Abstract
The establishment of robust T cell memory is critical for the development of novel vaccines for infections and cancers. Classical memory generated by CD8+ T cells is characterised by contracted populations homing to lymphoid organs. T cell “memory inflation”, as seen for example after cytomegalovirus infection, is the maintenance of expanded, functional, tissue-associated effector memory cell pools. Such memory pools may also be induced after adenovirus vaccination, and we recently defined common transcriptional and phenotypic features of these populations in mouse and man. However, the rules that govern which epitopes drive memory inflation compared to classical memory are not fully defined and thus it is not currently possible to direct this process. We used our adenoviral model of memory inflation to first investigate the role of the promoter and then the role of the epitope context in determining memory formation. Specifically, we tested the hypothesis that conventional memory could be converted to “inflationary” memory by simple presentation of the antigen in the form of “minigene” vectors. When epitopes from LacZ and MCMV that normally induce classical memory responses were presented as minigenes, they induced clear memory inflation. These data demonstrate that, regardless of the transgene promoter, the polypeptide context of a CD8+ T cell epitope may determine whether classical or inflating memory responses are induced. The ability to direct this process by the use of minigenes is relevant to the design of vaccines and understanding of immune responses to pathogens.
Introduction
T cell “memory inflation” is a striking immunological response, originally described in murine cytomegalovirus (MCMV) infection (1). Certain peptide-specific CD8+ T cell populations are noted to expand after an initial viral infection and remain dominant over the life span of the host (1-5). This is in contrast to what is seen in most other viral infections and vaccines, with the usual CD8+ T cell contraction to a central memory pool after the acute phase, or the exhaustion of any remaining peripheral CD8+ T cells (6-9). Critically, inflationary responses show a persistent effector memory (TEM) phenotype, homing to the periphery, and remaining functional. It has also been observed that “inflationary” epitopes show relative independence of the immunoproteasome, compared to epitopes inducing classic “non-inflating” memory (10). Whether certain key antigen presenting cells (APCs) are therefore responsible remains unknown, although there is increasing evidence that non-professional APCs are involved (11, 12).
We recently described an adenoviral model of memory inflation (13). This model is based upon a recombinant non-replicating human adenovirus serotype 5 (AdHu5). The transgene comprises a human CMV (HCMV) immediate early promoter and a LacZ open reading frame, encoding β-galactosidase (making the construct Ad-LacZ). Within β-galactosidase (β-gal), two Kb-restricted epitopes have been identified. The first is at position β-gal96-103, known as D8V (DAPIYTNV) (14), and the second is at β-gal497-504, or I8V (ICPMYARV) (15). These two epitopes elicit CD8+ T cell populations with a typical effector memory and central memory response respectively, when Ad-LacZ is immunised intravenously into a C57BL/6 mouse. The use of AdHu5 vectors in this setting is well established, with the role for specific qualities of the adenoviral vector itself in induction of sustained T cell memory being of critical importance. Specifically, other groups have observed the importance of the route and titre of the immunisation (16, 17) and the role of the adenovirus in delivering continued transgene expression (13, 18). A likely role of non-classical APCs in driving AdHu5 vector-induced immunity has also been described (19), similar to that described for the CMV model of memory inflation above.
The Ad-LacZ model has been shown to replicate fully what is seen in MCMV infection in terms of the phenotype, distribution, functionality, frequency and immunoproteasome-independency of the CD8+ T cell populations induced (13) as well as the transcriptional profile (20). To elaborate, we have identified a core set of transcriptional changes in “inflationary” T cell populations that emerge over time and which are shared by both adenovector-driven and MCMV-specific responses. Such transcriptional changes appear to be driven by a limited set of transcription factors, especially T-bet. Furthermore the changes observed are mirrored in human CMV-specific T cells and in CD8+ T cells induced by novel adenovirus vectors in human vaccine studies. These data indicate that there exists a programme for the induction/maintenance of effector memory CD8+ T cell memory pools, which can be induced by diverse stimuli in mouse and man. It suggests also that the mouse adenovector model can be readily used to explore the mechanisms driving this, which may be relevant to novel vaccines for Hepatitis C Virus (HCV), Respiratory Syncytial Virus and Ebola (21-23).
The Ad-LacZ vector contains an HCMV promoter. The balance between the concentration of adenoviral vector used and the choice of promoter can allow for marked differences in expression of the transgene (24-26). It is important to note that CMV possesses a very strong, ubiquitous promoter. However, the promoters of Rous Sarcoma Virus (RSV) (27) and mammalian Elongation Factor 1-alpha (EF1α) (28-30) are similarly ubiquitous and their use can be advantageous where a tailored approach is required, according to the target cell type and level of expression. As such, there is a good body of evidence for the use of these promoters in adenoviral vectors.
Here we first tested the hypothesis that the induction of inflation was strictly dependent on the CMV promoter used. It was necessary to evaluate this, since the promoter remains the only part of the adenoviral vector that is shared with CMV. It also allowed us to explore to what extent memory inflation was dependent on a very strong promoter associated with high-level antigen production (and in turn to address the hypothesis that the use of a weaker promoter would lead to loss of inflation).
We then tested the hypothesis that processing requirements provide a checkpoint, limiting the presentation and therefore the inflationary response to “non-inflationary” or classical epitopes. We tested this by removing the requirements for processing of the β-gal antigen for presentation of the D8V and I8V epitopes using “minigene” adenovectors, with the idea that this would allow for induction of memory inflation from both epitopes following vaccination. We looked to further test this hypothesis by also presenting a distinct virally derived epitope - the M45 epitope from MCMV (normally inducing a classical memory response) - in an adenoviral vector. We show that with “minigene” vectors we can transform the quality of T cell memory responses against these epitopes (I8V and M45) from classical to inflationary. This has implications both for our understanding of memory induction and more practically opens up potential simple approaches to modulate immunisation for CD8+ T cell induction for prophylaxis or therapy.
Materials and Methods
Animals
Experiments were performed in Oxford according to UK Home Office regulations (project licence number 30/2744,). Mice (females aged 6±2 weeks) were maintained in Specific Pathogen Free (SPF) conditions in individually ventilated cages and fed on a normal chow diet.
C57BL/6 mice were purchased from Harlan (UK). LMP7−/− mice (31) were re-derived with the help of Denise Jelfs and Richard Corderoy.
MCMV
Strain Smith (ATCC: VR194) was used, kindly provided by Professor U.H. Koszinoswki, Max von Pettenkofer Institute, Munich. MCMV was propagated and titrated on NIH 3T3 cells (ECACC, UK), and injected intravenously at a dose of 2×106 infectious units (iu) per mouse.
Adenoviral constructs
Replication-deficient recombinant adenovirus expressing the β-gal protein with an HCMV (short) promoter (Ad-LacZ) was used (13). Variant Ad-LacZ constructs were developed with The Viral Vectors Core Facility (VVCF), The Jenner Institute (Oxford, UK). These were a construct expressing the mammalian elongation factor 1α (EF1α) promoter and a construct with the HCMV long (including intron A) promoter. Further variant Ad-LacZ constructs were developed with The VVCF expressing the minigenes D8V/βgal96-103 and I8V/βgal497-504 only and the Ad-7aa-I8V and Ad-10aa-I8V constructs. Briefly, these were produced using a shuttle vector containing a promoter and transcription terminator with inserts as described (table I and II). These were recombined into the pAD/PL-DEST vector (Invitrogen, UK) using LR clonase (Invitrogen, UK). Recombinant plasmids were used to transfect TRex HEK293A cells (Invitrogen, UK) and purified as previously described (32).
Table I. Summary of AdHu5 promoter constructs.
| Vector | Promoter | Insert | P:I |
Used at (iu/mouse) |
|---|---|---|---|---|
|
Ad-LacZ (wildtype) |
HCMV (short) | β-gal | - | 2×109 |
|
Ad-LacZ (Jenner) |
HCMV (long) | β-gal | 30 | 1×109 |
|
Ad-LacZ (RSV – Kerafast) |
RSV | β-gal | 20.4 | 1×109 |
|
Ad-LacZ (EF1α) |
EF1α - mammalian | β-gal | 23 | 1×109 |
AdHu5 replication-deficient vectors used with a LacZ (encoding for the full β-galactosidase protein) insert and varying transgene promoters are shown. These are a long (with intron A) and short (without intron A) human IE CMV promoter, a Rous sarcoma virus (RSV) promoter and a mammalian elongation factor 1-alpha (EF1α) promoter. P:I ratios (particle number to infectivity) are shown alongside the viral titre in infectious units for which the virus was immunised into a single mouse.
Table II. Summary of AdHu5 processing constructs.
| Vector | Promoter | Insert | P:I | Used at (iu/mouse) |
|---|---|---|---|---|
| Ad-I8V | HCMV | β-gal497-504 | 17 | 1×108 |
| Ad-D8V | HCMV | β-gal96-103 | 23 | 1×108 |
| Ad-M45 | HCMV | M45985-993 | 25 | 1×108 |
| Ad-7aa-I8V | HCMV | β-gal490-504 | 20 | 1×108 |
| Ad-10aa-I8V | HCMV | β-gal487-504 | 60 | 1×108 |
| Ad-10aa-I8V-10aa | HCMV | β-gal487-514 | 42 | 1×108 |
| Ad-I8V-10aa | HCMV | β-gal497-514 | 28 | 1×108 |
|
Ad-I8V-D8V (Ad-ICD) |
HCMV | β-gal497-504- GGGCCCGGG – β-gal96-103 |
22 | 1×108 |
|
Ad-D8V-I8V (Ad-DAI) |
HCMV | β-gal96-103 - GGGCCCGGG – β-gal497-504 |
21 | 1×108 |
AdHu5 replication-deficient vectors used with their varying inserts are shown (all have a human IE CMV promoter). P:I ratios (particle number to infectivity) are shown alongside the viral titre in infectious units for which the virus was immunised into a single mouse.
An alternative Ad-LacZ construct with a Rous Sarcoma Virus (RSV) promoter was purchased from Kerafast (Boston, USA). Ad-M45 was purchased from Vector BioLabs (Pennsylvania, USA), expressing the M45985-993 minigene (HGIRNASFI) with an HCMV promoter. Additional constructs of Ad-10aa-I8V-10aa and Ad-I8V-10aa, Ad-ICD and Ad-DAI were also purchased from Vector BioLabs.
All AdHu5 vectors were evaluated in pilot experiments (data not shown) to define the optimum titre for intravenous immunisation across a range of 107 to 1010 iu/mouse. This was necessary based upon the previous reports of a narrow dose range, beyond which immune tolerance is seen (13, 17). Overall, we saw inflation across this range, albeit with some level of reduction in the tetramer-positive CD8+ T cell responses at the two extremes (107 and 1010) of that range. Tables I and II describe fully the individual vectors, including the viral titres used (all diluted into PBS at a volume of 200μL per immunisation).
Peptides
The D8V/βgal96-103 (DAPIYTNV) (14), I8V/βgal497-504 (ICPMYARV) (15), M45(985-993) (HGIRNASFI) and M38(316-323) (SSPPMFRV) peptides were purchased from Proimmune (Oxford, UK).
Tetrameric MHC class I peptide complexes
MHC class I monomers (H-2Kb) were kindly provided by the NIH Tetramer Core Facility, Emory University, USA: (DAPIYTNV (D8V) and SSPPMFRV (M38) tetramerised with streptavidin-PE and ICPMYARV (I8V) and HGIRNASFI (M45) tetramerised with streptavidin-APC). Cells were incubated with the indicated tetramer at 37°C for 20 minutes.
Antibodies
Anti-CD8a-eFluoro® 450, anti-CD127-PE-Cy7, anti-IFNγ-eFluoro® 450, anti-TNFα-FITC were obtained from eBioscience (San Diego, USA), anti-CD44-FITC, anti-CD62L-alexa700 were obtained from BD Biosciences (Oxford, UK), and anti-CD27-PerCP-Cy5.5 was obtained from Biolegend (San Diego, USA). Cells were incubated with the indicated antibodies at 4°C for 20 minutes.
Flow cytometry
Blood or organs were prepared as previously described (13). Cells were counted using a BD LSR II flow cytometer (Oxford, UK) and results were analysed using Flowjo software (Tree star, USA), the gating strategy as shown in supplementary figures 1 and 2. Intracellular cytokine staining was performed on splenocytes as previously described (13). Peptide-specific responses were assessed after stimulation with 10−5 M of the applicable peptide, alongside positive (PMA) and negative (medium alone) controls.
Statistical analysis
All data is presented as the mean result from individual groups, with error bars indicating the standard error of the mean (SEM). An unpaired two-tailed Students’ test was used. P values <0.05 were considered statistically significant. Statistical data analysis was performed using Graph-Pad Prism version 5.0a for MACs (GraphPad Software, San Diego, CA, USA).
Results
Induction of inflationary responses occurs independent of the CMV transgene promoter
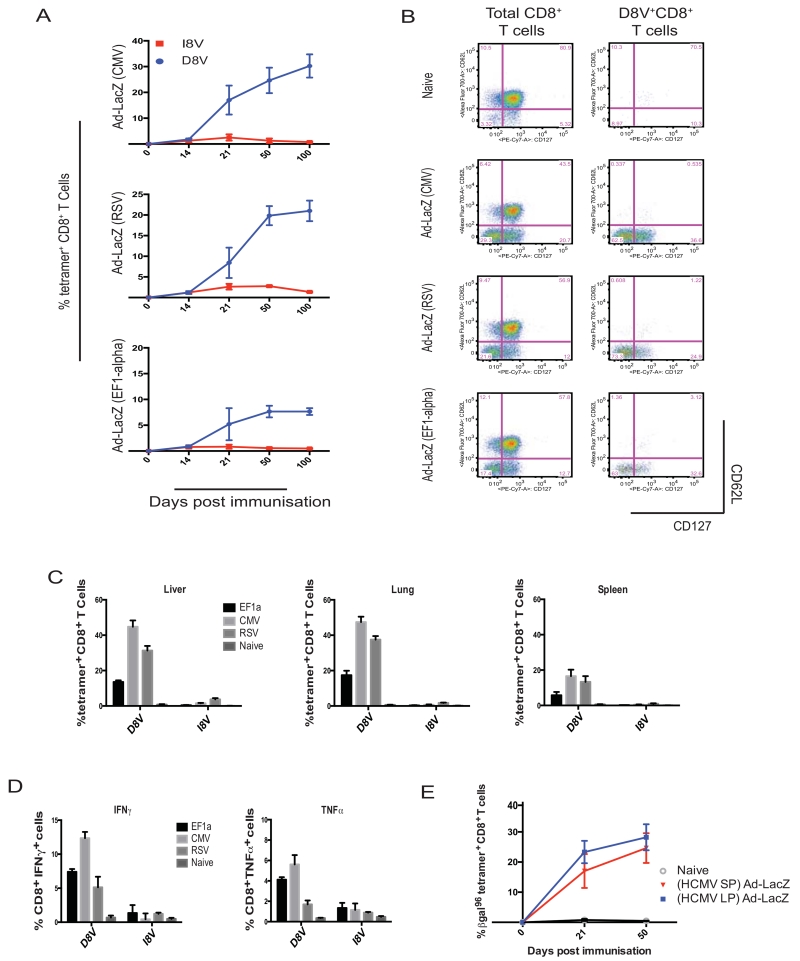
We first tested the hypothesis that the CMV promoter was necessary for induction of “CMV-like” memory inflation in the Ad-LacZ model. The CMV promoter was therefore replaced with alternative promoters from RSV or a mammalian EF1α promoter. Figure 1a presents results that demonstrate memory inflation following vaccination from both of the alternative Ad-LacZ constructs. Although the magnitude of the CD8+ T cell response to D8V (β-gal96-103) varied, according to the recognised promoter strength (33-35), memory responses induced by all constructs showed features of “inflation” (maintenance of expanded CD8+ T cell pools, with retained function, effector memory phenotype and homing to peripheral tissues). The quality of these inflating responses was consistent between all three Ad-LacZ vectors. All constructs elicited immune responses showing the effector memory phenotype typical of memory inflation: CD44hi, CD62Llo, CD27lo, CD127lo (13, 36) as demonstrated by representative individual flow cytometry plots in figure 1b (and the full phenotypic data in supplementary figure 3). The inflated CD8+ T cells were found in high numbers in peripheral tissues (figure 1c) and remained functional (figure 1d), as shown by the production of IFNγ and TNFα in intracellular cytokine stain assays. The difference between the tetramer-positive CD8+ T cell frequency in spleen (typically lower frequencies than in blood) and the fraction of those CD8+ T cells making IFNγ in vitro is similar to that noted in previous studies using both the Ad-lacZ model (13) and also in recent comparative studies using MCMV (20). The difference between the two measures (tetramer versus ICS) is not due to T cell exhaustion, as the adenovector-induced cells show no phenotypic or functional features of this (20), but may result from technical aspects of the in vitro stimulation and subsequent stringent gating strategies.
Figure 1. Inflation is seen following immunisation with Ad-LacZ vectors utilising different transgenic promoters.
C57BL/6 mice were immunised with Ad-LacZ constructs, which had either of an HCMV (short) promoter (2×109 iu/mouse), RSV (Rous sarcoma virus) promoter (1×109 iu/mouse) or an EF1-α (mammalian elongation factor 1-α) promoter (1×109 iu/mouse). A control group of naïve mice has been tested alongside all constructs. (A) Shows the tetramer-specific responses for D8V/βgal96 and I8V/βgal497, which were tracked in blood over a time course of day 14, 21, 50 and 100-post immunisation. The naïve control background responses are undetectable. (B) Demonstrates representative phenotyping of the D8V-specific populations in blood from day 100 immunised mice. (C) Demonstrates the distribution data at day 100-post immunisation. Individual D8V and I8V-specific responses are shown for each of the Ad-LacZ constructs with varying promoters in the liver, lung and spleen. (D) Demonstrates the functional (IFNγ and TNFα production in ICS) data at day 100-post immunisation (in splenocytes). (E) Demonstrates comparison of a long and short HCMV promoter (immunised at 1×109 and 2×109 iu/mouse respectively), with D8V tetramer-specific responses in blood shown at day 21 and 50-post immunisation (n=4/group with results showing the mean ± SEM. All work has been performed twice independently showing the same results).
Comparison was made between alternative HCMV promoters as well: long and short. These are with and without the intron A included, respectively (37). Our wildtype Ad-LacZ model contains a short HCMV promoter (lacking intron A). Figure 1e shows no significant difference in the kinetics of D8V (inflationary) tetramer-specific CD8+ T cells induced from these two vectors.
Overall these data clearly show that memory inflation can be induced regardless of the promoter used, and a CMV promoter is not a prerequisite. Interestingly, even with relatively weaker promoters, the distinct patterns of responsiveness seen with Ad-LacZ immunisation were maintained, with “inflation” seen for D8V and classic memory for I8V. This indicates that other factors inherent in the vector and antigen must influence the development of CD8+ T cell memory.
A minigene vector allows for inflation from a “non-inflating” epitope
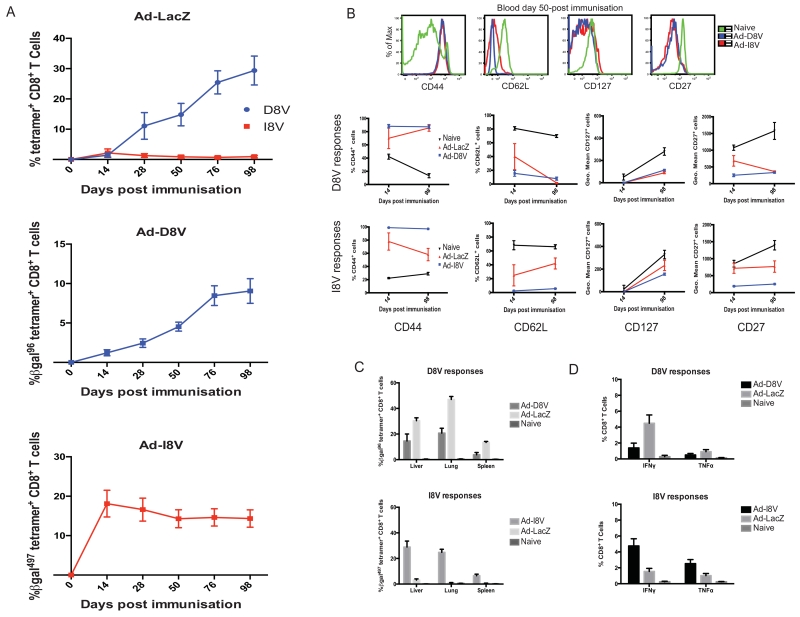
As highlighted, two major epitope-specific responses are observed following vaccination with Ad-LacZ: I8V (classical memory) and D8V (inflationary memory). Therefore, two AdHu5 vectors were developed, each containing one individual I8V or D8V epitope as a minigene (called Ad-I8V and Ad-D8V). Each of these contained an HCMV (long) promoter. Figure 2a presents results showing that an inflating response can be produced from the minigene vector (Ad-I8V) expressing the non-inflating epitope from Ad-LacZ (I8V). This is in contrast to the I8V peptide-specific CD8+ T cell response in full-length Ad-LacZ immunised mice, which demonstrates the expected classical memory (non-inflating) response. Figure 2b shows the minigene-induced CD8+ T cell phenotypes for I8V and D8V-specific responses, as assessed by a panel of CD44, CD62L, CD127 and CD27. Comparative Ad-LacZ and naive responses are shown. An early (day 14) and late (day 98) time-point is given for each phenotypic marker. These data demonstrate that inflationary responses from each of the Ad-LacZ (D8V), Ad-D8V (D8V) and Ad-I8V (I8V) possess an effector memory phenotype. Inflating populations from Ad-I8V and Ad-D8V are also clearly distributed in the periphery (figure 2c), and remain functional (figure 2d) as assessed by IFNγ and TNFα production in ICS assays. Overall, the features of these responses are as previously reported in the MCMV and Ad-LacZ models, where inflationary cells are present in the peripheral tissues, sustained at high frequencies at later time-points post infection within the host, and remain functional (13, 20).
Figure 2. An Ad-I8V minigene construct switches a non-inflating response to inflation.
C57BL/6 mice were immunised with either of Ad-LacZ (2×109 iu/mouse), Ad-D8V (1×108 iu/mouse) or Ad-I8V (1×108 iu/mouse) (or left naïve). Tetramer-specific responses were tracked in blood over a time course of day 14, 28, 50, 76 and 98-post immunisation. (A) Shows (left to right) the conventional Ad-LacZ immunisation, with the inflating D8V (blue) and the non-inflating I8V (red) responses; Ad-D8V immunisation; Ad-I8V immunisation. Naïve control responses are undetectable. (B) Representative results for the phenotypic markers (CD44, CD62L, CD127 and CD27) from tetramer-specific responses in blood at day 50-post immunisation in the minigene constructs, compared to naïve CD8+ T cells as well as the full phenotypic data at an early and late time-point. (C) Individual D8V and I8V-specific day 100 responses are shown for each of the constructs in the liver, lung and spleen. (D) IFNγ and TNFα production from peptide stimulated day 75-post immunisation splenocytes, from each of the 3 constructs alongside naïve controls. (n=5/group with results showing the mean ± SEM. All work has been performed at least twice independently showing the same results.)
Impact of modifications of antigen context at the N- and C-termini
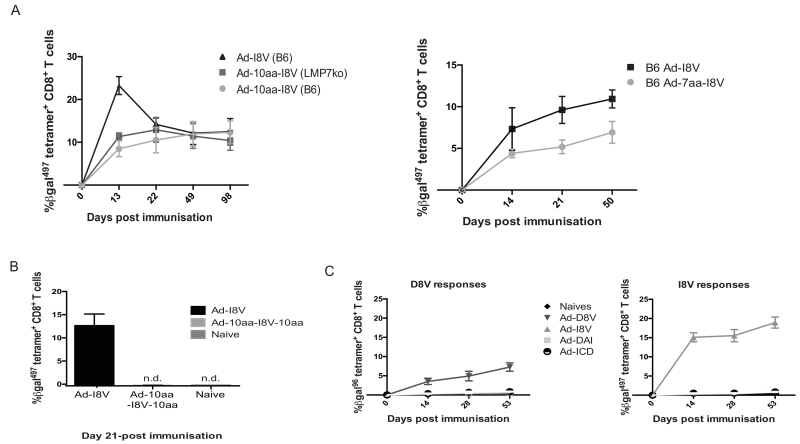
We next tested the impact of short N- and C-terminal additions of native β-gal sequence to the I8V epitope in minigene vectors, to assess whether a conversion back to classical memory responses would occur. Constructs containing I8V with short N-terminal extensions of 10 and 7 amino acids were immunised intravenously into C57BL/6 and, for the 10aa construct, LMP7−/− mice. Given the immunoproteasome-dependence of non-inflating/classical memory responses, vaccination of LMP7−/− mice was included to assess the impact of N-terminal extensions. Figure 3a presents the results that show 7 and 10 amino acid N-terminal extensions to be effectively trimmed, ultimately allowing for I8V responses as seen in Ad-I8V. Thus the minimal processing required of the peptide N-terminus appears to be well tolerated, LMP7 independence is maintained and induction of inflationary responses is sustained.
Figure 3. Short N-terminal extensions show effective trimming, whilst C-terminal extensions do not allow for tetramer-specific responses in vivo.
(A) I8V tetramer-specific responses from Ad-10aa-I8V (1×108 iu/mouse) (left) in both C57BL/6 (purple) and LMP7ko (red) mice, in blood, compared to Ad-I8V (blue) alone. Responses from Ad-7aa-I8V (1×108 iu/mouse) are also shown (right) from experiments in C57BL/6 mice only. (B) I8V tetramer-specific responses from Ad-10aa-I8V-10aa (1×108 iu/mouse) compared to Ad-I8V and naïve controls in blood at day 21-post immunisation. (C) Shows Ad-ICD (Ad-I8V-linker-D8V) and Ad-DAI (Ad-D8V-linker-I8V) (both immunised at 1×108 iu/mouse) responses in blood compared to Ad-D8V and Ad-I8V. No in vivo responses could be tracked from these dual constructs. (n=4/group with results showing the mean ± SEM.)
While additions at the N-terminus were very well tolerated, we observed that the minigene model consistently failed to induce immune responses using short C-terminally extended vectors. Minigene vectors containing additions of 10 amino acids on the C-terminus of I8V did not induce a detectable I8V peptide-specific response following immunisation. Figure 3b shows day 21 results for Ad-10aa-I8V-10aa. We were unable to track any I8V tetramer-specific responses in blood or organs at any time-point. Similarly with Ad-I8V-10aa (data not shown), no peptide-specific responses could be tracked in vivo from this short C-terminal extension construct.
We looked to further explore the impact of short N and C-terminal extensions through the construction of vectors containing both of the D8V and I8V epitopes in both orientations (D8V-I8V and I8V-D8V) with a short glycine-proline linker. (i.e. an Ad-I8V-linker-D8V or Ad-ICD and an Ad-D8V-linker-I8V vector or Ad-DAI). This linker was chosen based upon experience within malaria vaccine development (38). In this setting it was envisaged that both epitopes would be delivered to the same cell and processed simultaneously, allowing for an additional readout of competition between these two epitopes. Again, these minigene vectors failed to induce a detectable immune response. Figure 3c demonstrates these results, in which no peptide-specific responses could be identified ex vivo using tetramers.
Overall these data indicate that the minigene vector approach induces robust responses, even with short N-terminus extensions using the natural sequence. However, extensions at the C-terminus and/or alternative sequences may lead to loss of antigen production, likely through aberrant peptide processing.
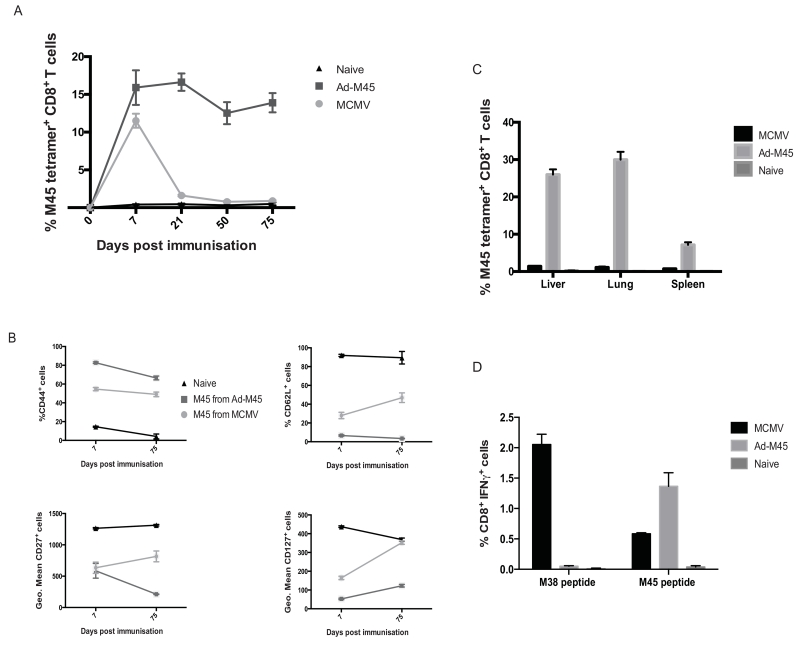
A minigene construct Ad-M45 also induces memory inflation
To test the processing context further using a viral epitope, an Ad-M45 construct was developed expressing the M45 epitope from MCMV. CD8+ T cell responses specific to the M45 epitope in C57BL/6 mice infected with MCMV exhibit a classical central memory phenotype (2). Figure 4a demonstrates that immunisation with an additional minigene-expressing adenoviral vector induces memory inflation in the epitope-specific T cell population. Phenotypic (figure 4b), distribution (figure 4c) and functional (figure 4d) assays showed the same pattern of inflation, as with the Ad-I8V construct. In contrast, infection with MCMV elicited the recognised M45 epitope classical memory response that expands at around day 7-post immunisation and has contracted back to a central memory response by around day 21-post immunisation.
Figure 4. M45, a further “non-inflating” epitope, can also show a switch to inflation in a minigene construct (Ad-M45).
C57BL/6 mice were immunised intravenously with Ad-M45 (1×108 iu/mouse) alongside control groups of MCMV-infected (2×106 iu/mouse) and naïve mice. (A) Time-course of M45-specific responses in blood for Ad-M45 compared to MCMV. (B) Phenotypic data for CD44, CD62L, CD27 and CD127 shown in blood at day 7 and day 75-post immunisation, compared to naive. (C) Distribution in peripheral tissues (liver, lung and spleen at day 75-post immunisation). (D) IFNγ production from peptide stimulated day 75-post immunisation splenocytes. (n=8/group – data pooled from 2 experiments - with results showing the mean ± SEM.)
“Co-inflation” between minigene vectors in a single host
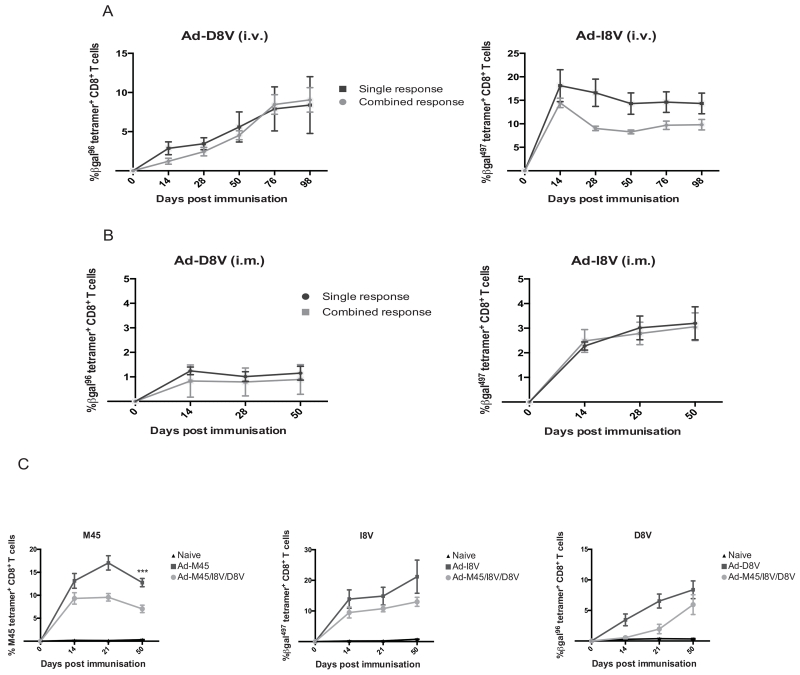
We finally tested whether a single host could accommodate multiple inflationary responses from minigene constructs. We started with Ad-I8V and Ad-D8V, and addressed whether in the presence of a dominant inflationary response, one would revert to classical memory. We immunised mice intravenously (figure 5a) with both Ad-I8V and Ad-D8V simultaneously. The results show that the inflating responses broadly occur in the same way in this mixed immunisation, as in a mouse immunised with a single construct. We next repeated this experiment using an intramuscular immunisation (figure 5b). The intramuscular route additionally allowed for the two minigene constructs to be given at different sites, but at the same time in the same host. We observed that inflation occurred using this combined minigene vaccination when the vectors were given at separate sites but also when given at the same site. Finally, in figure 5c, we performed an experiment to test co-immunisation of 3 minigene vectors (Ad-I8V, Ad-D8V and Ad-M45). Again we observed that memory inflation could be induced in parallel to 3 epitopes using the minigene approach, two of which induce classic non-inflating memory in their normal context. In this setting we do note some variation in the population sizes compared to single immunisation responses.
Figure 5. A single host is able to accommodate responses from multiple minigene constructs.
C57BL/6 mice were co-immunised with Ad-I8V and Ad-D8V or given a single immunisation (individual Ad-I8V or Ad-D8V) (all at 1×108 iu/mouse). (A) Tetramer-specific responses in blood for D8V and I8V in mice immunised intravenously with relevant constructs (B) Responses in blood following co-immunisation via the intramuscular route. Ad-I8V and Ad-D8V were combined in the same mouse, but administered (at the same time) at separate sites. (n=5/group with results showing the mean ± SEM. All work has been performed twice independently showing the same results.) (C) C57BL/6 mice were co-immunised with Ad-I8V, Ad-D8V and Ad-M45 (all at 1×108 iu/mouse) alongside individually immunised animals. (n=8/group – data pooled from 2 experiments - with results showing the mean ± SEM. Statistical analysis on M45: ***p <0.0005.)
These data indicate that co-induction of memory inflation is possible using the minigene approach, and that competition for presentation or for T cell expansion does not impact on the pathway of memory development. However, as these individual responses accumulate in a single host, there does seem to be some influence on the overall size of such a response.
Discussion
Induction of CD8+ T cell responses against pathogens and cancers is an important goal of modern immunology. One current approach of translational interest is the use of adenoviral vectors, which in human populations are very effective at priming strong and sustained CD8+ T cell responses. In studies of adenovectors for immunisation against HCV we have observed that such responses possess features of mature effector memory pools (20, 21, 39). Furthermore, there appears to be a close association at a transcriptional level between the features of such expanded adenovector-induced responses and those induced by the classical persistent virus MCMV. This core transcriptional programme is shared not only between T cells induced by the two vectors, but also shared between mouse and man. Thus adenoviral vectors may be harnessing a natural pathway for memory expansion normally observed in response to persistent herpesvirus infection. Since CMV-derived vectors show significant promise in protection against mucosal challenges (40), it is possible that adenovector vaccines could provide robust defense against complex viruses (such as HCV, Respiratory Syncytial Virus and Ebola), other pathogens (such as malaria) and also cancers (22, 23, 39, 41, 42).
One limitation of such an approach for memory induction is that it is clear that distinct patterns of memory may be induced using adenovectors, as they are against CMV. Specific epitopes undergo memory “inflation” whilst others, even when processed from the same antigen, may show classical contraction after the initial priming, with conversion to a central memory pool. Defining the rules governing this could allow us to promote the induction of effector memory pools in vaccinations and potentially impact on protection or therapeutic efficacy. Here we addressed whether the promoter used was critical, and explored whether by bypassing processing requirements we could drive epitopes towards an inflationary profile.
We began by testing whether the nature of the CMV immediate early promoter utilised in the transgenic expression cassette of Ad-LacZ could influence whether memory CD8+ T cell responses exhibited inflation. This was addressed by the use of Ad-LacZ constructs with either of an RSV or EF1α promoter in place of the HCMV promoter. In this work a simplified readout of ex vivo peptide-specific responses induced from these vectors (essentially present or not present) has been used. However, it is recognised that the intricacies of promoter choice and the concentration of protein expressed over time hold importance. This will obviously affect the efficacy and toxicity of adenovirus-based therapeutics. Also important to acknowledge are the strong innate and adaptive immune responses elicited by adenoviral vectors, which in turn can affect the efficacy of the promoter (26, 34).
Whilst all three Ad-LacZ constructs with differing promoters produce inflation, the magnitude of the response is noted to be quite different between the vectors. It seems likely that this is down to a combination of promoter strength, target cells and the immune responses to the adenoviral vector, in keeping with the literature (24, 33-35). In considering the strengths of individual promoters, it can be advantageous to tailor the type of promoter used and the viral titre of adenovirus immunised to optimise for the best host response (24). This may well be reflected in part in the initial studies of Ad-LacZ immunisation and the observation that only a very narrow dose range of the virus in vivo would lead to inflationary responses (13, 17). The strength of the HCMV promoter undoubtedly plays a critical role in the activity of the transgene, where over-activity in turn leads to the tolerisation described.
This work has additionally made some limited comparison of alternative HCMV promoters as well. The intron A region of the HCMV promoter has been shown to have a regulatory role on the enhancer region of the IE promoter (43). With both the “short” promoter (lacking intron A) and the “long” or native promoter (containing intron A) (37), responses to D8V and I8V showed comparable typical memory inflation and classical memory, respectively. In summary, these findings indicate that induction of CD8+ T cell memory inflation from our Ad-LacZ model is variable in the magnitude of response in relation to the choice of transgene promoter, but remains qualitatively the same regardless of the promoter used. Critically, it is not dependent upon the use of the cytomegalovirus immediate early promoter.
We next assessed the role of antigen processing and looked to use the Ad-LacZ model to address the question as to why it is that some epitopes lead to the production of inflationary CD8+ T cell populations, and not others. We observe that a simple “minimalist” approach in the vaccine construct reproducibly allows for memory inflation in response to previously “non-inflationary” epitopes. These data indicate that the quality of T cell memory is not a fixed property of the inducing vector or of the peptide epitope, but may be governed by the antigenic context. We also conclude that modification of the antigen context (and associated processing requirements) provides a critical tool to modulate the nature of memory induced by the same vector. We acknowledge that further work is required to investigate the exact requirements of peptide processing in this setting. This work has shown concordance with the well described lack of impact of short N-terminal extensions, likely due to N-terminal trimming in the endoplasmic reticulum (44). Short C-terminal extension of the I8V minigene did not provide evidence of a returned “non-inflationary” profile, since no I8V-specific responses were detectable in vivo. Additional constructs containing both of the I8V and D8V epitopes in a string (in both orientations) similarly were unable to induce any tetramer-specific responses in vivo. We suggest that this is likely due to aberrant expression and/or processing in the context of the C-terminus extension. We hypothesize that longer C-terminal extensions, or even a full length LacZ insert, might be required for natural processing of this epitope. These data may be important in considering translational use of such minigenes, as some care may be required in particular at the C-terminus, to present a minimal epitope. It could also impact on the use of epitope “strings” in this context.
Presenting these epitopes as minigenes bypasses the requirements for processing and likely allows for increased antigen production and presentation, in particular from non-professional antigen presenting cells that lack the immunoproteasome. We do observe the variation in the kinetic of the inflationary response from the minigene vectors of Ad-I8V and Ad-M45. Here we see a much greater initial response at day 14, which then reaches a lower plateau over the time-course of the immunisation, but remains as a sustained effector memory pool. This interesting feature reflects the heterogeneity of non-classical memory responses observed in MCMV (2, 7). Although they share a capacity for efficient processing and presentation over time, epitopes driving such responses will however vary in other key features such as avidity and off-rate. Given that the development of the inflationary memory pool is a continuous process dependent on recruitment and expansion on the one hand and cell death on the other, these additional features may impact on this dynamic equilibrium and thus the final “set-point” level of the memory inflation observed. Further studies using subtle modifications of an epitope presented within a minigene context to impact on binding or TCR contact could address this point experimentally in the future.
In addition to processing, competition between epitopes could also impact on memory development following adenovector vaccination. Here we can provide some insight into the ability of a single host to respond to both inflationary epitopes from the Ad-I8V and Ad-D8V minigene vectors. From these experiments, where there is presumably sufficient antigen (and sufficient APCs) for each epitope, we conclude that there is no competitive process between the two constructs and both responses can be accommodated in a single host. We make note of previously published data indicating later inflation of epitopes, which are initially “subdominant”, including recombinant epitopes (1). It may also be the case that epitope competition at other points in the antigen-processing pathway could influence the dominance of specific memory pools. However, the principle is clear that classical memory can be converted to inflationary memory even in the presence of other inflationary responses, although we acknowledge the variation in the magnitude of responses in mice where multiple minigenes have been immunised.
In addition to highlighting the point that the memory phenotype is dependent on antigen context, we propose that this work also holds an important potential translational element, given the data described above on adenoviral vaccines (21, 39) and CMV-vectored vaccines (1, 40). In this context our finding proves that a simple modification of the context of an epitope can allow for a clear switch in memory phenotype, and that such responses can be elicited in parallel. Future work within the model will focus on the protective capacity of these populations induced from minigene vectors, as well as extending the premise to other disease models. Overall, we believe that this approach holds significant potential for utilisation as a tool in vaccine development, in addition to furthering our understanding into the production of memory inflating CD8+ T cell responses.
Supplementary Material
Acknowledgments
We would like to acknowledge the Viral Vector Core Facility and specifically Alison Turner for her help in the production of the adenovirus vectors. We would also like to acknowledge the NIH for the tetramers used in this work.
This work was funded by the Wellcome Trust (099897/Z/12/A and 091663MA).
Abbreviations
- AdHu5
Human Adenovirus serotype 5
- β-gal
β-galactosidase
- EF1α
Elongation Factor 1-alpha
- RSV
Rous Sarcoma Virus
References
- 1.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 2.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 3.Komatsu H, Sierro S, A VC, Klenerman P. Population analysis of antiviral T cell responses using MHC class I-peptide tetramers. Clinical and experimental immunology. 2003;134:9–12. doi: 10.1046/j.1365-2249.2003.02266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillespie GM, Wills MR, Appay V, O’Callaghan C, Murphy M, Smith N, Sissons P, Rowland-Jones S, Bell JI, Moss PA. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. Journal of virology. 2000;74:8140–8150. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. Journal of virology. 2000;74:11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Hara GA, Welten SP, Klenerman P, Arens R. Memory T cell inflation: understanding cause and effect. Trends in immunology. 2012;33:84–90. doi: 10.1016/j.it.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. European journal of immunology. 2005;35:1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 8.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. The Journal of experimental medicine. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. The Journal of experimental medicine. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchinson S, Sims S, O’Hara G, Silk J, Gileadi U, Cerundolo V, Klenerman P. A dominant role for the immunoproteasome in CD8+ T cell responses to murine cytomegalovirus. PloS one. 2011;6:e14646. doi: 10.1371/journal.pone.0014646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torti N, Walton SM, Brocker T, Rulicke T, Oxenius A. Non-hematopoietic cells in lymph nodes drive memory CD8 T cell inflation during murine cytomegalovirus infection. PLoSpathogens. 2011;7:e1002313. doi: 10.1371/journal.ppat.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith CJ, Turula H, Snyder CM. Systemic hematogenous maintenance of memory inflation by MCMV infection. PLoS pathogens. 2014;10:e1004233. doi: 10.1371/journal.ppat.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolinger B, Sims S, O’Hara G, de Lara C, Tchilian E, Firner S, Engeler D, Ludewig B, Klenerman P. A new model for CD8+ T cell memory inflation based upon a recombinant adenoviral vector. J Immunol. 2013;190:4162–4174. doi: 10.4049/jimmunol.1202665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overwijk WW, Surman DR, Tsung K, Restifo NP. Identification of a Kb-restricted CTL epitope of beta-galactosidase: potential use in development of immunization protocols for “self” antigens. Methods. 1997;12:117–123. doi: 10.1006/meth.1997.0461. [DOI] [PubMed] [Google Scholar]
- 15.Oukka M, Cohen-Tannoudji M, Tanaka Y, Babinet C, Kosmatopoulos K. Medullary thymic epithelial cells induce tolerance to intracellular proteins. J Immunol. 1996;156:968–975. [PubMed] [Google Scholar]
- 16.Holst PJ, Orskov C, Thomsen AR, Christensen JP. Quality of the transgene-specific CD8+ T cell response induced by adenoviral vector immunization is critically influenced by virus dose and route of vaccination. J Immunol. 2010;184:4431–4439. doi: 10.4049/jimmunol.0900537. [DOI] [PubMed] [Google Scholar]
- 17.Krebs P, Scandella E, Odermatt B, Ludewig B. Rapid functional exhaustion and deletion of CTL following immunization with recombinant adenovirus. J Immunol. 2005;174:4559–4566. doi: 10.4049/jimmunol.174.8.4559. [DOI] [PubMed] [Google Scholar]
- 18.Finn JD, Bassett J, Millar JB, Grinshtein N, Yang TC, Parsons R, Evelegh C, Wan Y, Parks RJ, Bramson JL. Persistence of transgene expression influences CD8+ T-cell expansion and maintenance following immunization with recombinant adenovirus. Journal of virology. 2009;83:12027–12036. doi: 10.1128/JVI.00593-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassett JD, Yang TC, Bernard D, Millar JB, Swift SL, McGray AJ, VanSeggelen H, Boudreau JE, Finn JD, Parsons R, Evelegh C, Damjanovic D, Grinshtein N, Divangahi M, Zhang L, Xing Z, Wan Y, Bramson JL. CD8+ T-cell expansion and maintenance after recombinant adenovirus immunization rely upon cooperation between hematopoietic and nonhematopoietic antigen-presenting cells. Blood. 2011;117:1146–1155. doi: 10.1182/blood-2010-03-272336. [DOI] [PubMed] [Google Scholar]
- 20.Bolinger B, Sims S, Swadling L, O’Hara G, de Lara C, Baban D, Saghal N, Lee LN, Marchi E, Davis M, Newell E, Capone S, Folgori A, Barnes E, Klenerman P. Adenoviral Vector Vaccination Induces a Conserved Program of CD8 T Cell Memory Differentiation in Mouse and Man. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, Brown A, Antrobus R, Ammendola V, Naddeo M, O’Hara G, Willberg C, Harrison A, Grazioli F, Esposito ML, Siani L, Traboni C, Oo Y, Adams D, Hill A, Colloca S, Nicosia A, Cortese R, Klenerman P. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Science translational medicine. 2012;4:115ra111. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green CA, Scarselli E, Sande CJ, Thompson AJ, de Lara CM, Taylor KS, Haworth K, Del Sorbo M, Angus B, Siani L, Di Marco S, Traboni C, Folgori A, Colloca S, Capone S, Vitelli A, Cortese R, Klenerman P, Nicosia A, Pollard AJ. Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Science translational medicine. 2015;7:300ra126. doi: 10.1126/scitranslmed.aac5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledgerwood JE, DeZure AD, Stanley DA, Novik L, Enama ME, Berkowitz NM, Hu Z, Joshi G, Ploquin A, Sitar S, Gordon IJ, Plummer SA, Holman LA, Hendel CS, Yamshchikov G, Roman F, Nicosia A, Colloca S, Cortese R, Bailer RT, Schwartz RM, Roederer M, Mascola JR, Koup RA, Sullivan NJ, Graham BS. Chimpanzee Adenovirus Vector Ebola Vaccine - Preliminary Report. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1410863. [DOI] [PubMed] [Google Scholar]
- 24.Arita E, Kondoh M, Isoda K, Nishimori H, Yoshida T, Mizuguchi H, Yagi K. Evaluation of promoter strength in mouse and rat primary hepatocytes using adenovirus vectors. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2008;70:1–6. doi: 10.1016/j.ejpb.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Zheng C, Baum BJ. Evaluation of viral and mammalian promoters for use in gene delivery to salivary glands. Molecular therapy: the journal of the American Society of Gene Therapy. 2005;12:528–536. doi: 10.1016/j.ymthe.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Chen P, Tian J, Kovesdi I, Bruder JT. Promoters influence the kinetics of transgene expression following adenovector gene delivery. The journal of gene medicine. 2008;10:123–131. doi: 10.1002/jgm.1127. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, de Crombrugghe B, Pastan I. Identification of a functional promoter in the long terminal repeat of Rous sarcoma virus. Cell. 1980;22:787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]
- 28.Kim DW, Uetsuki T, Kaziro Y, Yamaguchi N, Sugano S. Use of the human elongation factor 1 alpha promoter as a versatile and efficient expression system. Gene. 1990;91:217–223. doi: 10.1016/0378-1119(90)90091-5. [DOI] [PubMed] [Google Scholar]
- 29.Uetsuki T, Naito A, Nagata S, Kaziro Y. Isolation and characterization of the human chromosomal gene for polypeptide chain elongation factor-1 alpha. The Journal of biological chemistry. 1989;264:5791–5798. [PubMed] [Google Scholar]
- 30.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic acids research. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fehling HJ, Swat W, Laplace C, Kuhn R, Rajewsky K, Muller U, von Boehmer H. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science. 1994;265:1234–1237. doi: 10.1126/science.8066463. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert SC, Schneider J, Hannan CM, Hu JT, Plebanski M, Sinden R, Hill AV. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine. 2002;20:1039–1045. doi: 10.1016/s0264-410x(01)00450-9. [DOI] [PubMed] [Google Scholar]
- 33.Zarrin AA, Malkin L, Fong I, Luk KD, Ghose A, Berinstein NL. Comparison of CMV, RSV, SV40 viral and Vlambda1 cellular promoters in B and T lymphoid and non-lymphoid cell lines. Biochimica et biophysica acta. 1999;1446:135–139. doi: 10.1016/s0167-4781(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 34.Schaack J, Bennett ML, Shapiro GS, DeGregori J, McManaman JL, Moorhead JW. Strong foreign promoters contribute to innate inflammatory responses induced by adenovirus transducing vectors. Virology. 2011;412:28–35. doi: 10.1016/j.virol.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren BZ, Lahn BT. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PloS one. 2010;5:e10611. doi: 10.1371/journal.pone.0010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 37.Sridhar S, Reyes-Sandoval A, Draper SJ, Moore AC, Gilbert SC, Gao GP, Wilson JM, Hill AV. Single-dose protection against Plasmodium berghei by a simian adenovirus vector using a human cytomegalovirus promoter containing intron A. Journal of virology. 2008;82:3822–3833. doi: 10.1128/JVI.02568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Draper SJ, Biswas S, Spencer AJ, Remarque EJ, Capone S, Naddeo M, Dicks MD, Faber BW, de Cassan SC, Folgori A, Nicosia A, Gilbert SC, Hill AV. Enhancing blood-stage malaria subunit vaccine immunogenicity in rhesus macaques by combining adenovirus, poxvirus, and protein-in-adjuvant vaccines. J Immunol. 2010;185:7583–7595. doi: 10.4049/jimmunol.1001760. [DOI] [PubMed] [Google Scholar]
- 39.Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, Halliday J, Kelly C, Bowen D, Fergusson J, Kurioka A, Ammendola V, Del Sorbo M, Grazioli F, Esposito ML, Siani L, Traboni C, Hill A, Colloca S, Davis M, Nicosia A, Cortese R, Folgori A, Klenerman P, Barnes E. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Science translational medicine. 2014;6:261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr., Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, de Barra E, Havelock T, Bowyer G, Poulton ID, de Cassan S, Longley R, Illingworth JJ, Douglas AD, Mange PB, Collins KA, Roberts R, Gerry S, Berrie E, Moyle S, Colloca S, Cortese R, Sinden RE, Gilbert SC, Bejon P, Lawrie AM, Nicosia A, Faust SN, Hill AV. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. The Journal of infectious diseases. 2015;211:1076–1086. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lubaroff DM, Konety BR, Link B, Gerstbrein J, Madsen T, Shannon M, Howard J, Paisley J, Boeglin D, Ratliff TL, Williams RD. Phase I clinical trial of an adenovirus/prostate-specific antigen vaccine for prostate cancer: safety and immunologic results. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:7375–7380. doi: 10.1158/1078-0432.CCR-09-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapman BS, Thayer RM, Vincent KA, Haigwood NL. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic acids research. 1991;19:3979–3986. doi: 10.1093/nar/19.14.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shastri N, Schwab S, Serwold T. Producing nature’s gene-chips: the generation of peptides for display by MHC class I molecules. Annual review of immunology. 2002;20:463–493. doi: 10.1146/annurev.immunol.20.100301.064819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.