Abstract
Treatment of acromegaly with monotherapy long-acting somatostatin analogues (LA-SSA) as primary treatment or after neurosurgery can only achieve complete normalization of insulin-like growth factor I (IGF-I) in roughly 40 % of patients. Recently, one of the acromegaly consensus groups has recommended switching to combined treatment of LA-SSA and pegvisomant (PEGV) in patients with partial response to LA-SSAs. This combination of LA-SSA and PEGV, a growth hormone receptor antagonist, can normalize IGF-I levels in virtually all patients, requiring that the adequate dose of PEGV is used. The required PEGV dose varies significantly between individual acromegaly patients. One of the advantages of the combination therapy is that tumor size control or even tumor shrinkage can be observed in a vast majority of patients. The main side effects of the combination treatment are gastrointestinal symptoms, lipohypertrophy and transient elevated liver transaminases. In this review we provide an overview of the efficacy and safety of the combined treatment of LA-SSAs with PEGV.
Keywords: Acromegaly, Somatostatin analogues, Growth hormone receptor antagonist, Pegvisomant, Growth hormone, Insulin-like growth factor I
Introduction
Acromegaly is a rare disease almost exclusively caused by a growth hormone (GH) secreting pituitary adenoma. This hypersecretion of GH and the associated elevated IGF-I levels result in increased morbidity and mortality [1]. Surgery is in general the first treatment modality, but is not always successful, as the majority of the patients have a macroadenoma [1]. The reported cure rates of transsphenoidal surgery vary widely, mainly depending on tumor size and invasiveness and the experience of the neurosurgeon [2]. Surgery of microadenomas (<1 cm in diameter) has an average cure rate of 78 %, whereas with macroadenomas (≥1 cm in diameter) the average cure rate ≤50 % [2]. However, these previous results were obtained 10 years ago. More recent data from daily practice were shown in a study with data gathered from the UK National Acromegaly Registry. This study reported cure rates between 20 and 40 % [3]. LA-SSAs are currently used as pre-surgical treatment, adjuvant treatment and as primary medical treatment. Multiple studies addressed the efficacy of LA-SSA, and have shown that LA-SSA treatment alone reached normalization of IGF-I levels in about half of the patients [4]. No differences between lanreotide SR and octreotide LAR were observed regarding tumor shrinkage and normalization of IGF-I levels [5]. An attractive way to control biochemical disease activity in patients that are uncontrolled on LA-SSA monotherapy is to add PEGV, because of the different modes of action of these two drugs. LA-SSA reduces hypersecretion of GH by binding to somatostatin receptors on the pituitary adenoma. PEGV is a pegylated recombinant analogue of GH and thereby functions as a GH antagonist. It acts by reducing the excessive GH actions in peripheral tissues and by blocking the increased production of IGF-I by the liver. Combined treatment as pre-surgical treatment to improve morbidity is very questionable, as there are yet no supporting data. Adjuvant treatment and primary medical treatment are suitable for LA-SSA combined with PEGV. However, in the current guidelines combination treatment is only recommended in partial responders to LA-SSA [6]. A recent Italian registration study reported that LA-SSA combined with PEGV is prescribed widely in Italy and more frequently in tertiary than in secondary referral hospitals (59 vs. 37 % of the patients, p < 0.001). In more experienced centers less adverse events were reported during PEGV treatment, however long-term efficacy rates were lower. The authors suggest for this latter finding an inadequate patient’s selection due to prevalence of more aggressive tumors in the tertiary referral centers.
The first clinical PEGV-trials observed high efficacy rates of 89–97 % depending on dose and duration of the treatment [7, 8]. The large observational ACROSTUDY™ could not confirm these high efficacy rates. However, it is noteworthy that this non-interventional safety surveillance study was not designed to address efficacy rates, as dose titration was not part of the study design [9]. The first study about combined treatment with both LA-SSA and PEGV was reported in 2005, which observed a high efficacy rate of 95 % with a lower required PEGV dose to achieve normalization of IGF-I levels compared to the first data reporting PEGV monotherapy with a similar efficacy rate [8, 10]. This high efficacy rate and lower required PEGV dose during combined treatment was confirmed in later reports [11–14]. This review will discuss the available literature on the combination treatment in acromegaly patients, including level of disease control, tumor size and side effects.
Efficacy of the combination therapy with LA-SSA and PEGV
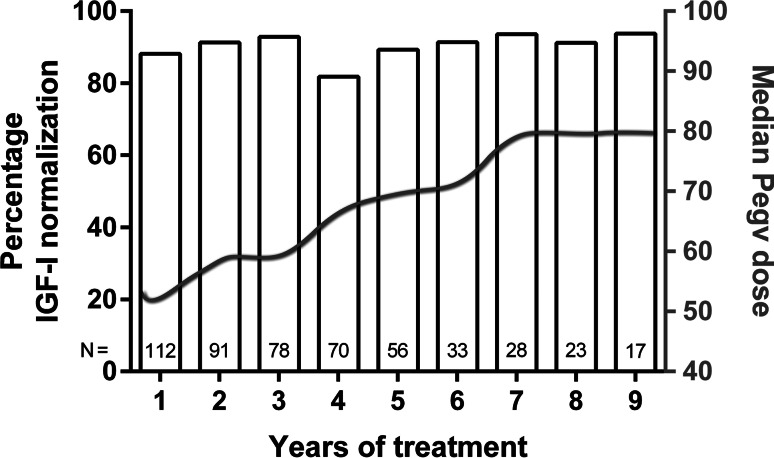
Meta-analyses of clinical trials showed that LA-SSAs alone normalize GH and IGF-I levels in about 50 % of patients [4]. However, due to selection bias this efficacy rate is probably an overestimation. In unselected treatment-naive patients an LA-SSA efficacy rate of 40 % seems to be more common [15, 16]. PEGV appears to be more effective, both during monotherapy as during co-treatment with LA-SSA [7, 8, 11–14, 17]. Table 1 shows a summary of studies reporting on efficacy of acromegaly patients using LA-SSA in combination with PEGV. The efficacy rates during a period of 9 years in a large Dutch cohort are shown in Fig. 1 [12]. Because PEGV is a competitive blocker of the GH receptor (GHR), pharmacology dictates that in principle it should be possible to control IGF-I levels in all patients with acromegaly, provided that the appropriate PEGV dose is used. LA-SSAs have direct and indirect effects that result in a GH-independent decrease of IGF-I production [18, 19]. A Danish study reported that PEGV serum levels increase by 20 % when combined with LA-SSA [20]. The appropriate PEGV dose varies among acromegaly patients, the Dutch cohort reported that patients using high dose of LA-SSA needed a median weekly PEGV dose of 80 mg (range: 30–300 mg) to achieve normal IGF-I levels in 97 % of the patients [12]. Monotherapy of PEGV requires a higher cumulative weekly dose of around 130 mg to achieve a similar normalization rate after 12 months of treatment [8]. In contrast to these reports, an Italian observational study reported no difference in median required PEGV dose to normalize IGF-I levels in patients using PEGV monotherapy compared to patients treated with the combination therapy [21]. However, these groups were not similar according to severity of the disease. The PEGV monotherapy group had significant lower GH and IGF-I levels at baseline compared to the combination group in this Italian observational study [21]. Observational registries such as the ACROSTUDY™ and German Pegvisomant Observational Study (GPOS) [9, 22], in which patients using PEGV are included regardless their concomitant medication, observed lower efficacy rates around 60 %, compared with the clinical trials of PEGV monotherapy and combination treatment mentioned previously [8, 12]. In the ACROSTUDY™ PEGV was combined with LA-SSA in 23 %, with dopamine agonist in 6 %, and a combination of the three agents in 4 % of patients [9]. After 5 years the mean weekly dose was 106 mg in patients with a normal IGF-I, and 113 mg in those with an elevated IGF-I [23]. However, these studies were not designed to evaluate efficacy and dose titration, but aimed for the evaluation of safety aspects, such as rare side effects. The lower efficacy rates in the observational registration studies might be explained by the relative lower dose of PEGV. To achieve efficacy rates of more than 90 % during PEGV monotherapy, the average expected weekly dose is probably above 120–130 mg.
Table 1.
Summary of studies reporting on combination treatment
| First author, year, (Ref) | Design | Aim of study | No. of patients | Disease control (%) | PEGV dose (mg/weekly) | Duration study (months) |
|---|---|---|---|---|---|---|
| Van der Lely et al. [24] | Case report | IGF-I normalization | 1 | 100 | 280 | 18 |
| Trainer et al. [17] | Randomized controlled trial | Primary end-point: AEs, secondary end-point: IGF-I normalization | 29 | 73 | 105 | 9 |
| Van der Lely et al. [14] | Prospective observational study | IGF-I normalization and AEs | 57 | 79 | 60 | 7 |
| Bianchi et al. [21] | Retrospective observational study | IGF-I normalization and AEs | 27 | 67 | 140 | 30 (median) |
| Neggers et al. [12] | Retrospective observational study | IGF-I normalization and AEs | 112 | 97 | 80 | 59 (median) |
Summary of studies reporting on LA-SSA combined with PEGV and the percentage of disease control (normalization of IGF-I levels) and the required PEGV dose in order to control IGF-I levels. The ACROSTUDY is not included in this table as it includes patients with monotherapy of PEGV and various other medical combinations with PEGV
LA-SSA long-acting somatostatin analogues, PEGV pegvisomant, IGF-I insulin like growth factor I, AEs adverse events
Fig. 1.
Efficacy of combination treatment. Percentages of patients with IGF-I < 1.2 ×ULN and median PEGV doses (grey line, right Y-axis) are shown for every individual year during 9 years of combination treatment, LA-SSA combined with PEGV. Median PEGV dose is in mg weekly. Cumulative numbers of the included patients at each treatment year are depicted at the bottom of every bar. All patients (n = 112) were treated for at least 1 year, 17 patients were treated for a maximum of 9 years of follow up. LA-SSA: Long-acting somatostatin analogues; PEGV pegvisomant, IGF-I insulin like growth factor I. This figure was reproduced with permission from [12]
Escape, defined as need to increase the dose of PEGV because of an increase in IGF-I levels, was reported in 34 % of a Spanish cohort [25]. The majority of patients were easily controlled with either an increase in PEGV dose, additional medical treatment or both. Whether this increase in the necessary dose of PEGV should be called an escape is questionable, as most patients were easily controlled and remained controlled on a higher dose of PEGV. A significant number of these patients escaped from PEGV within the first 6 months after discontinuation of LA-SSA. Presumably during this period these patients were actually still receiving combination treatment, due to the long half-life of LA-SSAs. The Dutch cohort could not observe this phenomenon during combination treatment in acromegaly patients [12].
Co-administration of the highest dose of LA-SSA on top of PEGV monotherapy appears to reduce the necessary mean PEGV dose by 50 % [14], although with a high individual variability [26]. An analysis including eight patients whose mean IGF-I levels were similar during PEGV monotherapy, showed that these patients were able to reduce their PEGV dose from 131.3 ± 36.2 to 62.5 ± 16.7 mg weekly [14]. PEGV dose reduction during combination treatment might improve cost-effectiveness of medical treatment in acromegaly and may reduce injection frequency for patients. However, there are no direct studies comparing cost-effectiveness of the median required weekly PEGV dose during mono- and combination treatment.
Improved quality of life by the addition of PEGV
Physicians tend to be mainly focused on biochemical parameters as GH and IGF-I levels during treatment of acromegaly. These parameters are definitely linked to a better outcome and a lower risk of morbidity and mortality [27, 28]. A recent French study has described long-term effects of PEGV on cardiorespiratory and metabolic comorbidities [29]. They reported that long-term PEGV treatment improves cardiac function measured by the left ventricular ejection fraction and the left ventricular mass index. PEGV also improved the apnea–hypopnea index. Normalized serum GH and IGF-I levels does not necessarily result in complete resolution of signs and symptoms [30, 31]. Neggers et al. reported on the results of a prospective, double blind, placebo controlled, crossover study, and demonstrated improved quality of life (QoL) in patients using LA-SSA combined with low-dose PEGV [31]. QoL was assessed by two acromegaly specific QoL-questionnaires, the AcroQoL and the PASQ. Improved QoL was observed without significant changes in IGF-I levels after addition of PEGV to LA-SSA therapy in patients with normalized IGF-I levels during monotherapy of LA-SSA. However, a study by Madsen et al. did not observe an improved QoL in patients using LA-SSA combined with a low dose of PEGV [32]. Two differences in the design of the studies might clarify these different outcomes in QoL; (1) Madsen et al. reduced the dose of LA-SSA by 50 % before adding low-dose PEGV treatment, while Neggers et al. did not change the LA-SSA dose; (2) disease specific QoL-questionnaires were used by Neggers et al. whereas Madsen et al. used QoL-questionnaires which were not disease specific.
An explanation for the observed improvement in QoL by the addition of low-dose PEGV could be a persistent systemic acromegaly disease activity during monotherapy of LA-SSA that has been hypothesized and was called ‘extra-hepatic acromegaly’ [33]. LA-SSA treatment selectively decreases hepatic-IGF-I production by around 20 % via direct and indirect mechanisms [18, 19, 34]. This might lead to an overestimation of the efficacy of LA-SSAs to normalize IGF-I via a reduction in the pathological GH secretion because of this GH-independent reduction of IGF-I levels. Therefore, GH actions on extra-hepatic tissues can remain elevated despite normalization of serum IGF-I levels. LA-SSA reduces portal insulin levels which decreases hepatic GHR expression only [34]. This results in a relatively hyper-GH-sensitive state of the other tissues. Patients report these still excessive GH actions on non-hepatic tissues such as edema, fatigue and headaches comparable to the side-effects of high-dose GH replacement therapy in GH deficient subjects. A clinical example is a Danish study which observed, despite similarly normalized IGF-I levels, that LA-SSA treatment compared with neurosurgery alone was associated with less suppressed GH levels and less symptom relief [35]. Blocking non-hepatic GH actions by low-dose PEGV could therefore be useful in treating this ‘extra-hepatic acromegaly’ [33]. Moreover, PEGV has also been shown to improve insulin resistance by several mechanisms [36–41], which is beneficial in the presence of LA-SSAs, which are known to reduce insulin secretion [42, 43].
Pituitary tumor volume
Recent prospective multicenter clinical trial observed tumor shrinkage in 63 % of primary treated patients with 120 mg Lanreotide Autogel administered every 28 days [44]. Tumor shrinkage was defined as clinically significant when ≥20 % tumor volume reduction was observed after 48 weeks of Lanreotide Autogel administration. While LA-SSA is successful in reducing tumor volume, previous concerns were raised whether PEGV might induce growth of the pituitary adenoma. Despite the fact that in a few cases an increase in tumor size during PEGV therapy was reported, there are no data suggesting that PEGV directly promotes tumor growth [14, 45, 46]. In the GPOS study, changes in tumor size were systematically monitored in 307 patients, from which 28 patients were treated with PEGV in combination with LA-SSA, however predominantly treated with PEGV monotherapy [47]. In eight out of 307 (2.7 %) an initial increase in tumor size was reported. In only three of these eight patients a real, but minor, increase in tumor size after PEGV treatment was observed [47]. In two of these patients a detectable rebound increase in tumor size after discontinuation of LA-SSA therapy was the probable reason of this increase in adenoma size. In a Spanish study in 75 patients, 5 (6.7 %) acromegaly patients were identified with an increase in pituitary tumor size [48]. All of these patients were pre-treated with LA-SSA and then switched to PEGV monotherapy. Noteworthy in this study is that the reference MRI was made just after LA-SSA was discontinued [48]. Therefore, the reported tumor size increases in this study may also be explained by the rebound phenomenon after cessation of LA-SSA treatment. In the Dutch cohort of acromegaly patients (n = 141) on combination therapy, growth of the adenoma has been reported in only one patient, while this growth was already observed before the addition of PEGV [12]. Moreover, during combined treatment tumor size shrinkage of more than 20 % of the largest diameter before and during combination treatment was observed in 17 % of the patients and the vast majority had a stable tumor volume. We, therefore, conclude that PEGV apparently does not influence the natural course of tumor growth, but ongoing alertness is required to monitor tumor size by repetitive pituitary imaging.
Side effects
PEGV treatment is generally well tolerated and is increasingly considered to be safe [9, 49]. As LA-SSAs are on the market since 1988, more data are available on side effects. Most commonly reported are gastrointestinal symptoms such as nausea, vomiting, abdominal pain, biliary sludge or gallstones and diarrhea [50]. Abnormal glucose metabolism and injection-site reactions are also described [50]. Lipohypertrophy and hepatotoxicity are the most frequently reported adverse events during long-term PEGV treatment. Lipohypertrophy is an increased subcutaneous fat deposition around the injection sites of PEGV. It is suggested that the blockade of GH receptors causes unopposed insulin effects and, therefore, promotes lipogenesis [51]. The symptom arises in 3–15 % of the PEGV users either during monotherapy or combination treatment and appeared to be reversible by a more frequent rotation of the injection-site [12, 14, 21, 25, 52]. However, in one of the Dutch patients during combined treatment cosmetic surgery was necessary in order to reduce the significant lipohypertrophy [12]. Large observational studies as ACROSTUDY™ and GPOS, in which patients using PEGV are included regardless their concomitant medication, reported 2.2 and 7.4 % lipohypertrophy respectively.
Hepatotoxicity can occur during both PEGV monotherapy and combination therapy. Cholestasis is a common side effect of LA-SSA, as it reduces the secretion of cholecystokinin and motility of the gallbladder and induces an increase in bile concentration. This results in an increased risk in development of sludge and gallstones [53, 54]. LA-SSA often induces asymptomatic cholelithiasis, but an acute cholecystitis has been observed in only a minority of these patients [55]. The PEGV-induced elevations in hepatocellular enzymes are usually mild and self-limiting, both during monotherapy PEGV as in combination with LA-SSA [21, 52]. Transient elevated transaminases (TET) of more than three times the upper limit of normal (>3 ×ULN) were observed in 11.3–13.5 % of the patients using LA-SSA combined with PEGV [12, 21]. One Italian study compared long-term treatment of PEGV alone with PEGV in combination with LA-SSA regarding to TET [21]. Incidence of TET during monotherapy of PEGV was reported in 14.3 % of the patients, while this incidence was 11.1 % in the combined group. In the Dutchcohort (N = 19/141) all cases were transient without PEGV dose adaptation or discontinuation of the drug, except for one patient [12]. More details about TET patients are shown in Table 2. The development of TET was not PEGV dose-dependent. The ACROSTUDY™ and GPOS reported lower incidence rates of TET >3 ×ULN, 2.5 and 5.2 % respectively. The most recent ACROSTUDY™ paper, the Italian experience as it includes only patients registered in Italy (n = 341), reported an increase of TET >5 ×ULN in 0.9 % of the patients after 3 months of PEGV use and TET normalized after PEGV was withdrawn.
Table 2.
Transient elevated transaminases during combination treatment
| Sex | Age | Time between start PEGV and TET (months) | PEGV dose during TET | Peak LFT (×ULN) | Follow up | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bili | Alk. Phos. | γ-GT | AST | ALT | ||||||
| Biering et al. [56] | ||||||||||
| 1 | M | 43 | 2.3 | 10 mg daily | NA | NA | NA | 3.1 | 4.9 | Drug withdrawn, norm. of TET |
| 2a | F | 44 | 2.2 | 15 mg daily | NA | NA | NA | 11.1 | 20.8 | Drug withdrawn, norm. of TET |
| Soto Moreno et al. [57] | ||||||||||
| 1 | F | 31 | 1.5 | 10 mg daily | 0.3 | 0.3 | 1.4 | 117 | 80 | Drug continued, norm. of TET |
| Neggers et al. [12] | ||||||||||
| 1 | M | 41 | 61.9 | 30 mg weekly | 0.7 | 0.6 | 2.0 | 4.0 | 4.7 | Drug continued, norm. of TET |
| 2 | M | 39 | 19.5 | 300 mg weekly | NA | 1.0 | 1.8 | 5.0 | 5.5 | Drug continued, norm. of TET |
| 3 | M | 60 | 2.7 | 40 mg weekly | 0.4 | 1.2 | 0.9 | 4.3 | 7.0 | Drug continued, norm. of TET |
| 4 | M | 60 | 58.3 | 160 mg weekly | 0.4 | 1.2 | 1.8 | 4.6 | 6.5 | Drug continued, norm. of TET |
| 5 | M | 45 | 3.2 | 80 mg weekly | 1.1 | 0.5 | 2.0 | 3.5 | 3.9 | Drug continued, norm. of TET |
| 6a | M | 51 | 3.9 | 60 mg weekly | 2.3 | 2.9 | 16.7 | 16.7 | 25.8 | Drug continued, norm. of TET |
| 7 | M | 59 | 3.5 | 60 mg weekly | 0.6 | 0.8 | 1.8 | 2.4 | 3.6 | Drug continued, norm. of TET |
| 8a | M | 29 | 18.8 | 40 mg weekly | 5.3 | 0.9 | 5.1 | 4.9 | 8.0 | Drug continued, norm. of TET |
| 9 | M | 30 | 0.7 | 80 mg weekly | NA | 0.9 | 0.8 | 3.1 | 1.2 | Drug continued, norm. of TET |
| 10 | M | 40 | 5.8 | 40 mg weekly | 1.6 | 1.1 | 4.6 | 3.1 | 4.3 | Drug continued, norm. of TET |
| 11 | M | 34 | 4.9 | 40 mg weekly | 2.0 | 1.4 | 8.8 | 9.5 | 8.3 | Drug continued, norm. of TET |
| 12 | M | 46 | 2.9 | 60 mg weekly | 0.9 | 1.2 | 4.8 | 6.3 | 13.2 | Drug continued, norm. of TET |
| 13a | M | 74 | 12.8 | 80 mg weekly | 1.3 | 2.6 | 11.8 | 5.6 | 3.7 | Drug continued, norm. of TET |
| 14 | M | 45 | 5.8 | 60 mg weekly | 1.3 | 1.3 | 6.9 | 13.7 | 26.1 | Drug withdrawn, norm. of TET |
| 15 | F | 48 | 15.4 | 80 mg weekly | 0.8 | 0.8 | 1.5 | 2.9 | 4.5 | Drug continued, norm. of TET |
| 16 | F | 44 | 3.4 | 20 mg weekly | 1.0 | 0.6 | 0.9 | 7.5 | 10.0 | Drug continued, norm. of TET Re-exposure to PEGV: caused a 2nd TET period |
| 17 | F | 54 | 5.5 | 60 mg weekly | 0.7 | 0.7 | 2.7 | 2.8 | 4.0 | Drug continued, norm. of TET |
| 18 | F | 41 | 4.8 | 60 mg weekly | NA | NA | 3.9 | NA | 4.6 | Drug continued, norm. of TET |
| 19 | F | 61 | 11.3 | 160 mg weekly | NA | 1.1 | 4.1 | 2.4 | 3.7 | Drug continued, norm. of TET |
| 20 | F | 67 | 2.2 | 20 mg weekly | NA | 0.9 | 2.3 | 2.8 | 3.7 | Drug continued, norm. of TET Re-exposure to PEGV: caused a 2nd TET period |
| 21 | F | 41 | 2.5 | 40 mg weekly | 1.3 | 0.7 | 1.3 | 8.4 | 11.7 | Drug continued, norm. of TET |
| 22 | F | 27 | 4.1 | 60 mg weekly | NA | 1.0 | 1.5 | 4.0 | 4.8 | Drug continued, norm. of TET |
Description of TET patients during medical treatment with LA-SSA combined with PEGV
aElevated transaminases due to proven cholecystolithiasis
LFT liver function tests, TET transient elevated transaminases, LA-SSA long-acting somatostatin analogues, PEGV pegvisomant, ×ULN times upper limit of normal, Bili total bilirubin, Alk. Phos alkaline phosphatase, γ-GT γ-glutamyltranspeptidase, AST aspartate aminotransaminase, ALT alanine aminotransferase, NA not available
More frequent out-patient clinic visits and, thereby, more frequent assessments of ALT and AST might explain the observed differences in the incidence of TET, as the elevations in transaminases are transient and will pass unnoticed when follow-up intervals are wider. A previously described association between TET and Gilbert’s polymorphism (UGT1A1*28) in a Spanish study (n = 36) [58], was not confirmed by the Dutch cohort (n = 141) [12].
Conclusion
Advantages of the combination treatment of LA-SSA and PEGV are high efficacy rates in acromegaly patients, provided that the appropriate PEGV dose is used, which seems to be lower in the combined treatment than during PEGV monotherapy. Tumor shrinkage and cessation of tumor volume was observed in the vast majority of the patients. Disadvantages of combining LA-SSA with PEGV is the economic burden of life-long administration of expensive medicaments and side effects of both drugs can occur. Furthermore, in patients treated with LA-SSA and PEGV, only IGF-I levels can be interpreted as some commercial GH assays are not able to distinguish between GH and PEGV. Although, the combination of LA-SSA and PEGV is generally well tolerated and side effects as lipohypertrophy and elevated transaminase are usually mild and transient, clinical attention towards side effects remain obligate. Therefore, we recommend to frequently rotate the PEGV injection-site as this reduces the risk of (serious) lipohypertrophy. With respect to the elevated transaminases >3 ×ULN, we recommend assessing liver enzymes levels with the same frequency as described by the label of PEGV. Cholelithiasis must be excluded by an ultrasound of the liver and gallbladder. In patients with TET >10 ×ULN, we also recommend performing a liver biopsy and discontinuing PEGV in case of drug-induced hepatitis. PEGV-induced tumor size increase has not been observed to date, but repetitive pituitary imaging remains mandatory.
Acknowledgments
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Abbreviations
- GH
Growth hormone
- IGF-I
Insulin-like growth factor I
- LA-SSA
Long-acting somatostatin analogue
- GHR
Growth hormone receptor
- PEGV
Pegvisomant
Compliance with ethical standards
Conflict of Interest
A.J. van der Lely and S.J.C.M.M. Neggers have received financial support for investigator-initiated research, unrestricted grants and speakers fees from Novartis, Pfizer, and Ipsen. A.J. van der Lely also received consultancy fees from Novartis and Pfizer. S.E. Franck and A. Muhammad have nothing to disclose.
Footnotes
S.E. Franck and A. Muhammad contributed equally and share first authorship.
References
- 1.Melmed S. Medical progress: acromegaly. N. Engl. J. Med. 2006;355:2558–2573. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 2.Holdaway IM. Treatment of acromegaly. Horm. Res. 2004;62(Suppl 3):79–92. doi: 10.1159/000080505. [DOI] [PubMed] [Google Scholar]
- 3.Bates PR, Carson MN, Trainer PJ, Wass JA, Group UKNARS Wide variation in surgical outcomes for acromegaly in the UK. Clin. Endocrinol. 2008;68:136–142. doi: 10.1111/j.1365-2265.2007.03012.x. [DOI] [PubMed] [Google Scholar]
- 4.Freda PU, Katznelson L, van der Lely AJ, Reyes CM, Zhao S, Rabinowitz D. Long-acting somatostatin analog therapy of acromegaly: a meta-analysis. J. Clin. Endocrinol. Metab. 2005;90:4465–4473. doi: 10.1210/jc.2005-0260. [DOI] [PubMed] [Google Scholar]
- 5.Amato G, Mazziotti G, Rotondi M, Iorio S, Doga M, Sorvillo F, Manganella G, Di Salle F, Giustina A, Carella C. Long-term effects of lanreotide SR and octreotide LAR on tumour shrinkage and GH hypersecretion in patients with previously untreated acromegaly. Clin. Endocrinol. 2002;56:65–71. doi: 10.1046/j.0300-0664.2001.01438.x. [DOI] [PubMed] [Google Scholar]
- 6.Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, van der Lely AJ, Strasburger CJ, Lamberts SW, Ho KK, Casanueva FF, Melmed S, Acromegaly Consensus G Expert consensus document: a consensus on the medical treatment of acromegaly. Nat. Rev. Endocrinol. 2014;10:243–248. doi: 10.1038/nrendo.2014.21. [DOI] [PubMed] [Google Scholar]
- 7.Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML, Besser GM, Scarlett JA, Thorner MO, Parkinson C, Klibanski A, Powell JS, Barkan AL, Sheppard MC, Malsonado M, Rose DR, Clemmons DR, Johannsson G, Bengtsson BA, Stavrou S, Kleinberg DL, Cook DM, Phillips LS, Bidlingmaier M, Strasburger CJ, Hackett S, Zib K, Bennett WF, Davis RJ. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N. Engl. J. Med. 2000;342:1171–1177. doi: 10.1056/NEJM200004203421604. [DOI] [PubMed] [Google Scholar]
- 8.van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L, Klibanski A, Herman-Bonert V, Melmed S, Vance ML, Freda PU, Stewart PM, Friend KE, Clemmons DR, Johannsson G, Stavrou S, Cook DM, Phillips LS, Strasburger CJ, Hackett S, Zib KA, Davis RJ, Scarlett JA, Thorner MO. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358:1754–1759. doi: 10.1016/S0140-6736(01)06844-1. [DOI] [PubMed] [Google Scholar]
- 9.van der Lely AJ, Biller BM, Brue T, Buchfelder M, Ghigo E, Gomez R, Hey-Hadavi J, Lundgren F, Rajicic N, Strasburger CJ, Webb SM, Koltowska-Haggstrom M. Long-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDY. J. Clin. Endocrinol. Metab. 2012;97:1589–1597. doi: 10.1210/jc.2011-2508. [DOI] [PubMed] [Google Scholar]
- 10.Feenstra J, de Herder WW, ten Have SM, van den Beld AW, Feelders RA, Janssen JA, van der Lely AJ. Combined therapy with somatostatin analogues and weekly pegvisomant in active acromegaly. Lancet. 2005;365:1644–1646. doi: 10.1016/S0140-6736(05)63011-5. [DOI] [PubMed] [Google Scholar]
- 11.Neggers SJ, de Herde WW, Janssen JA, Feelder RA, van der Lely AJ. Combined treatment for acromegaly with long-acting somatostatin analogs and pegvisomant: long-term safety for up to 4.5 years (median 2.2 years) of follow-up in 86 patients. Eur. J. Endocrinol. 2009;160:529–533. doi: 10.1530/EJE-08-0843. [DOI] [PubMed] [Google Scholar]
- 12.Neggers SJ, Franck SE, de Rooij FW, Dallenga AH, Poublon RM, Feelders RA, Janssen JA, Buchfelder M, Hofland LJ, Jorgensen JO, van der Lely AJ. Long-term efficacy and safety of pegvisomant in combination with long-acting somatostatin analogs in acromegaly. J. Clin. Endocrinol. Metab. 2014;99:3644–3652. doi: 10.1210/jc.2014-2032. [DOI] [PubMed] [Google Scholar]
- 13.Neggers SJ, van Aken MO, Janssen JA, Feelders RA, de Herder WW, van der Lely AJ. Long-term efficacy and safety of combined treatment of somatostatin analogs and pegvisomant in acromegaly. J. Clin. Endocrinol. Metab. 2007;92:4598–4601. doi: 10.1210/jc.2007-1234. [DOI] [PubMed] [Google Scholar]
- 14.van der Lely AJ, Bernabeu I, Cap J, Caron P, Colao A, Marek J, Neggers S, Birman P. Coadministration of lanreotide Autogel and pegvisomant normalizes IGF1 levels and is well tolerated in patients with acromegaly partially controlled by somatostatin analogs alone. Eur. J. Endocrinol. 2011;164:325–333. doi: 10.1530/EJE-10-0867. [DOI] [PubMed] [Google Scholar]
- 15.Annamalai AK, Webb A, Kandasamy N, Elkhawad M, Moir S, Khan F, Maki-Petaja K, Gayton EL, Strey CH, O’Toole S, Ariyaratnam S, Halsall DJ, Chaudhry AN, Berman L, Scoffings DJ, Antoun NM, Dutka DP, Wilkinson IB, Shneerson JM, Pickard JD, Simpson HL, Gurnell M. A comprehensive study of clinical, biochemical, radiological, vascular, cardiac, and sleep parameters in an unselected cohort of patients with acromegaly undergoing presurgical somatostatin receptor ligand therapy. J. Clin. Endocrinol. Metab. 2013;98:1040–1050. doi: 10.1210/jc.2012-3072. [DOI] [PubMed] [Google Scholar]
- 16.Caron PJ, Bevan JS, Petersenn S, Flanagan D, Tabarin A, Prevost G, Maisonobe P, Clermont A, On behalf of the PI Tumor shrinkage with lanreotide autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial. J. Clin. Endocrinol. Metab. 2013 doi: 10.1210/jc.2013-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trainer PJ, Ezzat S, D’Souza GA, Layton G, Strasburger CJ. A randomized, controlled, multicentre trial comparing pegvisomant alone with combination therapy of pegvisomant and long-acting octreotide in patients with acromegaly. Clin. Endocrinol. 2009;71:549–557. doi: 10.1111/j.1365-2265.2009.03620.x. [DOI] [PubMed] [Google Scholar]
- 18.Laursen T, Moller J, Fisker S, Jorgensen JO, Christiansen JS. Effects of a 7-day continuous infusion of octreotide on circulating levels of growth factors and binding proteins in growth hormone (GH)-treated GH-deficient patients. Growth Horm. IGF Res. 1999;9:451–457. doi: 10.1054/ghir.1999.0131. [DOI] [PubMed] [Google Scholar]
- 19.Pokrajac A, Frystyk J, Flyvbjerg A, Trainer PJ. Pituitary-independent effect of octreotide on IGF1 generation. Eur. J. Endocrinol. 2009;160:543–548. doi: 10.1530/EJE-08-0822. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen JO, Feldt-Rasmussen U, Frystyk J, Chen JW, Kristensen LO, Hagen C, Orskov H. Cotreatment of acromegaly with a somatostatin analog and a growth hormone receptor antagonist. J. Clin. Endocrinol. Metab. 2005;90:5627–5631. doi: 10.1210/jc.2005-0531. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi A, Valentini F, Iuorio R, Poggi M, Baldelli R, Passeri M, Giampietro A, Tartaglione L, Chiloiro S, Appetecchia M, Gargiulo P, Fabbri A, Toscano V, Pontecorvi A, De Marinis L. Long-term treatment of somatostatin analog-refractory growth hormone-secreting pituitary tumors with pegvisomant alone or combined with long-acting somatostatin analogs: a retrospective analysis of clinical practice and outcomes. J. Exp. Clin. Cancer Res. 2013;32:40. doi: 10.1186/1756-9966-32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber I, Buchfelder M, Droste M, Forssmann K, Mann K, Saller B, Strasburger CJ. German Pegvisomant I. Treatment of acromegaly with the GH receptor antagonist pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur. J. Endocrinol. 2007;156:75–82. doi: 10.1530/eje.1.02312. [DOI] [PubMed] [Google Scholar]
- 23.Trainer PJ. ACROSTUDY: the first 5 years. Eur. J. Endocrinol. 2009;161(Suppl 1):S19–S24. doi: 10.1530/EJE-09-0322. [DOI] [PubMed] [Google Scholar]
- 24.van der Lely AJ, Muller A, Janssen JA, Davis RJ, Zib KA, Scarlett JA, Lamberts SW. Control of tumor size and disease activity during cotreatment with octreotide and the growth hormone receptor antagonist pegvisomant in an acromegalic patient. J. Clin. Endocrinol. Metab. 2001;86:478–481. doi: 10.1210/jcem.86.2.7206. [DOI] [PubMed] [Google Scholar]
- 25.Sesmilo G, Resmini E, Bernabeu I, Aller J, Soto A, Mora M, Pico A, Fajardo C, Torres E, Alvarez-Escola C, Garcia R, Blanco C, Camara R, Gaztambide S, Salinas I, Pozo CD, Castells I, Villabona C, Biagetti B, Webb SM. Escape and lipodystrophy in acromegaly during pegvisomant therapy, a retrospective multicentre Spanish study. Clin. Endocrinol. 2014;81:883–890. doi: 10.1111/cen.12440. [DOI] [PubMed] [Google Scholar]
- 26.Neggers SJ, de Herder WW, Feelders RA, van der Lely AJ. Conversion of daily pegvisomant to weekly pegvisomant combined with long-acting somatostatin analogs, in controlled acromegaly patients. Pituitary. 2011;14:253–258. doi: 10.1007/s11102-010-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur. J. Endocrinol. 2008;159:89–95. doi: 10.1530/EJE-08-0267. [DOI] [PubMed] [Google Scholar]
- 28.Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J. Clin. Endocrinol. Metab. 2004;89:667–674. doi: 10.1210/jc.2003-031199. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn E, Maione L, Bouchachi A, Roziere M, Salenave S, Brailly-Tabard S, Young J, Kamenicky P, Assayag P, Chanson P. Long-term effects of pegvisomant on comorbidities in patients with acromegaly: a retrospective single-center study. Eur. J. Endocrinol. 2015;173:693–702. doi: 10.1530/EJE-15-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hua SC, Yan YH, Chang TC. Associations of remission status and lanreotide treatment with quality of life in patients with treated acromegaly. Eur. J. Endocrinol. 2006;155:831–837. doi: 10.1530/eje.1.02292. [DOI] [PubMed] [Google Scholar]
- 31.Neggers SJ, van Aken MO, de Herder WW, Feelders RA, Janssen JA, Badia X, Webb SM, van der Lely AJ. Quality of life in acromegalic patients during long-term somatostatin analog treatment with and without pegvisomant. J. Clin. Endocrinol. Metab. 2008;93:3853–3859. doi: 10.1210/jc.2008-0669. [DOI] [PubMed] [Google Scholar]
- 32.Madsen M, Poulsen PL, Orskov H, Moller N, Jorgensen JO. Cotreatment with pegvisomant and a somatostatin analog (SA) in SA-responsive acromegalic patients. J. Clin. Endocrinol. Metab. 2011;96:2405–2413. doi: 10.1210/jc.2011-0654. [DOI] [PubMed] [Google Scholar]
- 33.Neggers SJ, Kopchick JJ, Jorgensen JO, van der Lely AJ. Hypothesis: Extra-hepatic acromegaly: a new paradigm? Eur. J. Endocrinol. 2011;164:11–16. doi: 10.1530/EJE-10-0969. [DOI] [PubMed] [Google Scholar]
- 34.Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J. Clin. Endocrinol. Metab. 2000;85:4712–4720. doi: 10.1210/jcem.85.12.7017. [DOI] [PubMed] [Google Scholar]
- 35.Rubeck KZ, Madsen M, Andreasen CM, Fisker S, Frystyk J, Jorgensen JO. Conventional and novel biomarkers of treatment outcome in patients with acromegaly: discordant results after somatostatin analog treatment compared with surgery. Eur. J. Endocrinol. 2010;163:717–726. doi: 10.1530/EJE-10-0640. [DOI] [PubMed] [Google Scholar]
- 36.Rose DR, Clemmons DR. Growth hormone receptor antagonist improves insulin resistance in acromegaly. Growth Horm. IGF Res. 2002;12:418–424. doi: 10.1016/S1096-6374(02)00083-7. [DOI] [PubMed] [Google Scholar]
- 37.Drake WM, Rowles SV, Roberts ME, Fode FK, Besser GM, Monson JP, Trainer PJ. Insulin sensitivity and glucose tolerance improve in patients with acromegaly converted from depot octreotide to pegvisomant. Eur. J. Endocrinol. 2003;149:521–527. doi: 10.1530/eje.0.1490521. [DOI] [PubMed] [Google Scholar]
- 38.Barkan AL, Burman P, Clemmons DR, Drake WM, Gagel RF, Harris PE, Trainer PJ, van der Lely AJ, Vance ML. Glucose homeostasis and safety in patients with acromegaly converted from long-acting octreotide to pegvisomant. J. Clin. Endocrinol. Metab. 2005;90:5684–5691. doi: 10.1210/jc.2005-0331. [DOI] [PubMed] [Google Scholar]
- 39.Lindberg-Larsen R, Moller N, Schmitz O, Nielsen S, Andersen M, Orskov H, Jorgensen JO. The impact of pegvisomant treatment on substrate metabolism and insulin sensitivity in patients with acromegaly. J. Clin. Endocrinol. Metab. 2007;92:1724–1728. doi: 10.1210/jc.2006-2276. [DOI] [PubMed] [Google Scholar]
- 40.Higham CE, Rowles S, Russell-Jones D, Umpleby AM, Trainer PJ. Pegvisomant improves insulin sensitivity and reduces overnight free fatty acid concentrations in patients with acromegaly. J. Clin. Endocrinol. Metab. 2009;94:2459–2463. doi: 10.1210/jc.2008-2086. [DOI] [PubMed] [Google Scholar]
- 41.Urbani C, Sardella C, Calevro A, Rossi G, Scattina I, Lombardi M, Lupi I, Manetti L, Martino E, Bogazzi F. Effects of medical therapies for acromegaly on glucose metabolism. Eur. J. Endocrinol. 2013;169:99–108. doi: 10.1530/EJE-13-0032. [DOI] [PubMed] [Google Scholar]
- 42.Krentz AJ, Boyle PJ, Macdonald LM, Schade DS. Octreotide: a long-acting inhibitor of endogenous hormone secretion for human metabolic investigations. Metabolism. 1994;43:24–31. doi: 10.1016/0026-0495(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 43.Presti ME, Burton FR, Niehoff ML, Rioux J, Garvin PJ. Effect of octreotide on stimulated insulin release from pancreatic tissue slices. Pancreas. 1998;16:141–147. doi: 10.1097/00006676-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Caron PJ, Bevan JS, Petersenn S, Flanagan D, Tabarin A, Prevost G, Maisonobe P, Clermont A. Tumor shrinkage with lanreotide Autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial. J. Clin. Endocrinol. Metab. 2014;99:1282–1290. doi: 10.1210/jc.2013-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchfelder M, Schlaffer S, Droste M, Mann K, Saller B, Brubach K, Stalla GK, Strasburger CJ. The German ACROSTUDY: past and present. Eur. J. Endocrinol. 2009;161(Suppl 1):S3–S10. doi: 10.1530/EJE-09-0350. [DOI] [PubMed] [Google Scholar]
- 46.Neggers SJ, Biermasz NR, van der Lely AJ. What is active acromegaly and which parameters do we have? Clin. Endocrinol. 2012;76:609–614. doi: 10.1111/j.1365-2265.2012.04346.x. [DOI] [PubMed] [Google Scholar]
- 47.Buchfelder M, Weigel D, Droste M, Mann K, Saller B, Brubach K, Stalla GK, Bidlingmaier M, Strasburger CJ. Investigators of German Pegvisomant Observational S. Pituitary tumor size in acromegaly during pegvisomant treatment: experience from MR re-evaluations of the German Pegvisomant Observational Study. Eur. J. Endocrinol. 2009;161:27–35. doi: 10.1530/EJE-08-0910. [DOI] [PubMed] [Google Scholar]
- 48.Marazuela M, Paniagua AE, Gahete MD, Lucas T, Alvarez-Escola C, Manzanares R, Cameselle-Teijeiro J, Luque-Ramirez M, Luque RM, Fernandez-Rodriguez E, Castano JP, Bernabeu I. Somatotroph tumor progression during pegvisomant therapy: a clinical and molecular study. J. Clin. Endocrinol. Metab. 2011;96:E251–E259. doi: 10.1210/jc.2010-1742. [DOI] [PubMed] [Google Scholar]
- 49.Freda PU, Gordon MB, Kelepouris N, Jonsson P, Koltowska-Haggstrom M, van der Lely AJ. Long-term treatment with pegvisomant as monotherapy in patients with acromegaly: experience from acrostudy. Endocr. Pract. 2014 doi: 10.4158/EP14330.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grasso LF, Auriemma RS, Pivonello R, Colao A. Adverse events associated with somatostatin analogs in acromegaly. Expert. Opin. Drug Saf. 2015;14:1213–1226. doi: 10.1517/14740338.2015.1059817. [DOI] [PubMed] [Google Scholar]
- 51.Bonert VS, Kennedy L, Petersenn S, Barkan A, Carmichael J, Melmed S. Lipodystrophy in patients with acromegaly receiving pegvisomant. J. Clin. Endocrinol. Metab. 2008;93:3515–3518. doi: 10.1210/jc.2008-0833. [DOI] [PubMed] [Google Scholar]
- 52.Marazuela M, Lucas T, Alvarez-Escola C, Puig-Domingo M, de la Torre NG, de Miguel-Novoa P, Duran-Hervada A, Manzanares R, Luque-Ramirez M, Halperin I, Casanueva FF, Bernabeu I. Long-term treatment of acromegalic patients resistant to somatostatin analogues with the GH receptor antagonist pegvisomant: its efficacy in relation to gender and previous radiotherapy. Eur. J. Endocrinol. 2009;160:535–542. doi: 10.1530/EJE-08-0705. [DOI] [PubMed] [Google Scholar]
- 53.Lembcke B, Creutzfeldt W, Schleser S, Ebert R, Shaw C, Koop I. Effect of the somatostatin analogue sandostatin (SMS 201-995) on gastrointestinal, pancreatic and biliary function and hormone release in normal men. Digestion. 1987;36:108–124. doi: 10.1159/000199408. [DOI] [PubMed] [Google Scholar]
- 54.Moschetta A, Stolk MF, Rehfeld JF, Portincasa P, Slee PH, Koppeschaar HP, Van Erpecum KJ, Vanberge-Henegouwen GP. Severe impairment of postprandial cholecystokinin release and gall-bladder emptying and high risk of gallstone formation in acromegalic patients during Sandostatin LAR. Aliment. Pharmacol. Ther. 2001;15:181–185. doi: 10.1046/j.1365-2036.2001.00924.x. [DOI] [PubMed] [Google Scholar]
- 55.Shi YF, Zhu XF, Harris AG, Zhang JX, Dai Q. Prospective study of the long-term effects of somatostatin analog (octreotide) on gallbladder function and gallstone formation in Chinese acromegalic patients. J. Clin. Endocrinol. Metab. 1993;76:32–37. doi: 10.1210/jcem.76.1.8421099. [DOI] [PubMed] [Google Scholar]
- 56.Biering H, Saller B, Bauditz J, Pirlich M, Rudolph B, Johne A, Buchfelder M, Mann K, Droste M, Schreiber I, Lochs H, Strasburger CJ. German pegvisomant i. Elevated transaminases during medical treatment of acromegaly: a review of the German pegvisomant surveillance experience and a report of a patient with histologically proven chronic mild active hepatitis. Eur. J. Endocrinol. 2006;154:213–220. doi: 10.1530/eje.1.02079. [DOI] [PubMed] [Google Scholar]
- 57.Soto Moreno A, Guerrero Vazquez R, Venegas Moreno E, Palma Milla S, Castano JP, Leal Cerro A. Self-limited acute hepatotoxicity caused by pegvisomant. Pituitary. 2011;14:371–376. doi: 10.1007/s11102-009-0173-3. [DOI] [PubMed] [Google Scholar]
- 58.Bernabeu I, Marazuela M, Lucas T, Loidi L, Alvarez-Escola C, Luque-Ramirez M, Fernandez-Rodriguez E, Paniagua AE, Quinteiro C, Casanueva FF. Pegvisomant-induced liver injury is related to the UGT1A1*28 polymorphism of Gilbert’s syndrome. J. Clin. Endocrinol. Metab. 2010;95:2147–2154. doi: 10.1210/jc.2009-2547. [DOI] [PubMed] [Google Scholar]