and/or 100 nM KGF siRNA. H5N1-infected alveolar epithelium cocultured with MSCs with knock-down of Ang1, KGF, or both showed (A) less increase of AFC and (B) less decrease of APP than did cells cocultured with intact MSCs. Addition of 100 ng/mL recombinant human rhAng1 and rhKGF proteins to H5N1 virus–infected alveolar epithelium in the absence of MSCs reduced the H5N1 virus-mediated (A) AFC decrease and (B) APP increase. Dotted lines indicate AFC and APP in mock-infected cells. Data are the mean ± SE from three experiments. *P < 0.05; **P < 0.01.
Effect of MSC Therapy in H5N1 Virus-Infected Mice.
We assessed the effect of MSC treatment on the survival, body weight, lung alveolar protein permeability, alveolar fluid clearance, lung cellular infiltration, and lung pathology of H5N1 virus-infected mice. In young mice (6–8 wk of age) challenged with H5N1 486/97 virus and treated at day 5 p.i. with i.v. MSCs (5 × 105 cells in 100 μL), survival and weight loss were comparable with that with mouse fibroblast treatment (Fig. S5). However, survival (P = 0.032) (Fig. 5A) and body weight (P < 0.05) (Fig. 5B) were significantly greater in infected, MSC-treated aged mice (8–12 mo of age) than in aged controls, despite similar lung virus titers at days 7 and 10 p.i. (i.e., days 2 and 5 post-MSC treatment) (Fig. S6A).
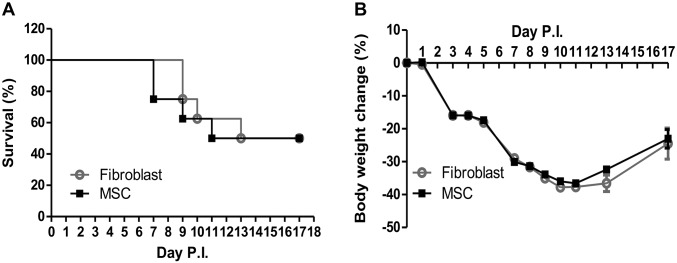
Fig. S5.
Effects of treatment with MSCs in a young animal model of highly pathogenic H5N1 influenza virus infection. All experiments were carried out in aged (6–8 wk) mice inoculated with A/Hong Kong/486/1997(H5N1) virus and injected i.v. with 5 × 105 MSCs/100 μL or mouse fibroblasts (control) on day 5 p.i. MSC treatment of young mice 5 d after inoculation with H5N1 influenza virus showed no significant improvement in (A) survival or (B) body weight change. Young mice infected with A/HongKong/486/1997 (H5N1) virus were i.v. injected with 5 × 105 MSCs/100 μL (n = 16) or with mouse fibroblasts (controls, n = 25).
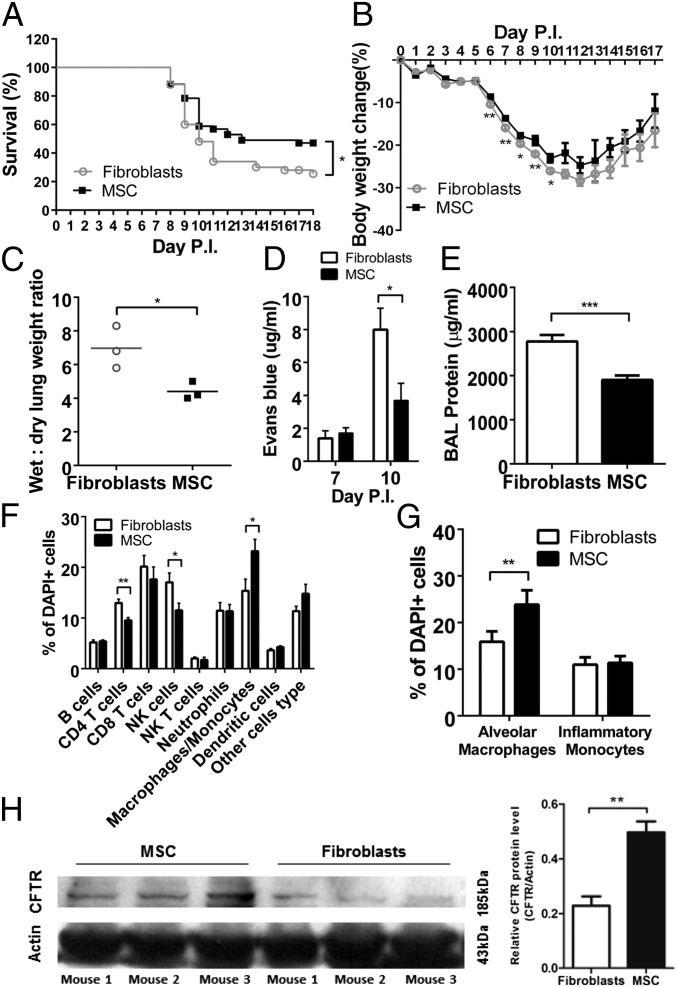
Fig. 5.
Effects of treatment with MSC in an aged animal model of highly pathogenic H5N1 influenza virus infection. All experiments were carried out in aged (8–12 mo) mice inoculated with A/Hong Kong/486/1997(H5N1) virus and injected i.v. with 5 × 105 MSC/100 μL or mouse fibroblasts (control) on day 5 p.i. (A) Survival of mice injected with MSC (n = 51) or mouse fibroblasts (n = 50). Data are the means from three experiments. (B) Percent body weight change of mice injected with MSCs (n = 79) or mouse fibroblasts (n = 83). Data are the mean from three experiments. (C) Wet-to-dry lung weight ratio of mice treated with MSCs (n = 3) or mouse fibroblasts (n = 3) on day 7 p.i. (D) Lung tissue concentration of Evans blue dye at days 7 and 10 p.i. in mice treated with MSCs (n = 4) or mouse fibroblasts (n = 4). (E) Total protein concentration (indicating lung injury) at day 7 p.i. in BAL fluid from mice treated with MSCs (n = 4) or mouse fibroblasts (n = 4). (F) Immune cell profile of BAL fluid on day 7 p.i. from mice treated with MSCs (n = 5) or mouse fibroblasts (n = 5). (G) Mean (± SD) percentage of alveolar macrophages and inflammatory monocytes at day 7 p.i. in BAL fluid from mice treated with MSCs (n = 5) or mouse fibroblasts (n = 5). Counts were repeated at least twice. (H) CFTR transporter protein at day 10 p.i. in lung lysates of virus-infected mice injected with MSCs (n = 3) or mouse fibroblasts (n = 3). In all comparisons, *P < 0.05; **P < 0.01; ***P < 0.005.
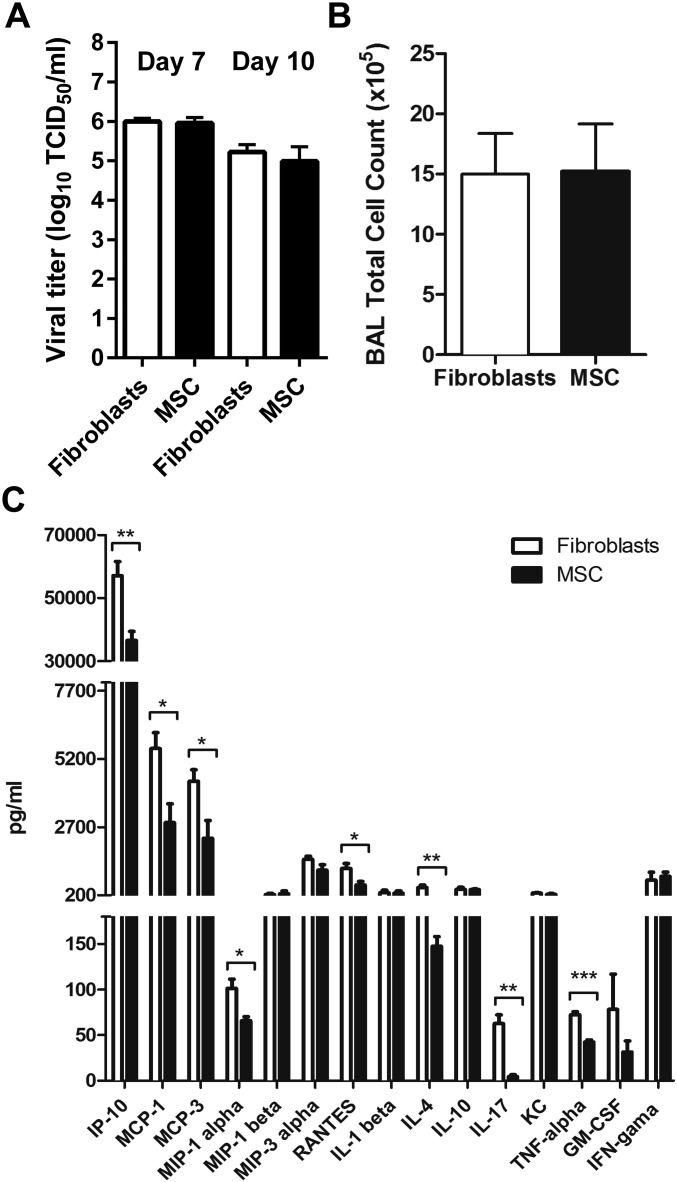
Fig. S6.
Effects of treatment with MSCs in an aged animal model of highly pathogenic H5N1 influenza virus infection. All experiments were carried out in aged (8–12 mo) mice inoculated with A/Hong Kong/486/1997(H5N1) virus and injected i.v. with 5 × 105 MSCs/100 μL or mouse fibroblasts (control) on day 5 p.i. (A) Comparable lung virus titers at days 7 and 10 p.i. in mice injected with MSCs (n = 3) or mouse fibroblasts (n = 3). (B) Mean (± SD) cell count at day 7 p.i. in BAL fluid of mice treated with MSCs (n = 5) or mouse fibroblasts (n = 5). Cell counts were repeated at least twice. (C) Concentration of major proinflammatory cytokines and chemokines in BAL fluid at day 7 p.i. in mice treated with MSCs (n = 4) or mouse fibroblasts (n = 4). In all comparisons, *P < 0.05; **P < 0.01; ***P < 0.005.
Comparison of the wet-to-dry lung weight indicated the net effect of alveolar fluid clearance whereas extravasation of Evans blue dye was an indicator of fluid leakage into the alveolar spaces. MSC-treated mice had a significantly lower wet-to-dry lung weight than control mice at day 7 p.i. (P = 0.023) (Fig. 5C). A significantly lower concentration of Evans blue dye in the lungs of MSC-treated vs. control mice at day 10 p.i. (P = 0.015) (Fig. 5D) suggested that MSC treatment reduces vascular leakage of plasma protein. The total protein concentration in the bronchoalveolar lavage (BAL) fluid was significantly less in MSC-treated than in control mice at day 7 p.i. (P = 0.004) (Fig. 5E).
MSC treatment did not affect the total number of cells in the BAL fluid (Fig. S6B). However, the BAL fluid at day 7 p.i. showed significantly fewer CD4+ T cells (P = 0.008) and NK cells (P = 0.039) and significantly more macrophages/monocytes (P = 0.028) after MSC treatment than after fibroblast treatment (Fig. 5F). After MSC treatment, the macrophage/monocyte population in the BAL fluid was predominantly alveolar macrophages with an M2 phenotype (CD11c+ Ly6Cint F4/80+) (14) (P = 0.023) (Fig. 5G).
The concentrations of most cytokine and chemokine proteins tested (IP10, MCP-1, MCP-3, M1P-1 alpha, RANTES, IL-4, IL-17, and TNF-alpha) were significantly lower in the BAL fluid of MSC-treated mice than in that of fibroblast-treated mice at day 7 p.i. (Fig. S6C). CFTR protein expression was significantly higher in the lung lysates of MSC-treated mice than in the fibroblast-treated mice at day 10 p.i., as shown by Western blot analysis (P = 0.007, Fig. 5H).
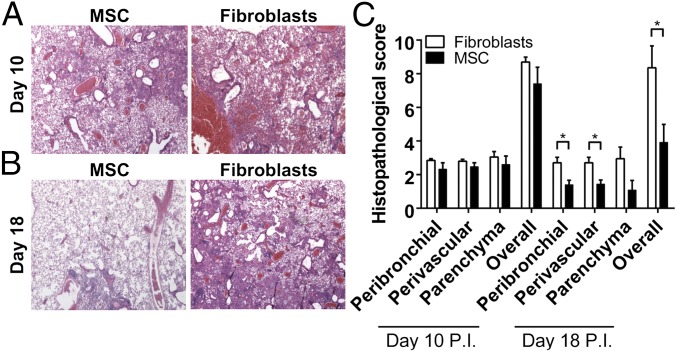
Mice infected with the H5N1 influenza viruses with or without MSC treatment showed comparable lung pathology (Fig. 6A) and histopathology scores (Fig. 6C) at day 10 p.i., but MSC-treated mice had significantly less lung pathology at day 18 p.i. (Fig. 6B). The peribronchial, perivascular, and overall histopathology scores of the MSC-treated mice were significantly lower than the fibroblast-treated mice at day 18 p.i. (P = 0.018, 0.023, and 0.015, respectively) (Fig. 6C). MSC-treated and control mice did not seem to differ in alveolar epithelial or endothelial cell proliferation.
Fig. 6.
Improved lung pathology and histopathology scores of aged mice treated with MSCs after inoculation with highly pathogenic H5N1 virus. (A and B) Lung tissue at (A) day 10 p.i. and (B) day 18 p.i. from H5N1 virus-infected mice after treatment with MSCs (n = 4) or mouse fibroblasts (n = 4) on day 5 p.i. Hematoxylin/eosin stain. (Magnification: 100×.) (C) Histopathology scores of peribronchiolar, perivascular, and parenchymal lung tissues at days 10 and 18 p.i. from influenza virus-infected mice treated with MSCs or mouse fibroblasts. *P < 0.05.
Discussion
Here, we demonstrated that soluble mediators released from influenza A H5N1 virus-infected alveolar epithelial cells impair alveolar fluid clearance and protein permeability by down-regulating alveolar sodium and chloride transporter proteins. This pathology was prevented or reduced by coculture with MSCs in vitro and by MSC treatment in vivo in aged mice.
Highly pathogenic H5N1 and H7N9 influenza viruses caused markedly greater impairment of AFC and APP in cultured human alveolar epithelial cells than did the seasonal H1N1 and H3N2 influenza viruses. This effect was caused largely by soluble mediators and growth factors released by H5N1 or H7N9 virus-infected, but not H1N1-infected, alveolar epithelial cells rather than by direct viral cytopathic effects or viral replication competence alone. Infection with H5N1, compared with seasonal H1N1, influenza virus also led to greater down-regulation of the Na,K-ATPase and CFTR transporter proteins in alveolar epithelium. Influenza virus infection was previously shown to impair alveolar fluid transport by inhibiting epithelial sodium channel activity through interaction of viral hemagglutinin (15) and matrix (16) proteins with the epithelial sodium channel. To our knowledge, ours is the first demonstration of impaired alveolar fluid clearance and protein permeability due to soluble mediators released by infected alveolar epithelium.
Alveolar fluid clearance was previously found to be impaired in patients with sepsis-induced acute injury; a similar in vitro experimental model of Escherichia coli endotoxin-induced acute lung injury showed that alveolar fluid clearance was impaired via proinflammatory cytokines and chemokines (10), demonstrating the physiological relevance of the model we used. In our study, this model comprised alveolar epithelium infected with the highly pathogenic H5N1 viruses that induce higher levels of proinflammatory cytokines and chemokines than does seasonal H1N1 virus. Activation of proinflammatory pathways was previously shown to result in down-regulation of sodium and chloride transporters responsible for vectorial fluid transport across the alveolar epithelium (17), increasing paracellular protein permeability. To our knowledge, ours is the first demonstration of this phenomenon in an experimental model of virus-infected alveolar epithelium.
We found that when MSC expression of the growth factors Ang1 and/or KGF was knocked down by siRNA, the MSCs were less able to attenuate the effects of H5N1 virus infection on alveolar AFC and APP. However, recombinant Ang1 and KGF only partially restored the attenuation of pathology. Therefore, secreted Ang1 and KGF account only partially for the therapeutic effect of MSCs on alveolar epithelial AFC and APP.
When mice were infected with H5N1 virus, MSC therapy increased survival and reduced weight loss only among the aged mice. At day 18 p.i., these mice had fewer lung lesions and a greater number of bronchoalveolar antiinflammatory M2 macrophages, which also enhance tissue repair (18), and proinflammatory cytokine and chemokine levels were low. However, their lung virus titers remained comparable with those in untreated mice; therefore, the observed phenotype did not reflect a direct antiviral effect of MSCs.
MSCs, alone or as an adjunct to antiviral therapy, were recently reported not to improve the survival of 7- to 10-wk-old C57BL/6 mice inoculated with A/PuertoRico/8/34 (mouse-adapted H1N1) or A/Mexico/4108/2009 (pandemic H1N1) influenza virus (13, 19). Our findings are similar; MSCs failed to improve survival (Fig. S5) or histopathology in young (6–8 wk of age) mice inoculated with H5N1 influenza viruses. MSC treatment was beneficial only in aged mice (8–12 mo of age). Age affects various MSC functions, including expression and secretion of soluble factors important in recovery from lung injury (20). Aged animals show lower expression of genes involved in cell activation and migration and of cytokine receptors (e.g., TNFR1 and TNFR2) and chemokine receptors (CCR7, CX3CR1, and CXCR5) involved in MSC migration and chemotaxis (21). Thus, younger animals have more potent endogenous MSC responses (22) whereas exogenous MSC therapy is more likely to exert an apparent benefit in older animals.
Age-related loss of lung repair capacity may also explain the progressive age-related increase in the clinical severity of severe acute respiratory syndrome (SARS) (23, 24) and pandemic H1N1 virus infection (25) and the higher mortality reported in older patients with acute respiratory distress syndrome (26). Age also determines the severity of SARS in both mouse and macaque experimental models (27, 28), and aged mice were more susceptible to exogenous endotoxin (LPS) in an animal model of sepsis-induced kidney injury (29). Although age-related changes may account for some of these findings, there may be other factors as well. We hypothesize that, in the context of severe influenza, aged and young mice differ in the mechanisms of both lung pathology and repair. As we previously reported, the pathogenic mechanisms of H5N1 viruses differ in important ways from the seasonal or pandemic H1N1 influenza viruses (30, 31). Likewise, the pathogenic mechanisms and the potential benefits of MSC therapy very likely depend on factors relating to both the virus and the host.
We did not investigate the effect of combining MSC therapy with antiviral therapy, as would be done clinically. Our primary interest at this stage was the impact of MSC therapy on the pathogenesis of severe influenza, which was best shown by using MSC therapy alone. Future studies should investigate the synergy of MSC with antivirals and the mechanisms underlying the interaction of age with the effects of MSC therapy.
In summary, infection with highly pathogenic H5N1 and H7N9 influenza viruses dysregulated alveolar fluid clearance and protein permeability in vitro, as observed in humans; these effects were mediated by infected cells’ release of soluble factors that down-regulate the sodium and chloride transporters. The ability of highly pathogenic influenza virus to modulate AFC and APP may provide useful parameters for assessing virulence. In vivo, MSC therapy diminished lung injury and increased survival in aged H5N1-infected mice. These preclinical data suggest that MSCs may provide a therapeutic benefit to humans with severe pulmonary illness caused by influenza viruses such as H5N1 and H7N9, especially older patients, who are at higher risk. The identification of alveolar epithelial sodium and chloride transporters as key pathogenic targets and the modulation of these pathogenic mechanisms by factors released by MSCs suggest additional therapeutic possibilities for the clinical management of viral acute lung injury.
Materials and Methods
Detailed descriptions of experiments are provided in SI Materials and Methods.
Influenza A Viruses.
The H5N1 influenza A viruses used were A/HongKong/483/97 (483/97), A/HongKong/486/97 (486/97), and A/Vietnam/3046/04 (3046/04); all were highly pathogenic viruses. The H7N9 influenza A virus was A/Shanghai/2/2013 (Sh2). The seasonal influenza A viruses were H1N1 [A/HongKong/54/98 (54/98) and A/Oklahoma/447/08 (OK447/08)] and H3N2 [A/HongKong/1174/99 (1174/99) and A/Oklahoma/370/05 (OK370/05)]. All viruses were human isolates. They were grown in Madin–Darby canine kidney (MDCK) cells and then aliquotted and stored at −80 °C. Viruses were titrated in MDCK cells [50% tissue culture infective dose (TCID50)].
Acute Lung Injury Coculture Model.
A previously described in vitro model (Fig. 1) (7) was adapted for use in a biosafety level-3 (BSL-3) laboratory and used to measure net AFC and APP during influenza virus infection. Alveolar epithelial cells were isolated from resected, nonmalignant lung tissues at Queen Mary Hospital, Hong Kong. The study was approved by the Institutional Review Board of the University of Hong Kong (IRB reference no. UW 09-394) and Hospital Authority, Hong Kong West Cluster. The cells were plated on 24-well transwell insets to establish an air–liquid interface. Net AFC was measured by adding FITC-labeled dextran (Sigma) to the cells 24 h after virus infection [multiplicity of infection (MOI) = 0.1] or after incubation with virus-free conditioned medium for 1 h. Cytomix (mixed proinflammatory cytokines in buffer; R&D Systems) served as positive control.
Bone marrow-derived human MSCs obtained from the Texas A&M Health Science Center were added to the basolateral chamber of the transwell to assess their effect on AFC and APP; human lung fibroblasts were used as controls. To assess the roles of the MSC-secreted soluble growth mediators Ang1 and KGF, MSCs were reverse-transfected with 300 nM Ang1 (121280; Ambion) and/or 100 nM KGF (10818; Ambion) silencing RNA (siRNA) duplex or with scrambled siRNA (negative control). The recombinant human proteins, 100 ng/mL rhAng-1 and 100 ng/mL rhKGF (R&D Systems), were used to compare the effect of defined soluble proteins on H5N1 virus-impaired AFC and APP.
Mouse Studies.
Female Balb/C mice aged 6–8 wk (young) or 8–12 mo (“aged”) were inoculated intranasally with 106 TCID50 of 486/97 influenza virus in 25 μL. At day 5 p.i., mice were injected i.v. with 5 × 105 human bone marrow-derived MSCs in 100 μL. NIH 3T3 mouse embryo fibroblasts (5 × 105) in 100 μL (ATCC) served as control. MSCs do not express major histocompatibility antigens and do not elicit a rejection response, and human MSCs have been used in previous studies on endotoxin-induced acute lung injury models (10). Survival and body weight were monitored for 18 d. At day 7 p.i., virus was titrated in three mouse lungs per group (MSC-treated, fibroblast-treated groups). BAL fluid was collected on day 7 p.i. for cytokine assay and immune cell profiling (SI Materials and Methods). At day 10 p.i., three mice per group were killed for measurement of pulmonary microvascular permeability and CFTR protein expression. On days 10 and 18 p.i., four mouse lungs per group were fixed for histopathology. The animal infection experiments were approved by the University of Hong Kong Animal Ethics Committee (CULATR 3445-14).
Measurement of Cytokines, Sodium and Chloride Transporter Proteins, and MSC-Secreted Soluble Proteins.
Total RNA and total cellular protein were extracted from virus-infected alveolar epithelial cells or mouse lungs. Cytokines, major transporter proteins, and Ang1 and KGF soluble proteins secreted by MSCs were measured as described in SI Materials and Methods.
Statistical Analysis.
Mean values from three independent experiments were compared by unpaired two-tailed t tests. One-way analysis of variance with a post hoc Bonferroni test was used as appropriate. Differences were considered significant at P < 0.05. All statistical analyses used GraphPad Prism version 6.0 software.
SI Materials and Methods
Virus Titration by TCID50 Assay.
A 96-well tissue culture plate of confluent MDCK cells was prepared 1 d before virus titration (TCID50). Cells were washed once with PBS, and medium was replenished with serum-free minimum essential medium (MEM) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 μg/mL tosylsulfonyl phenylalanylchloromethyl ketone (TPCK)-treated trypsin. Serial dilutions of virus supernatant, from 0.5 log to 7 log, were added to the cell plates in quadruplicate. The plates were observed daily for cytopathic effects. The virus dilution causing cytopathic effects in 50% of inoculated wells was estimated by using the Karber method.
Human Mesenchymal Stromal Cells.
Human MSCs derived from bone marrow were obtained from the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott and White. All MSCs used in this study fulfilled the standard definition of MSCs published by the International Society of Cellular Therapy. MSCs used in both in vitro and in vivo models were analyzed by flow cytometry before experiments and found to be >99% positive for the stem cell surface antigens CD73, CD90, and CD105, thereby meeting the defining criteria. MSCs were cultured in α-MEM without ribonucleosides or deoxyribonucleosides (GibcoBRL) containing 2 mM l-glutamine, 16.5% (vol/vol) HyClone FBS (SV30160.03; Thermo Fisher) and 1% each of penicillin and streptomycin.
Isolation of Human Alveolar Epithelial Cells.
Human alveolar epithelial cells were isolated as previously described (6) from resected, nonmalignant human lung tissue obtained from the Department of Cardiothoracic Surgery, Queen Mary Hospital, Hong Kong, as part of a study approved by the Institutional Review Board of the University of Hong Kong (IRB reference no. UW 09–394) and Hospital Authority, Hong Kong West Cluster.
Briefly, visible bronchi were removed, and the lung tissue was minced (using a tissue chopper) into pieces of <0.5 mm in size. Minced tissue was washed three times with Hank's balanced salt solution (HBSS) (Gibco) and 0.7 mM sodium bicarbonate (Gibco) at pH 7.4 to remove most of the macrophages and blood cells. The tissue was digested using a combination of 0.5% trypsin (Gibco) and 4 U/mL elastase (Worthington Biochemical Corp.) for 40 min at 37 °C in a shaking water bath. Digestion was stopped by adding DMEM/F12 medium (Gibco) with 40% FBS and 350 U/mL DNase I (Sigma). Cell clumps were dispersed by repeatedly pipetting the cell suspension for 10 min. A disposable cell strainer (gauze size 50 μm) (BD Bioscience) was used to remove large undigested tissue fragments. The single-cell suspension was centrifuged, and the pellet was resuspended in a 1:1 mixture of DMEM/F12 medium and small airway basal medium (SABM) (Lonza) supplemented with 0.5 ng/mL epidermal growth factor (hEGF), 500 ng/mL epinephrine, 10 μg/mL transferrin, 5 μg/mL insulin, 0.1 ng/mL retinoic acid, 6.5 ng/mL triiodothyronine, 0.5 μg/mL hydrocortisone, 30 μg/mL bovine pituitary extract, and 0.5 mg/mL BSA, with 5% FBS and 350 U/mL DNase I. The cell suspension was plated in tissue culture flasks and incubated in a 37 °C water-jacketed incubator at 5% CO2 for 90 min. The nonadherent cells were layered on a discontinuous cold Percoll density gradient (density 1.089 and 1.040 g/mL) and centrifuged at 25 × g for 20 min. The cell layer at the interface of the two gradients was collected and washed four times with BSS to remove the Percoll fluid. The cell suspension was incubated with magnetic beads coated with anti-CD14 antibodies (MACS CD14 MicroBeads) at room temperature for 20 min. After removal of the beads by using a magnet, cell viability was assessed by trypan blue dye exclusion. The alveolar epithelial cell suspension was resuspended in SAGM (Lonza Walkersville, Inc.) supplemented with 1% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin and plated at a cell density of 3 × 105 per cm2. The cells were maintained in a humidified atmosphere (5% CO2, 37 °C), and growth medium was changed daily, starting 60 h after plating the cells. When the cell layer approached 75% confluence, the alveolar epithelial cells were dissociated with trypsin, detached into Hanks’ buffered saline solution, and then seeded as described below in the transwell culture inserts.
In Vitro Model of Acute Lung Injury.
To study the effect of MSCs on alveolar fluid clearance and protein permeability impaired by influenza H5N1 virus infection in human alveolar epithelial cells, we adapted an in vitro model of acute lung injury by using a previously described coculture system (7); the protocol was modified for use in a BSL-3 laboratory. The alveolar epithelial cells were plated on the apical chamber of 24-well Costar Transwell inserts with a 0.4-μm pore size (Corning), at a density of 100,000 cells per well. The microporous transwell membrane established an air–liquid interface similar to that of human lung epithelium, and the plated cells were maintained in a humidified atmosphere (5% CO2, 37 °C). For coculture experiments with alveolar epithelial cells, MSCs or normal adult human lung fibroblasts (controls) (Lonza Inc.) were plated on the basolateral chamber of the transwell (Fig. 1) at a density of 100,000 cells per well (or with different MSC cell density: 1,000, 10,000, 50,000, and 500,000 MSCs) and had no direct contact with the apical chamber. The virus-free conditioned medium was the supernatant of H5N1 virus-infected alveolar epithelial cells, collected at 24 h postinfection (p.i.) and filtered through a 100-kDa-pore filter (Millipore) to remove virus particles. In the filtered supernatant, influenza viral matrix (M) gene expression was monitored by quantitative RT-PCR, and virus was titrated by TCID50 assay to ensure the absence of infectious virus. The virus-free supernatants were used to treat the influenza A virus-infected alveolar epithelial cells to observe the effect on alveolar fluid transport and protein permeability across the alveolar epithelial cell monolayers. Cytomix, a mixture of the cytokines IL-1β, TNF-α, and IFN-γ (R&D Systems), was used at 50 ng/mL as a positive control for lung injury.
Measurement of Alveolar Fluid Clearance and Alveolar Protein Permeability.
We measured net alveolar fluid transport from the apical chamber of the transwell culture insert (containing a monolayer of alveolar epithelial cells infected with influenza virus) to the basolateral chamber of the transwell at 24 h p.i. Transepithelial resistance was maintained at 1,500 Ω/cm2, and there was morphological evidence of tight junctions. Alveolar epithelial cells were either inoculated with the respective influenza A viruses at an MOI of 0.1 or incubated with the virus-free conditioned medium for 1 h. Then, 200 μL of FITC-labeled dextran (Sigma) with a size of 70 kDa was added to the cells (final concentration, 500 ng/μL). After 5 min, the initial sample of 20 μL was collected from the apical chamber. After 24 h of dextran incubation, another 20 μL was collected from the same compartment, and 100 μL was collected from the basolateral chamber as the final samples. The fluorescence of each sample was measured by a modulus fluorometer (Fluostar Optima, BMG Labtech).
The in vitro lung injury model and the measurement of net AFC and APP were described previously (7); the protocol was modified for use in a BSL-3 laboratory. The net alveolar fluid transport in μL⋅cm−2⋅h−1 was measured as [1 – (fluorescence reading in the initial sample/weight of the initial sample)/(fluorescence reading in the final sample/weight of the final sample)] × 100. The remaining fluid in the basolateral chamber was collected after 24 h of dextran incubation to measure protein permeability, based on the unidirectional flux of fluorescence-labeled dextran from the apical to the basolateral chamber of the transwell culture insert. The transepithelial resistance between the apical and basolateral chambers of the transwell was maintained at 1,500 Ω/cm2, and there was morphological evidence of tight junctions.
The Cytokine Responses of Influenza Virus-Infected Alveolar Epithelial Cells.
Alveolar epithelial cells were inoculated with influenza viruses at an MOI of 2. After 24 h, total RNA was extracted by using the RNeasy Mini Kit (Qiagen) and incubated with RNase-free DNase (Qiagen) according to the manufacturer’s instructions. cDNA was generated by reverse transcription using the PrimeScript RT Reagent Kit (Takara) according to the manufacturer’s instructions. The mRNA expression of cytokines (IFN-β, and IL-1β) and chemokines (RANTES, IP-10, MCP-1, IL-6, and IL8) and of sodium and chloride transporters (CFTR; α-, β-, and γ-ENaC; α1-, α2-, α3-, β1-, β2-, and β3-NaKATPase) by the infected cells was evaluated by using the SYBR Premix Ex Taq II kit (Takara) according to the manufacturer’s instructions. The primers for the proinflammatory cytokines and chemokines were described previously (6), and the primers for the other genes of interest were obtained from Sigma. The cDNA products were amplified by using gene-specific primers and quantified by using the 96-well format on an ABI ViiA 7 Real-Time PCR System (Applied Biosystems Inc.). Amplification data (cycle threshold values) were converted to relative gene copy number based on the linear regression of the standard curve. Each gene’s expression was calculated after normalization to beta-actin expression.
Protein Expression of Major Ion Transporters, Cytokines, and Growth Factors in Infected Alveolar Epithelial Cells, MSC Supernatants, and Mouse Lungs.
Total cellular protein was extracted from influenza virus-infected alveolar epithelial cells and prepared mouse lung tissue by using radioimmunoprecipitation assay (RIPA) lysis buffer (Sigma) (0.15 mL per well of cells and 0.4 mL per lung of mouse) with EDTA-free protease inhibitor (Roche). Each sample was denatured, and 20 μg was loaded on a 4–12% gradient self-cast Tris-Gly polyacrylamide gel with Tris-Gly SDS buffer and electrophoresed at 120 V for 80 min. The proteins were then transferred to a Hybond-P PVDF membrane (GE Healthcare) and incubated with 5% milk in Tris-buffered saline with Tween 20 (TBS-T) for 1 h. The membrane was incubated overnight with the specific primary antibodies to CFTR (Cell Signaling), α1-NaKATPase (Novus Biologicals), and beta-actin (Millipore). Secondary conjugate antibodies to the primary antibodies were used the following day. Protein bands were visualized by chemiluminescence (ECL+ reagent; Amersham Biosciences) and quantified by using NIH ImageJ software.
Bronchoalveolar lavage (BAL) (a washing procedure that recovered a fluid sample from the site of infection) was conducted at day 7 p.i. BAL fluid was analyzed quantitatively for cytokines (IP10, MCP-1, MCP-3, M1P-1α, M1P-1β, M1P-3α, RANTES, IL-1β, IL-4, IL-10, IL-17 KC, TNF-α, GM-CSF, and IFN-γ) by using a flow cytometry-based immunoassay (Flowcytomix Multiplex; Bender MedSystems) and protocols according to the manufacturer's instruction manual. The soluble proteins secreted by MSCs, comprising angiopoietin-1 (Ang-1) and keratinocyte growth factor (KGF), were measured in the supernatants of MSC monolayers by ELISA (R&D Systems) according to the manufacturer’s instructions.
siRNA Transfection of MSCs.
MSCs were transfected with siRNAs as previously described by Lee et al. (10), except that Lipofectamine LTX (Invitrogen) was used as the transfection reagent. Negative scrambled siRNA was transfected as a control.
Experimental Infection of Mice.
Female Balb/C mice aged 6–8 mo (“young”) or 8–12 mo (“aged”) were obtained from the Laboratory Animal Unit of The University of Hong Kong. They were divided into two groups, anesthetized with a mixture of ketamine 1.88 mg and xylazine 0.11 mg, and inoculated intranasally with 25 μL of 1.5 log LD50 (106 log TCID50) of the influenza H5N1 virus A/HongKong/486/97 (486/97). Acute respiratory distress syndrome (ARDS) was the main cause of disease and death in Balb/C mice experimentally infected with this H5N1 influenza virus strain. Some other H5N1 virus strains commonly used for experimental studies in mice are associated with virus dissemination to the brain, and encephalitis is the major cause of death, although this pathology is not observed in most human H5N1 disease (31). Mouse survival and body weight were monitored after inoculation. The animal infection experiments were approved by the University of Hong Kong Animal Ethics Committee and were performed in the biosafety level 3 (BSL-3) containment laboratories at The University of Hong Kong.
Evans Blue Dye Extravasation in Infected Mouse Lungs.
At day 10 p.i., pulmonary microvascular permeability was measured in all mouse groups by using Evans blue assay. Dye (20 mg/kg) dissolved in PBS was i.v. injected 3 h before mice were euthanized. The pulmonary vasculature was perfused with 1 mL of PBS, and the lungs were dissected from the thoracic cavity and surrounding mediastinal structures. The lung tissue was then homogenized in 1 mL of PBS, added to two volumes of deionized formamide, and incubated at 60 °C for 12 h. The supernatant was then separated from the lung tissue by centrifugation at 2,000 × g for 30 min. Evans blue dye in lung tissue was quantified by dual-wavelength spectrophotometric analysis, using the following formula: corrected absorbance at 620 nm = actual absorbance at 620 nm − [1.426 (absorbance at 740 nm) + 0.03]. The concentration of extravasated Evans blue dye in lung homogenates was calculated against a standard curve. A pathologist blinded to the experimental details histopathologically scored airway, vascular, and parenchymal inflammation on a 13-point scale in tissue sections prepared from formalin-fixed, paraffin-embedded lungs and stained with hematoxylin/eosin.
Measurement of Immune Cells in BAL Fluid.
The protein concentration of BAL fluid was measured by using the Pierce BCA Protein Assay kit (Thermo Scientific). After centrifugation and separation, cells in the BAL fluid were characterized by flow cytometry. After Fc receptor blocking (anti-CD16/CD32, BD Bioscience), cells were stained for 30 min on ice with one of three panels of monoclonal antibodies to innate and adaptive immune cells (all from Biolegend, unless otherwise indicated): panel 1, F4/80-PE, I-AE-PerCPCy5·5, CD11b-APCy7, Gr1-PECy7, IA8-APC, and CD11c-FITC; panel 2, CD3-APC, CD4-APCCy7, CD8-PerCPCy5·5, Dx5-FITC, γδT-PE, and B220-PECy7; panel 3, F4/80-PECy7, CD11b-APCCy7, I-AE-PerCPCy5·5, MMR-APC, IL-4Rα-PE, and iNOS-FITC (BD Bioscience). For intracellular staining, cells were fixed in fixation/permeabilization buffer (eBioscience) for 30 min on ice and stained with iNOS-FITC in Perm wash buffer (BD Bioscience). Cells were then fixed with 100 μL of 4% paraformaldehyde for 20 min on ice and washed and stained with DAPI in PBS for 10 min on ice. Samples were analyzed by flow cytometry on a FACS LSRII and analyzed with FlowJo software.
Statistical Analysis.
All in vitro experiments were performed independently, using at least three different mice; each experiment was conducted in duplicate. Results shown in the figures are the calculated mean and SEM from three independent experiments. Groups were compared by using an unpaired, two-tailed t test. One-way analysis of variance, with the post hoc Bonferroni test, was used as appropriate, and no corrections were needed. Differences were considered significant at P < 0.05.
Mouse survival and body weight loss were compared in three independent in vivo experiments by using the log-rank test and a linear regression model. Pooled data from three independent in vivo experiments were analyzed if there was no indication of heterogeneity; otherwise, the difference was accounted for in the model by adding an indicator variable for the experiment. The survival of mice in the MSC treatment and fibroblast control groups was compared by using the log rank test whereas body weight loss was compared by using linear regression models. Virus titers, Evans blue dye extravasation, and the histopathology scores were compared by using the Student t test with a threshold of P < 0.05 for statistical significance. All statistical analyses used GraphPad Prism version 6.0 and R version 3.0.2 (R Foundation for Statistical Computing) programs.
Ethics Statement.
The Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (IRB reference UW 09–394) approved the use of human tissue for the isolation of human alveolar epithelial cells in this study. All adult subjects provided written consent for use of their tissue samples in scientific studies.
The animal studies were approved by the Committee on the Use of Live Animals in Teaching and Research (CULATR 3445-14), LKS Faculty of Medicine, The University of Hong Kong and were performed in a biosafety level 3 laboratory. The committee reviewed and approved the animal care plan, and the protocol of this study followed the Guide for the Care and Use of Laboratory Animals of the National Research Council of the National Academies of Science (32).
Acknowledgments
Dr. Alan D. L. Sihoe and staff (Division of Cardiothoracic Surgery, Department of Surgery, Li Ka Shing Faculty of Medicine, The University of Hong Kong and Queen Mary Hospital) provided human lung tissues. Technical support was provided by Ms. Carol C. C. Ho, Mandy M. T. Ng, Joanne H. M. Fong, Sia Sin Fan, Candy Ho, Mr. Li Hung Sing, Dr. Chris K. P. Mok (Centre of Influenza Research, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong), and Mr. Kevin Fung (Department of Pathology, The University of Hong Kong). Research funding was provided by the US National Institute of Allergy and Infectious Diseases (NIAID) under CEIRS Contract HHSN272201400006C. The Research Grants Council of the Hong Kong Special Administrative Region, provided funding under the Theme Based Research Scheme (T11-705/14N) and General Research Fund (HKU761210M).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601911113/-/DCSupplemental.
References
- 1.Chan MC, et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrone LA, Plowden JK, García-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4(8):e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandun IN, et al. Factors associated with case fatality of human H5N1 virus infections in Indonesia: A case series. Lancet. 2008;372(9640):744–749. doi: 10.1016/S0140-6736(08)61125-3. [DOI] [PubMed] [Google Scholar]
- 4.Howard WA, Peiris M, Hayden FG. Report of the 'mechanisms of lung injury and immunomodulator interventions in influenza' workshop, 21 March 2010, Ventura, California, USA. Influenza Other Respir Viruses. 2011;5(6):453–454, e458–e475. doi: 10.1111/j.1750-2659.2011.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Riel D, et al. H5N1 virus attachment to lower respiratory tract. Science. 2006;312(5772):399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 6.Chan MC, et al. Tropism and innate host responses of a novel avian influenza A H7N9 virus: An analysis of ex-vivo and in-vitro cultures of the human respiratory tract. Lancet Respir Med. 2013;1(7):534–542. doi: 10.1016/S2213-2600(13)70138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JW, et al. Acute lung injury edema fluid decreases net fluid transport across human alveolar epithelial type II cells. J Biol Chem. 2007;282(33):24109–24119. doi: 10.1074/jbc.M700821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: Mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2(12):1016–1026. doi: 10.1016/S2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
- 10.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106(38):16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monsel A, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192(3):324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson JG, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol. 2014;307(5):L395–L406. doi: 10.1152/ajplung.00110.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kedzierski L, et al. Suppressor of cytokine signaling 4 (SOCS4) protects against severe cytokine storm and enhances viral clearance during influenza infection. PLoS Pathog. 2014;10(5):e1004134. doi: 10.1371/journal.ppat.1004134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunzelmann K, et al. Influenza virus inhibits amiloride-sensitive Na+ channels in respiratory epithelia. Proc Natl Acad Sci USA. 2000;97(18):10282–10287. doi: 10.1073/pnas.160041997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazrak A, et al. Influenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen species. FASEB J. 2009;23(11):3829–3842. doi: 10.1096/fj.09-135590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elia N, et al. Functional identification of the alveolar edema reabsorption activity of murine tumor necrosis factor-alpha. Am J Respir Crit Care Med. 2003;168(9):1043–1050. doi: 10.1164/rccm.200206-618OC. [DOI] [PubMed] [Google Scholar]
- 18.Mills CD, Ley K. M1 and M2 macrophages: The chicken and the egg of immunity. J Innate Immun. 2014;6(6):716–726. doi: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darwish I, et al. Mesenchymal stromal (stem) cell therapy fails to improve outcomes in experimental severe influenza. PLoS One. 2013;8(8):e71761. doi: 10.1371/journal.pone.0071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bustos ML, et al. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014;189(7):787–798. doi: 10.1164/rccm.201306-1043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Németh K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsui PT, Kwok ML, Yuen H, Lai ST. Severe acute respiratory syndrome: Clinical outcome and prognostic correlates. Emerg Infect Dis. 2003;9(9):1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung CW, Chiu WK. Clinical picture, diagnosis, treatment and outcome of severe acute respiratory syndrome (SARS) in children. Paediatr Respir Rev. 2004;5(4):275–288. doi: 10.1016/j.prrv.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JT, et al. The infection attack rate and severity of 2009 pandemic H1N1 influenza in Hong Kong. Clin Infect Dis. 2010;51(10):1184–1191. doi: 10.1086/656740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts A, et al. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol. 2005;79(9):5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smits SL, et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6(2):e1000756. doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119(10):2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peiris JS, Cheung CY, Leung CY, Nicholls JM. Innate immune responses to influenza A H5N1: Friend or foe? Trends Immunol. 2009;30(12):574–584. doi: 10.1016/j.it.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung YH, et al. Highly pathogenic avian influenza A H5N1 and pandemic H1N1 virus infections have different phenotypes in Toll-like receptor 3 knockout mice. J Gen Virol. 2014;95(Pt 9):1870–1879. doi: 10.1099/vir.0.066258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]