Abstract
Women are more vulnerable to stress- and fear-based disorders, such as anxiety and post-traumatic stress disorder. Despite the growing literature on this topic, the neural basis of these sex differences remains unclear, and the findings appear inconsistent. The neurobiological mechanisms of fear and stress in learning and memory processes have been extensively studied, and the crosstalk between these systems is beginning to explain the disproportionate incidence and differences in symptomatology and remission within these psychopathologies. In this review, we discuss the intersect between stress and fear mechanisms and their modulation by gonadal hormones and discuss the relevance of this information to sex differences in anxiety and fear-based disorders. Understanding these converging influences is imperative to the development of more effective, individualized treatments that take sex and hormones into account.
Keywords: sex difference, anxiety, fear extinction, fear conditioning, PTSD, estrogen, testosterone, progesterone, stress, estrogen, hormone, psychiatric disorders
Introduction
Women are twice as likely as men to develop stress- and anxiety-related psychiatric disorders (Kessler et al., 1995, 2006, 2009; Tolin & Foa, 2006). This sex bias may be attributed in part to a greater sensitivity to stressful and traumatic life experiences in women. Indeed, numerous studies have examined sex differences in the response to stress and have identified differences in the neural circuits that impact emotional reactivity (Goldstein et al., 2010; Kogler et al., 2014). However, how these mechanisms may be mediating sex differences in anxiety disorders remains unclear. It is often observed that individuals who suffer from anxiety have great difficulty forming memories and learning new or challenging tasks. Profound sex differences have been documented in laboratory experiments, where inducing stress or fear has also led to impaired learning and memory consolidation. Therefore, investigating the mechanisms of learning and memory that are differentially affected by stress, fear, or the combination of both may provide insight into the systems that mediate these sex differences.
In this review, we focus on processes of fear regulation (acquisition and extinction) to explore these sex differences and compare the findings to what has been reported in the stress literature. As gonadal hormones, such as estrogen and testosterone, are known to influence learning and memory processes as well, these factors will be included in our discussion. By describing the intersection of stress, fear, and gonadal hormones in learning and memory modulation, we aim to promote a better understanding of the factors that increase vulnerability to anxiety disorders to potentially improve the efficacy and efficiency of treatment.
Sex differences in anxiety disorders
Anxiety disorders are the most prevalent of mental disorders, with an estimated lifetime prevalence rate of about 16% world-wide and 20% in the U.S. alone (Kessler et al., 2005, 2009). Epidemiological reports consistently indicate that women are at about a two-fold higher risk for any anxiety-related disorder compared to men (Breslau et al., 1997; Foa & Street, 2001; Kessler et al., 1994, 1995, 2005; McLean et al., 2011; Nolen-Hoeksema & Girgus, 1994; Tolin & Foa, 2006). The higher incidence rate in women is maintained across all anxiety- and fear-based disorders, including social anxiety disorder, generalized anxiety disorder, panic disorder, specific phobia, and post-traumatic stress disorder (PTSD; Breslau, 2009; Breslau et al., 1997; Kessler et al., 1994, 1995). Although PTSD and obsessive compulsive disorder (OCD) are no longer classified as anxiety disorders in the DSM-V, we will include them in our discussion as they share important features with anxiety disorders and were defined as such in previous DSMs.
Women seem to be more negatively affected by symptoms of anxiety disorders, often experiencing symptoms to a greater degree (Altemus et al., 2014). Women comprise more than half of the population with generalized anxiety disorder and have a greater vulnerability to comorbid mental disorders that persists later in life (60 years of age and older; American Psychiatric Association, 2013; Bakish, 1999; van der Veen et al., 2014). Women are not only twice as likely to develop PTSD following a traumatic event, but they also experience more severe, debilitating, and persistent symptoms (Breslau et al., 1998; Holbrook, Hoyt, Stein, & Sieber, 2002; Seedat, Stein, & Carey, 2005). In individuals with panic disorder, this increased severity of symptoms is demonstrated by women, who experience a higher frequency of panic attacks than men (Kessler et al., 2006; Reed & Wittchen, 1998). These differences contribute to an overall worse quality of life for women suffering from anxiety disorders compared to men with these disorders (Breslau et al., 1998; Breslau, 2002; Frans et al., 2005; Holbrook et al., 2002; Kilpatrick et al., 2013; Perrin et al., 2014; Seedat et al., 2005). In addition to these pronounced differences in symptom severity, men and women also differentially express the characteristics and symptoms of anxiety disorders. For instance, women are more likely to show obsessive-compulsive disorder symptoms in the contamination/cleaning domain, whereas men exhibit more obsessive behaviors related to the sexual/religious dimension of OCD (Labad et al., 2008).
Epidemiological studies suggest that women may have a higher risk for developing anxiety disorders, or exacerbation of their present symptoms, during different phases of their reproductive lives, such as puberty, menses, pregnancy, postpartum, and menopause (Hickey, Bryant, & Judd, 2012; Pigott, 2003; Ross & McLean, 2006; van Veen, Jonker, van Vliet, & Zitman, 2009; Vesga-López et al., 2008). These periods of elevated risk coincide with times of drastic hormonal fluctuations, implicating a role for gonadal hormones in the onset, maintenance, and persistence of anxiety disorders in women. This sex-specific elevated risk for developing fear and anxiety disorders may be due to an inability to down regulate negative emotional responses to stress and fear (Campbell-Sills, Barlow, Brown, & Hofmann, 2006; Cover, Maeng, Lebrón-Milad, & Milad, 2014; Lebron-Milad & Milad, 2012; Mennin, Heimberg, Turk, & Fresco, 2005; Nolen-Hoeksema, 1991).
Neurobiology of fear extinction
Fear is a necessary and adaptive response that is critical for survival, but it can develop into debilitating psychopathology if it does not subside in the absence of threat. Pavlovian fear conditioning is a learning paradigm that is commonly used to investigate fear learning and memory processes. Fear learning occurs after several presentations of a neutral conditioned stimulus (CS), such as a light or tone, which is paired with an aversive unconditioned stimulus (US), such as a mild shock. The subject learns that the CS predicts the US and expresses conditioned responses (CRs) to subsequent CS presentations. The CR that is usually measured to assess fear is freezing behavior in rodents and skin conductance response (SCR) or fear-potentiated startle in humans. During fear extinction, the CS is repeatedly presented without the expected negative consequence; the subject learns that the CS no longer predicts the aversive US and exhibits a reduction in either freezing or SCR. As the neurobiology of fear extinction has been studied extensively and implicated in the etiology of anxiety and stress-related disorders (Bishop, 2007; Dias, Banerjee, Goodman, & Ressler, 2013; Hofmann, 2008), it is not surprising that stress and fear responses are mediated by overlapping neural circuits. Here, we focus on the fear extinction network, including the ventromedial prefrontal cortex (vmPFC), dorsal anterior cingulate cortex (dACC), amygdala, and hippocampus (Linnman et al., 2012; Linnman, Rougemont-Bücking, Beucke, Zeffiro, & Milad, 2011; Milad et al., 2007).
Relevance to anxiety disorders
The exaggerated fear response is a signature characteristic of anxiety- and stress-related disorders. This is especially true for individuals suffering from PTSD, who struggle to control fear elicited by stimuli associated with past traumatic events. Understanding the fear extinction network can inform research on anxiety disorders not only because of their shared neurobiology, but also because fear extinction processes model some of the core behavioral features of anxiety disorders (for review, see Graham & Milad, 2011; Maren, Phan, & Liberzon, 2013; Pitman et al., 2012). These shared characteristics allow findings from the rodent fear extinction model to be easily translated to clinical applications (Briscione, Jovanovic, & Norrholm, 2014; Milad & Quirk, 2012; Norrholm et al., 2011). For instance, PTSD patients exhibit poor extinction recall, which appears to be associated with disruptions in the fear extinction network, e.g. hyperactivity within the dACC and hypoactivity within the vmPFC (Etkin & Wager, 2007; Liberzon & Sripada, 2008; Milad et al., 2009; Pitman et al., 2012). Similarly, extinction recall and its neural correlates are disrupted in OCD patients (Milad et al., 2013). Although more studies are necessary to evaluate the implications of its use, the fear extinction model may be an effective transdiagnostic tool to detect susceptibility to anxiety disorders and predict recovery after a stressful life event in humans and rodents (Marin, Camprodon, Dougherty, & Milad, 2014). In addition to identifying biomarkers of vulnerability to anxiety disorders, another potential application of this model may also be to help assess the efficacy of treatment. Prolonged exposure therapy is one of the most effective forms of cognitive behavioral therapy for the treatment of anxiety disorders (Foa, 2000; Foa, 2011; McLean & Foa, 2013). This treatment induces extinction learning by exposing the individual to the stimulus that provokes their uncontrollable fear in the absence of any negative outcomes or danger. Fear extinction may be a good experimental model for investigating the neural mechanisms that underlie these treatments and identifying the dysfunctional target areas that make some individuals less responsive to therapy.
Rodent fear circuitry
The above described fear extinction network in humans was based and driven by numerous studies conducted on rodents. In rodents, the circuit modulating fear expression involves excitatory input from the prelimbic (PL) medial prefrontal cortex (mPFC) to the basolateral amygdala (BLA), which activates the central amygdala (CeA) for enhanced fear expression (Likhtik, Pelletier, Paz, & Paré, 2005; Sierra-Mercado, Padilla-Coreano, & Quirk, 2011; Sotres-Bayon, Bush, & LeDoux, 2004). The infralimbic (IL) area of the mPFC projects to, and activates inhibitory intercalated cells in, the amygdala. These cells connect the BLA to the CeA and inhibit fear output (Likhtik et al., 2005; Quirk, Likhtik, Pelletier, & Paré, 2003; Quirk & Mueller, 2008; Sierra-Mercado et al., 2011; Sotres-Bayon et al., 2004). The hippocampus interacts with this network in response to contextual cues and can induce or suppress fear memory expression depending on the context (Sotres-Bayon, Sierra-Mercado, Pardilla-Delgado, & Quirk, 2012). For instance, the hippocampus will activate IL to suppress fear when the CS is presented in the context in which it was extinguished (Corcoran & Maren, 2001).
Human fear circuitry
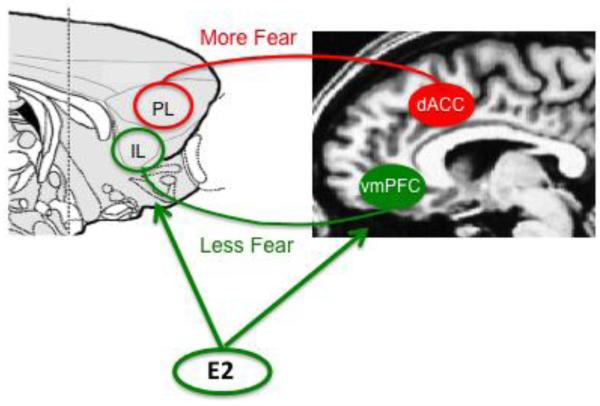
The rodent circuitry for fear conditioning and extinction appears to have functional homologies with that of humans (Milad & Quirk, 2012). Neuroimaging studies illustrating brain activations during fear conditioning and extinction suggest that the human dACC and vmPFC are homologous with the rodent PL and IL, respectively (Fig.1; Linnman et al., 2011, 2012). Extinction memory recall (as indicated by low SCR to the CS) was positively associated with increased activation of the vmPFC during presentation of the extinguished CS (Kalisch et al., 2006; Milad et al., 2007; Phelps, Delgado, Nearing, & LeDoux, 2004). In addition, the amygdala and hippocampus were also activated during these tasks, demonstrating similar functional roles as in rodents (Kalisch et al., 2006).
Figure 1.
Functional homologies within the fear extinction circuitry. The rodent prelimbic (PL) and infralimbic (IL) areas of the medial prefrontal cortex (mPFC) appear to be homologous with the human dorsal anterior cingulate cortex (dACC) and ventromedial prefrontal cortex (vmPFC), respectively. Based on rodent and neuroimaging studies, the PL and dACC are associated with high fear expression, whereas the IL and vmPFC are associated with fear suppression (Modified from Milad & Quirk, 2012). Estrogen appears to modulate activity within the vmPFC and IL to enhance fear extinction memory.
Effects of stress on fear circuitry
The neural nodes of the fear extinction network are sensitive to stress. Acute and chronic stress exposure can alter the structure and function of both the hippocampus and medial prefrontal cortex, with these changes often accompanied by behavioral consequences/impairment (Arnsten, 2009; Cook & Wellman, 2004; de Quervain, Roozendaal, & McGaugh, 1998; Luine, Villegas, Martinez, & McEwen, 1994; McEwen, 2005; Watanabe, Gould, & McEwen, 1992). For instance, acute inescapable forced swim stress induces dendritic retraction in IL neurons and impairs fear extinction, but not fear conditioning, in mice (Izquierdo, Wellman, & Holmes, 2006). Amygdala structure and function are also altered by stress (LeDoux, 2000; McGaugh, 2002). For instance, acute immobilization stress, as well as a single administration of glucocorticoids, induces neuronal hypertrophy within the basolateral amygdala and heightened anxiety (Kim et al., 2014; Mitra & Sapolsky, 2008; Mitra, Jadhav, McEwen, Vyas, & Chattarji, 2005).
This brain circuitry is also clinically relevant as patients with anxiety-related disorders and patients with PTSD, exhibit alterations in hippocampal and prefrontal volume as well as amygdala hyperactivity (Bremner et al., 1997; Coffey et al., 1993; Fani et al., 2015; Lebron-Milad et al., 2012; Machado-de-Sousa et al., 2014; Shin, Rauch, & Pitman, 2006). Interestingly, in both human and nonhuman animals, these brain regions not only subserve cognition and emotional processing, but they are also differentially activated between the sexes during stress and fear learning (Bangasser & Shors, 2010; Baran, Armstrong, Niren, & Conrad, 2010; Goldstein et al., 2010; Lebron-Milad et al., 2012).
Sex differences in fear extinction
Rodents
Given the marked sex differences observed in psychopathology, it is not surprising that the regions comprising the fear extinction network also exhibit sexual dimorphisms. Although sex differences in fear extinction have not been examined as thoroughly as sex differences in stress, converging findings in these two areas may still contribute to our understanding of the sex bias in anxiety disorders. The sexually dimorphic nature of the brain regions that respond to stress and fear learning (e.g. mPFC, amygdala, and hippocampus) may underlie the sex differences observed in fear conditioning and extinction, although the direction of these behavioral sex differences are not consistent. Some studies report that females do not perform as well as males in fear conditioning and extinction learning (Baker-Andresen, Flavell, Li, & Bredy, 2013; Baran et al., 2009, 2010; Fenton et al., 2014). Male rats exhibit more freezing to the conditioned stimulus in fewer trials during fear conditioning compared to females (Aguilar et al., 2003; Baran et al., 2009, 2010; Daviu, Andero, Armario, & Nadal, 2014; Maren, De Oca, & Fanselow, 1994; Pryce, Lehmann, & Feldon, 1999; Ribeiro et al., 2010). Moreover, male rats trained to avoid an aversive arm (bright light and loud noise) in a plus maze, made more entries into the arm when the aversive stimuli were no longer present during extinction, while female rats decreased aversive arm exploration, indicating failure to extinguish the behavior (Ribeiro et al., 2010). In a separate study, males performed better in a contextual fear conditioning task, while females expressed less fear and a higher extinction rate than males (Daviu et al., 2014). Baran and colleagues (2009), however, reported that although males and females acquired fear similarly, the female rats did not extinguish as well as males.
Humans
The mixed findings in rodents are also observed in human studies. Milad et al. (2006) demonstrated in healthy humans that although men exhibited a greater conditioned fear response compared to women during fear acquisition, they did not differ during extinction learning. In contrast, healthy women exhibited greater fear conditioning to a CS paired with viewing mock panic attacks, with increased electrodermal responding and distress ratings compared to men in an observational fear conditioning procedure (Kelly & Forsyth, 2007). Although the literature appears inconsistent in these findings, this may be due to differences in fear paradigm, strain, or species studied as well as not controlling for menstrual cycle phase or oral contraceptive use.
Sexually dimorphic circuitry
Differences in the neural mechanisms modulating fear may underlie these sex differences in fear learning and extinction (Baran et al., 2010; Ter Horst, Carobrez, van der Mark, de Kloet, & Oitzl, 2012). In a study examining the role of the medial prefrontal cortex (mPFC), rats were fear conditioned to a paired tone-footshock. During extinction training, males with and without mPFC lesions and sham-lesioned females successfully reduced freezing, whereas mPFC-lesioned female rats continued to freeze to the tone and failed to extinguish, an effect that persisted 24 hours later (Baran et al., 2010). Male rats with mPFC lesions showed impaired extinction recall, but like sham females, were able to reacquire extinction. Thus, the mPFC was necessary for extinction recall in males but for extinction acquisition in females (Baran et al., 2010). Others have reported no behavioral differences between males and females during fear conditioning and extinction (Milad et al., 2009). Despite a lack of perceived sex differences in performance, males and females may engage different brain regions to execute the same behavior. In a functional magnetic resonance imaging (fMRI) study, men and women exhibited equivalent SCR during fear conditioning and extinction, but showed differential activations within fear circuitry (Lebron-Milad et al., 2012). Women showed greater changes in activation than men within the amygdala and the dorsal and rostral anterior cingulate cortices during fear conditioning. During extinction recall, men exhibited greater rostral anterior cingulate cortex activations (Lebron-Milad et al., 2012). Interestingly, the brain regions in which sex differences were observed during fear conditioning and extinction overlap with those that are differentially activated by men and women during stress.
The sex differences observed across the various fear paradigms (in the absence or presence of stress) have important clinical implications, as reports of sex differences in fear conditioning and extinction in individuals with PTSD are beginning to emerge (Felmingham et al., 2010; Glover et al., 2012; Inslicht et al., 2013; Shvil et al., 2014). In one such study, enhanced fear conditioning was observed in women compared to men with PTSD (Inslicht et al., 2013). In contrast, neural activations and psychophysiological responses that were associated with poor extinction memory recall were exhibited in men but not in women with PTSD (Shvil et al., 2014). Because the clinical relevance of these sex differences in fear behaviors and circuitry, it is imperative that studies take sex into account when considering the neurobiological mechanisms underlying the pathophysiology of anxiety- and stress-related disorders.
Sex differences in stress
Rodents
Stress can differentially disrupt cognitive processes such as learning and memory as a function of sex. In a Pavlovian eyeblink conditioning paradigm, rats are trained to emit a well-timed eyeblink CR, associating a neutral white noise CS with an eyelid stimulation US. Acute stress exposure the day before training facilitates conditioned eyeblink responding in male rats, whereas females are impaired in this type of associative learning (Wood & Shors, 1998). The sex differences in the effect of stress on learning appear to be mediated by differences in the critical brain regions involved. The male stress effect relies on activity in the bed nucleus of the stria terminalis (BNST), whereas the female stress effect depends on mPFC activity (Bangasser, Santollo, & Shors, 2005; Bangasser & Shors, 2010; Maeng, Waddell, & Shors, 2010). These data indicate that the neural circuits that mediate the opposing effects of stress on eyeblink conditioning differ between males and females. Sex differences are also observed in the stress-induced changes in brain structure. Chronic restraint stress can induce different morphological changes in the prefrontal cortex and hippocampus in male versus female rats (Luine, 2002; Wellman, 2001).
Humans
Within the stress response circuitry in humans, including the hypothalamus, hippocampus, amygdala, brainstem, orbitofrontal cortex (OFC), mPFC, and anterior cingulate gyrus (ACG), activation is also sexually dimorphic (Goldstein et al., 2010). In one neuroimaging study, healthy men and women were presented with negative valence/high arousal images to examine whether there were sex differences in their brain responses. The data demonstrated that although men were similar to women in the low estrogen phase, the men had significantly higher activation within the hippocampus and ACG, with the greatest effect sizes in the mPFC and OFC, relative to high estrogen women (Goldstein et al., 2010). Moreover, in a psychosocial achievement stress test, women reported feeling more stress than men, which was associated with higher activation in limbic and attention-related brain structures (Kogler et al., 2014). Together, the data in both humans and rats demonstrate sex differences not only in the effects of stress on behavior, but also in the structure and function of the neurocircuitry mediating them. Interestingly, the critical brain regions that respond to stress are shared with those implicated in anxiety disorders and fear neurocircuitry, suggesting that these responses are interrelated and can modulate each other.
Sex differences in stress effects on fear processes
Literature on the effects of stress on fear conditioning and extinction also report sex differences (Farrell, Sengelaub, & Wellman, 2013; Zorawski, Blanding, Kuhn, & LaBar, 2006; Zorawski, Cook, Kuhn, & LaBar, 2005). In humans, a psychosocial stressor produced differential responding, both behavioral and in the critical brain regions of fear learning and expression, during a fear conditioning task in men and women taking oral contraceptives. Stressed men displayed reduced fear conditioning, which correlated with impaired fear responses in the anterior cingulate cortex and amygdala, whereas stressed women showed enhanced fear learning, which correlated with facilitated differential responses within these same brain regions (Merz et al., 2010, 2013). Similarly, pharmacological treatment with stress hormone cortisol impaired fear conditioning in men, but facilitated acquisition of fear in women (Merz et al., 2010; Stark et al., 2006). Fear extinction and recall were not assessed in these studies. In another study, stress exposure following fear acquisition reduced conditioned fear memory retrieval in men compared to women, but there were no sex differences in extinction (Bentz et al., 2013). However, in rodents, sex differences in both fear conditioning and extinction have been observed in response to stress. Chronic exposure to restraint stress impairs extinction recall in males, but it facilitates fear extinction recall and impairs fear acquisition in female rats (Baran et al., 2009). The effect of stress on fear conditioning appears to be mixed and may also be attributed to species differences, type of stressors, time and duration of stressful experience, and/or fear paradigms used. Despite these discrepancies, the differential effects of stress on males and females are consistently observed and thus highlight the need to further pursue these studies.
Gonadal hormones, stress, and fear extinction
Estrogen- Rats
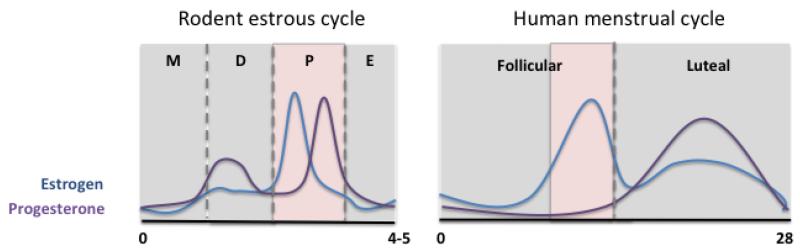
The inconsistent findings on sex differences in fear conditioning and extinction may be explained in part by the influence of gonadal hormones. Given the amount of evidence suggesting that sex hormones, and estrogen in particular, can modulate various learning and memory processes, the role of these hormones should be included in the study of sex differences in fear extinction learning and memory. As the primary gonadal hormone in females, estrogen will be the focus of this section. Moreover, it is important to note our discussion is centered on the activational effects of estrogen, which are more dynamic and occur throughout life, and not on its organizational effects, which are more permanent and occur during the early developmental hard-wiring of the brain (Arnold & Breedlove, 1985). The rodent estrous cycle consists of 4-5 days with 4 distinctive phases that are associated with varying levels of ovarian hormones: proestrus, estrus, metestrus, and diestrus (Fig. 2). Unfortunately, many rodent fear extinction studies do not control for estrous phase, overlooking the potential effects of the naturally fluctuating ovarian hormone levels on extinction learning and memory. Not accounting for estrous phase can mask underlying sex differences and mislead our understanding of important aspects of stress- and fear-related behaviors. For instance, Milad et al. (2009) did not observe sex differences in auditory cued fear conditioning, extinction, and recall in male and female rats when estrous phase was not considered. However, when the females were divided by the estrous phase during extinction training, sex differences in fear expression were observed in extinction recall. Specifically, females extinguished in the metestrus phase (low ovarian hormones) exhibited more freezing during recall compared to females extinguished in the proestrus phase (high ovarian hormones) as well as compared to male rats (Milad et al., 2009; Rey, Lipps, & Shansky, 2014). Moreover, exogenous administration of estrogen to metestrus females, prior to and within 4 hours after extinction training, enhanced fear extinction memory and reduced freezing during recall (Zeidan et al., 2011). The effect of estrogen on fear extinction was evident specifically during the retrieval of the extinction memory one day after training, which was associated with enhanced neuronal activity within the IL. Thus, it appears that estrogen modulates the consolidation of extinction memory and does so by increasing IL activity. In further support of these findings, endogenous estrogen facilitates synaptic potentiation within the IL, which would potentially allow for strengthening of extinction circuitry (Galvin & Ninan, 2014). The facilitatory role of estrogen on extinction has also been described in other tasks. Estrogen administration enhances extinction in a passive avoidance task in male Wistar rats (Rivas-Arancibia & Vazquez-Pereyra, 1994), as well as in conditioned taste aversion in both gonadectomized males and females (Yuan & Chambers, 1999). Moreover, intrahippocampal infusions of estrogen in ovariectomized female rats enhances contextual fear extinction (Chang et al., 2009). Together, these data demonstrate that estrogen is a potent neuromodulator of fear extinction learning and memory mechanisms. The molecular mechanisms underlying estrogen’s effect on extinction processes have been discussed in a recently published review (Cover et al., 2014; Glover, Jovanovic, & Norrholm, In press).
Figure 2.
Ovarian hormone levels fluctuate across the phases of the menstrual cycle. Estrogen levels peak during the proestrus phase of the estrous cycle and the late follicular phase of the woman’s menstrual cycle, times of enhanced extinction memory (shaded in pink). Left) Rodent estrous cycle. M: metestrus; D: diestrus; P: proestrus; E: estrus. Right) Human menstrual cycle.
Interestingly, estrogen appears to also enhance fear acquisition. For instance, ovariectomized female mice given estrogen exhibited enhanced fear conditioning (Jasnow, Schulkin, & Pfaff, 2006; Morgan & Pfaff, 2001), which was associated with increased corticotropin-releasing hormone (CRH) mRNA expression in the central amygdala (Jasnow et al., 2006). Similarly, fear-potentiated startle was facilitated by estrogen administration in ovariectomized female rats (Hiroi & Neumaier, 2006). In contrast, estrogen replacement in ovariectomized female rats reduced contextual fear conditioning (Gupta, Sen, Diepenhorst, Rudick, & Maren, 2001). Although some of these findings appear to challenge estrogen’s enhancement of fear extinction processes, it is important to note that these studies only examined the role of estrogen in males and ovariectomized females, neither of which experience steady variations in hormone levels. This makes it somewhat challenging to directly compare these findings with those in naturally cycling females. Moreover, given the overall learning and memory enhancement provided by estrogen administration, findings that report estrogen-facilitated fear conditioning should not be wholly unexpected. While they appear counterintuitive considering estrogen’s beneficial effects, they may simply be additional support for estrogen’s ability to increase learning capacity. Furthermore, estrogen depletion via ovariectomy may alter receptor density and function, producing varying dose dependent effects of estrogen replacement and making interpretation of the results difficult. This may be due to the inverted U-shaped effects of estrogen administration in which some doses may be optimal to produce protective effects, whereas other doses that are too low or high may induce impairments (Barha, Dalton, & Galea, 2010; Inagaki, Gautreaux, & Luine, 2010). In our studies, we have shown that naturally cycling high and low estrogen females exhibit no significant differences in fear conditioning, suggesting that high estrogen at normal physiological levels does not increase fear expression during conditioning and extinction, but does enhance extinction recall (Milad et al., 2009).
Estrogen- Humans
Similar to the female rodent, the hormonal milieu in women is constantly changing depending on reproductive stage throughout their lifespan. Estrogen’s influence on extinction retention in women follows the same pattern as in the rodent model as well, with low estrogen status associated with impaired extinction memory (Glover et al., 2013; Milad et al., 2010; Wegerer, Kerschbaum, Blechert, & Wilhelm, 2014). Women that underwent extinction training during the high estrogen luteal phase had comparable extinction recall to men, with both of these groups showing significantly better extinction memory than women trained during their low estrogen early follicular phase (Milad et al., 2010). Moreover, suppression of endogenous levels of estrogen in women taking hormonal contraceptives also impairs fear extinction, an effect that was also produced in female rodents (Graham & Milad, 2013). These alterations of extinction behavior in women taking hormonal contraceptives are associated with differences in activity of neural substrates within fear circuitry, e.g. increased differential activation to paired CS compared to unpaired CS in the amygdala, thalamus, anterior cingulate, and vmPFC during extinction (Merz et al., 2012).
Neural activity within the fear network also differs across the phases of the menstrual cycle in women (Goldstein et al., 2005; Milad et al., 2006; Protopopescu et al., 2005). Estrogen appears to enhance the functional activation of the vmPFC, the human homolog of the rodent IL, which is a critical structure in fear extinction (Protopopescu et al., 2005; Zeidan et al., 2011). Women in the late follicular-midcycle menstrual phase (high estrogen and progesterone) not only have better extinction retention than women in the early follicular menstrual phase (low estrogen and progesterone), but they also had significantly increased activation of the vmPFC during extinction learning and recall; vmPFC activation positively correlated with estrogen levels (Fig. 2; Zeidan et al., 2011). Interestingly, among women with low estrogen levels, women with PTSD exhibited higher fear-potentiated startle during extinction compared to women in the trauma-exposed control group, a distinction that was not observed in women with high estrogen levels (Glover et al., 2012). As mentioned previously, individuals with PTSD exhibit impaired extinction recall, which is associated with dysfunction in the vmPFC and thus may be related to activity during low estrogen states in women. Together, these findings highlight the vmPFC as a brain region that not only modulates fear, but is also affected by interactions between stress and estrogen and may be disrupted in anxiety disorders.
Estrogen- Stress
A similar pattern of stress sensitivity as in the modulation of fear appears to be associated with changing levels of ovarian hormones across the menstrual cycle and lifespan in women (Goldstein et al., 2005; Handa & Weiser, 2014; Jacobs et al., 2014; Ter Horst, Wichmann, Gerrits, Westenbroek, & Lin, 2009). Women are especially vulnerable to stress and disturbances in mood during drastic hormonal fluctuations (Brummelte & Galea, 2010). The higher prevalence of anxiety- and stress-related mental illness in women may also be associated with these changes, as it appears during puberty and lasts until menopause (Hyde, Mezulis, & Abramson, 2008; Kessler, McGonagle, Swartz, Blazer, & Nelson, 1993; Nolen-Hoeksema & Girgus, 1994; Silberg et al., 1999; Sonnenberg, Beekman, Deeg, & van Tilburg, 2000; Kaltiala-Heino, Kosunen, & Rimpelä, 2003; Piccinelli & Wilkinson, 2000). Just as for fear extinction recall, women taking hormonal contraceptives exhibit differences in emotional responding, such as blunted stress responses and memory for different aspects of an emotional story compared to those that are naturally cycling (Nielsen, Ahmed, & Cahill, 2014; Nielsen, Ertman, Lakhani, & Cahill, 2011; Nielsen, Segal, Worden, Yim, & Cahill, 2013). However, it is important to note that in this study, menstrual phase, which can also be influencing these effects, was not accounted for in the naturally cycling group.
Estrogen and stress effects on fear circuitry
These changes in stress sensitivity are also manifested in altered neural activity. During the low estrogen phase, healthy women presented with negative valence/high arousal images showed increased activation of brain regions in stress response circuitry, i.e. PFC, amygdala, hippocampus, paraventricular nucleus (PVN), and brainstem, compared to women in their high estrogen phase (Goldstein et al., 2005). A recent study examined the interaction of menstrual phase, stress, and fear during exposure to a psychosocial stressor prior to fear conditioning. These data demonstrated that there were no differential effects of stress on fear acquisition, but it enhanced extinction recall in women stressed in the high estrogen phase of their menstrual cycles. The opposite was seen in women stressed in their low estrogen phase, who exhibited impaired extinction memory (Antov & Stockhorst, 2014). Therefore, experiencing trauma in a low estrogen state may contribute to resistance to extinction and greater fear recovery during extinction recall. Because of their interconnected relationship with stress and fear mechanisms, ovarian hormones such as estrogen have been implicated in the etiology of anxiety disorders in women. At the very least, these data suggest that low levels or fluctuations in estrogen that occur in cycling women at the time of a traumatic event, in addition to their hormone status during therapy, can predict treatment outcomes.
As alluded to previously, the medial prefrontal cortex is one key brain region in the fear extinction network that is also sexually dimorphic (Baran et al., 2010; Goldstein et al., 2005; Maroun, 2013; Quirk, Russo, Barron, & Lebron, 2000) that is not only sensitive to estrogen levels (Merz et al., 2012; Zeidan et al., 2011), but is also responsive to stress (Arnsten, 2009; Garrett & Wellman, 2009; Maeng & Shors, 2013; Maeng et al., 2010; Shansky & Lipps, 2013; Shansky & Morrison, 2009). As such, this brain region seems to be a neurobiological point of intersection for estrogen, fear, and stress. Stress increases the number and length of apical dendritic branches in the medial prefrontal cortex, but this process is prevented by ovariectomy, suggesting that it may be dependent on the presence of estrogen (Garrett & Wellman, 2009). Acute stress disrupts classical eyeblink conditioning (Shors et al., 1998), an effect that is dependent on medial prefrontal cortex activity in low estrogen female rats but not in male rats (Maeng et al., 2010). Moreover, differential contributions of the PL and IL subregions of the mPFC to stress and fear have been identified (Akirav & Maroun, 2007; Fenton et al., 2014; Laurent & Westbrook, 2009; Maeng & Shors, 2013). Inactivation of the PL during acute forced swim stress exposure prevents the subsequent impairment in classical eyeblink conditioning, whereas the inactivation of the IL does not (Maeng & Shors, 2013). This suggests that stress may be engaging the PL to induce the negative effect of stress on associative learning. This may be related to the persistent PL activity observed in female rats that express high contextual fear during extinction and recall, although estrous phase was not accounted for in this study (Fenton et al., 2014). Together, these data suggest that the PL may be one brain target that is especially sensitive to stress and involved in impairments in fear extinction. Chronic restraint stress induces increases in dendritic branching in IL neurons that project to the BLA in estrogen-treated ovariectomized females (Shansky et al., 2010). This finding indicates that the IL-BLA pathway is sensitive to both stress and estrogen. The estrogen-enhanced IL plasticity may suggest increased pathway connectivity or functioning. Although fear learning and extinction were not assessed in this particular study, this effect on IL-BLA connectivity may be involved in the previously described stress-enhanced extinction recall in women stressed during their high estrogen phase (Antov & Stockhorst, 2014).
In males, the robust connections between the prefrontal cortex and the amygdala have been studied extensively in stress and fear modulation experiments (Garcia, Vouimba, Baudry, & Thompson, 1999; Laurent & Westbrook, 2008; Maroun & Richter-Levin, 2003; Quirk et al., 2003; Sotres-Bayon et al., 2004). However, this may be an especially critical circuit for stress and fear modulation in females (Maeng et al., 2010; Shansky et al., 2010). The amygdala also contains estrogen receptors (Jasnow et al., 2006; Shughrue, Scrimo, & Merchenthaler, 1998), and estrogen infusion influences amygdala function in fear and emotional responses (Frye & Walf, 2004). Disconnecting the mPFC and amygdala via contralateral lesions of these structures prevents the stress-induced suppression of eyeblink conditioning, indicating that the mPFC communicates with the amygdala to modify learning after stress in females (Maeng et al., 2010). Together, these data suggest that estrogen may be altering structure and function within the medial prefrontal cortex (via connections to the amygdala specifically in females) to modulate its role in stress and fear circuitry and the interactions between both systems.
HPA-HPG interactions
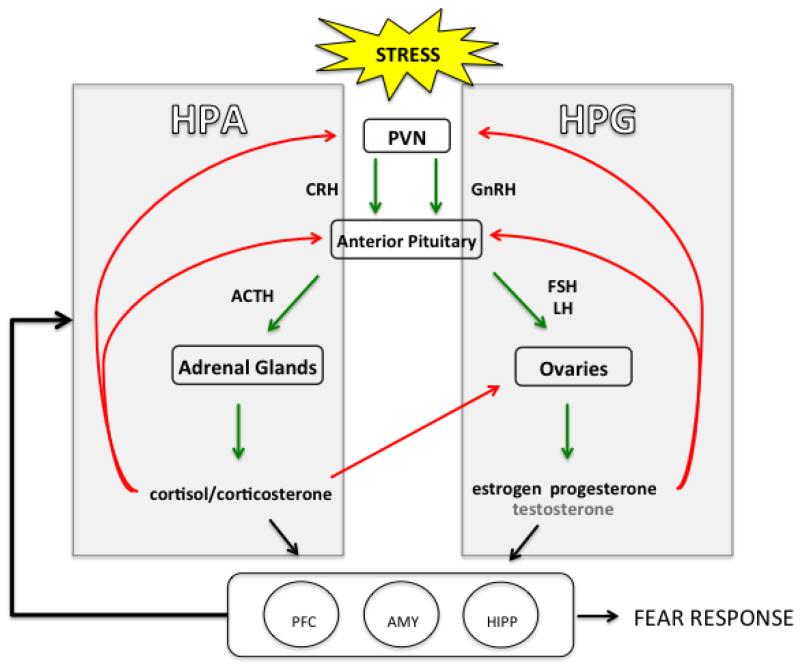
Stress exposure induces numerous responses, including those of the sympathetic nervous system (catecholamines) and immune system (cytokines). One of the principal outputs of the stress response is activation of the glucocorticoid system. Thus, stress, fear, and sex interactions may also be related to activity within the hypothalamic-pituitary-adrenal (HPA) axis, which modulates responses to stress and also appears to be dysregulated in anxiety and PTSD. There are indirect connections to the HPA axis that originate from the hippocampus, mPFC, and amygdala, regions described above in which stress, fear, and sex appear to interact-- essential structures in learning and fear circuitry that also contain receptors for stress hormones (Arnsten, 1997; Gray & Bingaman, 1996; McEwen, Weiss, & Schwartz, 1968; Patel, Katz, Karssen, & Lyons, 2008; Veldhuis, Van Koppen, Van Ittersum, & De Kloet, 1982). It has been reported that the mPFC has inhibitory control of HPA activity (Weinberg, Johnson, Bhatt, & Spencer, 2010). Interestingly, the HPA axis response to stress differs in males and females, with females appearing to have faster HPA reactivity and a higher release of stress hormones (Goel, Workman, Lee, Innala, & Viau, 2014; for review, see Kudielka & Kirschbaum, 2005). What may be critical to understanding these differences is the crosstalk between the HPA axis and the hypothalamic-pituitary-gonadal (HPG) axis, which regulates secretion of gonadal hormones such as estrogen, progesterone, and testosterone (Toufexis, Rivarola, Lara, & Viau, 2014). Estrogen and testosterone levels can regulate HPA responses with some reports that testosterone can inhibit HPA activity, whereas estrogen can enhance function of the stress axis, an effect also dependent on which estrogen receptor type is activated (Handa, Burgess, Kerr, & O’Keefe, 1994; Handa & Weiser, 2014; Kirschbaum et al., 1996). Moreover, exposure to stress, and more specifically stress-induced HPA activity, appears to inhibit estrogen and testosterone secretion (Lu et al., 2015; Toufexis et al., 2014). For instance, male rats exposed to predator odor stress showed reduced levels of testosterone (Fenchel et al., 2015). Female rats also exhibited a significant decrease in plasma levels of estrogen and testosterone following foot shock and chronic unpredictable mild stress (Lu et al., 2015). In females, the HPG axis regulates the menstrual/estrous cycle. HPG activity increases and decreases throughout the female lifespan, contributing to the hormonal fluctuations that occur during different reproductive life events (i.e. puberty, pregnancy, menopause), and thus, may play an important role in the neurocircuitry mediating the interactions between stress, estrogen, and fear. Both the HPA and HPG axes are influenced by similar factors such as trauma, and dysregulation in either system can lead to disturbances in emotional health, and in turn, learning and memory deficits (Dismukes, Johnson, Vitacco, Iturri, & Shirtcliff, 2014; Du et al., 2014; Toufexis et al., 2014). Given this relationship, crosstalk between the HPA and HPG circuitry may be critical to understanding the greater vulnerability to stress and anxiety disorders during the different hormonal phases across the female lifespan (Fig. 3).
Figure 3.
Female HPA-HPG circuitry. The HPA axis is activated by stress signals that travel from the paraventricular nucleus (PVN) of the hypothalamus to the pituitary gland and then to the adrenal glands for glucocorticoid release. In the HPG axis, the hypothalamus produces gonadotropin-releasing hormone, which binds to receptors within the anterior pituitary to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH then stimulate the gonads for the release of estrogen and progesterone, as well as a small amount of testosterone, in females. Red lines represent inhibitory action of negative feedback; green lines represent excitation. Estrogen levels can regulate HPA responses. HPA activity can inhibit estrogen secretion. The reciprocal modulation of gonadal hormone and stress response neurobiology may underlie the sex differences and influence of gonadal hormones in fear extinction and anxiety disorders. These systems are also indirectly connected to the hippocampus, mPFC, and amygdala, critical regions in fear circuitry that are affected by stress and estrogen.
Progesterone
Although much of the literature is on the role of estrogen in stress and fear learning and memory, progesterone is also an important female sex hormone involved in the menstrual/estrous cycle and pregnancy. High progesterone levels have been associated with enhanced memory for emotionally arousing stimuli in women (Ertman, Andreano, & Cahill, 2011). Endogenous progesterone levels also interact with stress exposure to regulate emotional memory. Women in a high progesterone state of their menstrual cycle had increased cortisol levels and better memory recall for negative images that were paired with the cold pressor stress task (Felmingham et al., 2010). Some report that progesterone and estrogen produce effects on arousal brain circuitry that interact or may even oppose each other. Increased amygdala activity during presentation of negative images was observed in women in the luteal phase when both estrogen and progesterone levels are high, despite previous reports of reduced amygdala activity during high estrogen states (Andreano & Cahill, 2010; Ferree, Kamat, & Cahill, 2011).
Progesterone may also be contributing to estrogen’s enhancing effects on extinction memory. Exogenous administration of progesterone facilitates extinction recall in female rats, similar to the effect of estrogen administration (Milad et al., 2009). However, progesterone did not have this effect in women (Milad et al., 2010). This may be due to species differences; however, progesterone has been shown to suppress HPA axis activity via conversion to its metabolite allopregnanolone (Biggio, Pisu, Biggio, & Serra, 2014). In fact, a number of studies have reported anxiolytic properties of allopregnanolone (Nillni, Toufexis, & Rohan, 2011; Pibiri, Nelson, Guidotti, Costa, & Pinna, 2008; D. J. Toufexis, Davis, Hammond, & Davis, 2004). Human imaging studies have shown that allopregnanolone is associated with reduced amygdala responsivity to aversive stimuli, further supporting the anxiolytic role of this hormone (Sripada et al., 2013; Sripada, Welsh, Marx, & Liberzon, 2014). Allopregnanolone is a positive modulator at GABA-A receptors, and women with PTSD have altered GABA-A receptor sensitivity as well as reduced cerebrospinal fluid levels of allopregnanolone (Möller, Bäckström, Nyberg, Söndergaard, & Helström, 2014; Rasmusson et al., 2006). Administration of ganaxolone, a synthetic analog of allopregnanolone, was shown to reduce anxiety-like behavior in the elevated plus maze, reduce high fear expression during contextual fear conditioning, and enhance fear extinction retention in mice (Pinna & Rasmusson, 2014). Given the evidence that progesterone and its neuroactive steroid metabolite allopregnanolone are involved in emotional memory formation and mood disorders (Bäckström et al., 2014), it is important to further examine their role in fear extinction mechanisms as potential therapeutic agents for anxiety disorders.
Testosterone
Testosterone also exerts effects on anxiety and fear processes that may be relevant in this discussion (McHenry, Carrier, Hull, & Kabbaj, 2014). Testosterone and its metabolites have been shown to possess anxiolytic properties, reducing anxiety behaviors and enhancing cognition in male rodents (Frye, Koonce, Edinger, Osborne, & Walf, 2008; Hodosy et al., 2012; McDermott, Liu, & Schrader, 2012). Findings for the role of testosterone in fear conditioning and extinction are not yet clear. Some report no role of testosterone in contextual fear conditioning (Anagnostaras et al., 1998), while others suggest a role of testosterone in cued fear conditioning (Chen et al., 2014). Testosterone is converted to estrogen in the brain via the enzyme aromatase. Thus, aromatase inhibitors, such as fadrozole, prevent estrogen synthesis. Interestingly, we have found that administration of fadrozole prior to extinction training impairs fear extinction recall in male rats, which can be rescued by estrogen administration (Graham & Milad, 2014). In a recent study, extinction learning was better in men with elevated testosterone to cortisol ratios (Pace-Schott et al., 2013), further implicating this hormone in fear extinction. As its presence is needed for the production of estrogen, testosterone may indirectly modulate fear extinction processes via this conversion.
Hypogonadal men with low testosterone have an increased risk for developing anxiety- and stress-related disorders (DiBlasio et al., 2008; Shores et al., 2004; Zarrouf, Artz, Griffith, Sirbu, & Kommor, 2009). The risk of developing pathological anxiety can also be reversed with testosterone administration, improving affect and reducing anxiety and depression (Kanayama, Amiaz, Seidman, & Pope, 2007; Pope, Cohane, Kanayama, Siegel, & Hudson, 2003; Wang et al., 1996; Zarrouf et al., 2009). Moreover, testosterone replacement in castrated male rodents ameliorates anxiety behaviors (Hodosy et al., 2012; Khakpai, 2014). Although there are mixed reports on the role of testosterone in PTSD, there is evidence suggesting that a single administration of testosterone to women may reduce the stress response and startle reflex, which is typically heightened in PTSD (Hermans et al., 2007; Hermans, Putman, Baas, Koppeschaar, & van Honk, 2006). Future studies will be necessary to examine the influence of testosterone on the mechanisms mediating fear extinction and its interactions with estrogen and other sex hormones.
Future of treatment for anxiety disorders
This line of research can significantly impact future treatment of anxiety disorders. More individualized, sex-specific treatments may not only ameliorate the sex bias in prevalence for these psychopathologies, but they could also: 1) eliminate symptoms, 2) reduce symptom severity to levels that allow proper life functioning, and 3) increase the duration of improved treatment outcomes to make them longer lasting or permanent. Further investigation of the mechanisms underlying the interactions between estrogen and antidepressants may also be warranted. For instance, chronic fluoxetine improves extinction recall in females but not in males and facilitates extinction learning in low estrogen rats while having no effect on high estrogen rats (Lebrón-Milad, Tsareva, Ahmed, & Milad, 2013). In addition to fear extinction, sex differences have also been reported in the effect of antidepressants on stress and associative learning, as well as their overall pharmacokinetics; studies suggest that females respond better to selective serotonin reuptake inhibitors (SSRIs), a response that may be attributed to the known interactions between estrogen and serotonin (Dalla, Pitychoutis, Kokras, & Papadopoulou-Daifoti, 2010; Damoiseaux, Proost, Jiawan, & Melgert, 2014; Keers & Aitchison, 2010; Kokras, Dalla, & Papadopoulou-Daifoti, 2011; Leuner, Mendolia-Loffredo, & Shors, 2004). Interestingly, some of these effects have been associated with naturally cycling female sex hormones, which may be influencing the differences in therapeutic response to antidepressant pharmacotherapy (Frackiewicz, Sramek, & Cutler, 2000). These data underscore the need to consider the impact that sex hormones may have on treatment options. Regimens that are scheduled around menstrual cycle phase, account for oral contraceptive use, and/or include the measurement of hormone levels prior to the start of treatment appear necessary.
Future research aimed at localizing and identifying cellular, molecular, and genetic mechanisms by which estrogen modulates fear extinction and anxiety can guide our treatment targets and ultimately improve the efficacy of clinical applications. Fairly recently, pituitary adenylate cyclase-activating polypeptide (PACAP), a stress- and fear-related peptide, was found to be associated with the diagnosis and symptoms of PTSD in women. This peptide appears to be modulated in the BNST by estrogen administration in ovariectomized female rats (Ressler et al., 2011). Further advances in this field could enable us to use the extinction-strengthening properties of estrogen (perhaps targeting critical regions such as the mPFC) without the potentially harmful side effects that typically accompany it, potentially enhancing the benefits of pharmacotherapy and/or prolonged exposure therapy. To this end, we must improve our understanding of the complex processes that underlie stress and fear responses and interact with sex hormones to contribute to the etiology of anxiety disorders.
Conclusions
The goals of this review are to (1) present current literature on hormonal influences on fear and stress mechanisms that may underlie sex differences in anxiety disorders and (2) highlight the gaps that need to be filled in order to enhance our understanding of the pathophysiology and treatment of anxiety disorders. Converging data from studies on stress, fear, and sex hormones indicate that heightened sensitivity to stress and impaired extinction memory consolidation are associated with low estrogen states in females. This may be related to vulnerability to psychopathology. Stressful life experiences can disrupt fear extinction, a behavioral process that models the psychopathology of PTSD and anxiety disorders. This negative effect of stress can be further amplified during low levels of, or fluctuations in, estrogen. Because stress is a major contributor to the development of anxiety disorders, stress and fear mechanisms should be studied together and not in isolation.
Although there is a substantial amount of literature on the effects of estrogen on stress and fear regulation, few studies have examined the role of other gonadal hormones. More studies are needed to understand the roles and interactions these hormones may have with the effects of estrogen in fear extinction memory consolidation. It is important to emphasize that although low estrogen levels are linked with poor extinction memory recall, the elevated risk for anxiety may be more dependent on fluctuations and less on the absolute “low” versus “high” levels of estrogen. Studies examining ovariectomized females may provide some initial information about the role of estrogen on the specific behavior being tested; however, conducting experiments using naturally cycling females may provide insight on the many interactions that take place in normal animals and thus offer more clinical relevance to the affected population, who are typically of child-bearing age. Moreover, the effects of estrogen can be dose-dependent and follow an inverted U shape in their interactions with stress and fear to influence subsequent learning and memory processes; estrogen levels that are too low or too high can have negative consequences (Arnsten, 2009; Shors & Leuner, 2003). This may explain some of the inconsistencies in the effects of estrogen in the literature, and should also illustrate why it is imperative to consider natural fluctuations, as they can determine whether estrogen treatment will have beneficial or harmful effects. These data highlight the intersection of stress, fear, and sex hormones to elucidate their role in the increased vulnerability of females to anxiety-related disorders (Fig. 3). We also highlight the medial prefrontal cortex and its communication with the amygdala as a potentially critical circuit that is affected by, and in turn modulates, these interactions. Based on the clinical implications of these data, it is evident that ovarian hormone levels should be taken into consideration when treating women for stress- and anxiety-related disorders.
Highlights.
Women are more vulnerable to anxiety and fear-related disorders than men.
Estrogen levels can modulate fear and stress responses in women and female rodents.
Stress affects the critical nodes of fear circuitry.
Interactions between sex hormones, stress, and fear should be further examined.
Treatment for anxiety and fear-based disorders should account for hormone status.
Acknowledgements
We appreciate the helpful and thoughtful comments on this manuscript from the members of the Behavioral Neuroscience Program in the Department of Psychiatry at Massachusetts General Hospital. Special thanks to Dr. Marie-France Marin and Kara K. Cover for editorial assistance. MRM is supported by 1R01MH097880-001.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar R, Gil L, Gray JA, Driscoll P, Flint J, Dawson GR, Tobeña A. Fearfulness and sex in F2 Roman rats: males display more fear though both sexes share the same fearfulness traits. Physiology & Behavior. 2003;78(4-5):723–732. doi: 10.1016/s0031-9384(03)00043-x. doi:10.1016/S0031-9384(03)00043-X. [DOI] [PubMed] [Google Scholar]
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plasticity. 2007;2007:30873. doi: 10.1155/2007/30873. doi:10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Frontiers in Neuroendocrinology. 2014;35(3):320–330. doi: 10.1016/j.yfrne.2014.05.004. doi:10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Author; Washington, D.C.: 2013. [Google Scholar]
- Anagnostaras SG, Maren S, DeCola JP, Lane NI, Gale GD, Schlinger BA, Fanselow MS. Testicular hormones do not regulate sexually dimorphic Pavlovian fear conditioning or perforant-path long-term potentiation in adult male rats. Behavioural Brain Research. 1998;92(1):1–9. doi: 10.1016/s0166-4328(97)00115-0. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53(4):1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. doi:10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antov MI, Stockhorst U. Stress exposure prior to fear acquisition interacts with estradiol status to alter recall of fear extinction in humans. Psychoneuroendocrinology. 2014;49:106–118. doi: 10.1016/j.psyneuen.2014.06.022. doi:10.1016/j.psyneuen.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Hormones and Behavior. 1985;19(4):469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. Journal of Psychopharmacology (Oxford, England) 1997;11(2):151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. doi:10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, van Wingen G. Allopregnanolone and mood disorders. Progress in Neurobiology. 2014;113:88–94. doi: 10.1016/j.pneurobio.2013.07.005. doi:10.1016/j.pneurobio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Baker-Andresen D, Flavell CR, Li X, Bredy TW. Activation of BDNF signaling prevents the return of fear in female mice. Learning & Memory (Cold Spring Harbor, N.Y.) 2013;20(5):237–240. doi: 10.1101/lm.029520.112. doi:10.1101/lm.029520.112. [DOI] [PubMed] [Google Scholar]
- Bakish D. The patient with comorbid depression and anxiety: the unmet need. The Journal of Clinical Psychiatry. 1999;60(Suppl 6):20–24. [PubMed] [Google Scholar]
- Bangasser DA, Santollo J, Shors TJ. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behavioral Neuroscience. 2005;119(6):1459–1466. doi: 10.1037/0735-7044.119.6.1459. doi:10.1037/0735-7044.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. Critical brain circuits at the intersection between stress and learning. Neuroscience and Biobehavioral Reviews. 2010;34(8):1223–1233. doi: 10.1016/j.neubiorev.2010.02.002. doi:10.1016/j.neubiorev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Conrad CD. Prefrontal cortex lesions and sex differences in fear extinction and perseveration. Learning & Memory (Cold Spring Harbor, N.Y.) 2010;17(5):267–278. doi: 10.1101/lm.1778010. doi:10.1101/lm.1778010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiology of Learning and Memory. 2009;91(3):323–332. doi: 10.1016/j.nlm.2008.11.005. doi:10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LA. Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35(2):547–559. doi: 10.1038/npp.2009.161. doi:10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz D, Michael T, Wilhelm FH, Hartmann FR, Kunz S, von Rohr IRR, de Quervain DJ-F. Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinology. 2013;38(7):1186–1197. doi: 10.1016/j.psyneuen.2012.12.018. doi:10.1016/j.psyneuen.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Biggio G, Pisu MG, Biggio F, Serra M. Allopregnanolone modulation of HPA axis function in the adult rat. Psychopharmacology. 2014;231(17):3437–3444. doi: 10.1007/s00213-014-3521-6. doi:10.1007/s00213-014-3521-6. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. doi:10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biological Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. Gender differences in trauma and posttraumatic stress disorder. The Journal of Gender-Specific Medicine: JGSM: The Official Journal of the Partnership for Women’s Health at Columbia. 2002;5(1):34–40. [PubMed] [Google Scholar]
- Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma, Violence & Abuse. 2009;10(3):198–210. doi: 10.1177/1524838009334448. doi:10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Archives of General Psychiatry. 1997;54(11):1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 1998;55(7):626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Briscione MA, Jovanovic T, Norrholm SD. Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Frontiers in Psychiatry. 2014;5:88. doi: 10.3389/fpsyt.2014.00088. doi:10.3389/fpsyt.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Galea LAM. Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Hormones and Behavior. 2010;58(5):769–779. doi: 10.1016/j.yhbeh.2010.07.012. doi:10.1016/j.yhbeh.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH, Brown TA, Hofmann SG. Acceptability and suppression of negative emotion in anxiety and mood disorders. Emotion (Washington, D.C.) 2006;6(4):587–595. doi: 10.1037/1528-3542.6.4.587. doi:10.1037/1528-3542.6.4.587. [DOI] [PubMed] [Google Scholar]
- Chang Y-J, Yang C-H, Liang Y-C, Yeh C-M, Huang C-C, Hsu K-S. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus. 2009;19(11):1142–1150. doi: 10.1002/hipo.20581. doi:10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- Chen L-S, Tzeng W-Y, Chuang J-Y, Cherng CG, Gean P-W, Yu L. Roles of testosterone and amygdaloid LTP induction in determining sex differences in fear memory magnitude. Hormones and Behavior. 2014;66(3):498–508. doi: 10.1016/j.yhbeh.2014.07.008. doi:10.1016/j.yhbeh.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WE, Weiner RD, Parashos IA, Djang WT, Webb MC, Spritzer CE. Quantitative cerebral anatomy in depression. A controlled magnetic resonance imaging study. Archives of General Psychiatry. 1993;50(1):7–16. doi: 10.1001/archpsyc.1993.01820130009002. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60(2):236–248. doi: 10.1002/neu.20025. doi:10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21(5):1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover KK, Maeng LY, Lebrón-Milad K, Milad MR. Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Translational Psychiatry. 2014;4:e422. doi: 10.1038/tp.2014.67. doi:10.1038/tp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic & Clinical Pharmacology & Toxicology. 2010;106(3):226–233. doi: 10.1111/j.1742-7843.2009.00516.x. doi:10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- Damoiseaux VA, Proost JH, Jiawan VCR, Melgert BN. Sex differences in the pharmacokinetics of antidepressants: influence of female sex hormones and oral contraceptives. Clinical Pharmacokinetics. 2014;53(6):509–519. doi: 10.1007/s40262-014-0145-2. doi:10.1007/s40262-014-0145-2. [DOI] [PubMed] [Google Scholar]
- Daviu N, Andero R, Armario A, Nadal R. Sex differences in the behavioural and hypothalamic-pituitary-adrenal response to contextual fear conditioning in rats. Hormones and Behavior. 2014 doi: 10.1016/j.yhbeh.2014.09.015. doi:10.1016/j.yhbeh.2014.09.015. [DOI] [PubMed] [Google Scholar]
- De Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394(6695):787–790. doi: 10.1038/29542. doi:10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Goodman JV, Ressler KJ. Towards new approaches to disorders of fear and anxiety. Current Opinion in Neurobiology. 2013;23(3):346–352. doi: 10.1016/j.conb.2013.01.013. doi:10.1016/j.conb.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBlasio CJ, Hammett J, Malcolm JB, Judge BA, Womack JH, Kincade MC, Derweesh IH. Prevalence and predictive factors for the development of de novo psychiatric illness in patients receiving androgen deprivation therapy for prostate cancer. The Canadian Journal of Urology. 2008;15(5):4249–4256. discussion 4256. [PubMed] [Google Scholar]
- Dismukes AR, Johnson MM, Vitacco MJ, Iturri F, Shirtcliff EA. Coupling of the HPA and HPG axes in the context of early life adversity in incarcerated male adolescents. Developmental Psychobiology. 2014 doi: 10.1002/dev.21231. doi:10.1002/dev.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Pang TY, Mo C, Renoir T, Wright DJ, Hannan AJ. The influence of the HPG axis on stress response and depressive-like behaviour in a transgenic mouse model of Huntington’s disease. Experimental Neurology. 2014;263C:63–71. doi: 10.1016/j.expneurol.2014.09.009. doi:10.1016/j.expneurol.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Ertman N, Andreano JM, Cahill L. Progesterone at encoding predicts subsequent emotional memory. Learning & Memory (Cold Spring Harbor, N.Y.) 2011;18(12):759–763. doi: 10.1101/lm.023267.111. doi:10.1101/lm.023267.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. American Journal of Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. doi:10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, Jovanovic T. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2015;64:249–259. doi: 10.1016/j.cortex.2014.11.006. doi:10.1016/j.cortex.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MR, Sengelaub DR, Wellman CL. Sex differences and chronic stress effects on the neural circuitry underlying fear conditioning and extinction. Physiology & Behavior. 2013;122:208–215. doi: 10.1016/j.physbeh.2013.04.002. doi:10.1016/j.physbeh.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. Journal of Abnormal Psychology. 2010;119(1):241–247. doi: 10.1037/a0017551. doi:10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- Fenchel D, Levkovitz Y, Vainer E, Kaplan Z, Zohar J, Cohen H. Beyond the HPA-axis: The role of the gonadal steroid hormone receptors in modulating stress-related responses in an animal model of PTSD. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2015 doi: 10.1016/j.euroneuro.2015.02.004. doi:10.1016/j.euroneuro.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Fenton GE, Pollard AK, Halliday DM, Mason R, Bredy TW, Stevenson CW. Persistent prelimbic cortex activity contributes to enhanced learned fear expression in females. Learning & Memory. 2014;21(2):55–60. doi: 10.1101/lm.033514.113. doi:10.1101/lm.033514.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree NK, Kamat R, Cahill L. Influences of menstrual cycle position and sex hormone levels on spontaneous intrusive recollections following emotional stimuli. Consciousness and Cognition. 2011;20(4):1154–1162. doi: 10.1016/j.concog.2011.02.003. doi:10.1016/j.concog.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB. Psychosocial treatment of posttraumatic stress disorder. The Journal of Clinical Psychiatry. 2000;61(Suppl 5):43–48. discussion 49-51. [PubMed] [Google Scholar]
- Foa EB. Prolonged exposure therapy: past, present, and future. Depression and Anxiety. 2011;28(12):1043–1047. doi: 10.1002/da.20907. doi:10.1002/da.20907. [DOI] [PubMed] [Google Scholar]
- Foa EB, Street GP. Women and traumatic events. The Journal of Clinical Psychiatry. 2001;62(Suppl 17):29–34. [PubMed] [Google Scholar]
- Frackiewicz EJ, Sramek JJ, Cutler NR. Gender differences in depression and antidepressant pharmacokinetics and adverse events. The Annals of Pharmacotherapy. 2000;34(1):80–88. doi: 10.1345/aph.18465. [DOI] [PubMed] [Google Scholar]
- Frans O, Rimmö P-A, Aberg L, Fredrikson M. Trauma exposure and post-traumatic stress disorder in the general population. Acta Psychiatrica Scandinavica. 2005;111(4):291–299. doi: 10.1111/j.1600-0447.2004.00463.x. doi:10.1111/j.1600-0447.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Edinger KL, Osborne DM, Walf AA. Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice. Hormones and Behavior. 2008;54(5):726–734. doi: 10.1016/j.yhbeh.2008.07.013. doi:10.1016/j.yhbeh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or Progesterone Administered Systemically or to the Amygdala Can Have Anxiety-, Fear-, and Pain-Reducing Effects in Ovariectomized Rats. Behavioral Neuroscience. 2004;118(2):306–313. doi: 10.1037/0735-7044.118.2.306. doi:10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Galvin C, Ninan I. Regulation of the mouse medial prefrontal cortical synapses by endogenous estradiol. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2014;39(9):2086–2094. doi: 10.1038/npp.2014.56. doi:10.1038/npp.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402(6759):294–296. doi: 10.1038/46286. doi:10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162(1):195–207. doi: 10.1016/j.neuroscience.2009.04.057. doi:10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological Psychiatry. 2012;72(1):19–24. doi: 10.1016/j.biopsych.2012.02.031. doi:10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Norrholm SD. Estrogen and Extinction of Fear Memories: Implications for Posttraumatic Stress Disorder Treatment. doi: 10.1016/j.biopsych.2015.02.007. In press. doi: http://dx.doi.org/10.1016/j.biopsych.2015.02.007. [DOI] [PMC free article] [PubMed]
- Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, Jovanovic T. Inhibition of fear is differentially associated with cycling estrogen levels in women. Journal of Psychiatry & Neuroscience: JPN. 2013;38(5):341–348. doi: 10.1503/jpn.120129. doi:10.1503/jpn.120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Workman JL, Lee TT, Innala L, Viau V. Sex differences in the HPA axis. Comprehensive Physiology. 2014;4(3):1121–1155. doi: 10.1002/cphy.c130054. doi:10.1002/cphy.c130054. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(2):431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. doi:10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. doi:10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. The Study of Fear Extinction: Implications for Anxiety Disorders. American Journal of Psychiatry. 2011;168(12) doi: 10.1176/appi.ajp.2011.11040557. doi:10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biological Psychiatry. 2013;73(4):371–378. doi: 10.1016/j.biopsych.2012.09.018. doi:10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learning & Memory (Cold Spring Harbor, N.Y.) 2014;21(7):347–350. doi: 10.1101/lm.034926.114. doi:10.1101/lm.034926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Critical Reviews in Neurobiology. 1996;10(2):155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Research. 2001;888(2):356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and Behavior. 1994;28(4):464–476. doi: 10.1006/hbeh.1994.1044. doi:10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Frontiers in Neuroendocrinology. 2014;35(2):197–220. doi: 10.1016/j.yfrne.2013.11.001. doi:10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 2007;32(8-10):1052–1061. doi: 10.1016/j.psyneuen.2007.08.006. doi:10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Koppeschaar HP, van Honk J. A single administration of testosterone reduces fear-potentiated startle in humans. Biological Psychiatry. 2006;59(9):872–874. doi: 10.1016/j.biopsych.2005.11.015. doi:10.1016/j.biopsych.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Hickey M, Bryant C, Judd F. Evaluation and management of depressive and anxiety symptoms in midlife. Climacteric: The Journal of the International Menopause Society. 2012;15(1):3–9. doi: 10.3109/13697137.2011.620188. doi:10.3109/13697137.2011.620188. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behavioural Brain Research. 2006;166(1):93–100. doi: 10.1016/j.bbr.2005.07.021. doi:10.1016/j.bbr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Hodosy J, Zelmanová D, Majzúnová M, Filová B, Malinová M, Ostatníková D, Celec P. The anxiolytic effect of testosterone in the rat is mediated via the androgen receptor. Pharmacology, Biochemistry, and Behavior. 2012;102(2):191–195. doi: 10.1016/j.pbb.2012.04.005. doi:10.1016/j.pbb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clinical Psychology Review. 2008;28(2):199–210. doi: 10.1016/j.cpr.2007.04.009. doi:10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook TL, Hoyt DB, Stein MB, Sieber WJ. Gender differences in long-term posttraumatic stress disorder outcomes after major trauma: women are at higher risk of adverse outcomes than men. The Journal of Trauma. 2002;53(5):882–888. doi: 10.1097/00005373-200211000-00012. doi:10.1097/01.TA.0000033749.65011.6A. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review. 2008;115(2):291–313. doi: 10.1037/0033-295X.115.2.291. doi:10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Hormones and Behavior. 2010;58(3):415–426. doi: 10.1016/j.yhbeh.2010.05.013. doi:10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, Neylan TC. Sex differences in fear conditioning in posttraumatic stress disorder. Journal of Psychiatric Research. 2013;47(1):64–71. doi: 10.1016/j.jpsychires.2012.08.027. doi:10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(21):5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. doi:10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Hormones and Behavior. 2006;49(2):197–205. doi: 10.1016/j.yhbeh.2005.06.005. doi:10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(37):9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. doi:10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Kosunen E, Rimpelä M. Pubertal timing, sexual behaviour and self-reported depression in middle adolescence. Journal of Adolescence. 2003;26(5):531–545. doi: 10.1016/s0140-1971(03)00053-8. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Amiaz R, Seidman S, Pope HG. Testosterone supplementation for depressed men: current research and suggested treatment guidelines. Experimental and Clinical Psychopharmacology. 2007;15(6):529–538. doi: 10.1037/1064-1297.15.6.529. doi:10.1037/1064-1297.15.6.529. [DOI] [PubMed] [Google Scholar]
- Keers R, Aitchison KJ. Gender differences in antidepressant drug response. International Review of Psychiatry (Abingdon, England) 2010;22(5):485–500. doi: 10.3109/09540261.2010.496448. doi:10.3109/09540261.2010.496448. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Forsyth JP. Sex differences in response to an observational fear conditioning procedure. Behavior Therapy. 2007;38(4):340–349. doi: 10.1016/j.beth.2006.10.007. doi:10.1016/j.beth.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Wang PS. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiologia E Psichiatria Sociale. 2009;18(1):23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. doi:10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Jin R, Ruscio AM, Shear K, Walters EE. The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2006;63(4):415–424. doi: 10.1001/archpsyc.63.4.415. doi:10.1001/archpsyc.63.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29(2-3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Khakpai F. The effect of opiodergic system and testosterone on anxiety behavior in gonadectomized rats. Behavioural Brain Research. 2014;263:9–15. doi: 10.1016/j.bbr.2014.01.013. doi:10.1016/j.bbr.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress. 2013;26(5):537–547. doi: 10.1002/jts.21848. doi:10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Yi JH, Choi K, Hong S, Shin KS, Kang SJ. Regional differences in acute corticosterone-induced dendritic remodeling in the rat brain and their behavioral consequences. BMC Neuroscience. 2014;15:65. doi: 10.1186/1471-2202-15-65. doi:10.1186/1471-2202-15-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Schommer N, Federenko I, Gaab J, Neumann O, Oellers M, Hellhammer DH. Short-term estradiol treatment enhances pituitary-adrenal axis and sympathetic responses to psychosocial stress in healthy young men. The Journal of Clinical Endocrinology & Metabolism. 1996;81(10):3639–3643. doi: 10.1210/jcem.81.10.8855815. doi:10.1210/jcem.81.10.8855815. [DOI] [PubMed] [Google Scholar]
- Kogler L, Gur RC, Derntl B. Sex differences in cognitive regulation of psychosocial achievement stress: Brain and behavior. Human Brain Mapping. 2014 doi: 10.1002/hbm.22683. doi:10.1002/hbm.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N, Dalla C, Papadopoulou-Daifoti Z. Sex differences in pharmacokinetics of antidepressants. Expert Opinion on Drug Metabolism & Toxicology. 2011;7(2):213–226. doi: 10.1517/17425255.2011.544250. doi:10.1517/17425255.2011.544250. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. doi:10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Labad J, Menchon JM, Alonso P, Segalas C, Jimenez S, Jaurrieta N, Vallejo J. Gender differences in obsessive-compulsive symptom dimensions. Depression and Anxiety. 2008;25(10):832–838. doi: 10.1002/da.20332. doi:10.1002/da.20332. [DOI] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learning & Memory (Cold Spring Harbor, N.Y.) 2008;15(9):657–666. doi: 10.1101/lm.1080108. doi:10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learning & Memory (Cold Spring Harbor, N.Y.) 2009;16(9):520–529. doi: 10.1101/lm.1474609. doi:10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Abbs B, Milad MR, Linnman C, Rougemount-Bücking A, Zeidan MA, Goldstein JM. Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biology of Mood & Anxiety Disorders. 2012;2(1):7. doi: 10.1186/2045-5380-2-7. doi:10.1186/2045-5380-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biology of Mood & Anxiety Disorders. 2012;2(1):3. doi: 10.1186/2045-5380-2-3. doi:10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrón-Milad K, Tsareva A, Ahmed N, Milad MR. Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behavioural Brain Research. 2013;253:217–222. doi: 10.1016/j.bbr.2013.07.024. doi:10.1016/j.bbr.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. doi:10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biological Psychiatry. 2004;56(12):964–970. doi: 10.1016/j.biopsych.2004.09.018. doi:10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Progress in Brain Research. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. doi:10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25(32):7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. doi:10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Rougemont-Bücking A, Beucke JC, Zeffiro TA, Milad MR. Unconditioned responses and functional fear networks in human classical conditioning. Behavioural Brain Research. 2011;221(1):237–245. doi: 10.1016/j.bbr.2011.02.045. doi:10.1016/j.bbr.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR. Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. The American Journal of Psychiatry. 2012;169(4):415–423. doi: 10.1176/appi.ajp.2011.10121780. doi:10.1176/appi.ajp.2011.10121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V. Sex differences in chronic stress effects on memory in rats. Stress (Amsterdam, Netherlands) 2002;5(3):205–216. doi: 10.1080/1025389021000010549. doi:10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Research. 1994;639(1):167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu X-Y, Zhu Q-B, Li J, Shi L-G, Wu J-L, Bao A-M. Sex differences in the stress response in SD rats. Behavioural Brain Research. 2015;284:231–237. doi: 10.1016/j.bbr.2015.02.009. doi:10.1016/j.bbr.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Machado-de-Sousa JP, Osório F. de L., Jackowski AP, Bressan RA, Chagas MHN, Torro-Alves N, Hallak JEC. Increased amygdalar and hippocampal volumes in young adults with social anxiety. PloS One. 2014;9(2):e88523. doi: 10.1371/journal.pone.0088523. doi:10.1371/journal.pone.0088523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Shors TJ. The stressed female brain: neuronal activity in the prelimbic but not infralimbic region of the medial prefrontal cortex suppresses learning after acute stress. Frontiers in Neural Circuits. 2013;7:198. doi: 10.3389/fncir.2013.00198. doi:10.3389/fncir.2013.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Waddell J, Shors TJ. The prefrontal cortex communicates with the amygdala to impair learning after acute stress in females but not in males. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(48):16188–16196. doi: 10.1523/JNEUROSCI.2265-10.2010. doi:10.1523/JNEUROSCI.2265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Research. 1994;661(1-2):25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews. Neuroscience. 2013;14(6):417–428. doi: 10.1038/nrn3492. doi:10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M-F, Camprodon JA, Dougherty DD, Milad MR. Device-based brain stimulation to augment fear extinction: implications for PTSD treatment and beyond. Depression and Anxiety. 2014;31(4):269–278. doi: 10.1002/da.22252. doi:10.1002/da.22252. [DOI] [PubMed] [Google Scholar]
- Maroun M. Medial prefrontal cortex: multiple roles in fear and extinction. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2013;19(4):370–383. doi: 10.1177/1073858412464527. doi:10.1177/1073858412464527. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(11):4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, Liu D, Schrader LA. Role of gonadal hormones in anxiety and fear memory formation and inhibition in male mice. Physiology & Behavior. 2012;105(5):1168–1174. doi: 10.1016/j.physbeh.2011.12.016. doi:10.1016/j.physbeh.2011.12.016. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism: Clinical and Experimental. 2005;54(5 Suppl 1):20–23. doi: 10.1016/j.metabol.2005.01.008. doi:10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220(5170):911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends in Neurosciences. 2002;25(9):456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Frontiers in Neuroendocrinology. 2014;35(1):42–57. doi: 10.1016/j.yfrne.2013.09.001. doi:10.1016/j.yfrne.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research. 2011;45(8):1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. doi:10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Foa EB. Dissemination and implementation of prolonged exposure therapy for posttraumatic stress disorder. Journal of Anxiety Disorders. 2013;27(8):788–792. doi: 10.1016/j.janxdis.2013.03.004. doi:10.1016/j.janxdis.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, Fresco DM. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behaviour Research and Therapy. 2005;43(10):1281–1310. doi: 10.1016/j.brat.2004.08.008. doi:10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35(1):33–46. doi: 10.1016/j.psyneuen.2009.07.009. doi:10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Neuronal correlates of extinction learning are modulated by sex hormones. Social Cognitive and Affective Neuroscience. 2012;7(7):819–830. doi: 10.1093/scan/nsr063. doi:10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT, Schweckendiek J, Klucken T, Vaitl D, Stark R. Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology. 2013;38(11):2529–2541. doi: 10.1016/j.psyneuen.2013.05.015. doi:10.1016/j.psyneuen.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, Wilhelm S. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70(6):608–618. doi: 10.1001/jamapsychiatry.2013.914. quiz 554. doi:10.1001/jamapsychiatry.2013.914. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience. 2006;120(6):1196–1203. doi: 10.1037/0735-7044.120.5.1196. doi:10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164(3):887–895. doi: 10.1016/j.neuroscience.2009.09.011. doi:10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. doi:10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. doi:10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. doi:10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168(3):652–658. doi: 10.1016/j.neuroscience.2010.04.030. doi:10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. doi:10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proceedings of the National Academy of Sciences. 2008;105(14):5573–5578. doi: 10.1073/pnas.0705615105. doi:10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller AT, Bäckström T, Nyberg S, Söndergaard HP, Helström L. Women with PTSD have a changed sensitivity to GABA-A receptor active substances. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3776-y. doi:10.1007/s00213-014-3776-y. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Hormones and Behavior. 2001;40(4):472–482. doi: 10.1006/hbeh.2001.1716. doi:10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Postlearning stress differentially affects memory for emotional gist and detail in naturally cycling women and women on hormonal contraceptives. Behavioral Neuroscience. 2014;128(4):482–493. doi: 10.1037/a0036687. doi:10.1037/a0036687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Ertman N, Lakhani YS, Cahill L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiology of Learning and Memory. 2011;96(2):378–384. doi: 10.1016/j.nlm.2011.06.013. doi:10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biological Psychology. 2013;92(2):257–266. doi: 10.1016/j.biopsycho.2012.10.007. doi:10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillni YI, Toufexis DJ, Rohan KJ. Anxiety sensitivity, the menstrual cycle, and panic disorder: a putative neuroendocrine and psychological interaction. Clinical Psychology Review. 2011;31(7):1183–1191. doi: 10.1016/j.cpr.2011.07.006. doi:10.1016/j.cpr.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100(4):569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychological Bulletin. 1994;115(3):424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear Extinction in Traumatized Civilians with Posttraumatic Stress Disorder: Relation to Symptom Severity. Biological Psychiatry. 2011;69(6):556–563. doi: 10.1016/j.biopsych.2010.09.013. doi:10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RMC, Vijayakumar S, Ahmed NAK, Verga PW, Orr SP, Milad MR. Extinction of conditioned fear is better learned and recalled in the morning than in the evening. Journal of Psychiatric Research. 2013;47(11):1776–1784. doi: 10.1016/j.jpsychires.2013.07.027. doi:10.1016/j.jpsychires.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33(3):360–367. doi: 10.1016/j.psyneuen.2007.12.003. doi:10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin M, Vandeleur CL, Castelao E, Rothen S, Glaus J, Vollenweider P, Preisig M. Determinants of the development of post-traumatic stress disorder, in the general population. Social Psychiatry and Psychiatric Epidemiology. 2014;49(3):447–457. doi: 10.1007/s00127-013-0762-3. doi:10.1007/s00127-013-0762-3. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. doi:10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(14):5567–5572. doi: 10.1073/pnas.0801853105. doi:10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. The British Journal of Psychiatry: The Journal of Mental Science. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Pigott TA. Anxiety disorders in women. The Psychiatric Clinics of North America. 2003;26(3):621–672. vi–vii. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Pinna G, Rasmusson AM. Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder. Frontiers in Cellular Neuroscience. 2014;8:256. doi: 10.3389/fncel.2014.00256. doi:10.3389/fncel.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Liberzon I. Biological studies of post-traumatic stress disorder. Nature Reviews. Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. doi:10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. The American Journal of Psychiatry. 2003;160(1):105–111. doi: 10.1176/appi.ajp.160.1.105. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, Stern E. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(44):16060–16065. doi: 10.1073/pnas.0502818102. doi:10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Lehmann J, Feldon J. Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacology, Biochemistry, and Behavior. 1999;64(4):753–759. doi: 10.1016/s0091-3057(99)00147-1. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(25):8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. doi:10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(16):6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biological Psychiatry. 2006;60(7):704–713. doi: 10.1016/j.biopsych.2006.03.026. doi:10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Reed V, Wittchen HU. DSM-IV panic attacks and panic disorder in a community sample of adolescents and young adults: how specific are panic attacks? Journal of Psychiatric Research. 1998;32(6):335–345. doi: 10.1016/s0022-3956(98)00014-4. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470(7335):492–497. doi: 10.1038/nature09856. doi:10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey CD, Lipps J, Shansky RM. Dopamine D1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal-amygdala circuits. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2014;39(5):1282–1289. doi: 10.1038/npp.2013.338. doi:10.1038/npp.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AM, Barbosa FF, Godinho MR, Fernandes VS, Munguba H, Melo TG, Silva RH. Sex differences in aversive memory in rats: possible role of extinction and reactive emotional factors. Brain and Cognition. 2010;74(2):145–151. doi: 10.1016/j.bandc.2010.07.012. doi:10.1016/j.bandc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Rivas-Arancibia S, Vazquez-Pereyra F. Hormonal modulation of extinction responses induced by sexual steroid hormones in rats. Life Sciences. 1994;54(21):PL363–367. doi: 10.1016/0024-3205(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: A systematic review. The Journal of Clinical Psychiatry. 2006;67(8):1285–1298. doi: 10.4088/jcp.v67n0818. [DOI] [PubMed] [Google Scholar]
- Seedat S, Stein DJ, Carey PD. Post-traumatic stress disorder in women: epidemiological and treatment issues. CNS Drugs. 2005;19(5):411–427. doi: 10.2165/00023210-200519050-00004. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cerebral Cortex (New York, N.Y.: 1991) 2010;20(11):2560–2567. doi: 10.1093/cercor/bhq003. doi:10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Lipps J. Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00123. doi:10.3389/fnhum.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: Effects of circuit, hormones and rest. Brain Research. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. doi:10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. doi:10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR. Increased incidence of diagnosed depressive illness in hypogonadal older men. Archives of General Psychiatry. 2004;61(2):162–167. doi: 10.1001/archpsyc.61.2.162. doi:10.1001/archpsyc.61.2.162. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. Journal of Affective Disorders. 2003;74(1):85. doi: 10.1016/s0165-0327(02)00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9(3):419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139(12):5267–5270. doi: 10.1210/endo.139.12.6525. doi:10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- Shvil E, Sullivan GM, Schafer S, Markowitz JC, Campeas M, Wager TD, Neria Y. Sex differences in extinction recall in posttraumatic stress disorder: a pilot fMRI study. Neurobiology of Learning and Memory. 2014;113:101–108. doi: 10.1016/j.nlm.2014.02.003. doi:10.1016/j.nlm.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. doi:10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, Eaves L. The influence of genetic factors and life stress on depression among adolescent girls. Archives of General Psychiatry. 1999;56(3):225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Sonnenberg CM, Beekman AT, Deeg DJ, van Tilburg W. Sex differences in late-life depression. Acta Psychiatrica Scandinavica. 2000;101(4):286–292. [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DEA, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learning & Memory (Cold Spring Harbor, N.Y.) 2004;11(5):525–535. doi: 10.1101/lm.79504. doi:10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76(4):804–812. doi: 10.1016/j.neuron.2012.09.028. doi:10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I. Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biological Psychiatry. 2013;73(11):1045–1053. doi: 10.1016/j.biopsych.2012.12.008. doi:10.1016/j.biopsych.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Welsh RC, Marx CE, Liberzon I. The neurosteroids allopregnanolone and dehydroepiandrosterone modulate resting-state amygdala connectivity. Human Brain Mapping. 2014;35(7):3249–3261. doi: 10.1002/hbm.22399. doi:10.1002/hbm.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Vaitl D. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. NeuroImage. 2006;32(3):1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. doi:10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiology & Behavior. 2009;97(2):239–249. doi: 10.1016/j.physbeh.2009.02.036. doi:10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- Ter Horst JP, Carobrez AP, van der Mark MH, de Kloet ER, Oitzl MS. Sex differences in fear memory and extinction of mice with forebrain-specific disruption of the mineralocorticoid receptor. The European Journal of Neuroscience. 2012;36(8):3096–3102. doi: 10.1111/j.1460-9568.2012.08237.x. doi:10.1111/j.1460-9568.2012.08237.x. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychological Bulletin. 2006;132(6):959–992. doi: 10.1037/0033-2909.132.6.959. doi:10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Davis C, Hammond A, Davis M. Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(45):10280–10287. doi: 10.1523/JNEUROSCI.1386-04.2004. doi:10.1523/JNEUROSCI.1386-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis D, Rivarola MA, Lara H, Viau V. Stress and the reproductive axis. Journal of Neuroendocrinology. 2014;26(9):573–586. doi: 10.1111/jne.12179. doi:10.1111/jne.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Veen DC, van Zelst WH, Schoevers RA, Comijs HC, Voshaar RCO. Comorbid anxiety disorders in late-life depression: results of a cohort study. International Psychogeriatrics / IPA. 2014:1–9. doi: 10.1017/S1041610214002312. doi:10.1017/S1041610214002312. [DOI] [PubMed] [Google Scholar]
- Van Veen JF, Jonker BW, van Vliet IM, Zitman FG. The effects of female reproductive hormones in generalized social anxiety disorder. International Journal of Psychiatry in Medicine. 2009;39(3):283–295. doi: 10.2190/PM.39.3.e. [DOI] [PubMed] [Google Scholar]
- Veldhuis HD, Van Koppen C, Van Ittersum M, De Kloet ER. Specificity of the adrenal steroid receptor system in rat hippocampus. Endocrinology. 1982;110(6):2044–2051. doi: 10.1210/endo-110-6-2044. doi:10.1210/endo-110-6-2044. [DOI] [PubMed] [Google Scholar]
- Vesga-López O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Archives of General Psychiatry. 2008;65(7):805–815. doi: 10.1001/archpsyc.65.7.805. doi:10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, Swerdloff RS. Testosterone replacement therapy improves mood in hypogonadal men--a clinical research center study. The Journal of Clinical Endocrinology and Metabolism. 1996;81(10):3578–3583. doi: 10.1210/jcem.81.10.8855804. doi:10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992;588(2):341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiology of Learning and Memory. 2014;116:145–154. doi: 10.1016/j.nlm.2014.10.001. doi:10.1016/j.nlm.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Johnson DC, Bhatt AP, Spencer RL. Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience. 2010;168(3):744–756. doi: 10.1016/j.neuroscience.2010.04.006. doi:10.1016/j.neuroscience.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of Neurobiology. 2001;49(3):245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(7):4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan DL, Chambers KC. Estradiol accelerates extinction of a conditioned taste aversion in female and male rats. Hormones and Behavior. 1999;36(1):1–16. doi: 10.1006/hbeh.1999.1520. doi:10.1006/hbeh.1999.1520. [DOI] [PubMed] [Google Scholar]
- Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. Journal of Psychiatric Practice. 2009;15(4):289–305. doi: 10.1097/01.pra.0000358315.88931.fc. doi:10.1097/01.pra.0000358315.88931.fc. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry. 2011;70(10):920–927. doi: 10.1016/j.biopsych.2011.05.016. doi:10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorawski M, Blanding NQ, Kuhn CM, LaBar KS. Effects of stress and sex on acquisition and consolidation of human fear conditioning. Learning & Memory (Cold Spring Harbor, N.Y.) 2006;13(4):441–450. doi: 10.1101/lm.189106. doi:10.1101/lm.189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorawski M, Cook CA, Kuhn CM, LaBar KS. Sex, stress, and fear: individual differences in conditioned learning. Cognitive, Affective & Behavioral Neuroscience. 2005;5(2):191–201. doi: 10.3758/cabn.5.2.191. [DOI] [PubMed] [Google Scholar]