Abstract
Cryptococcus gattii is a resurgent fungal pathogen that primarily infects immunocompetent hosts. Thus, it poses an increasingly significant impact on global public health; however, the mechanisms underlying its pathogenesis remain largely unknown. We conducted a detailed characterization of the deubiquitinase Ubp5 in the biology and virulence of C. gattii using the hypervirulent strain R265, and defined its properties as either distinctive or shared with C. neoformans. Deletion of the C. gattii Ubp5 protein by site-directed disruption resulted in a severe growth defect under both normal and stressful conditions (such as high temperature, high salt, cell wall damaging agents, and antifungal agents), similar to the effects observed in C. neoformans. However, unlike C. neoformans, the C. gattii ubp5Δ mutant displayed a slight enhancement of capsule and melanin production, indicating the evolutionary convergence and divergence of Ubp5 between these two sibling species. Attenuated virulence of the Cg-ubp5Δ mutant was not solely due to its reduced thermotolerance at 37°C, as shown in both worm and mouse survival assays. In addition, the assessment of fungal burden in mammalian organs further indicated that Ubp5 was required for C. gattii pulmonary survival and, consequently, extrapulmonary dissemination. Taken together, our work highlights the importance of deubiquitinase Ubp5 in the virulence composite of both pathogenic cryptococcal species, and it facilitates a better understanding of C. gattii virulence mechanisms.
Introduction
Cryptococcosis is one of most prominent invasive fungal diseases; it can invade both immunocompromised and immunocompetent hosts and often manifests as life-threatening meningoencephalitis. Among its two major pathogenic agents, Cryptococcus neoformans (Cn) is known to mainly infect the immunocompromised population and is responsible for the vast majority of cases of cryptococcosis globally[1]. The other agent, Cryptococcus gattii (Cg), was originally believed to be restricted to healthy individuals in tropical and subtropical countries such as Australia and Papua New Guinea[2, 3]. The outbreak of C. gattii cryptococcosis in temperate regions such as Vancouver Island, British Columbia, and the Pacific Northwest has redrawn public attention to this resurgent fungal pathogen[4, 5].
As the sibling species of C. neoformans, C. gattii is also an encapsulated budding yeast, but it exhibits distinct morphological, biochemical, and ecological patterns. For example, C. gattii yields both round and bacilliform cells, and it is consistently found inhabiting decaying trees but not bird droppings like C. neoformans[6, 7]. Although these pathogens are not routinely discriminated in clinical practice, their interspecific differences are significant for the clinical manifestation and management of infection. Brain infection caused by C. gattii is associated with an increased number of cryptococcomas, more neurological complications, and a slower response to therapy, and it usually requires additional diagnostic follow-ups and more frequent neurosurgical intervention, as compared with infection with C. neoformans[8]. The unique pattern of C. gattii in terms of its epidemiological and clinical features may be largely due to its distinctive mechanisms of pathogenesis. Previous studies have suggested that C. gattii infection results in defective induction of host immune responses, such as the arrested migration of neutrophils and the reduced expression of several protective pro-inflammatory cytokines[9, 10]. Furthermore, C. gattii also displays some divergent virulence-regulatory mechanisms compared with C. neoformans, such as the antioxidant superoxide dismutase (Sod1) and trehalose-6-phosphate synthase (Tps1 and Tps2)[11–14]. It is clear that C. gattii may rely on the variegated expression of virulence genes or some unknown but unique virulence traits to adapt to the host environment in vivo. A complete understanding of its unique mechanisms of pathogenesis is essential for allowing an accurate diagnosis and more appropriate intervention strategies in C. gattii infection, and these mechanisms remain to be further elucidated.
Ubiquitination is a critical reversible post-translational modification for regulating cell growth and physiology in eukaryotes[15]. Ubiquitin homeostasis is mainly determined by the processing of its precursors and its recycling from substrates via deubiquitinases (DUBs). DUBs are a conserved superfamily of proteases that are involved in a variety of biological processes, such as the cell cycle, signal transduction, and the stress response[16], and they have recently emerged as attractive targets in anticancer therapy[17]. For example, the deubiquitinase Usp7 has been linked to human hematopoietic tumors based on its ability to regulate the degradation of the tumor suppressor p53[18]. In model fungi, DUBs have also been reported to be essential for several cellular functions such as nutrient sensing, sexual reproduction, and stress responses[19–21]. However, few studies have reported the roles of deubiquitinase in the virulence of human fungal pathogens. Using a systematic genetic analysis, Liu et al. first demonstrated that some DUBs might be involved in melanization and pathogenesis in C. neoformans[22]. Thus, from the remaining DUBs, we further identified Ubp5, which is essential for sexual reproduction, the stress response, and the virulence composite in C. neoformans[23]. Interestingly, the same deubiquitinase gene has also been shown to be up-regulated in several hypervirulent C. gattii isolates from the Vancouver Island outbreak, the expression profiles of which display a significant correlation with the cryptococcal intracellular proliferation rate inside macrophage-like cells[24]. Hence, we hypothesize that deubiquitinase Ubp5 may possess a divergent function in the pathogenesis of C. gattii.
In the present study, we evaluated the biological functions of Ubp5 in Cryptococcus gattii using the hypervirulent strain R265 as a model. Deletion of Ubp5 in C. gattii revealed a severe growth defect under both normal and stressful conditions, and it also attenuated virulence in non-vertebrate and mammalian hosts. In contrast to the findings for C. neoformans, enhanced capsule production and melanin synthesis were observed in the C. gattii ubp5Δ mutant, indicating that the utilization of Ubp5 has evolved for distinct regulatory purposes in the virulence composite of these sibling species. Taken together, our study demonstrates the functional convergence and divergence of Ubp5 among pathogenic Cryptococcus species, facilitating a better understanding of C. gattii virulence mechanisms.
Results
Characterization of the C. gattii gene UBP5
The C. gattii gene UBP5 (CNBG_6153) displayed approximately 87% nucleotide identity to UBP5 from C. neoformans var. grubii (CNAG_05650) or C. neoformans var. neoformans (CNBL2960). A phylogenetic analysis of the protein alignment was performed using the deubiquitinase Ubp5 orthologs of the C. neoformans species complex and 10 other fungal species. This protein was classified into distinct clades of basidiomycetous yeasts, ascomycetous yeasts, molds, and zygomycetous molds, consistent with their evolutionary relationship (S1 Fig). Among the basidiomycetes, C. neoformans var. grubii and C. neoformans var. neoformans belonged to the same species, which was distinct from C. gattii. Interestingly, the Ubp5 orthologs of C. gattii and C. neoformans var. grubii formed one sister clade, suggesting an evolutionary divergence among the pathogenic cryptococcal species. Analysis of the predicted C. gattii protein Ubp5 revealed the presence of MATH (amino acids 55 to 206), UCH (amino acids 208 to 525), and USP7 (amino acids 631 to 1103) motifs. These motifs and their arrangement were common in Ubp5 orthologs from all of the analyzed fungi, and the three domains displayed identities of approximately 98%-99% in the C. neoformans species complex, indicating that the protein structure of deubiquitinase Ubp5 was evolutionarily conserved.
Ubp5 is required for cell propagation of C. gattii
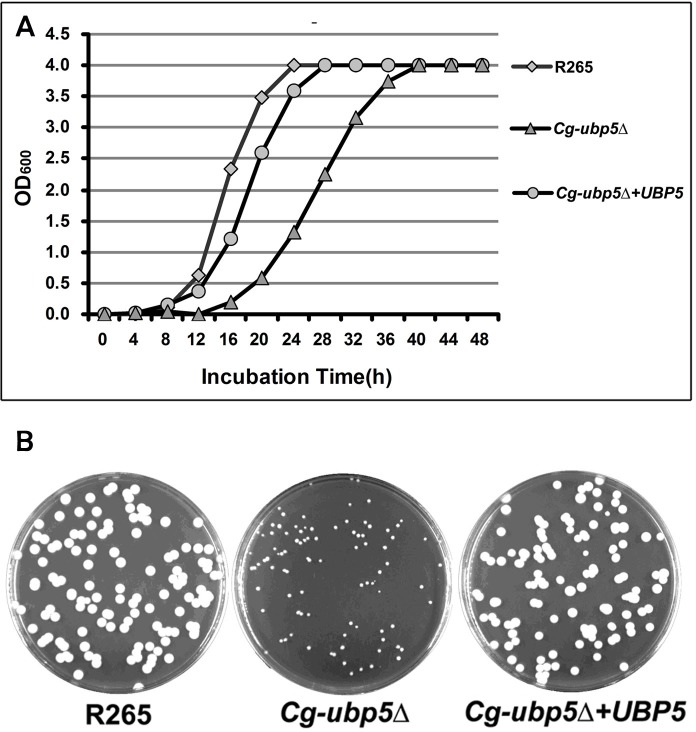
To determine the biological functions of deubiquitinase Ubp5 in C. gattii, we constructed the Cg-ubp5Δ mutant and its reconstituted strain Cg-ubp5Δ+UBP5 on the background of the R265 hypervirulent isolate. Similarly to C. neoformans[23], the lack of Ubp5 significantly delayed the growth of C. gattii, even on rich medium at 30°C. As shown in Fig 1A, the Cg-ubp5Δ mutant required an incubation of approximately 40 hours to achieve stationary phase, while the WT strain rapidly entered stationary phase by 24 hours. The reconstituted strain of CgUBP5 displayed a partially restored growth rate similar to the WT strain. We also compared the colony sizes of these three strains after a five-day incubation on YPD agar at 30°C. The remarkable differences in colony size further confirmed the decreased growth rate exhibited by the Cg-ubp5Δ mutant (Fig 1B), suggesting that Ubp5 was involved in the propagation of C. gattii.
Fig 1. Growth curve assay and colony size assessment.
A. The WT, mutant, and reconstituted strains were grown in YPD broth overnight at 30°C. Next, 106 cells of each strain were transferred to 30 mL fresh YPD broth in flasks and incubated at 30°C. The OD600 values were measured for each group at four-hour intervals. The growth rate of the Cg-ubp5Δ mutant was significantly reduced compared with the other two strains. B. One hundred cells from each strain were spread onto YPD agar, incubated for 5 days at 30°C and photographed.
C. gattii Ubp5 is involved in fungal tolerance to multiple stressors in vitro
Next, we evaluated the effect of UBP5 disruption on stress responses in C. gattii. Similarly to the Cn-ubp5Δ mutant[23], the Cg-ubp5Δ mutant strains displayed enhanced susceptibility to various stressors in vitro (Fig 2). The results revealed that the Cg-ubp5Δ mutant was hypersensitive to high temperature, exhibiting a partial growth defect at 37°C and complete fungistasis at 39°C. Similar phenotypes were also observed in the mutant strain following exposure to osmotic shock or cell membrane/wall damaging agents. In response to oxidative and nitrosative damage, the Cg-ubp5Δ mutant strains exhibited slight sensitivity, but it did not differ from the effects observed on YNB medium, suggesting that Ubp5 might not be directly involved in stress tolerance to peroxide and nitric oxide in C. gattii. Contrasting results have been obtained in C. neoformans, in which Ubp5 was essential for cryptococcal resistance to both H2O2 and NO[23]. In addition, we also tested the impact of the deletion of UBP5 on the susceptibility of C. gattii to several antifungal drugs. In comparison to the WT strain, the Cg-ubp5Δ mutant strains exhibited a 2-fold reduction in the MIC for amphotericin B and at least a 4-fold reduction in the MICs of other common antifungal agents such as flucytosine, terbinafine, and azoles (Table 1). Interestingly, reconstitution of Cg-UBP5 failed to restore the survival of C. gattii at 39°C in spite of restoring its tolerance to the other in vitro stressors, which might be due to damage caused by ectopic integration and/or repeated biolistic transformations. These data indicate that deubiquitinase Ubp5 positively regulates the fungal stress response, but with a subtle distinction, in both C. neoformans and C. gattii species.
Fig 2. C. gattii Ubp5 is involved in fungal responses to various stressors.
The R265, Cg-ubp5Δ, and Cg-ubp5Δ+UBP5 strains were grown on YPD broth to saturation at 30°C and then serially diluted 10-fold (1–106 dilutions). 3 μL suspension of 108 cells/mL were spotted on YPD or YNB agar (containing different stress-inducing agents), incubated for five days and photographed.
Table 1. Antifungal susceptibility assay.
| Strains | MICs (μg/mL) | |||||
|---|---|---|---|---|---|---|
| Amphotericin B | Fluconazole | Flucytosine | Itroconazole | Terbinafine | Voriconazole | |
| R265 | 0.5 | 16 | 32 | 0.5 | 2 | 0.25 |
| Cg-ubp5Δ | 0.25 | 4 | 8 | 0.0625 | 0.25 | 0.0313 |
| Cg-ubp5Δ+UBP5 | 0.5 | 16 | 32 | 0.5 | 4 | 0.25 |
| ATCC22019 | 1 | 0.5 | 0.5 | 0.25 | 8 | 0.06 |
Deletion of UBP5 enhances capsule and melanin production in C. gattii
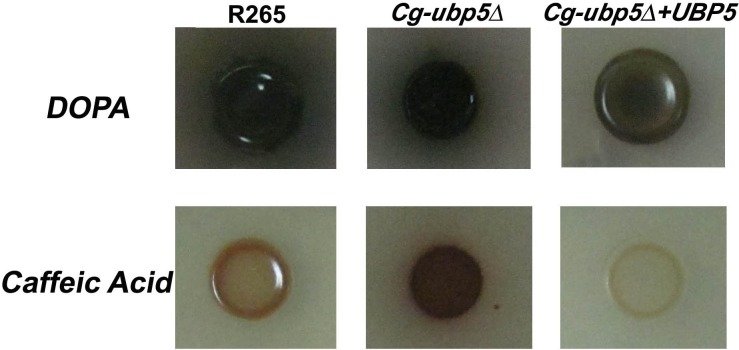
We tested whether the deletion of UBP5 could influence other known pathogenic factors, such as the polysaccharide capsule and melanin production in C. gattii. Unlike the Cn-ubp5Δ mutant[23], deletion of Ubp5 led to a slight enlargement in the size of the C. gattii capsule in DMEM when grown in the presence of CO2 (Fig 3A). A minimum of 50 cells from each strain were measured, and the average capsule size (relative volume) of the Cg-ubp5Δ strain was 96.2% compared with 91.0% and 92.3% in the WT and reconstituted strains, respectively (Fig 3B, P<0.001). Furthermore, deletion of Ubp5 had a similar effect on the pathogenic factor laccase (Fig 4). The C. gattii ubp5Δ strain displayed slight hypermelanization and produced leaky melanin around the colonies compared with the WT strain when incubated on L-DOPA medium at 30°C for 5 days. A similar difference in melanin production was more evident on caffeic acid medium, which revealed a sharp distinction in the melanin phenotype due to Ubp5 deletion in C. neoformans. We also noted that the complemented strain showed less melanization than the WT strain in both L-DOPA and caffeic acid, which might be attributed to trancriptional alteration of UBP5 and/or secondary mutation caused by ectopic integration. Taken together, these data establish a distinct role for Ubp5 in capsule or melanin production in C. gattii.
Fig 3. UBP5 mutation enhances capsule production in C. gattii.
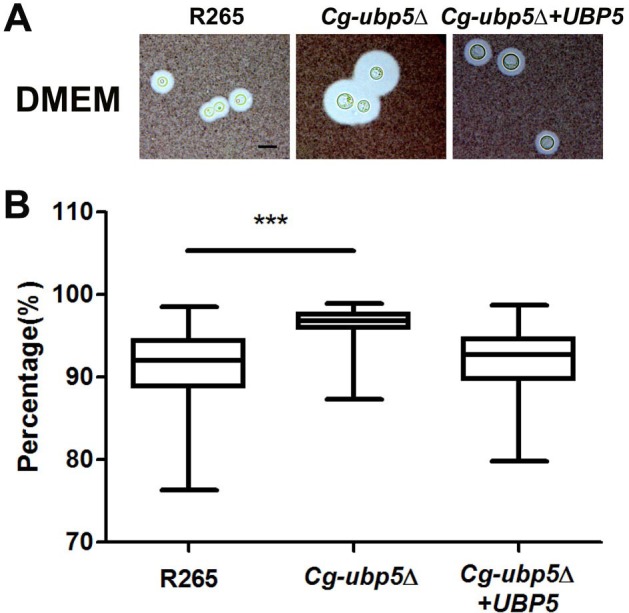
A. The WT, mutant, and reconstituted strains were incubated on DMEM medium for capsule induction at 37°C for three days. Capsules were assessed by India ink staining and visualization at 100X magnification (scale bar = 10 μm). B. Relative capsule volume on DMEM medium. Relative capsule volume = (Total Volume–Packed Volume)/Total Volume (N = 50). Wilcoxon test was performed to examine the capsule difference between R265 and Cg-ubp5Δ strains. The results revealed enhanced capsule production in the Cg-ubp5Δ mutant strain (P<0.01).
Fig 4. Ubp5 negatively regulates melanin production in C. gattii.
Strains grown in YPD broth were washed twice with PBS buffer, and a 5 μL suspension of 107 cells/mL was spotted on L-DOPA and Caffeic Acid media and incubated for 5 days at 30°C for melanin induction.
Role of Upb5 on C. gattii parasitism inside macrophages
Cryptococcus spp. have been generally accepted as facultative intracellular pathogens, and the hypervirulence of some C. gattii strains has been closely associated with their potent proliferative capacity inside macrophages[25, 26]. Thus, we assessed the ability of the Cg-ubp5Δ mutant to parasitize macrophages by co-incubating them with activated macrophages. Co-culture with activated J774A.1 macrophages revealed a 65.7% reduction in the intracellular survival of the mutant strain after 24 h compared with the background strain R265 (P<0.0001) (Fig 5). Reconstitution of CgUBP5 completely restored the intracellular proliferation of the mutant inside macrophages. Similar results were obtained in repeated experiments, suggesting that deubiquitinase Ubp5 is essential for C. gattii survival inside macrophages.
Fig 5. Ubp5 mediates the survival and proliferation of C. gattii in macrophages.

Activated J774A.1 macrophages were infected with R265, Cg-ubp5Δ, and Cg-ubp5Δ+UBP5 strains of C. gattii. After 2 hours of coincubation at 37°C with 5% CO2, the extracellular yeasts were removed, and the co-cultures were incubated for 24 hours under the same conditions. The macrophages were lysed, and the samples were then incubated on YPD agar at 30°C for 4 days to quantify the cryptococcal colonies. Each strain was assayed four times (average ± SEM, P<0.0001).
C. gattii Upb5 is essential for virulence in mammals
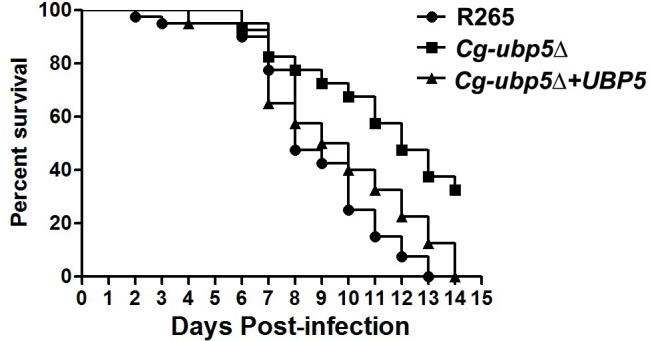
To gain insight into the overall impact of the C. gattii Ubp5 deletion on the total virulence composite, we first performed a survival assay using the murine inhalation model. Immunocompetent BALB/c mice were inoculated intranasally with 105 cells of R265, the Cg-ubp5Δ strain, or the reconstituted strain Cg-ubp5Δ+UBP5. As shown in Fig 6A, mice infected with the WT strain R265 survived for 33 days, and the average survival time was 29±3.73 days. The group infected with the reconstituted strain displayed a similar survival pattern, in which all of the mice were sacrificed by day 43, and the average survival time was 28±5.75 days (P = 0.157). In contrast, mice in the Cg-ubp5Δ mutant group did not die even at 80 days after infection, suggesting a significant attenuation of C. gattii virulence due to the deletion of Ubp5 (P<0.001).
Fig 6. Deletion of Ubp5 attenuates the virulence of C. gattii in a murine inhalation model.

A. Survival curve of mouse inhalational cryptococcosis with R265, Cg-ubp5Δ, and Cg-ubp5Δ+UBP5 strains over 80 days. All of the mice infected with R265 and Cg-ubp5Δ+UBP5 were sacrificed, but all of the mice in the Cg-ubp5Δ group survived (P<0.001). B&C. Fungal burden in the lung and brain. Organs were removed at 4, 7, 14, and 21 days post-infection in the three groups.
To investigate the potential impact of Ubp5 deletion on alveolar delivery or cryptococcal migration from the lungs, we next evaluated fungal burdens in the lung and brain in the above three groups at 4, 7, 14, and 21 days post-infection. Total lung CFU analyses at different time points post-infection revealed a high cryptococcal burden in the WT group (Fig 6B & 6C). However, the C. gattii strain lacking UBP5 resulted in significantly reduced pulmonary fungal burden at different time points after infection (P<0.001), which also displayed a gradual downward trend with an extended duration of infection. Furthermore, no viable colonies were found in the brain of mice infected with the Cg-ubp5Δ strain, in contrast to the other groups. The mice infected with reconstituted strain Cg-ubp5Δ+UBP5 displayed a slight reduction in CFU in both the lung and brain compared with the WT strain but a significant increase in CFU compared with the Cg-ubp5Δ strain (P<0.001, Fig 6B & 6C), suggesting that ectopic integration of UBP5 partially restored the virulence of the ubp5Δ mutant. These data indicate that C. gattii requires Ubp5 to parasitize the lung and disseminate into other organs, especially the central nervous system.
Impact of Upb5 on the C. elegans model
Since deletion of UBP5 enhanced the susceptibility of C. gattii to high temperature, we wondered whether attenuated in vivo virulence of ubp5Δ mutant was only attributed to its reduced thermotolerance. Caenorhabditis elegans provides an important model of pathogenesis at room temperature that can be utilized to exclude the potential effect of high temperature sensitivity on cryptococcal virulence[27, 28]. In the C. elegans/C. gattii system, the Cg-ubp5Δ mutant (LT50 = 12 days) was less virulent than the WT (LT50 = 8 days) or reconstituted (LT50 = 9 days) strains, as determined by survival analysis (P<0.001, Fig 7). This finding was consistent with results obtained in the murine inhalation model with these strains. Our data suggest that the lack of UBP5 may attenuate the virulence of C. gattii independently of its influence on high-temperature tolerance.
Fig 7. Survival analysis in the C. elegans model.
Forty C. elegans per group were fed on lawns of the WT, mutant, or reconstituted strain. Ubp5 deletion attenuated the virulence of C. gattii (P<0.001).
Discussion
C. gattii is known as the major cryptococcal pathogen in immunocompetent hosts worldwide other than in China[1, 28]. This pathogen, which was previously considered to be endemic in tropical and sub-tropical regions, has received global scientific interest due to its association with fatal outbreaks in humans and mammals, as well as its expanding geographical range[5, 29]. Experimental studies investigating the mechanisms underlying the pathogenicity of C. gattii are scarce. In the present study, we conducted a detailed characterization of the deubiquitinase Ubp5 in the biology and virulence of C. gattii using the hypervirulent strain R265, and we defined its properties as either distinctive or shared with C. neoformans.
The first phenotype observed for the Cg-ubp5Δ strain was its poor growth performance under both stressful and normal conditions. Similarly to its function in C. neoformans, the deubiquitinase Ubp5 of C. gattii positively regulated its responses to a variety of stressors in vitro, such as high temperature, high salt content, and antifungal drugs, among others. There may be several explanations for the role of Ubp5 in stress responses. First, many misfolded or damaged proteins accumulate inside the cryptococcal cell following prolonged exposure to various stressors, and this phenomenon relies in part on the ubiquitin-mediated degradation pathway for the maintenance of cellular homeostasis. The ubiquitin-proteasome pathway is critical for regulating various cellular processes, especially the stress response, in various eukaryotic species such as Saccharomyces. cerevisiae, Schizosaccharomyces. pombe, and Candida. candida[30–32]. In C. neoformans, several ubiquitin-system genes, such as UBI4 (polyubiquitin) and UBC6 (ubiquitin conjugating enzyme), also display significant transcriptional changes under stressful conditions[33, 34]. As a core component of the ubiquitin-proteasome system, deubiquitinase is essential for maintaining the dynamic balance of ubiquitin by processing ubiquitin precursors or proofreading ubiquitin-protein conjugation, and thus, it participates in the stress responses of fungi[35, 36]. Second, ubiquitination and deubiquitination might be an important modification mechanism in some signaling pathways associated with the stress response in fungi. For example, the HOG pathway is negatively regulated via ubiquitin-mediated degradation of the upstream component Ssk1 in S. cerevisiae[37]. In C. neoformans, many genes encoding ubiquitin-conjugating enzymes are significantly up-regulated in some HOG pathway mutants, while some genes encoding components (such as MAPKKK and Cpb1) of the MAPK and Ca2+/calcineurin signaling pathways also display significant transcriptional changes in the Cn-ubp5Δ mutant[23, 33]. The relationship of deubiquitinase with various signaling pathways remains to be further illuminated in Cryptococcus spp. Finally, the reduced stress tolerance of the Cg-ubp5Δ mutant was also associated with its slower proliferation rate, even in rich media. Yeast cell growth is a complex biological process that relies on the coordination of multiple factors, such as cell division, cell size, nutrients and energy metabolism[38, 39]. In S. cerevisiae, deubiquitination is an important modification mechanism that is involved in energy uptake and the cell cycle[21, 40]. Cg-Ubp5 might exploit similar strategies to regulate cell growth in C. gattii.
However, deletion of the deubiquitinase Ubp5 led to subtle differences in fungal susceptibility to some stressors between C. gattii and C. neoformans. For example, the C. gattii ubp5Δ strain was less sensitive to oxidative and NO stress but was more susceptible to cell wall/membrane damaging agents compared with the Cn-ubp5Δ mutant in C. neoformans[23]. Cryptococcus spp. rely on multiple signaling pathways and regulatory mechanisms to respond to each stressor[41], and the deubiquitinase Ubp5 might act on distinct substrates in its two major pathogens to differentially regulate the stress response. However, it might also be due to the functional similarities and redundancies of the DUB protein family such that selective environmental pressure could drive the microevolution of the function of Ubp5 in Cryptococcus spp.
The functional difference in Ubp5 between the two sibling species was more significant in terms of the expression of other pathogenic factors. In C. neoformans, CnUbp5 positively regulated both melanin and capsule production, which might be associated with its roles in regulating copper ion metabolism or polysaccharide attachment to the cell wall[23]. Interestingly, deletion of CgUbp5 led to opposite phenotypes (enhanced melanin and capsule production) in C. gattii, which further confirmed the functional reconfiguring of the homologous gene in cryptococcal evolution. Cryptococcus spp. exploit a similar mechanism for both capsule and melanin production, in which vesicles containing the protein components are excreted into the extracellular space and the components are then attached to the cell wall[42–45]. Several factors that participate in cell wall remodeling, such as chitin and chitosan, are also involved in capsule or melanin assembly[46–48]. For example, lack of chitosan in C. neoformans led to a "leaky melanin" phenotype like Cg-ubp5Δ mutant in this study[48]. Considering the hypersusceptibility of the Cg-ubp5Δ mutant to Congo Red, we speculate that CgUbp5 might indirectly regulate capsule or melanin production via its role in cell wall synthesis; however, this hypothesis requires further exploration.
The Cryptococcus neoformans species complex utilizes multiple pathogenic factors to overcome the hostile environment in vivo and cause damage to the host[49]. Each pathogenic factor potentially provides a different relative contribution to the overall virulence phenotype of this organism[50]. In C. gattii, deletion of deubiquitinase CgUbp5 resulted in significantly attenuated virulence in a mammalian host, although the Cg-ubp5Δ mutant displayed slightly enhanced production of capsule and melanin. Excluding the effect of the reduced thermotolerance, the survival assay in C. elegans model further confirmed the role of CgUbp5 in regulating the virulence composite of C. gattii. The results of our study suggest that the deubiquitinase Ubp5 is essential for the overall virulence phenotype in both C. gattii and C. neoformans but with some common and/or specialized mechanisms[23].
It is believed that cryptococcal spores or desiccated yeast cells are first inhaled into the lungs and then disseminate into extrapulmonary regions in the central nervous system when the host is immunocompromised[51, 52]. In the present study, the fungal burden in a murine model suggested that C. gattii lacking deubiquitinase Ubp5 was easily cleared by host pulmonary defense responses. This phenomenon was closely associated with the decreased survival rate of the Cg-ubp5Δ mutant inside macrophages. Alveolar macrophages have been demonstrated to be the first line of host defense, a primary haven for latent infection, and also to function as a Trojan horse for hematogenous dissemination during cryptococcal infection[25]. Enhanced intracellular replication within host macrophages is an important feature of the hypervirulent C. gattii strains recovered from the Vancouver Island outbreak, which are characterized by the upregulation of multiple genes including CgUBP5[24]. Consistent with this perspective, our work further indicates that CgUbp5 is required for the survival of C. gattii in the pulmonary space and, thus, its extrapulmonary dissemination.
In summary, our work supports the importance of deubiquitinase Ubp5 in the virulence composite of C. gattii strain R265. Ubp5 was found to be involved in cellular propagation, the stress response, capsule and melanin production, and thus pathogenicity of C. gattii in both non-vertebrate and vertebrate models. Furthermore, our results revealed the evolutionary convergence and divergence of Ubp5 in these two major cryptococcal pathogens to a certain extent, suggesting that C. gattii exploit some specialized mechanisms to adapt to environments in vivo and in vitro. However, the detailed mechanism by which Ubp5 deletion affects host immunological responses during cryptococcal infection remains unknown. It will be important to explore the potential of deubiquitinase as an anticryptococcal target.
Materials and Methods
Strains and media
R265, a VGIIa clinical C. gattii isolate from the Vancouver Island outbreak[4], was used as a background strain in this study. Mutant and complemented strains of R265 were constructed by biolistic transformation. All of the strains were maintained on non-selective yeast extract peptone dextrose solid medium (YPD, 1% yeast extract, 2% peptone, 2% dextrose, and 2% agar). Selective media containing geneticin (G418) or nourseothricin were used for the screening of mutant or reconstituted strains as previously reported[23].
Construction of mutant and reconstituted C. gattii strains
The primers used in this study are listed in S1 Table. For gene deletion, overlap PCR was employed to generate the knock-out cassette of Cg-UBP5, including the flanking fragments and NEO resistance gene[53]. The purified PCR products were precipitated onto gold microparticles and introduced into R265 cells by biolistic transformation[13]. Stable transformants were screened using selective medium that included G418 and then confirmed via diagnostic PCR, DNA sequencing, and Southern blot analysis. Southern blot examination was performed as previously described[23].
A whole DNA fragment of Cg-UBP5 containing the ORF, promoter and terminator region was amplified from R265 genomic DNA for mutant complementation. The purified PCR fragments were linked to the digested plasmid pCH233 by Xba I using the Infusion®EcoDryTM Cloning System (Clontec, Mountain View, US). The linked fragments were then introduced into the mutants by biolistic transformation. Stable colonies were screened on selective medium containing nourseothricin, and finally confirmed by diagnostic PCR and Southern blot analysis.
In vitro phenotypic assays
The yeast cells were cultured to saturation in YPD broth at 30°C, washed twice with 1×PBS buffer, and then quantified using a Countstar Automated Cell Counter. To evaluate the stress response of C. gattii strains, the cells were serially diluted 10-fold (1–106 dilutions). 3 μL suspension of 108 cells/mL were spotted on different stress media, incubated for five days and then photographed. For the high temperature stress test, the yeast cells were incubated at 30°C, 37°C and 39°C on YPD agar. For the oxidative and NO stress test, the cells were incubated on Yeast Nitrogen Base (YNB) agar containing 2 mM H2O2 or 0.75 mM NaNO2 (pH 4.0). To evaluate the response to high salt and osmotic stress, 1.5 M NaCl, 1.5 M KCl or 1.5 M sorbitol was added to the YPD agar. To assess cell wall/membrane integrity stress, 0.5% Congo Red or 0.02% SDS was added to the YPD agar.
The antifungal susceptibility test was performed as previously reported[23]. The MICs of common antifungal agents (including amphotericin B, flucytosine, terbinafine, and azoles) against the R265, Cg-ubp5Δ, and Cg-ubp5Δ+UBP5 strains were determined using the Clinical and Laboratory Standards Institute broth microdilution reference method (CLSI, 2002), and Candida parapsilosis ATCC22019 served as a quality control strain.
To measure capsule production, fungal cells were incubated on DMEM medium for three days in the presence of 5% CO2 at 37°C[54]. The capsule was stained with India ink and visualized by microscopy. The relative capsule volume was calculated for at least 50 cells for each strain according to the following formula: (Total Volume–Packed Volume)/Total Volume. To analyze melanin production, a 5 μL suspension of 107 cells/mL for each strain was spotted on L-DOPA and Caffeic Acid medium[55] and then incubated for 5 days at 30°C.
Macrophage killing assays
J744.A1. macrophage cells were used to assay the intracellular survival of different C. gattii strains as previously described[23, 56]. In brief, each strain was incubated overnight at 30°C and then opsonized with cryptococcal monoclonal antibody (C66441M, bought from Meridian Life Science, Inc. Saco, US). A total of 106 yeast cells were co-incubated with 105 J744.A1. cells that had been activated with interferon-gamma and lipopolysaccharide in 96-well tissue culture plates. After a 2-hour co-culture, the extracellular yeasts were washed away with PBS buffer, and fresh DMEM medium was added. After 24 hours of incubation, the macrophages were lysed with 0.5% SDS, and viable fungal cells were calculated by quantitative culture on YPD agar at 30°C for 3 days.
Virulence assays in vivo
BALB/c mice were intranasally infected according to an established protocol[23]. For the survival assay, ten mice per group were challenged with 105 CFU of the mutant (Cg-ubp5Δ), wild-type(WT) (R265), or complemented (Cg-ubp5Δ+UBP5) strain in 50 μL of PBS. All of the mice were monitored daily for signs of infection and sacrificed via carbon dioxide euthanasis based on predetermined endpoints such as weight loss (≥15%), neurological symptoms, and an inability to access food or water. To assess the organ fungal burden, lungs and brains were removed from the sacrificed mice (12 mice per group) after 3, 7, 14, and 21 days.
A Caenorhabditis elegans (C. elegans) model was also used to evaluate the virulence of each strain under room temperature as previous reported[27]. Briefly, a total of 40 standard C. elegans strain N2 Bristol in each group were incubated to the young adult developmental stage on a lawn of Escherichia coli OP50. Subsequently, they were transferred to plates containing WT, mutant, or complemented strains. The viability of C. elegans was determined every day by microscopy.
Statistics
The data obtained for the mouse and C. elegans model survival assays were plotted as Kaplan-Meier survival curves and analyzed with the log-rank test using SPSS 18.0 software. The LF50 (time for half of the worms to die) was also calculated to estimate survival differences in the C. elegans model. The remaining statistical analyses were conducted with the student’s t test or Mann-Whitney test. The results were considered statistically significant when the P value was less than 0.05.
Ethics Statement
The animal studies were proved by the Committee on Ethics of Biomedicine Research, Second Military Medical University, and carried out in strict accordance with the recommendations in the Regulations for the Administration of Affairs concerning Experimental Animals of the State Science and Technology Commission (China). Animal model was established under isofluorane anesthesia, and all efforts were made to minimize animal suffering and distress.
Supporting Information
The alignment of predicted Ubp5 orthologs from various fungal species was performed using the DNASTAR 6.13 ClustalW multiple-sequence alignment. The organism sources and accession numbers (NCBI database) for the protein sequences are as follows: C. gattii R265, KGB80315; C. gattii WM276, XP_003197136; C. neoformans var. grubii (CnVG) H99, AFR99081; C. neoformans var. neoformans (CnVN) JEC21, XP_572460; Ustilago maydis, XP_758786; Candida albicans, KGQ89526; Clavispora lusitaniae, XP_002617519; Candida glabrata, XP_449943; Saccharomyces cerevisiae, EWH16885; Aspergillus fumigatus, XP_748018; Talaromyces marneffei, XP_002147746; Colletotrichum fioriniae, XP_007599895; Fusarium graminearum, XP_009255591; Mucor circinelloides, EPB84371.
(TIF)
(DOC)
Acknowledgments
We sincerely acknowledge the kind gift R265 strain from Prof. Xiaorong Lin (Texas A&M University, College Station, Texas, USA).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Key Basic Research Programs of China (2013CB531601, WL), the Shanghai Municipal Natural Science Foundation (12JC1411000, 12ZR1454400 and 14495800500, WF), the National Natural Science Foundation of China (81401651, 81471926 and 31170139, WL), and Shanghai Key Laboratory of Molecular Medical Mycology (14DZ2272900, WL).
References
- 1.Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A. Cryptococcus: from human pathogen to model yeast Washington, DC: ASM Press; 2011. [Google Scholar]
- 2.Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, et al. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis. 2000;31(2):499–508. Epub 2000/09/15. 10.1086/313992 . [DOI] [PubMed] [Google Scholar]
- 3.Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39(2):155–68. Epub 2001/05/11. . [PubMed] [Google Scholar]
- 4.Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A. 2004;101(49):17258–63. Epub 2004/12/02. 10.1073/pnas.0402981101 ; PubMed Central PMCID: PMCPmc535360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Springer DJ, Chaturvedi V. Projecting global occurrence of Cryptococcus gattii. Emerg Infect Dis. 2010;16(1):14–20. Epub 2009/12/25. 10.3201/eid1601.090369 ; PubMed Central PMCID: PMCPmc2874352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaturvedi V, Chaturvedi S. Cryptococcus gattii: a resurgent fungal pathogen. Trends in microbiology. 2011;19(11):564–71. Epub 2011/09/02. 10.1016/j.tim.2011.07.010 ; PubMed Central PMCID: PMCPmc3205261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue C, Tada Y, Dong X, Heitman J. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell host & microbe. 2007;1(4):263–73. Epub 2007/11/17. 10.1016/j.chom.2007.05.005 . [DOI] [PubMed] [Google Scholar]
- 8.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50(3):291–322. Epub 2010/01/06. 10.1086/649858 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwer AE, Siddiqui AA, Kester MI, Sigaloff KC, Rajanuwong A, Wannapasni S, et al. Immune dysfunction in HIV-seronegative, Cryptococcus gattii meningitis. J Infect. 2007;54(3):e165–8. Epub 2006/11/18. 10.1016/j.jinf.2006.10.002 . [DOI] [PubMed] [Google Scholar]
- 10.Dong ZM, Murphy JW. Effects of the two varieties of Cryptococcus neoformans cells and culture filtrate antigens on neutrophil locomotion. Infect Immun. 1995;63(7):2632–44. Epub 1995/07/01. ; PubMed Central PMCID: PMCPmc173353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox GM, Harrison TS, McDade HC, Taborda CP, Heinrich G, Casadevall A, et al. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun. 2003;71(1):173–80. Epub 2002/12/24. ; PubMed Central PMCID: PMCPmc143417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narasipura SD, Ault JG, Behr MJ, Chaturvedi V, Chaturvedi S. Characterization of Cu,Zn superoxide dismutase (SOD1) gene knock-out mutant of Cryptococcus neoformans var. gattii: role in biology and virulence. Mol Microbiol. 2003;47(6):1681–94. Epub 2003/03/08. . [DOI] [PubMed] [Google Scholar]
- 13.Ngamskulrungroj P, Himmelreich U, Breger JA, Wilson C, Chayakulkeeree M, Krockenberger MB, et al. The trehalose synthesis pathway is an integral part of the virulence composite for Cryptococcus gattii. Infect Immun. 2009;77(10):4584–96. Epub 2009/08/05. 10.1128/iai.00565-09 ; PubMed Central PMCID: PMCPmc2747965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petzold EW, Himmelreich U, Mylonakis E, Rude T, Toffaletti D, Cox GM, et al. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect Immun. 2006;74(10):5877–87. Epub 2006/09/22. 10.1128/iai.00624-06 ; PubMed Central PMCID: PMCPmc1594924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clague MJ, Urbe S. Ubiquitin: same molecule, different degradation pathways. Cell. 2010;143(5):682–5. Epub 2010/11/30. 10.1016/j.cell.2010.11.012 . [DOI] [PubMed] [Google Scholar]
- 16.Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nature reviews Molecular cell biology. 2009;10(8):550–63. Epub 2009/07/25. 10.1038/nrm2731 . [DOI] [PubMed] [Google Scholar]
- 17.Nicholson B, Marblestone JG, Butt TR, Mattern MR. Deubiquitinating enzymes as novel anticancer targets. Future oncology (London, England). 2007;3(2):191–9. Epub 2007/03/27. 10.2217/14796694.3.2.191 ; PubMed Central PMCID: PMCPmc2291548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheon KW, Baek KH. HAUSP as a therapeutic target for hematopoietic tumors (review). International journal of oncology. 2006;28(5):1209–15. Epub 2006/04/06. . [PubMed] [Google Scholar]
- 19.Auesukaree C, Damnernsawad A, Kruatrachue M, Pokethitiyook P, Boonchird C, Kaneko Y, et al. Genome-wide identification of genes involved in tolerance to various environmental stresses in Saccharomyces cerevisiae. Journal of applied genetics. 2009;50(3):301–10. Epub 2009/07/30. 10.1007/bf03195688 . [DOI] [PubMed] [Google Scholar]
- 20.Enyenihi AH, Saunders WS. Large-scale functional genomic analysis of sporulation and meiosis in Saccharomyces cerevisiae. Genetics. 2003;163(1):47–54. Epub 2003/02/15. ; PubMed Central PMCID: PMCPmc1462418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahana A. The deubiquitinating enzyme Dot4p is involved in regulating nutrient uptake. Biochem Biophys Res Commun. 2001;282(4):916–20. Epub 2001/05/16. 10.1006/bbrc.2001.4669 . [DOI] [PubMed] [Google Scholar]
- 22.Liu OW, Chun CD, Chow ED, Chen C, Madhani HD, Noble SM. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell. 2008;135(1):174–88. Epub 2008/10/16. 10.1016/j.cell.2008.07.046 ; PubMed Central PMCID: PMCPmc2628477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang W, Price MS, Toffaletti DL, Tenor J, Betancourt-Quiroz M, Price JL, et al. Pleiotropic effects of deubiquitinating enzyme Ubp5 on growth and pathogenesis of Cryptococcus neoformans. PLoS One. 2012;7(6):e38326 Epub 2012/06/22. 10.1371/journal.pone.0038326 PONE-D-12-11810 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma H, Hagen F, Stekel DJ, Johnston SA, Sionov E, Falk R, et al. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc Natl Acad Sci U S A. 2009;106(31):12980–5. Epub 2009/08/05. 10.1073/pnas.0902963106 ; PubMed Central PMCID: PMCPmc2722359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coelho C, Bocca AL, Casadevall A. The intracellular life of Cryptococcus neoformans. Annual review of pathology. 2014;9:219–38. Epub 2013/09/21. 10.1146/annurev-pathol-012513-104653 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma H, May RC. Virulence in Cryptococcus species. Adv Appl Microbiol. 2009;67:131–90. Epub 2009/02/28. 10.1016/s0065-2164(08)01005-8 . [DOI] [PubMed] [Google Scholar]
- 27.Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc Natl Acad Sci U S A. 2002;99(24):15675–80. Epub 2002/11/20. 10.1073/pnas.232568599 ; PubMed Central PMCID: PMCPmc137775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang W, Fa Z, Liao W. Epidemiology of Cryptococcus and cryptococcosis in China. Fungal Genet Biol. 2015;78:7–15. Epub 2014/12/03. 10.1016/j.fgb.2014.10.017 . [DOI] [PubMed] [Google Scholar]
- 29.Byrnes EJ 3rd, Bartlett KH, Perfect JR, Heitman J. Cryptococcus gattii: an emerging fungal pathogen infecting humans and animals. Microbes and infection / Institut Pasteur. 2011;13(11):895–907. Epub 2011/06/21. 10.1016/j.micinf.2011.05.009 ; PubMed Central PMCID: PMCPmc3318971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finley D, Ozkaynak E, Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell. 1987;48(6):1035–46. Epub 1987/03/27. . [DOI] [PubMed] [Google Scholar]
- 31.Leach MD, Stead DA, Argo E, MacCallum DM, Brown AJ. Molecular and proteomic analyses highlight the importance of ubiquitination for the stress resistance, metabolic adaptation, morphogenetic regulation and virulence of Candida albicans. Mol Microbiol. 2011;79(6):1574–93. Epub 2011/01/29. 10.1111/j.1365-2958.2011.07542.x ; PubMed Central PMCID: PMCPmc3084552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogiso Y, Sugiura R, Kamo T, Yanagiya S, Lu Y, Okazaki K, et al. Lub1 participates in ubiquitin homeostasis and stress response via maintenance of cellular ubiquitin contents in fission yeast. Mol Cell Biol. 2004;24(6):2324–31. Epub 2004/03/03. ; PubMed Central PMCID: PMCPmc355854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko YJ, Yu YM, Kim GB, Lee GW, Maeng PJ, Kim S, et al. Remodeling of global transcription patterns of Cryptococcus neoformans genes mediated by the stress-activated HOG signaling pathways. Eukaryot Cell. 2009;8(8):1197–217. Epub 2009/06/23. 10.1128/ec.00120-09 ; PubMed Central PMCID: PMCPmc2725552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Upadhya R, Campbell LT, Donlin MJ, Aurora R, Lodge JK. Global transcriptome profile of Cryptococcus neoformans during exposure to hydrogen peroxide induced oxidative stress. PLoS One. 2013;8(1):e55110 Epub 2013/02/06. 10.1371/journal.pone.0055110 ; PubMed Central PMCID: PMCPmc3557267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura Y, Tanaka K. Regulatory mechanisms involved in the control of ubiquitin homeostasis. Journal of biochemistry. 2010;147(6):793–8. Epub 2010/04/27. 10.1093/jb/mvq044 . [DOI] [PubMed] [Google Scholar]
- 36.Komada M. Controlling receptor downregulation by ubiquitination and deubiquitination. Current drug discovery technologies. 2008;5(1):78–84. Epub 2008/06/10. . [DOI] [PubMed] [Google Scholar]
- 37.Sato N, Kawahara H, Toh-e A, Maeda T. Phosphorelay-regulated degradation of the yeast Ssk1p response regulator by the ubiquitin-proteasome system. Mol Cell Biol. 2003;23(18):6662–71. Epub 2003/08/29. ; PubMed Central PMCID: PMCPmc193698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutteridge A, Pir P, Castrillo JI, Charles PD, Lilley KS, Oliver SG. Nutrient control of eukaryote cell growth: a systems biology study in yeast. BMC biology. 2010;8:68 Epub 2010/05/26. 10.1186/1741-7007-8-68 ; PubMed Central PMCID: PMCPmc2895586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramanathan A, Schreiber SL. Multilevel regulation of growth rate in yeast revealed using systems biology. Journal of biology. 2007;6(2):3 Epub 2007/05/03. 10.1186/jbiol56 ; PubMed Central PMCID: PMCPmc2373900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozza WP, Zhuang Z. Biochemical characterization of a multidomain deubiquitinating enzyme Ubp15 and the regulatory role of its terminal domains. Biochemistry. 2011;50(29):6423–32. Epub 2011/06/30. 10.1021/bi200529z . [DOI] [PubMed] [Google Scholar]
- 41.Bahn YS, Jung KW. Stress signaling pathways for the pathogenicity of Cryptococcus. Eukaryot Cell. 2013;12(12):1564–77. Epub 2013/10/01. 10.1128/ec.00218-13 ; PubMed Central PMCID: PMCPmc3889573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenman HC, Casadevall A. Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol. 2012;93(3):931–40. Epub 2011/12/17. 10.1007/s00253-011-3777-2 ; PubMed Central PMCID: PMCPmc4318813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reese AJ, Doering TL. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol Microbiol. 2003;50(4):1401–9. Epub 2003/11/19. . [DOI] [PubMed] [Google Scholar]
- 44.Eisenman HC, Nosanchuk JD, Webber JB, Emerson RJ, Camesano TA, Casadevall A. Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry. 2005;44(10):3683–93. Epub 2005/03/09. 10.1021/bi047731m . [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7(1):58–67. Epub 2007/11/28. 10.1128/ec.00370-07 ; PubMed Central PMCID: PMCPmc2224146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banks IR, Specht CA, Donlin MJ, Gerik KJ, Levitz SM, Lodge JK. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot Cell. 2005;4(11):1902–12. Epub 2005/11/10. 10.1128/ec.4.11.1902-1912.2005 ; PubMed Central PMCID: PMCPmc1287864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fonseca FL, Nimrichter L, Cordero RJ, Frases S, Rodrigues J, Goldman DL, et al. Role for chitin and chitooligomers in the capsular architecture of Cryptococcus neoformans. Eukaryot Cell. 2009;8(10):1543–53. Epub 2009/07/21. 10.1128/ec.00142-09 ; PubMed Central PMCID: PMCPmc2756858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker LG, Specht CA, Donlin MJ, Lodge JK. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot Cell. 2007;6(5):855–67. Epub 2007/04/03. 10.1128/ec.00399-06 ; PubMed Central PMCID: PMCPmc1899242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kronstad J, Saikia S, Nielson ED, Kretschmer M, Jung W, Hu G, et al. Adaptation of Cryptococcus neoformans to mammalian hosts: integrated regulation of metabolism and virulence. Eukaryot Cell. 2012;11(2):109–18. Epub 2011/12/06. 10.1128/ec.05273-11 ; PubMed Central PMCID: PMCPmc3272904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClelland EE, Bernhardt P, Casadevall A. Estimating the relative contributions of virulence factors for pathogenic microbes. Infect Immun. 2006;74(3):1500–4. Epub 2006/02/24. 10.1128/iai.74.3.1500-1504.2006 ; PubMed Central PMCID: PMCPmc1418678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect Immun. 2009;77(8):3491–500. Epub 2009/05/20. 10.1128/iai.00334-09 ; PubMed Central PMCID: PMCPmc2715683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kronstad JW, Attarian R, Cadieux B, Choi J, D'Souza CA, Griffiths EJ, et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nature reviews Microbiology. 2011;9(3):193–203. Epub 2011/02/18. 10.1038/nrmicro2522 ; PubMed Central PMCID: PMCPmc4698337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu G, Kronstad JW. Gene disruption in Cryptococcus neoformans and Cryptococcus gattii by in vitro transposition. Current genetics. 2006;49(5):341–50. Epub 2006/01/07. 10.1007/s00294-005-0054-x . [DOI] [PubMed] [Google Scholar]
- 54.May RC, Park Y-D, Shin S, Panepinto J, Ramos J, Qiu J, et al. A Role for LHC1 in Higher Order Structure and Complement Binding of the Cryptococcus neoformans Capsule. PLoS Pathogens. 2014;10(5):e1004037 10.1371/journal.ppat.1004037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vidotto V, Aoki S, Ponton J, Quindos G, Koga-Ito CY, Pugliese A. A new caffeic acid minimal synthetic medium for the rapid identification of Cryptococcus neoformans isolates. Rev Iberoam Micol. 2004;21(2):87–9. Epub 2004/11/13. . [PubMed] [Google Scholar]
- 56.Alby K, Bennett RJ. Interspecies pheromone signaling promotes biofilm formation and same-sex mating in Candida albicans. Proc Natl Acad Sci U S A. 2011;108(6):2510–5. Epub 2011/01/26. 10.1073/pnas.1017234108 ; PubMed Central PMCID: PMCPmc3038756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The alignment of predicted Ubp5 orthologs from various fungal species was performed using the DNASTAR 6.13 ClustalW multiple-sequence alignment. The organism sources and accession numbers (NCBI database) for the protein sequences are as follows: C. gattii R265, KGB80315; C. gattii WM276, XP_003197136; C. neoformans var. grubii (CnVG) H99, AFR99081; C. neoformans var. neoformans (CnVN) JEC21, XP_572460; Ustilago maydis, XP_758786; Candida albicans, KGQ89526; Clavispora lusitaniae, XP_002617519; Candida glabrata, XP_449943; Saccharomyces cerevisiae, EWH16885; Aspergillus fumigatus, XP_748018; Talaromyces marneffei, XP_002147746; Colletotrichum fioriniae, XP_007599895; Fusarium graminearum, XP_009255591; Mucor circinelloides, EPB84371.
(TIF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.