Abstract
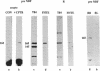
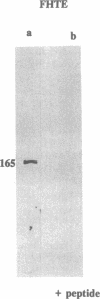
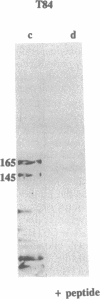
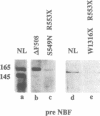
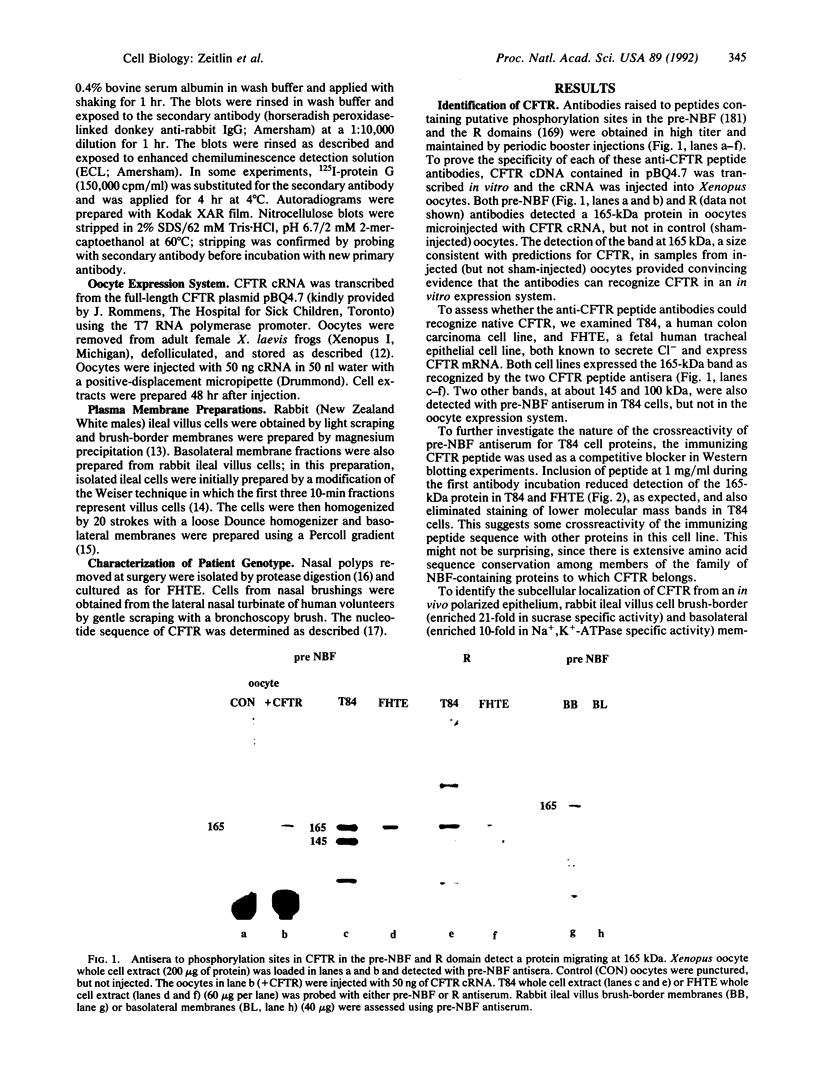
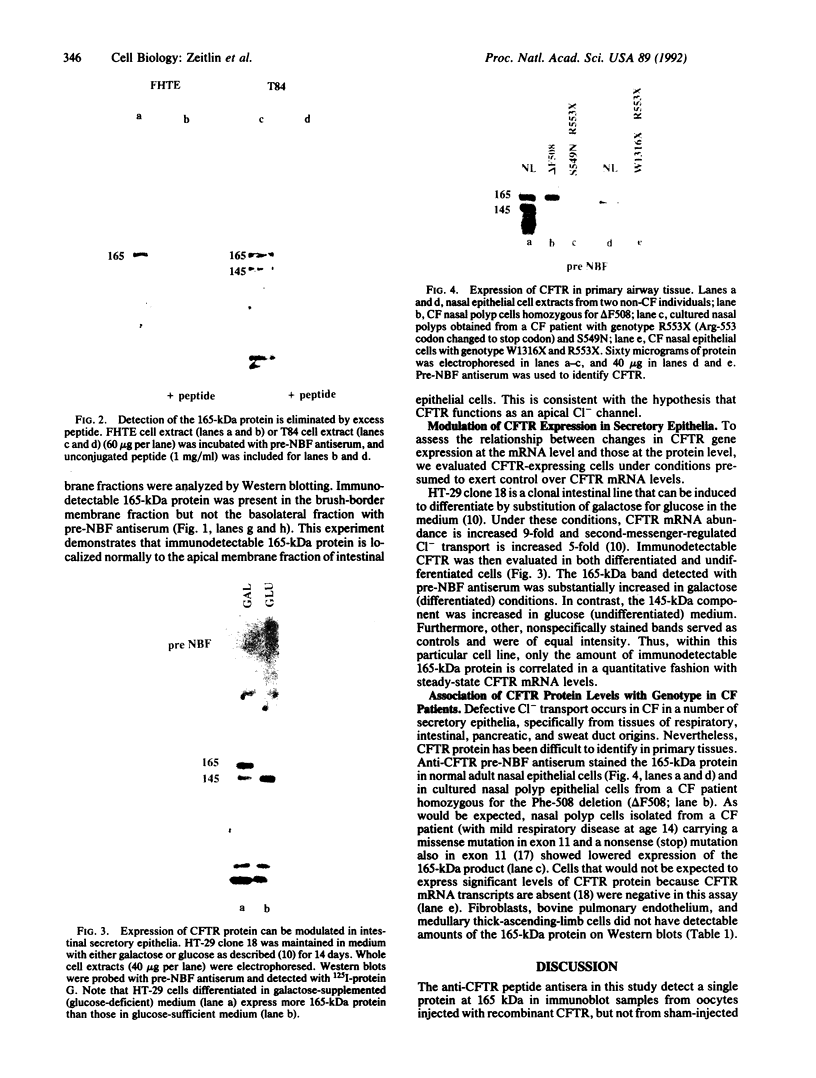
The gene responsible for the lethal disorder cystic fibrosis encodes a 1480-amino acid glycoprotein, CFTR. Using polyclonal antibodies directed against separate phosphorylation sites in the pre-nucleotide-binding fold (exon 9) and the R domain (exon 13), we have identified a 165-kDa protein in Xenopus laevis oocytes injected with recombinant CFTR cRNA transcribed from the full-length CFTR plasmid pBQ4.7. A protein of the same mobility was also detected with Western blotting techniques in whole cell extracts of cells that express CFTR mRNA (T84, FHTE, HT-29), including biopsied human nasal and bronchial tissue. Immunodetectable 165-kDa protein was concentrated in the apical membrane fraction of ileal villus tissue. We also report that the 165-kDa protein levels can be modulated pharmacologically, and these levels are appropriately correlated with second-messenger-regulated Cl- efflux. Thus, native or recombinant CFTR can be recognized by these anti-CFTR peptide polyclonal antibodies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991 Feb 8;251(4994):679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Cohen M. E., Reinlib L., Watson A. J., Gorelick F., Rys-Sikora K., Tse M., Rood R. P., Czernik A. J., Sharp G. W., Donowitz M. Rabbit ileal villus cell brush border Na+/H+ exchange is regulated by Ca2+/calmodulin-dependent protein kinase II, a brush border membrane protein. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8990–8994. doi: 10.1073/pnas.87.22.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. E., Wesolek J., McCullen J., Rys-Sikora K., Pandol S., Rood R. P., Sharp G. W., Donowitz M. Carbachol- and elevated Ca(2+)-induced translocation of functionally active protein kinase C to the brush border of rabbit ileal Na+ absorbing cells. J Clin Invest. 1991 Sep;88(3):855–863. doi: 10.1172/JCI115387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I., Maloney P. C., Zeitlin P. L., Guggino W. B., Hyde S. C., Turley H., Gatter K. C., Harris A., Higgins C. F. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9262–9266. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting G. R., Kasch L. M., Rosenstein B. J., Zielenski J., Tsui L. C., Antonarakis S. E., Kazazian H. H., Jr A cluster of cystic fibrosis mutations in the first nucleotide-binding fold of the cystic fibrosis conductance regulator protein. Nature. 1990 Jul 26;346(6282):366–369. doi: 10.1038/346366a0. [DOI] [PubMed] [Google Scholar]
- Gruenert D. C., Basbaum C. B., Welsh M. J., Li M., Finkbeiner W. E., Nadel J. A. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Lu L., Montrose-Rafizadeh C., Hwang T. C., Guggino W. B. A delayed rectifier potassium current in Xenopus oocytes. Biophys J. 1990 Jun;57(6):1117–1123. doi: 10.1016/S0006-3495(90)82632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
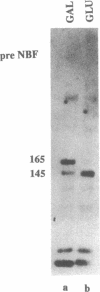
- Montrose-Rafizadeh C., Guggino W. B., Montrose M. H. Cellular differentiation regulates expression of Cl- transport and cystic fibrosis transmembrane conductance regulator mRNA in human intestinal cells. J Biol Chem. 1991 Mar 5;266(7):4495–4499. [PubMed] [Google Scholar]
- Rich D. P., Gregory R. J., Anderson M. P., Manavalan P., Smith A. E., Welsh M. J. Effect of deleting the R domain on CFTR-generated chloride channels. Science. 1991 Jul 12;253(5016):205–207. doi: 10.1126/science.1712985. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Zeitlin P. L., Lu L., Rhim J., Cutting G., Stetten G., Kieffer K. A., Craig R., Guggino W. B. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991 Apr;4(4):313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]