Abstract
Embryonic implantation comprises a dynamic and complicated series of events, which takes place only when the maternal uterine endometrium is in a receptive state. Blastocysts reaching the uterus communicate with the uterine endometrium to implant within a narrow time window. Interplay among various signalling molecules and transcription factors under the control of ovarian hormones is necessary for successful establishment of pregnancy. However, the molecular mechanisms that allow embryonic implantation in the receptive endometrium are still largely unknown. Here, we show that Sry-related HMG box gene-17 (Sox17) heterozygous mutant female mice exhibit subfertility due to implantation failure. Sox17 was expressed in the oviduct, uterine luminal epithelium, and blood vessels. Sox17 heterozygosity caused no appreciable defects in ovulation, fertilisation, blastocyst formation, and gross morphology of the oviduct and uterus. Another group F Sox transcription factor, Sox7, was also expressed in the uterine luminal and glandular epithelium relatively weakly. Despite uterine Sox7 expression, a significant reduction in the number of implantation sites was observed in Sox17 heterozygous mutant females due to haploinsufficiency. Our findings revealed a novel role of Sox17 in uterine receptivity to embryo implantation.
Embryonic implantation in the uterus is an essential event that allows progression of embryogenesis beyond the blastocyst stage and takes place during the fifth day of development in mice1. Blastocysts reach the uterus, hatch from the zona pellucida, and thereafter implant into the uterus, only when the uterus is in a receptive state. The uterus is composed of endometrium and myometrium. Of these, the endometrium dynamically changes during pregnancy. Invagination of the luminal epithelium occurs at the antimesometrial side to form a crypt structure, wherein the blastocyst settles. Following blastocyst attachment, decidualisation of the surrounding stromal cells takes place, allowing the embryos to develop further. In humans and rodents, uterine receptivity is primarily coordinated by the ovarian hormones progesterone and estrogen1,2. Under the influence of these hormones, interplay between signalling molecules and transcription factors orchestrates these dynamic events, leading to successful embryonic implantation1.
Sry-related HMG box gene 17 (Sox17), together with Sox7 and Sox18 comprises the group F Sox proteins3. These proteins share conserved amino acid sequences in the DNA-binding high mobility group (HMG) box domain and play similar roles in several developmental events, such as cardiovascular development4,5,6,7,8, haematopoiesis9,10, endoderm formation11, and human primordial germ cell specification12. Sox17 is expressed in the uterine luminal epithelium during the implantation period with an elevated level at the embryo attachment site13. However, the role of Sox17 in implantation has not been addressed in previous studies. In this study, we showed that Sox17 was expressed in the oviduct and uterine luminal and glandular epithelium and that Sox17 heterozygous mutant females exhibited subfertility due to implantation failure. The results from this study suggested a novel role of Sox17 in uterine receptivity and embryonic implantation.
Results
Sox17 was expressed in female reproductive organs
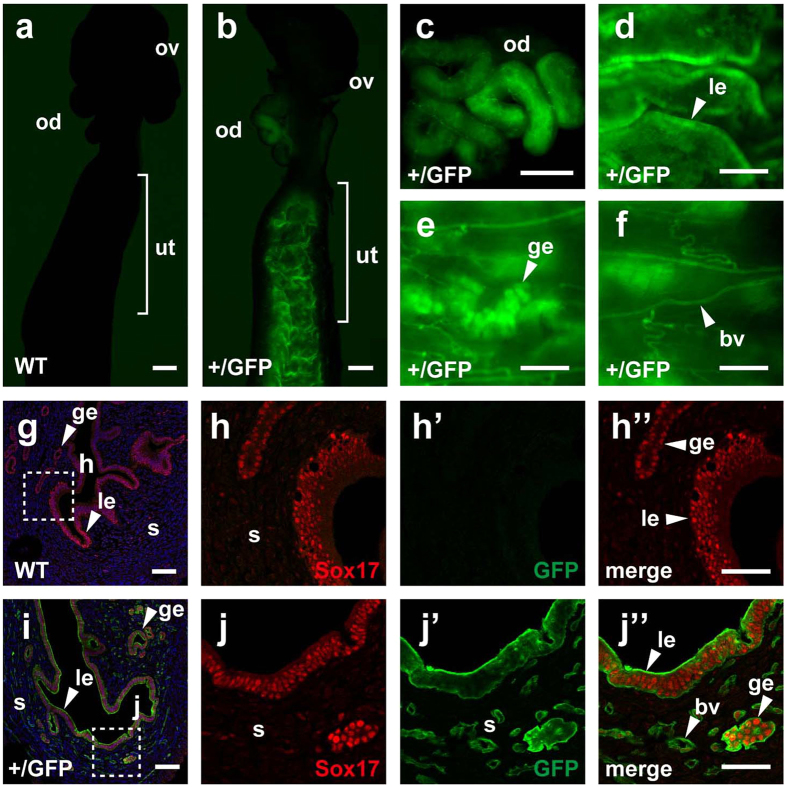
To examine Sox17 expression in female reproductive organs, we first used Sox17-green fluorescent protein (GFP) knock-in mice, which express GFP under the control of the Sox17 promoter9. In nonpregnant, Sox17-GFP heterozygous mutant females (Sox17+/GFP), GFP fluorescence was observed in the oviduct and uterine endometrium (Fig. 1b–d), whereas wild-type (WT) female mice showed no discernible fluorescence in these organs under the same conditions of image acquisition (Fig. 1a). In the uterine endometrium, both the luminal and glandular epithelium expressed GFP (Fig. 1d,e). Blood vessels also expressed GFP (Fig. 1f). Uterine expression of GFP was stronger than that observed in the blood vessels.
Figure 1. Expression of Sox17 in the oviduct and uterus.
(a–f) Fluorescence emitted from GFP without staining in nonpregnant, WT and Sox17+/GFP female reproductive organs. (g–j”) Immunofluorescent staining of GFP (green) and Sox17 (red) in the WT (g–h”) and Sox17+/GFP (i–j”) uterus. Nuclei are stained with Hoechst (blue). Enlarged images corresponding to dotted boxes in (g) and (i) are shown in (h–h”) and (j–j”), respectively. Abbreviations: ov, ovary; od, oviduct; ut, uterus; le, luminal epithelium; ge, glandular epithelium; s, stroma; and bv, blood vessels. Scale bars, 500 μm for (a–c); 200 μm for (d–f); 100 μm for (g,i); and 50 μm for (h”,j”).
To examine whether uterine expression of GFP in Sox17+/GFP mice coincided with that of endogenous Sox17, we performed co-immunofluorescent staining using anti-Sox17 and anti-GFP antibodies. In the WT uterus, Sox17 staining was detected in the luminal and glandular epithelium (Fig. 1g–h”). In the Sox17+/GFP uterus, GFP staining coincided with Sox17 staining (Fig. 1i–j”), confirming the reliability of Sox17-GFP for monitoring Sox17 expression. Fluorescence emitted from GFP without staining was also detectable (Supplementary Fig. S1). However, GFP staining was more distinct and clearly showed GFP-positive tubular structures in the stroma (Fig. 1j–j”). These Sox17-GFP-positive cells were likely to be capillary blood vessels. These microscopic analyses suggest that Sox17 was expressed in the oviduct and uterine luminal epithelium, with weak expression in the blood vessels of nonpregnant, female mice. Importantly, Sox17+/GFP mice had no appreciable defects in uterine morphology, including the blood vessels.
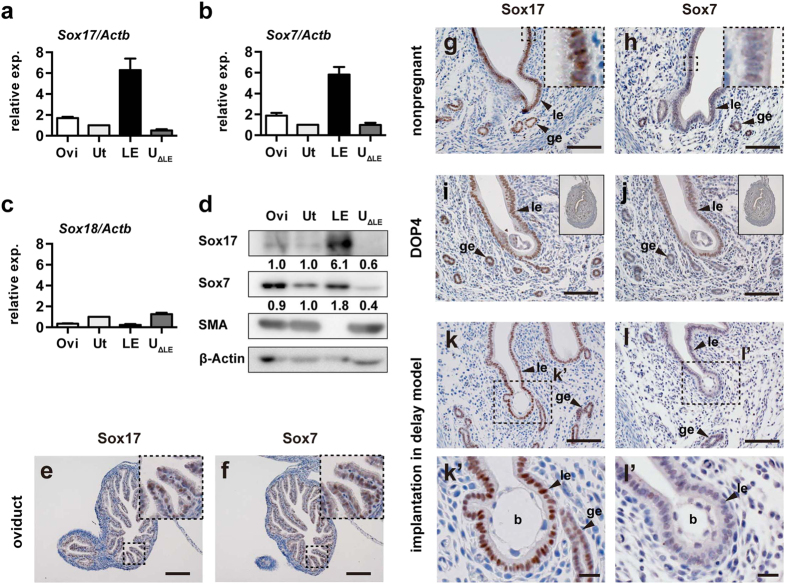
Next, we examined the expression of Sox17 together with other Sox-F group transcription factors, Sox7 and Sox18, in the oviduct, uterus, and uterine tissue fractions at day-of-pregnancy (DOP) 4 in natural pregnancy. Here, DOP 1 was the day at which the vaginal plug was formed, and DOP 4 was the day at which the uterus became receptive to implantation. First, we performed quantitative reverse transcription polymerase chain reaction (RT-PCR), in which Actb was used as an internal control, and the uterus was used as a calibrator (Fig. 2a–c). Similar results were also obtained using Gapdh as an internal control (Supplementary Fig. S2). In the oviduct, Sox17, Sox7, and a lesser amount of Sox18 transcripts were detected; the expression levels were 1.7-, 1.9-, and 0.3-fold higher, respectively, than those detected in the uterus (n = 5; Fig. 2a,b). In the uterine luminal epithelium, Sox17 and Sox7 transcripts were abundant, exhibiting 6.3- and 5.8-fold higher expression, respectively, than that observed in the uterus (n = 5; Fig. 2a,b). In contrast, Sox18 transcript levels in the luminal epithelium were negligible (Fig. 2c). Moreover, Sox18 transcripts were undetectable in one of the five samples from the luminal epithelium.
Figure 2. Expression of Sox17, Sox7, and Sox18 in the oviduct and uterus receptive to implantation.
(a–c) Relative expression levels of Sox17 (a), Sox7 (b), and Sox18 (c) transcripts in WT oviduct and uterine tissues at 18:00–20:00 on DOP 4 were determined by real-time RT-PCR. Relative expression levels (2−ΔΔCT) were analysed by the comparative CT method (mean ± standard error of mean [SEM]). Actb was used as an internal control. The uterus was used as a calibrator. (d) Western blot analysis to show Sox17 and Sox7 protein levels in the WT oviduct and uterine tissues prepared at DOP 4 simultaneously with RNA samples. Lack of smooth muscle actin (SMA) expression indicates proper preparation of the luminal epithelium. β-Actin was used as a loading control. The numbers beneath the Sox17 and Sox7 bands represent relative signal levels (Ut = 1.0), in which β-Actin was used for normalisation. (e,f) Immunohistochemistry of Sox17 and Sox7 in the WT oviduct at DOP 4. Enlarged images corresponding to the dotted boxes are shown in insets. (g,h) Immunohistochemistry of Sox17 and Sox7 in nonpregnant, WT uteri. Enlarged images corresponding to the small dotted boxes are shown in insets. (i,j) Immunohistochemistry of Sox17 and Sox7 in the WT uteri at DOP 4. Low magnification images of the cross-sectioned uteri are shown in insets. (k–l’) Immunohistochemistry of Sox17 and Sox7 in the WT uteri at 6 h after administration of P4 + E2 in the delayed implantation model. Enlarged images corresponding to dotted boxes in (k,l) are shown in (k’,l’), respectively. Abbreviations: Ovi, oviduct; Ut, uterus; LE(le), luminal epithelium; UΔLE, uterine tissues after removal of LE; ge, glandular epithelium; and b, blastocyst. Scale bars, 100 μm for (e–l); and 20 μm for (k’,l’).
Next, we performed western blotting to examine Sox17 and Sox7 protein levels. Proper preparation of the luminal epithelium was ensured by absence of smooth muscle actin (Fig. 2d). Similar to the PCR analysis, the blot was quantified by normalisation with β-actin and divided by the value from the uterus (i.e., uterine expression = 1.0). In the quantification, the amounts of Sox17 and Sox7 proteins were 6.1- and 1.8-fold higher than those observed in the uterus. These data implied that, among the Sox-F proteins, Sox17 was mainly expressed in the luminal epithelium.
To examine the tissues expressing Sox17 and Sox7 in the oviduct and uterus, we conducted immunohistochemistry (Fig. 2e–l’). In the oviduct, staining for both Sox17 and Sox7 was clearly detected at a comparable level (Fig. 2e,f). In the nonpregnant WT uterus, staining for Sox17 and Sox7 was found in the luminal and glandular epithelium (Fig. 2g,h). Consistent with their function as transcription factors, the staining for Sox17 and Sox7 was localised exclusively in the nuclei (insets in Fig. 2g,h). Similar patterns of Sox17 and Sox7 staining were also observed at the implantation site in the DOP 4 uteri after natural mating (Fig. 2i,j, and Supplementary Fig. S1) and in a delayed implantation model (Fig. 2k,l). The latter was used to ensure the timing of implantation. Consistent with a previous report13, Sox17 showed moderately elevated expression at the embryo attachment site (Fig. 2k,k’). These data suggested that Sox17 and Sox7 were expressed in the oviduct and uterine luminal epithelium receptive to implantation.
Sox17 heterozygous mutant females exhibit subfertility
While breeding Sox17-GFP mice, we noticed that the number of pups from Sox17+/GFP female and WT male pairs was fewer than that from WT female and Sox17+/GFP male pairs. For quantitative analysis, we counted the cumulative number of pups while breeding Sox17-GFP mice for 12 months (Fig. 3a). WT female and Sox17+/GFP male pairs produced an average of 105.0 ± 14.3 pups (n = 4), whereas Sox17+/GFP female and WT male pairs produced an average of 16.4 ± 8.8 pups (n = 5). These numbers include the pups that died soon after birth. The difference was statistically significant as determined by t-tests (p = 0.0141). Notably, Sox17+/GFP females had a smaller litter size and tended to become infertile early in adulthood.
Figure 3. Subfertility of Sox17+/GFP female mice.
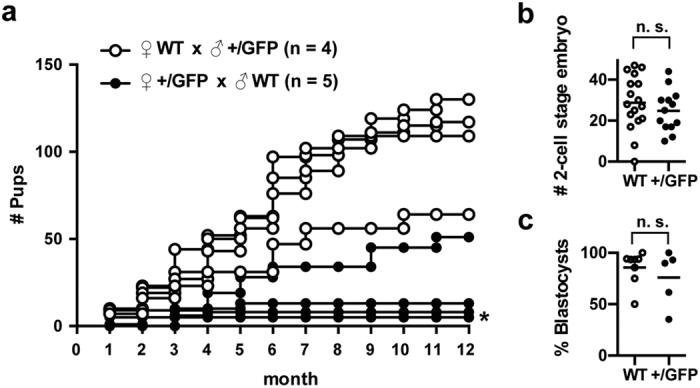
(a) Cumulative number of pups from WT females mated with Sox17+/GFP males (○) (n = 4) and from Sox17+/GFP females mated with WT males (●) (n = 5). (b) Number of 2-cell stage embryos collected from WT and Sox17+/GFP females after superovulation treatment. (c) Percent of blastocysts that were developed normally from 2-cell stage embryos collected from WT and Sox17+/GFP females after mating with WT males. Abbreviation: n.s., not significant. An asterisk (*) shows two lines that are completely overlapped.
Since Sox17 haploinsufficiency results in perinatal lethality in C57BL/6 background14, we also examined the genotype of the pups after weaning. WT to +/GFP ratio was 161:139 for the pups produced by WT females and 39:37 for the pups produced by Sox17+/GFP females. Chi-square test revealed that WT and +/GFP pups were born approximately at the expected Mendelian ratio of 1:1 for both the mating groups (p = 0.204 and 0. 815 from WT and Sox17+/GFP females, respectively), indicating that perinatal lethality of Sox17+/GFP pups was not a major reason for the subfertility observed in Sox17+/GFP females.
Subsequently, to examine the influence of Sox17 heterozygosity on the ovulation, fertilisation, and early development, we collected 2-cell stage embryos from superovulated, WT and Sox17+/GFP females and found that the number of the embryos was comparable (Fig. 3b). These 2-cell stage embryos were further cultured in vitro and formed blastocysts at a similar rate (Fig. 3c). These data suggested that ovulation, fertilisation and embryonic development to the blastocyst stage were phenotypically normal and were not affected by Sox17 heterozygosity.
Sox17 heterozygous mutant females were defective in implantation
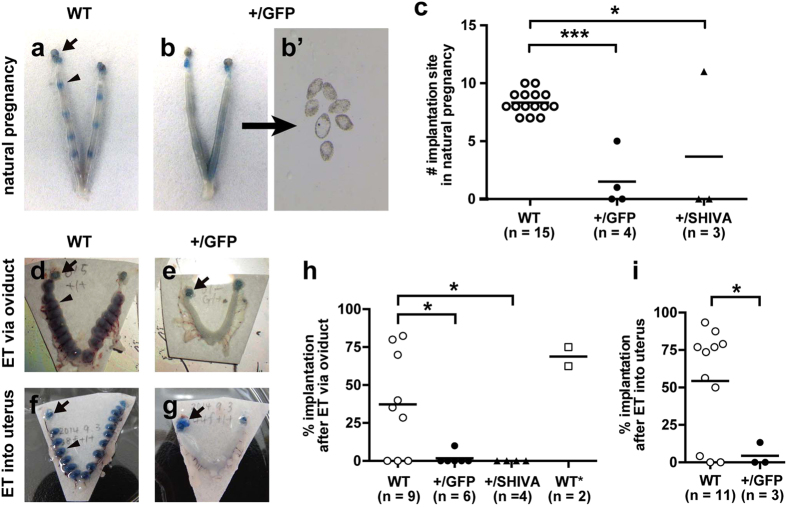
To investigate when the embryos were lost in Sox17+/GFP females, we counted the number of implantation sites at DOP 5, which was the day blastocysts were implanted into the uterus. WT females showed an average of 8.3 ± 0.3 (n = 15) implantation sites (Fig. 4a,c). In contrast, Sox17+/GFP females showed an average of 1.5 ± 1.2 (n = 4) implantation sites (Fig. 4b,c). The difference was statistically significant (p < 0.0001 by t-tests). Notably, unimplanted blastocysts were recovered from the Sox17+/GFP uteri with no implantation sites (Fig. 4b’), clearly indicating the normal function of the oviduct and normal development of the blastocyst. Since GFP expressed in Sox17+/GFP female mice may exert adverse effects on the number of implantation sites, we also counted the number of implantation sites using Sox17 point-mutant mice (SHIVA)15, with a point mutation that alters the 72nd Met to Ala in the DNA-binding region of the HMG domain. Sox17+/SHIVA female mice showed an average of 3.7 ± 3.7 implantation sites (n = 3; p = 0.0077 by t-tests; Fig. 4c), confirming that GFP expression was not responsible for the implantation failure observed in Sox17+/GFP. These results indicated that implantation was defective in Sox17 heterozygous mutant females.
Figure 4. Defective implantation in Sox17 heterozygous mutant females.
(a,b) Blue dye staining showing implantation sites in WT (a) and Sox17+/GFP (b) uteri at DOP 5 after natural mating. Arrows and arrowheads show the ovaries and implantation sites, respectively. (b’) Unimplanted blastocysts recovered from Sox17+/GFP uterus by flushing. (c) Number of implantation site in females of the indicated genotype at DOP 5 after natural mating. Dots and bars show sample and mean values, respectively. (d–g) Blue dye staining showing implantation sites in WT (d,f) and Sox17+/GFP (e,g) uteri at DOP 7 after embryo transfer (ET). WT embryos were transferred either to the oviduct (d,e) or the uterus (f,g). (h,i) Implantation rates at DOP 6 or 7 in embryo-transferred females of the indicated genotype. WT embryos were transferred via the oviduct (h) or in the uterus (i), except for WT*, to which approximately a 1:1 mixture of WT and Sox17+/GFP embryos were transferred. *p < 0.05; **p < 0.01; ***p < 0.001 as per one-way analysis of variance Dunnett’s multiple comparisons test (c,h) and t-tests (i).
To directly demonstrate the failure in implantation, we performed embryo transfer and examined the implantation rate. In embryo transfer, we used WT embryos to exclude the possibility that the implantation failure was caused by problems with the embryo. In addition, the implantation site was observed at DOP 6 or 7 to examine whether the smaller number of implantation sites observed at DOP 5 in Sox17 heterozygous mutant females could be explained by factors other than a delay in implantation.
First, we transferred WT embryos into the oviduct. WT female mice showed an average implantation rate of 37.4% ± 11.3% (n = 9; Fig. 4d,h). In contrast, Sox17+/GFP female mice showed a much lower average implantation rate (1.7% ± 1.7%, n = 6; Fig. 4e,h). The difference was statistically significant (p = 0.0273 by t-tests). Sox17+/SHIVA female mice showed no implantation sites (n = 4; Fig. 4h). We also transferred approximately a 1:1 mixture of WT and Sox17+/GFP embryos to WT female mice to examine whether Sox17+/GFP embryos could be implanted successfully. Female mice with transferred embryos showed an average implantation rate of 68.8% ± 6.3% (n = 2). This number was comparable to the results of WT embryo transfer, suggesting that Sox17 heterozygosity in embryos was not responsible for implantation failure. This was also supported by the normal number of pups from the mating of WT female and Sox17+/GFP male mice (Fig. 3a).
Secondly, we transferred WT embryos directly to the uterus. WT females showed an average implantation rate of 54.3% ± 10.9% (n = 11; Fig. 4f,i), whereas Sox17+/GFP females showed a much lower average implantation rate (4.4% ± 4.4%, n = 3; Fig. 4g,i). This difference was statistically significant (p = 0.0394 by t-tests). These results suggested that the smaller number of implantation sites was not due to delays in implantation and further suggested that embryonic implantation into the uterus was defective in Sox17 heterozygous mutant females.
Discussion
In this study, we showed expression of Sox17 in the oviduct and uterine luminal and glandular epithelium, female subfertility, and decreased numbers of implantation sites in Sox17 heterozygous mutant females. In addition to Sox17, we also found expression of Sox7 in the uterine endometrium. However, the fact that most of the Sox17 heterozygous mutant females showed subfertility and implantation failure and that Sox17 protein was predominant in the luminal epithelium led to the conclusion that Sox17 was a major player in embryonic implantation among Sox-F proteins.
The observed implantation failure may have been caused by haploinsufficiency of the Sox17 gene. Sox17 is known to exhibit haploinsufficiency in bile duct formation, which results in biliary atresia and hepatitis in C57BL/6 background mice14. This Sox17 haploinsufficiency may result from lack of redundancy by other Sox-F proteins in the gallbladder and bile duct epithelia. It seems that Sox17 exhibits haploinsufficiency in implantation in a similar manner. However, we also observed that some Sox17 heterozygous mutant females had relatively normal litter sizes in mating and numbers of implantation sites. Functional redundancy among the Sox-F transcription factors has been reported in various events, namely, early cardiovascular development6,7, postnatal angiogenesis5, and haematopoiesis9,10 in mice. In addition, Sox7 and Sox17 modify the Sox18-null phenotype in the lymphatic vasculature in a strain-specific manner16. In a similar way, it is possible that compensatory increase in Sox7 and/or Sox18 expression may occur in Sox17 heterozygous mutant females depending on the genetic background. Because we used a hybrid of C57BL/6 and ICR mice in this study, there may be individual differences in genetic background; this may be one reason for the presence of some outliers among the mutants.
Role of Sox17 in implantation remained elusive. However, there are some clues to how Sox17 may function during implantation. ChIP-Seq analysis revealed Sox17 as a direct transcriptional target of the progesterone receptor in the mouse uterus17, suggesting the role of Sox17 as a mediator of progesterone functions in early pregnancy. In addition to the progesterone, Leukaemia inhibitory factor (LIF) is an essential factor for implantation18. LIF regulates expression of a number of genes critical for implantation, such as the muscle segment homeobox (Msh) gene family member Msx1 and Indian hedgehog19,20,21,22. Sox17 is also reported as one of the genes that are upregulated after LIF treatment21. Therefore, progesterone together with LIF is likely to induce Sox17 expression at the timing of implantation. Intriguingly, Sox17 showed moderately enhanced expression at the blastocyst attachment site (Fig. 2i,k’ and Wallingford et al.13), implicating the direct role of Sox17 in blastocyst attachment. Of interest, Wnt/β-catenin signalling is also active at the implantation site23. Previous studies have reported that Wnt/β-catenin signalling induces Sox1724,25; moreover, interaction of β-catenin with Sox17 may enhance the activity of Sox17 for induction of its target genes26. Therefore, expression of Sox17 together with Wnt/β-catenin signalling may be critical for embryonic implantation.
The mechanism of embryonic implantation is diverse across mammalian species, including humans and mice27,28,29. For example, mice produce many offspring in a single birth, whereas humans produce a single offspring in a single birth; additionally mouse implantation is eccentric (i.e., the blastocyst lies in a uterine crypt), whereas human implantation is interstitial (i.e., the blastocyst is completely embedded within the endometrium)27. However, availability of sophisticated genetic engineering has made the mouse an attractive model for human implantation and contributed to reveal the molecular basis of implantation, including elucidation of LIF as a critical factor for implantation18,30. Evidence from human studies suggests that Sox17 may also play a role in implantation. In a public database “The Human Protein Atlas”31,32, RNA expression of SOX17 in the endometrium is reported at a medium level (19 Fragments Per Kilobase gene model and Million reads [FPKM] in RNA-Seq), whereas RNA expressions of SOX7 and SOX18 in the endometrium are at low levels (4 and 2 FPKM, respectively). Therefore, it is suggested that SOX17 is the principal Sox-F protein expressed in the human uterus.
Another line of evidence implicates the involvement of SOX17 in successful pregnancy in humans. In exogenously administrated hormone-stimulated cycles aimed to conduct in vitro fertilisation and embryo transfer in humans, advanced endometrial maturation in histological dating compared to the expected chronological date is usually observed, in which, advanced maturation exceeding 3 days never results in successful pregnancy33,34. Microarray analysis revealed that SOX17 is one of the genes upregulated in the endometrium with 2–3 days advanced maturation (i.e., possible to achieve pregnancy) compared to those with 4 days advanced maturation (i.e., unable to achieve pregnancy)35, suggesting the relevance of SOX17 to pregnancy.
In this study, we found a novel role of uterine Sox17 in embryonic implantation by investigating both bulk knockout (Sox17+/GFP) and a point mutant (Sox17+/SHIVA). Further studies of changes in the localisation, expression, and molecular interactions of uterine Sox17 during the estrus cycle and pregnancy and the use of conditional knockout in the luminal epithelium will lead to clarification of the role of uterine Sox17 in implantation and ultimately contribute to improve infertility treatment in humans.
Methods
Animals and ethical statement
Sox17 GFP knock-in mice (Sox17tm1Sjm)9, and mutant mice carrying a point mutation in the Sox17 locus (SHIVA)15 were used in this study. Sox17+/GFP mice in the C57BL/6 background are also known to exhibit haploinsufficiency in bile duct formation, which leads to perinatal death due to biliary atresia and hepatitis14. For this reason, we used Sox17 GFP knock-in mice in a mixed background of C57BL/6 and ICR. Mice were housed in environmentally controlled, specific pathogen-free rooms in the Center for Experimental Animals of Tokyo Medical and Dental University (TMDU). All experiments were carried out in accordance with the approved guidelines by the institutional committees for animal and recombinant DNA experiments at TMDU. All experimental protocols were approved by the Institutional Animal Care and Use Committee of TMDU (Nos 0130082C, 0140007A, 0150259C2, and 0160024C2).
Superovulation and in vitro culture of embryos
Female mice of 8- to 12-week old were superovulated according to the standard procedure36. Pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG) were obtained from ASKA pharmaceutical (Tokyo, Japan) and used at a concentration of 7.5 IU, each. Two-cell stage embryos were collected according to the standard procedure36, and cultured at 37 °C, 5% CO2 in a drop of KSOM medium (ARK Resource, Kumamoto, Japan) covered with a layer of liquid paraffin (Nacalai Tesque, Kyoto, Japan).
Implantation site counting
Female mice were naturally mated with male mice. For females with vaginal plug, implantation sites were visualised by intravenous injection of 1% solution of Chicago sky blue dye (Sigma-Aldrich, MO, USA) at 13:00–17:00 on DOP 536.
Embryo transfer and implantation rate
Female mice were mated with vasectomised males to induce pseudopregnancy. At DOP 1, cryopreserved embryos at the pronuclear or 2-cell stage were thawed and transferred to the oviduct36. Alternatively, embryos at the morula or blastocyst stage were transferred to the uterus at DOP 336. The implantation rate was calculated as the number of implantation sites divided by the number of transferred embryos × 100 (%).
Delayed implantation
Delayed implantation was performed as previously described by Ma et al.37. Embryo transfer and ovariectomy were conducted at DOP 2. Progesterone (P4) (Sigma-Aldrich) was administrated at DOP 5 and 6. At DOP 7, both P4 and estrogen (E2) (Sigma-Aldrich) was administrated to induce implantation. The uteri were fixed at 6 h after P4 + E2 administration.
Preparation of the luminal epithelium
The luminal epithelium at DOP 4 was prepared by incubating the uteri in Hank’s balanced salt solution with pancreatin (Sigma-Aldrich) and dispase (Thermo Fisher, MA, USA) at 4 °C for 1 h and successively at room temperature for 1 h.
Quantitative RT-PCR
Total RNA was prepared from the naturally mated C57BL/6 female mice at 18:00–20:00 on DOP 4 using TRIzol® in combination with a PureLink® RNA mini kit and on-column DNase digestion (Thermo Fisher). Reverse transcription was performed using a SuperScript® III first-strand synthesis system for RT-PCR (Thermo Fisher) with random hexamers. Real-time PCR was performed by StepOne™ with TaqMan® Fast Advanced Master Mix (Thermo Fisher). The TaqMan® gene expression assays used in this study included Sox17 (Mm00488363_m1), Sox7 (Mm00776876_m1), Sox18 (Mm00656049_gH), Actb (Mm02619580_g1), and Gapdh (Mm99999915_g1). Data were analysed by the comparative CT (ΔΔCT) method, in which Actb or Gapdh served as an internal control, and uterus data were used as a calibrator38. We confirmed that the target genes and the internal control genes had similar PCR efficiencies, which were close to 100% (Supplementary Fig. S2).
Western blotting
Protein samples were prepared from the naturally mated C57BL/6 female mice at 18:00–20:00 on DOP 4, separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels, and transferred to polyvinylidene fluoride (PVDF) membranes. The primary antibodies used in this study included goat anti-Sox17 (R&D Systems, AF1924; 1:500), goat anti-Sox7 (R&D Systems, AF2766; 1:500), mouse anti-α-smooth muscle actin (Sigma-Aldrich, A2547; 1:20,000), and mouse anti-β-actin (Sigma-Aldrich, A1978, 1:2,000). The secondary antibodies used in this study included horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG (Jackson ImmunoResearch, PA, USA) and HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch). Amersham™ ECL Select™ (GE Healthcare, IL, USA) was used for detection.
Tissue processing
Animals were fixed by perfusion of 4% paraformaldehyde (PFA)/phosphate-buffered saline (PBS) after blood removal and female reproductive organs were excised. For observation of whole-mount fluorescence, organs were placed in PBS and photographed by Axio Zoom V16 microscope (Carl Zeiss). For immunostaining, the uteri were further fixed in 4% PFA at 4 °C overnight and embedded in paraffin or optimal cutting temperature (OCT) compound (Sakura Finetek Japan, Tokyo, Japan). After blocking with Tris-NaCl-blocking (TNB) buffer (Perkin Elmer, MA, USA), sections were incubated with primary antibodies diluted with PBS containing 0.1% Triton X-100 or Can Get Signal® solution A (Toyobo, Osaka, Japan). The primary antibodies and dilutions used in this study included chicken anti-GFP (Abcam, ab13970; 1:1,000), goat anti-Sox17 (R&D Systems, AF1924; 1:500), and goat anti-Sox7 (R&D Systems, AF2766; 1:500)7,39. Secondary antibodies included Alexa 488-conjugated donkey anti-chicken IgG, Cy3-conjugated bovine anti-goat IgG, and Biotin-SP donkey anti-goat IgG (Jackson ImmunoResearch). Heat-induced antigen retrieval in sodium citrate was conducted for immunohistochemistry of paraffin sections. For counterstaining, Meyer’s Haematoxylin (Wako Pure Chemical Industries, Osaka, Japan) or Hoechst 33258 were used. Specimens were mounted with Permount® (FALMA, Tokyo, Japan) or ProLong® Gold antifade reagent (Thermo Fisher) and photographed by BX53 microscope equipped with DP80 camera (Olympus) or by SP8 confocal microscope (Leica microsystems).
Statistical analysis
All statistical analyses were conducted using Prism 6 software (GraphPad Software, CA, USA). For all analyses, p < 0.05 was considered statistically significant. In implantation site counting, females with no implantation site due to failure in ovulation, fertilisation, and embryonic development were excluded from the statistical analysis.
Additional Information
How to cite this article: Hirate, Y. et al. Mouse Sox17 haploinsufficiency leads to female subfertility due to impaired implantation. Sci. Rep. 6, 24171; doi: 10.1038/srep24171 (2016).
Supplementary Material
Acknowledgments
We would like to thank M.M. Thet, Y. Kikuchi, and H. Takahashi for their excellent technical assistance and animal care; Y. Saijoh and S. Morrison for providing the Sox17 mutant mice. We are also grateful to the staff at the Center for Experimental Animals, Tokyo Medical and Dental University for housing the mice used for this study. This work was supported by JSPS KAKENHI Grant Numbers 24500485 and 15H04282 to M.K.-A., 25440112 to Y.H., 25830060 to M.K. and 24228005 to Y.K.
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.H., H.S., M.K., Y.K. and M.K.-A. designed the experiments; Y.H., H.S., M.K., H.M.T. and H.I. performed the experiments; P.N. produced mutant mice (SHIVA); and Y.H., H.S., M.K.-A. wrote the manuscript. All authors read and approved the final manuscript. Y.H., S.H. and M.K. contributed equally to this work.
References
- Cha J., Sun X. & Dey S. K. Mechanisms of implantation: strategies for successful pregnancy. Nat. Med. 18, 1754–1767, doi: 10.1038/nm.3012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. et al. Navigating the site for embryo implantation: biomechanical and molecular regulation of intrauterine embryo distribution. Mol. Aspects. Med. 34, 1024–1042, doi: 10.1016/j.mam.2012.07.017 (2013). [DOI] [PubMed] [Google Scholar]
- Schepers G. E., Teasdale R. D. & Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev. cell 3, 167–170 (2002). [DOI] [PubMed] [Google Scholar]
- Pennisi D. et al. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat. genet. 24, 434–437, doi: 10.1038/74301 (2000). [DOI] [PubMed] [Google Scholar]
- Matsui T. et al. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J. Cell Sci. 119, 3513–3526, doi: 10.1242/jcs.03081 (2006). [DOI] [PubMed] [Google Scholar]
- Sakamoto Y. et al. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem. Biophys. Res. Commun. 360, 539–544, doi: 10.1016/j.bbrc.2007.06.093 (2007). [DOI] [PubMed] [Google Scholar]
- Zhou Y., Williams J., Smallwood P. M. & Nathans J. Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina. PloS one 10, e0143650, doi: 10.1371/journal.pone.0143650 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermkens D. M. et al. Sox7 controls arterial specification in conjunction with hey2 and efnb2 function. Development 142, 1695–1704, doi: 10.1242/dev.117275 (2015). [DOI] [PubMed] [Google Scholar]
- Kim I., Saunders T. L. & Morrison S. J. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130, 470–483, doi: 10.1016/j.cell.2007.06.011 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuhisa I. et al. Sox17-mediated maintenance of fetal intra-aortic hematopoietic cell clusters. Mol. Cell. Biol. 34, 1976–1990, doi: 10.1128/MCB.01485-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai-Azuma M. et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129, 2367–2379 (2002). [DOI] [PubMed] [Google Scholar]
- Irie N. et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell 160, 253–268, doi: 10.1016/j.cell.2014.12.013 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford M. C., Angelo J. R. & Mager J. Morphogenetic analysis of peri-implantation development. Dev. Dyn. 242, 1110–1120, doi: 10.1002/dvdy.23991 (2013). [DOI] [PubMed] [Google Scholar]
- Uemura M. et al. Sox17 haploinsufficiency results in perinatal biliary atresia and hepatitis in C57BL/6 background mice. Development 140, 639–648, doi: 10.1242/dev.086702 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere S. et al. Sox17 regulates liver lipid metabolism and adaptation to fasting. PloS One 9, e104925, doi: 10.1371/journal.pone.0104925 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking B. et al. Sox7 and Sox17 are strain-specific modifiers of the lymphangiogenic defects caused by Sox18 dysfunction in mice. Development 136, 2385–2391, doi: 10.1242/dev.034827 (2009). [DOI] [PubMed] [Google Scholar]
- Rubel C. A. et al. Research resource: Genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol. Endocrinol. 26, 1428–1442, doi: 10.1210/me.2011-1355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. L. et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79, doi: 10.1038/359076a0 (1992). [DOI] [PubMed] [Google Scholar]
- Daikoku T. et al. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol. endocrinol. 18, 1238–1250, doi: 10.1210/me.2003-0403 (2004). [DOI] [PubMed] [Google Scholar]
- Daikoku T. et al. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev. Cell 21, 1014–1025, doi: 10.1016/j.devcel.2011.09.010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario G. X. et al. The LIF-mediated molecular signature regulating murine embryo implantation. Biol. Reprod. 91, 66, doi: 10.1095/biolreprod.114.118513 (2014). [DOI] [PubMed] [Google Scholar]
- Wakitani S., Hondo E., Phichitraslip T., Stewart C. L. & Kiso Y. Upregulation of Indian hedgehog gene in the uterine epithelium by leukemia inhibitory factor during mouse implantation. J. Reprod. Dev. 54, 113–116 (2008). [DOI] [PubMed] [Google Scholar]
- Mohamed O. A. et al. Uterine Wnt/beta-catenin signaling is required for implantation. Proc. Nat. Acad. Sci. USA 102, 8579–8584, doi: 10.1073/pnas.0500612102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormish J. D., Sinner D. & Zorn A. M. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev. Dyn. 239, 56–68, doi: 10.1002/dvdy.22046 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P. & Harley V. R. Acquisition of SOX transcription factor specificity through protein-protein interaction, modulation of Wnt signalling and post-translational modification. Int. J. Biochem. Cell Biol. 42, 400–410, doi: 10.1016/j.biocel.2009.10.017 (2010). [DOI] [PubMed] [Google Scholar]
- Sinner D., Rankin S., Lee M. & Zorn A. M. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development 131, 3069–3080, doi: 10.1242/dev.01176 (2004). [DOI] [PubMed] [Google Scholar]
- Wimsatt W. A. Some comparative aspects of implantation. Biol. Reprod. 12, 1–40 (1975). [DOI] [PubMed] [Google Scholar]
- Lee K. Y. & DeMayo F. J. Animal models of implantation. Reproduction 128, 679–695, doi: 10.1530/rep.1.00340 (2004). [DOI] [PubMed] [Google Scholar]
- Wang H. & Dey S. K. Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7, 185–199, doi: 10.1038/nrg1808 (2006). [DOI] [PubMed] [Google Scholar]
- Cullinan E. B. et al. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc. Nat. Acad. Sci. USA 93, 3115–3120 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M. et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 28, 1248–1250, doi: 10.1038/nbt1210-1248 (2010). [DOI] [PubMed] [Google Scholar]
- Uhlen M. et al. Tissue-based map of the human proteome. Science 347, 1260419, doi: 10.1126/science.1260419 (2015). [DOI] [PubMed] [Google Scholar]
- Ubaldi F. et al. Endometrial evaluation by aspiration biopsy on the day of oocyte retrieval in the embryo transfer cycles in patients with serum progesterone rise during the follicular phase. Fertil. Steril. 67, 521–526 (1997). [DOI] [PubMed] [Google Scholar]
- Kolibianakis E. et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil. Steril. 78, 1025–1029 (2002). [DOI] [PubMed] [Google Scholar]
- Van Vaerenbergh I. et al. In GnRH antagonist/rec-FSH stimulated cycles, advanced endometrial maturation on the day of oocyte retrieval correlates with altered gene expression. Hum. Reprod. 24, 1085–1091, doi: 10.1093/humrep/den501 (2009). [DOI] [PubMed] [Google Scholar]
- Behringer R., Gertsenstein M., Nagy K. V. & Nagy A. Manipulating the mouse embryo: a laboratory manual, 4th ed., Ch. 6, 195–235 (Cold Spring Harbor Laboratory Press, 2014). [Google Scholar]
- Ma W. G., Song H., Das S. K., Paria B. C. & Dey S. K. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc. Nat. Acad. Sci. USA 100, 2963–2968, doi: 10.1073/pnas.0530162100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T. D. & Livak K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008). [DOI] [PubMed] [Google Scholar]
- Wat M. J. et al. Mouse model reveals the role of SOX7 in the development of congenital diaphragmatic hernia associated with recurrent deletions of 8p23.1. Hum. Mol. Genet. 21, 4115–4125, doi: 10.1093/hmg/dds241 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.