Abstract
Rationale: Although research supports a sleep-disordered breathing and atrial fibrillation association, prospective data examining sleep-disordered breathing predicting incident atrial fibrillation are lacking.
Objectives: To investigate sleep-disordered breathing indices as predictors of incident atrial fibrillation.
Methods: A cohort (n = 843) of ambulatory older men without prevalent atrial fibrillation was assessed for baseline sleep indices: apnea–hypopnea index, central sleep apnea (central apnea index, ≥5 vs. <5), central sleep apnea or Cheyne-Stokes respiration, obstructive apnea–hypopnea index, and percentage of sleep time with less than 90% oxygen saturation. Incident clinically symptomatic adjudicated or self-reported atrial fibrillation outcome was ascertained (mean follow-up, 6.5 ± 0.7 yr). We used logistic regression models adjusted for age, race, body mass index, cardiopulmonary disease, alcohol use, pacemaker, cholesterol, cardiac medications, and alternate apnea type for obstructive and central apnea. Age interaction terms and median age-stratified analyses were performed.
Measurements and Main Results: Central sleep apnea (odds ratio [OR], 2.58; 95% confidence interval [CI], 1.18–5.66) and Cheyne-Stokes respiration with central sleep apnea (OR, 2.27; 95% CI, 1.13–4.56), but not obstructive apnea or hypoxemia, predicted incident atrial fibrillation. Central apnea, Cheyne-Stokes respiration, and sleep-disordered breathing–age interaction terms were significant (P < 0.05). Unlike the case with younger participants, among participants aged 76 years or older (albeit with small atrial fibrillation counts), atrial fibrillation was related to central apnea (OR, 9.97; 95% CI, 2.72–36.50), Cheyne-Stokes respiration with central apnea (OR, 6.31; 95% CI, 1.94–20.51), and apnea–hypopnea index (OR, 1.22; 95% CI, 1.08–1.39 [per 5-unit increase]).
Conclusions: In older men, central apnea and Cheyne-Stokes respiration predicted increased atrial fibrillation risk, with findings being strongest in older participants in whom overall sleep-disordered breathing also increased atrial fibrillation risk.
Keywords: atrial fibrillation, cohort study, sleep-disordered breathing
At a Glance Commentary
Scientific Knowledge on the Subject
Obstructive sleep apnea has been linked with atrial fibrillation (AF) in clinic-based, cross-sectional, and retrospective studies.
What This Study Adds to the Field
In a large, population-based, multicenter, prospective study with careful collection of sleep measures involving a cohort of older men vulnerable to AF development, central sleep apnea and Cheyne-Stokes respiration were associated with incident AF. In the older (age ≥76 yr) but not the younger subgroup, all sleep-disordered breathing indices, including both obstructive and central measures, were associated with incident AF.
Sleep-disordered breathing (SDB) is a highly prevalent physiologic stressor that has been implicated in the burden of atrial fibrillation (AF) (1–3). SDB is characterized by repetitive breathing cessation accompanied by physiologic stressors that include intermittent hypoxia, hypercapnia, autonomic nervous system fluctuations, and intrathoracic pressure swings, which can predispose individuals to enhanced arrhythmogenicity. AF is highly prevalent, particularly with increasing age, as evidenced by a threefold increase between the sixth and eighth decades of life (4). The large population burden of AF is highlighted by a progressively increased prevalence, estimated to reach 10 million in the United States by 2050, and contributes to substantial health care costs and morbidity (4–7).
Cross-sectional epidemiologic data have identified a strong association between AF and SDB (8, 9). The plausibility of a causal relationship is supported by data from a case–crossover study that identified an increase in paroxysmal AF events immediately following SDB events, suggesting that respiratory disturbances may trigger AF events (10). However, an important limitation of existing community-based epidemiologic studies is their cross-sectional nature, which precludes understanding of the temporal relationship between exposure (SDB and its subtypes) and outcome (AF), thereby limiting ability to inform causality (8, 9). In large, retrospective, clinic-based studies with a focus on obstructive sleep apnea (OSA), researchers have identified (1) an association of nocturnal oxygen desaturation and incident AF only in participants younger than 65 years of age (11) and (2) a dose–response relationship of OSA severity and hypoxemia with incident AF (12). Existing data also suggest that central events may more significantly associate with atrial arrhythmogenesis, while obstructive respiratory events may more strongly contribute to ventricular arrhythmogenesis (9). Although rigorously conducted randomized controlled trials are lacking, data from retrospective studies identify the impact of primarily OSA (but not central sleep apnea [CSA]) treatment on reduction of the recurrence of AF after cardioversion or ablation, and they allude to a potential causal relationship (13–17).
Although existing studies support an association of SDB and AF, it remains unclear whether SDB serves as a causal risk factor in the development of AF or vice versa. Gaining insight into the role of initiators of AF such as SDB is key to the development of AF prevention strategies and has been identified as a critical knowledge gap by several national AF prevention and treatment expert working groups (18, 19). Furthermore, understanding the SDB characteristics (i.e., obstructive, central, and hypoxemic) that contribute to atrial arrhythmogenesis may allow honing of prevention and treatment to target the specific physiologic determinants of abnormal sleep-related breathing that lead to AF development.
To further characterize the relationship of SDB and its subtypes with AF, we examined SDB and AF development in a prospective, large-scale, multicenter, community-based study of older men, a group particularly vulnerable to AF and its morbidity. To do so, we systematically collected polysomnography (PSG) data and used standardized methods of clinically symptomatic AF identification, and we also analyzed self-reported AF data (20). We hypothesized that SDB would predict incident AF and postulated that central apnea would represent the strongest SDB determinant of predicting future AF (8, 11). Some of the results of these studies were reported previously in the form of an abstract (21).
Methods
Participants and Study Design
This prospective observational study involved participants in the MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study, an ancillary study of the MrOS (Osteoporotic Fractures in Men) Study. The MrOS investigators enrolled 5,994 community-living men aged 65 years and older who were able to ambulate without assistance and were without a history of bilateral hip replacement. They recruited participants at six centers (Birmingham, AL; Minneapolis, MN; the Monongahela Valley, near Pittsburgh, PA; Palo Alto, CA; Portland, OR; and San Diego, CA) (22, 23). The MrOS study design, methods, and demographics were published previously (see online supplement) (9, 22, 23).
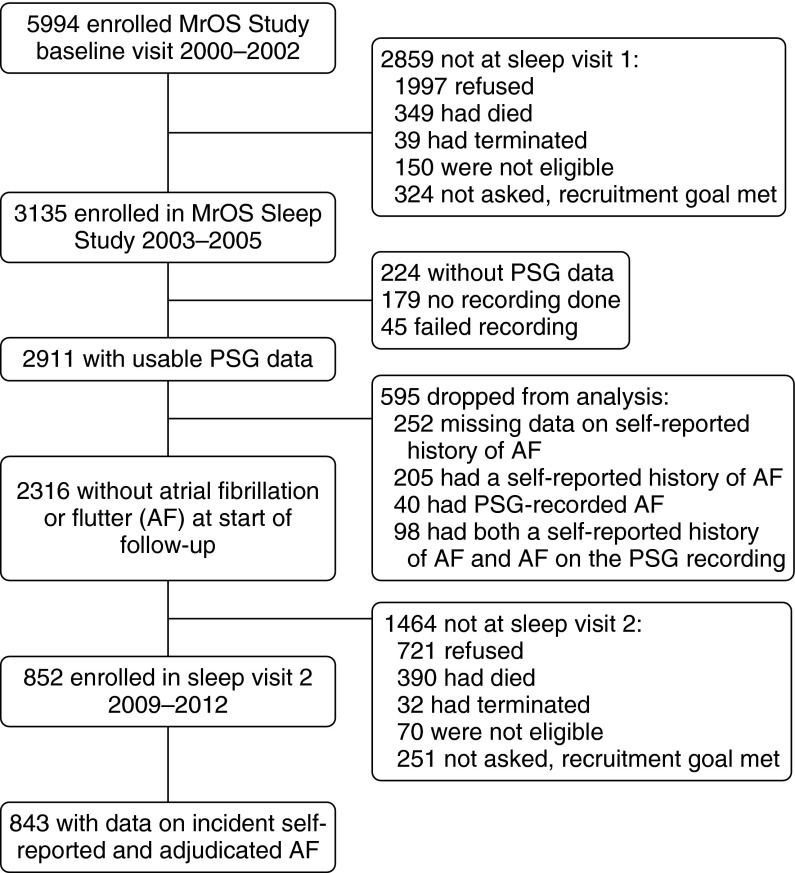
The MrOS Sleep Study researchers recruited 3,135 participants between December 2003 and March 2005 for a comprehensive sleep assessment. Of the 3,135 MrOS Sleep Study participants recruited, 179 did not participate in PSG secondary to refusal or contemporaneous treatment for SDB, and 45 men had failed sleep studies (1.5%), thus leaving 2,911 participants. Of these, 2,316 did not have PSG-identified or self-reported prevalent AF at the first sleep visit. A subgroup of men (n = 852) participated in a second sleep visit between November 2009 and March 2012. The analytic cohort consisted of study participants with acceptable PSG at the initial sleep visit, data on incident adjudicated and self-reported AF (n = 843), and mean follow-up time of 6.5 ± 0.7 years (Figure 1). Additional recruitment information is provided in the online supplement.
Figure 1.
MrOS (Osteoporotic Fractures in Men) Study design: recruitment, attrition, and retention. PSG = polysomnography.
Each site and the study coordinating center received ethical approval from their institutional review boards. Written informed consent was obtained from all participants.
PSG Data
Unattended home PSG (Safiro ambulatory EEG system; Compumedics, Abbotsford, Australia) was performed. It consisted of recording of C3/A2 and C4/A1 electroencephalograms, bilateral electrooculograms, a bipolar submental electromyogram, thoracic and abdominal inductance plethysmography, airflow (oronasal thermocouple and nasal pressure cannula), finger pulse oximetry, lead I ECG, body positions, and bilateral leg movements (9). Indices per hour of sleep included the apnea–hypopnea index (AHI; measure of the number of obstructive and central apneas and hypopneas with ≥3% oxygen desaturation) (24), obstructive apnea–hypopnea index (OAHI; measure of the number of obstructive apneas and hypopneas with ≥3% desaturation), and central apnea index (CAI; measure of the number of central apneas). Cheyne-Stokes respiration (CSR) was characterized by a minimum consecutive 10-minute period of a crescendo–decrescendo respiratory pattern culminating in a nadir of central apneas with typical cycle length. The percentage of total sleep time (TST) spent below an oxygen saturation of 90% defined sleep-related hypoxemia.
Outcome Measures
AF was defined as either incident adjudicated AF or self-reported AF (25–27). Adjudicated clinically symptomatic AF—defined by symptoms, hospitalization, prolongation of hospitalization, or requiring invasive procedure—was identified by surveying for incident events followed by central adjudication using a standard protocol (see online supplement). Self-reported AF was ascertained at each sleep visit by asking participants if they had ever been diagnosed with AF or atrial flutter.
Covariate Measures
Covariate measures, including demographics, medical history, alcohol use, cardiovascular disease (CVD) medications (calcium channel blockers, nonophthalmic β-blockers, cardiac glycosides, or antiarrhythmic medications such as cardiac sodium channel blockers and potassium channel blockers), body mass index (BMI), cholesterol level, and pacemaker presence were assessed (28). CVD was defined by history of myocardial infarction, angina, angioplasty, congestive heart failure (CHF), and/or coronary artery bypass graft surgery. A full description of covariate collection is provided in the online supplement.
Statistical Analysis
PSG parameters were expressed as continuous and/or categorical variables, the latter informed by standard cutoffs (29) or distributional characteristics. SDB was defined per 5-unit AHI increase and alternatively as AHI greater than or equal to 15 versus less than 15 based upon clinical convention (29) and as OSA per 5-unit OAHI increase. CSA was defined as CAI of 5 or greater versus CAI less than 5 and CSA-CSR as CAI of 5 or greater or CSR. TST spent below an oxygen saturation of 90% was defined per category, given its skewed nature (<1%, 1 to <3.5%, 3.5 to <10%, and ≥10%).
Participant characteristics were summarized as mean ± SD or count and percentage, and they were compared by AHI category using χ2 tests for categorical variables, t tests for normally distributed continuous variables, and Wilcoxon rank-sum tests for continuous variables with skewed distributions. SDB or nocturnal hypoxemia and subsequent AF risk associations were assessed by logistic regression. The results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). Models are presented as minimally adjusted (age, clinic, and race) and multivariable adjusted (age; clinic; race; BMI; total cholesterol; self-reported history of CVD, hypertension, diabetes, stroke, alcohol use, and chronic obstructive pulmonary disease; pacemaker placement; use of CVD medications; and OAHI in the CSA and CSA-CSR models and CAI in the OSA model). We assessed SDB by age interaction (significant if P < 0.10) and stratified by median age (<76 yr, ≥76 yr) (8). Sensitivity analyses included separate analyses excluding participants with a history of CHF or incident CHF.
All significance levels reported are two-sided, and all analyses were conducted using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
Study Population
Of 843 participants in the final analytic study sample, 86% were white. Their average BMI was 27 ± 4 kg/m2. Compared with those in our analysis subset, the 390 men who died before sleep visit 2 had higher rates of CAI of 5 or greater (6% vs. 8%; P = 0.13) and higher rates of CSA-CSR (8% vs. 11%; P = 0.07), although these differences did not reach statistical significance. As expected for this age range, 45% had hypertension. Although 25% had some form of CVD, only 3% of participants had a self-reported history of heart failure (Table 1). Compared with those with AHI less than 15, those with AHI of 15 or greater (42% of participants) had a significantly higher BMI, more prevalent hypertension, and a higher percentage of TST with hypoxemia (Table 1). Compared with the 843 men in our analytic cohort, the 1,473 men with PSG data without a history of AF who did not participate in the second examination were similar, on average, in SDB indices and BMI and had similar rates of chronic obstructive lung disease, stroke, diabetes, and pacemaker placement (P > 0.05). However, these men were older by 2.5 years, on average, and had a higher percentage of hypertension, CVD, CHF, and cardiac medication use as well as lower alcohol intake (P < 0.05).
Table 1.
Baseline Characteristics of Participants
| Characteristic | Overall (N = 843) | AHI <15 (n = 491) | AHI ≥15 (n = 352) | P Value |
|---|---|---|---|---|
| Age, yr | 75 ± 5 | 74 ± 5 | 75 ± 5 | 0.50 |
| Race/ethnicity | 0.89 | |||
| White | 726 (86) | 419 (85) | 307 (87) | |
| African American | 36 (4) | 22 (5) | 14 (4) | |
| Asian | 40 (5) | 25 (5) | 15 (4) | |
| Other | 41 (5) | 25 (5) | 16 (4) | |
| Body mass index, kg/m2 | 27 ± 4 | 27 ± 4 | 28 ± 4 | <0.0001 |
| Diabetes | 101 (12) | 56 (11) | 45 (13) | 0.54 |
| Hypertension | 383 (45) | 202 (41) | 181 (51) | 0.003 |
| Cardiovascular disease* | 208 (25) | 115 (23) | 93 (26) | 0.32 |
| Heart failure | 25 (3) | 12 (2) | 13 (4) | 0.29 |
| Stroke | 22 (3) | 16 (3) | 6 (2) | 0.16 |
| Chronic obstructive pulmonary disease or emphysema | 33 (4) | 18 (4) | 15 (4) | 0.66 |
| Pacemaker placement | 17 (2) | 8 (2) | 9 (3) | 0.34 |
| Total cholesterol, mg/dl | 197 ± 34 | 196 ± 33 | 198 ± 36 | 0.45 |
| Taking cardiovascular medications | 272 (32) | 150 (31) | 122 (35) | 0.21 |
| Alcohol use, drinks/wk | 0.61 | |||
| <1 | 384 (46) | 229 (47) | 155 (44) | |
| 1–13 | 393 (47) | 226 (46) | 167 (48) | |
| ≥14 | 60 (7) | 32 (7) | 28 (8) | |
| Obstructive apnea–hypopnea index | 16 ± 14 | 7 ± 4 | 29 ± 13 | <0.0001 |
| Central apnea index ≥5 | 48 (6) | 4 (1) | 44 (13) | <0.0001 |
| Cheyne-Stokes respiration with central sleep apnea | 68 (8) | 12 (2) | 56 (16) | <0.0001 |
| Percentage of total sleep time with SaO2 <90% | <0.0001 | |||
| <1% | 416 (49) | 339 (69) | 77 (22) | |
| 1 to <3.5% | 236 (28) | 108 (22) | 128 (36) | |
| 3.5 to <10% | 100 (12) | 23 (5) | 77 (22) | |
| ≥10% | 91 (11) | 21 (4) | 70 (20) |
Definition of abbreviation: AHI = apnea–hypopnea index.
For continuous variables, means and SDs are presented; for categorical variables, number and percentage are given. P values were generated from t tests for normally distributed continuous variables, Wilcoxon rank-sum tests for skewed variables, and χ2 tests for categorical data.
Cardiovascular disease includes myocardial infarction, angina, heart failure, coronary bypass surgery, and angioplasty.
Sleep-disordered Breathing Indices and Incidence of Atrial Fibrillation
There were 99 cases of AF (12%) reported during the follow-up period. SDB variables were analyzed in relation to AF incidence (Table 2). Overall, SDB defined by AHI level was not a significant predictor of incident AF when considered as a continuous or dichotomous variable. Alternatively, CSA (OR, 2.58; 95% CI, 1.18–5.66) and CSA-CSR (OR, 2.27; 95% CI, 1.13–4.56) were significantly related to incident AF in the multivariable model including heart failure and OSA severity. Hypoxemia, as defined by percentage of TST with saturation less than 90%, was a significant predictor of AF in minimally adjusted analyses (3.5% to <10% vs. <1%: OR, 1.97; 95% CI, 1.07–3.63) but not in fully adjusted analyses. Indices of OSA were not significantly related to incident AF.
Table 2.
Adjusted Associations of Sleep-disordered Breathing Predictors and Incident Atrial Fibrillation or Flutter
| Predictor | Events/Total | Minimally Adjusted OR* (95% CI) | Multivariable Adjusted OR† (95% CI) |
|---|---|---|---|
| AHI per 5-unit increase | 99/843 | 1.05 (0.98–1.13) | 1.01 (0.94–1.10) |
| AHI <15 | 49/491 | 1.00 (reference) | 1.00 (reference) |
| AHI ≥15 | 50/352 | 1.47 (0.95–2.27) | 1.15 (0.72–1.84) |
| OAHI per 5-unit increase‡ | 99/843 | 1.04 (0.97–1.12) | 0.98 (0.91–1.07) |
| CAI <5 | 88/795 | 1.00 (reference) | 1.00 (reference) |
| CAI ≥5‡ | 11/48 | 2.34 (1.14–4.77) | 2.58 (1.18–5.66) |
| No CSA-CSR | 85/775 | 1.00 (reference) | 1.00 (reference) |
| CSA-CSR‡ | 14/68 | 2.10 (1.11–3.97) | 2.27 (1.13–4.56) |
| Percentage of total sleep time with SaO2 <90% | |||
| <1% | 43/416 | 1.00 (reference) | 1.00 (reference) |
| 1% to <3.5% | 24/236 | 0.93 (0.55–1.60) | 0.83 (0.47–1.47) |
| 3.5% to <10% | 19/100 | 1.97 (1.07–3.63) | 1.64 (0.83–3.24) |
| ≥10% | 13/91 | 1.31 (0.65–2.64) | 1.01 (0.46–2.24) |
Definition of abbreviations: AHI = apnea–hypopnea index; CAI = central apnea index; CI = confidence interval; CSA-CSR = Cheyne-Stokes respirations or central sleep apnea; OAHI = obstructive apnea–hypopnea index; OR = odds ratio.
OAHI adjustment is continuous; CAI adjustment is CAI ≥5. Bold denotes statistically significant values.
Adjusted for age, clinic, and race.
Adjusted for age, clinic, race, body mass index, history of cardiovascular disease, hypertension, diabetes, stroke, chronic obstructive pulmonary disease, pacemaker placement, total cholesterol, use of cardiovascular medications, and alcohol use.
Multivariable models were further adjusted by alternate apnea type: Models with CAI and CSA-CSR predictors were further adjusted by OAHI; the model with the OAHI predictor was further adjusted by CAI.
Secondary Analyses
There was a statistically significant interaction of CSA, CSA-CSR, and AHI indices with age for the incident AF outcome (P < 0.05). In multivariable adjusted stratified analyses (Table 3), SDB was significantly associated with AF development in men aged 76 years or older. The OR for AHI per 5-unit increase was 1.22 (95% CI, 1.08–1.39), and that for AHI greater than or equal to 15 was 2.64 (95% CI, 1.16–6.00). Similar to the case in primary analyses, CSA indices remained significant predictors of incident AF in adjusted analyses, with an observed stronger magnitude of association. The OR for CAI greater than or equal to 5 was 9.97 (95% CI, 2.72–36.50), and that for CSA-CSR was 6.31 (95% CI, 1.94–20.51); however, this analysis was hampered by low participant counts in the category for both CAI and CSA-CSR. Although OSA as defined using OAHI as a continuous variable was a significant predictor of incident AF in minimally adjusted models, this association lost significance in the fully adjusted model. Consistent with findings from the primary analyses, hypoxemia was not significantly associated with incident AF. After excluding those men who had a history of CHF or who developed CHF during follow-up, the results remained significant in multivariable models for CSA-CSR (OR, 2.56; 95% CI, 1.17–5.58) and were similar in magnitude to and marginally significant for CSA (OR, 2.42; 95% CI, 0.98–5.96) (P = 0.05).
Table 3.
Age-stratified Adjusted Associations of Sleep-disordered Breathing Predictors and Incident Atrial Fibrillation or Flutter
| Predictor | Events/Total | Minimally Adjusted OR* (95% CI) | Multivariable Adjusted OR† (95% CI) | ||
|---|---|---|---|---|---|
| Age <76 yr | |||||
| AHI per 5-unit increase | 57/529 | 0.95 (0.86–1.06) | 0.92 (0.82–1.04) | ||
| AHI <15 | 33/308 | 1.00 (reference) | 1.00 (reference) | ||
| AHI ≥15 | 24/221 | 0.96 (0.54–1.70) | 0.79 (0.42–1.49) | ||
| OAHI per 5-unit increase‡ | 57/529 | 0.95 (0.85–1.06) | 0.92 (0.82–1.04) | ||
| CAI <5 | 53/500 | 1.00 (reference) | 1.00 (reference) | ||
| CAI ≥5‡ | 4/29 | 1.33 (0.44–3.99) | 1.22 (0.34–4.41) | ||
| No CSA-CSR | 51/486 | ||||
| CSA-CSR‡ | 6/43 | 1.36 (0.55–3.40) | 1.45 (0.52–4.07) | ||
| Percentage of total sleep time with SaO2 <90% | |||||
| <1% | 29/276 | 1.00 (reference) | 1.00 (reference) | ||
| 1% to <3.5% | 11/139 | 0.69 (0.33–1.45) | 0.61 (0.28–1.35) | ||
| 3.5% to <10% | 12/61 | 2.13 (0.99–4.57) | 1.74 (0.71–4.24) | ||
| ≥10% | 5/53 | 0.78 (0.27–2.23) | 0.57 (0.18–1.82) | ||
| Age ≥76 yr | |||||
| AHI per 5-unit increase | 42/314 | 1.20 (1.08–1.33) | 1.22 (1.08–1.39) | ||
| AHI <15 | 16/183 | 1.00 (reference) | 1.00 (reference) | ||
| AHI ≥15 | 26/131 | 2.91 (1.43–5.90) | 2.64 (1.16–6.00) | ||
| OAHI per 5-unit increase‡ | 42/314 | 1.17 (1.05–1.30) | 1.13 (0.98–1.29) | ||
| CAI <5 | 35/295 | 1.00 (reference) | 1.00 (reference) | ||
| CAI ≥5‡ | 7/19 | 4.86 (1.72–13.72) | 9.97 (2.72–36.50) | ||
| No CSA-CSR | 34/289 | 1.00 (reference) | 1.00 (reference) | ||
| CSA-CSR‡ | 8/25 | 3.88 (1.50–10.07) | 6.31 (1.94–20.51) | ||
| Percentage of total sleep time with SaO2 <90% | |||||
| <1% | 14/140 | 1.00 (reference) | 1.00 (reference) | ||
| 1% to <3.5% | 13/97 | 1.34 (0.58–3.09) | 1.69 (0.65–4.40) | ||
| 3.5% to <10% | 7/39 | 1.93 (0.67–5.51) | 2.24 (0.69–7.30) | ||
| ≥10% | 8/38 | 2.38 (0.87–6.52) | 2.68 (0.77–9.34) |
For definition of abbreviations, see Table 2
The P value for interaction was significant for all variables (P < 0.10). Interactions with age as a categorical variable (above or below median): AHI continuous, P = 0.01; AHI ≥15, P = 0.04; OAHI, P = 0.02. Interactions of age as a continuous variable: CAI ≥5, P = 0.04; CSA-CSR, P = 0.02; percentage of total sleep time with SaO2 <90%, P = 0.08. Bold denotes statistically significant values.
Adjusted for age, clinic, and race.
Adjusted for age, clinic, race, body mass index, history of cardiovascular disease, hypertension, diabetes, stroke, chronic obstructive pulmonary disease, pacemaker placement, total cholesterol, use of cardiovascular medications, and alcohol use.
Multivariable models were further adjusted by alternate apnea type: Models with CAI and CSA-CSR predictors were further adjusted by OAHI; the model with the OAHI predictor was further adjusted by CAI.
Discussion
In this prospective, multicenter study of community-dwelling older men, indices of CSA, including CSR, were significantly associated with incident AF even after accounting for confounders, including cardiovascular risk and disease. Additionally, there was effect modification by age such that all SDB indices were significant predictors of incident AF. Overall, SDB as defined by AHI, as well as central SDB as defined by CSA and CSA-CSR, significantly predicted AF in participants older than the median age (≥76 yr) even after consideration of cardiopulmonary risk. The robustness of these findings persisted in sensitivity analyses excluding those with self-reported heart failure. Although these prospective findings do not prove a causal relationship of CSA with AF, they highlight the potential role of CSA as a marker of AF development (19).
Our finding of a stronger relationship of central apnea than OSA with AF is consistent with previously published cross-sectional results and identifies CSA as a predictor of AF development (9). Unlike the prior cross-sectional epidemiologic work, AF ascertainment in the present study was based on incident events identified either through adjudication or by self-report and not exclusively based on AF identified by ECG monitoring during the MrOS Sleep Study. A preponderance of data elucidates specific underlying mechanisms related to SDB that contribute to atrial arrhythmogenicity. Autonomic dysfunction, a well-recognized corollary of SDB characterized by enhanced vagal activity during and sympathetic activation subsequent to the respiratory events, increases the likelihood of AF by shortening the atrial effective refractory period and predisposes individuals to focal atrial firing (“adrenergic AF”), respectively (30, 31). SDB-related intermittent hypoxia represents a potent stimulus of autonomic function activation that creates regions of heterogeneous myocyte excitability, thereby increasing AF propensity (32). On one hand, substantial negative intrathoracic pressure alterations observed in OSA may lead to an abrupt decrease in left atrial volume and reduction in left ventricular systolic performance, augmenting AF development (33). On the other hand, some data suggest that the reverse may be true (i.e., that AF may lead to SDB). Paroxysms of AF have been described as occurring subsequent to discrete apneic events plausibly due to immediate effects of tachycardia-induced left ventricular dysfunction and diastolic dysfunction, leading to reduction of cardiac output and increased pulmonary vascular pressure and hence resulting in breathing instability (34, 35). The findings of the present study provide further evidence to support the importance of central apnea pathophysiology leading to AF.
Mechanistically, CSA is characterized by ventilatory system instability that results in hypocapnia, which may contribute to cardiac electrical instability (36). An underlying increase in central and peripheral chemoresponsiveness may contribute to augmented sympathetic activity and autonomic imbalance, which are predisposing factors for AF (37–39). It is possible that CSA is a marker of augmented respiratory chemoreflexes and autonomic nervous system dysfunction rather than the primary impetus for AF development. However, heart failure is associated with autonomic dysfunction and altered chemosensitivity, so the causal directionality of these associations is unclear (40). Furthermore, as chemoresponsiveness wanes with age, the extent of its role in AF in older patients is unclear (41). Our observation of a relationship of CSA and AF independent of heart failure is corroborated by findings from clinical studies in which researchers reported increased AF in those with central apnea compared with obstructive apnea, even in the absence of heart failure (42). Previous work suggesting that central apnea events directly trigger AF paroxysms also supports a role for CSA in AF development (34). Further evidence implicating CSA as a true pathogenic factor and not simply a marker of underlying cardiac dysfunction is provided by improvement in heart transplant–free survival in heart failure patients whose CSA was effectively suppressed with positive airway pressure treatment (43). These benefits did not appear to be due to direct positive airway pressure–related improvement in hemodynamics, but rather seemed to result from the reversal of central apnea pathophysiology (i.e., sympathetic nervous system activation secondary to apnea and arousal from sleep) (43, 44). We recognize, however, that we cannot definitively exclude the possibility that CSA may represent a marker of heart disease leading to AF (40, 45). Recent data demonstrate increased mortality in reduced ejection fraction heart failure with CSA treatment by adaptive servoventilation, an advanced positive airway pressure modality (46). However, given the contrasting pathophysiologic states of heart failure and AF, implications of the reversal of CSA and associated hypoxia and/or sympathetic activation via positive pressure or nonpositive pressure modalities in AF remain unclear and warrant further investigation.
In the present study, stronger associations of SDB with AF were observed in the older study participants. This contrasts with several prior epidemiologic studies demonstrating weaker associations of adverse cardiac outcomes and SDB in older than in younger individuals (8, 11). Differences among studies with regard to age modification are related to differences in age ranges compared. In the present study, the older cohort had a mean age of 76 years, a group in which the effects of advanced age and SDB may be multiplicative. For example, the pathophysiologic insults of SDB, including intermittent hypoxia, autonomic imbalance, and intrathoracic pressure swings, may have more pronounced effects on atrial arrhythmogenesis when superimposed on the aged heart, which has an electrophysiologic substrate different from that of younger individuals (47–51). Enhanced impulse generation due to increased automaticity, triggered activity, and reentrant circuits within the atria and pulmonary veins are considered to be responsible for AF initiation and maintenance in the elderly and at a specific age threshold may be more likely to occur as a pathophysiologic response to SDB-induced physiologic stress (48, 50, 52).
Unlike prior studies, this work does not show an association with OSA, except in the oldest of the old subgroup. This difference may be secondary to different susceptibilities in the very aged (mean age, 75 yr) compared with those in cohorts that include significant numbers of middle-aged adults (mean age, 49 yr) (11).
This study does not show a relationship between hypoxemia and AF development, a finding that was somewhat unanticipated. A large, single-center, clinic-based study showed that degree of hypoxemia was associated in fully adjusted models with development of AF in individuals younger than 65 years of age but not in those aged 65 years or older (11). Another retrospective cohort study (mean age, 59 yr) affirmed a link between incident AF and hypoxemia but did not find effect modification by age (12). In our cohort (all aged >65 yr), even the younger subgroup did not show a statistically significant relationship of hypoxemia with the development of AF. This may be secondary to different mechanisms of AF development in the elderly compared with younger individuals and may potentially be due to limited power, as an association of hypoxemia and AF was observed in minimally but not fully adjusted models.
Strengths of this study include the prospective design and high rate of follow-up completers (99% for the adjudicated AF). Standardized protocols and procedures were used to maximize data integrity. A systematic consideration of several metrics of SDB, including central apnea and overnight hypoxemia, was conducted. Limitations of this study include possible residual confounding. Potentially representing overestimates of true risk, small AF event counts in age-stratified analyses led to large CIs, which may be secondary to small differences in AF incidence. A potential limitation is survivorship bias, given the higher prevalence of central SDB in the subset who died (n = 390) and did not participate in the second examination; however, exclusion of these participants would be expected to bias the results toward the null. Because this study focused on a cohort of community-dwelling elderly men, the findings may not be generalizable to younger individuals or to women. Measures of cardiac function such as echocardiography were not included, which precludes examination of cardiac function mediation of SDB and AF relationships. Use of self-reported measures of cardiac comorbidity, including heart failure, limited our ability to adjust for potential confounding related to undiagnosed heart disease. Interestingly, the overall higher percentage of participants with CSA-CSR relative to the percentage of participants with self-reported heart failure or stroke suggests the possibility of underreporting of these comorbidities versus central apnea physiology, which is idiopathic or attributable to other factors. In future investigations focused on the underlying pathways that link SDB and AF, researchers should examine the role of cardiac function and structure through morphologic analysis, the interplay between CSA and respiratory chemoreflexes and autonomic dysfunction, and the role of biochemical mediators (i.e., systemic inflammation and oxidative stress). In addition, further characterization of phenotypes of CSA by analysis of apnea, ventilation, and overall cycle length may provide further insight into the mechanism of this phenomenon (53, 54). Although we cannot exclude the possibility that CSA represents a marker of altered chemosensitivity and autonomic dysfunction mediating AF risk, these findings provide a basis on which to stratify risk and target individuals with CSA for AF risk reduction, and they potentially support investigation of CSA pathophysiology reversal on AF development.
This study highlights the importance of CSA and CSR in the development of incident AF, with results strengthened in the older subgroup of an elderly cohort of men. These findings suggest that CSA serves as an important potential prevention and therapeutic target for AF, particularly in older individuals, the latter representing a group particularly susceptible to AF development and accompanying morbidity.
Acknowledgments
Acknowledgment
The authors thank the investigators in the MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study: Coordinating Center, California Pacific Medical Center Research Institute and University of California, San Francisco: K. L. Stone (principal investigator), D. C. Bauer (coinvestigator), S. R. Cummings (coinvestigator), N. Goldschlager (coinvestigator), P. Varosy (coinvestigator), K. Yaffe (coinvestigator), P. M. Cawthon (coinvestigator), R. Fullman (project director), R. Benard, T. Blackwell, L. Concepcion, J. Diehl, S. Ewing, C. Fox, M. Jaime-Chavez, E. Kwan, S. Litwack, W. Liu, L. Y. Lui, J. Schneider, R. Scott, D. Tanaka, and J. Ziarno; Administrative Center, Oregon Health & Science University: E. Orwoll (principal investigator), K. Phipps (coinvestigator), L. Marshall (coinvestigator), J. Babich Blank (project director), L. Lambert, B. Chan, and D. Neevel; Participating Centers, University of Alabama at Birmingham: C. E. Lewis (principal investigator), J. Shikany (coinvestigator), P. Johnson (project director), C. Oden, S. House, N. Webb, K. Hardy, S. Felder, J. Wilkoff, J. King, T. Johnsey, M. Young, J. Smith, C. Sassaman, C. Collier, and C. Atkins; University of Minnesota: K. Ensrud (principal investigator), H. Fink (coinvestigator), D. King (program manager), N. Michaels (assistant program manager), N. Nelson (clinic coordinator), C. Bird, D. Blanks, F. Imker-Witte, K. Moen, M. Paudel, and M. Slindee; Stanford University: M. Stefanick (principal investigator), A. Hoffman (coinvestigator), K. Kent, B. Malig, and S. Wong; University of Pittsburgh: J. Cauley (principal investigator), J. Zmuda (coinvestigator), M. Danielson (study administrator), L. Harper (project director), L. Buck (clinic coordinator), M. Nasim, D. Cusick, M. Gorecki, N. Watson, C. Bashada, and C. Newman; University of California, San Diego: E. Barrett-Connor (principal investigator), S. Ancoli-Israel (coinvestigator), T. Dam (coinvestigator), M. L. Carrion-Petersen (project director), P. Miller, and N. Kamantigue; Central Sleep Reading Center: S. Redline (principal investigator), S. Surovec (project administrator), N. Scott (chief polysomnologist), N. Johnson (programmer analyst), J. Arnold (polysomnologist), R. Nawabit (polysomnologist), J. Romaniuk (polysomnologist), and S. Seicean (polysomnologist).
Footnotes
The Osteoporotic Fractures in Men (MrOS) Study is supported by the National Institutes of Health (NIH). The following institutes provide support: the National Institute on Aging, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Advancing Translational Sciences, and the NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The NHLBI provides funding for the MrOS Sleep ancillary study Outcomes of Sleep Disorders in Older Men under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. R.M. is supported by NHLBI grants R21 HL108226 and R01 HL109493. The sponsors did not participate in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Author Contributions: A.M.M. and R.M. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; A.M.M., T.B., P.H.S., K.L.S., P.M.C., W.H.S., P.D.V., S.R., and R.M. made substantial contributions to the design and analysis plan of the study; T.B. conducted the statistical analysis and edited the manuscript; A.M.M. was involved in the data analysis and interpretation of results and also drafted the manuscript. All authors revised the manuscript draft critically and gave final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201508-1523OC on November 23, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mannarino MR, Di Filippo F, Pirro M. Obstructive sleep apnea syndrome. Eur J Intern Med. 2012;23:586–593. doi: 10.1016/j.ejim.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Menezes AR, Lavie CJ, DiNicolantonio JJ, O’Keefe J, Morin DP, Khatib S, Milani RV. Atrial fibrillation in the 21st century: a current understanding of risk factors and primary prevention strategies. Mayo Clin Proc. 2013;88:394–409. doi: 10.1016/j.mayocp.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6:557–566. doi: 10.1586/ers.12.44. [DOI] [PubMed] [Google Scholar]
- 4.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 6.Kim MH, Lin J, Hussein M, Kreilick C, Battleman D. Cost of atrial fibrillation in United States managed care organizations. Adv Ther. 2009;26:847–857. doi: 10.1007/s12325-009-0066-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen LY, Shen W-K. Epidemiology of atrial fibrillation: a current perspective. Heart Rhythm. 2007;4(3 Suppl):S1–S6. doi: 10.1016/j.hrthm.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehra R, Stone KL, Varosy PD, Hoffman AR, Marcus GM, Blackwell T, Ibrahim OA, Salem R, Redline S. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169:1147–1155. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monahan K, Storfer-Isser A, Mehra R, Shahar E, Mittleman M, Rottman J, Punjabi N, Sanders M, Quan SF, Resnick H, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009;54:1797–1804. doi: 10.1016/j.jacc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 12.Cadby G, McArdle N, Briffa T, Hillman DR, Simpson L, Knuiman M, Hung J. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest. 2015;148:945–952. doi: 10.1378/chest.15-0229. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Wang ZW, Li J, Ge X, Guo LZ, Wang Y, Guo WH, Jiang CX, Ma CS. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace. 2014;16:1309–1314. doi: 10.1093/europace/euu066. [DOI] [PubMed] [Google Scholar]
- 14.Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC, Hylek EM, Mahaffey KW, Freeman JV, Chang P, et al. ORBIT-AF Investigators. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am Heart J. 2015;169:647–654.e2. doi: 10.1016/j.ahj.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K, Kramer DB, Zimetbaum PJ, Buxton AE, Josephson ME, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300–305. doi: 10.1016/j.jacc.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman ASM, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 17.Patel D, Mohanty P, Di Biase L, Shaheen M, Lewis WR, Quan K, Cummings JE, Wang P, Al-Ahmad A, Venkatraman P, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol. 2010;3:445–451. doi: 10.1161/CIRCEP.109.858381. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, et al. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Wagoner DR, Piccini JP, Albert CM, Anderson ME, Benjamin EJ, Brundel B, Califf RM, Calkins H, Chen PS, Chiamvimonvat N, et al. Progress toward the prevention and treatment of atrial fibrillation: a summary of the Heart Rhythm Society Research Forum on the Treatment and Prevention of Atrial Fibrillation, Washington, DC, December 9–10, 2013. Heart Rhythm. 2015;12:e5–e29. doi: 10.1016/j.hrthm.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naderi S, Wang Y, Miller AL, Rodriguez F, Chung MK, Radford MJ, Foody JM. The impact of age on the epidemiology of atrial fibrillation hospitalizations. Am J Med. 2014;127:158.e1–7. doi: 10.1016/j.amjmed.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.May AM, Blackwell T, Stone P, Stone KL, Cawthon PM, Loparo K, Varosi P, Redline SS, Mehra R Osteoporotic Fractures in Men (MrOS) Study Group. Sleep disordered breathing determinants of incident atrial fibrillation in an elderly male cohort [abstract] Am J Respir Crit Care Med. 2014;189(Meeting Abstracts):A2403. [Google Scholar]
- 22.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, et al. Design and baseline characteristics of the Osteoporotic Fractures in Men (MrOS) Study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Redline S, Budhiraja R, Kapur V, Marcus CL, Mateika JH, Mehra R, Parthasarthy S, Somers VK, Strohl KP, Sulit LG, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169–200. [PubMed] [Google Scholar]
- 25.Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009;103:1572–1577. doi: 10.1016/j.amjcard.2009.01.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 27.Soliman EZ, Howard G, Meschia JF, Cushman M, Muntner P, Pullicino PM, McClure LA, Judd S, Howard VJ. Self-reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2011;42:2950–2953. doi: 10.1161/STROKEAHA.111.621367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 29.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research: the report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 30.Bayés de Luna A, Bayés Genís A, Guindo J, Viñolas X, Boveda S, Torner P, Oter R, Sobral J, Sztajzel J. Mechanisms favoring and triggering atrial fibrillation [in French] Arch Mal Coeur Vaiss. 1994;87(1 Spec No):19–25. [PubMed] [Google Scholar]
- 31.Tse HF, Lau CP. Electrophysiological properties of the fibrillating atrium: implications for therapy. Clin Exp Pharmacol Physiol. 1998;25:293–302. doi: 10.1111/j.1440-1681.1998.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 32.Rossi VA, Stradling JR, Kohler M. Effects of obstructive sleep apnoea on heart rhythm. Eur Respir J. 2013;41:1439–1451. doi: 10.1183/09031936.00128412. [DOI] [PubMed] [Google Scholar]
- 33.Orban M, Bruce CJ, Pressman GS, Leinveber P, Romero-Corral A, Korinek J, Konecny T, Villarraga HR, Kara T, Caples SM, et al. Dynamic changes of left ventricular performance and left atrial volume induced by the Mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation, and heart failure. Am J Cardiol. 2008;102:1557–1561. doi: 10.1016/j.amjcard.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rupprecht S, Hutschenreuther J, Brehm B, Figulla H-R, Witte OW, Schwab M. Causality in the relationship between central sleep apnea and paroxysmal atrial fibrillation. Sleep Med. 2008;9:462–464. doi: 10.1016/j.sleep.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Goldin JM, Naughton MT. Obstructive sleep apnoea induced atrial fibrillation. Intern Med J. 2006;36:136–137. doi: 10.1111/j.1445-5994.2006.01000.x. [DOI] [PubMed] [Google Scholar]
- 36.Javaheri S, Corbett WS. Association of low PaCO2 with central sleep apnea and ventricular arrhythmias in ambulatory patients with stable heart failure. Ann Intern Med. 1998;128:204–207. doi: 10.7326/0003-4819-128-3-199802010-00006. [DOI] [PubMed] [Google Scholar]
- 37.Fioranelli M, Piccoli M, Mileto GM, Sgreccia F, Azzolini P, Risa MP, Francardelli RL, Venturini E, Puglisi A. Analysis of heart rate variability five minutes before the onset of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1999;22:743–749. doi: 10.1111/j.1540-8159.1999.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 38.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol (1985) 1999;86:1264–1272. doi: 10.1152/jappl.1999.86.4.1264. [DOI] [PubMed] [Google Scholar]
- 39.Tomita T, Takei M, Saikawa Y, Hanaoka T, Uchikawa S, Tsutsui H, Aruga M, Miyashita T, Yazaki Y, Imamura H, et al. Role of autonomic tone in the initiation and termination of paroxysmal atrial fibrillation in patients without structural heart disease. J Cardiovasc Electrophysiol. 2003;14:559–564. doi: 10.1046/j.1540-8167.2003.02462.x. [DOI] [PubMed] [Google Scholar]
- 40.Mansfield D, Kaye DM, Brunner La Rocca H, Solin P, Esler MD, Naughton MT. Raised sympathetic nerve activity in heart failure and central sleep apnea is due to heart failure severity. Circulation. 2003;107:1396–1400. doi: 10.1161/01.cir.0000056520.17353.4f. [DOI] [PubMed] [Google Scholar]
- 41.Peterson DD, Pack AI, Silage DA, Fishman AP. Effects of aging on ventilatory and occlusion pressure responses to hypoxia and hypercapnia. Am Rev Respir Dis. 1981;124:387–391. doi: 10.1164/arrd.1981.124.4.387. [DOI] [PubMed] [Google Scholar]
- 42.Leung RS, Huber MA, Rogge T, Maimon N, Chiu KL, Bradley TD. Association between atrial fibrillation and central sleep apnea. Sleep. 2005;28:1543–1546. doi: 10.1093/sleep/28.12.1543. [DOI] [PubMed] [Google Scholar]
- 43.Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, et al. CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 44.Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152:473–479. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 45.Heistad DD, Wheeler RC, Mark AL, Schmid PG, Abboud FM. Effects of adrenergic stimulation on ventilation in man. J Clin Invest. 1972;51:1469–1475. doi: 10.1172/JCI106943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho M-P, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anyukhovsky EP, Sosunov EA, Chandra P, Rosen TS, Boyden PA, Danilo P, Jr, Rosen MR. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc Res. 2005;66:353–363. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 48.Dun W, Boyden PA. Aged atria: electrical remodeling conducive to atrial fibrillation. J Interv Card Electrophysiol. 2009;25:9–18. doi: 10.1007/s10840-008-9358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kojodjojo P, Kanagaratnam P, Markides V, Davies DW, Peters N. Age-related changes in human left and right atrial conduction. J Cardiovasc Electrophysiol. 2006;17:120–127. doi: 10.1111/j.1540-8167.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 50.Sakabe K, Fukuda N, Nada T, Shinohara H, Tamura Y, Wakatsuki T, Nishikado A, Oki T. Age-related changes in the electrophysiologic properties of the atrium in patients with no history of atrial fibrillation. Jpn Heart J. 2003;44:385–393. doi: 10.1536/jhj.44.385. [DOI] [PubMed] [Google Scholar]
- 51.Sakabe K, Fukuda N, Soeki T, Shinohara H, Tamura Y, Wakatsuki T, Nishikado A, Oki T. Relation of age and sex to atrial electrophysiological properties in patients with no history of atrial fibrillation. Pacing Clin Electrophysiol. 2003;26:1238–1244. doi: 10.1046/j.1460-9592.2003.t01-1-00174.x. [DOI] [PubMed] [Google Scholar]
- 52.Mirza M, Strunets A, Shen WK, Jahangir A. Mechanisms of arrhythmias and conduction disorders in older adults. Clin Geriatr Med. 2012;28:555–573. doi: 10.1016/j.cger.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solin P, Roebuck T, Swieca J, Walters EH, Naughton MT. Effects of cardiac dysfunction on non-hypercapnic central sleep apnea. Chest. 1998;113:104–110. doi: 10.1378/chest.113.1.104. [DOI] [PubMed] [Google Scholar]
- 54.Hall MJ, Xie A, Rutherford R, Ando S, Floras JS, Bradley TD. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996;154:376–381. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]