ATP13A2 (OMIM 610513) is a lysosomal P-type ATPase implicated in neurological disorders including Kufor-Rakeb Syndrome and Parkinson's disease, providing protection against stressors such as α-synuclein and heavy metal toxicity incited by Mn2+ and Zn2+. To date, the molecular function underlying ATP13A2's protection remains uncertain, despite significant advances in its cellular contributions, with notable roles of ATP13A2 in lysosomal function, autophagy, mitochondrial maintenance and exosome release (reviewed in1).
Our recent work2 uncovered a remarkable role of the N-terminus of ATP13A2, which compared to other P-type ATPases, contains an unusual hydrophobic stretch that first was predicted as an additional N-terminal transmembrane segment. However, we demonstrated that the N-terminus does not traverse the membrane, but rests on the cytosolic membrane surface of late endo-/lysosomes where it may serve as a docking platform for lipids and proteins2 (Fig. 1). In fact, we show that the signaling lipids phosphatidic acid (PA) and phosphatidylinositol(3,5)bisphosphate (PI(3,5)P2) interact with the N-terminal region. Biochemical evidence further indicated that ATP13A2 may reside in an inactive autophosphorylated state and that both lipids stimulate catalytic autophosphorylation, suggesting that the lipids unlock ATP13A2 activity. This mechanism becomes relevant in a cellular model of PD, where we demonstrated that ATP13A2 activity provides protection to rotenone-induced mitochondrial stress, which depends on the availability of PA and PI(3,5)P22 (Fig. 1).
Figure 1.
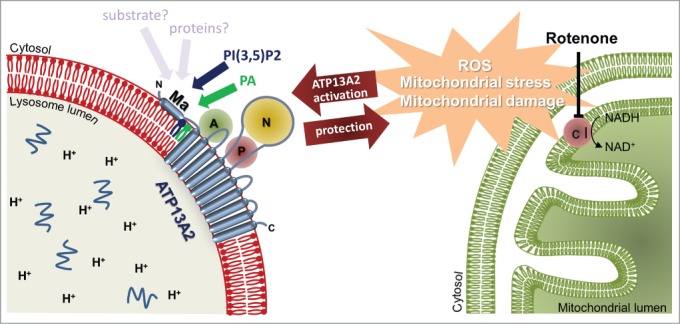
ATP13A2 elicits protective effects against mitochondrial stress. The activity of the mitochondrial complex I (c I), residing on the inner membrane of the mitochondria is inhibited by rotenone. This elevates the production of reactive oxygen species (ROS), inducing mitochondrial stress and mitochondrial damage. Overexpression of the lysosomal P5-type ATPase ATP13A2 elicits a protective effect on the cell against this ROS-induced mitochondrial stress. Protection was shown to depend on the availability of the signaling lipids PA and PI(3,5)P2, which appear to interact with ATP13A2 via its N-terminal membrane-associated region.
Lyso-PA, bis(monoacylglycero)phosphate and lyso-phosphatidylcholine do not interact with the N-terminus of ATP13A2, indicating a high specificity towards PA and PI(3,5)P2, notably two lipids involved in endo-/lysosomal pathways that are implicated in neurodegeneration.2 PA is a conical phospholipid with a small anionic head group inducing a negative membrane curvature and promoting membrane fission and fusion. PA is also a local signaling lipid e.g. produced via the hydrolysis of phosphatidylcholine by phospholipase D (PLD). Of interest, PLD1 regulates α-synuclein clearance via autophagy pathways, which might depend on PA-mediated ATP13A2 activation.2
The low-abundance phosphoinositide PI(3,5)P2 predominantly exists in endo-/lysosomal compartments, functioning as an organelle tag. Mutations in the PI(3,5)P2 5-phosphatase FIG4 trigger Charcot-Marie-Tooth Type 4J disease and Yunis-Varon syndrome, which are marked by neurodegeneration. PI(3,5)P2-deficiency in mice carrying mutations in Vac14, an activator of PIKFYVE, a lipid kinase which generates PI(3,5)P2 from PI(3)P, also results in neurodegeneration. PI(3,5)P2 regulates endo-/lysosome morphology, acidification, trafficking, membrane fusion/fission events and is implicated in autophagy. The cellular functions of PI(3,5)P2 might partially relate to ATP13A2, since loss of ATP13A2 results in neurodegeneration, an increased lysosomal pH and impaired autophagy.2
We further show that ATP13A2 mediates protection against rotenone-induced mitochondrial stress requiring both catalytic activity of ATP13A2 and the lipids PA and PI(3,5)P2. This hints to a lipid-dependent activation of ATP13A2 that is important for mitochondrial homeostasis and/or clearance2 (Fig. 1). In fact, PA and PI(3,5)P2 may be markers of stress regulating mitochondrial fragmentation and clearance. The levels of PA control mitochondrial fragmentation and elongation,3 and suppressed by the phosphatase Ptpmt1 under basal conditions, PI(3,5)P2 increases under mitochondrial dysfunction, which induces mitochondrial fragmentation.4
Together, PA and PI(3,5)P2 are important for endo-/lysosomal membrane dynamics and mitochondrial homeostasis, suggesting that ATP13A2 might be implicated in vesicular transport, fusion or fission events that are coupled to mitochondrial homeostasis. Moreover, many of the interacting genes of YPK9, the ATP13A2 ortholog in yeast,1 and many of the established interacting proteins of the human ATP13A25 play a role in vesicular transport or mitochondrial function. So, how does ATP13A2 confer protection against mitochondrial stress and what would be the transport function of ATP13A2? The presented biochemical evidence of the catalytic autophosphorylation reaction refutes any direct stimulation of activity by Zn2+ or Mn2+, suggesting that ATP13A2 may not directly transport heavy metals.2 Instead, the tight connection with lipids and vesicular processes rather suggests that ATP13A2 might be a putative lipid flippase, resembling the closely related P4-type lipid flippases that regulate lipid signaling and/or membrane curvature. Such a function might explain ATP13A2's pleiotropic effects on exosome release, Zn2+ and Mn2+ homeostasis and toxicity, mitochondrial clearance and α-synuclein detoxification.1,2
Several reports show that the catalytic activity of ATP13A2 is required to protect cells against various insults like mitochondrial stress, α-synuclein toxicity and metal exposure (Zn2+, Mn2+ and Fe3+).1,6 It remains to be established whether all these various cytoprotective effects depend on the same N-terminal lipid switch. Besides lipid binding, the similarity of the N-terminus with the P1B-type heavy metal pumps may hint to a role of the membrane-associated N-terminus in substrate recognition and protein interactions2 (Fig. 1).
In conclusion, our data highlight the importance of the ATP13A2 N-terminus for lipid interactions, autophosphorylation and cell viability. In a variety of model systems ATP13A2 protects against α-synuclein-, heavy metal- or mitochondrial stress-induced toxicity. Since ATP13A2 may accumulate predominantly in an inactive state, targeting the N-terminus may offer a modality to therapeutically activate these pro-survival characteristics of ATP13A2.
References
- 1.van Veen S, et al.. Front Mol Neurosci 2014; 7:48; PMID:24904274; http://dx.doi.org/ 10.3389/fnmol.2014.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holemans T, et al.. Proc Natl Acad Sci U S A 2015; 112:9040-9045; PMID:26134396; http://dx.doi.org/ 10.1073/pnas.1508220112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba T, et al.. J Biol Chem 2014; 289:11497-11511; PMID:24599962; http://dx.doi.org/ 10.1074/jbc.M113.531921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen J, et al.. Mol Cell Biol 2011; 31:4902-4916; PMID:21986498; http://dx.doi.org/ 10.1128/MCB.05629-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usenovic M, et al.. Hum Mol Genet 2012;21:3785-3794; PMID:22645275; http://dx.doi.org/ 10.1093/hmg/dds206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinaldi DE, et al.. Biochim Biophys Acta 2015; 1848:1646-1655; PMID:25912790; http://dx.doi.org/ 10.1016/j.bbamem.2015.04.008 [DOI] [PubMed] [Google Scholar]


