Abstract
The conditions and the specificity by which an antibody binds to its target protein in routinely fixed and embedded tissues are unknown. Direct methods, such as staining in a knock-out animal or in vitro peptide scanning of the epitope, are costly and impractical. We aimed to elucidate antibody specificity and binding conditions using tissue staining and public genomic and immunological databases by comparing human and pig—the farmed mammal evolutionarily closest to humans besides apes. We used a database of 146 anti-human antibodies and found that antibodies tolerate partially conserved amino acid substitutions but not changes in target accessibility, as defined by epitope prediction algorithms. Some epitopes are sensitive to fixation and embedding in a species-specific fashion. We also find that half of the antibodies stain porcine tissue epitopes that have 60% to 100% similarity to human tissue at the amino acid sequence level. The reason why the remaining antibodies fail to stain the tissues remains elusive. Because of its similarity with the human, pig tissue offers a convenient tissue for quality control in immunohistochemistry, within and across laboratories, and an interesting model to investigate antibody specificity.
Keywords: antigen, epitope, immunohistochemistry, swine, quality control
Introduction
Diagnostic immunohistochemistry (IHC) is a technique stably embedded in the daily practice of human pathology worldwide. The vast majority of IHC tests are done on tissue which has been fixed in formalin, embedded in paraffin (FFPE), and stored at room temperature. The introduction of a heat-mediated process for the retrieval of the immune reactivity of the tissue (antigen retrieval; AR) (Shi et al. 1991) has further consolidated this practice. Nowadays, IHC is the most versatile of the companion diagnostics needed for individualized therapy (Taylor 2014).
The diagnostic use of IHC requires standardization by adoption of common analytical protocols (both pre-analytical and analytical) and quality control (QC) programs, as suggested by the College of American Pathologists (Fitzgibbons et al. 2014; Goldstein et al. 2007; Hardy et al. 2013; Robb et al. 2014). In Europe, at least two initiatives (NordicQC in Denmark and NEQAS in UK) run voluntary QC tests for IHC. QC tests are run on human tissue remnants from the operating theater, which are used as external control tissue samples; although, there are severe procurement and quality limitations for normal noble tissues such as brain, brainstem, heart, and some endocrine organs (pituitary, parathyroid). In addition, ethical considerations and nation-specific rules restrict the exchange of material across Europe (Riegman and van Veen 2011; van Veen et al. 2006).
Despite the widespread use of this technique, the most difficult task is to validate the staining in situ, given that a surprisingly high number of antibodies are not specific for the intended target (Bradbury and Plückthun 2015). At the beginning, immunizing an animal with a biochemically purified abundant antigen and staining with a relatively low-level sensitivity method was enough to produce results with acceptable specificity. However, the advent of the Human Genome project brought the need to produce specific antibodies against molecularly discovered antigen-carrying proteins, often expressed at low level and bearing sequence domains that are shared with other unrelated proteins.
The introduction of methods of synthetic peptide immunization with unique sequences from the desired protein as well as monoclonal antibody production effective in FFPE tissues (Jones et al. 1993) has consolidated the presence of IHC in daily practice. Antibodies raised against synthetic peptides may recognize continuous epitopes in FFPE material (Jones et al. 1993; Sompuram et al. 2006). Indeed, indirect evidence from an analysis of rabbit antisera produced against recombinant sequences of 50–150 amino acids showed that target-specific components of antisera directed against linear epitopes correctly identifies denatured targets on a western blot, whereas those against conformational epitopes do not (Forsström et al. 2015).
Furthermore, the binding of anti-peptide antibodies may be inhibited through competition with the immunizing peptide. However, this does not guarantee specificity (Holmseth et al. 2012), and specificity is therefore implied by a complex combination of indirect evidence (Bordeaux et al. 2010; Smith and Womack 2014). Then again, probing a knock-out experimental animal or employing a gene-silenced cell line, while the most stringent, are often impractical and costly solutions.
Ideally, one would need an identically processed tissue as that of the diagnostic human biopsy, which contains controlled and known variations of the antibody epitope and abundant similar background noise-producing bystander proteins to mimic the staining conditions in the human material. The use of an animal substitute for this task may represent a convenient solution for exploration. The extremely low probability that two unrelated but antigenically identical proteins may be represented in another species preserving the similarity of the unrelated sequences will make a differential immunostaining across the two species the evidence that the antibody is not recognizing an epitope uniquely identifying a protein. On the other hand, conserved proteins performing an identical function in related species may be found identically distributed and therefore similarly stained in the same tissues.
The domestic pig (Sus scrofa) is farmed across Europe for human consumption. Overall, the pig genome is 84.1% homologous to the human genome (Fang et al. 2012), one of the closest taxa after the primates. The pig and human genomes diverged 97 million years ago; yet the porcine genome has extensive similarities with the human genome and, thus, it represents an interesting disease model (Groenen et al. 2012). About 250 genes were gained or lost in each species after divergence, most notably the interferon-associated genes and olfactory genes, among others (Fang et al. 2012; Groenen et al. 2012). Genes conserved in the pig related to the cardiovascular function or drug response are the most studied.
The aim of this study was, therefore, to test human diagnostic antibodies on similar, but not identical, swine orthologous targets in order to understand how antibodies bind to FFPE tissue, as well as determine whether these are continuous linear or discontinuous epitopes, which are the epitope variations permissive for binding, and whether porcine tissue could be used for QC in IHC.
Materials & Methods
Tissues and Ethics Statement
Mature, 8-month-old castrated pigs (Sus scrofa domesticus) and 10-year-old bulls (Bos taurus) were sacrificed in abattoirs certified by the local Health Authority (ASL).
Formalin-fixed, paraffin-embedded (FFPE) fully anonymous human leftover material is exempt from the San Gerardo Institutional Review Board (IRB) approval as per Hospital regulations (ASG-DA-050 Donazione di materiale biologico a scopo di ricerca e/o sperimentazione, May 2012).
The specimens were fixed overnight at room temperature in phosphate-buffered 10% formalin, pH 7.2–7.4 (Bio-Optica, Milano, Italy). The tissue was dehydrated through a graded alcohol series, transferred to D-limonene (Bioclear; Bio-Optica) and embedded in paraffin. Three-µm sections were cut and placed on positively charged glass slides, baked, deparaffinized in xylene, and then rehydrated through a graded alcohol series and brought to water until further use.
Selected specimens were snap-frozen, sectioned in a cryostat (Leica Microsystems, Milan, Italy) and acetone-fixed.
Routine immunostaining was performed along diagnostic runs on a modified pig “Multi-sausage” tissue block (Battifora 1986) containing a human specimen relevant for the staining protocol as a control.
Immunohistochemistry (IHC) Protocols
Inclusion criteria for antibodies were: i) performance on FFPE material, ii) directed at non-polymorphic antigens in the human, iii) relevant for diagnosis or research, and iv) well characterized in terms of specificity. Antibodies against human pathogens and gene mutation-specific were excluded.
Sections were stained as per individual antibody dilutions and protocols established in our laboratory for diagnostic human IHC on a Dako Autostainer Link 48 (Dako, Glostrup, Denmark) with established protocols as suggested by the manufacturer. None required retrieval with proteases.
For individual manual stains, see Supporting Information S1.
Surveys of the reactivity of antibodies raised against human antigens on swine tissue fixed by routine methods have been published previously (Brodersen et al. 1998; Chianini et al. 2001; Debeer et al. 2013; Driessen et al. 2002; Faldyna et al. 2007; Jacobsen et al. 1993; Lauweryns and Van Ranst 1987; Lauweryns et al. 1987; Sierralta and Thole 1996; Tanimoto and Ohtsuki 1996).
Scoring Criteria
Results were scored for subcellular distribution (nuclear, cytoplasmic, membranous) and combinations of subcellular staining and intensity. In addition, the architectural distribution of the stained cells within structured tissues was noted (e.g., proliferating germinal centers versus mantle or interfollicular areas, basal cells in intestinal glands versus apical luminal cells, nerve cells and fibers within smooth muscle layers, among others).
A stain was scored positive if (1) the stain was qualitatively and quantitatively different from the occasional homogeneous faint and diffuse non-specific negative control staining; and (2) both the expected subcellular localization and the architectural distribution were maintained in the porcine tissue, compared with the reference human reactivity. For antigens ubiquitously expressed or known to have a tissue- or subunit-dependent variegation (e.g., S-100 (Takahashi et al. 1984)), putative taxonomic variations were accepted, if the subcellular distribution and at least some of the human pattern was maintained in the pig.
Sequence Retrieval and Alignment
The epitope sequence, provided by the manufacturer or collected from the published literature, was used for the human–pig amino acid (aa) sequence comparison and is reported in Table 1. In the absence of a known sequence, a 100–300-aa sequence was used if the epitope was generically listed as “C-” or “N-terminus”. In the absence of either, the whole protein sequence was used. Only the shorter of these three choices was used for the analysis. Details and additional information is contained in Supporting Information Table S1.
Table 1.
Targeted Proteins, Antibodies Used, Sequences, Similarity and Results.
| Target | Source | Antibody | Clone | Epitope | UNIPROT Human | UNIPROT Swine | Similarity Hu/Sw | Tested | Class |
|---|---|---|---|---|---|---|---|---|---|
| Actin, alpha skeletal muscle | 11 | IgG1 | HHF35 | unknown | P68133 | P68137 | 100% | POS | Basic cytoskeletal |
| Actin, aortic smooth muscle | 5 | IgG2a | 1A4 | 10 N-term aa | P62736 | C7AI81 | 100% | POS | Basic cytoskeletal |
| ALK (CD246) | 4 | Rb mAb | D5F3 | C-term (cytoplasmic dom. aa 1060–1620) | Q9UM73 | K7GQT6 | 83% | NEG | Receptor signaling |
| ALK (anaplastic lymphoma kinase; CD246) | 5 | IgG3 | ALK1 | aa 1359–1460 (419–520 chimera) | Q9UM73 | K7GQT6 | 83% | NEG | Receptor signaling |
| ALK (CD246) | 9 | IgG1 | 5A4 | aa 419–520 (chimera) | Q9UM73 | K7GQT6 | 99% | NEG | Receptor signaling |
| ALK (CD246) | 11 | Rb mAb | SP8 | tyrosine kinase catalytic domain & C-terminus | Q9UM73 | K7GQT6 | 91% | NEG | Receptor signaling |
| AMACR | 5 | Rb mAb | 13H4 | unknown | Q9UHK6 | F1SP14 | 78% | NEG | Cytoplasmic; misc |
| BCL-2 | 5 | IgG1 | Bcl-2-100 | aa 41–54 | P10415 | A5A790 | 100% | POS | Apoptosis-related |
| BCL6 | 5 | IgG1 | PG-B6p | aa 3–484 | P41182 | F1FSH8 | 93% | NEG | TF |
| BCL6 | 6 | IgG2b | LN22 | aa 1–350 | P41182 | F1FSH8 | 93% | POS | TF |
| BCL6 | 9 | Rb poly | BCL6 (N3) | aa 3–484 | P41182 | F1FSH8 | 93% | POS | TF |
| BRCA-1 | 7 | IgG1 | MS110 | aa 1–304 | Q3B891 | A5A751 | 82% | NEG | Cell cycle; DNA replication |
| CA125 | 5 | IgG1 | M11 | unknown | N/A | N/A | N/A | NEG | Cell–cell interaction |
| Calcitonin | 5 | Rb poly | unknown | P01258 | A6P7L6 | 61% | POS | Cytoplasmic; misc | |
| Calponin 2 | 5 | IgG1 | CALP | unknown | Q99439 | Q08094 | 92% | POS | Basic cytoskeletal |
| Calretinin | 5 | IgG1 | DAK-Calret 1 | unknown | P22676 | F1S3E7 | 97% | NEG | Cytoplasmic; misc |
| CCND1 (Cyclin D1) | 11 | Rb mAb | SP4 | C-term (100 aa) | P24385 | F1RY77 | 98% | NEG | Cell cycle; DNA replication |
| CD10 | 5 | IgG1 | 56C6 | external domain (52–750) | P08473 | K7GMJ2 | 94% | POS | Receptor signaling |
| CD117 (c-kit) | 5 | Rb poly | aa 963–976 (C-term) | P10721 | Q2HWD6 | 90% | POS | Receptor signaling | |
| CD138 | 5 | IgG1 | MI15 | ectodomain (aa 105–112) | P18827 | M5DFN4 | 77% | NEG | Cell–cell interaction |
| CD14 | 10 | Rb poly | HPA002127 | aa 229–370 | P08571 | A2SW51 | 72% | NEG | Cell–cell interaction |
| CD141 | 1 | Rb mAb | EPR4051 | C-term (150 aa) | P07204 | B3STX8 | 69% | NEG | Receptor signaling |
| CD15 | 5 | IgM | C3D-1 | Lewis-X | N/A | N/A | N/A | NEG | Cell–cell interaction |
| CD16 | 6 | IgG2a | 2H7 | External domain (aa 17–208) | P08637 | Q28942 | 60% | NEG | Cell–cell interaction |
| CD16 | 9 | IgG1 | DJ130c | unknown | P08637 | Q28942 | 63% | NEG | Cell–cell interaction |
| CD163 | 11 | IgG1 | 10D6 | Domain 1–4 (N-term) | Q86VB7 | Q28942 | 90% | NEG | Cell–cell interaction |
| CD163L1 | 9 | Rb mAb | EPR6539 | Intracellular | Q9NR16 | J9T9K7 | 55% | NEG | Cell–cell interaction |
| CD1a | 5 | IgG1 | O10 | unknown | P06126 | A0ZPR3 | 63% | NEG | Cell–cell interaction; Immun |
| CD2 | 11 | IgG1 | TS2/18 | unknown | P06729 | F1SAX9 | 60% | NEG | Cell–cell interaction; Immun |
| CD20 | 5 | IgG2a | L26 | Intracellular (aa 1–56; aa 106–120; aa 210–297) | P11836 | I3LDX9 | 73% | NEG | Cell–cell interaction; Immun |
| CD23 | 5 | IgG1 | DAK-CD23 | aa 48–248 | P06734 | B8YM31 | 64% | NEG | Cell–cell interaction; Immun |
| CD271 (NGF-R p75) | 1 | Rb mAb | EP1039Y | unknown | P08138 | F1RVT6 | 72% | POS | Receptor signaling |
| CD3 epsilon | 5 | Rb poly | poly | aa 156–168 | P07766 | P79264 | 92% | POS | Cell–cell interaction; Immun |
| CD30 | 5 | IgG1 | Ber-H2 | unknown | P28908 | F1RF73 | 58% | NEG | Receptor signaling |
| CD31 | 5 | IgG1 | JC70A | unknown | P16284 | Q95242 | 58% | NEG | Cell–cell interaction |
| CD34 | 1 | Rb mAb | EP373Y | C-term (100 aa) | P28906 | K7GKN6 | 93% | NEG | Cell–-cell interaction |
| CD34 | 5 | IgG1 | QBEnd10 | Class II CD34 | P28906 | K7GKN6 | 63% | NEG | Cell–cell interaction |
| CD4 | 6 | IgG1 | 1F6 | External domain | P01730 | Q6R3N4 | 59% | NEG | Cell–cell interaction; Immun |
| CD43 | 5 | IgG1 | DF-T1 | unknown | P16150 | D9MNC9 | 50% | NEG | Cell–cell interaction |
| CD44 | 10 | Rb poly | aa 176–313 | P16070 | F1SGT4 | 55% | NEG | Cell–cell interaction | |
| CD45 | 5 | IgG1 | PD726 + 2B11 | multiple | P08575 | Q6SZ85 | 43% | NEG | Receptor signaling |
| CD45 | 9 | IgG1 | Bra-55 | Extracellular (aa 24–575) | P08575 | Q6SZ85 | 20% | NEG | Receptor signaling |
| CD5 | 5 | IgG1 | 4C7 | External domain aa 25–372 | P06127 | F1RIA2 | 62% | NEG | Cell–cell interaction; Immun |
| CD5 | 5 | IgG1 | CD5/54/F6 | aa 474–495 | P06127 | F1RIA2 | 91% | POS | Cell–cell interaction; Immun |
| CD56 | 5 | IgG1 | 123C3.D5 | unknown | P13591 | K7GMV4 | 97% | POS | Cell–cell interaction |
| CD57 | 5 | IgM | TB01 | unknown | Q96E93 | F1SLW9 | 70% | POS* | Cell–cell interaction |
| CD68 | 5 | IgG1 | KP1 | unknown | P34810 | F1S4M0 | 72% | NEG | Cytoplasmic; misc |
| CD68 | 5 | IgG3 | PGM1 | unknown | P34810 | F1S4M0 | 69% | NEG | Cytoplasmic; misc |
| CD68 | 9 | Rb poly | CD68 (H-255) | aa 100–354 | P34810 | F1S4M0 | 69% | POS | Cytoplasmic; misc |
| CD7 | 11 | IgG1 | MEM-186 | unknown | P09564 | M5DNE0 | 47% | NEG | Cell–cell interaction; Immun |
| CD79a | 5 | IgG1 | JCB117 | Extracellular (aa 33–143^) | P11912 | K7GM80 | 93% | POS (subpop) | Cell–cell interaction; Immun |
| CD79a | 10 | IgG1 | HM47 | aa 208–222 | P11912 | K7GM80 | 93% | POS | Cell–cell interaction; Immun |
| CD8 alpha | 5 | IgG1 | C8/144B | 13 C-term aa | P01732 | F1SVD3 | 59% | NEG | Cell–cell interaction; Immun |
| CD99 (HBA 71) | 5 | IgG1 | 12E7 | unknown | P14209 | F1RQ20 | 37% | NEG | Cell–cell interaction |
| CDKN1A (p21) | 11 | IgG2b | CP74 | aa 1–80 | P38936 | I3LK35 | 83% | NEG | Cell cycle; DNA replication |
| CDKN1B (p27) | 11 | IgG1 | DCS-72.F6 | aa 83–204 | P46527 | I3LIR2 | 81% | POS | Cell cycle; DNA replication |
| CDKN1B (p27) | 11 | Rb poly | C-term (100 aa) | P46527 | I3LIR2 | 86% | POS# | Cell cycle; DNA replication | |
| CDKN2A (p16) | 9 | IgG2a | F-12 | unknown | P42771 | Q9TSY1 | 55% | NEG | Cell cycle; DNA replication |
| CDKN2A (p16) | 9 | IgG2a | JC8 | unknown | P42771 | Q9TSY1 | 55% | NEG | Cell cycle; DNA replication |
| CDX2 | 1 | IgG1 | CDX2-88 | unknown | Q99626 | D0V4H7 | 96% | POS | TF |
| CEA | 5 | IgG1 | II-7 | unknown | P06731 | K7GKS4 | 66% | NEG | Cell–cell interaction |
| CFTR | 9 | IgG1 | M3A7 | aa 1370–1380 | P13569 | Q6PQZ2 | 91% | NEG | Receptor signaling |
| Chromogranin A | 5 | IgG2b | DAK-A3 | aa 210–439 | P10645 | F1SD66 | 72% | POS | Cytoplasmic; misc |
| Cleaved Caspase 3 | 4 | Rb poly | Asp175 | P42574 | Q95ND5 | 90% | POS | Apoptosis-related | |
| Cleaved Caspase 3 | 4 | Rb mAb | 5A1E | P42574 | Q95ND5 | 90% | POS | Apoptosis-related | |
| Cleaved Notch | 4 | Rb mAb | D3B8 | Val1744 | P46531 | F1SB08 | 50% | NEG | TF |
| Cleaved Notch | 4 | Rb poly | Val1744 | P46531 | F1SB08 | 50% | NEG | TF | |
| Cleaved PARP | 4 | Rb mAb | D64E10 | Asp214 | P09874 | I3LDH3 | 94% | NEG | TF |
| CTNNB1 | 2 | IgG1 | #14 | aa 571–781 (mouse) | B4DGU4 | Q8WNW4 | 99% | POS | Cell–cell interaction |
| Cytokeratin-A 34BETA E12 | 5 | IgG1 | 34bE12 | Ker 1, 5, 10, 14 | N/A | N/A | N/A | POS | Basic cytoskeletal |
| Cytokeratins AE1-AE3 | 5 | IgG1 (pooled) | AE1-AE3 | multiple | N/A | N/A | N/A | POS | Basic cytoskeletal |
| Cytokeratins 5/6 | 5 | IgG1 | D5/16 B4 | unknown | N/A | N/A | N/A | POS | Basic cytoskeletal |
| Cytokeratins 8/18 | 11 | IgG2a | 5D3 | unknown | N/A | N/A | N/A | NEG | Basic cytoskeletal |
| Desmin | 11 | IgG1 | D33 | unknown | P17661 | P02540 | 98% | POS | Basic cytoskeletal |
| E-Cadherin | 5 | IgG1 | NCH-38 | unknown | P12830 | C6EVT4 | 84% | POS | Cell–cell interaction |
| E2-2/TCF4 | 10 | IgG2a | 6H5-3 | aa 31–331 | P15884 | F1S1Z1 | 99% | POS | TF |
| E2A/E47/TCF3 | 10 | Rb poly | N-649 | aa 1–649 | P15923 | F1SDI1 | 71% | NEG | TF |
| EMA | 5 | IgG2a | E29 | APDTRP repeat on Mucin-1 | P15941 | F1RGR9 | 40% | POS¥ | Cell–cell interaction |
| Emerin | 9 | Rb poly | (FL-254) | aa 3–254 | P50402 | I3LHY0 | 50% | POS | Cell cycle; DNA replication |
| ER alpha | 5 | IgG1 | 1D5 | N-term (aa 24–575) | P03372 | Q29040 | 92% | POS | TF |
| Foxp3 | 1 | IgG1 | 236A/E7 | unknown | Q9BZS1 | Q6BBQ1 | 90% | NEG | TF |
| FRMD6 | 10 | Rb poly | unknown | Q96NE9-3 | F1SFF5 | 96% | POS | Cell–cell interaction | |
| GFAP | 5 | Rb poly | unknown | P14136 | F1RR02 | 93% | POS | Basic cytoskeletal | |
| gH2AX | 4 | Rb mAb | phospho-epitope | N/A | N/A | N/A | POS | Apoptosis-related | |
| gH2AX | 7 | IgG1 | JBW301 | phospho-epitope | N/A | N/A | N/A | POS | Apoptosis-related |
| HER2/ErbB2 | 5 | Rb poly | unknown | P04626 | K7GS43 | 91% | NEG | Receptor signaling | |
| HER2/ErbB2 | 11 | IgG1 | e2-4001 | intracellular (aa 676–1255) | P04626 | K7GS43 | 94% | NEG | Receptor signaling |
| HER2/ErbB2 | 11 | Rb mAb | SP3 | extracellular (aa 23–652) | P04626 | K7GS43 | 89% | NEG | Receptor signaling |
| HTR2B | 10 | Rb poly | aa 240–326 | P41595 | F1SMV8 | 91% | POS | Receptor signaling | |
| ID1 | 3 | Rb mAb | BCH-1/195-14 | unknown | P41134 | B3W6M6 | 91% | NEG | TF |
| ID2 | 3 | Rb mAb | BCH-3/9-2-8 | unknown | Q02363 | Q2VIU1 | 98% | POS | TF |
| ID3 | 3 | Rb mAb | BCH-4/17-3 | unknown | Q02535 | B3W6M8 | 96% | NEG | TF |
| Inhibin alpha | 5 | IgG2a | R1 | aa 1–32 | P05111 | P04087 | 83% | POS | Cytoplasmic; misc |
| IRF4 | 1 | Rb mAb | EP5699 | unknown | Q15306 | A0MZ86 | 93% | POS | TF |
| IRF8 | 10 | Rb poly | aa 90–211 | Q02556 | Q6T5D3 | 88% | POS | TF | |
| Ki-67 | 5 | IgG1 | MIB 1 | aa 1101–1112 (FKELF) | P46013 | I3LNN3 | 40% | POS | Cell cycle; DNA replication |
| Ki-67 | 8 | IgG2a | UMAB107 | aa 1160–1493 | P46013 | I3LNN3 | 45% | POS | Cell cycle; DNA replication |
| Ki-67 | 11 | Rb mAb | SP6 | C-term (150 aa) | P46013 | I3LNN3 | 92% | NEG# | Cell cycle; DNA replication |
| KRT18 (Cytokeratin 18) | 1 | Rb mAb | EPR1626 | C-term (100 aa) | P05783 | F1SGG1 | 93% | NEG | Basic cytoskeletal |
| KRT18 (Cytokeratin 18) | 5 | IgG1 | DC-10 | unknown | P05783 | F1SGG1 | 79% | NEG | Basic cytoskeletal |
| KRT19 (Cytokeratin 19) | 9 | Goat poly | M-17 | C-term (mouse) | P08727 | F1S0J8 | 84% | NEG | Basic cytoskeletal |
| KRT20 (Cytokeratin 20) | 5 | IgG2a | Ks 20.8 | unknown | P35900 | Q29218 | 75% | NEG | Basic cytoskeletal |
| KRT7 (Cytokeratin 7) | 1 | Rb mAb | EPR1619Y | N-term (200 aa) | P08729 | F1SGI7 | 57% | POS | Basic cytoskeletal |
| KRT7 (Cytokeratin 7) | 5 | IgG1 | OV-TL 12/30 | unknown | P08729 | F1SGI7 | 65% | POS | Basic cytoskeletal |
| MART1 (Melan A) | 5 | IgG1 | A103 | unknown | Q16655 | F1SMM1 | 73% | NEG | Cytoplasmic; misc |
| MCM5 | 9 | IgG2b | E-10 | aa 1-30 | P33992 | I3LR86 | 77% | POS | Cell cycle; DNA replication |
| Mel-18/PCGF2 | 9 | Goat poly | MEL-18 (C20) | C-term | P35226 | F2Z5D1 | 99% | NEG | Cell cycle; DNA replication |
| MMR MLH1 | 5 | IgG1 | ES05 | unknown (210 aa) | P40692 | D3K5L8 | 91% | NEG | Cell cycle; DNA replication |
| MMR MSH2 | 11 | IgG1 | FE11 | C-term | P43246 | D3K5K3 | 95% | POS | Cell cycle; DNA replication |
| MMR MSH6 | 1 | Rb mAb | EPR3945 | C-term | P52701 | I3LHZ9 | 91% | POS | Cell cycle; DNA replication |
| MMR PMS2 | 9 | Rb poly | (C-20) | C-term (300 aa) | P54278 | F1RFM9 | 78% | POS | Cell cycle; DNA replication |
| Mucin-2 | 9 | IgG1 | Ccp58 | 5 tandem repeats | N/A | N/A | N/A | NEG | Cell–cell interaction |
| Mucin-6 | 9 | IgG1 | CLH5 | tandem repeats | N/A | N/A | N/A | NEG | Cell–cell interaction |
| MYC | 1 | Rb mAb | Y69 | N-term | P01106 | Q29031 | 94% | POS | TF |
| MYC | 9 | Rb poly | (N-262) | aa 1–262 | P01106 | Q29031 | 94% | POS | TF |
| Myeloperoxidase | 5 | Rb poly | unknown | P05164 | K7GRV6 | 87% | POS | Cytoplasmic; misc | |
| Napsin A | 1 | Rb mAb | EPR6257 | aa 60–90 | O96009 | F1RH37 | 84% | NEG | Cytoplasmic;misc |
| NF Pool | 5 | IgG1 | 2F11 | unknown | N/A | N/A | N/A | POS | Basic cytoskeletal |
| NKX2-1 (TTF-1) | 5 | IgG1 | 8G7G3/1 | unknown | P43699 | F1SHK3 | 97% | POS | TF |
| Pax5 | 6 | IgG1 | 1EW | unknown | Q02548 | F1ST83 | 97% | NEG | TF |
| Pax5 | 11 | Rb mAb | SP34 | C-term (aa 251–261) | Q02548 | F1ST83 | 63% | POS | TF |
| Perforin | 11 | IgG1 | 5B10 | C-term (100 aa) | P14222 | F1SUB6 | 75% | POS | Cytoplasmic; misc |
| Pmel 17 (HMB 45) | 5 | IgG1 | HMB45 | unknown | P40967 | Q4LE84 | 79% | NEG | Cytoplasmic; misc |
| PRDM1 / Blimp-1 | 4 | rat IgG2a | 6D3 | aa 255–395 (mouse) | O75626 | F1RYP9 | 74% | NEG | TF |
| PRG Progesterone receptor | 5 | IgG1 | PGR 636 | unknown | P06401 | D0EWS6 | 85% | NEG | TF |
| Pro-opiomelanocortin (ACTH) | 5 | IgG1 | 02A3 | aa 1–39 (N-term) | P01189 | P01192 | 100% | NEG | Cytoplasmic; misc |
| Prolactin (PRL) | 5 | Rb poly | unknown | P01236 | P01238 | 79% | POS | Cytoplasmic; misc | |
| PSA | 11 | Rb poly | unknown | P07288 | P00752 | 59% | NEG | Cytoplasmic; misc | |
| PTEN | 4 | Rb mAb | 138G6 | C-term | P60484 | B8XSI6 | 99% | POS | Receptor signaling |
| S-100 alpha chain | 11 | IgG2a | 4C4.9 | unknown | P23297 | K7GQ84 | 73% | POS | Cytoplasmic; misc |
| S-100 alpha+beta | 5 | Rb poly | unknown | N/A | N/A | N/A | POS | Cytoplasmic; misc | |
| Somatotropin (Growth hormone) | 5 | Rb poly | unknown | P01241 | P01248 | 68% | POS | Cytoplasmic; misc | |
| Synaptophysin | 5 | IgG1 | DAK-SYNAP | C-term | P08247 | F1RW46 | 94% | POS | Cytoplasmic; misc |
| TdT | 5 | Rb poly | unknown | P04053 | F1SBG2 | 86% | POS | Cell cycle; DNA replication | |
| TFF3 (intestinal trefoil factor) | 9 | IgG1 | H-425 | unknown | Q07654 | Q29183 | 80% | POS | Cell–cell interaction |
| Thyroglobulin | 11 | Rb poly | unknown | P01266 | D1KKB3 | 74% | POS | Cytoplasmic; misc | |
| Thyrotropin subunit beta (TSH) | 11 | IgG1 | ZMTS2 | unknown | P01222 | P01224 | 89% | NEG | Cytoplasmic; misc |
| TP53 | 5 | IgG2b | DO-7 | aa 1–45 | P04637 | Q9TUB2 | 46% | POS | Cell cycle; DNA replication |
| TP53 | 12 | IgG1 | Pab 1801 | aa 32–79 | P04637 | Q9TUB2 | 72% | NEG | Cell cycle; DNA replication |
| TP63 | 5 | IgG2a | 4A4 | aa 1–205 (N-term) | Q9H3D4 | I3LP4 | 99% | POS | Cell cycle; DNA replication |
| TP63 | 9 | Rb poly | H-137 | aa 15–151 | Q9H3D4 | I3LP4 | 98% | POS | Cell cycle; DNA replication |
| TP63 p40 (DN63) | 7 | Rb poly | aa 5–17 | Q9H3D4-2 | I3LPD4 | 99% | POS | Cell cycle; DNA replication | |
| Vimentin | 5 | IgG1 | V9 | unknown | P08670 | P02543 | 98% | POS | Basic cytoskeletal |
| vWF (von Willebrand Factor VIII) | 5 | Rb poly | unknown | P04275 | Q28833 | 75% | POS | Cytoplasmic; misc | |
| WT1 Protein | 5 | IgG1 | 6F-H2 | aa 1–181 | P19544 | O62651 | 99% | POS* | TF |
| ZEB1 | 10 | Rb poly | aa 498–627 | P37275 | F1RWD4 | 84% | POS | TF |
All antibodies listed are from mouse, except when differently noted [rabbit (Rb); rat; goat]. The epitope is listed if known. Note that the % similarity between human and pig is calculated for the shortest sequence known for the pig, for the full-length protein if the epitope is unknown. Ventral and diffuse prostate and testis were obtained from a bull. Under the “Tested” column: *Differences in selected tissues; see text; #Non-specific staining, see text. ¥Different cell type stained. Abbreviations: poly, polyclonal; mAb, monoclonal antibody; N-term, N-terminal; C-term, C-terminal; POS, positive; NEG, negative; TF, transcription factor; Immun, immunity; misc, miscellaneous.
Human protein sequences and respective codes were obtained by querying UNIPROT (Universal Protein Resource) (www.uniprot.org) (last accessed on March 24, 2015, tutorial at http://www.uniprot.org/help/). The sequence was queried in UNIPROT for the Sus taxa, the animal sequence aligned and inspected for the degree of similarity. In addition, the human sequence in FASTA format was aligned with BLAST (Basic Local Alignment Search Tool) (http://blast.ncbi.nlm.nih.gov) with default settings (last accessed on March 24, 2015, tutorial at ftp://ftp.ncbi.nlm.nih.gov/pub/factsheets/HowTo_BLASTGuide.pdf) for the taxa required (human, swine, rat, others). The sequence was blasted, filtered for the taxonomic species and aligned in order to obtain the percent identity. Sequence alignments of discrepant cases (antibody positive / low identity or vice versa) were reviewed and visually inspected. The term “partially conserved” will be used throughout for substitutions of amino acids between groups of strongly similar properties with scoring >0.5 in the Gonnet PAM 250 matrix (see http://www.uniprot.org/help/sequence-alignments) (Mount 2008).
UniProtKB/Swiss-Prot database was preferred over UniProtKB/TrEMBL entries whenever available. Antibodies directed against multiple proteins (e.g., basic keratins) or carbohydrates were not considered for alignment.
The protein codes and the links are available as a supplementary Excel table (Supporting Information Table S1).
Epitope prediction modeling was used in selected cases with the IEDB Analysis Resource platform (http://tools.immuneepitope.org/bcell/) (last accessed on March 24, 2015, tutorial at http://tools.immuneepitope.org/bcell/help/). Human and porcine sequences were modeled with three algorithms (Emini surface accessibility scale, Kolaskar and Tongaonkar antigenicity scale and BepiPred Linear Epitope Prediction) in order to predict their epitope spatial disposition and accessibility; the latter was usually given a positive score on the y-axis. The data were imported in an Excel spreadsheet and a graph generated for the region of interest.
Virtual Whole-Slide Imaging
Stained slide images were acquired with an Aperio CS whole slide scanner (Leica Microsystems, Italy) at 20× and 40×. Individual single stain images in light microscopy were acquired with the ImageScope software (Aperio), optimized for contrast with Adobe Photoshop CS3 (Adobe Systems Incorporated, San Jose, CA), and mounted with Adobe Illustrator.
Results
Overall Similarity and Impact on FFPE-Stained Samples
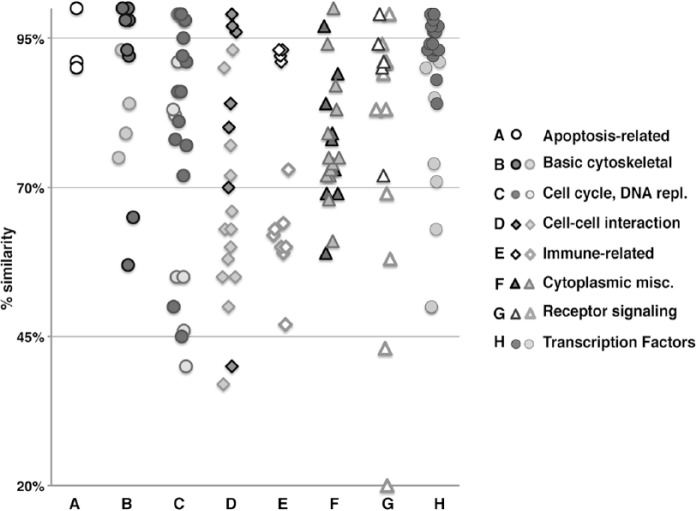
The average similarity score (mean ± SD) for the full list of proteins that were evaluated with a panel of antibodies was 79.2% ± 17.4% (range, 20% CD45 to 100% actins, BCL2), and this percentage was conserved among antibodies routinely used for human diagnostics or reagents used for research projects. Similar percentages were reported in broader, genome-wide comparisons of human and pig sequences (Dawson 2012).
Positive results, scored as specified, were recorded for 74/146 individual antibodies (50.7%). The positive-staining antibodies tended to cluster in the higher similarity group of targets (Table 1).
The proteins against which the antibodies were raised were representative of a broad collection of targets, heterogeneous in function, and cellular and subcellular localization. In order to gain insight into whether there was any preferential reactivity by target, we subdivided the targets into broad categories, and each group was plotted according to the degree of similarity and the positive or negative result in FFPE (Fig. 1). As reported previously (Fang et al. 2012; Groenen et al. 2012), some groups diverged more than others during evolution (e.g., the immune-associated molecules); however, within each group, FFPE-reactive antibodies were related to a higher degree of similarity of the target (Fig. 1). None of the secondary reagents used reacted with the swine tissue (not shown).
Figure 1.
Degree of similarity (human to porcine) versus FFPE staining using anti-human antibodies, categorized by protein class type. The human proteins against which antibodies have been raised are grouped by broad classes and plotted on a similarity scale. Within each class, darker symbols represents FFPE-staining antibodies, lighter ones non-staining antibodies.
Specificity and Differential Reactivity for Human versus Porcine Tissues
The correlation between the degree of similarity and the positive staining of fixed material (Fig. 1) was accompanied by a generally highly conserved distribution of staining within the tissue at the cellular and subcellular levels (with few exceptions, see below) (see examples in Supporting information Fig. S1).
Four out of 74 positively staining antibodies (5.4%) either presented additional reactivity or lacked some stained cell types in the human-pig comparison. These are illustrated in Figures 2 through 4. These results were replicated on frozen sections (not shown).
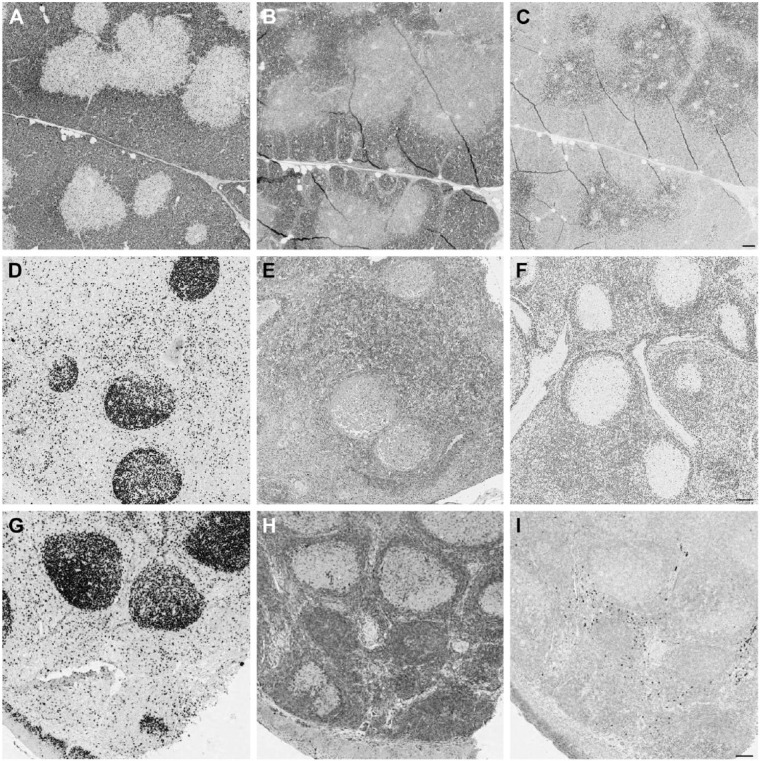
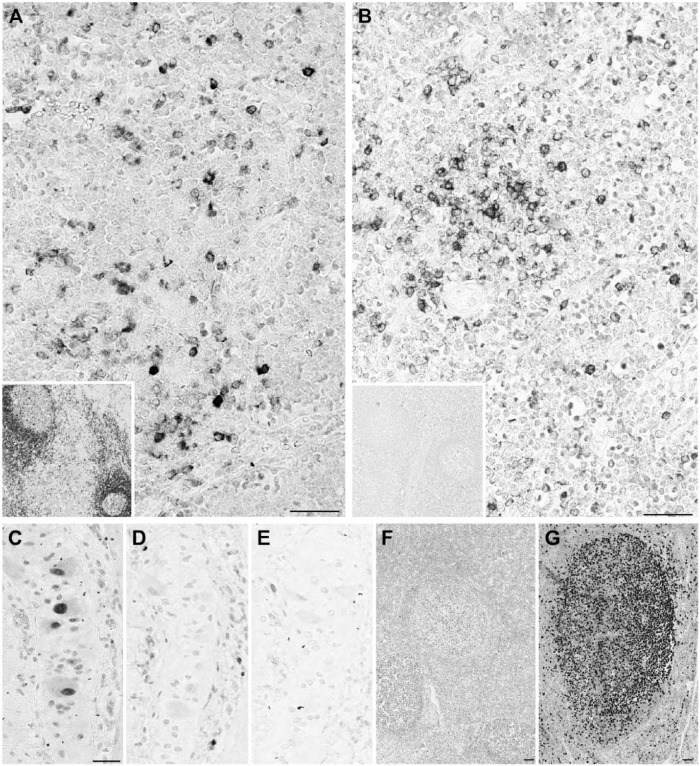
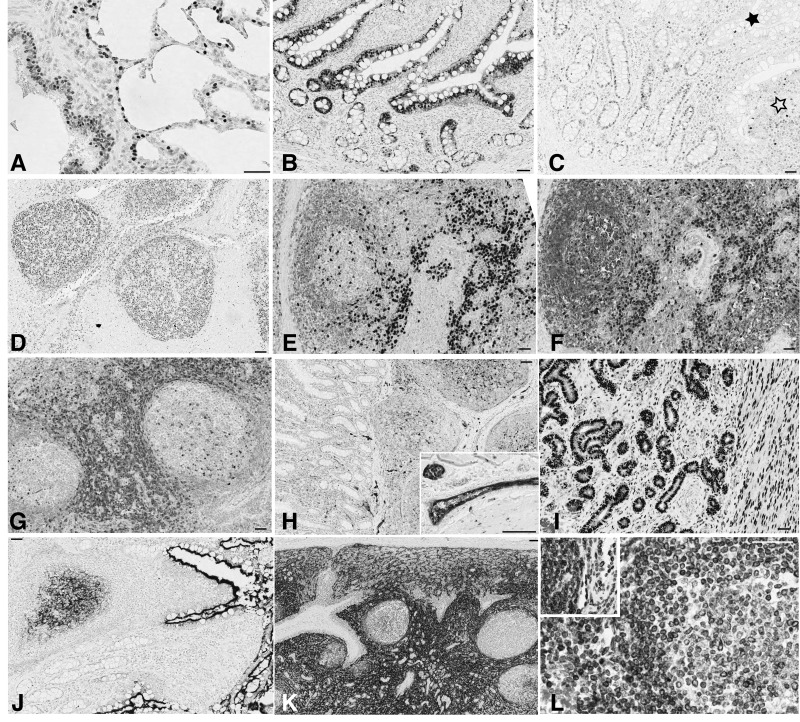
Figure 2.

Differential reactivity for anti-human antibodies on swine tissue. (A, B) CD57 decorates follicular helper T cells in human tonsil (A), but not in porcine (B). Swine neural cells in Auerbach plexi are stained (inset). (C, D) Wilms’ tumor 1 (WT1) stains the cytoplasm and nuclei (arrows) in the human kidney glomerulus (C) but only the nuclei are stained in the porcine kidney (D). Scale, 100 µm.
Figure 4.
CDKN1B (p27) antibodies stain opposite compartments in swine thymus. Porcine thymus (A, B and C), lymph node (D, E and F) and human tonsil (G, H and I) are stained with Ki-67 MIB 1 antibody (A, D and G), mouse anti-p27 (B, E and H) and rabbit anti-p27 (C, F and I). Note the opposite staining pattern of the mouse (B) and rabbit (C) reagents in the thymus. In the peripheral swine and human tissues, proliferating germinal center cells are unstained. Note the reversed staining intensity of both anti-p27 antibodies with porcine and human tissue. Double staining for Ki-67 and both anti-p27 antibodies showed that proliferating corticothymocytes were mutually exclusively labeled by the rabbit antibody and co-expressed CDKN1B with the mouse reagent; this co-expression is the expected distribution (Nagahama et al. 2001). Scale, 100 µm
Figure 3.
Heterogeneous staining patterns of anti-human antibodies on swine. (A) CD79a HM57 antibody stains scattered lymphocytes in the swine thymic medulla and all lymph nodal B cells (inset). (B) CD79a JCB11 antibody stains scattered lymphocytes in the swine thymic medulla but none in the lymph node (inset). (C) Ki-67 SP6 antibody stains the nuclei in an Auerbach neural plexus. (D, E) The same nuclei are unstained with anti-Ki-67 MIB 1 (D) and anti-MCM5 mouse antibody (E). (F, G) Ki-67 SP6 antibody weakly stains proliferating nuclei in porcine lymph node (F) but strongly in human tonsil (G). The C-terminal 250-aa sequence of human Ki-67 past the 16 repeated motifs (the immunogen for this monoclonal) has less than 40% similarity with the swine counterpart. Scale, 100 µm
Human Diagnostic IHC Control Use of Swine Tissue
The pig may be a convenient source of positive external control for important diagnostic antibodies. We focused on targets important for diagnostics or therapy and difficult to standardize in QC schemes. About half of routinely used human diagnostic antibodies, including antibodies against ER, MYC, and Ki-67 (Supporting Information Fig. S1), were found to stain porcine FFPE tissue in an identical fashion to that of human tissue.
Next, we focused on antigens for which no normal tissue is currently used as a control. Anaplastic lymphoma kinase (ALK) protein is aberrantly expressed in hematolymphoid and solid tumor cancers, where genetic lesions cause overexpression (Roskoski 2013). Besides this, ALK has been shown to be expressed as a transcript in the human small intestine (Morris et al. 1994; Tennstedt et al. 2014) and as a protein in the central nervous system and pons (Pulford et al. 1997). Public repositories of cDNA microarray data (BioGPS, http://biogps.org) (last accessed on October 2nd, 2014) report ALK expression in adrenal tissue (GeneAtlas U133A, gcrma; probe 208212_s_at), in astrocytes (Primary Cell Atlas; probe 208212_s_at) and heterogeneously at low levels in normal tissue, including the brain (Barcode on normal tissues).
We tested four different antibodies on three samples of human and one sample of swine small intestine; swine brain cortex, brainstem and cerebellum; and swine and bovine adrenal and failed to convincingly detect a specific signal for ALK. The reason why the ALK gene is transcribed, but not translated in normal adult tissues is unknown.
Similarly, we could not detect Her2 signal with three antibodies on pig breast and heart, which differs from that reported in the human (Fuchs et al. 2003).
Epitope Mapping across Species
For 84 antibodies, the entire epitope has been mapped, the region identified in broader terms (C- or N-term) or the epitope can be inferred from the immunizing peptide: We considered this group as informative. Forty-four antibodies in this group recognized FFPE material, significantly for targets above a 60% similarity limit (40/70 ≥60% similar vs 4/14 <60%; Chi square p= 1.66×10-9): This may represent a useful threshold to identify antibodies positive on FFPE material across species.
To gain insight into the amino acid substitutions within the epitope, we selected and examined a small group of antibodies whose epitope was relatively short (less than 24 aa; Fig. 5). All of the non-identical sequences bearing conserved amino acid substitutions allowed binding of the human-specific antibodies. The CD8 epitope, which has numerous non-conserved amino acid substitutions (Fig. 5) did not. Thus, the linear composition of an FFPE-proof epitope would allow changes in the sequence as long as these were completely or partially conserved.
Figure 5.
Epitope composition of six anti-human antibodies positive on swine formalin-fixed, paraffin-embedded (FFPE) material. Human (bold, top) and swine (light, bottom) sequence alignments for six protein epitopes are shown, with the antibody name at the top of each alignment. The position of the first amino acid in the human sequence is reported at the top left. The CD79a/JCB117 epitope has been split into two parts for graphic reasons. The only negatively staining antibody is CD8. Boxes highlight non-conserved substitutions. The legend is drawn from http://www.uniprot.org/help/sequence-alignments and http://www.ebi.ac.uk/Tools/msa/clustalo/help/faq.html#24. The color-coding sequence display was obtained by sequence alignment with Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) (last accessed on March 24, 2015).
Three antibodies positive on FFPE, for which the degree of similarity of the target was below 60% and thus expected to be negative, were further investigated, revealing possible cross-reactivity with proteins of the same biological function.
Keratin 7 has no direct homologous sequence in the pig; the nearest homology is to Keratin 75 (65% identity, 85% coverage), and the antibody labelled stratified epithelium but also mesothelial spleen-lining cells, consistent with keratin and analogous to that of human keratin 7 staining.
No porcine sequence close to the human Emerin could be found by BLAST search, despite the identical staining pattern. However, the human sequence has a low-coverage, 50% homology with a swine protein of similar nuclear membrane distribution, Inner nuclear membrane protein Man1, which itself is 93% similar to the human counterpart (Supporting Information Fig. S1); cross-reactivity with porcine Man1 may explain an identical immunostaining pattern.
The anti EMA (Muc-1) antibody we used (E29) is directed against an APDTRP epitope (Price et al. 1998), sensitive to glycosylation, and broadly distributed: It did not stain epithelial cells but positively identified sparse mononuclear cells. We classified it as positive; however, the type of antigen and the different tissue distribution in the pig suggest cross-reactivity via similar glycosylation of unrelated proteins.
No preferential representation of antibody type (polyclonal, rabbit monoclonal, mouse isotype) was noticed in the low-similarity binders in this group.
Among the antibodies negative on FFPE swine material, the CD20 L26 antibody is directed against a non-defined cytoplasmic portion of the MS4A1 protein; the three cytoplasmic portions of the protein, aa 1–56, 106–120 and 210–297 have, respectively, 75%, 60% and 81% similarity with swine MS4A1. Syndecan-1 (CD138), which shows 77% similarity in human and swine, has lower similarity (67%) in the aa 100–140 ectodomain, the region where the MI15 and B-B4 antibodies compete for binding (Dore et al. 1998).
Antibodies staining human tissue may not recognize FFPE swine tissue if they are directed against a swine-restricted conformational epitope, which does not survive fixation and embedding, or because the epitope is missing altogether. Frozen sections from unfixed material should provide epitopes in a native form and would thereby allow an investigation as to why this group of reagents did not stain the routinely treated porcine material. To this end, 19 antibodies were tested: all were negative on FFPE material, 13 are directed at proteins with ≥75% similarity, and 3 were used as positive controls. These antibodies were tested on frozen sections from porcine small intestine (Supporting Information Table 1). Only 3 antibodies of the 19 reacted positively: anti-Keratin 20, anti-cleaved PARP and anti-BCL6 clone PGB6p.
Epitope Projection Comparison between Human and Pig
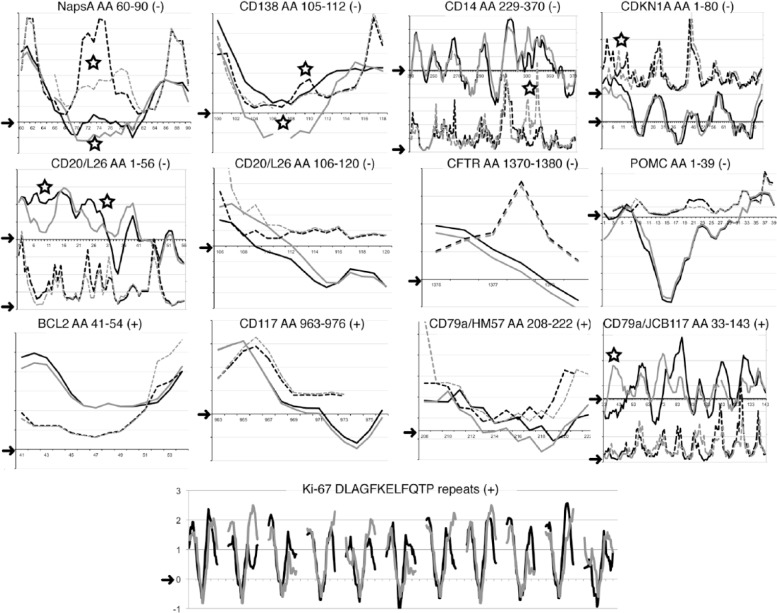
Raw similarity percentage of the linear sequence of the porcine counterpart of the human epitope may not fully explain the binding of an antibody raised against another mammal. To further elucidate the conditions for binding of anti-human antibodies in pig tissue, 13 sequences identified by seven negative and five positively staining antibodies (as controls) were plotted and compared via the linear epitope prediction algorithms. The seven human-restricted sequences were chosen among the ones with 70% similarity or greater and shorter than 24 amino acids. Five out of the seven negative antibodies showed a noticeable difference in the conformation of the linear antigenic sequence in the pig with at least one modeling method (Fig. 6). This was not observed for those antibodies that showed positive staining. Eleven known Ki-67 repeats in the human and in the pig were aligned, despite the partially conserved amino acid substitutions in the pig (Fig. 5).
Figure 6.
Epitope prediction for 13 sequences from 7 negative and 5 positively staining antibodies. The individual amino acid (aa) score obtained with two algorithms (BepiPred: continuous line; Emini: dashed line) for each antibody target sequence is plotted on the y-axis for the human (black) and the pig (grey). For graphic clarity, two different scales (left and right) are occasionally produced in the same graph for each model. Arrow on the y-axis points to the 0 value above which the algorithm predicts the epitope (see Supporting Information for individual plots). Each amino acid position number is listed on the x-axis. The antibody and clone, the amino acid target human sequence and the staining results (+ or -) is listed at the top of each graph. Eleven Ki-67 repeats BepiPred scores were plotted by aligning the human and corresponding porcine dodecapeptide sequences, flanked by 13 amino acids and an empty space for graphic rendering. The open star marks the areas of noticeable differences between human and pig with at least one prediction model. The top two rows depict antibodies negative on FFPE and frozen swine material: in all but three (CD20 AA 106-120, CFTR, POMC), noticeable conformational changes in the pig may highlight an inaccessible sequence for the swine counterpart. The bottom two rows depict positively staining antibodies. A conformational change at the N-terminus of the CDKN1A and of the CD79a-JB117 epitope may not affect the binding to the remaining sequence. Notice that the known Ki-67 target sequence FKELF, at the center of each repeat, has a negative score with the BepiPred model. Scores obtained with the Kolaskar and Tongaonkar antigenicity scale are not shown because this has been found to be informative in few cases. Sequence gaps and terminal missing sequences with the Emini algorithm are shown. The CD20 aa 210–297 cytoplasmic sequence, which is not informative, is not shown.
Because the prediction algorithms were unable to completely explain the failure of binding of some highly homologous epitopes, we re-examined the sequence details, starting with the anti-Ki-67 MIB 1 motif, recognized in humans, pig, dog, but not in rat or mouse (Endl and Gerdes 2000). The BepiPred algorithm successfully aligned the anti-Ki-67 motifs of human, pig and rat (Supporting Information Fig. S2). However, inspection of these sequences revealed a non-conserved amino acid sequence in the rat and mouse motifs that was not present in the corresponding porcine or dog motifs. This non-conserved amino acid substitution is close to the central core of the motif, and has been described as deleterious for this anti-Ki-67 antibody binding (Kubbutat et al. 1994). Therefore, amino acid changes known to alter the epitope recognition, as shown by peptide screening, may not be taken into account by the algorithms we have employed.
Re-examination of the amino acid alignments for an additional two highly homologous epitopes showed three non-conserved amino acid substitutions in the porcine Proopiomelanocortin (POMC) within the first 8 amino acids and one at aa 23. However, Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), which also did not show positive staining in the pig, had only one partially conserved substitution in the 10-amino acid epitope and an uninformative profile with the algorithms (Fig. 6 and Supporting Information).
Thus, besides non-conserved amino acid substitutions, there are other unknown factors which prevent the binding of human-specific antibodies to FFPE porcine epitopes and these cannot be entirely predicted by the available algorithms.
For 62 antibodies, no epitope specificity was available, they were directed against determinants shared by two or more proteins (e.g. acidic keratins), or they were simply polyclonal antibodies raised against pooled antigens (e.g. S-100). This group of reagents was therefore considered not informative for the epitope analysis part of the study, except for contributing to a trend for antibodies to stain preferably targets with higher similarity: only 3 out of the 23 antibodies that were positive on swine tissues were directed at targets less than 70% similar (Chi square p= 0.022).
Discussion
By using naturally occurring amino acid variations introduced during evolution in related species, we have shown that antibodies tolerate partially conserved amino acid substitutions but fail to stain near-identical sequences that harbor subtle changes in epitope accessibility or show non-conserved substitutions. This understanding was possible to discern whenever a reasonably detailed information of the putative epitope was available. These results have been previously obtained only in vitro by synthetic peptide scanning (Geysen et al. 1984; Kubbutat et al. 1994). Starting with the reactivity on human FFPE material—considered the “default” status (i.e. 100% staining, 100% specific)—we generated a catalog of information that applies to each antibody applied to FFPE swine tissues in general and to FFPE-specific antibodies as a whole.
FFPE-Proof Antibodies Are Largely Interpretable by a Linear Epitope Model and Accommodate Epitope Variations
Epitopes can be either linear or continuous. Because we applied epitope modeling, which assumes a linear composition, and we found that these models can interpret the tissue reactivity of the antibodies, our findings reinforce the assumption that these antibodies largely recognize linear epitopes in porcine and, by extension, human fixed and embedded tissue. The suggestion that FFPE antigens are linear has been made previously; they all survive FFPE processing, high heat during antigen retrieval (AR), and even 2-mercaptoethanol and SDS treatments (Gendusa et al. 2014), and are therefore denaturation-resistant. FFPE-proof antibodies are generated against synthetic peptides (Jones et al. 1993; Mason et al. 1989) or denatured antigens (Wang et al. 2005), another suggestion that they detect linear continuous epitopes, rather that conformation-dependent, discontinuous ones (Barlow et al. 1986; Fowler et al. 2011; Kringelum et al. 2013).
For some antibodies, the immunogenic sequence is known. Antibodies in this group bound targets with an overall similarity as low as 60%. This suggests that antibodies selected to react on FFPE tissue allow quite some variation in the sequence composition of the target, taking into account that the score derives from a matrix calculation for individual amino acid substitutions, not the simple identical/non-identical ratio.
Some epitopes, perfectly aligned according to the algorithms and highly similar to the human, were not detected in fixed or frozen swine material: We do not have a good explanation for this observation. Alternative splicing may be one explanation, but the pig genomic database does not have enough information to further investigate this possibility. Post-transcriptional protein modifications or complexes with other proteins may be a cause; the mosaic staining by the CD79a antibody JCB117 may be a good example.
One last additional reason for the differential reactivities of the antibodies on human and swine FFPE material may be the idiosyncrasy of the paratope for a unique binding milieu, going undetected by still imprecise modeling tools (Ponomarenko and Bourne 2007). Part of the required binding sequences may be located on adjacent protein loops, as shown for some therapeutic antibodies (de Weers et al. 2011); swine-specific changes in these sequences may prevent FFPE staining.
For these reasons, it will be impossible to predict whether an anti-human antibody will bind a related mammal, except by making an educated guess by epitope sequence similarity and staining the tissue.
We ruled out a suboptimal AR condition for the negative staining, because some of the highly similar (>80%) protein targets such as CD34, Cytokeratins, Mucin, Napsin A, Progesterone Receptor, were negative despite being abundantly expressed and detectable even without AR.
The epitope sequences did survive fixation, embedding and AR, which reverses many of the formalin-induced chemical bonds that prevent antibody access to the epitope. Not all bonds are resolved; among these may be bonds further stabilized by dehydration (Fowler et al. 2008), as shown by human FFPE-proof targets detected on frozen section only in the pig. Interestingly, only one out of three antibodies raised against the same N-terminal half of the BCL6 protein was fixation-sensitive in the pig, further suggesting that the stable, AR-insensitive bond had a local effect but did not modify the neighboring binding sites or the overall antigenic sequence conformation of porcine N-terminal BCL6, another hint at the linearity of the FFPE epitopes.
Porcine Tissue Can Be a Source of Reference FFPE Material for Human Quality Control
None of the 146 antibodies tested yielded any sort of “background” or non-specific staining; only four of the 74 that did stain resulted in a tissue or cellular staining pattern that could be classified as substantially different from that in human tissue, namely EMA, WT1, CD57 and the Ki-67 antibody SP6. The specificity of WT1 cytoplasmic staining in human endothelial cells is discussed (Carpentieri et al. 2002), and was absent in the swine tissue, suggesting that the cytoplasmic staining is spurious. Non-specific (cytoplasmic) staining of Ki-67 antibodies in non-human cells has been described previously (Falini et al. 1989).
For the vast majority of positively staining antibodies, swine tissue provided a histological and immunohistochemical staining identical to human tissue.
Swine tissue from abattoirs is thus a human-like material that can be used as an external control tissue and is nearly ubiquitous, cheap, freely exchangeable and free of the ethical constraints that limit the use of human tissue. This is most important for noble organs (brain, brainstem, heart) or small tissues (endocrine glands, ganglia) that are not commonly available in sufficient quantity as a healthy human control tissue. In addition, swine tissues may be used to standardize the detection of therapy-modifying targets (estrogen receptors (Sierralta and Thole 1996), MYC (Kluk et al. 2012), for example) in daily practice for antibodies proven to stain the pig.
Antibodies directed against mutated proteins (V600E BRAF (Capper et al. 2011), R132H IDH1 (Capper et al. 2010)), pathogens (EBV, KSHV, TB, among others) or proteins overexpressed because of a tumor-specific genetic modification (e.g., promoter swapping by chromosomal translocation for ALK or gene amplification for Her2) still need human pathological tissue or cell blocks from cultured cell lines. For some antigens that are structurally more divergent during evolution (CD30, immune receptors (Dawson 2012)), either different taxa may be investigated or human tissue still may be needed.
A minority of antibodies recognizing the FFPE porcine tissue showed a substantial differential reactivity, justifying in those few cases a concern about the monospecificity of the antibody, which is worth further investigation. To the contrary, the specificity of the vast majority of the remaining reagents currently used for diagnosis on human samples, accommodating epitope variations at least 50% of the time, is an encouraging finding; these antibodies may be used on occasionally heavily mutated targets, such as melanoma and lung cancer (Schumacher and Schreiber 2015), because of non-synonymous mutations. As we add more antibodies tested on both species, we keep finding reliable antibodies and, rarely, suspicious, unexpected staining (data not shown).
Not every manufacturer details the specificity of the epitope against which the antibody is directed, often because it is considered proprietary information, despite abundant evidence that, for a single immunogen, there are a variety of unique individual epitopes, each one recognized by a monoclonal antibody or an antiserum (Geysen et al. 1984). We have shown, however, that the availability of that information may help in choosing one reagent over another for the use across species or for validation studies (Bordeaux et al. 2010; Smith and Womack 2014). Ideally, the datasheet accompanying an antibody vial should carry the complete immunogen sequence and information on whether the antibody recognizes FFPE mammalian tissue other than human, preferably obtainable from abattoirs.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
We wish to thank Gloria Arrigoni for technical help, Dr. Franco Ferrario for continuous support, “Nonno” Dino Bellaria, Drs. Mauro Fava, Nicola Brambilla and Stefano Ibba, DVMs, for arranging the procurement of animal samples, Brendan Collins (Abcam Inc.) for providing data on antibody EPR6257 specificity, all the Laboratory Technicians for their professional contribution.
Footnotes
Author’s Contributions: GC and MMB designed the experiments. MB, AF, DDA, AGR procured and examined essential animal tissue. CRS, RG, LR, LT, AM performed immunohistochemical tests and histopathology preparations. MB, SV, CRS, RG scored the IHC preparations and annotated the results. MMB performed the genomic analysis and protein structure analysis and prediction. GC and MMB wrote the manuscript. All authors have read and approved the final manuscript.
Competing Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Carla Rossana Scalia, Rossella Gendusa and Maddalena Bolognesi are funded by a GlaxoSmithKline clinical research with the Azienda Ospedaliera San Gerardo (HGS1006-C1121) and Fondazione per la Ricerca Scientifica Termale (FoRST), IV call grants (Project ‘‘Lymphopoiesis In Secondary Lymphoid Tissue’’). The Aperio Scanscope was provided through a grant from the Regione Lombardia (Call for Independent Research, DDG 6716 del 1/7/2009). This project has been supported by Departmental Hospital funds.
References
- Barlow DJ, Edwards MS, Thornton JM. (1986). Continuous and discontinuous protein antigenic determinants. Nature 322:747-748. [DOI] [PubMed] [Google Scholar]
- Battifora H. (1986). The multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab Invest 55:244-248. [PubMed] [Google Scholar]
- Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, Anagnostou V, Rimm D. (2010). Antibody validation. Biotech 48:197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury A, Plückthun A. (2015). Reproducibility: Standardize antibodies used in research. Nature 518:27-29. [DOI] [PubMed] [Google Scholar]
- Brodersen R, Bijlsma F, Gori K, Jensen KT, Chen W, Dominguez J, Haverson K, Moore PF, Saalmüller A, Sachs D, Slierendrecht WJ, Stokes C, Vainio O, Zuckermann F, Aasted B. (1998). Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species. Vet Immunol Immunopathol 64:1-13. [DOI] [PubMed] [Google Scholar]
- Capper D, Preusser M, Habel A, Sahm F, Ackermann U, Schindler G, Pusch S, Mechtersheimer G, Zentgraf H, von Deimling A. (2011). Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol 122:11-19. [DOI] [PubMed] [Google Scholar]
- Capper D, Sahm F, Hartmann C, Meyermann R, von Deimling A, Schittenhelm J. (2010). Application of mutant IDH1 antibody to differentiate diffuse glioma from nonneoplastic central nervous system lesions and therapy-induced changes. Am J Surg Path 34:1199-1204. [DOI] [PubMed] [Google Scholar]
- Carpentieri DF, Nichols K, Chou PM, Matthews M, Pawel B, Huff D. (2002). The expression of WT1 in the differentiation of rhabdomyosarcoma from other pediatric small round blue cell tumors. Mod Pathol 15:1080-1086. [DOI] [PubMed] [Google Scholar]
- Chianini F, Majó N, Segalés J, Dominguez J, Domingo M. (2001). Immunohistological study of the immune system cells in paraffin-embedded tissues of conventional pigs. Vet Immunol Immunopathol 82:245-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson H. (2012). A Comparative Assessment of the Pig, Mouse and Human Genomes. In, CRC Press, 323-342. [Google Scholar]
- de Weers M, Tai Y-T, van der Veer MS, Bakker JM, Vink T, Jacobs DCH, Oomen LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson KC, Lokhorst HM, van de Winkel JGJ, Parren PWHI. (2011). Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840-1848. [DOI] [PubMed] [Google Scholar]
- Debeer S, Le Luduec J-B, Kaiserlian D, Laurent P, Nicolas J-F, Dubois B, Kanitakis J. (2013). Comparative histology and immunohistochemistry of porcine versus human skin. Eur J Dermatol 23:456-466. [DOI] [PubMed] [Google Scholar]
- Dore JM, Morard F, Vita N, Wijdenes J. (1998). Identification and location on syndecan-1 core protein of the epitopes of B-B2 and B-B4 monoclonal antibodies. FEBS Lett 426:67-70. [DOI] [PubMed] [Google Scholar]
- Driessen A, Van Ginneken C, Creemers J, Lambrichts I, Weyns A, Geboes K, Ectors N. (2002). Histological and immunohistochemical study of the lymphoid tissue in the normal stomach of the gnotobiotic pig. Virchows Archiv 441:589-598. [DOI] [PubMed] [Google Scholar]
- Endl E, Gerdes J. (2000). The Ki-67 protein: fascinating forms and an unknown function. Exp Cell Res 257:231-237. [DOI] [PubMed] [Google Scholar]
- Faldyna M, Samankova P, Leva L, Cerny J, Oujezdska J, Rehakova Z, Sinkora J. (2007). Cross-reactive anti-human monoclonal antibodies as a tool for B-cell identification in dogs and pigs. Vet Immunol Immunopathol 119:56-62. [DOI] [PubMed] [Google Scholar]
- Falini B, Flenghi L, Fagioli M, Stein H, Schwarting R, Riccardi C, Manocchio I, Pileri S, Pelicci PG, Lanfrancone L. (1989). Evolutionary conservation in various mammalian species of the human proliferation-associated epitope recognized by the Ki-67 monoclonal antibody. J Histochem Cytochem 37:1471-1478. [DOI] [PubMed] [Google Scholar]
- Fang X, Mou Y, Huang Z, Li Y, Han L, Zhang Y, Feng Y, Chen Y, Jiang X, Zhao W, Sun X, Xiong Z, Yang L, Liu H, Fan D, Mao L, Ren L, Liu C, Wang J, Li K, Wang G, Yang S, Lai L, Zhang G, Li Y, Wang J, Bolund L, Yang H, Wang J, Feng S, Li S, Du Y. (2012). The sequence and analysis of a Chinese pig genome. GigaScience 1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons PL, Bradley LA, Fatheree LA, Alsabeh R, Fulton RS, Goldsmith JD, Haas TS, Karabakhtsian RG, Loykasek PA, Marolt MJ, Shen SS, Smith AT, Swanson PE. (2014). Principles of Analytic Validation of Immunohistochemical Assays: Guideline From the College of American Pathologists Pathology and Laboratory Quality Center. Arch Path Lab Med 138:1432-1433. [DOI] [PubMed] [Google Scholar]
- Forsström B, Bisławska Axnäs B, Rockberg J, Danielsson H, Bohlin A, Uhlén M. (2015). Dissecting antibodies with regards to linear and conformational epitopes. PLoS ONE 10:e0121673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CB, Evers DL, O’Leary TJ, Mason JT. (2011). Antigen retrieval causes protein unfolding: evidence for a linear epitope model of recovered immunoreactivity. J Histochem Cytochem 59:366-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CB, O’Leary TJ, Mason JT. (2008). Modeling formalin fixation and histological processing with ribonuclease A: effects of ethanol dehydration on reversal of formaldehyde cross-links. Lab Invest 88:785-791. [DOI] [PubMed] [Google Scholar]
- Fuchs IB, Landt S, Bueler H, Kuehl U, Coupland S, Kleine-Tebbe A, Lichtenegger W, Schaller G. (2003). Analysis of HER2 and HER4 in human myocardium to clarify the cardiotoxicity of trastuzumab (Herceptin). Br Cancer Res Treat 82:23-28. [DOI] [PubMed] [Google Scholar]
- Gendusa R, Scalia CR, Buscone S, Cattoretti G. (2014). Elution of High Affinity (>10-9 KD) Antibodies from Tissue Sections: Clues to the Molecular Mechanism and Use in Sequential Immunostaining. J Histochem Cytochem 62:519-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen HM, Meloen RH, Barteling SJ. (1984). Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. PNAS 81:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein NS, Hewitt SM, Taylor CR, Yaziji H, Hicks DG, Standardization MoA-HCOI (2007). Recommendations for improved standardization of immunohistochemistry. In Appl Immunohistochem Mol Morphol. 124-133. [DOI] [PubMed] [Google Scholar]
- Groenen MAM, Archibald AL, Uenishi H, Tuggle CK, Takeuchi Y, Rothschild MF, Rogel-Gaillard C, Park C, Milan D, Megens H-J, Li S, Larkin DM, Kim H, Frantz LAF, Caccamo M, Ahn H, Aken BL, Anselmo A, Anthon C, Auvil L, Badaoui B, Beattie CW, Bendixen C, Berman D, Blecha F, Blomberg J, Bolund L, Bosse M, Botti S, Bujie Z, Bystrom M, Capitanu B, Carvalho-Silva D, Chardon P, Chen C, Cheng R, Choi S-H, Chow W, Clark RC, Clee C, Crooijmans RPMA, Dawson HD, Dehais P, De Sapio F, Dibbits B, Drou N, Du Z-Q, Eversole K, Fadista J, Fairley S, Faraut T, Faulkner GJ, Fowler KE, Fredholm M, Fritz E, Gilbert JGR, Giuffra E, Gorodkin J, Griffin DK, Harrow JL, Hayward A, Howe K, Hu Z-L, Humphray SJ, Hunt T, Hornshøj H, Jeon J-T, Jern P, Jones M, Jurka J, Kanamori H, Kapetanovic R, Kim J, Kim J-H, Kim K-W, Kim T-H, Larson G, Lee K, Lee K-T, Leggett R, Lewin HA, Li Y, Liu W, Loveland JE, Lu Y, Lunney JK, Ma J, Madsen O, Mann K, Matthews L, Mclaren S, Morozumi T, Murtaugh MP, Narayan J, Nguyen DT, Ni P, Oh S-J, Onteru S, Panitz F, Park E-W, Park H-S, Pascal G, Paudel Y, Perez-Enciso M, Ramirez-Gonzalez R, Reecy JM, Rodriguez-Zas S, Rohrer GA, Rund L, Sang Y, Schachtschneider K, Schraiber JG, Schwartz J, Scobie L, Scott C, Searle S, Servin B, Southey BR, Sperber G, Stadler P, Sweedler JV, Tafer H, Thomsen B, Wali R, Wang J, Wang J, White S, Xu X, Yerle M, Zhang G, Zhang J, Zhang J, Zhao S, Rogers J, Churcher C, Schook LB. (2012). Analyses of pig genomes provide insight into porcine demography and evolution. Nature 491:393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy LB, Fitzgibbons PL, Goldsmith JD, Eisen RN, Beasley MB, Souers RJ, Nakhleh RE. (2013). Immunohistochemistry Validation Procedures and Practices: A College of American Pathologists Survey of 727 Laboratories. Arch Path Lab Med 137:19-25. [DOI] [PubMed] [Google Scholar]
- Holmseth S, Zhou Y, Follin-Arbelet VV, Lehre KP, Bergles DE, Danbolt NC. (2012). Specificity controls for immunocytochemistry: the antigen preadsorption test can lead to inaccurate assessment of antibody specificity. J Histochem Cytochem 60:174-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen CN, Aasted B, Broe MK, Petersen JL. (1993). Reactivities of 20 anti-human monoclonal antibodies with leucocytes from ten different animal species. Vet Immunol Immunopathol 39:461-466. [DOI] [PubMed] [Google Scholar]
- Jones M, Cordell JL, Beyers AD, Tse AG, Mason DY. (1993). Detection of T and B cells in many animal species using cross-reactive anti-peptide antibodies. J Immunol 150:5429-5435. [PubMed] [Google Scholar]
- Kluk MJ, Chapuy B, Sinha P, Roy A, Dal Cin P, Neuberg DS, Monti S, Pinkus GS, Shipp MA, Rodig SJ. (2012). Immunohistochemical Detection of MYC-driven Diffuse Large B-Cell Lymphomas. PLoS ONE 7:e33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelum JV, Nielsen M, Padkjær SB, Lund O. (2013). Structural analysis of B-cell epitopes in antibody:protein complexes. Mol Immunol 53:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbutat MH, Key G, Duchrow M, Schlüter C, Flad HD, Gerdes J. (1994). Epitope analysis of antibodies recognising the cell proliferation associated nuclear antigen previously defined by the antibody Ki-67 (Ki-67 protein). J Clin Path 47:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauweryns JM, Van Ranst L. (1987). Leu-7 immunoreactivity in human, monkey, and pig bronchopulmonary neuroepithelial bodies and neuroendocrine cells. J Histochem Cytochem 35:687-691. [DOI] [PubMed] [Google Scholar]
- Lauweryns JM, Van Ranst L, Lloyd RV, O’Connor DT. (1987). Chromogranin in bronchopulmonary neuroendocrine cells. Immunocytochemical detection in human, monkey, and pig respiratory mucosa. J Histochem Cytochem 35:113-118. [DOI] [PubMed] [Google Scholar]
- Mason DY, Cordell J, Brown M, Pallesen G, Ralfkiaer E, Rothbard J, Crumpton M, Gatter KC. (1989). Detection of T cells in paraffin wax embedded tissue using antibodies against a peptide sequence from the CD3 antigen. J Clin Path 42:1194-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. (1994). Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 263:1281-1284. [DOI] [PubMed] [Google Scholar]
- Mount DW. (2008). Using PAM Matrices in Sequence Alignments. CSH protocols 2008:pdb top38. [DOI] [PubMed] [Google Scholar]
- Nagahama H, Hatakeyama S, Nakayama K, Nagata M, Tomita K, Nakayama K. (2001). Spatial and temporal expression patterns of the cyclin-dependent kinase (CDK) inhibitors p27Kip1 and p57Kip2 during mouse development. Anat Embryol 203:77-87. [DOI] [PubMed] [Google Scholar]
- Ponomarenko JV, Bourne PE. (2007). Antibody-protein interactions: benchmark datasets and prediction tools evaluation. BMC Struct Biol 7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MR, Rye PD, Petrakou E, Murray A, Brady K, Imai S, Haga S, Kiyozuka Y, Schol D, Meulenbroek MF, Snijdewint FG, von Mensdorff-Pouilly S, Verstraeten RA, Kenemans P, Blockzjil A, Nilsson K, Nilsson O, Reddish M, Suresh MR, Koganty RR, Fortier S, Baronic L, Berg A, Longenecker MB, Hilgers J. (1998). Summary report on the ISOBM TD-4 Workshop: analysis of 56 monoclonal antibodies against the MUC1 mucin. San Diego, Calif, November 17-23, 1996. Tumour Biol 19 Suppl 1:1-20. [DOI] [PubMed] [Google Scholar]
- Pulford K, Lamant L, Morris SW, Butler LH, Wood KM, Stroud D, Delsol G, Mason DY. (1997). Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 89:1394-1404. [PubMed] [Google Scholar]
- Riegman PHJ, van Veen E-B. (2011). Biobanking residual tissues. Hum Genetics 130:357-368. [DOI] [PubMed] [Google Scholar]
- Robb JA, Gulley ML, Fitzgibbons PL, Kennedy MF, Cosentino LM, Washington K, Dash RC, Branton PA, Jewell SD, Lapham RL. (2014). A call to standardize preanalytic data elements for biospecimens. Arch Path Lab Med 138:526-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr (2013). Anaplastic lymphoma kinase (ALK): Structure, oncogenic activation, and pharmacological inhibition. Pharmacological Research 68:68-94. [DOI] [PubMed] [Google Scholar]
- Schumacher TN, Schreiber RD. (2015). Neoantigens in cancer immunotherapy. Science 348:69-74. [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL. (1991). Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741-748. [DOI] [PubMed] [Google Scholar]
- Sierralta WD, Thole HH. (1996). Retrieval of estradiol receptor in paraffin sections of resting porcine uteri by microwave treatment. Immunostaining patterns obtained with different primary antibodies. Histochem Cell Biol 105:357-363. [DOI] [PubMed] [Google Scholar]
- Smith NR, Womack C. (2014). A matrix approach to guide IHC-based tissue biomarker development in oncology drug discovery. J Pathol 232:190-198. [DOI] [PubMed] [Google Scholar]
- Sompuram SR, Vani K, Hafer LJ, Bogen SA. (2006). Antibodies immunoreactive with formalin-fixed tissue antigens recognize linear protein epitopes. Am J Clin Pathol 125:82-90. [PubMed] [Google Scholar]
- Takahashi K, Isobe T, Ohtsuki Y, Sonobe H, Takeda I, Akagi T. (1984). Immunohistochemical localization and distribution of S-100 proteins in the human lymphoreticular system. Am J Pathol 116:497-503. [PMC free article] [PubMed] [Google Scholar]
- Tanimoto T, Ohtsuki Y. (1996). Evaluation of antibodies reactive with porcine lymphocytes and lymphoma cells in formalin-fixed, paraffin-embedded, antigen-retrieved tissue sections. Am J Vet Res 57:853-859. [PubMed] [Google Scholar]
- Taylor CR. (2014). Predictive biomarkers and companion diagnostics. The future of immunohistochemistry: “in situ proteomics” or just a “stain”? Appl Immunohistochem Mol Morphol 22:555-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennstedt P, Strobel G, Bölch C, Grob T, Minner S, Masser S, Simon R. (2014). Patterns of ALK expression in different human cancer types. J Clin Pathol 67:477-481. [DOI] [PubMed] [Google Scholar]
- van Veen EB, Riegman PHJ, Dinjens WNM, Lam KH, Oomen MHA, Spatz A, Mager R, Ratcliffe C, Knox K, Kerr D, van Damme B, Van De Vijver M, van Boven H, Morente MM, Alonso S, Kerjaschki D, Pammer J, Lopez-Guerrero JA, Llombart Bosch A, Carbone A, Gloghini A, Teodorovic I, Isabelle M, Passioukov A, Lejeune S, Therasse P, Oosterhuis JW. (2006). TuBaFrost 3: regulatory and ethical issues on the exchange of residual tissue for research across Europe. Eur J Cancer 42:2914-2923. [DOI] [PubMed] [Google Scholar]
- Wang X, Campoli M, Cho HS, Ogino T, Bandoh N, Shen J, Hur SY, Kageshita T, Ferrone S. (2005). A method to generate antigen-specific mAb capable of staining formalin-fixed, paraffin-embedded tissue sections. J Immunol Methods 299:139-151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.