Abstract
The gold standard in yeast population genomics has been the model organism Saccharomyces cerevisiae. However, the exploration of yeast species outside the Saccharomyces genus is essential to broaden the understanding of genome evolution. Here, we report the analyses of whole-genome sequences of nineisolates from the recently described yeast species Lachancea quebecensis. The genome of one isolate was assembled and annotated, and the intraspecific variability within L. quebecensis was surveyed by comparing the sequences from the eight other isolates to this reference sequence. Our study revealed that these strains harbor genomes with an average nucleotide diversity of π = 2 × 10−3 which is slightly lower, although on the same order of magnitude, as that previously determined for S. cerevisiae (π = 4 × 10−3). Our results show that even though these isolates were all obtained from a relatively isolated geographic location, the same ecological source, and represent a smaller sample size than is available for S. cerevisiae, the levels of divergence are similar to those observed in this model species. This divergence is essentially linked to the presence of two distinct clusters delineated according to geographic location. However, even with relatively similar ranges of genome divergence, L. quebecensis has an extremely low global phenotypic variance of 0.062 compared with 0.59 previously determined in S. cerevisiae.
Keywords: genome evolution, Lachancea quebecensis, phenotype, intraspecific diversity
Introduction
The recent advent of affordable genome sequencing has opened an unprecedented door to understanding the molecular mechanisms driving genome evolution among closely related organisms. Although sequence data provide a method to obtain a thorough understanding of diversification in a variety of eukaryotes, yeasts are the best models for in-depth genomic studies. Indeed, due to the small and compact nature of the genomes of the Saccharomycotina yeasts, the relative ease in obtaining high-quality sequence data, and the vast number of natural isolates available, this group of organisms is well suited for intraspecific analysis and population genomic research (Dujon 2010; Liti and Schacherer 2011).
Although the most studied groups among the Saccharomycotina are within the Saccharomyces genus (Hittinger et al. 2010; Almeida et al. 2014), with the best known being Saccharomyces cerevisiae (Liti et al. 2009; Schacherer et al. 2009; Wang et al. 2012; Cromie et al. 2013; Skelly et al. 2013; Strope et al. 2015), it is essential to broaden the view of genomic diversity among yeasts. To ensure a thorough understanding of different forces driving diversification, a range of species should be investigated. The genus Lachancea harbors multiple species, characterized by genomic histories that comprise a continuum of rearrangements, ranging from mostly collinear to significantly reshuffled genomes. Isolates are found all over the world and inhabit many niches including soil, plants, and insects as well as processed food and beverages (Naumova et al. 2007).
Recent studies have paved the way for the genus Lachancea to be a model system for the exploration of intraspecific diversity in both mitochondrial (mt) and nuclear genomes in non-Saccharomyces yeasts (Friedrich et al. 2012, 2015; Jung et al. 2012; Freel, Friedrich, et al. 2014). An analysis of the intraspecific diversity among the mt genomes of Lachancea kluyveri (Jung et al. 2012) suggested that there has been recent rapid evolution resulting in variable genomes, while the largest intraspecific survey of mt genomes in yeast to date revealed that Lachancea thermotolerans harbors highly conserved sequences (Freel, Friedrich, et al. 2014). These studies demonstrated that different levels of purifying selection could influence genomes of closely related species. Furthermore, the first interspecific study of nuclear genome evolution outside of the genus Saccharomyces, focused on L. kluyveri, was recently reported (Friedrich et al. 2015) and revealed that even within a single genome different evolutionary patterns can exist.
In the genus Lachancea, the most recently described species is Lachancea quebecensis (Freel et al. 2015). Thus far, the majority of isolates from this species have been obtained from Québec, Canada, while two additional strains have also been reported from Japan (Naumova et al. 2007). First identified as a population related to L. thermotolerans, these isolates reportedly have demonstrated an ability to grow on melibiose, distinguishing them from their sister species (Lachance and Kurtzman 2011). Although there are limited isolates available for this species, this lineage represents an opportunity to explore the genomes of a species very closely related to L. thermotolerans, ultimately enhancing the utility of the genus Lachancea as a model for genome evolution, population genomics, and mechanisms driving speciation. Additionally, the currently available L. quebecensis strains mainly originated from the same general geographic location and were all isolated from tree bark, allowing the possibility for fine-scale comparison of isolates from the same ecological niche.
Here, we analyzed the genomes of nine L. quebecensis isolates that support the recent delineation of this species and confirm that this lineage is most closely related to L. thermotolerans. The genome sequences of the nine strains provided an opportunity for the analysis of a novel species and investigation of the divergence among isolates from a novel Lachancea species. Interestingly, we found that these genomes from isolates inhabiting a single environmental niche and relatively restricted geographic location harbor levels of genome diversity equal to that found in S. cerevisiae, while the phenotypic diversity is not as variable based on the value of the global phenotypic variance across all conditions tested. Our results highlight the importance of investigating various natural populations of yeast in order to obtain a global view of genome evolution in the Saccharomycotina.
Materials and Methods
Yeast Strains, Flow Cytometry (FC), and Electrophoretic Karyotyping
All strains were generously provided by Christian Landry and were originally isolated using previously described methods (Charron et al. 2014). Samples were prepared for DNA content analysis using flow cytometry and pulse field gel electrophoresis using previously described protocols (Freel et al. 2015).
Genomic DNA Extraction and Illumina High-Throughput Sequencing
The nine L. quebecensis isolates were grown overnight at 30 °C in 20 ml of YPD (10 g yeast extract, 20 g peptone, and 20 g glucose per liter of distilled water) medium to early stationary phase before cells were harvested by centrifugation. Total genomic DNA was subsequently extracted using the MasterPure Yeast DNA purification kit (Cat No. MPY80200) according to the manufacturer’s instructions (Epicentre, Germany). For all genomes, 480-bp insert libraries were produced and sequenced on an Illumina HiSeq2000 platform. A total of 100-bp paired-end reads were obtained and trimmed according to quality criteria with Cutadapt (Martin 2011).
Reference Genome Annotation of Strain LL2013_164
Because there was no reference genome for L. quebecensis available, SOAPdenovo2, version r240 (Luo et al. 2012) was used to assemble the LL2013_164 reads, with a k-mer size of 63. The assembly was annotated with the Amadea Annotation transfer tool (Isoft, France), and considered as the reference genome for the species. Functional annotation was performed as in L. lanzarotensis (Sarilar et al. 2015) and Cyberlindnera fabianii (Freel, Sarilar, et al. 2014).
The final assembly of this reference genome consisted of 119 scaffolds larger than 1 kb, revealing a genome size of 10,535,401 bp that contains 47,783 ambiguous sites (maximum scaffold size: 1,366,161 bp, mean scaffold size: 8,750 bp, N50: 453 kb [7 scaffolds], N80: 222 kb [18 scaffolds]).
Population Genomic Analysis
The eight other sequences were compared with this reference genome to obtain insights into genomic intraspecific variability. The clean reads were mapped with Burrows-Wheeler Aligner (BWA) (version 0.7, option -I -q 25 -n 8 -o 2) (Li and Durbin 2009) and single nucleotide polymorphisms (SNPs) and short indels were identified on the basis of the mpileup files generated by SAMtools (version 0.1.18) (Li et al. 2009).
To obtain a view of the phylogenetic relationships among the different isolates, a neighbor-joining tree of the 9 strains using the 69,346 polymorphic positions detected in the whole population was constructed with the software package SplitsTree (Huson and Bryant 2006). Branch lengths are proportional to the number of segregating sites that differentiate each node. An estimation of π, the average pairwise nucleotide diversity, was calculated with Variscan (Hutter et al. 2006).
Synteny conservation analysis of L. quebecensis LL2013_164 (CBS 14088), L. thermotolerans CBS 6340T, and Lachancea waltii CBS 6430T was performed with SynChro (Drillon et al. 2014).
Phenotyping of Lachancea quebecensis
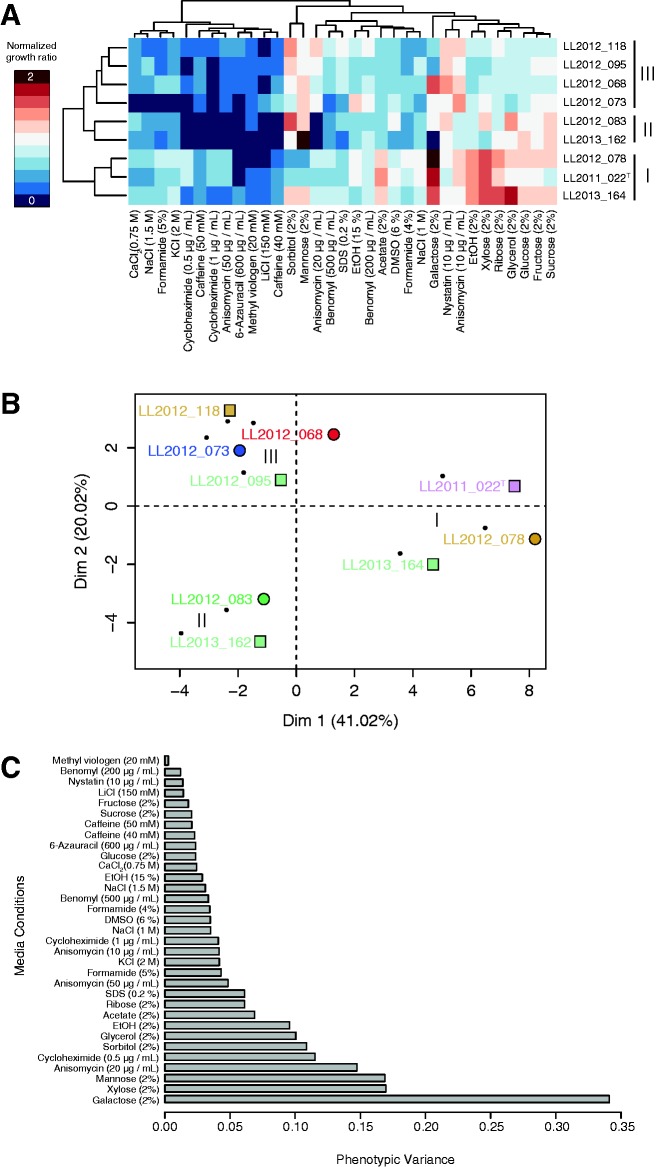
All L. quebecensis isolates in this study were subjected to growth on a total of 33 different media types, the specific composition of each medium can be found in figure 1. The conditions tested were in standard YPD with the addition of the relevant stressor. In media testing carbon utilization, 2% glucose was substituted with 2% of the following carbon sources: Sorbitol, mannose, EtOH (ethanol), acetate, galactose, xylose, ribose, glycerol, glucose, fructose, and sucrose. To prepare the master matrices, the isolates were grown in a 96-well format in liquid YPD overnight. After overnight growth, the colonies were pinned on Singer PlusPlates of YPD media using the Singer RoToR HDA Microbial Pinning Robot (Singer Instruments, Somerset, UK) and allowed to grow for approximately 30 h. The plate with 96 colonies was then used to construct 384 colony matrices on YPD, where each original colony was plated in 4 replicates. After an additional 30 h, the 384 matrices were pinned onto the various media types as well as control plates with YPD. All plates were scanned after 24, 40, and 48 h of growth at 30 °C using an 8-bit gray scale and resolution of 300 dpi. The colony size from each condition was used as a measure of growth and compared with that on the standard YPD control plates. Images were analyzed using the Gitter package in R in order to obtain growth ratios for all isolates on all media types. Data from the 40-h time point was used to construct the heat maps and calculate global phenotypic variance. Heatmaps and Principal Component Analysis (PCA) analyses were generated with R also using the growth ratio data, which was also used to calculate the global and individual condition phenotypic variance (RCoreTeam 2013) using the ggplot2 and FactorMineR packages.
Fig. 1.—
Phenotypic diversity among the 9 Lachancea quebecensis strains on 33 different media after 40 h of growth at 30 °C. The three phenotypic groups discussed are indicated by Roman numerals (I, II, and III). Growth ratios calculated from colony size on the media of interest and that on YPD media were used for all comparative analyses. (A) Heatmap of the 9 strains that were grown in 33 different media including multiple carbon sources (which replaced the 2% glucose in standard YPD media) as well as other compounds that induced stress on various cellular functions. (B) A PCA analysis of the 9 L. quebecensis isolates analyzed. (C) Phenotypic variance in the 9 L. quebecensis strains across all 33 media conditions tested.
Results and Discussion
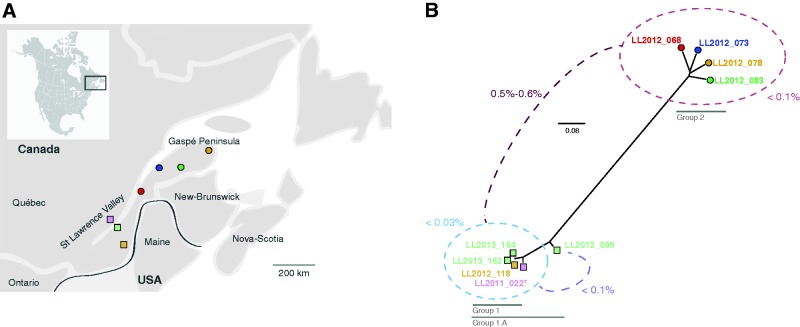
Nine L. quebecensis isolates were used in our study. These strains were determined to be haploid, based on flow cytometry analysis, and share very similar electrophoretic karyotypes which demonstrate that there is variability in the size of the largest chromosome (supplementary fig. S1, Supplementary Material online). All strains belong to the recently described species L. quebecensis and are most closely related to L. thermotolerans (Freel et al. 2015). They were all obtained from tree bark (either maple, oak, or birch) found in essentially two locations: Either the Gaspé Peninsula or Saint Lawrence Valley, in the province of Québec, Canada (table 1 and fig. 2) (Charron et al. 2014).
Table 1.
Description of the Nine Lachancea quebecensis Strains Studied
| Strain ID | Year Isolated | Geographical Origin | Ecological Origin | Phylogenetic Groupa | FC |
|---|---|---|---|---|---|
| LL2011_022T (CBS 14138) | 2011 | Saint Lawrence Valley, Québec, Canada | Maple tree bark | Group 1 (<0.005%) | Haploid |
| LL2012_068 | 2012 | Gaspé Peninsula, Québec, Canada | Oak tree bark | Group 2 (<0.1%) | Haploid |
| LL2012_073 | 2012 | Gaspé Peninsula, Québec, Canada | Maple tree bark | Group 2 (<0.1%) | Haploid |
| LL2012_078 | 2012 | Gaspé Peninsula, Québec, Canada | Maple tree bark | Group 2 (<0.1%) | Haploid |
| LL2012_083 | 2012 | Gaspé Peninsula, Québec, Canada | Maple tree bark | Group 2 (<0.1%) | Haploid |
| LL2012_095 | 2012 | Saint Lawrence Valley, Québec, Canada | Maple tree bark | Group 1A (<0.1%) | Haploid |
| LL2012_118 | 2012 | Saint Lawrence Valley, Québec, Canada | Maple tree bark | Group 1 (<0.005%) | Haploid |
| LL2013_162 | 2013 | Mont-St-Anne, Québec, Canada | Birch tree bark | Group 1 (<0.005%) | Haploid |
| LL2013_164 (CBS 14088) | 2013 | Mont-St-Anne, Québec, Canada | Maple tree bark | Group 1 (<0.005%) | Haploid |
aSee figure 2 for the delineation of the phylogenetic groups.
Fig. 2.—
(A) Map of strain origins. Five strains were obtained from sites in the Saint Lawrence Valley area (three squares), while the remaining four strains were isolated from the Gaspé Peninsula (four circles). (B) Strain relationships based on the 69,346 polymorphic positions identified in nine genomes of Lachancea quebecensis. The divergence among isolates in each group is less than 0.1%.
Genome Sequencing, Assembly, and Annotation
The nine L. quebecensis isolates were sequenced with Illumina HiSeq2000 technology, with an average mean theoretical coverage of 75X. The reads of isolate LL2013_164 (CBS 14088) were used for complete de novo assembly. We performed annotation on the 51 scaffolds larger than 5 kb, which had a cumulative size of 10,225,810 bp and a Guanine-Cytosine (GC) content of 46.6%. This GC content is comparable with what is found in L. thermotolerans (47.3%) (Souciet et al. 2009); however, it is higher than the average from other Lachancea species, including L. kluyveri (41.5%) (Payen et al. 2009; Souciet et al. 2009; Friedrich et al. 2015) and L. lanzarotensis (44.3%) (Sarilar et al. 2015).
Genome annotation was performed using the Amadea Annotation transfer tool (Isoft) with L. kluyveri (Souciet et al. 2009) as the reference genome, followed by manual curation. This strategy allowed for the identification of 5,075 putative protein-coding genes and 71 pseudogenes. Spliceosomal introns were predicted in 323 genes (6.4% of the genes), only 11 of which were interrupted by 2 introns. Whenever possible, functional annotation was transferred from S. cerevisiae, RefSeq protein sequences, or experimentally validated proteins from other Lachancea species. During this process, 3,892 proteins showed at least 50% sequence similarity with S. cerevisiae. Finally, all but 70 putative proteins have a homolog in Saccharomycotina yeasts. A single case of interkingdom horizontal transfer specific to L. quebecensis was predicted (LAQU0S32e00122g), which provides this species with a putative FMN-dependent (flavin mononucleotide-dependent) NADH-azoreductase (nicotinamide adenine dinucleotide-azoreductase).
Gene Content Variation Compared with the Closest Related Species: Lachancea thermotolerans
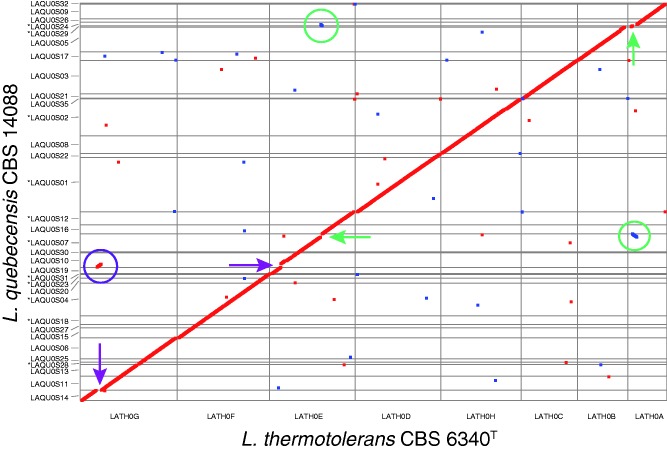
Lachancea thermotolerans is the species most closely related to L. quebecensis. The genome of L. thermotolerans CBS 6340T is slightly larger than that of CBS 14088 by approximately 165 kb, and harbors 102 additional genes. Structural comparison between the CBS 6340T and CBS 14088 genomes revealed that their chromosomes are highly collinear. Only three major synteny breakpoints were detected, two of which corresponded to a reciprocal translocation involving scaffolds LAQU0S24 and LAQU0S07 (fig. 3). We identified the genes flanking these breakpoints (fig. 3). The third synteny breakpoint involved scaffold LAQU0S10; however, it is difficult to determine if it is a true reciprocal translocation as the breakpoints in L. thermotolerans correspond to scaffold ends in L. quebecensis. Further comparison with the L. waltii strain CBS 6430T revealed that both rearrangements occurred in the L. quebecensis branch. At least 4,827 genes were found to be syntenic homologs with a mean sequence similarity of 92.8%. The majority of the remaining 248 genes have a nonsyntenic homolog in L. thermotolerans. Among the 73 exceptions, some are noteworthy: For instance, LAQU0S45e00144g, an MEL1 homolog encoding α-galactosidase which enables the fermentation of melibiose; LAQU0S10e01904g, a triphenylmethane reductase-like protein, mostly found in bacteria but also with a Kluyveromyces lactis homolog (KLLA0C19547g); and LAQU0S33e00144g, a putative gamma-glutamyltranspeptidase involved in amino acid transport.
Fig. 3.—
Whole-genome dot-plot comparison between Lachancea quebecensis CBS 14088 and Lachancea thermotolerans CBS 6340T. Only scaffolds with at least five orthologous gene pairs were considered. The six-frame translations of both genomes were compared via MUMmer 3.0. Homologous regions are plotted as squares, which are color coded according to their relative orientation: Red for parallel orientation and blue for antiparallel orientation. Synteny breakpoints and chromosomal rearrangements are highlighted with circles and arrows, respectively. The green circles indicate the reciprocal translocation that occurred in L. quebecensis between homologs of KLTH0A01804g and KLTH0A01826g, as well as KLTH0E10010g and KLTH0E10054g. The purple circle highlights the third major synteny breakpoint, which involved the homologous regions KLTH0G04730g–KLTH0G04752g and KLTH0E02200g–KLTH0E02310g. (*) indicate that the scaffolds were reverse complemented, gene order is well conserved however the strand sequence was inverted.
Intraspecific Genetic Diversity across the Lachancea quebecensis Isolates
To survey the intraspecific variability within L. quebecensis, we compared the sequence of the eight other L. quebecensis isolates to the newly assembled reference genome. A total of 243,358 SNPs distributed across 69,346 polymorphic positions were identified, with the number of SNPs ranging from 41 to 59,109 per isolate (table 2). There were only 41 and 127 SNPs identified between the reference genome (LL2013_164) and two other isolates, LL2013_162 and LL2012_118, respectively. This demonstrates that these strains are highly similar. However, it is interesting to note that while LL2013_164 and LL2012_118 were both isolated from maple tree bark, they were isolated from different samples collected in different years from two distinct collection sites (table 1). Additionally, LL2013_164 and LL2013_162, which are incredibly similar genetically, were collected from distinct sites and isolated from maple and birch tree bark, respectively (table 1). This demonstrates that nearly clonal isolates of L. quebecensis are present in the population sampled in Québec. Among the SNPs identified, 9,407 correspond to singletons, that is, are found only in one genome. The phylogenetic relationship was constructed based on these polymorphic positions (fig. 2) and revealed the presence of two main clusters that are in accordance with the geographical origins of the strains. Indeed, one cluster is composed of isolates originating from the Gaspé Peninsula, while the other harbors strains from the Saint Lawrence Valley (fig. 2). Links between phylogenetic cluster and geographic origin have been observed in the closely related L. kluyveri (Friedrich et al. 2015), while the ecological origin was linked to some clusters in S. cerevisiae (Liti et al. 2009; Schacherer et al. 2009). Between these two main clusters, the genetic divergence ranges from 0.5% to 0.6% (fig. 2), which is approximately what was reported in S. cerevisiae (Schacherer et al. 2009). Within each of the two subclusters, the divergence is less than 0.1%. However, among the five isolates from the Saint Lawrence Valley, varying levels of divergence are observed among three that share less than 0.005% (LL2013_164, LL2013_162, and LL2012_118), while further including LL2011_022T leads to a level of 0.03%, and among all five isolates there is a maximum of 0.1% divergence observed (when LL2012_095 is also considered). Although this phylogeny was constructed with the genomes from only nine isolates, which were all obtained from the same ecological source and general geographic location, we observed the same level of diversity seen in S. cerevisiae (Liti et al. 2009; Schacherer et al. 2009; Wang et al. 2012; Cromie et al. 2013), even though the samples included form two clear clusters in contrast to the continuum revealed by the S. cerevisiae phylogeny.
Table 2.
SNPs and Indels Per Strain in Comparison with the Reference (LL2013_164) Genome
| Strain ID | No. of SNPs | SNPs/kb in Genes | SNPs/kb in Intergenic Regions | No. of Small Indels |
|---|---|---|---|---|
| LL12011_022T (CBS 14138) | 2,335 | 0.2 | 0.6 | 32 |
| LL2012_068 | 59,109 | 4.4 | 10.1 | 360 |
| LL2012_073 | 58,528 | 4.4 | 10.0 | 447 |
| LL2012_078 | 57,726 | 4.3 | 9.9 | 624 |
| LL2012_083 | 57,069 | 4.3 | 9.7 | 3,726 |
| LL2012_095 | 8,423 | 0.7 | 1.6 | 601 |
| LL2012_118 | 127 | 0.03 | 0.2 | 61 |
| LL2013_162 | 41 | 0.02 | 0.1 | 83 |
Global L. quebecensis genetic diversity estimated by the average pairwise divergence (π = 2 × 10−3) demonstrated that this species harbors similar values to those estimated for S. cerevisiae (π = 4 × 10−3) (Schacherer et al. 2009) and S. pombe (π = 3 × 10−3) (Jeffares et al. 2015). However, it must be noted that these strains represent a very limited data set composed of only nine genome sequences from isolates obtained from a single geographic region (Québec, Canada) and ecological niche (tree bark). However, it is an order of magnitude lower than both the most closely related species, L. kluyveri (π = 17 × 10−3) (Friedrich et al. 2015) as well as the more distantly related Saccharomyces uvarum (π = 12 × 10−3) (Almeida et al. 2014). These differences could be attributed in part to the fact that L. quebecensis is represented currently by a dramatically smaller set of isolates sequenced (9) in comparison with 28 and 54 in the L. kluyveri and S. uvarum studies, respectively. The dichotomy observed between the two Lachancea species is probably due to the fact that all L. quebecensis isolates are from the same geographical region and ecological niche, essentially representing a subpopulation-level sampling of this species, while the L. kluyveri isolates were derived from diverse global geographical locations and were obtained from various environmental sources including tree exudate, soil, and Drosophila. Indeed, analysis of individual subpopulations from Europe and Asia were characterized by dramatically lower values of π equal to 0.6 × 10−3 and 7.5 × 10−3, respectively (Friedrich et al. 2015). The following four L. quebecensis strains were previously identified: Two originated from Japan and were isolated from leaves (NBRC 10066) and flowers (NBRC 10067) (Naumova et al. 2007), one was obtained from black knot on a cherry tree in Québec (UWOPS 79-139), and one from Drosophila sp. in Ontario (UWOPS 82-231). It would be interesting to obtain additional whole-genome sequences to determine the full extent of the diversity within the currently known isolates of this species.
Phenotypic Diversity Observed among Lachancea quebecensis Isolates
In order to survey the overall phenotypic diversity of these 9 isolates, they were grown in a total of 33 conditions (fig. 1), which tested the ability of the strains to survive in a range of conditions. The media assessed growth on a variety of carbon and nitrogen sources as well as compounds that affect different cellular functions including protein stability (formamide, KCl, and sodium dodecyl sulfate (SDS)), osmotic stress (NaCl), sterol biosynthesis (nystatin), transcription (6-azauracil), and translation (anisomycin and cycloheximide). When all conditions were considered, the isolates formed three phenotypic clusters (fig. 1). Each cluster was a mix of representatives from both phylogenetic subgroups shown in figure 3 and strains among each group share levels of genome divergence of approximately 0.6%. The first group (I) is composed of three isolates, and demonstrated an enhanced growth rate on galactose (2%), ethanol (2%), xylose (2%), and also tolerated a higher level of osmotic stress (NaCl 1.5 M) in comparison with the other groups. The second (II) and third groups (III) share more similar profiles, with group II distinguished in particular by poor growth on anisomycin at 20 µg/ml. The global phenotypic variance among all the 33 conditions tested was 0.067 (fig. 1), which is an order of magnitude lower than what was observed for S. cerevisiae (0.59) as well as Saccharomyces paradoxus (0.35) (Warringer et al. 2011). Although these global values for S. cerevisiae and S. paradoxus were determined across larger sample sizes of 38 and 35 isolates, respectively, even in small subpopulations phenotypic variance is high. For example, analysis of three S. cerevisiae isolates from Malaysia yielded a value of approximately 0.5, while four strains of S. paradoxus from the Far East harbor a value of 0.35 (Warringer et al. 2011). It is difficult to compare phenotypic variation among different laboratories and to previous studies due to changes in methodology and available tools as well as media conditions tested. Furthermore, it might be expected that divergent species differentially utilize various media types as in nature they likely inhabit various ecological niches. Assessing the phenotypic variance for species across multiple conditions is an attempt to broadly summarize the phenotypic diversity in a population. The values obtained across 33 media conditions for the 9 L. quebecensis isolates studied here are consistently low with the highest levels in variation observed on galactose (fig. 1). All strains exhibited reduced growth in the presence of a suite of compounds associated with various cellular stresses. However, an additional analysis with these 12 conditions removed resulted in the same clustering pattern (supplementary fig. S2A and B, Supplementary Material online). The lack of correlation between genotype and phenotype was surprising considering the extremely low level of nucleotide divergence (<0.005%) observed between some of the isolates in the Saint Lawrence Valley clade (LL2012_162 and LL2012_164). However, it must be noted that although three groups can be distinguished, overall the nine strains share relatively similar phenotypic profiles. Using the global phenotypic variance to compare species as was previously done by Warringer et al. is not a definitive method to define phenotypic variation; however, it is an initial attempt to broadly summarize trait variation in different populations (Warringer et al. 2011). As divergent populations and phenotypes of yeasts are explored, better tools to assess and compare multiple groups of isolates and data sets will be developed, continually elucidating a better understanding of yeast diversity and genome evolution.
Conclusion
The genomes of the nine L. quebecensis isolates obtained in this study confirm the relationship of this species to the closely related sister species L. thermotolerans. These strains were obtained from approximately the same geographic location and environmental niche and while they were isolated from different samples, the close proximity of their origins likely biases the view of genome diversity in L. quebecensis. It could prove interesting to continue the exploration of this species by sequencing the other few isolates currently known. A sample size of nine genomes from strains isolated from tree bark all generally located in the same geographical area harbor the same level of genetic diversity as has been observed in S. cerevisiae isolated from global locations and a wide range of ecological niches. The L. quebecensis diversity is essentially explained by the presence of two phylogenetic groups that reflect a slight difference in geographic distribution. Although there is a relatively similar range of genome divergence within the L. quebecensis and S. cerevisiae populations, the global phenotypic variance is dramatically lower in L. quebecensis.
Supplementary Material
Supplementary figures S1 and S2 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
We thank Christian Landry for generously providing the Lachancea quebecensis isolates. This work was supported by an ANR grant (2010-BLAN-1606 to J.S. and C.N.) and ANR Young Investigator grant (2011-JSV6-004-01 to J.S.). We also thank the Université de Strasbourg (IdEx 2012 Attractivité) for their financial support and the BioImage platform (IBMP-CNRS, Strasbourg) for their support.
Literature Cited
- Almeida P, et al. 2014. A Gondwanan imprint on global diversity and domestication of wine and cider yeast Saccharomyces uvarum. Nat Commun. 5:4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron G, Leducq JB, Bertin C, Dubé AK, Landry CR. 2014. Exploring the northern limit of the distribution of Saccharomyces cerevisiae and Saccharomyces paradoxus in North America. FEMS Yeast Res. 14(2):281–288. [DOI] [PubMed] [Google Scholar]
- Cromie GA, et al. 2013. Genomic sequence diversity and population structure of Saccharomyces cerevisiae assessed by RAD-seq. G3 (Bethesda) 3(12):2163–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drillon G, Carbone A, Fischer G. 2014. SynChro: a fast and easy tool to reconstruct and visualize synteny blocks along eukaryotic chromosomes. PLoS One 9(3):e92621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B. 2010. Yeast evolutionary genomics. Nat Rev Genet. 11(7):512–524. [DOI] [PubMed] [Google Scholar]
- Freel KC, Charron G, Leduc LG, Landry CR, Schacherer J. 2015. Lachancea quebecensis sp. nov., a yeast species consistently isolated from tree bark in the Canadian province of Québec. Int J Syst Evol Microbiol. 65(10):3392–3399. [DOI] [PubMed] [Google Scholar]
- Freel KC, Friedrich A, Hou J, Schacherer J. 2014. Population genomic analysis reveals highly conserved mitochondrial genomes in the yeast species Lachancea thermotolerans. Genome Biol Evol. 6(10):2586–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freel KC, Sarilar V, et al. 2014. Genome sequence of the yeast Cyberlindnera fabianii (Hansenula fabianii). Genome Announc. 2(4):e0063800614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich A, Jung P, Reisser C, Fischer G, Schacherer J. 2015. Population genomics reveals chromosome-scale heterogeneous evolution in a protoploid yeast. Mol Biol Evol. 32(1):184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich A, Jung PP, Hou J, Neuvéglise C, Schacherer J. 2012. Comparative mitochondrial genomics within and among yeast species of the Lachancea genus. PLoS One 7(10):e47834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittinger CT, et al. 2010. Remarkably ancient balanced polymorphisms in a multi-locus gene network. Nature 464(7285):54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 23(2):254–267. [DOI] [PubMed] [Google Scholar]
- Hutter S, Vilella AJ, Rozas J. 2006. Genome-wide DNA polymorphism analyses using VariScan. BMC Bioinformatics 7:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffares DC, et al. 2015. The genomic and phenotypic diversity of Schizosaccharomyces pombe. Nat Genet.. 47:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung PP, Friedrich A, Reisser C, Hou J, Schacherer J. 2012. Mitochondrial genome evolution in a single protoploid yeast species. G3 (Bethesda) 2(9):1103–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachance MA, Kurtzman CP. 2011. Lachancea Kurtzman (2003) In: The yeasts, a taxonomic study. 5th ed. Kurtzman CP, Fell JW, Boekhout T, editors. Elsevier, Amsterdam (The Netherlands) pp. 511–519. [Google Scholar]
- Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25(14):1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25(16):2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G, Schacherer J. 2011. The rise of yeast population genomics. C R Biol. 334(8–9):612–619. [DOI] [PubMed] [Google Scholar]
- Liti G, et al. 2009. Population genomics of domestic and wild yeasts. Nature 458(7236):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, et al. 2012. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17(1):10–12. [Google Scholar]
- Naumova ES, Serpova EV, Naumov GI. 2007. Molecular systematics of Lachancea yeasts. Biochemistry (Mosc) 72(12):1356–1362. [DOI] [PubMed] [Google Scholar]
- Payen C, et al. 2009. Unusual composition of a yeast chromosome arm is associated with its delayed replication. Genome Res. 19(10):1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RCoreTeam. 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing: Vienna (Austria). [Google Scholar]
- Sarilar V, Devillers H, Freel KC, Schacherer J, Neuvéglise C. 2015. Draft genome sequence of Lachancea lanzarotensis CBS 12615(T), an ascomycetous yeast isolated from grapes. Genome Announc. 3(2):e00292–e00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacherer J, Shapiro JA, Ruderfer DM, Kruglyak L. 2009. Comprehensive polymorphism survey elucidates population structure of Saccharomyces cerevisiae. Nature 458(7236):342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly DA, et al. 2013. Integrative phenomics reveals insight into the structure of phenotypic diversity in budding yeast. Genome Res. 23(9):1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souciet JL, et al. 2009. Comparative genomics of protoploid Saccharomycetaceae. Genome Res. 19(10):1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strope PK, et al. 2015. The 100-genomes strains, an S. cerevisiae resource that illuminates its natural phenotypic and genotypic variation and emergence as an opportunistic pathogen. Genome Res. 25:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Liu WQ, Liti G, Wang SA, Bai FY. 2012. Surprisingly diverged populations of Saccharomyces cerevisiae in natural environments remote from human activity. Mol Ecol. 21(22):5404–5417. [DOI] [PubMed] [Google Scholar]
- Warringer J, et al. 2011. Trait variation in yeast is defined by population history. PLoS Genet. 7(6):e1002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.