Abstract
Background
Fludarabine, cyclophosphamide and rituximab (FCR) results in durable responses in patients with previously untreated chronic lymphocytic leukemia (CLL). Previous reports suggest that in patients with relapsed CLL a dose-intensified rituximab regimen increases response rates compared to standard-dose rituximab. It is unknown whether rituximab intensification of the FCR regimen will result in improved response rates and patient outcomes in patients with previously untreated CLL.
Methods
We conducted a single-arm study to evaluate the safety and efficacy of a modified FCR regimen with multiple-dose rituximab (FCR3) in 65 patients with previously untreated CLL. Results were compared to an historical cohort treated with FCR.
Results
The overall response rate to FCR3 was 97%, with 75% of patients achieving a complete remission. Minimal residual disease negativity was achieved in 62% of patients by flow cytometry. Median time to progression (TTP) was 81 months, and median overall survival (OS) was not reached, with 58% of patients still alive at a median survivor follow-up of 9.7 years. Grade 3-4 neutropenia, grade 3-4 thrombocytopenia and major infection were observed with 45%, 5% and 1.9% of FCR3 courses, respectively. Therapy-related myelodysplastic syndrome (t-MDS) or acute myelogenous leukemia (t-AML) developed in 7 patients (11%) (P <0.01 compared to the historical FCR cohort).
Conclusions
In patients with previously untreated CLL, FCR3 resulted in similar response rates, TTP and OS compared to a historical cohort of patients treated with FCR. FCR3 was associated with an increased incidence of t-MDS/AML.
Keywords: Chronic lymphocytic leukemia, chemoimmunotherapy, rituximab, FCR, therapy-related AML, therapy-related MDS
Introduction
The development of the anti-CD20 monoclonal antibody, rituximab, has improved the outcomes of patients with chronic lymphocytic leukemia (CLL). Combination chemotherapy with fludarabine and cyclophosphamide (FC) results in complete remission (CR) rates of up to 38% in patients with previously untreated CLL.1-3 In a phase 2 trial, the addition of rituximab to this regimen resulted in an overall response rate (ORR) of 95% and a CR rate of 70%.4, 5 In a randomized phase 3 trial, the combination of fludarabine, cyclophosphamide and rituximab (FCR) was associated with improved progression-free survival (PFS) and overall survival (OS) as compared to that seen with FC in treatment-naïve patients with CLL.6
Single-agent rituximab has also been shown to have activity in patients with both treatment-naïve and relapsed CLL.7-9 Notably, the response rates in CLL are significantly lower than those seen in other indolent lymphoproliferative disorders such as follicular lymphoma.8 Possible explanations for this phenomenon include the relatively low expression of CD20 on CLL cells10 and altered pharmacodynamics in patients with CLL due to a high intravascular tumor burden.8 In the latter scenario, the high amount of circulating malignant CLL cells may lead to an “antibody sink,” causing rapid clearance of rituximab from the blood and leading to decreased efficacy.
In an effort to increase the response rate of single-agent rituximab in CLL, some investigators employed dose-intensification strategies. A dose-dense regimen of rituximab given three times a week for 4 weeks at the standard dose of 375 mg/m2 resulted in an ORR of 45% in a cohort of mostly pretreated patients with CLL.11 In another trial, escalating doses of rituximab from 500 mg/m2 to 2250 mg/m2 were given weekly for 4 weeks to previously treated patients with CLL.12 An ORR of 36% was achieved, and there was evidence of increased response rates in patients treated with higher rituximab doses. In both of these studies, the dose-intensified rituximab regimens increased response rates compared to those seen in historical controls and were associated with an acceptable toxicity profile.
Given improved response rates with dose-intensified regimens of single-agent rituximab, we designed a trial to investigate whether increasing the number of rituximab doses given with FCR chemoimmunotherapy would improve outcomes in patients with untreated CLL. Results were compared to historical data using FCR. We hypothesized that a modified FCR regimen with multiple-dose rituximab (FCR3) would result in higher CR rates without a significant increase in toxicity.
Methods
Patients
This phase 2 study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center (MDACC) and registered on www.clinicaltrials.gov with a trial identifier of NCT00794820. All patients provided informed consent according to institutional guidelines and the Declaration of Helsinki. Patients were required to have progressive CLL with an indication for treatment according to the National Cancer Institute Working Group (NCIWG) guidelines.13 Patients of all ages were eligible. All patients were required to have an Eastern Cooperative Oncology Group performance status of 0 to 3, serum creatinine <2 mg/dL and bilirubin <2 mg/dL, unless renal or liver dysfunction was thought to be due to organ infiltration by lymphocytes. Prognostic markers including presence or absence of Zap-70, immunoglobulin heavy-chain variable gene (IGHV) mutation status, standard metaphase cytogenetic analysis, and fluorescent in situ hybridization (FISH) were performed on the bone marrow at baseline in the majority of patients.
Therapy
The FCR3 regimen consisted of six 28-day cycles of intravenous fludarabine (25 mg/m2 per day) and cyclophosphamide (250 mg/m2 per day) on days 2-4 for cycle 1 and on days 1-3 for cycles 2-6. For the first cycle, rituximab was administered at a dose of 375 mg/m2 on day 1 and at 500 mg/m2 on days 2-3. For cycles 2-6, the rituximab was administered at a dose of 500 mg/m2 on days 1-3. Premedication for rituximab infusion with acetaminophen, diphenhydramine and/or steroids was recommended and provided at the discretion of the treating physician. Courses were delayed at the discretion of the treating physician if needed to allow for adequate recovery of neutrophil and platelet counts. Dose reductions for fludarabine and cyclophosphamide, but not rituximab, were made if a patient experienced prolonged grade 3 or 4 hematologic toxicity, infection or organ dysfunction. Prophylaxis against tumor lysis syndrome, herpes simplex virus (HSV) reactivation and Pneumocystis jiroveci pneumonia (PJP), as well as the use of myeloid growth factors were administered at the discretion of the treating physician.
Response Assessment
Patients were evaluated with bone marrow aspiration and biopsy and with 4-color flow cytometry for residual CD5- and CD19- coexpressing lymphocytes with light-chain restriction at end of the third and sixth cycle and then every 12 months until evidence of disease progression. Minimal residual disease (MRD) negativity was defined as <1% of CD5- and CD19- coexpressing cells with a normal κ-λ ratio, a cutoff that was standard at the time at which this trial was conducted but that differs from the recent consensus definition for MRD negativity.14 Computed tomography scans were not used for response assessment. Patients were evaluated for response according to the response criteria defined by the NCIWG.13
FCR Comparison Group
Patients enrolled at MDACC in a previously published clinical trial of FCR for patients with untreated CLL were used as a historical comparison group (n = 300).4, 5 The median follow-up for the FCR cohort at the time of this analysis was 119 months.
Statistical Considerations
The primary objective of this study was to determine the CR rate of FCR3 in treatment-naïve patients with CLL. Secondary objectives were to evaluate the OS, time-to-progression (TTP), ORR, MRD-negativity rate and toxicity of the FCR3 regimen. TTP was defined as the time from the initiation of treatment to primary refractory disease or CLL progression. TTP was censored for therapy-related myelodysplastic syndrome (t-MDS) or acute myelogenous leukemia (t-AML) and death in remission. t-MDS/AML was diagnosed in accordance with consensus guidelines.15 OS was defined as the time from the initiation of treatment to last follow-up date or death. The data cutoff for this analysis was October 1, 2014. TTP and OS were calculated using Kaplan-Meier estimates, and survival estimates were compared using the log-rank test. Responses were assessed by pretreatment characteristics and compared using the Fisher exact test (2-tailed). All analyses were performed on GraphPad Prism version 6.0.
Results
Patient Characteristics
A total of 65 patients with untreated CLL were enrolled between January 2004 and March 2005. Baseline characteristics of the patients are summarized in Table 1. The median age was 59 (range 27-82) and the majority of patients were men (80%). Nineteen patients (29%) had advanced Rai stage disease (stage III-IV). IGHV was unmutated in 74% of evaluable patients, and Zap-70 was positive in 54%. Of those patients on whom FISH was performed, deletion of 11q was detected in 14 patients (29%), one of whom also carried a 17p deletion. The median time from diagnosis to treatment was 20 months (range 0-121 months).
Table 1. Baseline Patient Characteristics.
| Pretreatment characteristics | Value |
|---|---|
| Age (y) | 59 (27-82) |
| WBC (K/μL) | 92.4 (7.9-363) |
| ALC (K/μL) | 79.5 (3.7-323.9) |
| Hemoglobin (g/dL) | 12.8 (8.5-15.2) |
| Platelets (K/μL) | 156 (31-353) |
| β2-microglobulin (mg/L) | 3.8 (1.6-10.1) |
| Gender | |
| Male | 52 (80) |
| Female | 13 (20) |
| Rai stage | |
| 0-II | 46 (71) |
| III-IV | 19 (29) |
| CD38 expression (n=61) | |
| <30% | 43 (70) |
| ≥30% | 18 (30) |
| IGHV mutational status (n=62) | |
| Mutated (≥2%) | 16 (26) |
| Unmutated (<2%) | 46 (74) |
| Zap-70 flow (n=39) | |
| Negative (<20%) | 18 (46) |
| Positive (≥20%) | 21 (54) |
| FISH, bone marrow (n=48) | |
| Negative | 14 (29) |
| Single abnormality | |
| del13q | 10 (21) |
| Trisomy 12 | 8 (17) |
| del11q | 3 (6) |
| Multiple abnormalities | |
| del11q and del13q | 10 (21) |
| del11q and del17p | 1 (2) |
| Trisomy 12 and del13q | 2 (4) |
Values are median (range) or n (%)
WBC, white blood cells; ALC, absolute lymphocyte count; IGHV, immunoglobulin variable heavy chain; FISH, fluorescent in situ hybridization
Response to Therapy
Sixty-four of 65 patients were evaluable for assessment of response to FCR3. Among the patients evaluable for response, the ORR was 97% (Table 2). Forty-eight patients (75%) achieved a CR, 10 (16%) achieved an nPR, 4 (6%) achieved a PR and 2 (3%) did not respond to FCR3. These response rates were not significantly different from the CR, nPR and PR in patients treated with FCR (72%, 10% and 12%, respectively). Of the two patients whose CLL did not respond to FCR3, one patient with 17p deletion developed cytopenias from progressive CLL after 2 cycles of FCR3 and died 6 months after study enrollment. The other patient whose chromosomal status was unknown also developed cytopenias due to CLL progression after 4 cycles of FCR3 and died from sepsis 7 months after study enrollment. One patient died 2 months after study enrollment due to sepsis after receiving 3 cycles of therapy and was not evaluable for response.
Table 2. Response Rates and Long-Term Outcomes with FCR3.
| Response | N (%) | Flow MRD negative, n/N (%) | Median TTP (months) | Median OS (months) | 9-year survival (%) |
|---|---|---|---|---|---|
| All patients | 65 (100) | 37/60 (62) | 81 | Not reached | 58 |
| Any response (CR + nPR + PR) | 62 (97) | 37/59 (63) | 82 | Not reached | 61 |
| CR | 48 (75) | 34/46 (74) | 86 | Not reached | 65 |
| nPR | 10 (16) | 1/9 (11) | 49 | Not reached | 60 |
| PR | 4 (6) | 2/4 (50) | -- | 41 | 25 |
MRD, minimal residual disease; TTP, time to progression; OS, overall survival; CR, complete response; nPR, nodular partial response; PR, partial response
Responses to FCR3 by patient pretreatment characteristics are listed in Table 3. Only a pre-treatment β2-microglobuln <3.8 mg/L was predictive of achieving a CR (P <0.01). No significant association was found between CR rates and patient age, Rai stage, IGVH mutation or Zap-70 status or chromosomal abnormalities by FISH. The one patient with 17p deletion was the only patient with known chromosomal status whose CLL did not respond to FCR3.
Table 3. Response Rates to FCR3 by Pretreatment Characteristics.
| Patient characteristic | N | ORR, N (%) | CR, N (%) | P-value1 |
|---|---|---|---|---|
|
| ||||
| All | 64 | 62 (97) | 48 (75) | --- |
|
| ||||
| Age (y) | 0.51 | |||
| <55 | 19 | 19 (100) | 16 (84) | |
| 55-69 | 36 | 35 (97) | 26 (72) | |
| ≥70 | 9 | 8 (89) | 6 (67) | |
|
| ||||
| WBC (K/μL) | 0.77 | |||
| <100 | 34 | 33 (97) | 25 (74) | |
| ≥100 | 30 | 29 (97) | 23 (77) | |
|
| ||||
| β2-microglobulin (mg/L) | <0.01 | |||
| ≤3.8 | 34 | 34 (100) | 30 (88) | |
| >3.8 | 30 | 28 (93) | 18 (60) | |
|
| ||||
| Rai stage | 0.11 | |||
| 0-II | 46 | 45 (98) | 37 (80) | |
| III-IV | 18 | 17 (94) | 11 (61) | |
|
| ||||
| CD38 expression | 0.33 | |||
| <30% | 42 | 40 (95) | 33 (79) | |
| ≥30% | 18 | 18 (100) | 12 (67) | |
|
| ||||
| IGHV mutational status | 0.90 | |||
| Mutated (≥2%) | 16 | 16 (100) | 12 (75) | |
| Unmutated (<2%) | 45 | 43 (96) | 33 (73) | |
|
| ||||
| Zap-70 flow | 0.28 | |||
| Negative (<20%) | 18 | 18 (100) | 14 (78) | |
| Positive (≥20%) | 21 | 20 (95) | 23 (62) | |
|
| ||||
| FISH, bone marrow | ||||
| Negative | 13 | 13 (100) | 10 (77) | |
| del13q | 10 | 10 (100) | 9 (90) | 0.29 |
| Trisomy 122 | 10 | 10 (100) | 8 (80) | |
| del11q3 | 13 | 13 (100) | 11 (85) | |
| del17p | 1 | 0 (0) | 0 (0) | |
P-values are comparing CR rates vs. any response less than CR.
Includes 2 patients with concomitant del13q.
Includes 10 patients with concomitant del13q and excludes the one patient with concomitant del17p.
ORR, overall response rate; CR, complete remission; WBC, white blood cells; IGHV, immunoglobulin variable heavy chain; FISH, fluorescent in situ hybridization
Sixty patients were tested for MRD with flow cytometry on bone marrow aspirate samples after 6 cycles of therapy or after their last cycle of therapy if fewer than 6 cycles were administered (Table 2). Thirty-seven patients (62%) had <1% CD5- and CD19- coexpressing cells (MRD negativity), 14 patients (23%) had 1 to 5% residual CLL cells and 9 patients (15%) had >5% residual CLL cells by flow cytometry. Of the patients who achieved a CR, 34 of 46 evaluable patients (74%) were negative for MRD. Among those patients who became MRD negative, the median time to loss of MRD negativity was 49.2 months. The MRD negativity rate was not significantly different between patients treated with FCR and FCR3 when all patients were included (71% with FCR, P = 0.17) or when only those patients in CR were included (72% with FCR, P = 0.19).
Response Duration and Survival
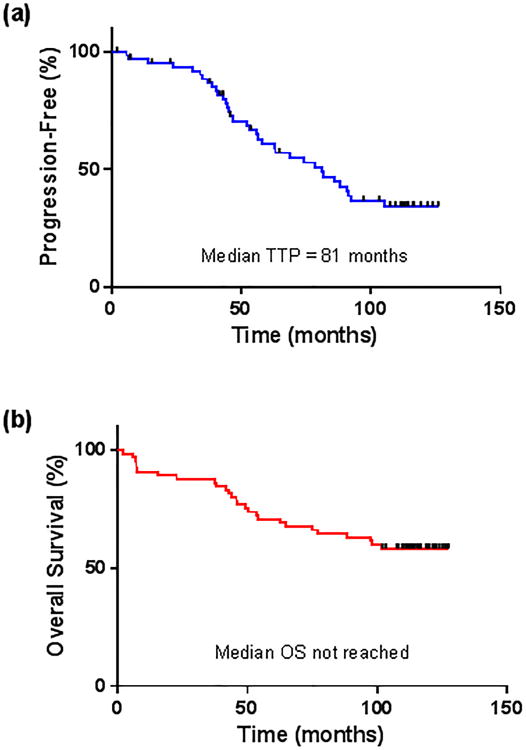
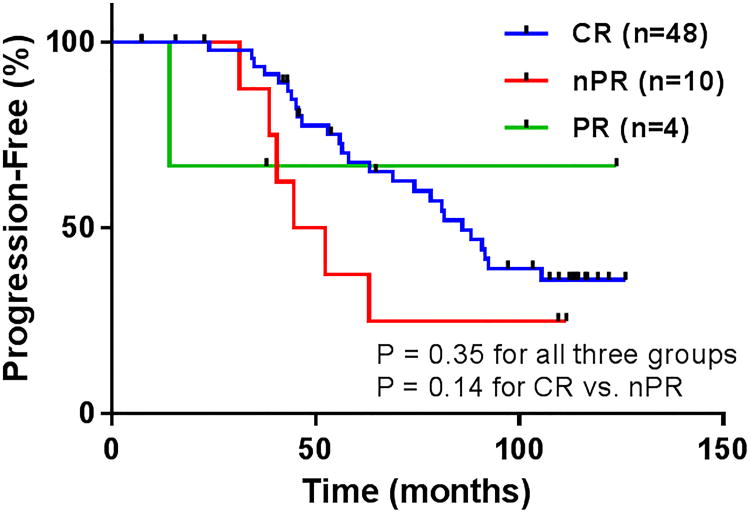
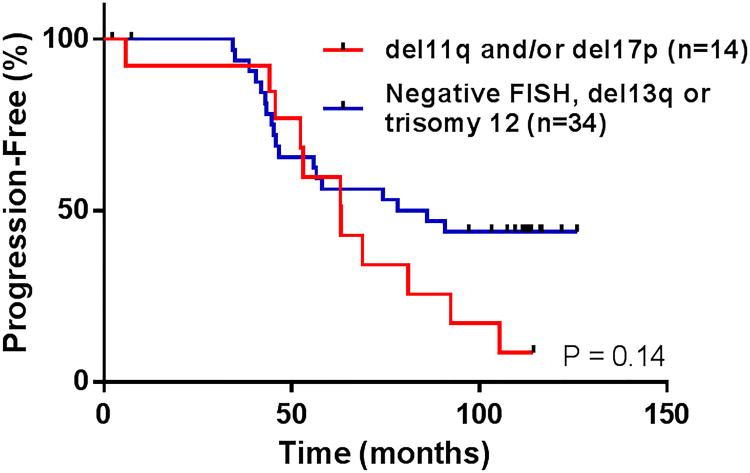
The median TTP for the entire FCR3 cohort was 81 months (Figure 1A), which was similar to the historical FCR population which had a median TTP of 84 months (P = 0.63). The median TTP for patients achieving CR, nPR and PR were 86 months, 49 months and not evaluable, respectively (P = 0.14 for CR vs. nPR) (Table 2, Figure 2). Sixteen patients (25%) are still alive without relapsed disease at the time of this analysis. The median TTP for patients with unfavorable chromosomal abnormalities by FISH (i.e. deletion 11q and/or deletion 17p) was 63 months, compared to 82 months in patients with favorable chromosomal abnormalities or who were negative by FISH (P = 0.14) (Figure 3). There was a trend toward prolonged TTP in patients with mutated IGVH versus those were unmutated (median TTP not reached vs. 74 months, respectively, P = 0.09). No significant difference was observed in TTP between patients who were MRD-negative versus MRD-positive when all patients were analyzed or when only patients who achieved a CR were included in the analysis.
Figure 1. (a) Time to progression (TTP) and (b) overall survival (OS) for the entire FCR3 cohort.
Figure 2.
Time to progression (TTP) by response to FCR3.
Figure 3.
Effect of fluorescent in situ hybridization (FISH) chromosomal abnormalities on time to progression (TTP) after FCR3.
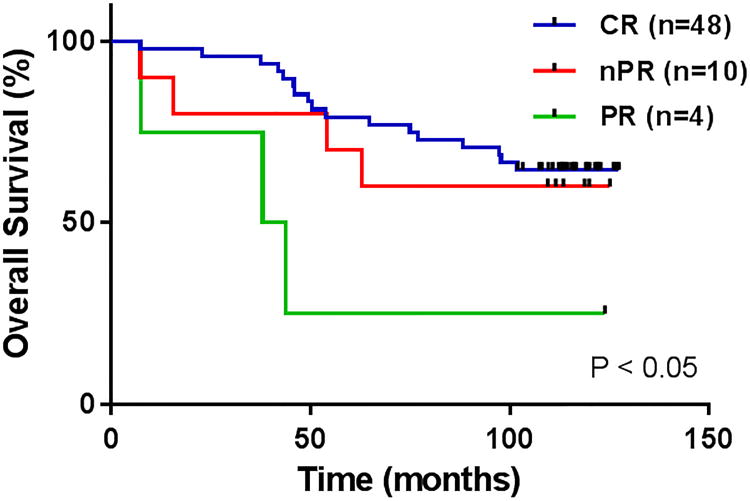
At a median survivor follow-up of 9.7 years, median OS has not been reached with 58% of patients still alive (Figure 1B). Thirty-one (65%) of those patients who achieved CR, 6 patients (60%) who achieved nPR and 1 patient (25%) who achieved PR are still alive (P < 0.05) (Table 2, Figure 4). The one patient who achieved PR and is still alive underwent stem cell transplantation after FCR3 therapy. In the historical FCR group, median OS has not yet been reached with 55% of patients still alive at a median survivor follow-up of 11.5 years (P = 0.58 compared to OS with FCR3).
Figure 4.
Overall survival (OS) by response to FCR3.
Treatment Characteristics and Toxicity
Fifty patients (77%) completed the prescribed six courses of therapy, and the mean number of treatment courses administered was 5.5 (range 2-6). The most common cause of premature discontinuation of therapy was persistent cytopenias, which developed in 5 patients (8%). Other reasons for discontinuation were resistant disease (n = 2), infection (n = 3), hepatitis B reactivation (n = 1), interstitial pneumonitis (n = 1), persistent splenomegaly requiring splenectomy (n = 1) (no CLL on pathology), metastatic gastric cancer (n=1) and cutaneous anaplastic large-cell lymphoma (n = 1).
Grade 3-4 neutropenia was observed in 19% and 26% of FCR3 cycles, respectively. Fifty-eight patients (89%) experienced at least one episode of grade 3-4 neutropenia, and the median incidence of grade 3-4 neutropenia per patient was 2 (range 1-6). Growth factor support with granulocyte (G-CSF) or granulocyte macrophage colony-stimulating factor (GM-CSF) was provided to 41 patients (63%) during one or more cycles. Grade 3-4 thrombocytopenia was observed in 5% of courses. A total of 14 patients (22%) experienced at least one episode of grade 3-4 thrombocytopenia. No patients experienced major bleeding. Rates of grade 3-4 neutropenia and thrombocytopenia were not significantly different from those seen with FCR.
All patients received HSV prophylaxis with valacyclovir, which is standard practice at our institution. Twenty-two patients (34%) received PJP prophylaxis with trimethoprim-sulfamethoxazole. Minor infections such as urinary tract infections, cellulitis, sinusitis or upper respiratory infections occurred in 14% of courses. Only 1.9% of courses were associated with major infection, including septicemia (n = 5) and pneumonia (n = 2). There were no cases of HSV or PJP infections. One patient developed hepatitis B reactivation after cycle 4, which prompted treatment discontinuation. This patient developed only mild transaminase elevation and responded well to anti-viral medications.
Secondary Malignancies
t-MDS developed in 6 patients and t-AML developed in one patient. t-MDS/AML occurred in 11% of study participants at a median of 32 months after initiating FCR3 treatment (range 22-92 months). In the historical FCR cohort, t-MDS/AML developed in 2.7% of patients (P <0.01). The mean age (± SEM) at study enrollment of patients who developed t-MDS/AML after FCR3 was 62.7 (± 4.2) compared to 58.6 (± 1.3) in patients who did not develop t-MDS/AML (P = 0.30). Five of the 7 patients received all 6 cycles of FCR3, and 2 patients received only 4 cycles. The mean number of cycles received by patients who developed t-MDS/AML was not significantly different than that of patients who did not (5.4 vs. 5.6 cycles, respectively, P = 0.99). No difference was observed in baseline IGVH mutation status, abnormalities by FISH or the number of courses associated with grade 3-4 neutropenia among those patients who developed t-MDS/AML compared to those who did not.
Six patients developed t-MDS/AML without receiving any further chemotherapy (5 who were in CR and one who was in PR at the time of t-MDS/AML diagnosis). None had a history of any prior radiation or cytotoxic chemotherapy. The other patient developed t-MDS 7.5 years after study enrollment. This patient's CLL progressed 4 years after completing 6 cycles of FCR3. This patient was then observed for 1 year and then was subsequently treated with rituximab and lenalidomide for 2.5 years prior to developing t-MDS. All 6 patients with t-MDS had deletions of all or part of chromosome 5 and/or 7, and the one patient with t-AML had trisomy 8. Baseline karyotype was available for 5 patients who developed t-MDS/AML. None of these patients harbored baseline cytogenetic abnormalities other than those known to be associated with CLL. The median OS for patients who developed t-MDS/AML was 10 months from the time of t-MDS/AML diagnosis (range 3-21 months). All patient diagnosed with t-MDS/AML had died at the time of this analysis.
Richter's syndrome (RS) developed in 3 patients (5%) at the time of disease progression. All of these patients were in CR prior to the diagnosis of RS. One patient developed RS 41 months after initiating FCR3 and died 10 months after the RS diagnosis, and one developed RS 43 months after FCR3 and died 6 months later. The third patient was diagnosed with RS 105 months after FCR3 and was still alive at most recent follow-up 8 months after RS diagnosis. There was no significant difference between RS rates in the FCR3 and FCR cohorts (3% in FCR, P = 0.61).
Malignancies other than t-MDS/AML and RS developed in 6 patients (9%). Observed malignancies included cutaneous anaplastic T-cell lymphoma, gastric adenocarcinoma, lung adenocarcinoma, Merkel cell carcinoma, squamous cell carcinoma (SCC) of the oropharynx and SCC of the penis, all of which occurred in 1 patient each. The median time to the diagnosis of a secondary malignancy (excluding t-MDS/AML and RS) was 14.7 months (range 4.5 to 98.7 months).
Discussion
Chemoimmunotherapy with a regimen such as FCR is the standard of care for younger, physically-fit patients with previously untreated CLL.14 Nearly all patients respond to FCR and most achieve a CR, although the majority of patients eventually develop relapsed disease.5 Patients who achieve a CR and those who are MRD-negative after FCR have a longer disease-free interval than those who achieve lesser responses.5, 17, 18 In a large randomized, controlled trial, the addition of rituximab to FC significantly increased CR and MRD-negativity rates and resulted in marked improvements in PFS and OS as compared to FC alone in patients with previously untreated CLL.6, 17 In patients with relapsed CLL, dose-intensified rituximab regimens increase response rates compared to standard-dose rituximab.11, 12 This observation prompted our group to evaluate the effect of a modified FCR regimen with multiple doses of rituximab in patients with previously untreated CLL.
Despite prior reports of increased responses with dose-intensified rituximab, FCR3 did not result in significant improvements in response rates, MRD negativity, TTP or OS, as compared to an historical FCR cohort. It is possible that different mechanisms underlie the increased efficacy observed with dose-intensified single-agent rituximab and that observed when rituximab is combined with chemotherapy (e.g. FCR vs. FC). Given the relatively low CD20 expression on CLL cells10, higher doses of rituximab may be needed to induce adequate antibody-dependent cellular cytotoxicity when administered as a single agent. In contrast, rituximab has been shown to potentiate the cytotoxic effects of chemotherapy agents such as fludarabine19-21, and this synergism likely contributes to the efficacy of rituximab-based combination regimens for CLL, compared to chemotherapy alone. The lack of improved response rates with FCR3 compared to FCR suggests that when rituximab is used in combination with chemotherapy there may be a ceiling beyond which higher cumulative doses of rituximab do not further potentiate the cytotoxic effects of the backbone chemotherapy regimen.
The FCR3 regimen was well-tolerated and had an early safety profile comparable to standard FCR chemoimmunotherapy, as previously reported.4 The majority of patients (77%) received all 6 cycles of FCR. Despite increased doses of rituximab given in the FCR3 regimen, the incidences of neutropenia, thrombocytopenia and major infections were not increased. The rate of RS was also similar to that seen in patients treated with FCR.
t-MDS/AML developed in 7 patients (11%) who received FCR3 and accounted for 26% of all study deaths. The development of t-MDS/AML was not associated with patient age or with the number of cycles of FCR3 administered. The frequency of t-MDS/AML in patients treated with FCR3 was 4-fold higher than that observed in the historical FCR cohort (2.7%), a difference which reached statistical significance. Our group at MDACC previously reported a rate of t-MDS/AML in patients treated with FCR-based therapy as high as 5.1%, although notably this analysis included data from patients treated with FCR3 as well as FCR plus concomitant GM-CSF, both of which were associated with higher rates of t-MDS/AML than FCR alone.22 Despite the significant increase in t-MDS/AML with FCR3, OS was not decreased compared to that seen in the FCR cohort.
It should be noted that definitive conclusions about the relationship between FCR3 and the development the t-MDS/AML are precluded by the small sample size of this study and the comparison to an historical control group. To our knowledge, a statistically significant association between higher cumulative doses of rituximab and the development of t-MDS/AML has not previously been reported. The addition of rituximab to FC in the CLL8 trial did not result in increased rates of t-MDS/AML.23 Similarly, in a single-arm study of 67 patients with CLL given rituximab maintenance after upfront rituximab, fludarabine, cyclophosphamide and mitoxantrone, no cases of t-MDS/AML were reported after a median follow-up of over 4 years.24
In contrast, the addition of rituximab to high-dose chemotherapy has been associated with an increased rate of solid tumors in patients with lymphoma in a large series.25 In this series, there was a numerically higher cumulative incidence of t-MDS/AML in patients who received rituximab, although this did not reach statistical significance. A randomized trial evaluating rituximab maintenance versus observation in patients with CLL after either first- or second-line chemoimmunotherapy also suggested an increase in secondary malignancies in the rituximab maintenance arm compared to the observation arm (6% vs. <1%, respectively), although the rates of t-MDS/AML were not reported.26
The mechanism by which an increased cumulative dose of rituximab might have caused the apparent increased incidence of t-MDS/AML in the present study is unclear. B-cells have been shown to exert anti-tumor effects through both antibody-mediated cytotoxicity of malignant cells and antigen-presentation to effector T-cells.27, 28 It is therefore feasible that the immunosuppressive and B-cell depleting effects of rituximab might impair immune surveillance and predispose to secondary malignancies. Alternatively, rituximab might potentiate the cytotoxic effect of the backbone FC chemotherapy on normal hematopoietic progenitors and predispose to secondary hematologic malignancies.19-21 It is also possible that frequent use of growth factors may have played a role in the higher rate of t-MDS/AML in the present study. In a previous trial, the routine addition of GM-CSF to FCR resulted in a numerically higher rate of t-MDS/AML compared to FCR alone (6% vs. 3%, respectively), although this difference did not reach statistical significance.29 The use of growth factors in the historical FCR cohort was not recorded, and therefore our ability to draw conclusions about the role of growth factors in the development of t-MDS/AML is limited.
In conclusion, the addition of multiple doses of rituximab to FCR failed to significantly improve response rates or long-term outcomes in patients with previously untreated CLL. FCR3 had similar short-term toxicity to FCR, though resulted in an apparent increased rate of secondary hematologic malignancies. Further studies are needed to evaluate this finding.
Acknowledgments
Funding Source: Research support from Genentech
Funding info: P30 CA016672
Footnotes
Financial Disclosures: None
References
- 1.Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 2.Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 3.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 4.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 5.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 7.Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21:1746–1751. doi: 10.1200/JCO.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 9.Huhn D, von Schilling C, Wilhelm M, et al. Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood. 2001;98:1326–1331. doi: 10.1182/blood.v98.5.1326. [DOI] [PubMed] [Google Scholar]
- 10.Almasri NM, Duque RE, Iturraspe J, Everett E, Braylan RC. Reduced expression of CD20 antigen as a characteristic marker for chronic lymphocytic leukemia. Am J Hematol. 1992;40:259–263. doi: 10.1002/ajh.2830400404. [DOI] [PubMed] [Google Scholar]
- 11.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 14.Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21:956–964. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. [Google Scholar]
- 16.Network NCC. Non-Hodgkin's Lymphomas (Version 5.2014) [accessed December 1, 2014]; Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.
- 17.Bottcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30:980–988. doi: 10.1200/JCO.2011.36.9348. [DOI] [PubMed] [Google Scholar]
- 18.Strati P, Keating MJ, O'Brien SM, et al. Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood. 2014;123:3727–3732. doi: 10.1182/blood-2013-11-538116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alas S, Bonavida B, Emmanouilides C. Potentiation of fludarabine cytotoxicity on non-Hodgkin's lymphoma by pentoxifylline and rituximab. Anticancer Res. 2000;20:2961–2966. [PubMed] [Google Scholar]
- 20.Chow KU, Sommerlad WD, Boehrer S, et al. Anti-CD20 antibody (IDEC-C2B8, rituximab) enhances efficacy of cytotoxic drugs on neoplastic lymphocytes in vitro: role of cytokines, complement, and caspases. Haematologica. 2002;87:33–43. [PubMed] [Google Scholar]
- 21.Di Gaetano N, Xiao Y, Erba E, et al. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. Br J Haematol. 2001;114:800–809. doi: 10.1046/j.1365-2141.2001.03014.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Tang G, Medeiros LJ, et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol. 2012;25:237–245. doi: 10.1038/modpathol.2011.158. [DOI] [PubMed] [Google Scholar]
- 23.Fischer K, Bahlo J, Fink AM, et al. Extended Follow up of the CLL8 Protocol, a randomized phase-III trial of the German CLL study group (GCLLSG) comparing fludarabine and cyclophosphamide (FC) to FC plus rituximab (FCR) for previously untreated patients with chronic lymphocytic leukemia (CLL): results on survival, progression-free survival, delayed neutropenias and secondary malignancies confirm superiority of the FCR regimen. American Society of Hematology. 2012 [Google Scholar]
- 24.Abrisqueta P, Villamor N, Terol MJ, et al. Rituximab maintenance after first-line therapy with rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) for chronic lymphocytic leukemia. Blood. 2013;122:3951–3959. doi: 10.1182/blood-2013-05-502773. [DOI] [PubMed] [Google Scholar]
- 25.Tarella C, Passera R, Magni M, et al. Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol. 2011;29:814–824. doi: 10.1200/JCO.2010.28.9777. [DOI] [PubMed] [Google Scholar]
- 26.Greil R, Obrtlikova P, Smolej L, et al. Rituximab maintenance after chemoimmunotherapy induction in 1st and 2nd line improves progression free survival: planned interim analysis of the international randomized AGMT-CLL8/a maintenance trial. American Society of Hematology. 2014 [Google Scholar]
- 27.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol. 2009;183:3195–3203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Lao X, Pan Q, et al. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res. 2011;17:4987–4995. doi: 10.1158/1078-0432.CCR-11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strati P, Ferrajoli A, Lerner S, et al. Fludarabine, cyclophosphamide and rituximab plus granulocyte macrophage colony-stimulating factor as frontline treatment for patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2014;55:828–833. doi: 10.3109/10428194.2013.819574. [DOI] [PMC free article] [PubMed] [Google Scholar]