Abstract
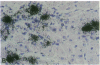
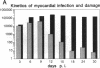
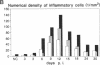
Coxsackievirus B3-induced myocarditis in different immunocompetent mouse strains was used as a model to investigate interrelationships between virus replication and development of chronic enteroviral heart disease. Using in situ hybridization to detect enteroviral RNA, we show that heart muscle infection is not only detected in acute myocarditis but is also detected during the chronic phase of the disease. Coxsackievirus B3 could evade immunological surveillance in a host-dependent fashion, thus inducing a persistent infection of the myocardium in association with ongoing inflammation. Patterns of acute and persistent myocardial infection were quantitatively assessed in one representative mouse strain (A.CA/SnJ, H-2f) by applying computer-assisted digital image processing; these patterns were then related to the extent of myocardial tissue damage as well as to inflammation. We observed a strong correlation, both spatial and temporal, between viral replication and development of myocardial lesions, indicating that acute and chronic myocardial injuries are a consequence of multifocal organ infection. Analysis of strand-specific in situ hybridization revealed that viral replication in persistent infection is restricted at the level of RNA synthesis. The described procedure for quantitating organ infection provides a powerful tool for evaluating virus-host interactions and will be of particular interest to those studying human enterovirus-induced cardiomyopathies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelmann W. H. Viral myocarditis and its sequelae. Annu Rev Med. 1973;24:145–152. doi: 10.1146/annurev.me.24.020173.001045. [DOI] [PubMed] [Google Scholar]
- Cash E., Chamorro M., Brahic M. Minus-strand RNA synthesis in the spinal cords of mice persistently infected with Theiler's virus. J Virol. 1988 May;62(5):1824–1826. doi: 10.1128/jvi.62.5.1824-1826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro M., Aubert C., Brahic M. Demyelinating lesions due to Theiler's virus are associated with ongoing central nervous system infection. J Virol. 1986 Mar;57(3):992–997. doi: 10.1128/jvi.57.3.992-997.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godeny E. K., Gauntt C. J. In situ immune autoradiographic identification of cells in heart tissues of mice with coxsackievirus B3-induced myocarditis. Am J Pathol. 1987 Nov;129(2):267–276. [PMC free article] [PubMed] [Google Scholar]
- Herskowitz A., Wolfgram L. J., Rose N. R., Beisel K. W. Coxsackievirus B3 murine myocarditis: a pathologic spectrum of myocarditis in genetically defined inbred strains. J Am Coll Cardiol. 1987 Jun;9(6):1311–1319. doi: 10.1016/s0735-1097(87)80471-0. [DOI] [PubMed] [Google Scholar]
- Hohenadl C., Klingel K., Mertsching J., Hofschneider P. H., Kandolf R. Strand-specific detection of enteroviral RNA in myocardial tissue by in situ hybridization. Mol Cell Probes. 1991 Feb;5(1):11–20. doi: 10.1016/0890-8508(91)90033-g. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Canu A., Hofschneider P. H. Coxsackie B3 virus can replicate in cultured human foetal heart cells and is inhibited by interferon. J Mol Cell Cardiol. 1985 Feb;17(2):167–181. doi: 10.1016/s0022-2828(85)80019-5. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Viral heart disease. Springer Semin Immunopathol. 1989;11(1):1–13. doi: 10.1007/BF00197080. [DOI] [PubMed] [Google Scholar]
- Klump W. M., Bergmann I., Müller B. C., Ameis D., Kandolf R. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5' uridine residues are regained during plus-strand RNA synthesis. J Virol. 1990 Apr;64(4):1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie K., Blay R., Haisch C., Lodge A., Weller A., Huber S. Clinical and experimental aspects of viral myocarditis. Clin Microbiol Rev. 1989 Apr;2(2):191–203. doi: 10.1128/cmr.2.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Viral persistence. Cell. 1989 Feb 24;56(4):517–520. doi: 10.1016/0092-8674(89)90573-4. [DOI] [PubMed] [Google Scholar]
- Pomeroy C., Hilleren P. J., Jordan M. C. Latent murine cytomegalovirus DNA in splenic stromal cells of mice. J Virol. 1991 Jun;65(6):3330–3334. doi: 10.1128/jvi.65.6.3330-3334.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovin B. H., Harris K. P., Morrison A., Klahr S., Schreiner G. F. Renal cortical release of a specific macrophage chemoattractant in response to ureteral obstruction. Lab Invest. 1990 Aug;63(2):213–220. [PubMed] [Google Scholar]
- Seko Y., Tsuchimochi H., Nakamura T., Okumura K., Naito S., Imataka K., Fujii J., Takaku F., Yazaki Y. Expression of major histocompatibility complex class I antigen in murine ventricular myocytes infected with Coxsackievirus B3. Circ Res. 1990 Aug;67(2):360–367. doi: 10.1161/01.res.67.2.360. [DOI] [PubMed] [Google Scholar]
- Werner S., Klump W. M., Schönke H., Hofschneider P. H., Kandolf R. Expression of coxsackievirus B3 capsid proteins in Escherichia coli and generation of virus-specific antisera. DNA. 1988 Jun;7(5):307–316. doi: 10.1089/dna.1.1988.7.307. [DOI] [PubMed] [Google Scholar]