Abstract
Elevated whole blood serotonin, or hyperserotonemia, was the first biomarker identified in autism spectrum disorder (ASD) and is present in more than 25% of affected children. The serotonin system is a logical candidate for involvement in ASD due to its pleiotropic role across multiple brain systems both dynamically and across development. Tantalizing clues connect this peripheral biomarker with changes in brain and behavior in ASD, but the contribution of the serotonin system to ASD pathophysiology remains incompletely understood. Studies of whole blood serotonin levels in ASD and in a large founder population indicate greater heritability than for the disorder itself and suggest an association with recurrence risk. Emerging data from both neuroimaging and postmortem samples also indicate changes in the brain serotonin system in ASD. Genetic linkage and association studies of both whole blood serotonin levels and of ASD risk point to the chromosomal region containing the serotonin transporter (SERT) gene in males but not in females. In ASD families with evidence of linkage to this region, multiple rare SERT amino acid variants lead to a convergent increase in serotonin uptake in cell models. A knock-in mouse model of one of these variants, SERT Gly56Ala, recapitulates the hyperserotonemia biomarker and shows increased brain serotonin clearance, increased serotonin receptor sensitivity, and altered social, communication, and repetitive behaviors. Data from other rodent models also suggest an important role for the serotonin system in social behavior, in cognitive flexibility, and in sensory development. Recent work indicates that reciprocal interactions between serotonin and other systems, such as oxytocin, may be particularly important for social behavior. Collectively, these data point to the serotonin system as a prime candidate for treatment development in a subgroup of children defined by a robust, heritable biomarker.
Keywords: Genetic, monoamine, reuptake, platelet, neurodevelopment, multisensory
Findings from multiple domains of research have implicated the serotonin system in autism spectrum disorder (ASD). Initial findings more than fifty years ago identified elevated whole blood serotonin levels, termed hyperserotonemia, in a subset of children with autism. Since then, work on the serotonin system in ASD has extended intermittently as new tools have become available, from pharmacology to genetics to neuroimaging. Emerging findings, driven largely by advances in mouse models, point to the importance of serotonin for social function, repetitive behavior, and sensory development. With the increasing recognition of heterogeneity in ASD, biological markers will play an important role in identifying subsets of children who are more likely to share common risk or benefit from the same treatments. The combination of a robust biomarker with a rich understanding of serotonin neurobiology suggests that the serotonin system is particularly ripe for treatment development in a subset of children with ASD.
Brief overview of the serotonin system
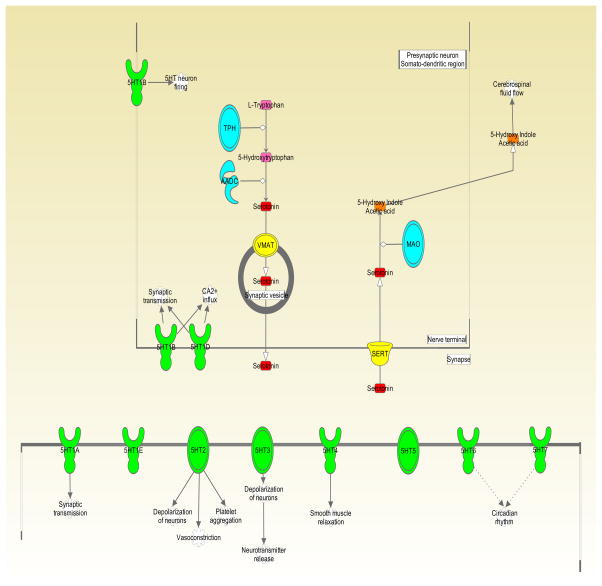
For millions of years, serotonin (5-hydroxytryptamine, 5-HT) has existed as a signaling molecule across phylogeny (Hay-Schmidt, 2000). It is produced from the essential amino acid, tryptophan, via a two-step synthetic pathway. In the first step, tryptophan is converted to 5-hydroxytryptophan (5-HTP) by the rate-limiting enzyme in 5-HT synthesis, tryptophan hydroxylase (Figure 1). There are two isoforms of tryptophan hydroxylase, TPH1 and TPH2, which are primarily responsible for 5-HT synthesis in the periphery and central nervous system (CNS), respectively (Lovenberg et al., 1967, Walther et al., 2003). In the final step, the intermediate product, 5-HTP, is converted to 5-HT by aromatic acid decarboxylase (AADC). Degradation of 5-HT primarily occurs by the mitochondrial bound protein monoamine oxidase A (MAOA), leading to the production of the metabolite, 5-hydroxyindoleacetic acid (5-HIAA). Importantly, serotonin also serves as an intermediate substrate for melatonin synthesis.
Figure 1. Overview of the Serotonin System.
This cartoon depicts the serotonin synthesis, release, reuptake, and degradation, as well as the canonical roles of a subset of serotonin receptors. TPH = tryptophan hydroxylase. AADC = aromatic acid decarboxylase. VMAT = vesicular monoamine transporter. SERT = serotonin transporter. MAO = monoamine oxidase. 5HT1-7 = serotonin receptor subgroups (oval shape) and individual receptors (goblet shape). Figure created using Ingenuity Pathway Analysis (Qiagen, Valencia, CA).
Though more recognized for its role as a neurotransmitter, the vast majority of 5-HT produced in the body is located in the periphery. Specifically, enterochromaffin cells that line the lumen of the gastrointestinal tract are the primary source of peripheral 5-HT, which is then taken up by platelets as they pass through enteric circulation (Anderson et al., 1987b). Interestingly, peripheral production of 5-HT expands during pregnancy, with the pancreas contributing to maternal 5-HT levels and the placenta producing 5-HT that contributes to fetal forebrain 5-HT levels (Kim et al., 2010, Bonnin et al., 2011), suggesting an additional developmental role for peripheral 5-HT.
Although gut-derived 5-HT influences numerous aspects of peripheral physiology, it is unable to cross the mature blood brain barrier and interact with neural tissue (Hardebo and Owman, 1980). Consequently, 5-HT found in the brain is produced by TPH2-expressing serotonergic neurons in the midbrain and hindbrain. Serotonergic neurons are organized into nine discrete clusters (B1–B9), collectively known as the raphe nuclei (Dahlstroem and Fuxe, 1964). While the more caudal raphe nuclei (B1–B5) project to the peripheral nervous system (PNS), the rostral groups (B6–B9), the dorsal and median raphe nuclei, primarily send their projections to forebrain structures (Conrad et al., 1974, O’Hearn and Molliver, 1984). Despite their relatively small number, serotonergic neurons innervate a broad collection of brain regions (Jacobs and Azmitia, 1992), allowing 5-HT to influence neural circuitry underlying a myriad of behaviors.
Reflective of its seemingly ubiquitous presence in the brain and periphery, 5-HT exerts its effects through 14 genetically distinct receptor subtypes (Hannon and Hoyer, 2008, Millan et al., 2008). Moreover, mRNA editing and alternative splicing adds another layer of complexity to an already diverse signaling system (Burns et al., 1997, Kishore and Stamm, 2006). While a thorough description of 5-HT receptor function in the brain and periphery is beyond the scope of this review, it should be clearly noted that the unique temporal and spatial patterns of 5-HT receptor expression implicate 5-HT as a key developmental molecule (Lidow and Rakic, 1992, Bonnin et al., 2006).
Arguably the most crucial aspect of 5-HT homeostasis is the clearance of extracellular 5-HT. In addition to terminating 5-HT receptor signaling, reuptake of 5-HT back into the cell helps recycle the monoamine for future use (Blakely and Edwards, 2012). The serotonin transporter (SERT, 5-HTT) is an integral plasma membrane protein that actively transports 5-HT in a Na+/Cl−-coupled dependent and antidepressant-sensitive manner (Blakely et al., 1991). In addition to being found on presynaptic terminals at serotonergic synapses in the brain, SERT is expressed at numerous sites in the periphery, including in the platelet (Qian et al., 1995). Since the discovery of SERT, there has been considerable interest in understanding its role in psychiatric disorders. Initially, this interest was driven by the efficacy of selective serotonin reuptake inhibitors (SSRIs), which bind to SERT and block 5-HT uptake, in conditions such as depression, anxiety, and obsessive-compulsive disorder (Vaswani et al., 2003). Genetic studies have now implicated SERT gene variation in moderating risk of depression and other neuropsychiatric symptoms following environmental stressors, particularly during childhood (Caspi et al., 2002, Caspi et al., 2003). As highlighted in other reviews, genetic and pharmacological studies have pointed to the critical role of SERT and the serotonin system during neurodevelopment (Suri et al., 2014, Kepser and Homberg, 2015).
Serotonin and neurodevelopment
Serotonin has now been linked to developmental processes such as cell proliferation, migration and differentiation, but the most definitive evidence of a developmental role for 5-HT first emerged from studies of the rodent somatosensory system. Barrel field architecture within rodent primary somatosensory cortex is the cortical representation of peripheral snout whiskers (Welker, 1971). Both 5-HT immunostaining and 3[H]-Citalopram binding identified a transient serotonergic innervation of the barrel cortex and other primary sensory areas that peaked during the first postnatal week of life and disappeared by postnatal day 21 (P21) in rodents (Fujimiya et al., 1986, D’Amato et al., 1987). The 5-HT in barrel cortex is actually contained in glutamatergic thalamocortical axons that lack synthetic capacity but take up extracellular 5-HT via SERT (Lebrand et al., 1996). It remains unclear, however, how 5-HT captured by TCAs plays a functional role during neurodevelopment.
Several groups have manipulated 5-HT levels in the developing brain to elucidate 5-HT-mediated mechanisms of sensory map formation. Decreased or ablated 5-HT during development causes, at most, subtle changes in barrel cortex formation, including delayed sensory map maturation (Bennett-Clarke et al., 1994, Bennett-Clarke et al., 1995, Persico et al., 2000, van Kleef et al., 2012). In contrast, manipulations that increase 5-HT levels during development show a 5-HT1B-dependent disruption of barrel field architecture (Cases et al., 1996, Salichon et al., 2001). Further, thalamocortical 5-HT1B signaling also modulates responsiveness to axon guidance cues (Bonnin et al., 2007). Beyond structural disruption, indirect measures of somatosensory function in various serotonin transporter mouse models indicate altered processing of whisker stimulation (Esaki et al., 2005, Dawson et al., 2009). In parallel, other groups have found that serotonin also impacts visual and auditory cortex development and function (Salichon et al., 2001, Simpson et al., 2011).
Emerging evidence also points to a broader role for 5-HT extending beyond sensory brain regions. Ansorge and colleagues demonstrated that exposure to a serotonin reuptake inhibitor early in postnatal life results in increased anxiety-like behavior in adult mice (Ansorge et al., 2004), with evidence that this effect is mediated by the medial prefrontal cortex (Rebello et al., 2014). Genetic ablation of SERT also alters other aspects of brain development, including overall brain growth (Page et al., 2009) and interneuron migration into the cortex (Riccio et al., 2009) (Figure 2). The importance of SERT specifically and 5-HT generally in neurodevelopment converges with accumulating evidence pointing to abnormalities in the peripheral and central serotonin system in autism spectrum disorder.
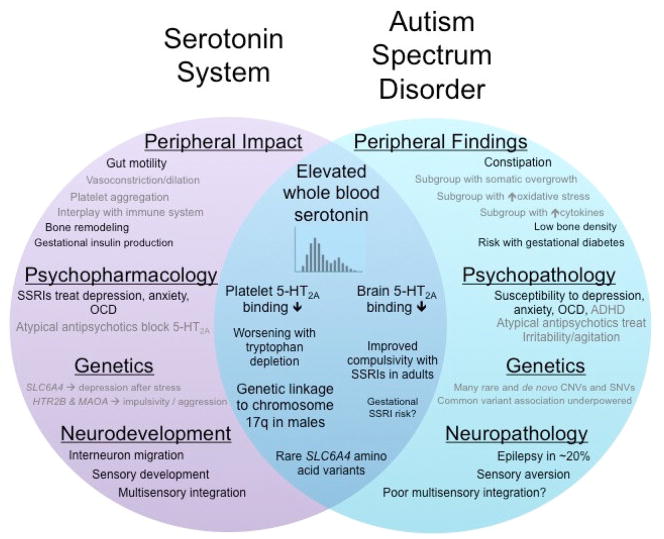
Figure 2. Venn Diagram of the Serotonin System and Autism Spectrum Disorder.
Key roles or research findings within the serotonin system are shown in the purple circle on the left. Research or clinical findings in autism spectrum disorder are depicted in the teal circle on the right. The overlapping blue region demonstrates findings directly related to serotonin in ASD. Some findings in ASD, such as constipation, overlap with known roles of the serotonin system, such as gut motility – even though they have not been directly connected – and these are shown in black type in parallel positions within the corresponding circles. Other findings, such as somatic overgrowth or regulation of vasoconstriction, so no such overlap, and these are shown in gray type.
Hyperserotonemia: the first biomarker in autism spectrum disorder
The identification of hyperserotonemia as a biomarker in autism spectrum disorder (ASD) preceded the broad acceptance of 5-HT as a neurotransmitter (Folk and Long, 1988). In 1961, Schain and Freedman first reported that six children in a cohort of 23 individuals with a diagnosis of “infantile autism” had dramatically increased blood 5-HT levels (Schain and Freedman, 1961). The 5-HT contained in whole blood is now understood to be almost completely contained in platelets (Anderson et al., 1987a). In contrast with platelet stores of 5-HT, only 1% of blood 5-HT is contained in plasma, and it is unclear if plasma 5-HT levels are changed in autism (Cook et al., 1988a, Spivak et al., 2004).
The description of hyperserotonemia occurred in an era when autism was rarely diagnosed and was attributed by many to poor parenting. Only sixteen years earlier, Leo Kanner had published his initial case series of children with abnormal social behavior and repetitive patterns of behavior (Kanner, 1943). The diagnostic criteria and terms for autism have evolved substantially over the years, with the current definition of autism as a spectrum disorder (American Psychiatric Association, 2013) reflecting the substantial heterogeneity and unclear boundaries of these symptoms. Even while the diagnosis has climbed from very rare to more than 1% of school-aged children (Developmental Disabilities Monitoring Network, 2014), rates of hyperserotonemia, typically defined as whole blood 5-HT levels above the 95th percentile in the control population, have remained stable in ASD. A meta-analysis indicated that this biomarker is present in more than 25% of the ASD population (Gabriele et al., 2014). Of note, probands with ASD from multiplex families (more than one affected child) have been found to have higher whole blood 5-HT levels than probands from simplex families (Piven et al., 1991). Interestingly, elevated whole blood serotonin has been reported in children with obsessive-compulsive disorder (OCD), which shares some symptoms with ASD such as repetitive behaviors, who have a family history of OCD, but not in those without a family history (Hanna et al., 1991).
Initial debate focused on whether hyperserotonemia was unique to autism. While there were a few initial reports of elevated serotonin levels in intellectual disability, these occurred when ASD was rarely diagnosed in the severely affected or non-verbal population (Partington et al., 1973, Hanley et al., 1977a). Subsequent work has found a weak inverse correlation between whole blood 5-HT and measures of intelligence but has not identified significantly increased rates of hyperserotonemia in intellectual disability (Cook et al., 1988b, Cook et al., 1990, Mulder et al., 2004, Weiss et al., 2004b, Gabriele et al., 2014). One study in an ethnically more homogeneous population suggested a bimodal distribution of whole blood 5-HT levels in ASD, with one group similar to the normal distribution of controls and the second mode higher than the others (Mulder et al., 2004). Despite its strong and specific association with ASD, no prospective study has yet assessed whether hyperserotonemia may predict ASD risk in infants, including baby siblings of children with ASD.
Several groups have attempted to find clinical correlates of hyperserotonemia in ASD but with mixed results for symptoms such as stereotypic and self-injurious behavior and with some inconsistency across studies (McBride et al., 1998, Mulder et al., 2004, Kolevzon et al., 2010, Sacco et al., 2010). The relationship between hyperserotonemia and other ASD symptoms or frequently co-occurring disorders has been underexplored, including systems that are known to relate to 5-HT function, such as sensory function and gastrointestinal symptoms.
Potential mechanisms of hyperserotonemia
The potential mechanisms underlying platelet 5-HT levels have been explored both in ASD families and in the general population. Essentially, elevated 5-HT levels in platelets could be due to one of four mechanisms: increased 5-HT production by enterochromaffin cells in the intestine, increased uptake of 5-HT into the platelet, decreased metabolic breakdown of 5-HT, or altered platelet release (Cook et al., 1993a). Most studies have focused on the platelet, rather than on the source of 5-HT in the gut. We recently identified a small positive correlation between higher whole blood 5-HT levels and more gastrointestinal symptoms in children with ASD (Marler et al., In Press), although it isn’t possible to assess the nature of this relationship. Decreased blood tryptophan levels have been described in a few but not all studies in ASD, which could be consistent with altered 5-HT synthesis but could also reflect dietary or other metabolic changes (Anderson et al., 1987c, D’Eufemia et al., 1995, Croonenberghs et al., 2000, Boccuto et al., 2013). To date, no studies have directly evaluated the relationship between 5-HT levels in the platelet and gut 5-HT production or content.
Most platelet studies in ASD have focused on the serotonin transporter. Studies with various SERT ligands have yielded little evidence of changes in specific binding, either in children with ASD or in family members (Anderson et al., 1984, Cook et al., 1993b, Anderson et al., 2002). Two studies have found a positive correlation between the maximal velocity (Vmax) of platelet 5-HT uptake and whole blood 5-HT levels in either participants with ASD or their family members (Cook et al., 1993b, Anderson et al., 2002). Neither study found a significant difference in 5-HT uptake between hyperserotonemic and non-hyperserotonemic participants; although they had less power for this analysis than for the correlation.
A few studies have also evaluated other elements of the platelet 5-HT system, including serotonin receptor binding. In separate studies, decreased platelet binding of the 5-HT receptor agonist LSD (3H- or 125I-labeled) has been identified in participants with ASD and first-degree relatives of hyperserotonemic probands (McBride et al., 1989, Cook et al., 1993b); although one study using a different ligand found no difference (Perry et al., 1991). The primary metabolite of serotonin, 5-HIAA, is also increased in the urine in a few ASD studies, with little evidence that there is altered metabolism of 5-HT (Hanley et al., 1977b, Minderaa et al., 1994, Mulder et al., 2010). Interestingly, one study reported that N-acetylserotonin (NAS), which is a precursor in melatonin synthesis, is also increased in ASD, but neither NAS nor melatonin show a correlation with 5-HT levels despite a robust negative correlation between NAS and melatonin levels (Pagan et al., 2014).
In addition to platelet physiological and biochemical studies, genetic approaches have also been applied to understand the control of whole blood 5-HT levels. Evidence for heritability of whole blood 5-HT levels initially emerged from correlations between whole blood serotonin levels in ASD probands and their parents and siblings (Kuperman et al., 1985, Abramson et al., 1989, Cook et al., 1990, Leventhal et al., 1990, Leboyer et al., 1999). A large study of whole blood 5-HT levels in a Hutterite founder population confirmed that whole blood serotonin levels are strongly heritable in an unaffected population, with both additive and dominance components that sum up to a broad heritability of 0.99 (Abney et al., 2001), substantially higher than estimates of heritability for ASD itself. In a small twin study, the maximum rate of 5-HT uptake was also found to be highly heritable (Meltzer and Arora, 1988).
Based upon its high heritability, linkage and association techniques were applied to map 5-HT levels as a quantitative trait in the Hutterite population, identifying significant association at a functional polymorphism in the integrin β3 subunit gene (ITGB3) as well as suggestive association at the Vitamin D receptor gene (Weiss et al., 2004a). Recently, vitamin D was shown to regulate 5-HT synthesis (Patrick and Ames, 2014), in further support of the relationship between vitamin D and 5-HT. Follow-up analyses on the quantitative trait loci revealed that ITGB3, as well as the serotonin transporter gene (SLC6A4), were primarily associated with whole blood 5-HT levels in males (Weiss et al., 2005). Carneiro and colleagues subsequently demonstrated physical and functional interaction between the corresponding proteins in controlling platelet 5-HT uptake as well as aggregation (Carneiro et al., 2008). Remarkably, three studies have reported gene-gene interaction between ITGB3 and SLC6A4 in association with autism (Weiss et al., 2006, Coutinho et al., 2007, Mei et al., 2007), supporting the idea that whole blood 5-HT may serve as an intermediate phenotype for mapping autism susceptibility genes. Candidate gene association studies in ASD have also identified an association between SLC6A4 polymorphisms and whole blood 5-HT levels or SERT function (Coutinho et al., 2004, Coutinho et al., 2007, Cross et al., 2008).
Complementing analyses in the human population, the Blakely laboratory reported that whole blood 5-HT levels are also heritable in recombinant inbred BxD mouse strains that comprise multiple congenic lines derived from an initial cross of C57BL/6J with DBA/2J, with a heritability estimate of 0.60 (Ye et al., 2014). Genetic mapping identified two loci with suggestive evidence of linkage with whole blood 5-HT levels in the BxD population, again with evidence of sexual dimorphism in control of this quantitative trait (Ye et al., 2014). Denser mapping of sequence variation in the Hutterite population and more statistical power in the BxD analyses would be necessary to more accurately assess the consistency of findings between human and mouse studies.
Of note, in relation to the consistency of hyperserotonemia as a biomarker, the serotonin system has been largely unrepresented in the list of high confidence ASD genes identified by de novo, gene-disrupting mutations in two or more children with ASD (De Rubeis et al., 2014, Iossifov et al., 2014). This could be taken as evidence that the serotonin system is less important than previously thought; however, the high heritability of whole blood 5-HT could suggest that individuals with hyperserotonemia are less likely to have a de novo event and more likely to fit the common, inherited variant model still favored by epidemiological data (Gaugler et al., 2014). It is also possible that hyperserotonemia is less well represented in the group of patients with intellectual disability and/or epilepsy that shows an over-representation of de novo, gene-disrupting variants (Robinson et al., 2014, Sanders et al., 2015). These ideas could be tested by assessing the contribution of de novo mutations in individuals with or without hyperserotonemia.
The central serotonin system in autism spectrum disorder
Accumulating findings indicate that the brain 5-HT system is also altered in ASD. Based upon the peripheral findings of increased platelet 5-HT, one might hypothesize that increased 5-HT uptake or storage in the presynaptic neuron would lead to decreased synaptic 5-HT. Obviously, it is not possible to get a direct assessment of synaptic 5-HT in humans. One surrogate measure is to assess brain 5-HT synthesis, which appears to follow an altered developmental pattern in autism (Chugani et al., 1999, Shoaf et al., 2000). Other studies have considered serotonin receptor or transporter binding. Paralleling platelet binding studies, two neuroimaging studies have found decreased 5-HT2 receptor binding: a SPECT study in adults with Asperger’s syndrome (Murphy et al., 2006) and a PET study in parents of children with autism (Goldberg et al., 2009). A postmortem study found decreases in both 5-HT2A and 5-HT1A binding in ASD (Oblak et al., 2013). Consistent findings showing decreased 5-HT2 receptor binding in platelet, neuroimaging, and post-mortem studies support the idea that peripheral alterations in the serotonin system may be an important marker of central abnormalities in autism.
Findings have been less consistent for the serotonin transporter. Two reports have found decreased binding to SERT in ASD: a SPECT study in children with autism (Makkonen et al., 2008) and a PET study in young adults with autism (Nakamura et al., 2010). Another report found no changes in a PET study of SERT binding in adults with Asperger’s disorder (Girgis et al., 2011). One postmortem study found decreased SERT binding in deep layers of the fusiform gyrus but no difference in superficial layers or in the posterior cingulate cortex (Oblak et al., 2013). In contrast, the Azmitia laboratory described an increase in axons showing SERT immunoreactivity in postmortem tissue from individuals with autism spectrum disorder, spanning two to twenty-nine years old (Azmitia et al., 2011). While it is tempting to view this as contradicting the neuroimaging data, the antibody recognizes a different protein domain than the binding ligand, and the Azmitia analyses were focused on immunoreactive axons rather than total SERT binding. In sum, it remains uncertain whether the serotonin transporter is altered in ASD at a group level, let alone whether any alteration is due to changes in the number or projections of serotonergic neurons or to changes in SERT expression specifically.
Genes in the serotonin system have also been studied in relation to structural or functional MRI findings in ASD. Besides hyperserotonemia, one of the most consistent biomarker findings in ASD is an altered developmental trajectory of brain growth, with increased brain size during childhood that normalizes over time (Courchesne et al., 2001). In the first ASD neuroimaging study to examine the functional SERT gene (SLC6A4) promoter polymorphism (5-HTTLPR), Wassink and colleagues found that the low SERT expressing short allele was significantly associated with increased cerebral cortex grey matter volume in children with autism (Wassink et al., 2007).
In contrast, a subsequent study examining 5-HTTLPR effects on cortical grey matter volume in adults with Asperger’s disorder reported no significant genotype associations (Raznahan et al., 2009). In addition to basic structural MRI measures, emerging literature indicates that SLC6A4 variants may impact the connectivity and function of neural circuits in ASD. The Monk laboratory reported that affected children and adolescents with high-expressing 5-HTTLPR genotypes had stronger connectivity of the default network, a broadly distributed, interconnected group of brain regions that are active during wakeful rest, with the opposite finding in typically developing children (Wiggins et al., 2012). They have also explored 5-HTTLPR genotype effects on amygdala responsiveness in ASD, based upon previous reports in healthy adults (Hariri et al., 2002). Using fMRI to examine potential SERT genotype differences in amygdala habituation to face stimuli in children and adolescents with ASD, they found that affected individuals with low SERT expressing genotypes had decreased amygdala habituation upon repeated exposure to faces (Wiggins et al., 2014).
Finally, limited pharmacology studies also point to the central 5-HT system in autism. Tryptophan depletion, which is expected to cause decreased synaptic 5-HT, leads to worsened repetitive behaviors and irritability in autism (McDougle et al., 1993, McDougle et al., 1996). Neuroimaging studies show differential effects of tryptophan depletion on brain region activity by fMRI in the ASD population in contrast to typical controls, particularly in response to presentation of emotional faces (Daly et al., 2012, Daly et al., 2014). Adult studies suggested that serotonin reuptake inhibitors relieve symptoms of irritability and rigid-compulsive behavior in autism (Gordon et al., 1993, Hollander et al., 2005, Hollander et al., 2012); however studies in children have been less supportive, perhaps because of greater adverse events in children or because of methodological difficulties (King et al., 2009, King et al., 2013). More consistent data supports the use of risperidone and aripiprazole (McCracken et al., 2002, McDougle et al., 2005), atypical antipsychotic medications with antagonism at multiple monoamine receptors, including the serotonin receptor 5-HT2A.
Some studies have also examined prenatal exposure to serotonin reuptake inhibitors (SRIs) in relation to ASD risk. It is very difficult to disentangle exposure from the indications for SRI use, such as depression, anxiety disorders, or OCD, each of which is more common in family members of children with ASD (Harrington et al., 2013). An initial small study in a California population reported association of prenatal exposure to SRIs with ASD risk; although they also described more severe psychiatric history in women taking SRIs (Croen et al., 2011). Subsequently, a large study in the Swedish registry supported this association (Rai et al., 2013), whereas a large study in the Danish population found no significant association (Hviid et al., 2013). Another smaller study found no significant effect of SRI exposure overall but with some secondary analyses suggesting risks specific to male fetuses or exposure during the first trimester (Harrington et al., 2014). Overall, it is difficult to assess whether these mixed data indicate a contribution of SRI exposure to ASD risk or whether this represents an association driven by a separate, common factor, such as maternal psychiatric history (Levitt, 2011).
Across all of these studies of the central serotonin system in relation to ASD, from neuroimaging to pharmacology, investigators have not evaluated whether there is a relationship with peripheral 5-HT measures. Since hyperserotonemia appears to define a subgroup of children, it would be very helpful to know if this subgroup drives 5-HT receptor binding differences or altered response to tryptophan depletion observed in ASD. Accounting for the peripheral biomarker could also potentially clarify ambiguous or contradictory results.
Genetic linkage and association studies of SLC6A4 in ASD
Genetic findings in ASD have largely centered on the serotonin transporter gene SLC6A4, initially based upon its position as the primary candidate gene for the hyperserotonemia biomarker. Unfortunately, very few of these studies have included measurement of whole blood 5-HT levels as a heritable biomarker in ASD. Family-based association studies involving common SLC6A4 variation were inconsistent, with the largest studies finding association with the 5-HTTLPR short allele (Devlin et al., 2005). A recent analysis indicated that mixed effects could explain some of this inconsistency, with risk associated with the long allele in mothers and the short allele in probands (Kistner-Griffin et al., 2011). The first linkage study in ASD found the strongest single-point evidence of linkage for an intron 2 polymorphism in SLC6A4 (International Molecular Genetic Study of Autism Consortium, 1998), and two subsequent linkage studies have identified significant evidence of linkage at the chromosome 17q11 region harboring SLC6A4 (Yonan et al., 2003). Two groups subsequently found that linkage signals on 17q strengthened when considering only families with affected males but no affected females (Stone et al., 2004, Sutcliffe et al., 2005).
Common polymorphisms in SLC6A4 did not appear to explain this linkage signal (McCauley et al., 2004), leading Sutcliffe and colleagues to focus on rare variants. In families with evidence of linkage near SLC6A4 (Sutcliffe et al., 2005), they identified multiple rare variants of SERT, including Gly56Ala and the novel SERT variants, Ile425Leu, Phe465Leu, and Leu550Val, located in highly conserved transporter transmembrane domains. Interestingly, the Ile425Leu variant displayed a unique segregation pattern indicative of the male-biased linkage signal, with the maternally transmitted Leu425 allele present in two affected sons as well as three unaffected daughters. Furthermore, another rare SERT mutation located at the same protein residue, Ile425Val, was previously observed in two unrelated families with a history of OCD and other psychiatric comorbidities, including ASD (Ozaki et al., 2003). The most common rare variant, Gly56Ala, was enriched in families with linkage to 17q11 but not in families without evidence of linkage, a finding corroborated by a subsequent screen of SLC6A4 in a case-control association study (Sakurai et al., 2008).
Interestingly, affected carriers of the Gly56Ala, Ile425Leu, Phe465Leu, and Leu550Val variants exhibited increased rigid-compulsive behaviors as measured by the Autism Diagnostic Interview (ADI-R). The Gly56Ala variant had a unique association with ADI-R ratings of sensory aversion in affected individuals (Sutcliffe et al., 2005). In support of these findings, we found association between high-expressing SLC6A4 genotypes and tactile hypersensitivity, the most common form of sensory aversion, in children with ASD (Schauder et al., 2015). Aberrant sensory behavior could be related to the reported role of SERT in the establishment of sensory-related circuits during neurodevelopment (Persico et al., 2001, Salichon et al., 2001, Esaki et al., 2005).
In addition to genetic and behavioral analyses, the Blakely laboratory examined the impact of the Ala56 variant on SERT function in lymphocytes taken from genotyped study participants. Strikingly, lymphocytes expressing the Ala56 variant displayed significantly increased 5-HT transport at basal conditions. 5-HT transport in Ala56 lymphocytes was also insensitive to regulation by p38 mitogen-activated protein kinase (MAPK) and protein kinase G (PKG) activators, which normally enhance SERT function (Blakely et al., 2005). Moreover, these findings were subsequently confirmed in heterologous cell systems devoid of potential genetic modifiers of SERT (Prasad et al., 2009).
The SERT Ala56 mouse as a model of ASD risk and hyperserotonemia
As the most common of the SERT amino acid variants, the Ala56 allele was the natural initial target for a genetic knock-in mouse model (Veenstra-VanderWeele et al., 2012). As long hypothesized, altered SERT function in these animals led to a significant increase in whole blood 5-HT levels in comparison to wildtype littermate controls. Paralleling findings in cell models, these animals also showed increased 5-HT clearance in the brain, as well as increased basal, p38-MAPK-dependent phosphorylation of SERT. Both 5-HT1A and 5-HT2A receptors showed hypersensitivity to agonist drugs, suggesting a homeostatic response to chronic decreases in synaptic 5-HT levels. Consistent with increased sensitivity of 5-HT1A receptors, electrophysiology studies in SERT Ala56 midbrain slides revealed greater inhibition of neuronal firing with bath application of 5-HT, in comparison to wildtype littermate controls. Surprisingly, 5-HT-sensitive neurons in SERT Ala56 animals showed diminished baseline firing in this preparation, suggesting that heightened receptor sensitivity may overcompensate for diminished synaptic 5-HT, at least in this preparation (Veenstra-VanderWeele et al., 2012).
SERT Ala56 mice also exhibited changes in social and repetitive behavior. The common three-chamber test of sociability was complicated by the inbred strain background used (129S6/S4), with some animals showing minimal exploration of the apparatus, regardless of genotype. When mice with fewer than four chamber entries were excluded from the analysis, wildtype littermate controls showed significant preference for the social chamber, whereas the mutant animals did not. Two other tests supported a change in social behavior in these animals. First, the tube test for dominance revealed that SERT Ala56 animals were much more likely to back away when confronted with an animal of the opposite genotype. Second, SERT Ala56 pups separated from their dam at P7 vocalized much less than their wildtype littermate controls. Further, SERT Ala56 mice showed a repetitive cage lid climbing/hanging behavior in their home cage, with some animals showing over a thousand separate bouts of this behavior in twenty-four hours. Finally, the mutant animals showed altered prepulse inhibition, with a significant genotype by prepulse intensity interaction effect, reflecting increased startle response at lower prepulse intensities (Veenstra-VanderWeele et al., 2012).
The challenge of assessing social and cognitive behaviors on the original 129S6/S4 inbred strain background motivated a backcross of the SERT Ala56 allele onto a pure C57BL/6J (B6) inbred strain background. Since the B6 SERT sequence differs from 129S SERT at two amino acids (Glu39Gly and Arg152Lys) (Carneiro et al., 2009), the native 129S SERT was also backcrossed in parallel with the mutant SERT backcross, to greater than 99% congenic status (Kerr et al., 2013). Unfortunately, no statistically significant differences in synaptosome 5-HT uptake, whole blood 5-HT, or 5-HT1A or 5-HT1B receptor sensitivity were observed in B6 SERT Ala56 mice, indicating a background strain dependence of the mutant serotonin transporter phenotype. Not surprisingly, most of the behavioral phenotypes were also non-significant, with the exception of the tube test for dominance, which showed consistent effects. Ultrasonic vocalizations at P7 were actually increased in the mutant animals on the B6 background, which is difficult to interpret. Overall, these findings suggest that the Ala56 variant has less impact on SERT function on the B6 background, perhaps owing to altered regulatory partners in B6 animals, which express two other SERT variants that impact function (Carneiro et al., 2009). The observed differences across strains may enable mapping of modifier loci that could be relevant in humans.
There are a number of logical next steps in the SERT Ala56 animals. One key question is whether the brain and behavioral phenotypes in these animals are developmental or dynamic. As has been found for the SERT null mouse, it is quite possible that enhanced SERT function during development leads to altered sensory and medial prefrontal cortex development (Rebello et al., 2014, Chen et al., 2015). As noted above, the somatosensory system would be of particular interest, as would multisensory integration, which is abnormal in some children with ASD (Stevenson et al., 2014). The developmental impact could extend to maternal effects of altered SERT function, as suggested by association studies (Kistner-Griffin et al., 2011) and as observed in other models of altered 5-HT synthesis or receptor function (Cote et al., 2007, Gleason et al., 2010). The original linkage and association findings implicated the SERT gene region specifically in males (Sutcliffe et al., 2005, Weiss et al., 2005), and published studies thus far have not examined which observed phenotypes extend to female animals. Potential rescue experiments could target SERT regulation in presynaptic neurons or 5-HT receptors in post-synaptic neurons but could also target the consequences of developmental alterations due to increased SERT activity (Rebello et al., 2014, Chen et al., 2015). Finally, in addition to opportunities to probe the neural mechanisms of autism-relevant behavior, these animals also offer the potential to understand the mechanisms underlying peripheral changes in the 5-HT system in ASD, including platelets but also other organ functions impacted by 5-HT including gut motility and bone density (Yadav et al., 2008, Gorrindo et al., 2012, Gershon, 2013, Neumeyer et al., 2013, Margolis et al., 2014).
Other rodent models of altered 5-HT function relevant to ASD
At this point, knockout of most of the known genes in the 5-HT system has been reported. Hundreds of papers have now been published describing the various phenotypes of the SERT knockout mouse (Bengel et al., 1998), including increased anxiety-like behavior, decreased aggression, and disruption of brain architecture in various regions, including the sensory cortex, paralleling the MAOA knockout described above, and the amygdala (Murphy and Lesch, 2008). Despite seeing decreased platelet 5-HT levels, the opposite of what is observed in the hyperserotonemia of autism, these mice also show decreased sociability in the three-chamber test, potentially reflecting the confound of anxiety-like behavior (Moy et al., 2008). Interestingly, when bred with mice haploinsufficient for Pten, a putative autism susceptibility gene, SERT heterozygous knockout mice show an exacerbation of macrocephaly and decreased sociability (Page et al., 2009). These findings may be consistent with the idea that SERT activity may be constrained within a certain range for normal function and behavior, with elevated or diminished SERT function leading to vulnerability to autism-like behavior.
A number of other ASD-relevant phenotypes have been reported in mice lacking critical proteins in the 5-HT system. For example, mice lacking tryptophan hydroxylase 2, responsible for central 5-HT synthesis, show decreased ultrasonic vocalizations, decreased sniffing of social odors, apparent deficits in social memory, and cognitive inflexibility (Kane et al., 2012, Del’Guidice et al., 2014, Mosienko et al., 2015). Mice lacking MAOA, responsible for breaking down 5-HT, show decreased social approach, decreased ultrasonic vocalizations, and impaired reversal learning (Bortolato et al., 2013). Mice lacking 5-HT1B show increased aggression, with some suggestion that 5-HT1A has opposite effects (Saudou et al., 1994, Olivier et al., 1995). Interestingly, mice lacking 5-HT1A also show elevated platelet 5-HT levels that emerge in the second week of life (Janusonis et al., 2006). Female mice lacking the 5-HT3A receptor show decreased reciprocal social interaction, and both male and female animals show decreased social transmission of food preference (Smit-Rigter et al., 2010).
Beyond the enzymes, transporters, and receptors that are typically considered as part of the 5-HT system, a number of transcription factors and regulatory partners have emerged. As noted above, the integrin β3 gene was associated with whole blood 5-HT levels in the human population. Mice lacking integrin β3, which impacts SERT function both in platelets and in the brain (Carneiro et al., 2008, Whyte et al., 2014), appear to show a deficit in social memory, and exhibit repetitive grooming behavior (Carter et al., 2011). Using translational profiling of 5-HT neurons, Dougherty and colleagues identified CELF6 as a differentially expressed gene that was disrupted in one proband with ASD (Dougherty et al., 2013). In addition to decreased whole brain 5-HT levels, mice lacking Celf6 show decreased ultrasonic vocalizations and a perseverative-like phenotype on a hole board assay (Dougherty et al., 2013).
Mouse models of ASD risk with abnormal 5-HT
Curiously, despite consistent reports of hyperserotonemia in autism, as well as data suggesting that whole blood serotonin levels are strongly heritable, few labs have assessed this biomarker in genetic mouse models of ASD. Mouse models of 15q11-q13 duplication and Smith-Lemli-Opitz syndromes show alterations in the central 5-HT system (Waage-Baudet et al., 2003, Nakatani et al., 2009, Farook et al., 2012, Korade et al., 2013). The BTBR inbred mouse strain, which shows many autism-relevant behavioral phenotypes, shows decreased baseline SERT binding throughout the brain and increased 5-HT1A activity in the hippocampus (Gould et al., 2011, Gould et al., 2014). Further, short-term tryptophan supplementation as well as acute treatment with the serotonin reuptake inhibitor fluoxetine or the 5-HT1A partial agonist buspirone result in increased sociability in BTBR mice (Chadman, 2011, Gould et al., 2011, Gould et al., 2014, Zhang et al., 2015).
Interestingly, abnormalities in the 5-HT system have been reported in mice lacking Gtf2ird1, a key gene in the region deleted in Williams-Beuren syndrome (WBS), which includes intellectual disability and a hypersociable personality in many affected children. In addition to decreased anxiety-like behavior and some evidence of increased social interaction, animals lacking Gtf2ird1 show increased levels of 5-HIAA in amygdala and frontal cortex and enhanced sensitivity to 5-HT1A stimulation in the prefrontal cortex (Young et al., 2008, Proulx et al., 2010). Two reports have described hyperserotonemia in a few patients with WBS and co-occurring ASD, suggesting that the 5-HT system could contribute to or modulate the WBS phenotype (Reiss et al., 1985, Tordjman et al., 2013).
A few reports indicate that the 5-HT system is perturbed in environmental models of ASD. The most robust environmental risk factor to be modeled in rodents is prenatal valproic acid exposure, which has been assessed primarily in rats. Reports of altered brain and gut 5-HT system function in these animals have been intriguing, with most studies reporting decreased 5-HT levels or innervation in various brain regions (Miyazaki et al., 2005, Tsujino et al., 2007, Winter et al., 2008, Dufour-Rainfray et al., 2010, de Theije et al., 2014).
Maternal immune activation or viral exposure has also become a popular rodent model. Of note, while exposure to infection in utero clearly contributes to risk of schizophrenia, it remains unclear when and how – and even whether – maternal immune activation contributes directly to ASD risk (Atladottir et al., 2012, Brown, 2012, Langridge et al., 2013, Zerbo et al., 2013a, Zerbo et al., 2013b). In a mouse model of maternal immune activation, Hsiao and colleagues described elevated serum 5-HT (Hsiao et al., 2013), although it is somewhat difficult to interpret serum 5-HT levels in relation to ASD, where platelet-free plasma levels appear unchanged (Anderson et al., 1987a, Anderson, 2007, Anderson et al., 2012). Other papers reported that maternal immune activation or virus exposure resulted in abnormal development of 5-HT neurons or decreased brain 5-HT levels (Miller et al., 2013b, Ohkawara et al., 2015). More epidemiological research is needed to better understand the right way to interpret these models, but substantial evidence suggests bidirectional interplay between serotonin and the immune system, including evidence that inflammation triggers increased SERT function (Baganz and Blakely, 2013).
Interplay between the serotonin and oxytocin systems
The serotonin system intersects with many other signaling systems, both peripherally and in the brain, with oxytocin (OT) as one of the most obviously relevant to the social deficits observed in ASD. Biomarker and genetic data for OT and its receptor have been promising but inconsistent in ASD and in relation to various measures of human social behavior (Modahl et al., 1998, Jacob et al., 2007, Hammock et al., 2012, Miller et al., 2013a, Parker et al., 2014, Skuse et al., 2014). Intranasal OT has gained momentum as a potential treatment for ASD but with similarly promising but inconsistent results thus far (Gordon et al., 2013, Aoki et al., 2014, Preti et al., 2014, Aoki et al., 2015, Auyeung et al., 2015, Guastella et al., 2015). Evidence points to multiple intersections of the 5-HT and OT systems, and interactions between these systems influence behaviors such as sociability, aggression, and anxiety that are relevant to ASD (Dolen, 2015). Interactions between genes or polymorphisms in the 5-HT and OT systems have been associated with ASD or related behaviors (Montag et al., 2011, Thanseem et al., 2012, Nyffeler et al., 2014), while animal models have been particularly useful for elucidating the mechanisms of 5-HT and OT interactions in specific brain regions and how they impact behaviors.
Examining the effects of “entactogens,” drugs that elicit social behavior, in animal models can reveal particular neural mechanisms crucial for social behavior. In particular, studies of MDMA (3,4-methylenedioxymethamphetamine), which causes reverse transport of 5-HT via SERT, have implicated interactions between the 5-HT and OT systems. Male rats given MDMA demonstrated increased plasma OT levels, which were blocked by systemic pre-administration of a 5-HT1A receptor antagonist (Thompson et al., 2007). In a related experiment, MDMA also increased time the rats spent in social interaction, and intracerebroventricular pre-administration of an OT receptor antagonist blocked the effect (Thompson et al., 2007). Similarly, male mice given MDMA showed increased sociability and locomotor activity, behaviors which were blocked by a 5-HT1A or OT receptor antagonist (Kuteykin-Teplyakov and Maldonado, 2014). Furthermore, MDMA-induced changes in c-Fos expression were reduced in hypothalamic OT-positive cells following pre-administration of a 5-HT1A receptor antagonist in male rats (Hunt et al., 2011). Collectively, these findings indicate that the 5-HT system impacts social behavior via OT release.
Other work has investigated the reciprocal impact of the OT system on 5-HT release. Using a transgenic reporter, Yoshida and colleagues (2009) localized OT receptor gene expression in 5-HT cells in the raphe. Based upon this observation, they demonstrated that administration of OT directly into the median raphe led to increased 5-HT release. Further, central administration of OT led to increased time spent in the center of the open field test, an indication of anxiolysis, which was prevented by a 5-HT2A/2C receptor antagonist (Yoshida et al., 2009). In humans, intranasal OT results in increased 5-HT1A receptor availability as measured by PET in the dorsal raphe and a number of target regions, suggesting decreased, rather than increased, 5-HT release (Mottolese et al., 2014). Further work will be necessary to better understand the impact of OT on 5-HT release across species, especially given the limitations of indirect measurement in human studies.
Dölen and colleagues (2013) demonstrated that input from both the 5-HT and OT systems to the nucleus accumbens are essential for establishing social conditioned place preference (Panksepp and Lahvis, 2007). They found that presynaptic OT receptors on dorsal raphe projections within the accumbens induce release of 5-HT and, further, that the activation of the post-synaptic 5-HT1B receptors is necessary to establish social preference (Dölen et al., 2013). Another study used conditional knockout mice to demonstrate that male mice lacking the OT receptor on 5-HT neurons showed lower frequency of aggression than wild type mice; whereas female mice did not exhibit genotype effects on behavior (Pagani et al., 2015). This sex difference is important, but few other studies in mice have included female animals in their analyses.
Predictably, the interactions between 5-HT and OT also influence development of these systems and behaviors. When OT was administered to prairie vole pups on postnatal day 1, it led to greater density of 5-HT axons by weaning, in a brain region-specific manner (Eaton et al., 2012). Conversely, when a non-selective 5-HT agonist was given to rats or voles prenatally, beginning from gestational day 12, fewer OT cells were evident in the paraventricular nucleus of the hypothalamus in adulthood, and time spent in typical affiliative social behaviors was lower than in saline-treated animals (McNamara et al., 2008, Martin et al., 2012). A similar treatment given to male and female rats pre- and postnatally revealed that the effects on the number of OT or 5-HT receptor positive cells were age- and sex-dependent, and effects on juvenile play behavior were only seen in females, not males (Madden and Zup, 2014). One area for further research includes development of multisensory integration, with evidence that both 5-HT and OT play critical roles in cross-modal plasticity within the sensory cortex (Jitsuki et al., 2011, Zheng et al., 2014).
Interestingly, one study reported a correlation between whole blood 5-HT and plasma OT levels in children and adolescents with ASD (Hammock et al., 2012). Serotonin levels correlated negatively with age and with OT levels. A parallel experiment in the same report demonstrated that 5-HT levels were higher in juvenile mice lacking the OT receptor, compared with their wildtype littermates (Hammock et al., 2012). This suggests that the OT system also affects the peripheral 5-HT system. Importantly, it does not rule out a reciprocal role for 5-HT affecting the OT system, as has been shown in the brain. This work suggests that, in addition to further elucidating effects of changes in specific neural systems, animal models can be useful for understanding potential biomarkers for ASD (Hammock et al., 2012).
Next steps to understand the contribution of the serotonin system to ASD
Despite more than fifty years of research, the hyperserotonemia biomarker has yet to fully reveal its relationship with ASD risk and pathophysiology. The pleiotropic effects of 5-HT, both peripherally and in the brain, present a challenge to developing a simple model of its contribution. One central question is whether its contribution is developmental, dynamic, or both. For instance, the impact of altered 5-HT uptake or breakdown on sensory development is clearly developmental and may not relate to targeted treatments in adulthood. In contrast, short-term tryptophan depletion or SRI administration in adult rats and humans alters reversal learning and other cognitive measures relevant to the repetitive or compulsive behavior observed in ASD (Clarke et al., 2004, Cools et al., 2008, Worbe et al., 2015).
In addition to the temporal dynamics of its influence as both a morphogen and a neurotransmitter, we need a better understanding of the regional specificity of serotonin’s effects on ASD-relevant behavior. A variety of techniques, from the cre-mediated excision (Chen et al., 2015) to optogenetic, pharmacogenetic, or viral silencing (Dölen et al., 2013), allow either temporary or permanent manipulation of the 5-HT system with varying levels of temporal and spatial control. These approaches can also better examine the impact of the peripheral 5-HT system on brain development and behavior. For instance, the placenta is the key source of 5-HT for early forebrain development, using tryptophan from the maternal circulation as a substrate for 5-HT synthesis (Bonnin et al., 2011). By walking back and forth between rodent models, human epidemiology, and cognitive neuroscience using short-term manipulation of the 5-HT system, we can further dissect the developmental and spatial impacts of the serotonin system on the social, communication, repetitive, and sensory behaviors underlying ASD.
More complete understanding of the temporal and spatial influences of altered 5-HT function on ASD-relevant behavior may yield new opportunities for rescue experiments, but our existing knowledge already points to potential targets. On the presynaptic side, the influence of p38-MAPK and PKG signaling in the SERT Ala56 animals suggests that it may be possible to tune down SERT function without blocking it entirely (Veenstra-VanderWeele et al., 2012). Drugs targeting serotonin receptors, particularly 5-HT1A and 5-HT2A, have shown promise for increasing social interaction or decreasing cognitive rigidity (File et al., 1996, Edwards et al., 2006, Boulougouris and Robbins, 2010, Gould et al., 2011, Amodeo et al., 2014).
Beyond considering what targets are logical within the serotonin system, it is critical that we start to measure biomarkers that define ASD subgroups when conducting studies of novel treatments. For instance, the intersection between the serotonin and oxytocin systems suggests that studies of intranasal oxytocin should likely measure whole blood 5-HT levels in participants. Given the results of Dölen and colleagues (2013), it is possible that participants with hyperserotonemia could have decreased synaptic 5-HT and therefore not benefit from oxytocin administration. Measuring serotonin levels adds little burden to treatment studies that are collecting blood to monitor drug metabolism and safety. Remarkably, whole blood 5-HT levels have not even been reported for trials of serotonin reuptake inhibitors or for studies of tryptophan depletion, each of which was motivated in large part by the hyperserotonemia biomarker. By subgrouping participants using a robust, heritable biomarker, future studies can identify individuals who may be more likely to benefit from a treatment, or more likely to experience adverse events. Potentially, narrowing in on a more specific population could rescue clinical trials that would otherwise be viewed as negative.
Highlights.
Elevated whole blood serotonin is a well-replicated biomarker found in 25% of children with autism spectrum disorder.
In addition to a well-defined role in adults, serotonin modulates neurodevelopment, including sensory development.
The serotonin transporter gene is associated with whole blood serotonin levels and with autism risk but only in males.
A serotonin transporter knock-in mouse shows elevated blood serotonin levels and altered social and repetitive behavior.
The serotonin system is a prime candidate for treatment development in children with this heritable biomarker.
Acknowledgments
Critical insights into the serotonin system and its relationship with autism spectrum disorder were provided by Ed Cook, Randy Blakely, George Anderson, Suma Jacob, Ana Carneiro, and Carissa Cascio. Support for this work was provided by NIH MH094604, New York State Psychiatric Institute, the Mortimer D. Sackler Chair Fund, and the Columbia University Department of Psychiatry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Biomedical conflicts of interest: Dr. Veenstra-VanderWeele has consulted with Roche Pharmaceuticals, Novartis, and SynapDx and has had research funding from Roche Pharmaceuticals, Novartis, SynapDx, Seaside Therapeutics, Forest, and Sunovion. He receives an honorarium for editorial work from Springer and John Wiley and Sons.
Contributor Information
Christopher L. Muller, Email: christopher.l.muller@vanderbilt.edu.
Allison M.J. Anacker, Email: aanacke@nyspi.columbia.edu.
Jeremy Veenstra-VanderWeele, Email: veenstr@nyspi.columbia.edu.
References
- Abney M, McPeek MS, Ober C. Broad and narrow heritabilities of quantitative traits in a founder population. Am J Hum Genet. 2001;68:1302–1307. doi: 10.1086/320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson RK, Wright HH, Carpenter R, Brennan W, Lumpuy O, Cole E, Young SR. Elevated blood serotonin in autistic probands and their first-degree relatives. Journal of Autism and Developmental Disorders. 1989;19:397–407. doi: 10.1007/BF02212938. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. DSM-5. [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Risperidone and the 5-HT2A receptor antagonist M100907 improve probabilistic reversal learning in BTBR T + tf/J mice. Autism Res. 2014;7:555–567. doi: 10.1002/aur.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GM. Measurement of plasma serotonin in autism. Pediatr Neurol. 2007;36:138. doi: 10.1016/j.pediatrneurol.2006.11.007. author reply 138–139. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Feibel FC, Cohen DJ. Determination of serotonin in whole blood, platelet-rich plasma, platelet-poor plasma and plasma ultrafiltrate. Life Sci. 1987a;40:1063–1070. doi: 10.1016/0024-3205(87)90568-6. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Feibel FC, Cohen DJ. Determination of serotonin in whole blood, platelet-rich plasma, platelet-poor plasma and plasma ultrafiltrate. Life sciences. 1987b;40:1063–1070. doi: 10.1016/0024-3205(87)90568-6. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Freedman DX, Cohen DJ, Volkmar FR, Hoder EL, McPhedran P, Minderaa RB, Hansen CR, Young JG. Whole blood serotonin in autistic and normal subjects. Journal of Child Psychology, Psychiatry and Allied Disciplines. 1987c;28:885–900. doi: 10.1111/j.1469-7610.1987.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Gutknecht L, Cohen DJ, Brailly-Tabard S, Cohen JH, Ferrari P, Roubertoux PL, Tordjman S. Serotonin transporter promoter variants in autism: functional effects and relationship to platelet hyperserotonemia. Molecular psychiatry. 2002;7:831–836. doi: 10.1038/sj.mp.4001099. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Hertzig ME, McBride PA. Brief report: Platelet-poor plasma serotonin in autism. J Autism Dev Disord. 2012;42:1510–1514. doi: 10.1007/s10803-011-1371-1. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Minderaa RB, van Benthem PP, Volkmar FR, Cohen DJ. Platelet imipramine binding in autistic subjects. Psychiatry research. 1984;11:133–141. doi: 10.1016/0165-1781(84)90097-0. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Watanabe T, Abe O, Kuwabara H, Yahata N, Takano Y, Iwashiro N, Natsubori T, Takao H, Kawakubo Y, Kasai K, Yamasue H. Oxytocin’s neurochemical effects in the medial prefrontal cortex underlie recovery of task-specific brain activity in autism: a randomized controlled trial. Mol Psychiatry. 2015;20:447–453. doi: 10.1038/mp.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Yahata N, Watanabe T, Takano Y, Kawakubo Y, Kuwabara H, Iwashiro N, Natsubori T, Inoue H, Suga M, Takao H, Sasaki H, Gonoi W, Kunimatsu A, Kasai K, Yamasue H. Oxytocin improves behavioural and neural deficits in inferring others’ social emotions in autism. Brain. 2014;137:3073–3086. doi: 10.1093/brain/awu231. [DOI] [PubMed] [Google Scholar]
- Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130:e1447–1454. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Lombardo MV, Heinrichs M, Chakrabarti B, Sule A, Deakin JB, Bethlehem RA, Dickens L, Mooney N, Sipple JA, Thiemann P, Baron-Cohen S. Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Translational psychiatry. 2015;5:e507. doi: 10.1038/tp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Singh JS, Whitaker-Azmitia PM. Increased serotonin axons (immunoreactive to 5-HT transporter) in postmortem brains from young autism donors. Neuropharmacology. 2011;60:1347–1354. doi: 10.1016/j.neuropharm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Baganz NL, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem Neurosci. 2013;4:48–63. doi: 10.1021/cn300186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4- methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter- deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Lane RD, Rhoades RW. Fenfluramine depletes serotonin from the developing cortex and alters thalamocortical organization. Brain Res. 1995;702:255–260. doi: 10.1016/0006-8993(95)00867-5. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Lane RD, Rhoades RW. Effect of serotonin depletion on vibrissa-related patterns of thalamic afferents in the rat’s somatosensory cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14:7594–7607. doi: 10.1523/JNEUROSCI.14-12-07594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Defelice LJ, Galli A. Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology. 2005;20:225–231. doi: 10.1152/physiol.00013.2005. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Edwards RH. Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccuto L, Chen CF, Pittman AR, Skinner CD, McCartney HJ, Jones K, Bochner BR, Stevenson RE, Schwartz CE. Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol Autism. 2013;4:16. doi: 10.1186/2040-2392-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Peng W, Hewlett W, Levitt P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience. 2006;141:781–794. doi: 10.1016/j.neuroscience.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nature neuroscience. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Godar SC, Alzghoul L, Zhang J, Darling RD, Simpson KL, Bini V, Chen K, Wellman CL, Lin RC, Shih JC. Monoamine oxidase A and A/B knockout mice display autistic-like features. Int J Neuropsychopharmacol. 2013;16:869–888. doi: 10.1017/S1461145712000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72:1272–1276. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Carneiro AM, Airey DC, Thompson B, Zhu CB, Lu L, Chesler EJ, Erikson KM, Blakely RD. Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2047–2052. doi: 10.1073/pnas.0809449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118:1544–1552. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter MD, Shah CR, Muller CL, Crawley JN, Carneiro AM, Veenstra-VanderWeele J. Absence of preference for social novelty and increased grooming in integrin beta3 knockout mice: initial studies and future directions. Autism Res. 2011;4:57–67. doi: 10.1002/aur.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97:586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye R, Gargus JJ, Blakely RD, Dobrenis K, Sze JY. Disruption of Transient Serotonin Accumulation by Non-Serotonin-Producing Neurons Impairs Cortical Map Development. Cell Rep. 2015 doi: 10.1016/j.celrep.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, Chugani HT. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Annals of neurology. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Conrad LC, Leonard CM, Pfaff DW. Connections of the median and dorsal raphe nuclei in the rat: an autoradiographic and degeneration study. J Comp Neurol. 1974;156:179–205. doi: 10.1002/cne.901560205. [DOI] [PubMed] [Google Scholar]
- Cook E, Arora R, Anderson G, Berry-Kravis E, Yan S-Y, Yeoh H, Sklena P, Charak D, Leventhal B. Platelet serotonin studies in hyperserotonemic relatives of children with autistic disorder. Life sciences. 1993a;52:2005–2015. doi: 10.1016/0024-3205(93)90685-v. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Arora RC, Anderson GM, Berry-Kravis EM, Yan SY, Yeoh HC, Sklena PJ, Charak DA, Leventhal BL. Platelet serotonin studies in hyperserotonemic relatives of children with autistic disorder. Life sciences. 1993b;52:2005–2015. doi: 10.1016/0024-3205(93)90685-v. [DOI] [PubMed] [Google Scholar]
- Cook EH, Leventhal BL, Freedman DX. Free serotonin in plasma: Autistic children and their first-degree relatives. Biological Psychiatry. 1988a;24:488–491. doi: 10.1016/0006-3223(88)90192-8. [DOI] [PubMed] [Google Scholar]
- Cook EH, Leventhal BL, Freedman DX. Serotonin and measured intelligence. Journal of Autism and Developmental Disorders. 1988b;18:553–559. doi: 10.1007/BF02211873. [DOI] [PubMed] [Google Scholar]
- Cook EH, Leventhal BL, Heller W, Metz J, Wainwright M, Freedman DX. Autistic children and their first-degree relatives: Relationships between serotonin and norepinephrine levels and intelligence. Journal of Neuropsychiatry and Clinical Neurosciences. 1990;2:268–274. doi: 10.1176/jnp.2.3.268. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A. 2007;104:329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Coutinho AM, Oliveira G, Morgadinho T, Fesel C, Macedo TR, Bento C, Marques C, Ataide A, Miguel T, Borges L, Vicente AM. Variants of the serotonin transporter gene (SLC6A4) significantly contribute to hyperserotonemia in autism. Mol Psychiatry. 2004;9:264–271. doi: 10.1038/sj.mp.4001409. [DOI] [PubMed] [Google Scholar]
- Coutinho AM, Sousa I, Martins M, Correia C, Morgadinho T, Bento C, Marques C, Ataide A, Miguel TS, Moore JH, Oliveira G, Vicente AM. Evidence for epistasis between SLC6A4 and ITGB3 in autism etiology and in the determination of platelet serotonin levels. Human genetics. 2007;121:243–256. doi: 10.1007/s00439-006-0301-3. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J, Delmeire L, Verkerk R, Lin AH, Meskal A, Neels H, Van der Planken M, Scharpe S, Deboutte D, Pison G, Maes M. Peripheral markers of serotonergic and noradrenergic function in post-pubertal, caucasian males with autistic disorder. Neuropsychopharmacology. 2000;22:275–283. doi: 10.1016/S0893-133X(99)00131-1. [DOI] [PubMed] [Google Scholar]
- Cross S, Kim SJ, Weiss LA, Delahanty RJ, Sutcliffe JS, Leventhal BL, Cook EH, Jr, Veenstra-Vanderweele J. Molecular genetics of the platelet serotonin system in first-degree relatives of patients with autism. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:353–360. doi: 10.1038/sj.npp.1301406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Eufemia P, Finocchiaro R, Celli M, Viozzi L, Monteleone D, Giardini O. Low serum tryptophan to large neutral amino acids ratio in idiopathic infantile autism. Biomedicine and Pharmacotherapy. 1995;49:288–292. doi: 10.1016/0753-3322(96)82645-X. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Blue ME, Largent BL, Lynch DR, Ledbetter DJ, Molliver ME, Snyder SH. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci U S A. 1987;84:4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstroem A, Fuxe K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta physiologica Scandinavica Supplementum SUPPL. 1964;232:231–255. [PubMed] [Google Scholar]
- Daly E, Ecker C, Hallahan B, Deeley Q, Craig M, Murphy C, Johnston P, Spain D, Gillan N, Gudbrandsen M, Brammer M, Giampietro V, Lamar M, Page L, Toal F, Schmitz N, Cleare A, Robertson D, Rubia K, Murphy DG. Response inhibition and serotonin in autism: a functional MRI study using acute tryptophan depletion. Brain. 2014;137:2600–2610. doi: 10.1093/brain/awu178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EM, Deeley Q, Ecker C, Craig M, Hallahan B, Murphy C, Johnston P, Spain D, Gillan N, Brammer M, Giampietro V, Lamar M, Page L, Toal F, Cleare A, Surguladze S, Murphy DG. Serotonin and the neural processing of facial emotions in adults with autism: an fMRI study using acute tryptophan depletion. Arch Gen Psychiatry. 2012;69:1003–1013. doi: 10.1001/archgenpsychiatry.2012.513. [DOI] [PubMed] [Google Scholar]
- Dawson N, Ferrington L, Olverman HJ, Harmar AJ, Kelly PA. Sex influences the effect of a lifelong increase in serotonin transporter function on cerebral metabolism. Journal of neuroscience research. 2009;87:2375–2385. doi: 10.1002/jnr.22062. [DOI] [PubMed] [Google Scholar]
- De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, Singh T, Klei L, Kosmicki J, Shih-Chen F, Aleksic B, Biscaldi M, Bolton PF, Brownfeld JM, Cai J, Campbell NG, Carracedo A, Chahrour MH, Chiocchetti AG, Coon H, Crawford EL, Curran SR, Dawson G, Duketis E, Fernandez BA, Gallagher L, Geller E, Guter SJ, Hill RS, Ionita-Laza J, Jimenz Gonzalez P, Kilpinen H, Klauck SM, Kolevzon A, Lee I, Lei I, Lei J, Lehtimaki T, Lin CF, Ma’ayan A, Marshall CR, McInnes AL, Neale B, Owen MJ, Ozaki N, Parellada M, Parr JR, Purcell S, Puura K, Rajagopalan D, Rehnstrom K, Reichenberg A, Sabo A, Sachse M, Sanders SJ, Schafer C, Schulte-Ruther M, Skuse D, Stevens C, Szatmari P, Tammimies K, Valladares O, Voran A, Li-San W, Weiss LA, Willsey AJ, Yu TW, Yuen RK, Cook EH, Freitag CM, Gill M, Hultman CM, Lehner T, Palotie A, Schellenberg GD, Sklar P, State MW, Sutcliffe JS, Walsh CA, Scherer SW, Zwick ME, Barett JC, Cutler DJ, Roeder K, Devlin B, Daly MJ, Buxbaum JD Study DDD, Homozygosity Mapping Collaborative for A, Consortium UK. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, Garssen J, Kraneveld AD, Oozeer R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun. 2014;37:197–206. doi: 10.1016/j.bbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Del’Guidice T, Lemay F, Lemasson M, Levasseur-Moreau J, Manta S, Etievant A, Escoffier G, Dore FY, Roman FS, Beaulieu JM. Stimulation of 5-HT2C receptors improves cognitive deficits induced by human tryptophan hydroxylase 2 loss of function mutation. Neuropsychopharmacology. 2014;39:1125–1134. doi: 10.1038/npp.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Developmental Disabilities Monitoring Network. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- Devlin B, Cook EH, Jr, Coon H, Dawson G, Grigorenko EL, McMahon W, Minshew N, Pauls D, Smith M, Spence MA, Rodier PM, Stodgell C, Schellenberg GD, Network CG. Autism and the serotonin transporter: the long and short of it. Mol Psychiatry. 2005;10:1110–1116. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- Dolen G. Autism: Oxytocin, serotonin, and social reward. Soc Neurosci. 2015;10:450–465. doi: 10.1080/17470919.2015.1087875. [DOI] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty JD, Maloney SE, Wozniak DF, Rieger MA, Sonnenblick L, Coppola G, Mahieu NG, Zhang J, Cai J, Patti GJ, Abrahams BS, Geschwind DH, Heintz N. The disruption of Celf6, a gene identified by translational profiling of serotonergic neurons, results in autism-related behaviors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:2732–2753. doi: 10.1523/JNEUROSCI.4762-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour-Rainfray D, Vourc’h P, Le Guisquet AM, Garreau L, Ternant D, Bodard S, Jaumain E, Gulhan Z, Belzung C, Andres CR, Chalon S, Guilloteau D. Behavior and serotonergic disorders in rats exposed prenatally to valproate: a model for autism. Neuroscience letters. 2010;470:55–59. doi: 10.1016/j.neulet.2009.12.054. [DOI] [PubMed] [Google Scholar]
- Eaton JL, Roache L, Nguyen KN, Cushing BS, Troyer E, Papademetriou E, Raghanti MA. Organizational effects of oxytocin on serotonin innervation. Developmental psychobiology. 2012;54:92–97. doi: 10.1002/dev.20566. [DOI] [PubMed] [Google Scholar]
- Edwards DJ, Chugani DC, Chugani HT, Chehab J, Malian M, Aranda JV. Pharmacokinetics of buspirone in autistic children. J Clin Pharmacol. 2006;46:508–514. doi: 10.1177/0091270006286903. [DOI] [PubMed] [Google Scholar]
- Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci U S A. 2005;102:5582–5587. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farook MF, DeCuypere M, Hyland K, Takumi T, LeDoux MS, Reiter LT. Altered serotonin, dopamine and norepinepherine levels in 15q duplication and Angelman syndrome mouse models. PLoS One. 2012;7:e43030. doi: 10.1371/journal.pone.0043030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci. 1996;16:4810–4815. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk GE, Jr, Long JP. Serotonin as a neurotransmitter: a review. Comp Biochem Physiol C. 1988;91:251–257. doi: 10.1016/0742-8413(88)90193-4. [DOI] [PubMed] [Google Scholar]
- Fujimiya M, Kimura H, Maeda T. Postnatal development of serotonin nerve fibers in the somatosensory cortex of mice studied by immunohistochemistry. J Comp Neurol. 1986;246:191–201. doi: 10.1002/cne.902460205. [DOI] [PubMed] [Google Scholar]
- Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2014;24:919–929. doi: 10.1016/j.euroneuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, Mahajan M, Manaa D, Pawitan Y, Reichert J, Ripke S, Sandin S, Sklar P, Svantesson O, Reichenberg A, Hultman CM, Devlin B, Roeder K, Buxbaum JD. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46:881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Slifstein M, Xu X, Frankle WG, Anagnostou E, Wasserman S, Pepa L, Kolevzon A, Abi-Dargham A, Laruelle M, Hollander E. The 5-HT(2A) receptor and serotonin transporter in Asperger’s disorder: A PET study with [(1)(1)C]MDL 100907 and [(1)(1)C]DASB. Psychiatry Res. 2011;194:230–234. doi: 10.1016/j.pscychresns.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason G, Liu B, Bruening S, Zupan B, Auerbach A, Mark W, Oh JE, Gal-Toth J, Lee F, Toth M. The serotonin1A receptor gene as a genetic and prenatal maternal environmental factor in anxiety. Proc Natl Acad Sci U S A. 2010;107:7592–7597. doi: 10.1073/pnas.0914805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, Anderson GM, Zwaigenbaum L, Hall GB, Nahmias C, Thompson A, Szatmari P. Cortical serotonin type-2 receptor density in parents of children with autism spectrum disorders. J Autism Dev Disord. 2009;39:97–104. doi: 10.1007/s10803-008-0604-4. [DOI] [PubMed] [Google Scholar]
- Gordon C, State R, Nelson J, Hamburger S, Rapoport J. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Archives of General Psychiatry. 1993;50:441–447. doi: 10.1001/archpsyc.1993.01820180039004. [DOI] [PubMed] [Google Scholar]
- Gordon I, Vander Wyk BC, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, Zagoory-Sharon O, Leckman JF, Feldman R, Pelphrey KA. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110:20953–20958. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Res. 2012;5:101–108. doi: 10.1002/aur.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Burke TF, Osorio MD, Smolik CM, Zhang WQ, Onaivi ES, Gu TT, DeSilva MN, Hensler JG. Enhanced novelty-induced corticosterone spike and upregulated serotonin 5-HT1A and cannabinoid CB1 receptors in adolescent BTBR mice. Psychoneuroendocrinology. 2014;39:158–169. doi: 10.1016/j.psyneuen.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Gray KM, Rinehart NJ, Alvares GA, Tonge BJ, Hickie IB, Keating CM, Cacciotti-Saija C, Einfeld SL. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J Child Psychol Psychiatry. 2015;56:444–452. doi: 10.1111/jcpp.12305. [DOI] [PubMed] [Google Scholar]
- Hammock E, Veenstra-VanderWeele J, Yan Z, Kerr TM, Morris M, Anderson GM, Carter CS, Cook EH, Jacob S. Examining autism spectrum disorders by biomarkers: example from the oxytocin and serotonin systems. J Am Acad Child Adolesc Psychiatry. 2012;51:712–721. e711. doi: 10.1016/j.jaac.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley HG, Stahl SM, Freedman DX. Hyperserotonemia and amine metabolites in autistic and retarded children. Arch Gen Psychiatry. 1977a;34:521–531. doi: 10.1001/archpsyc.1977.01770170031002. [DOI] [PubMed] [Google Scholar]
- Hanley HG, Stahl SM, Freedman DX. Hyperserotonemia and amine metabolites in autistic and retarded children. Arch Gen Psychiatry. 1977b;34:521–531. doi: 10.1001/archpsyc.1977.01770170031002. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Yuwiler A, Cantwell DP. Whole blood serotonin in juvenile obsessive-compulsive disorder. Biological Psychiatry. 1991;29:738–744. doi: 10.1016/0006-3223(91)90193-p. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hardebo JE, Owman C. Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain interface. Annals of neurology. 1980;8:1–31. doi: 10.1002/ana.410080102. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Serotonin hypothesis of autism: implications for selective serotonin reuptake inhibitor use during pregnancy. Autism Res. 2013;6:149–168. doi: 10.1002/aur.1288. [DOI] [PubMed] [Google Scholar]
- Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics. 2014;133:e1241–1248. doi: 10.1542/peds.2013-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay-Schmidt A. The evolution of the serotonergic nervous system. Proceedings Biological sciences/The Royal Society. 2000;267:1071–1079. doi: 10.1098/rspb.2000.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Phillips A, Chaplin W, Zagursky K, Novotny S, Wasserman S, Iyengar R. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30:582–589. doi: 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]
- Hollander E, Soorya L, Chaplin W, Anagnostou E, Taylor BP, Ferretti CJ, Wasserman S, Swanson E, Settipani C. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am J Psychiatry. 2012;169:292–299. doi: 10.1176/appi.ajp.2011.10050764. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GE, McGregor IS, Cornish JL, Callaghan PD. MDMA-induced c-Fos expression in oxytocin-containing neurons is blocked by pretreatment with the 5-HT-1A receptor antagonist WAY 100635. Brain research bulletin. 2011;86:65–73. doi: 10.1016/j.brainresbull.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med. 2013;369:2406–2415. doi: 10.1056/NEJMoa1301449. [DOI] [PubMed] [Google Scholar]
- International Molecular Genetic Study of Autism Consortium. A full genome screen for autism with evidence for linkage to a region on chromosome 7q. Human Molecular Genetics. 1998;7:571–578. doi: 10.1093/hmg/7.3.571. [DOI] [PubMed] [Google Scholar]
- Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, Smith JD, Paeper B, Nickerson DA, Dea J, Dong S, Gonzalez LE, Mandell JD, Mane SM, Murtha MT, Sullivan CA, Walker MF, Waqar Z, Wei L, Willsey AJ, Yamrom B, Lee YH, Grabowska E, Dalkic E, Wang Z, Marks S, Andrews P, Leotta A, Kendall J, Hakker I, Rosenbaum J, Ma B, Rodgers L, Troge J, Narzisi G, Yoon S, Schatz MC, Ye K, McCombie WR, Shendure J, Eichler EE, State MW, Wigler M. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neuroscience letters. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiological reviews. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Janusonis S, Anderson GM, Shifrovich I, Rakic P. Ontogeny of brain and blood serotonin levels in 5-HT receptor knockout mice: potential relevance to the neurobiology of autism. J Neurochem. 2006;99:1019–1031. doi: 10.1111/j.1471-4159.2006.04150.x. [DOI] [PubMed] [Google Scholar]
- Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, Sano A, Yuzaki M, Zukin RS, Ziff EB, Kessels HW, Takahashi T. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–792. doi: 10.1016/j.neuron.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Angoa-Perez M, Briggs DI, Sykes CE, Francescutti DM, Rosenberg DR, Kuhn DM. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS One. 2012;7:e48975. doi: 10.1371/journal.pone.0048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kepser LJ, Homberg JR. The neurodevelopmental effects of serotonin: a behavioural perspective. Behav Brain Res. 2015;277:3–13. doi: 10.1016/j.bbr.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Kerr TM, Muller CL, Miah M, Jetter CS, Pfeiffer R, Shah C, Baganz N, Anderson GM, Crawley JN, Sutcliffe JS, Blakely RD, Veenstra-Vanderweele J. Genetic background modulates phenotypes of serotonin transporter Ala56 knock-in mice. Mol Autism. 2013;4:35. doi: 10.1186/2040-2392-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, Yang K, Honig G, van der Hart M, Kishimoto N, Wang J, Yagihashi S, Tecott LH, Watada H, German MS. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BH, Dukes K, Donnelly CL, Sikich L, McCracken JT, Scahill L, Hollander E, Bregman JD, Anagnostou E, Robinson F, Sullivan L, Hirtz D. Baseline factors predicting placebo response to treatment in children and adolescents with autism spectrum disorders: a multisite randomized clinical trial. JAMA Pediatr. 2013;167:1045–1052. doi: 10.1001/jamapediatrics.2013.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, Donnelly CL, Anagnostou E, Dukes K, Sullivan L, Hirtz D, Wagner A, Ritz L. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66:583–590. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore S, Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 2006;311:230–232. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- Kistner-Griffin E, Brune CW, Davis LK, Sutcliffe JS, Cox NJ, Cook EH., Jr Parent-of-origin effects of the serotonin transporter gene associated with autism. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2011;156:139–144. doi: 10.1002/ajmg.b.31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolevzon A, Newcorn JH, Kryzak L, Chaplin W, Watner D, Hollander E, Smith CJ, Cook EH, Jr, Silverman JM. Relationship between whole blood serotonin and repetitive behaviors in autism. Psychiatry research. 2010;175:274–276. doi: 10.1016/j.psychres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Folkes OM, Harrison FE. Behavioral and serotonergic response changes in the Dhcr7-HET mouse model of Smith-Lemli-Opitz syndrome. Pharmacol Biochem Behav. 2013 doi: 10.1016/j.pbb.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Beeghly JH, Burns TL, Tsai LY. Serotonin relationships of autistic probands and their first-degree relatives. Journal of the American Academy of Child Psychiatry. 1985;24:186–190. doi: 10.1016/s0002-7138(09)60446-5. [DOI] [PubMed] [Google Scholar]
- Kuteykin-Teplyakov K, Maldonado R. Looking for prosocial genes: ITRAQ analysis of proteins involved in MDMA-induced sociability in mice. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2014;24:1773–1783. doi: 10.1016/j.euroneuro.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Langridge AT, Glasson EJ, Nassar N, Jacoby P, Pennell C, Hagan R, Bourke J, Leonard H, Stanley FJ. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS One. 2013;8:e50963. doi: 10.1371/journal.pone.0050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboyer M, Philippe A, Bouvard M, Guilloud-Bataille M, Bondoux D, Tabuteau F, Feingold J, Mouren-Simeoni M, Launay J. Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives. Biological Psychiatry. 1999;45:158–163. doi: 10.1016/s0006-3223(97)00532-5. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Leventhal BL, Cook EH, Jr, Morford M, Ravitz A, Freedman DX. Relationships of whole blood serotonin and plasma norepinephrine within families. J Autism Dev Disord. 1990;20:499–511. doi: 10.1007/BF02216055. [DOI] [PubMed] [Google Scholar]
- Levitt P. Serotonin and the autisms: a red flag or a red herring? Arch Gen Psychiatry. 2011;68:1093–1094. doi: 10.1001/archgenpsychiatry.2011.98. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Rakic P. Scheduling of monoaminergic neurotransmitter receptor expression in the primate neocortex during postnatal development. Cereb Cortex. 1992;2:401–416. doi: 10.1093/cercor/2.5.401. [DOI] [PubMed] [Google Scholar]
- Lovenberg W, Jequier E, Sjoerdsma A. Tryptophan hydroxylation: measurement in pineal gland, brainstem, and carcinoid tumor. Science. 1967;155:217–219. doi: 10.1126/science.155.3759.217. [DOI] [PubMed] [Google Scholar]
- Madden AM, Zup SL. Effects of developmental hyperserotonemia on juvenile play behavior, oxytocin and serotonin receptor expression in the hypothalamus are age and sex dependent. Physiology & behavior. 2014;128:260–269. doi: 10.1016/j.physbeh.2014.01.036. [DOI] [PubMed] [Google Scholar]
- Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol. 2008;50:593–597. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- Margolis KG, Stevanovic K, Li Z, Yang QM, Oravecz T, Zambrowicz B, Jhaver KG, Diacou A, Gershon MD. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut. 2014;63:928–937. doi: 10.1136/gutjnl-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler S, Ferguson BJ, Lee EB, Peters B, Williams KC, McDonnell E, Macklin EA, Levitt P, Gillespie CH, Anderson GM, Margolis KG, Beversdorf DQ, Veenstra-VanderWeele J. Whole blood serotonin levels and gastrointestinal symptoms in autism spectrum disorder. J Autism Dev Disord. doi: 10.1007/s10803-015-2646-8. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MM, Liu Y, Wang Z. Developmental exposure to a serotonin agonist produces subsequent behavioral and neurochemical changes in the adult male prairie vole. Physiology & behavior. 2012;105:529–535. doi: 10.1016/j.physbeh.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride PA, Anderson GM, Hertzig ME, Snow ME, Thompson SM, Khait VD, Shapiro T, Cohen DJ. Effects of diagnosis, race, and puberty on platelet serotonin levels in autism and mental retardation. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:767–776. doi: 10.1097/00004583-199807000-00017. [DOI] [PubMed] [Google Scholar]
- McBride PA, Anderson GM, Hertzig ME, Sweeney JA, Kream J, Cohen DJ, Mann JJ. Serotonergic responsivity in male young adults with autistic disorder. Arch Gen Psychiatry. 1989;46:205–212. doi: 10.1001/archpsyc.1989.01810030019003. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Dowd M, Amin T, Steele A, Blakely RD, Folstein SE, Haines JL, Sutcliffe JS. Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid-compulsive subset of autism. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2004;127B:104–112. doi: 10.1002/ajmg.b.20151. [DOI] [PubMed] [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, Arnold LE, Lindsay R, Nash P, Hollway J, McDougle CJ, Posey D, Swiezy N, Kohn A, Scahill L, Martin A, Koenig K, Volkmar F, Carroll D, Lancor A, Tierney E, Ghuman J, Gonzalez NM, Grados M, Vitiello B, Ritz L, Davies M, Robinson J, McMahon D. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- McDougle C, Naylor S, Cohen D, Aghajanian G, Heninger G, Price L. Effects of tryptophan depletion in drug-free adults with autistic disorder. Archives of General Psychiatry. 1996;53:993–1000. doi: 10.1001/archpsyc.1996.01830110029004. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Naylor ST, Goodman WK, Volkmar FR, Cohen DJ, Price LH. Acute tryptophan depletion in autistic disorder: a controlled case study. Biol Psychiatry. 1993;33:547–550. doi: 10.1016/0006-3223(93)90011-2. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, Arnold LE, Posey DJ, Martin A, Ghuman JK, Shah B, Chuang SZ, Swiezy NB, Gonzalez NM, Hollway J, Koenig K, McGough JJ, Ritz L, Vitiello B. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162:1142–1148. doi: 10.1176/appi.ajp.162.6.1142. [DOI] [PubMed] [Google Scholar]
- McNamara IM, Borella AW, Bialowas LA, Whitaker-Azmitia PM. Further studies in the developmental hyperserotonemia model (DHS) of autism: social, behavioral and peptide changes. Brain Res. 2008;1189:203–214. doi: 10.1016/j.brainres.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Mei H, Cuccaro ML, Martin ER. Multifactor dimensionality reduction-phenomics: a novel method to capture genetic heterogeneity with use of phenotypic variables. Am J Hum Genet. 2007;81:1251–1261. doi: 10.1086/522307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Arora RC. Genetic control of serotonin uptake in blood platelets: a twin study. Psychiatry research. 1988;24:263–269. doi: 10.1016/0165-1781(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, Mannoury la Cour C. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends in pharmacological sciences. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Miller M, Bales KL, Taylor SL, Yoon J, Hostetler CM, Carter CS, Solomon M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: sex differences and associations with symptoms. Autism Res. 2013a;6:91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM, Zhu Y, Bucher C, McGinnis W, Ryan LK, Siegel A, Zalcman S. Gestational flu exposure induces changes in neurochemicals, affiliative hormones and brainstem inflammation, in addition to autism-like behaviors in mice. Brain Behav Immun. 2013b;33:153–163. doi: 10.1016/j.bbi.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Minderaa R, Anderson G, Volkmar F, Akkerhuis G, Cohen D. Noradrenergic and adrenergic functioning in autism. Biological Psychiatry. 1994;36:237–241. doi: 10.1016/0006-3223(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Narita N, Narita M. Maternal administration of thalidomide or valproic acid causes abnormal serotonergic neurons in the offspring: implication for pathogenesis of autism. Int J Dev Neurosci. 2005;23:287–297. doi: 10.1016/j.ijdevneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biological Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Montag C, Fiebach CJ, Kirsch P, Reuter M. Interaction of 5-HTTLPR and a variation on the oxytocin receptor gene influences negative emotionality. Biological psychiatry. 2011;69:601–603. doi: 10.1016/j.biopsych.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Mosienko V, Beis D, Alenina N, Wohr M. Reduced isolation-induced pup ultrasonic communication in mouse pups lacking brain serotonin. Mol Autism. 2015;6:13. doi: 10.1186/s13229-015-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottolese R, Redoute J, Costes N, Le Bars D, Sirigu A. Switching brain serotonin with oxytocin. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8637–8642. doi: 10.1073/pnas.1319810111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social Approach in Genetically-Engineered Mouse Lines Relevant to Autism. Genes Brain Behav. 2008 doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kemperman RF, Oosterloo-Duinkerken A, Minderaa RB, Kema IP. Urinary excretion of 5-hydroxyindoleacetic acid, serotonin and 6-sulphatoxymelatonin in normoserotonemic and hyperserotonemic autistic individuals. Neuropsychobiology. 2010;61:27–32. doi: 10.1159/000258640. [DOI] [PubMed] [Google Scholar]
- Murphy DG, Daly E, Schmitz N, Toal F, Murphy K, Curran S, Erlandsson K, Eersels J, Kerwin R, Ell P, Travis M. Cortical serotonin 5-HT2A receptor binding and social communication in adults with Asperger’s syndrome: an in vivo SPECT study. Am J Psychiatry. 2006;163:934–936. doi: 10.1176/ajp.2006.163.5.934. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, Tsuchiya KJ, Sugihara G, Iwata Y, Suzuki K, Matsuzaki H, Suda S, Sugiyama T, Takei N, Mori N. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Archives of general psychiatry. 2010;67:59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, Tomonaga S, Watanabe Y, Chung YJ, Banerjee R, Iwamoto K, Kato T, Okazawa M, Yamauchi K, Tanda K, Takao K, Miyakawa T, Bradley A, Takumi T. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeyer AM, Gates A, Ferrone C, Lee H, Misra M. Bone density in peripubertal boys with autism spectrum disorders. J Autism Dev Disord. 2013;43:1623–1629. doi: 10.1007/s10803-012-1709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyffeler J, Walitza S, Bobrowski E, Gundelfinger R, Grunblatt E. Association study in siblings and case-controls of serotonin- and oxytocin-related genes with high functioning autism. Journal of molecular psychiatry. 2014;2:1. doi: 10.1186/2049-9256-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain research bulletin. 1984;13:709–726. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Oblak A, Gibbs TT, Blatt GJ. Reduced serotonin receptor subtypes in a limbic and a neocortical region in autism. Autism Res. 2013;6:571–583. doi: 10.1002/aur.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara T, Katsuyama T, Ida-Eto M, Narita N, Narita M. Maternal viral infection during pregnancy impairs development of fetal serotonergic neurons. Brain Dev. 2015;37:88–93. doi: 10.1016/j.braindev.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van Oorschot R, Hen R. Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry. 1995;28(Suppl 2):80–90. doi: 10.1055/s-2007-979624. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Pagan C, Delorme R, Callebert J, Goubran-Botros H, Amsellem F, Drouot X, Boudebesse C, Le Dudal K, Ngo-Nguyen N, Laouamri H, Gillberg C, Leboyer M, Bourgeron T, Launay JM. The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders. Translational psychiatry. 2014;4:e479. doi: 10.1038/tp.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani JH, Williams Avram SK, Cui Z, Song J, Mezey E, Senerth JM, Baumann MH, Young WS. Raphe serotonin neuron-specific oxytocin receptor knockout reduces aggression without affecting anxiety-like behavior in male mice only. Genes, brain, and behavior. 2015;14:167–176. doi: 10.1111/gbb.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci U S A. 2009;106:1989–1994. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Garner JP, Libove RA, Hyde SA, Hornbeak KB, Carson DS, Liao CP, Phillips JM, Hallmayer JF, Hardan AY. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci U S A. 2014;111:12258–12263. doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partington MW, Tu JB, Wong CY. Blood serotonin levels in severe mental retardation. Dev Med Child Neurol. 1973;15:616–627. doi: 10.1111/j.1469-8749.1973.tb05172.x. [DOI] [PubMed] [Google Scholar]
- Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. 2014;28:2398–2413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- Perry BD, Cook EH, Jr, Leventhal BL, Wainwright MS, Freedman DX. Platelet 5-HT2 serotonin receptor binding sites in autistic children and their first-degree relatives. Biol Psychiatry. 1991;30:121–130. doi: 10.1016/0006-3223(91)90165-i. [DOI] [PubMed] [Google Scholar]
- Persico AM, Altamura C, Calia E, Puglisi-Allegra S, Ventura R, Lucchese F, Keller F. Serotonin depletion and barrel cortex development: impact of growth impairment vs. serotonin effects on thalamocortical endings. Cerebral cortex. 2000;10:181–191. doi: 10.1093/cercor/10.2.181. [DOI] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall SF, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Tsai G, Nehme E, Coyle JT, Folstein SE. Platelet serotonin, a possible marker for familial autism. Journal of Autism and Developmental Disorders. 1991;21:51–59. doi: 10.1007/BF02206997. [DOI] [PubMed] [Google Scholar]
- Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti A, Melis M, Siddi S, Vellante M, Doneddu G, Fadda R. Oxytocin and autism: a systematic review of randomized controlled trials. J Child Adolesc Psychopharmacol. 2014;24:54–68. doi: 10.1089/cap.2013.0040. [DOI] [PubMed] [Google Scholar]
- Proulx E, Young EJ, Osborne LR, Lambe EK. Enhanced prefrontal serotonin 5-HT(1A) currents in a mouse model of Williams-Beuren syndrome with low innate anxiety. J Neurodev Disord. 2010;2:99–108. doi: 10.1007/s11689-010-9044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Melikian HE, Rye DB, Levey AI, Blakely RD. Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ. 2013;346:f2059. doi: 10.1136/bmj.f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Pugliese L, Barker GJ, Daly E, Powell J, Bolton PF, Murphy DG. Serotonin transporter genotype and neuroanatomy in autism spectrum disorders. Psychiatric genetics. 2009;19:147–150. doi: 10.1097/YPG.0b013e32832a505a. [DOI] [PubMed] [Google Scholar]
- Rebello TJ, Yu Q, Goodfellow NM, Caffrey Cagliostro MK, Teissier A, Morelli E, Demireva EY, Chemiakine A, Rosoklija GB, Dwork AJ, Lambe EK, Gingrich JA, Ansorge MS. Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. J Neurosci. 2014;34:12379–12393. doi: 10.1523/JNEUROSCI.1020-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Feinstein C, Rosenbaum KN, Borengasser-Caruso MA, Goldsmith BM. Autism associated with Williams syndrome. J Pediatr. 1985;106:247–249. doi: 10.1016/s0022-3476(85)80296-1. [DOI] [PubMed] [Google Scholar]
- Riccio O, Potter G, Walzer C, Vallet P, Szabo G, Vutskits L, Kiss JZ, Dayer AG. Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol Psychiatry. 2009;14:280–290. doi: 10.1038/mp.2008.89. [DOI] [PubMed] [Google Scholar]
- Robinson EB, Samocha KE, Kosmicki JA, McGrath L, Neale BM, Perlis RH, Daly MJ. Autism spectrum disorder severity reflects the average contribution of de novo and familial influences. Proc Natl Acad Sci U S A. 2014;111:15161–15165. doi: 10.1073/pnas.1409204111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco R, Curatolo P, Manzi B, Militerni R, Bravaccio C, Frolli A, Lenti C, Saccani M, Elia M, Reichelt KL, Pascucci T, Puglisi-Allegra S, Persico AM. Principal pathogenetic components and biological endophenotypes in autism spectrum disorders. Autism Res. 2010;3:237–252. doi: 10.1002/aur.151. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Reichert J, Hoffman EJ, Cai G, Jones HB, Faham M, Buxbaum JD. A large-scale screen for coding variants in SERT/SLC6A4 in autism spectrum disorders. Autism Res. 2008;1:251–257. doi: 10.1002/aur.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, Murtha MT, Bal VH, Bishop SL, Dong S, Goldberg AP, Jinlu C, Keaney JF, 3rd, Klei L, Mandell JD, Moreno-De-Luca D, Poultney CS, Robinson EB, Smith L, Solli-Nowlan T, Su MY, Teran NA, Walker MF, Werling DM, Beaudet AL, Cantor RM, Fombonne E, Geschwind DH, Grice DE, Lord C, Lowe JK, Mane SM, Martin DM, Morrow EM, Talkowski ME, Sutcliffe JS, Walsh CA, Yu TW, Autism Sequencing C, Ledbetter DH, Martin CL, Cook EH, Buxbaum JD, Daly MJ, Devlin B, Roeder K, State MW. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 2015;87:1215–1233. doi: 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou F, Amara D, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot M-C, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- Schauder KB, Muller CL, Veenstra-VanderWeele J, Cascio CJ. Genetic Variation in Serotonin Transporter Modulates Tactile Hyperresponsiveness in ASD. Res Autism Spectr Disord. 2015;10:93–100. doi: 10.1016/j.rasd.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaf SE, Carson RE, Hommer D, Williams WA, Higley JD, Schmall B, Herscovitch P, Eckelman WC, Linnoila M. The suitability of [11C]-alpha-methyl-L-tryptophan as a tracer for serotonin synthesis: studies with dual administration of [11C] and [14C] labeled tracer. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:244–252. doi: 10.1097/00004647-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, Rodriguez-Porcel F, Paul IA, Merzenich M, Lin RC. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci U S A. 2011;108:18465–18470. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, Lehtimaki T, Binder EB, Young LJ. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc Natl Acad Sci U S A. 2014;111:1987–1992. doi: 10.1073/pnas.1302985111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit-Rigter LA, Wadman WJ, van Hooft JA. Impaired Social Behavior in 5-HT(3A) Receptor Knockout Mice. Frontiers in behavioral neuroscience. 2010;4:169. doi: 10.3389/fnbeh.2010.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak B, Golubchik P, Mozes T, Vered Y, Nechmad A, Weizman A, Strous RD. Low platelet-poor plasma levels of serotonin in adult autistic patients. Neuropsychobiology. 2004;50:157–160. doi: 10.1159/000079108. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Schneider BC, Eberly HE, Woynaroski TG, Camarata SM, Wallace MT. Multisensory temporal integration in autism spectrum disorders. J Neurosci. 2014;34:691–697. doi: 10.1523/JNEUROSCI.3615-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JL, Merriman B, Cantor RM, Yonan AL, Gilliam TC, Geschwind DH, Nelson SF. Evidence for sex-specific risk alleles in autism spectrum disorder. American journal of human genetics. 2004;75:1117–1123. doi: 10.1086/426034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri D, Teixeira C, Cagliostro MC, Mahadevia D, Ansorge MS. Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanseem I, Anitha A, Nakamura K, Suda S, Iwata K, Matsuzaki H, Ohtsubo M, Ueki T, Katayama T, Iwata Y, Suzuki K, Minoshima S, Mori N. Elevated transcription factor specificity protein 1 in autistic brains alters the expression of autism candidate genes. Biological psychiatry. 2012;71:410–418. doi: 10.1016/j.biopsych.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146:509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Cohen D, Kermarrec S, Carlier M, Touitou Y, Saugier-Veber P, Lagneaux C, Chevreuil C, Verloes A. Presence of autism, hyperserotonemia, and severe expressive language impairment in Williams-Beuren syndrome. Mol Autism. 2013;4:29. doi: 10.1186/2040-2392-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino N, Nakatani Y, Seki Y, Nakasato A, Nakamura M, Sugawara M, Arita H. Abnormality of circadian rhythm accompanied by an increase in frontal cortex serotonin in animal model of autism. Neurosci Res. 2007;57:289–295. doi: 10.1016/j.neures.2006.10.018. [DOI] [PubMed] [Google Scholar]
- van Kleef ES, Gaspar P, Bonnin A. Insights into the complex influence of 5-HT signaling on thalamocortical axonal system development. The European journal of neuroscience. 2012;35:1563–1572. doi: 10.1111/j.1460-9568.2012.8096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Progress in neuro-psychopharmacology & biological psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage-Baudet H, Lauder JM, Dehart DB, Kluckman K, Hiller S, Tint GS, Sulik KK. Abnormal serotonergic development in a mouse model for the Smith-Lemli-Opitz syndrome: implications for autism. Int J Dev Neurosci. 2003;21:451–459. doi: 10.1016/j.ijdevneu.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Hazlett HC, Epping EA, Arndt S, Dager SR, Schellenberg GD, Dawson G, Piven J. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Archives of general psychiatry. 2007;64:709–717. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Abney M, Cook EH, Jr, Ober C. Sex-specific genetic architecture of whole blood serotonin levels. American journal of human genetics. 2005;76:33–41. doi: 10.1086/426697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Ober C, Cook EH., Jr ITGB3 shows genetic and expression interaction with SLC6A4. Human genetics. 2006;120:93–100. doi: 10.1007/s00439-006-0196-z. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Veenstra-VanderWeele J, Abney M, Newman DL, Kim S-J, Dytch H, McPeek MS, Cheng S, Cook EH, Jr, Ober C. Genome-wide association study identifies ITGB3 as a QTL for whole blood serotonin. European Journal of Human Genetics. 2004a doi: 10.1038/sj.ejhg.5201239. advance online publication. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Veenstra-Vanderweele J, Newman DL, Kim SJ, Dytch H, McPeek MS, Cheng S, Ober C, Cook EH, Jr, Abney M. Genome-wide association study identifies ITGB3 as a QTL for whole blood serotonin. Eur J Hum Genet. 2004b;12:949–954. doi: 10.1038/sj.ejhg.5201239. [DOI] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971;26:259–275. [PubMed] [Google Scholar]
- Whyte A, Jessen T, Varney S, Carneiro AM. Serotonin transporter and integrin beta 3 genes interact to modulate serotonin uptake in mouse brain. Neurochemistry international. 2014;73:122–126. doi: 10.1016/j.neuint.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, Bedoyan JK, Carrasco M, Welsh RC, Martin DM, Lord C, Monk CS. The impact of serotonin transporter genotype on default network connectivity in children and adolescents with autism spectrum disorders. NeuroImage Clinical. 2012;2:17–24. doi: 10.1016/j.nicl.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Swartz JR, Martin DM, Lord C, Monk CS. Serotonin transporter genotype impacts amygdala habituation in youth with autism spectrum disorders. Social cognitive and affective neuroscience. 2014;9:832–838. doi: 10.1093/scan/nst039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C, Reutiman TJ, Folsom TD, Sohr R, Wolf RJ, Juckel G, Fatemi SH. Dopamine and serotonin levels following prenatal viral infection in mouse--implications for psychiatric disorders such as schizophrenia and autism. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2008;18:712–716. doi: 10.1016/j.euroneuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Palminteri S, Savulich G, Daw ND, Fernandez-Egea E, Robbins TW, Voon V. Valence-dependent influence of serotonin depletion on model-based choice strategy. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Carneiro AM, Airey D, Sanders-Bush E, Williams RW, Lu L, Wang J, Zhang B, Blakely RD. Evaluation of heritable determinants of blood and brain serotonin homeostasis using recombinant inbred mice. Genes, brain, and behavior. 2014;13:247–260. doi: 10.1111/gbb.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonan AL, Alarcon M, Cheng R, Magnusson PK, Spence SJ, Palmer AA, Grunn A, Juo SH, Terwilliger JD, Liu J, Cantor RM, Geschwind DH, Gilliam TC. A genomewide screen of 345 families for autism-susceptibility loci. American journal of human genetics. 2003;73:886–897. doi: 10.1086/378778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EJ, Lipina T, Tam E, Mandel A, Clapcote SJ, Bechard AR, Chambers J, Mount HT, Fletcher PJ, Roder JC, Osborne LR. Reduced fear and aggression and altered serotonin metabolism in Gtf2ird1-targeted mice. Genes Brain Behav. 2008;7:224–234. doi: 10.1111/j.1601-183X.2007.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013a;43:25–33. doi: 10.1007/s10803-012-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 2013b doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WQ, Smolik CM, Barba-Escobedo PA, Gamez M, Sanchez JJ, Javors MA, Daws LC, Gould GG. Acute dietary tryptophan manipulation differentially alters social behavior, brain serotonin and plasma corticosterone in three inbred mouse strains. Neuropharmacology. 2015;90:1–8. doi: 10.1016/j.neuropharm.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JJ, Li SJ, Zhang XD, Miao WY, Zhang D, Yao H, Yu X. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat Neurosci. 2014;17:391–399. doi: 10.1038/nn.3634. [DOI] [PubMed] [Google Scholar]