ABSTRACT
Inhibitory leukocyte immunoglobulin-like receptors (LILRBs 1-5) transduce signals via intracellular immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that recruit protein tyrosine phosphatase non-receptor type 6 (PTPN6 or SHP-1), protein tyrosine phosphatase non-receptor type 11 (PTPN11 or SHP-2), or Src homology 2 domain-containing inositol phosphatase (SHIP), leading to negative regulation of immune cell activation. Certain of these receptors also play regulatory roles in neuronal activity and osteoclast development. The activation of LILRBs on immune cells by their ligands may contribute to immune evasion by tumors. Recent studies found that several members of LILRB family are expressed by tumor cells, notably hematopoietic cancer cells, and may directly regulate cancer development and relapse as well as the activity of cancer stem cells. LILRBs thus have dual concordant roles in tumor biology – as immune checkpoint molecules and as tumor-sustaining factors. Importantly, the study of knockout mice indicated that LILRBs do not affect hematopoiesis and normal development. Therefore LILRBs may represent ideal targets for tumor treatment. This review aims to summarize current knowledge on expression patterns, ligands, signaling, and functions of LILRB family members in the context of cancer development.
Keywords: cancer, CD85, HLA, ILT, immunoglobulin-like transcript, immunoreceptor tyrosine-based activation motif, Immunoreceptor tyrosine-based inhibitory motifs, ITAM, ITIM, leukemia, leukocyte immunoglobulin-like receptor subfamily B, leukocyte immunoglobulin-like receptor, LILRB, LIR, MHC, phosphatase, SHIP, SHP-1, SHP-2, signal transduction
Introduction
Immunoreceptor tyrosine-based inhibitory motif (ITIM) was first described in 1995. This conserved motif consists of 6 amino acids (S/I/V/LxYxxI/V/L) located in the cytoplasmic portion of certain transmembrane receptors.1 Proteome-wide analysis identified 109 human ITIM-containing receptors.2 Conformational changes induced by ligand binding results in Src kinase-mediated phosphorylation of tyrosine in the ITIM, which in turn leads to the recruitment of SH2 domain-containing phosphatases. The ITIM-containing receptors bind tyrosine phosphatases SHP-1 or SHP-2 with the exception of immunoglobulin (Ig) G Fc receptor II-B (FcγRIIB), which only recruits the inositol-phosphatase SHIP.3-5 The first amino acid of ITIM affects binding specificity; isoleucine at the first position (IxYxxL/V) favors SHP-1 binding, whereas leucine (LxYxxL/V) favors SHIP.6 Phosphatase activation usually inhibits immune cell activation. Therefore these receptors are classified as immune inhibitory receptors. The downstream signaling of ITIM remains largely undefined, although known substrates of SHP-1 include the activated immunoreceptor tyrosine-based activation motif (ITAM), spleen tyrosine kinase (Syk), Src, zeta-chain associated protein kinase 70-kDa (ZAP70), Lck/Yes-related novel protein tyrosine kinase (Lyn), phosphatidylinositol-4-phosphate 3-kinase (PI3K), phospholipase C gamma (PLC-γ), and Vav 1 guanine nucleotide exchange factor (Vav1).7-10
In contrast to the immune inhibitory ITIM, the activated immunoreceptor tyrosine-based activation motif, abbreviated ITAM, results in immune activation. The ITAM has a conserved amino acid sequence of YxxL/Ix(6-8)YxxL/I and is located in the cytoplasmic tail of membrane proteins. ITAM transmits signals from various membrane receptors including B cell receptors, T cell receptors, activating leukocyte Ig-like receptors (LILRs), certain activating natural killer (NK) cell receptors, and Fc receptors to name a few.11 Like ITIM-containing receptors, ligand binding of the ITAM-related receptor triggers Src kinase-mediated tyrosine phosphorylation within the ITAM, followed by recruitment and activation of tyrosine kinases (Syk in myeloid cells or ZAP-70 in lymphoid cells), usually resulting in immune activation.11
The leukocyte Ig-like receptor subfamily B (LILRB) is a group of type I transmembrane glycoproteins with extracellular Ig-like domains that bind ligands and intracellular ITIMs. This important group of ITIM-containing receptors contains 5 members, LILRB1 to LILRB5, also called CD85J, CD85D, CD85A, CD85K, and CD85C, respectively, or leukocyte Ig-like receptors (LIR1, LIR2, LIR3, LIR5, and LIR8, respectively). LILRBs 1–4 were also named Ig-like transcripts (ILT2, ILT4, ILT5, ILT3, respectively). Several LILRBs were cloned in 1997.12-15 In 2001, the names LILRB and LILRA were officially given to the inhibitory receptors and the activating receptors, respectively.16 These receptors are encoded in a region called leukocyte receptor complex at chromosomal region 19q13.4 in human.12,17 The domain organizations of the LILRBs are depicted schematically in Figure 1A.
Figure 1.
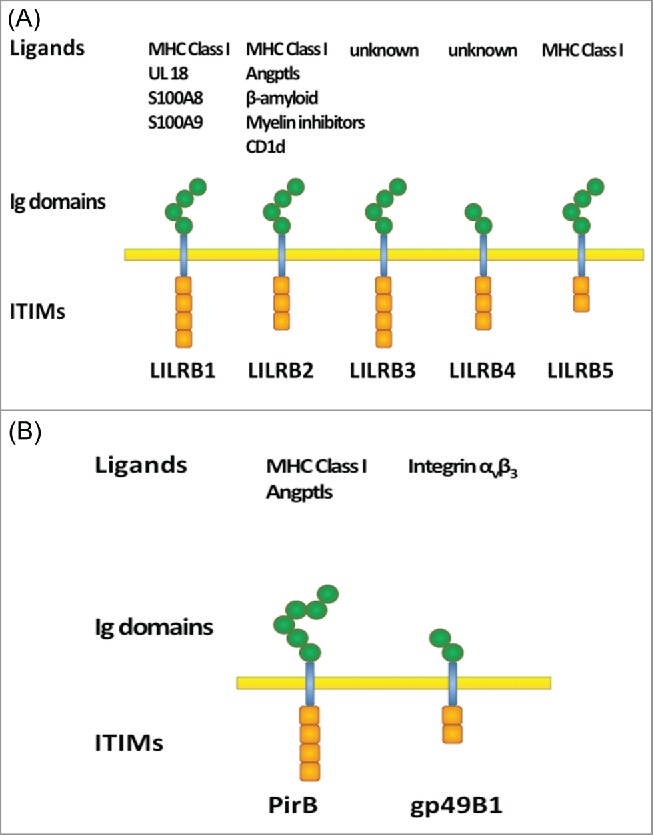
Domain structure of (A) human LILRBs and (B) mouse orthologs. Extracellular Ig-domains are depicted as hexagons and intracellular ITIMs are depicted as boxes.
LILRBs are primate and human specific, with only 2 mouse orthologs, paired immunoglobulin-like receptor B (PirB) 18 and gp49B1,19 known so far (Fig. 1B). Due to rapid evolution of LILRBs, animal models are of limited value, and the biological function and clinical significance of these receptors are not well understood. LILRBs were reported to be predominantly expressed in hematopoietic lineage cells and to suppress activation of various types of immune cells. LILRBs expressed on osteoclasts were reported to regulate osteoclastogenesis,20 and LILRB2 on hematopoietic stem cells (HSCs) supports ex vivo expansion of HSCs.21,22 LILRBs expressed on neurons regulate axon regeneration and have been implicated in neuropathology of Alzheimer's disease.23,24 Because the immune-suppressive function of LILRBs is similar to that of immune checkpoint proteins such as CTLA4 and PD-1,25 LILRBs are considered to be immune checkpoint factors.26 Importantly, several groups including ours recently showed that LILRBs and a related ITIM-containing receptor LAIR127-30 are expressed on and have tumor-promoting functions in various hematopoietic and solid cancer cells.21,31,32-46,47 Therefore, in addition to the role in immune checkpoints, which is indirectly tumor-supportive, LILRBs are also capable of directly sustaining cancer development. There are excellent recent reviews of structural, functional, and genetic features of LILRBs and related molecules and their functions in immune system-related diseases.3,5,48-50 In this report, we aim to review the evidence that implicates LILRB family members in cancer development.
Ligands for ITIM-containing receptors
Known ligands for ITIM-containing receptors can be roughly divided into 3 groups: membrane-bound proteins (e.g., major histocompatibility complex (MHC) Class I or human leukocyte antigen (HLA) Class I molecules for LILRB1, 2, and 5),51-53,54 extracellular matrix proteins (collagens for LAIR1),27 and soluble proteins (e.g., antibodies for FcγRIIB).55 Some of the ligands and signaling pathways for LILRBs have been identified,12,21,23,24,52-54,56 but many uncertainties remain. LILRB1 and LILRB2 bind classical and non-classical MHC molecules.12,51,52 Several non-MHC or non-HLA ligands also bind to LILRBs 1 and 2, including S100A8 and S100A9 for LILRB1,57 and CD1d,56 several angiopoietin-like proteins (Angptls),21,22 oligomeric β-amyloid,24 myelin inhibitors reticulon 4 (RTN4, Nogo66), myelin associated glycoprotein (MAG), and oligodendrocyte myelin glycoprotein (OMgp) for LILRB2.23 No ligands have been identified for LILRB3 or 4. Relatively little is known about LILRB5, but, recently, evidence that HLA-Class I heavy chains are LILRB5 ligands was reported.54 The known ligands for PirB, the mouse ortholog of LILRB2/3, include MHC class I and Angptls.20,21,58,59 gp49B1, the mouse ortholog of LILRB4, reportedly interacts with mouse integrin αvβ3.60 Human integrin αvβ3 does not bind to LILRB4, however. What is known about LILRBs ligands is summarized in Figure 1.
Relevance to cancer
The interactions between ligands and LILRBs are proposed to serve as immune checkpoints, although certain LILRBs act on a broader array of immune cell types than the classical immune checkpoint proteins CTLA4 and PD-1.26 Upon stimulation by ligands such as HLA-G on tumor cells, LILRBs inhibit immune activation thus indirectly supporting tumor development. What is surprising is that LILRBs and related receptors are expressed by tumor cells and appear to have direct tumor-sustaining activity. Multiple pieces of evidence suggest that LILRBs and related receptors directly support development of certain tumors. First, LILRBs are up-regulated or specifically expressed in some cancer cells. For example, LILRB4 is expressed at higher levels on primary human acute myeloid leukemia (AML) cells, especially M5 subtype AML cells, than on normal counterparts.31,61 LILRBs, a related receptor LAIR1, and a number of ITIM-containing receptors are upregulated in Philadelphia chromosome positive acute lymphocytic leukemia (Ph+ B-ALL) cells compared to normal pre-B cells.46 LILRB4 is not expressed by normal B cells but is expressed in about 50% of B cell chronic lymphocytic leukemia (B-CLL) cells.40 LILRBs are also specifically expressed or up-regulated on lung cancer, gastric cancer, breast cancer, and pancreas cancer cells.32-34,38,41,42 Second, the expression of LILRBs correlates with survival of AML and Ph+ B-ALL patients.31,46 Third, silencing of LILRB2, 3, or 4 in human AML cell lines inhibits cell growth in vitro.31 Finally, inhibition of expression of LAIR1 abolishes leukemia development in different xenograft models.31,46 While LILRBs support tumor development, individual knockout of PirB or gp49B1 (the known mouse orthologs of human LILRBs) or of LAIR1 failed to induce overt defects in normal hematopoiesis.21,62-65 Because LILRBs act as both immune checkpoint molecules and tumor sustaining factors and do not affect hematopoiesis and normal development, they have potential as targets for tumor treatment.
LILRB1
LILRB1 (also known as CD85J, ILT2, LIR1, and MIR7) has 4 ITIMs on the cytoplasmic side, and its extracellular portion has 4 immunoglobulin domains.12,15 LILRB1 is the most widely distributed of the LILRBs with expression on certain NK cells, monocytes/macrophages, eosinophils and basophils, dendritic cells (DCs), subsets of T cells, B cells,5,12,13,15 decidual macrophages,66 progenitor mast cells (but not mature mast cells),67 and osteoclasts.20 LILRB1 is expressed uniformly on monocytes and B cells, but the expression of LILRB1 on NK cells varies between individuals due to a significant degree of diversity within the LILRB1 locus,68 promoter choice, and translational repression.69 Various ligands are known to interact with LILRB1, including HLA class I molecules (e.g., HLA-A, HLA-B, HLA-C, HLA-E, HLA-F, and HLA-G) with affinities in μM range.51,53,70,71 Of note, LILRB1 binds more strongly to HLA-G than to classical HLA class I molecules 53 and dimerized HLA-G induces more efficient LILRB1 signaling than the monomeric form.72 LILRB1 also binds UL18, an HLA class I homologue that is encoded by human cytomegalovirus 12 with more than 1000-fold higher affinity than that for regular HLAs.73 LILRB1 interacts with the α3 domain and β2-microglobulin of class I proteins and the analogous region of UL18 53,73,74 but does not bind HLA-B27 lacking β2-microglobulin.75 In addition, S100A8 and S100A9, 2 calcium-binding proteins, interact with LILRB1.57
Activation of LILRB1 transduces a negative signal that down-regulates the immune response and cytotoxicity, exerting inhibitory effects on NK cells, monocytes/macrophages, DCs, T cells, B cells, osteoblasts, and other cells. However, LILRB1 was also reported to be an activating receptor in NK cells, macrophages, DCs, T cells, and some cancer cells under certain contexts.
NK cells
LILRB1 expressed on NK cells can inhibit immune activity of these cells.12,13,76-78 The binding of HLA-G to LILRB1 up-regulates LILRB1 expression in NK cells, antigen-presenting cells, and T cells,79 and the ligand/LILRB1 interaction inhibits the polarization of NK-cell lytic granules by blocking accumulation of microtubule organizing center and F-actin at the area of contact, intracellular Ca2+ mobilization, and IFN-γ production.80 HLA-G binding to LILRB1 on NK and macrophages also inhibits cytotoxicity and inflammation toward trophoblasts, circumventing undesired anti-fetus immune responses during pregnancy.81,82 LILRB1 also regulates roles of NK cells in cancer immunotherapy. With the progression of breast cancer, the expression of LILRB1 on NK cells increases with concomitant decrease in functions of NK cells.83,84 Blockage of LILRB1 can restore the cytotoxicity function of NK cells in triple negative breast cancer.84 LILRB1 may act cooperatively with other receptors, such as killer cell Ig-like receptor (KIR) in NK cell line NK92, to exert inhibitory effects.85 Inhibition of both NKG2A and LILRB1 induce significant killing of AML and ALL cells by resting KIR-deficient NK cells, suggesting that NKG2A, LILRB1, and KIR might be promising NK cell targets for treatment of acute leukemias.86 Interestingly, LILRB1 may also mediate activating signaling through its immunoreceptor tyrosine-based switch motif (ITSM).87 NK cell-mediated inhibition of HIV-1 replication in monocyte-derived DCs is mediated by the interaction between LILRB1 on the NK cells with S100A9, a non-HLA class I calcium-binding protein, which is expressed on the DCs.57,88
Monocytes/macrophages
LILRB1 can inhibit monocyte activation signals. The binding of HLA-DR to LILRB1 inhibits Ca2+ mobilization in monocytes.13 Co-ligation of LILRB1 with Fc receptor I (CD64) decreases tyrosine phosphorylation of the Fc receptor γ chain and Syk molecules and inhibits intracellular Ca2+ mobilization.89 The expression of LILRB1 decreases in activated decidual macrophages and increases in activated decidual CD4+ T cells, leading to the secretion of IL-4, a cytokine critical for successful pregnancy, from CD4+ T cells.90 Interestingly, upregulated HLA-G on human breast cancer cells may interact with LILRB1-expressing CD68+ cells and CD8+ cells to aid infiltration into breast cancer tissues, contributing to tumor development.91 In contrast, HLA-G homodimer binds LILRB1 on CD14+ macrophages and induces cytokine secretion 87; in this scenario LILRB1 acts as an activating receptor.
DCs
The level of LILRB1 decreases following DC activation by CpG-DNA and inflammatory stimuli.92 Both up-regulation 93 and down-regulation 94 of LILRB1 are observed during the differentiation from monocyte precursors to DCs. Continuous ligation of LILRB1 during DC differentiation confers a distinctive cell phenotype profile, decreases the susceptibility to CD95-mediated cell death, and inhibits cytokine secretion and the immunostimulatory function of DCs.93 In immature human monocyte-derived DCs, crosslinking of LILRB1 inhibits osteoclast-associated receptor-mediated intracellular Ca2+ mobilization, cytokine production, T cell proliferation, and resistance to survival factor deprivation.95 Trophoblast HLA-G may down-regulate allogeneic T cell proliferation by binding with antigen-presenting cells.96 Urenda et al. observed that the levels of circulating plasmacytoid DCs (pDCs) are correlated with disease activity in systemic lupus erythematosus (SLE) patients and that the expression of LILRB1 is diminished in both pDCs and myeloid DCs (mDCs) from these patients, suggesting that lack of LILRB1 may underlie the defective immune-regulation in SLE patients.97 HIV-1-infected elite controllers (who maintain undetectable HIV-1 replication in the absence of antiviral therapy) exhibit strong and selective upregulation of LILRB1 and LILRB3, which significantly increase the antigen-presentation abilities of circulating mDCs.98 LILRB1 also mediates cytokine secretion by mDCs.98 The complex effects of LILRB1 may be partially due to high levels of polymorphism and mutation of its gene.98
T cells
Although LILRB1 can only be detected on the surface of a subset of T cells,12,13,99,100 it is expressed in the cytoplasm of all human T lymphocytes.101 The expression of LILRB1 is increased on anti-viral CD8 T cells during chronic infection.102 Soluble anti-LILRB1 stimulates, but crosslinked antibody inhibits, proliferation and functions of antigen-specific T cells.102-104 LILBR1 plays a negative regulatory effect on the activation of T cells by inhibiting phosphorylation of linker for activation of T cells (LAT) and of ERK1/2.105 LILRB1 competes with CD8 in binding to HLA class I molecules and modulates the activity of CD8+ T cells.53 The HLA-G/LILRB1 interaction on myeloid-derived suppressor cells (MDSCs) expands the population of MDSCs, which inhibited the proliferation and functions of T cells.106 In a transgenic mouse model in which LILRB1 is expressed on T, B, NK, and natural killer T cells, the interaction of LILRB1 and H-2Db, a murine MHC class I molecule, results in impaired development and function of T cells.107 Tumor-cell-expressed HLA-G interacts with LILRB1 on Vγ9Vδ2 T cells or CD8+ T cells to inhibit cytotoxicity of these T cells.108 LILRB1 also mediates activation signaling. For example, interaction of cytomegalovirus protein UL18 and LILRB1 on CD8+ T cells causes non-MHC-restricted lysis of virus-infected cells by resting and activated CD8+ T cells 109 and IFN-γ production by T cells.110
B cells
Crosslinking of LILRB1 inhibits antigen-induced B cell activation and suppresses antibody production.13,111 Interaction between HLA-G and LILRB1 inhibits B cell differentiation, chemotaxis, Ig secretion, and proliferation by G0/G1 arrest through dephosphorylation of AKT, GSK-3β, c-Raf, and Foxo proteins.112 Apoptotic LILRB1+ B cells may contribute to the overwhelming cytokine release and the impairment of the immune memory of malaria patients.113
Osteoclasts
LILRB1, like LILRBs 2-4, is expressed on immature osteoclasts. During osteoclastogenesis, LILRBs are tyrosine phosphorylated and constitutively associate with SHP-1. LILRB1, LILRB3, and LILRB4 inhibit differentiation of osteoclasts.20
Cancer development
As summarized above, tumor cell-expressed HLA-G can interact with LILRB1 on various types of immune cells possibly enabling immune evasion. LILRB1 is directly expressed on certain cancer cells, including AML cells (especially in monocytic AML cells),31 neoplastic B cells (including B cell leukemia, B cell lymphoma, and multiple myeloma cells 35,36), T cell leukemia and lymphoma cells,37 and gastric cancer cells.38 There is evidence that LILRB1 protects primary cutaneous CD8+ and CD56+ T cell lymphomas from cell death 37 and that its expression on human gastric cancer cells contributes to enhanced tumor growth.38 In contrast, binding of soluble or nanoparticle-aggregated HLA-G with LILRB1 on neoplastic B cells inhibits cell proliferation.35 In addition, blocking of LILRB1 on myeloma or lymphoblastic cells in culture using neutralizing antibodies did not affect cell lysis mediated by NK cells.39 Context-dependent LILRB1 function in cancer biology warrants further investigation.
LILRB2
LILRB2 (also known as CD85D, ILT4, LIR2, and MIR10) contains 4 extracellular immunoglobulin domains, a transmembrane domain, and 3 cytoplasmic ITIMs. It is expressed on hematopoietic stem cells, monocytes, macrophages, dendritic cells, basophils in some individuals,21,52,89 decidual macrophages,66 mast cell progenitors,67 endothelial cells,114 and osteoclasts 20 but not on lymphoid cells. LILRB2 binds to multiple types of ligands, notably HLA class I molecules,52 CD1d,56 Angptls,21,22 myelin inhibitors (including Nogo66, MAG, and OMgp 23), and β-amyloid.24 Unlike LILRB1, LILRB2 does not require β2-microglobulin in the complex to bind HLA ligands.75 Cis interaction between LILRB2 and HLA ligands on the same cell has been reported.115 We demonstrated that multimeric Angptls are superior to HLA-G in terms of binding and activating LILRB2.22
Studies on the function of LILRB2 have shown that this receptor plays a physiological role in several tissues. In hematopoietic lineages, LILRB2 has been associated with down modulation of immune response through various mechanisms. Crosslinking of LILRB2 with FcγR in vitro led to inhibition of FcR-mediated signaling in monocytes 89 and to serotonin release in a LILRB2-transfected basophilic cell line.52 Up-regulation of LILRB2 induced dendritic cell tolerance.116 Investigations into the role of LILRB2 in HIV have demonstrated that stronger binding between LILRB2 and HLA class I molecules is positively associated with viral replication, suggesting that this interaction leads to a blunted immune response.117 LILRB2 and LILRB4 are up-regulated in antigen presenting cells in response to Salmonella infection, suggesting a role of these receptors in balancing the inflammatory response in face of bacterial infection.118 Our lab has shown that LILRB2 contributes to ex vivo expansion of HSCs likely through inhibition of differentiation.21 LILRB2 is also expressed and activated on immature osteoclasts during osteoclastogenesis.20 In neurologic tissues, LILRB2 suppresses axonal regeneration via binding to myelin inhibitors 23 and promotes the development of Alzheimer's disease through interaction with β-amyloid.24
LILRB2 plays various roles in cancer biology as well. Expression of LILRB2 has been reported in various cancer cells including AML, especially the monocytic subtype,31 some chronic lymphoblastic leukemia (CLL),40 primary ductal and lobular breast cancer,41 and human non-small cell lung cancer.42 32-34 By contrast, LILRB2 is not expressed by normal lymphoid cells or normal breast tissues.40 41 PirB, the mouse ortholog of LILRB2 and LILRB3, is expressed on MLL-AF9 AML cells including AML stem cells.21 The functional role of LILRB2 is still under investigation, but in lung cancer, LILRB2 supports cancer cell development and survival.32
LILRB3
LILRB3 (also called CD85A, ILT5, LIR3, HL9) contains 4 extracellular immunoglobulin domains, a transmembrane domain, and 4 cytoplasmic ITIMs. Expression of LILRB3 has been reported on monocytes, monocyte-derived osteoclasts, neutrophils, eosinophils, basophils, osteoclasts,20 and progenitor mast cells.67 There is significant polymorphism in the gene encoding LILRB3.48
The ligand for LILRB3 is unknown, and relatively little is known about the function of LILRB3. In human basophils, co-ligation of LILRB3 with FcεRI inhibits Fc receptor-mediated cell activities in vitro.119 When LILRB3 is co-ligated with LILRA2 or IgE receptors on basophils, release of histamines, interleukin-4, and cysteinyl leukotrienes is inhibited.5,48 LILRB3 is also proposed to be an inhibitor of allergic inflammation and a contributor to uncontrolled immune responses and autoimmunity.48 Polymorphisms in LILRB3 are linked to graft-versus-host disease in animal models and to the allergic response.48 As do several other LILRBs, LILRB3 inhibits differentiation of osteoclasts.20
Certain myeloid leukemia, B lymphoid leukemia, and myeloma cells express LILRB3.43 Of note, the observed co-expression of LILRB3 with stem cell marker CD34 and with myeloma marker CD138 suggests a role in cancer development.43 Indeed, inhibition of LILRB3 expression in human leukemia cell lines blocks cell growth.31 Antibodies against LILRB3 induce cytotoxicity of LILRB3-expressing cells via complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity.43 LILRB3 thus is a potential target for anti-cancer therapy.
LILRB4
LILRB4 (also known as CD85K, ILT3, LIR5, HM18) is unique among LILRB family members in that it contains only 2 extracellular immunoglobulin domains; it also has a transmembrane domain and 3 ITIMs. Expression of LILRB4 has been reported on dendritic cells, monocytes and macrophages,12,14,120 14,121 progenitor mast cells,67 endothelial cells,122 and osteoclasts.20 Interestingly, the gene encoding LILRB4 is one of the most polymorphic of all receptor-encoding genes with at least 15 known single-nucleotide polymorphisms 123; the significance of this polymorphism is unclear. LILRB4 is conformationally and electrostatically unsuitable for MHC binding,124 and the ligand for human LILRB4 is unknown.
Monocytes
LILRB4 is expressed on monocytes and can be upregulated by IFNβ and vitamin D3 during central nervous system inflammation.125 Upon crosslinking of LILRB4 to HLA-DR, SHP-1 is recruited to LILRB4 through the 2 ITIMs and inhibits tyrosine phosphorylation of downstream cellular signaling, which inhibits Ca2+ mobilization in monocytes.14 Similarly, co-ligation of CD11b or FcγRIII with LILRB4 inhibits monocyte activation.14 Crosslinking of FcγRI with LILRB4 also significantly reduces FcγRI-induced TNFα production and phosphorylation of Lck, Syk, LAT, ERK, and c-Cbl via recruitment of phosphatases other than SHP-1.126
T cells
Literature showed that LILRB4 from other cell types was capable of inhibiting activation of T cells, on which an unknown ligand for LILRB4 may be expressed. DCs that express high levels of LILRB4 and LILRB2 promote conversion of alloreactive CD4+CD45RO+CD25+ T cells to regulatory T cells (Treg).116 Both membrane-bound and soluble LILRB4 inhibit T cell proliferation and induce differentiation of CD8+ T suppressor cells (Ts) in vitro 127 and in vivo.44 Injection of soluble LILRB4 protects allogeneic human pancreatic islet transplantation 128 and prevents graft-vs.-host disease 129 via induction of Ts cells. Secretion of cytokines, such as IL-1α, IL-1β, IL-6, IFN-γ, and IL-17A, from DCs,130 and the transcriptional factor BCL6 131 are important for Ts induction by LILRB4. On the other hand, LILRB4 expression in Treg cells can be negatively regulated by casein kinase 2, and LILRB4+ Treg cells show attenuated T cell receptor-mediated signaling.132
Cancer development
LILRB4 expressed on immune cells may facilitate tumor immune escape. In humanized mouse experiments, both membrane-bound and soluble LILRB4, mainly produced by tumor-associated macrophages, support cancer cell escape from immune suppression through induction of CD8+ T suppressor cells.44 LILRB4 inhibits T cell proliferation via induction of anergy of CD4+ helper T cells and differentiation of T suppressor cells, suggesting that a ligand or counter-receptor for LILRB4 is expressed on T cells.127 LILRB4 is also expressed on MDSCs in human lung cancer patients, and shorter survival of patients is associated with elevated MDSC numbers and higher LILRB4 expression.133
LILRB4 is also expressed on surfaces of several types of cancer cells. Dobrowolska et al. found that LILRB4 is expressed in all M4 and M5 monocytic AML cells and that it is co-expressed with leukemia stem cell markers CD34 and CD117 in 39% and 50% cases, respectively.61 Although LILRB4 is not expressed by normal B cells, it was detected in 23 out of 47 patients with chronic lymphoblastic leukemia (CLL) cells with more lymphoid tissue involvement; LILRB4 levels may thus be able to predict the prognosis of CLL.40 In solid tumors, Zhang et al. found that LILRB4 is moderately expressed in some gastric cancer cells and tissues; together with LILRB1, it may inhibit NK cell-mediated cytotoxicity to gastric cancer cells.38 More than 40% of patients with certain solid organ tumor such as colorectal carcinoma, pancreatic carcinoma, and melanoma have soluble LILRB4 that can inhibit T cell immunity in vitro.44,45 The supportive role of LILRB4 in solid cancers was evidenced by restoration of T cell responses upon treatment with anti-LILRB4 or by depletion of LILRB4 in serum.44 An animal model of spontaneous ovarian cancer also showed that increased LILRB4 expression is associated with tumor development and progression.134
LILRB5
Expression of LILRB5 (also known as CD85C and LIR8) has been reported in subpopulations of monocytes, NK cells, and mast cell granules.12,67 A very recent study showed that LILRB5 specifically binds to HLA-B7 and HLA-B27 heavy chains.54 Due to relative paucity of studies on LILRB5, the functional role of this receptor is not clear. One study suggested that LILRB5 present in the mononuclear phagocytic system of liver might play a role in clearance of creatine kinase.135 Within human mast cells, LILRB5 is expressed in cytoplasmic granules that are released after crosslinking of high-affinity IgE receptors, which hints at a possible role in mast cell inflammatory response.67 LILRB5 is unique among LILRBs in that it is the only LILRB that is not highly expressed by M5 AML cells, and its expression level does not correlate with the overall survival of AML patients based on analysis of TCGA database of AML patients (https://tcga-data.nci.nih.gov/tcga/).
PirB
PirB is the mouse ortholog of LILRB2/3; it contains 3 functional ITIMs. It is expressed on many hematopoietic cells, such as HSCs, DCs, macrophages, neutrophils and eosinophils, B cells, and osteoclasts. Its ligands include MHCI and Angptls,20,21,58,59 and PirB can interact in cis with MHCI expressed on the same cell.115 PirB regulates cell activity via direct recruitment of tyrosine phosphatases SHP-1 and SHP-2 for downstream signaling.5,21,62,136 Along with the well-documented activity of PirB in regulation of hematopoietic cells, there are also studies that show that PirB influences neuron and osteoclast activities and leukemia development in AML.5,20,21,47,62,64,136-145
DCs
Within DCs, the roles of PirB have been relatively well studied with effects including regulating cytokine-mediated signaling, inducing peripheral tolerance within the graft-versus-host disease context, and facilitating DC maturation.64,136,137 PirB suppresses type I interferon secretion in plasmacytoid DCs.64 When PirB is ectopically expressed in DCs, there is a significant decrease in morbidity and mortality within an allograft graft-vs.-host disease model with the DCs exerting a suppressive function on alloreactive T cells.137 In apparent contradiction, the knockout of PirB was found to impair the maturation of DCs with a hypothesized alteration of cell signaling involving granulocyte-macrophage colony stimulating factor.138
Macrophages
The roles of PirB on macrophages appear to be diverse and depend on organs. In the hematopoietic system, differentiation of macrophage precursors and MDSCs is regulated by PirB expression.47 The deficiency of PirB in MDSCs biases differentiation toward M1 macrophages, which significantly stifles tumor growth and prevents metastasis in an LL2 tumor model.47 PirB inhibits the activity of intestinal macrophages to prevent the progression of inflammatory diseases such as Crohn's disease and ulcerative colitis; the knockout of PirB leads to an increased susceptibility to induced colitis.139 PirB is also a negative regulator in alveolar macrophages and suppresses IL-4 induction of pulmonary fibrosis.140
Other hematopoietic cells
PirB is highly expressed in eosinophils with an unexpected contribution to both inhibitory and activating pathways.142 PirB reduces eotaxin-induced chemotaxis yet promotes chemotaxis response to chitin-induced inflammation.142 Increased PirB expression results upon differentiation of myeloid lineage cells and B cells.146 PirB is also expressed on mouse HSCs and binds with Angptls to support ex vivo expansion of adults HSCs.21 PirB is generally not expressed on mature T cells, but its ectopic expression in peripheral T cells contributes to the suppression of type 1 helper T cell immune response. The exclusion of PirB from mature T cells might allow prompt immune responses.141
Osteoclasts
The deletion of PirB in prefusion osteoclasts led to an accelerated rate of osteoclast formation in vitro, and the study authors concluded that PirB negatively regulates osteoclast development.20 However, PirB-deficient mice show no signs of osteoporosis in models, possibly resulting from involvements of other factors.20
Neurons
PirB regulates neural plasticity in the visual cortex. It appears to act as a negative regulator in the nervous system. It stabilizes neuronal networks by minimizing structural changes, which correlates with the slower learning rates with age.143-145 Blocking activity of PirB in cortical pyramidal neurons led to enhanced ocular dominance plasticity.143-145 The deletion of PirB in a mouse stroke model resulted in more rapid recovery.143
Cancer development
PirB supports the development of AML in mouse models by maintaining self-renewal and inhibiting differentiation of these cancer cells.21 In addition, PirB on MDSCs cancer cells suppresses differentiation of myeloid-derived suppressor cell into M1 macrophages, which in turn inhibits regulatory T cell activities and tumor development.47
gp49B1
gp49B1, or mouse LILRB4, is expressed on macrophages, mast cells, neutrophils, NK cells, and T cells.147-151 It contains 2 extracellular Ig-like domains and 2 cytoplasmic ITIMs. Integrin αvβ3 is the only known ligand of gp49B1.60 The ITIMs of gp49B1 interact with SHIP, SHP-1, and SHP-2,152-154 and recruitment of SHP-1 to gp49B1 leads to inhibition of mast cell activation.154 Co-ligation of gp49B1 and FcγRI blocked IgE-mediated mast cell activation.19 Interaction of gp49B1 with integrin αvβ3 also inhibits mast cell activation 60 and CD40-mediated antibody production by memory and marginal zone B cells.155 The gp49b-deficient mice 63,156 exhibit no development abnormality but show hypersensitivity of mast cells to ovalbumin-challenged anaphylaxis,156 a great elevation of SCF-induced mast cell activation,157 and increased neutrophil-dependent vascular injury induced by LPS.149 The activation of mast cells and neutrophil infiltration in gp49b-deficient mice parallel increases in secretion of cytokines, such as IL-1β, MIP-1α, and MIP-2, upon type-II collagen antibody and LPS treatment.158 Moreover, the combination of ovalbumin challenge with LPS sensitization induces gp49B1 expression on DCs. gp49B1 deficiency induces significant T helper cell type 2 immune response and pulmonary inflammation,159 resulting from elevated chemokine (C-C motif) receptor 7 (CCR7) on gp49B1-deficient DCs and increased secretion of CCL21 by lung lymphatic vessels.160
LILRB signaling in cancer cells
Signaling through LILRBs starts with phosphorylation of tyrosine in ITIMs by Src kinases and results in activation of ITIMs and recruitment of SH2 domain-containing phosphatases SHP-1/SHP-2 or inositol-phosphatase SHIP.3-5 Studies on mouse ortholog PirB furthered our understanding of the interaction between ITIM phospho-tyrosines and phosphatases.62,136 Nevertheless, the signaling cascades downstream of LILRBs are not well characterized. ITIM-containing receptors likely have diverging signaling branches instead of the linear signaling cascades seen in the Janus kinase and signal transducer and activator of transcription (JAK/STAT) pathway. This may partially result from the large number of substrates of ITIM-recruited phosphatases including ITAMs, Src, Syk, ZAP70, Lyn, PI3K, PLC-γ, and Vav1.7-10 This list is far from complete, and additional substrates likely interact with ITIMs. In addition, as reported in a number of studies, phosphatase-independent activities of SHP-1 and SHP-2 may take part in certain contexts.31,161,162 Therefore, interaction of LILRBs with diverse ligands in different cells may result in activation of distinct signaling pathways. Here we summarize recent progress on analysis of LILRB signaling in cancer cells.
Signaling pathways of LILRB2 and PirB in cancer cells
PirB is associated with SHP-1 and SHP-2. Defective PirB signaling diminishes phosphorylation of SHP-1 and SHP-2 in AML cells.21 In human cord blood CD34+ cells, binding of the ligand Angptl to LILRB2 induces phosphorylation of CAMKIV. Concordantly, p-CAMKIV levels are decreased in PirB-deficient AML cells.21 The interaction between Angptl2 and LILRB2 also plays an important role in non-small cell lung cancer (NSCLC).32-34 One study found that LILRB2 drives B7-H3 expression via PI3K/AKT/mTOR signaling and that LILRB2 and B7-H3 co-expression is correlated with poor prognosis in NSCLC.34 In a different study, inhibition of LILRB2 drastically decreased proliferation, colony formation, and migration of NSCLC cells, whereas Angptl2 binding to LILRB2 supported lung cancer development via the SHP2/CAMKI/CREB axis.32 These findings suggest LILRB2 signaling may become a therapeutic target for certain AML subtypes and lung cancer.
LAIR1 signaling in leukemia cells
LAIR1 is a type I transmembrane glycoprotein with identical domain organization to LILRBs: LAIR1 has one extracellular Ig-like domain and 2 intracellular ITIMs that can recruit SHP-1 and SHP-2 upon activation.27-30 LAIR1 is expressed on various hematopoietic cell lineages including CD34+ progenitor cells.163 Of note, LAIR1 appears to be dispensable for normal hematopoiesis.31,46,65 The function of LAIR1 in different leukemias has not been completely clarified. In CLL, downregulation of LAIR1 correlates with increased risk of disease.164,165 Antibody engagement with LAIR1 blocks AKT and NF-κB activation in CLL cells, leading to decreased cell proliferation.165 In AML cells lines, the engagement of LAIR1 inhibits proliferation of blasts, induces apoptosis, and prevents nuclear translocation of NF-κB.165-167 Most recently, 2 independent studies using in vitro and xenograft experiments showed that LAIR1 deficiency retards development of AML and B-ALL.31,46 These same studies indicated that lack of LAIR1 expression leads to remission of leukemia and significantly longer survival time in different leukemia mouse models including MLL-AF9 (AML),21,168,169 AML1-ETO9a (AML),170 BCR-ABL (B-ALL),46 and N-Myc (B-ALL).171 Importantly, LAIR1 deficiency blocks leukemia development in primary or serial transplantation, suggesting that LAIR1 is critical for maintenance of the activity of AML stem cells.31
Interestingly, we found out that SHP-1, but not SHP-2, mediates LAIR1 signaling in AML cells and prevents exhaustion of AML stem cells in vitro and in vivo. SHP-1 is a negative signaling molecule for normal myeloid differentiation. However, it acts as a phosphatase-independent adaptor to recruit CAMKI for activation of the downstream transcription factor CREB in MLL-AF9-transformed AML cells. The LAIR1/SHP-1/CAMKI/CREB axis thus represents an appealing target for AML treatment.31
In the case of Ph+ B-ALL, as demonstrated by Müschen's group, LAIR1, along with some other ITIM-containing receptors, supports development of leukemic cells.46 LAIR1 mediates dephosphorylation of Syk by SHP-1 and SHIP. Hyperactive Syk tyrosine kinase activity is necessary and sufficient to induce death of these B-ALL cells. This suggests that the basic mechanism of negative selection of overactivated B cells still remains functional in transformed B-ALL cells. Therefore, the use of a negative B cell selection strategy might become a new strategy in overcoming drug resistance in Ph+ B-ALL.46
Perspectives and future work
LILRBs and related receptor LAIR1 support cancer cell survival and self-renewal in various types of cancer and represent attractive therapeutic targets. These ITIM-containing receptors support the development, drug resistance, relapse, or cancer stem cell activity of various types of cancer, although exact downstream signaling pathway may differ. It is noteworthy that independent studies demonstrated that SHP-1 was a key downstream regulator that is sufficient to promote the cancerous phenotype in AML and B-ALL. Therefore SHP-1 may act as a main signaling mediator downstream of ITIM-containing receptors and may exert its function via phosphatase-dependent or -independent mechanisms (Fig. 2). A number of questions regarding ITIM-containing receptors need to be answered before we can fully understand the biology of these receptors and apply this knowledge to cancer diagnosis and treatment.
Figure 2.
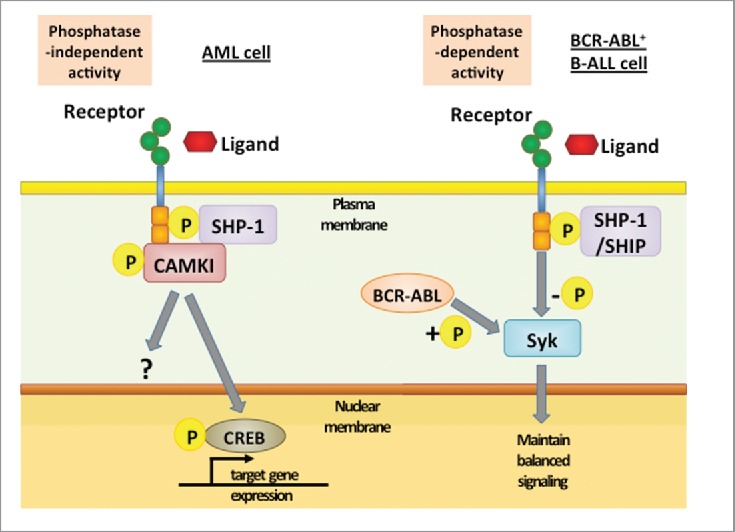
Downstream signaling of ITIM-containing receptor in different leukemia cells. In AML cells, binding of ligands or interaction between receptors activates LILRBs and results in tyrosine phosphorylation in ITIMs. This event is followed by recruitment of SHP-1, which acts as a phosphatase-independent scaffolding protein and forms a complex with the kinase CAMKI. CAMKI activation then induces phosphorylation and nuclear translocation of transcription factor CREB. In contrast, in BCR-ABL+ B-ALL cells, BCR-ABL-induced phosphorylation of Syk is balanced by SHP-1- and SHIP-mediated dephosphorylation. By preventing hyperphosphorylation of Syk, ITIM-containing receptors enable over-activated malignant B cells to avoid negative selection.
Identification of ligands
Identification of ligands for LILRBs is a key step in understanding the biology of these receptors and how LILRBs function in tumor immune escape and cancer development and relapse. The ligands for LILRB3 and LILRB4 have not been identified yet. Given the fact that LILRB2 and LAIR1 each have multiple ligands, it will not be surprising if other ITIM-containing receptors have multiple binding partners. As the affinity of LILRB1 and LILRB2 for MHC-I is of low (μM) affinity, high-affinity ligands, co-ligands, or binding proteins of these 2 receptors may yet be identified. Several experimental techniques could be useful in identification of LILRB ligands such as expression cloning, crosslinking followed by co-immunoprecipitation and mass spectroscopy, protein arrays, and candidate screening. Newer techniques such as cell microarrays 172 and ligand-based receptor capture technologies 173 could also prove helpful in this effort.
Context dependent signaling
Why can inhibitory receptors support cancer cell activity? The tumor-supportive role of SHP-1 in certain acute leukemias may provide mechanistic insight into this question. A role for SHP-1 in AML development is supported by several lines of evidence: a) human LAIR1 is mainly associated with SHP-1 but not SHP-2 174; b) SHP-1 suppresses differentiation in some leukemia cells,175 which is in line with the reported anti-differentiation activity of LAIR1 176; c) the deletion of SHP-1 was found to cause an increase in reactive oxidation species, inactivation of other phosphatases, and a drastic reduction in colony forming ability by upregulation of Arf and p53 46; and d) elevated SHP-1 expression has been reported to be correlated with the chronic phase of CML and with AML progression,177 and SHP-1 inhibits apoptosis in freshly isolated leukemia cells.178 SHP-1 is capable of binding to Grb2 in a phosphatase-independent manner 161; however, the CAMKI recruitment of SHP-1 represents a different phosphatase-independent mechanism that appears to sustain AML stem cell activity,31 and SHP-1 utilizes a phosphatase-dependent mechanism to support Ph+ B-ALL.46 In contrast, SHP-1 is a negative regulator of growth of normal hematopoietic progenitors and overexpression of SHP-1 inhibits growth of cancer cell lines.179-183 LAIR1 signaling negatively regulates myeloid leukemia and CLL.165,166 Multiple studies have also reported decreased expression of SHP-1 in CML, AML, and pediatric acute lymphoblastic leukemia patients leading to the notion that loss of SHP-1 might be a critical step in development of leukemia 184,185 with poorer prognosis for patients.177,186 It will be critical to determine the cell or tumor-stage specificity of the tumor-supportive and tumor-suppressive activities of SHP-1.
The possibility that SHP-1, SHP-2, and SHIP have overlapping but distinct roles in ITIM-containing receptor-mediated signaling in different cells is intriguing. It is generally agreed that SHP-2 plays a positive signaling role in the hematopoietic system, whereas SHP-1 is a negative regulator of cell signaling. However, SHP-1, but not SHP-2, mediates LAIR1 signaling to support AML development.31 SHP-2 is also known to act as an oncogene or as a tumor-suppressor depending on the type of cancers.187 Whereas SHP-1 appears to be solely responsible for the tumor-promoting function of LAIR1 in AML cells,31 both SHP-1 and SHIP support the development of Ph+ B-ALL.46 It is reasonable to assume that the large number of substrates for these phosphatases and divergent downstream signaling contribute to the complexity. Identification of the cell and tumor contexts of SHP-1-, SHP-2-, and SHIP-mediated signaling and their respective substrates or interacting proteins and the full spectrum of LILRB downstream signaling in different cell types will be critical.
LILRBs and related ITIM-containing receptors in solid cancer
The tumor-supportive role of LILRBs extends beyond hematopoietic malignancies. Indeed, expression of LILRB1, 2, and 4 are reported in non-hematopoietic cancers such as gastric cancer, breast cancer, lung cancer, and pancreatic cancer.32-34,38,41,42 Given the number of ITIM-containing receptors, each tumor type and subtype may express unique combinations. An improved understanding of ITIM-containing receptor biology will be critical as we seek to understand their individual and combined effects in these cancer cells.
Potential therapeutic approaches targeting LILRBs
The dual roles as immune checkpoint molecules and tumor-sustaining factors and a lack of apparent function in normal development and hematopoiesis suggest that LILRBs are ideal targets for treating cancer. Although LILRBs have been reported to regulate axon development 23 and osteoclast differentiation,20 the potential side effects of LILRB inhibition may not be a big hurdle. For instance, a design of drug (such as antibody) that cannot cross blood-brain-barrier would spare the central neuronal system, and it has been shown that inhibition of LILRBs does not alter osteoclastogenesis in vivo.20 Potential approaches to inhibition of LILRB signaling include targeting different segments of these signaling pathways. A possible approach would be to use recombinant soluble extracellular domains of these receptors or blocking antibodies to competitively block activity. Antibody conjugate therapeutics 188 that directly target LILRB-expressing malignant cells are also possible options. In addition, it will be interesting to test whether chimeric antigen receptors 189 engineered to target LILRBs and other ITIM-containing receptors are effective in treating hematopoietic malignancies. In parallel, inhibitors specific to phosphatase-dependent and/or independent SHP-1 activity have great potential. Finally, cloning of novel high-affinity ligands for LILRBs will provide novel insights into targeting signaling through these receptors.
Conclusion
The identification of LILRBs and their downstream signaling as potential therapeutic targets has reshaped our views regarding how cancer develops, how cancer cells differ from other cells, and how to treat this difficult disease. The studies reviewed here suggest that some leukemia cells have unique signaling pathways downstream of ITIM-containing receptors. These inhibitory receptors may enable the cancer cells to survive conventional therapies, resulting in tumor relapse. Because inhibition of the signaling of certain LILRBs directly blocks cancer growth and unleashes immune checkpoints that may suppress tumorigenesis, but does not disturb normal development, these receptors may represent ideal targets for treating cancer. The blockade of inhibitory receptor signaling in combination with conventional therapies may prove to be an effective strategy for elimination of leukemia cells as well as other types of cancer cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
We would like to thank the NIH (1R01CA172268), the Leukemia & Lymphoma Society (1024-14 and TRP-6024-14), the Robert A. Welch Foundation (I-1834), the Cancer Prevention and Research Institute of Texas (RP140402 and DP150056), and the March of Dimes Foundation (#1-FY14-201) for generous support.
Acknowledgments
We thank Drs. John E. Coligan, Eric O. Long, and Susan K. Pierce at NIAID for insightful discussions. We regret that we have been unable to cite many relevant primary references due to space limitations.
References
- 1. Daeron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S, Fridman WH. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity 1995; 3:635-46; PMID:7584153; http://dx.doi.org/ 10.1016/1074-7613(95)90134-5 [DOI] [PubMed] [Google Scholar]
- 2. Staub E, Rosenthal A, Hinzmann B. Systematic identification of immunoreceptor tyrosine-based inhibitory motifs in the human proteome. Cell Signal 2004; 16:435-56; PMID:14709333; http://dx.doi.org/ 10.1016/j.cellsig.2003.08.013 [DOI] [PubMed] [Google Scholar]
- 3. Takai T, Nakamura A, Endo S. Role of PIR-B in autoimmune glomerulonephritis. J Biomed Biotechnol 2011; 2011:275302; PMID:20976309; http://dx.doi.org/ 10.1155/2011/275302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev 2008; 224:11-43; PMID:18759918; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00666.x [DOI] [PubMed] [Google Scholar]
- 5. Katz HR. Inhibition of inflammatory responses by leukocyte Ig-like receptors. Adv Immunol 2006; 91:251-72; PMID:16938543; http://dx.doi.org/ 10.1016/S0065-2776(06)91007-4 [DOI] [PubMed] [Google Scholar]
- 6. Bruhns P, Vely F, Malbec O, Fridman WH, Vivier E, Daeron M. Molecular basis of the recruitment of the SH2 domain-containing inositol 5-phosphatases SHIP1 and SHIP2 by fcgamma RIIB. J Biol Chem 2000; 275:37357-64; PMID:11016922; http://dx.doi.org/ 10.1074/jbc.M003518200 [DOI] [PubMed] [Google Scholar]
- 7. Huang ZY, Hunter S, Kim MK, Indik ZK, Schreiber AD. The effect of phosphatases SHP-1 and SHIP-1 on signaling by the ITIM- and ITAM-containing Fcgamma receptors FcgammaRIIB and FcgammaRIIA. J Leukocyte Biol 2003; 73:823-9; PMID:12773515; http://dx.doi.org/ 10.1189/jlb.0902454 [DOI] [PubMed] [Google Scholar]
- 8. Binstadt BA, Brumbaugh KM, Dick CJ, Scharenberg AM, Williams BL, Colonna M, Lanier LL, Kinet JP, Abraham RT, Leibson PJ. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity 1996; 5:629-38; PMID:8986721; http://dx.doi.org/ 10.1016/S1074-7613(00)80276-9 [DOI] [PubMed] [Google Scholar]
- 9. Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat Med 2002; 8:61-7; PMID:11786908; http://dx.doi.org/ 10.1038/nm0102-61 [DOI] [PubMed] [Google Scholar]
- 10. Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol 2003; 23:6291-9; PMID:12917349; http://dx.doi.org/ 10.1128/MCB.23.17.6291-6299.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol 2009; 10:340-7; PMID:19295630; http://dx.doi.org/ 10.1038/ni.1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borges L, Hsu ML, Fanger N, Kubin M, Cosman D. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J Immunol (Baltimore, Md : 1950) 1997; 159:5192-6; PMID:9548455 [PubMed] [Google Scholar]
- 13. Colonna M, Navarro F, Bellon T, Llano M, Garcia P, Samaridis J, Angman L, Cella M, Lopez-Botet M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med 1997; 186:1809-18; PMID:9382880; http://dx.doi.org/ 10.1084/jem.186.11.1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cella M, Dohring C, Samaridis J, Dessing M, Brockhaus M, Lanzavecchia A, Colonna M. A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J Exp Med 1997; 185:1743-51; PMID:9151699; http://dx.doi.org/ 10.1084/jem.185.10.1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samaridis J, Colonna M. Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur J Immunol 1997; 27:660-5; PMID:9079806; http://dx.doi.org/ 10.1002/eji.1830270313 [DOI] [PubMed] [Google Scholar]
- 16. Martin AM, Kulski JK, Witt C, Pontarotti P, Christiansen FT. Leukocyte Ig-like receptor complex (LRC) in mice and men. Trend Immunol 2002; 23:81-8; PMID:11929131; http://dx.doi.org/ 10.1016/S1471-4906(01)02155-X [DOI] [PubMed] [Google Scholar]
- 17. Wende H, Colonna M, Ziegler A, Volz A. Organization of the leukocyte receptor cluster (LRC) on human chromosome 19q13.4. Mammalian Gen 1999; 10:154-60; PMID:9922396; http://dx.doi.org/ 10.1007/s003359900961 [DOI] [PubMed] [Google Scholar]
- 18. Kubagawa H, Burrows PD, Cooper MD. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc Natl Acad Sci U S A 1997; 94:5261-6; PMID:9144225; http://dx.doi.org/ 10.1073/pnas.94.10.5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katz HR, Vivier E, Castells MC, McCormick MJ, Chambers JM, Austen KF. Mouse mast cell gp49B1 contains two immunoreceptor tyrosine-based inhibition motifs and suppresses mast cell activation when coligated with the high-affinity Fc receptor for IgE. Proc Natl Acad Sci U S A 1996; 93:10809-14; PMID:8855262; http://dx.doi.org/ 10.1073/pnas.93.20.10809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mori Y, Tsuji S, Inui M, Sakamoto Y, Endo S, Ito Y, Fujimura S, Koga T, Nakamura A, Takayanagi H, et al. . Inhibitory immunoglobulin-like receptors LILRB and PIR-B negatively regulate osteoclast development. J Immunol 2008; 181:4742-51; PMID:18802077; http://dx.doi.org/ 10.4049/jimmunol.181.7.4742 [DOI] [PubMed] [Google Scholar]
- 21. Zheng J, Umikawa M, Cui C, Li J, Chen X, Zhang C, Hyunh H, Kang X, Silvany R, Wan X, et al. . Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature 2012; 485:656-60; PMID:22660330; http://dx.doi.org/ 10.1038/nature11095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deng M, Lu Z, Zheng J, Wan X, Chen X, Hirayasu K, Sun H, Lam Y, Chen L, Wang Q, et al. . A motif in LILRB2 critical for Angptl2 binding and activation. Blood 2014; 124:924-35; PMID:24899623; http://dx.doi.org/ 10.1182/blood-2014-01-549162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 2008; 322:967-70; PMID:18988857; http://dx.doi.org/ 10.1126/science.1161151 [DOI] [PubMed] [Google Scholar]
- 24. Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, Shatz CJ. Human LilrB2 is a beta-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science 2013; 341:1399-404; PMID:24052308; http://dx.doi.org/ 10.1126/science.1242077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015; 161:205-14; PMID:25860605; http://dx.doi.org/ 10.1016/j.cell.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carosella ED, Rouas-Freiss N, Roux DT, Moreau P, LeMaoult J. HLA-G: An Immune Checkpoint Molecule. Adv Immunol 2015; 127:33-144; PMID:26073983; http://dx.doi.org/ 10.1016/bs.ai.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 27. Olde Nordkamp MJ, van Eijk M, Urbanus RT, Bont L, Haagsman HP, Meyaard L. Leukocyte-associated Ig-like receptor-1 is a novel inhibitory receptor for surfactant protein D. J Leukoc Biol 2014; 96:105-11; PMID:24585933; http://dx.doi.org/ 10.1189/jlb.3AB0213-092RR [DOI] [PubMed] [Google Scholar]
- 28. Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 1997; 7:283-90; PMID:9285412; http://dx.doi.org/ 10.1016/S1074-7613(00)80530-0 [DOI] [PubMed] [Google Scholar]
- 29. Poggi A, Pella N, Morelli L, Spada F, Revello V, Sivori S, Augugliaro R, Moretta L, Moretta A. p40, a novel surface molecule involved in the regulation of the non-major histocompatibility complex-restricted cytolytic activity in humans. Eur J Immunol 1995; 25:369-76; PMID:7875198; http://dx.doi.org/ 10.1002/eji.1830250210 [DOI] [PubMed] [Google Scholar]
- 30. Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, Lisman T, Sonnenberg A, Lenting PJ, et al. . Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med 2006; 203:1419-25; PMID:16754721; http://dx.doi.org/ 10.1084/jem.20052554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang X, Lu Z, Cui C, Deng M, Fan Y, Dong B, Han X, Xie F, Tyner JW, Coligan JE, et al. . The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat Cell Biol 2015; 17:665-77; PMID:25915125; http://dx.doi.org/ 10.1038/ncb3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Yu X, Xie J, Zhan M, Yu Z, Xie L, Zeng H, Zhang F, Chen G, Yi X, et al. . ANGPTL2/LILRB2 signaling promotes the propagation of lung cancer cells. Oncotarget 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang L, Geng T, Guo X, Liu J, Zhang P, Yang D, Li J, Yu S, Sun Y. Co-expression of immunoglobulin-like transcript 4 and angiopoietin-like proteins in human non-small cell lung cancer. Mol Med Rep 2015; 11:2789-96; PMID:25482926 [DOI] [PubMed] [Google Scholar]
- 34. Zhang P, Yu S, Li H, Liu C, Li J, Lin W, Gao A, Wang L, Gao W, Sun Y. ILT4 drives B7-H3 expression via PI3K/AKT/mTOR signalling and ILT4/B7-H3 co-expression correlates with poor prognosis in non-small cell lung cancer. FEBS Lett 2015; 589:2248-56; PMID:26149216 [DOI] [PubMed] [Google Scholar]
- 35. Naji A, Menier C, Maki G, Carosella ED, Rouas-Freiss N. Neoplastic B-cell growth is impaired by HLA-G/ILT2 interaction. Leukemia 2012; 26:1889-92; PMID:22441169; http://dx.doi.org/ 10.1038/leu.2012.62 [DOI] [PubMed] [Google Scholar]
- 36. Harly C, Peyrat MA, Netzer S, Dechanet-Merville J, Bonneville M, Scotet E. Up-regulation of cytolytic functions of human Vdelta2-gamma T lymphocytes through engagement of ILT2 expressed by tumor target cells. Blood 2011; 117:2864-73; PMID:21233315; http://dx.doi.org/ 10.1182/blood-2010-09-309781 [DOI] [PubMed] [Google Scholar]
- 37. Urosevic M, Kamarashev J, Burg G, Dummer R. Primary cutaneous CD8+ and CD56+ T-cell lymphomas express HLA-G and killer-cell inhibitory ligand, ILT2. Blood 2004; 103:1796-8; PMID:14592815; http://dx.doi.org/ 10.1182/blood-2003-10-3372 [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y, Lu N, Xue Y, Zhang M, Li Y, Si Y, Bian X, Jia Y, Wang Y. Expression of immunoglobulin-like transcript (ILT)2 and ILT3 in human gastric cancer and its clinical significance. Mol Med Rep 2012; 5:910-6; PMID:22246571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heidenreich S, Zu Eulenburg C, Hildebrandt Y, Stubig T, Sierich H, Badbaran A, Eiermann TH, Binder TM, Kroger N. Impact of the NK cell receptor LIR-1 (ILT-2/CD85j/LILRB1) on cytotoxicity against multiple myeloma. Clin Dev Immunol 2012; 2012:652130; PMID:22844324; http://dx.doi.org/ 10.1155/2012/652130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Colovai AI, Tsao L, Wang S, Lin H, Wang C, Seki T, Fisher JG, Menes M, Bhagat G, Alobeid B, et al. . Expression of inhibitory receptor ILT3 on neoplastic B cells is associated with lymphoid tissue involvement in chronic lymphocytic leukemia. Cytometry B Clin Cytom 2007; 72:354-62; PMID:17266150; http://dx.doi.org/ 10.1002/cyto.b.20164 [DOI] [PubMed] [Google Scholar]
- 41. Liu J, Wang L, Gao W, Li L, Cui X, Yang H, Lin W, Dang Q, Zhang N, Sun Y. Inhibitory receptor immunoglobulin-like transcript 4 was highly expressed in primary ductal and lobular breast cancer and significantly correlated with IL-10. Diagn Pathol 2014; 9:85; PMID:24762057; http://dx.doi.org/ 10.1186/1746-1596-9-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun Y, Liu J, Gao P, Wang Y, Liu C. Expression of Ig-like transcript 4 inhibitory receptor in human non-small cell lung cancer. Chest 2008; 134:783-8; PMID:18625675; http://dx.doi.org/ 10.1378/chest.07-1100 [DOI] [PubMed] [Google Scholar]
- 43. Pfistershammer K, Lawitschka A, Klauser C, Leitner J, Weigl R, Heemskerk MH, Pickl WF, Majdic O, Bohmig GA, Fischer GF, et al. . Allogeneic disparities in immunoglobulin-like transcript 5 induce potent antibody responses in hematopoietic stem cell transplant recipients. Blood 2009; 114:2323-32; PMID:19617579; http://dx.doi.org/ 10.1182/blood-2008-10-183814 [DOI] [PubMed] [Google Scholar]
- 44. Suciu-Foca N, Feirt N, Zhang QY, Vlad G, Liu Z, Lin H, Chang CC, Ho EK, Colovai AI, Kaufman H, et al. . Soluble Ig-like transcript 3 inhibits tumor allograft rejection in humanized SCID mice and T cell responses in cancer patients. J Immunol 2007; 178:7432-41; PMID:17513794; http://dx.doi.org/ 10.4049/jimmunol.178.11.7432 [DOI] [PubMed] [Google Scholar]
- 45. Cortesini R. Pancreas cancer and the role of soluble immunoglobulin-like transcript 3 (ILT3). JOP 2007; 8:697-703; PMID:17993722 [PubMed] [Google Scholar]
- 46. Chen Z, Shojaee S, Buchner M, Geng H, Lee JW, Klemm L, Titz B, Graeber TG, Park E, Tan YX, et al. . Signalling thresholds and negative B-cell selection in acute lymphoblastic leukaemia. Nature 2015; 521:357-61; PMID:25799995; http://dx.doi.org/ 10.1038/nature14231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ma G, Pan PY, Eisenstein S, Divino CM, Lowell CA, Takai T, Chen SH. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity 2011; 34:385-95; PMID:21376641; http://dx.doi.org/ 10.1016/j.immuni.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hirayasu K, Arase H. Functional and genetic diversity of leukocyte immunoglobulin-like receptor and implication for disease associations. J Hum Gen 2015; PMID:26040207 [DOI] [PubMed] [Google Scholar]
- 49. Barrow AD, Trowsdale J. The extended human leukocyte receptor complex: diverse ways of modulating immune responses. Immunol Rev 2008; 224:98-123; PMID:18759923; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00653.x [DOI] [PubMed] [Google Scholar]
- 50. Trowsdale J, Jones DC, Barrow AD, Traherne JA. Surveillance of cell and tissue perturbation by receptors in the LRC. Immunol Rev 2015; 267:117-36; PMID:26284474; http://dx.doi.org/ 10.1111/imr.12314 [DOI] [PubMed] [Google Scholar]
- 51. Willcox BE, Thomas LM, Bjorkman PJ. Crystal structure of HLA-A2 bound to LIR-1, a host and viral major histocompatibility complex receptor. Nat Immunol 2003; 4:913-9; PMID:12897781; http://dx.doi.org/ 10.1038/ni961 [DOI] [PubMed] [Google Scholar]
- 52. Colonna M, Samaridis J, Cella M, Angman L, Allen RL, O'Callaghan CA, Dunbar R, Ogg GS, Cerundolo V, Rolink A. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J Immunol (Baltimore, Md : 1950) 1998; 160:3096-100; PMID:9531263 [PubMed] [Google Scholar]
- 53. Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, Allan DS, Makadzange A, Rowland-Jones S, Willcox B, et al. . Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A 2003; 100:8856-61; PMID:12853576; http://dx.doi.org/ 10.1073/pnas.1431057100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang Z, Hatano H, Shaw J, Olde Nordkamp M, Jiang G, Li D, Kollnberger S. The Leukocyte Immunoglobulin-Like Receptor Family Member LILRB5 Binds to HLA-Class I Heavy Chains. PLoS One 2015; 10:e0129063; PMID:26098415; http://dx.doi.org/ 10.1371/journal.pone.0129063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fridman WH. Fc receptors and immunoglobulin binding factors. FASEB J 1991; 5:2684-90; PMID:1916092 [DOI] [PubMed] [Google Scholar]
- 56. Li D, Wang L, Yu L, Freundt EC, Jin B, Screaton GR, Xu XN. Ig-like transcript 4 inhibits lipid antigen presentation through direct CD1d interaction. J Immunol 2009; 182:1033-40; PMID:19124746; http://dx.doi.org/ 10.4049/jimmunol.182.2.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arnold V, Cummings JS, Moreno-Nieves UY, Didier C, Gilbert A, Barre-Sinoussi F, Scott-Algara D. S100A9 protein is a novel ligand for the CD85j receptor and its interaction is implicated in the control of HIV-1 replication by NK cells. Retrovirology 2013; 10:122; PMID:24156302; http://dx.doi.org/ 10.1186/1742-4690-10-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Torii I, Oka S, Hotomi M, Benjamin WH, Jr., Takai T, Kearney JF, Briles DE, Kubagawa H. PIR-B-deficient mice are susceptible to Salmonella infection. J Immunol 2008; 181:4229-39; http://dx.doi.org/ 10.4049/jimmunol.181.6.4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Takai T. Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology 2005; 115:433-40; PMID:16011512; http://dx.doi.org/ 10.1111/j.1365-2567.2005.02177.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Castells MC, Klickstein LB, Hassani K, Cumplido JA, Lacouture ME, Austen KF, Katz HR. gp49B1-alpha(v)beta3 interaction inhibits antigen-induced mast cell activation. Nat Immunol 2001; 2:436-42; PMID:11323698 [DOI] [PubMed] [Google Scholar]
- 61. Dobrowolska H, Gill KZ, Serban G, Ivan E, Li Q, Qiao P, Suciu-Foca N, Savage D, Alobeid B, Bhagat G, et al. . Expression of immune inhibitory receptor ILT3 in acute myeloid leukemia with monocytic differentiation. Cytometry B Clin Cytom 2013; 84:21-9; PMID:23027709; http://dx.doi.org/ 10.1002/cyto.b.21050 [DOI] [PubMed] [Google Scholar]
- 62. Blery M, Kubagawa H, Chen CC, Vely F, Cooper MD, Vivier E. The paired Ig-like receptor PIR-B is an inhibitory receptor that recruits the protein-tyrosine phosphatase SHP-1. Proc Natl Acad Sci U S A 1998; 95:2446-51; PMID:9482905; http://dx.doi.org/ 10.1073/pnas.95.5.2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rojo S, Stebbins CC, Peterson ME, Dombrowicz D, Wagtmann N, Long EO. Natural killer cells and mast cells from gp49B null mutant mice are functional. Mol Cell Biol 2000; 20:7178-82; PMID:10982834; http://dx.doi.org/ 10.1128/MCB.20.19.7178-7182.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mitsuhashi Y, Nakamura A, Endo S, Takeda K, Yabe-Wada T, Nukiwa T, Takai T. Regulation of plasmacytoid dendritic cell responses by PIR-B. Blood 2012; 120:3256-9; PMID:22948046; http://dx.doi.org/ 10.1182/blood-2012-03-419093 [DOI] [PubMed] [Google Scholar]
- 65. Tang X, Tian L, Esteso G, Choi SC, Barrow AD, Colonna M, Borrego F, Coligan JE. Leukocyte-associated Ig-like receptor-1-deficient mice have an altered immune cell phenotype. J Immunol 2012; 188:548-58; PMID:22156345; http://dx.doi.org/ 10.4049/jimmunol.1102044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hunt JS, Petroff MG, Morales P, Sedlmayr P, Geraghty DE, Ober C. HLA-G in reproduction: studies on the maternal-fetal interface. Human immunology 2000; 61:1113-7; PMID:11137215; http://dx.doi.org/ 10.1016/S0198-8859(00)00195-6 [DOI] [PubMed] [Google Scholar]
- 67. Tedla N, Lee CW, Borges L, Geczy CL, Arm JP. Differential expression of leukocyte immunoglobulin-like receptors on cord-blood-derived human mast cell progenitors and mature mast cells. J Leukoc Biol 2008; 83:334-43; PMID:17998301; http://dx.doi.org/ 10.1189/jlb.0507314 [DOI] [PubMed] [Google Scholar]
- 68. Davidson CL, Li NL, Burshtyn DN. LILRB1 polymorphism and surface phenotypes of natural killer cells. Hum Immunol 2010; 71:942-9; PMID:20600445; http://dx.doi.org/ 10.1016/j.humimm.2010.06.015 [DOI] [PubMed] [Google Scholar]
- 69. Lamar DL, Weyand CM, Goronzy JJ. Promoter choice and translational repression determine cell type-specific cell surface density of the inhibitory receptor CD85j expressed on different hematopoietic lineages. Blood 2010; 115:3278-86; PMID:20194892; http://dx.doi.org/ 10.1182/blood-2009-09-243493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lepin EJ, Bastin JM, Allan DS, Roncador G, Braud VM, Mason DY, van der Merwe PA, McMichael AJ, Bell JI, Powis SH, et al. . Functional characterization of HLA-F and binding of HLA-F tetramers to ILT2 and ILT4 receptors. Eur J Immunol 2000; 30:3552-61; PMID:11169396; http://dx.doi.org/ 10.1002/1521-4141(200012)30:12%3c3552::AID-IMMU3552%3e3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- 71. Allan DS, Lepin EJ, Braud VM, O'Callaghan CA, McMichael AJ. Tetrameric complexes of HLA-E, HLA-F, and HLA-G. J Immun Methods 2002; 268:43-50; PMID:12213342; http://dx.doi.org/ 10.1016/S0022-1759(02)00199-0 [DOI] [PubMed] [Google Scholar]
- 72. Shiroishi M, Kuroki K, Ose T, Rasubala L, Shiratori I, Arase H, Tsumoto K, Kumagai I, Kohda D, Maenaka K. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J Biol Chem 2006; 281:10439-47; PMID:16455647; http://dx.doi.org/ 10.1074/jbc.M512305200 [DOI] [PubMed] [Google Scholar]
- 73. Chapman TL, Heikeman AP, Bjorkman PJ. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity 1999; 11:603-13; PMID:10591185; http://dx.doi.org/ 10.1016/S1074-7613(00)80135-1 [DOI] [PubMed] [Google Scholar]
- 74. Chapman TL, Heikema AP, West AP, Jr., Bjorkman PJ. Crystal structure and ligand binding properties of the D1D2 region of the inhibitory receptor LIR-1 (ILT2). Immunity 2000; 13:727-36; PMID:11114384; http://dx.doi.org/ 10.1016/S1074-7613(00)00071-6 [DOI] [PubMed] [Google Scholar]
- 75. Allen RL, Raine T, Haude A, Trowsdale J, Wilson MJ. Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J Immunol 2001; 167:5543-7; http://dx.doi.org/ 10.4049/jimmunol.167.10.5543 [DOI] [PubMed] [Google Scholar]
- 76. Vitale M, Castriconi R, Parolini S, Pende D, Hsu ML, Moretta L, Cosman D, Moretta A. The leukocyte Ig-like receptor (LIR)-1 for the cytomegalovirus UL18 protein displays a broad specificity for different HLA class I alleles: analysis of LIR-1 + NK cell clones. Inter Immunol 1999; 11:29-35; PMID:10050671; http://dx.doi.org/ 10.1093/intimm/11.1.29 [DOI] [PubMed] [Google Scholar]
- 77. Navarro F, Llano M, Bellon T, Colonna M, Geraghty DE, Lopez-Botet M. The ILT2(LIR1) and CD94/NKG2A NK cell receptors respectively recognize HLA-G1 and HLA-E molecules co-expressed on target cells. Eur J Immunol 1999; 29:277-83; PMID:9933109; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199901)29:01%3c277::AID-IMMU277%3e3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 78. Morel E, Bellón T. HLA class I molecules regulate IFN-γ production induced in NK cells by target cells, viral products, or immature dendritic cells through the inhibitory receptor ILT2/CD85j. J Immunol 2008; 181:2368-81; PMID:18684926; http://dx.doi.org/ 10.4049/jimmunol.181.4.2368 [DOI] [PubMed] [Google Scholar]
- 79. LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J 2005; 19:662-4; PMID:15670976 [DOI] [PubMed] [Google Scholar]
- 80. Favier B, Lemaoult J, Lesport E, Carosella ED. ILT2/HLA-G interaction impairs NK-cell functions through the inhibition of the late but not the early events of the NK-cell activating synapse. FASEB J 2010; 24:689-99; PMID:19841038; http://dx.doi.org/ 10.1096/fj.09-135194 [DOI] [PubMed] [Google Scholar]
- 81. Ponte M, Cantoni C, Biassoni R, Tradori-Cappai A, Bentivoglio G, Vitale C, Bertone S, Moretta A, Moretta L, Mingari MC. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc Natl Acad Sci U S A 1999; 96:5674-9; PMID:10318943; http://dx.doi.org/ 10.1073/pnas.96.10.5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Petroff MG, Sedlmayr P, Azzola D, Hunt JS. Decidual macrophages are potentially susceptible to inhibition by class Ia and class Ib HLA molecules. J Reproduct Immunol 2002; 56:3-17; PMID:12106880; http://dx.doi.org/ 10.1016/S0165-0378(02)00024-4 [DOI] [PubMed] [Google Scholar]
- 83. Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Goncalves A, Andre P, Romagne F, Thibault G, et al. . Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121:3609-22; PMID:21841316; http://dx.doi.org/ 10.1172/JCI45816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Roberti MP, Juliá EP, Rocca YS, Amat M, Bravo AI, Loza J, Coló F, Loza CM, Fabiano V, Maino M. Overexpression of CD85j in TNBC patients inhibits Cetuximab-mediated NK-cell ADCC but can be restored with CD85j functional blockade. Eur J Immunol 2015; 45:1560-9; PMID:25726929; http://dx.doi.org/ 10.1002/eji.201445353 [DOI] [PubMed] [Google Scholar]
- 85. Kirwan SE, Burshtyn DN. Killer cell Ig-like receptor-dependent signaling by Ig-like transcript 2 (ILT2/CD85j/LILRB1/LIR-1). J Immunol 2005; 175:5006-15; http://dx.doi.org/ 10.4049/jimmunol.175.8.5006 [DOI] [PubMed] [Google Scholar]
- 86. Godal R, Bachanova V, Gleason M, McCullar V, Yun GH, Cooley S, Verneris MR, McGlave PB, Miller JS. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Trans 2010; 16:612-21; PMID:20139023; http://dx.doi.org/ 10.1016/j.bbmt.2010.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li C, Houser BL, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci U S A 2009; 106:5767-72; PMID:19304799; http://dx.doi.org/ 10.1073/pnas.0901173106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Scott-Algara D, Arnold V, Didier C, Kattan T, Pirozzi G, Barre-Sinoussi F, Pancino G. The CD85j+ NK cell subset potently controls HIV-1 replication in autologous dendritic cells. PloS one 2008; 3:e1975; PMID:18398485; http://dx.doi.org/ 10.1371/journal.pone.0001975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fanger NA, Cosman D, Peterson L, Braddy SC, Maliszewski CR, Borges L. The MHC class I binding proteins LIR-1 and LIR-2 inhibit Fc receptor-mediated signaling in monocytes. Eur J Immunol 1998; 28:3423-34; PMID:9842885; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199811)28:11%3c3423::AID-IMMU3423%3e3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- 90. Lombardelli L, Aguerre-Girr M, Logiodice F, Kullolli O, Casart Y, Polgar B, Berrebi A, Romagnani S, Maggi E, Le Bouteiller P. HLA-G5 induces IL-4 secretion critical for successful pregnancy through differential expression of ILT2 receptor on decidual CD4+ T cells and macrophages. J Immunol 2013; 191:3651-62; PMID:23997222; http://dx.doi.org/ 10.4049/jimmunol.1300567 [DOI] [PubMed] [Google Scholar]
- 91. Lefebvre S, Antoine M, Uzan S, McMaster M, Dausset J, Carosella ED, Paul P. Specific activation of the non-classical class I histocompatibility HLA-G antigen and expression of the ILT2 inhibitory receptor in human breast cancer. J Pathol 2002; 196:266-74; PMID:11857488; http://dx.doi.org/ 10.1002/path.1039 [DOI] [PubMed] [Google Scholar]
- 92. Ju XS, Hacker C, Scherer B, Redecke V, Berger T, Schuler G, Wagner H, Lipford GB, Zenke M. Immunoglobulin-like transcripts ILT2, ILT3 and ILT7 are expressed by human dendritic cells and down-regulated following activation. Gene 2004; 331:159-64; PMID:15094202; http://dx.doi.org/ 10.1016/j.gene.2004.02.018 [DOI] [PubMed] [Google Scholar]
- 93. Young NT, Waller EC, Patel R, Roghanian A, Austyn JM, Trowsdale J. The inhibitory receptor LILRB1 modulates the differentiation and regulatory potential of human dendritic cells. Blood 2008; 111:3090-6; PMID:18094328; http://dx.doi.org/ 10.1182/blood-2007-05-089771 [DOI] [PubMed] [Google Scholar]
- 94. Fedoric B, Krishnan R. Rapamycin downregulates the inhibitory receptors ILT2, ILT3, ILT4 on human dendritic cells and yet induces T cell hyporesponsiveness independent of FoxP3 induction. Immunol Lett 2008; 120:49-56; PMID:18652845; http://dx.doi.org/ 10.1016/j.imlet.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 95. Tenca C, Merlo A, Merck E, Bates EE, Saverino D, Simone R, Zarcone D, Trinchieri G, Grossi CE, Ciccone E. CD85j (leukocyte Ig-like receptor-1/Ig-like transcript 2) inhibits human osteoclast-associated receptor-mediated activation of human dendritic cells. J Immunol 2005; 174:6757-63; http://dx.doi.org/ 10.4049/jimmunol.174.11.6757 [DOI] [PubMed] [Google Scholar]
- 96. Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol 2007; 37:1924-37; PMID:17549736; http://dx.doi.org/ 10.1002/eji.200737089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Monsivais-Urenda A, Gomez-Martin D, Santana-de-Anda K, Cruz-Martinez J, Alcocer-Varela J, Gonzalez-Amaro R. Defective expression and function of the ILT2/CD85j regulatory receptor in dendritic cells from patients with systemic lupus erythematosus. Hum Immunol 2013; 74:1088-96; PMID:23756160; http://dx.doi.org/ 10.1016/j.humimm.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 98. Huang J, Burke PS, Cung TD, Pereyra F, Toth I, Walker BD, Borges L, Lichterfeld M, Yu XG. Leukocyte immunoglobulin-like receptors maintain unique antigen-presenting properties of circulating myeloid dendritic cells in HIV-1-infected elite controllers. J Virol 2010; 84:9463-71; PMID:20631139; http://dx.doi.org/ 10.1128/JVI.01009-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Banham AH, Colonna M, Cella M, Micklem KJ, Pulford K, Willis AC, Mason DY. Identification of the CD85 antigen as ILT2, an inhibitory MHC class I receptor of the immunoglobulin superfamily. J Leukocyte Biol 1999; 65:841-5; PMID:10380908 [DOI] [PubMed] [Google Scholar]
- 100. Young NT, Uhrberg M, Phillips JH, Lanier LL, Parham P. Differential expression of leukocyte receptor complex-encoded Ig-like receptors correlates with the transition from effector to memory CTL. J Immunol 2001; 166:3933-41; http://dx.doi.org/ 10.4049/jimmunol.166.6.3933 [DOI] [PubMed] [Google Scholar]
- 101. Saverino D, Fabbi M, Ghiotto F, Merlo A, Bruno S, Zarcone D, Tenca C, Tiso M, Santoro G, Anastasi G, et al. . The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and down-regulates their functions. J Immunol 2000; 165:3742-55; http://dx.doi.org/ 10.4049/jimmunol.165.7.3742 [DOI] [PubMed] [Google Scholar]
- 102. Ince MN, Harnisch B, Xu Z, Lee SK, Lange C, Moretta L, Lederman M, Lieberman J. Increased expression of the natural killer cell inhibitory receptor CD85j/ILT2 on antigen-specific effector CD8 T cells and its impact on CD8 T-cell function. Immunology 2004; 112:531-42; PMID:15270723; http://dx.doi.org/ 10.1046/j.1365-2567.2004.01907.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Saverino D, Merlo A, Bruno S, Pistoia V, Grossi CE, Ciccone E. Dual effect of CD85/leukocyte Ig-like receptor-1/Ig-like transcript 2 and CD152 (CTLA-4) on cytokine production by antigen-stimulated human T cells. J Immunol (Baltimore, Md : 1950) 2002; 168:207-15; PMID:11751964; http://dx.doi.org/ 10.4049/jimmunol.168.1.207 [DOI] [PubMed] [Google Scholar]
- 104. Merlo A, Saverino D, Tenca C, Grossi CE, Bruno S, Ciccone E. CD85/LIR-1/ILT2 and CD152 (Cytotoxic T Lymphocyte Antigen 4) Inhibitory Molecules Down-Regulate the Cytolytic Activity of Human CD4+ T-Cell Clones Specific forMycobacterium tuberculosis. Infect Immun 2001; 69:6022-9; PMID:11553539; http://dx.doi.org/ 10.1128/IAI.69.10.6022-6029.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dietrich J, Cella M, Colonna M. Ig-like transcript 2 (ILT2)/leukocyte Ig-like receptor 1 (LIR1) inhibits TCR signaling and actin cytoskeleton reorganization. J Immunol 2001; 166:2514-21; PMID:11160312; http://dx.doi.org/ 10.4049/jimmunol.166.4.2514 [DOI] [PubMed] [Google Scholar]
- 106. Zhang W, Liang S, Wu J, Horuzsko A. Human Inhibitory Receptor ILT2 Amplifies CD11b+ Gr1+ Myeloid-Derived Suppressor Cells that Promote Long-Term Survival of Allografts. Transplantation 2008; 86:1125; PMID:18946352; http://dx.doi.org/ 10.1097/TP.0b013e318186fccd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Liang S, Zhang W, Horuzsko A. Human ILT2 receptor associates with murine MHC class I molecules in vivo and impairs T cell function. Eur J Immunol 2006; 36:2457-71; PMID:16897816; http://dx.doi.org/ 10.1002/eji.200636031 [DOI] [PubMed] [Google Scholar]
- 108. Lesport E, Baudhuin J, Sousa S, LeMaoult J, Zamborlini A, Rouas-Freiss N, Carosella ED, Favier B. Inhibition of human gamma delta [corrected] T-cell antitumoral activity through HLA-G: implications for immunotherapy of cancer. Cell Mol Life Sci 2011; 68:3385-99; PMID:21337044; http://dx.doi.org/ 10.1007/s00018-011-0632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Saverino D, Ghiotto F, Merlo A, Bruno S, Battini L, Occhino M, Maffei M, Tenca C, Pileri S, Baldi L, et al. . Specific recognition of the viral protein UL18 by CD85j/LIR-1/ILT2 on CD8+ T cells mediates the non-MHC-restricted lysis of human cytomegalovirus-infected cells. J Immunol 2004; 172:5629-37; http://dx.doi.org/ 10.4049/jimmunol.172.9.5629 [DOI] [PubMed] [Google Scholar]
- 110. Wagner CS, Riise GC, Bergstrom T, Karre K, Carbone E, Berg L. Increased expression of leukocyte Ig-like receptor-1 and activating role of UL18 in the response to cytomegalovirus infection. J Immunol 2007; 178:3536-43; http://dx.doi.org/ 10.4049/jimmunol.178.6.3536 [DOI] [PubMed] [Google Scholar]
- 111. Merlo A, Tenca C, Fais F, Battini L, Ciccone E, Grossi CE, Saverino D. Inhibitory receptors CD85j, LAIR-1, and CD152 down-regulate immunoglobulin and cytokine production by human B lymphocytes. Clin Diagn Laborat Immunol 2005; 12:705-12; PMID:15939744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Naji A, Menier C, Morandi F, Agaugue S, Maki G, Ferretti E, Bruel S, Pistoia V, Carosella ED, Rouas-Freiss N. Binding of HLA-G to ITIM-bearing Ig-like transcript 2 receptor suppresses B cell responses. J Immunol 2014; 192:1536-46; http://dx.doi.org/ 10.4049/jimmunol.1300438 [DOI] [PubMed] [Google Scholar]
- 113. Kalmbach Y, Boldt AB, Fendel R, Mordmuller B, Kremsner PG, Kun JF. Increase in annexin V-positive B cells expressing LILRB1/ILT2/CD85j in malaria. Eur Cytokine Net 2006; 17:175-80; PMID:17194637 [PubMed] [Google Scholar]
- 114. Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, Marboe C, Mancini D, Cortesini R, Suciu-Foca N. Alloantigen specific CD8+CD28- FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Inter Immunol 2004; 16:1055-68; PMID:15226269; http://dx.doi.org/ 10.1093/intimm/dxh107 [DOI] [PubMed] [Google Scholar]
- 115. Masuda A, Nakamura A, Maeda T, Sakamoto Y, Takai T. Cis binding between inhibitory receptors and MHC class I can regulate mast cell activation. J Exp Med 2007; 204:907-20; PMID:17420263; http://dx.doi.org/ 10.1084/jem.20060631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, Mancini D, Suciu-Foca N. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol 2003; 11:245-58; PMID:12967778; http://dx.doi.org/ 10.1016/S0966-3274(03)00058-3 [DOI] [PubMed] [Google Scholar]
- 117. Bashirova AA, Martin-Gayo E, Jones DC, Qi Y, Apps R, Gao X, Burke PS, Taylor CJ, Rogich J, Wolinsky S, et al. . LILRB2 interaction with HLA class I correlates with control of HIV-1 infection. PLoS genetics 2014; 10:e1004196; PMID:24603468; http://dx.doi.org/ 10.1371/journal.pgen.1004196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Brown DP, Jones DC, Anderson KJ, Lapaque N, Buerki RA, Trowsdale J, Allen RL. The inhibitory receptor LILRB4 (ILT3) modulates antigen presenting cell phenotype and, along with LILRB2 (ILT4), is upregulated in response to Salmonella infection. BMC Immunol 2009; 10:56; PMID:19860908; http://dx.doi.org/ 10.1186/1471-2172-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sloane DE, Tedla N, Awoniyi M, Macglashan DW, Jr., Borges L, Austen KF, Arm JP. Leukocyte immunoglobulin-like receptors: novel innate receptors for human basophil activation and inhibition. Blood 2004; 104:2832-9; PMID:15242876; http://dx.doi.org/ 10.1182/blood-2004-01-0268 [DOI] [PubMed] [Google Scholar]
- 120. Arm JP, Nwankwo C, Austen KF. Molecular identification of a novel family of human Ig superfamily members that possess immunoreceptor tyrosine-based inhibition motifs and homology to the mouse gp49B1 inhibitory receptor. J Immunol 1997; 159:2342-9 [PubMed] [Google Scholar]
- 121. Vlad G, Chang CC, Colovai AI, Berloco P, Cortesini R, Suciu-Foca N. Immunoglobulin-like transcript 3: A crucial regulator of dendritic cell function. Hum Immunol 2009; 70:340-4; PMID:19275918; http://dx.doi.org/ 10.1016/j.humimm.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 122. Kim-Schulze S, Seki T, Vlad G, Scotto L, Fan J, Colombo PC, Liu J, Cortesini R, Suciu-Foca N. Regulation of ILT3 gene expression by processing of precursor transcripts in human endothelial cells. Am J Transplant 2006; 6:76-82; PMID:16433759; http://dx.doi.org/ 10.1111/j.1600-6143.2005.01162.x [DOI] [PubMed] [Google Scholar]
- 123. Chang CC, Silvia EA, Ho EK, Vlad G, Suciu-Foca N, Vasilescu ER. Polymorphism and linkage disequilibrium of immunoglobulin-like transcript 3 g ene. Hum Immunol 2008; 69:284-90; PMID:18486764; http://dx.doi.org/ 10.1016/j.humimm.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 124. Cheng H, Mohammed F, Nam G, Chen Y, Qi J, Garner LI, Allen RL, Yan J, Willcox BE, Gao GF. Crystal structure of leukocyte Ig-like receptor LILRB4 (ILT3/LIR-5/CD85k): a myeloid inhibitory receptor involved in immune tolerance. J Biol Chem 2011; 286:18013-25; PMID:21454581; http://dx.doi.org/ 10.1074/jbc.M111.221028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Waschbisch A, Sanderson N, Krumbholz M, Vlad G, Theil D, Schwab S, Maurer M, Derfuss T. Interferon beta and vitamin D synergize to induce immunoregulatory receptors on peripheral blood monocytes of multiple sclerosis patients. PloS one 2014; 9:e115488; PMID:25551576; http://dx.doi.org/ 10.1371/journal.pone.0115488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lu HK, Rentero C, Raftery MJ, Borges L, Bryant K, Tedla N. Leukocyte Ig-like receptor B4 (LILRB4) is a potent inhibitor of FcgammaRI-mediated monocyte activation via dephosphorylation of multiple kinases. J Biol Chem 2009; 284:34839-48; PMID:19833736; http://dx.doi.org/ 10.1074/jbc.M109.035683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kim-Schulze S, Scotto L, Vlad G, Piazza F, Lin H, Liu Z, Cortesini R, Suciu-Foca N. Recombinant Ig-like transcript 3-Fc modulates T cell responses via induction of Th anergy and differentiation of CD8+ T suppressor cells. J Immunol 2006; 176:2790-8; PMID:16493035; http://dx.doi.org/ 10.4049/jimmunol.176.5.2790 [DOI] [PubMed] [Google Scholar]
- 128. Vlad G, D'Agati VD, Zhang QY, Liu Z, Ho EK, Mohanakumar T, Hardy MA, Cortesini R, Suciu-Foca N. Immunoglobulin-like transcript 3-Fc suppresses T-cell responses to allogeneic human islet transplants in hu-NOD/SCID mice. Diabetes 2008; 57:1878-86; PMID:18420485; http://dx.doi.org/ 10.2337/db08-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Vlad G, Stokes MB, Liu Z, Chang CC, Sondermeijer H, Vasilescu ER, Colovai AI, Berloco P, D'Agati VD, Ratner L, et al. . Suppression of xenogeneic graft-versus-host disease by treatment with immunoglobulin-like transcript 3-Fc. Hum Immunol 2009; 70:663-9; PMID:19501624; http://dx.doi.org/ 10.1016/j.humimm.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 130. Chang CC, Liu Z, Vlad G, Qin H, Qiao X, Mancini DM, Marboe CC, Cortesini R, Suciu-Foca N. Ig-like transcript 3 regulates expression of proinflammatory cytokines and migration of activated T cells. J Immunol 2009; 182:5208-16; http://dx.doi.org/ 10.4049/jimmunol.0804048 [DOI] [PubMed] [Google Scholar]
- 131. Chang CC, Vlad G, D'Agati VD, Liu Z, Zhang QY, Witkowski P, Torkamani AA, Stokes MB, Ho EK, Cortesini R, et al. . BCL6 is required for differentiation of Ig-like transcript 3-Fc-induced CD8+ T suppressor cells. J Immunol 2010; 185:5714-22; http://dx.doi.org/ 10.4049/jimmunol.1001732 [DOI] [PubMed] [Google Scholar]
- 132. Ulges A, Klein M, Reuter S, Gerlitzki B, Hoffmann M, Grebe N, Staudt V, Stergiou N, Bohn T, Bruhl TJ, et al. . Protein kinase CK2 enables regulatory T cells to suppress excessive TH2 responses in vivo. Nat Immunol 2015; 16:267-75; PMID:25599562; http://dx.doi.org/ 10.1038/ni.3083 [DOI] [PubMed] [Google Scholar]
- 133. de Goeje PL, Bezemer K, Heuvers ME, Dingemans AC, Groen HJ, Smit EF, Hoogsteden HC, Hendriks RW, Aerts JG, Hegmans JP. Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer. Oncoimmunology 2015; 4:e1014242; PMID:26140237; http://dx.doi.org/ 10.1080/2162402X.2015.1014242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Khan MF, Bahr JM, Yellapa A, Bitterman P, Abramowicz JS, Edassery SL, Basu S, Rotmensch J, Barua A. Expression of Leukocyte Inhibitory Immunoglobulin-like Transcript 3 Receptors by Ovarian Tumors in Laying Hen Model of Spontaneous Ovarian Cancer. Trans Oncol 2012; 5:85-91; PMID:22496924; http://dx.doi.org/ 10.1593/tlo.11328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Dube MP, Zetler R, Barhdadi A, Brown AM, Mongrain I, Normand V, Laplante N, Asselin G, Zada YF, Provost S, et al. . CKM and LILRB5 are associated with serum levels of creatine kinase. Circul Cardiov Gen 2014; 7:880-6; PMID:25214527; http://dx.doi.org/ 10.1161/CIRCGENETICS.113.000395 [DOI] [PubMed] [Google Scholar]
- 136. Maeda A, Kurosaki M, Ono M, Takai T, Kurosaki T. Requirement of SH2-containing protein tyrosine phosphatases SHP-1 and SHP-2 for paired immunoglobulin-like receptor B (PIR-B)-mediated inhibitory signal. J Exp Med 1998; 187:1355-60; PMID:9547347; http://dx.doi.org/ 10.1084/jem.187.8.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhao J, Luo Y, Wang X, Zhou H, Li Q, You Y, Zou P. Prevention of murine acute graft-versus-host disease by recipient-derived paired immunoglobulin-like receptor B lentivirus-transfected dendritic cells. Acta Haematol 2010; 124:134-40; PMID:20881379; http://dx.doi.org/ 10.1159/000315553 [DOI] [PubMed] [Google Scholar]
- 138. Ujike A, Takeda K, Nakamura A, Ebihara S, Akiyama K, Takai T. Impaired dendritic cell maturation and increased T(H)2 responses in PIR-B(-/-) mice. Nat Immunol 2002; 3:542-8; PMID:12021780; http://dx.doi.org/ 10.1038/ni801 [DOI] [PubMed] [Google Scholar]
- 139. Munitz A, Cole ET, Beichler A, Groschwitz K, Ahrens R, Steinbrecher K, Willson T, Han X, Denson L, Rothenberg ME, et al. . Paired immunoglobulin-like receptor B (PIR-B) negatively regulates macrophage activation in experimental colitis. Gastroenterol 2010; 139:530-41; PMID:20398663; http://dx.doi.org/ 10.1053/j.gastro.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Karo-Atar D, Moshkovits I, Eickelberg O, Konigshoff M, Munitz A. Paired immunoglobulin-like receptor-B inhibits pulmonary fibrosis by suppressing profibrogenic properties of alveolar macrophages. Am J Resp Cell Mol Biol 2013; 48:456-64; PMID:23258232; http://dx.doi.org/ 10.1165/rcmb.2012-0329OC [DOI] [PubMed] [Google Scholar]
- 141. Imada M, Masuda K, Satoh R, Ito Y, Goto Y, Matsuoka T, Endo S, Nakamura A, Kawamoto H, Takai T. Ectopically expressed PIR-B on T cells constitutively binds to MHC class I and attenuates T helper type 1 responses. Int Immunol 2009; 21:1151-61; PMID:19684158; http://dx.doi.org/ 10.1093/intimm/dxp081 [DOI] [PubMed] [Google Scholar]
- 142. Munitz A, McBride ML, Bernstein JS, Rothenberg ME. A dual activation and inhibition role for the paired immunoglobulin-like receptor B in eosinophils. Blood 2008; 111:5694-703; PMID:18316626; http://dx.doi.org/ 10.1182/blood-2007-12-126748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bochner DN, Sapp RW, Adelson JD, Zhang S, Lee H, Djurisic M, Syken J, Dan Y, Shatz CJ. Blocking PirB up-regulates spines and functional synapses to unlock visual cortical plasticity and facilitate recovery from amblyopia. Sci Trans Med 2014; 6:258ra140; http://dx.doi.org/ 10.1126/scitranslmed.3010157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Djurisic M, Vidal GS, Mann M, Aharon A, Kim T, Ferrao Santos A, Zuo Y, Hubener M, Shatz CJ. PirB regulates a structural substrate for cortical plasticity. Proc Natl Acad Sci U S A 2013; 110:20771-6; PMID:24302763; http://dx.doi.org/ 10.1073/pnas.1321092110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science 2006; 313:1795-800; PMID:16917027; http://dx.doi.org/ 10.1126/science.1128232 [DOI] [PubMed] [Google Scholar]
- 146. Kubagawa H, Chen CC, Ho LH, Shimada TS, Gartland L, Mashburn C, Uehara T, Ravetch JV, Cooper MD. Biochemical nature and cellular distribution of the paired immunoglobulin-like receptors, PIR-A and PIR-B. J Exp Med 1999; 189:309-18; PMID:9892613; http://dx.doi.org/ 10.1084/jem.189.2.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Katz HR, LeBlanc PA, Russell SW. Two classes of mouse mast cells delineated by monoclonal antibodies. Proc Natl Acad Sci U S A 1983; 80:5916-8; PMID:6577460; http://dx.doi.org/ 10.1073/pnas.80.19.5916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. LeBlanc PA, Biron CA. Mononuclear phagocyte maturation: a cytotoxic monoclonal antibody reactive with postmonoblast stages. Cell Immunol 1984; 83:242-54; PMID:6198098; http://dx.doi.org/ 10.1016/0008-8749(84)90303-4 [DOI] [PubMed] [Google Scholar]
- 149. Zhou JS, Friend DS, Feldweg AM, Daheshia M, Li L, Austen KF, Katz HR. Prevention of lipopolysaccharide-induced microangiopathy by gp49B1: evidence for an important role for gp49B1 expression on neutrophils. J Exp Med 2003; 198:1243-51; PMID:14557414; http://dx.doi.org/ 10.1084/jem.20030906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wang LL, Chu DT, Dokun AO, Yokoyama WM. Inducible expression of the gp49B inhibitory receptor on NK cells. J Immunol 2000; 164:5215-20; http://dx.doi.org/ 10.4049/jimmunol.164.10.5215 [DOI] [PubMed] [Google Scholar]
- 151. Gu X, Laouar A, Wan J, Daheshia M, Lieberman J, Yokoyama WM, Katz HR, Manjunath N. The gp49B1 inhibitory receptor regulates the IFN-gamma responses of T cells and NK cells. J Immunol 2003; 170:4095-101; http://dx.doi.org/ 10.4049/jimmunol.170.8.4095 [DOI] [PubMed] [Google Scholar]
- 152. Kuroiwa A, Yamashita Y, Inui M, Yuasa T, Ono M, Nagabukuro A, Matsuda Y, Takai T. Association of tyrosine phosphatases SHP-1 and SHP-2, inositol 5-phosphatase SHIP with gp49B1, and chromosomal assignment of the gene. J Biol Chem 1998; 273:1070-4; PMID:9422771; http://dx.doi.org/ 10.1074/jbc.273.2.1070 [DOI] [PubMed] [Google Scholar]
- 153. Wang LL, Blasioli J, Plas DR, Thomas ML, Yokoyama WM. Specificity of the SH2 domains of SHP-1 in the interaction with the immunoreceptor tyrosine-based inhibitory motif-bearing receptor gp49B. J Immunol 1999; 162:1318-23 [PubMed] [Google Scholar]
- 154. Lu-Kuo JM, Joyal DM, Austen KF, Katz HR. gp49B1 inhibits IgE-initiated mast cell activation through both immunoreceptor tyrosine-based inhibitory motifs, recruitment of src homology 2 domain-containing phosphatase-1, and suppression of early and late calcium mobilization. J Biol Chem 1999; 274:5791-6; PMID:10026201; http://dx.doi.org/ 10.1074/jbc.274.9.5791 [DOI] [PubMed] [Google Scholar]
- 155. Fukao S, Haniuda K, Nojima T, Takai T, Kitamura D. gp49B-mediated negative regulation of antibody production by memory and marginal zone B cells. J Immunol 2014; 193:635-44; http://dx.doi.org/ 10.4049/jimmunol.1302772 [DOI] [PubMed] [Google Scholar]
- 156. Daheshia M, Friend DS, Grusby MJ, Austen KF, Katz HR. Increased severity of local and systemic anaphylactic reactions in gp49B1-deficient mice. J Exp Med 2001; 194:227-34; PMID:11457897; http://dx.doi.org/ 10.1084/jem.194.2.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Feldweg AM, Friend DS, Zhou JS, Kanaoka Y, Daheshia M, Li L, Austen KF, Katz HR. gp49B1 suppresses stem cell factor-induced mast cell activation-secretion and attendant inflammation in vivo. Eur J Immunol 2003; 33:2262-8; PMID:12884301; http://dx.doi.org/ 10.1002/eji.200323978 [DOI] [PubMed] [Google Scholar]
- 158. Zhou JS, Friend DS, Lee DM, Li L, Austen KF, Katz HR. gp49B1 deficiency is associated with increases in cytokine and chemokine production and severity of proliferative synovitis induced by anti-type II collagen mAb. Eur J Immunol 2005; 35:1530-8; PMID:15827966; http://dx.doi.org/ 10.1002/eji.200425895 [DOI] [PubMed] [Google Scholar]
- 159. Breslow RG, Rao JJ, Xing W, Hong DI, Barrett NA, Katz HR. Inhibition of Th2 adaptive immune responses and pulmonary inflammation by leukocyte Ig-like receptor B4 on dendritic cells. J Immunol 2010; 184:1003-13; http://dx.doi.org/ 10.4049/jimmunol.0900877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Fanning LB, Buckley CC, Xing W, Breslow RG, Katz HR. Downregulation of key early events in the mobilization of antigen-bearing dendritic cells by leukocyte immunoglobulin-like Receptor B4 in a mouse model of allergic pulmonary inflammation. PloS One 2013; 8:e57007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Minoo P, Zadeh MM, Rottapel R, Lebrun JJ, Ali S. A novel SHP-1/Grb2-dependent mechanism of negative regulation of cytokine-receptor signaling: contribution of SHP-1 C-terminal tyrosines in cytokine signaling. Blood 2004; 103:1398-407; PMID:14551136; http://dx.doi.org/ 10.1182/blood-2003-07-2617 [DOI] [PubMed] [Google Scholar]
- 162. Dance M, Montagner A, Salles JP, Yart A, Raynal P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal 2008; 20:453-9; PMID:17993263; http://dx.doi.org/ 10.1016/j.cellsig.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 163. Meyaard L. LAIR and collagens in immune regulation. Immunol Lett 2010; 128:26-8; PMID:19836418; http://dx.doi.org/ 10.1016/j.imlet.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 164. Perbellini O, Falisi E, Giaretta I, Boscaro E, Novella E, Facco M, Fortuna S, Finotto S, Amati E, Maniscalco F, et al. . Clinical significance of LAIR1 (CD305) as assessed by flow cytometry in a prospective series of patients with chronic lymphocytic leukemia. Haematologica 2014; 99:881-7; PMID:24415628; http://dx.doi.org/ 10.3324/haematol.2013.096362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Poggi A, Catellani S, Bruzzone A, Caligaris-Cappio F, Gobbi M, Zocchi MR. Lack of the leukocyte-associated Ig-like receptor-1 expression in high-risk chronic lymphocytic leukaemia results in the absence of a negative signal regulating kinase activation and cell division. Leukemia 2008; 22:980-8; PMID:18288129; http://dx.doi.org/ 10.1038/leu.2008.21 [DOI] [PubMed] [Google Scholar]
- 166. Poggi A, Pellegatta F, Leone BE, Moretta L, Zocchi MR. Engagement of the leukocyte-associated Ig-like receptor-1 induces programmed cell death and prevents NF-kappaB nuclear translocation in human myeloid leukemias. Eur J Immunol 2000; 30:2751-8; PMID:11069054; http://dx.doi.org/ 10.1002/1521-4141(200010)30:10%3c2751::AID-IMMU2751%3e3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- 167. Zocchi MR, Pellegatta F, Pierri I, Gobbi M, Poggi A. Leukocyte-associated Ig-like receptor-1 prevents granulocyte-monocyte colony stimulating factor-dependent proliferation and Akt1/PKB alpha activation in primary acute myeloid leukemia cells. Eur J Immunol 2001; 31:3667-75; PMID:11745387; http://dx.doi.org/ 10.1002/1521-4141(200112)31:12%3c3667::AID-IMMU3667%3e3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- 168. Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. . Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 2006; 442:818-22; PMID:16862118; http://dx.doi.org/ 10.1038/nature04980 [DOI] [PubMed] [Google Scholar]
- 169. Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell 2006; 10:257-68; PMID:17045204; http://dx.doi.org/ 10.1016/j.ccr.2006.08.020 [DOI] [PubMed] [Google Scholar]
- 170. Yan M, Kanbe E, Peterson LF, Boyapati A, Miao Y, Wang Y, Chen IM, Chen Z, Rowley JD, Willman CL, et al. . A previously unidentified alternatively spliced isoform of t(8;21) transcript promotes leukemogenesis. Nat Med 2006; 12:945-9; PMID:16892037; http://dx.doi.org/ 10.1038/nm1443 [DOI] [PubMed] [Google Scholar]
- 171. Sugihara E, Shimizu T, Kojima K, Onishi N, Kai K, Ishizawa J, Nagata K, Hashimoto N, Honda H, Kanno M, et al. . Ink4a and Arf are crucial factors in the determination of the cell of origin and the therapeutic sensitivity of Myc-induced mouse lymphoid tumor. Oncogene 2011; PMID:21986948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, Avril M, Brazier AJ, Freeth J, Jespersen JS, Nielsen MA, et al. . Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 2013; 498:502-5; PMID:23739325; http://dx.doi.org/ 10.1038/nature12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Frei AP, Jeon OY, Kilcher S, Moest H, Henning LM, Jost C, Pluckthun A, Mercer J, Aebersold R, Carreira EM, et al. . Direct identification of ligand-receptor interactions on living cells and tissues. Nat Biotech 2012; 30:997-1001; PMID:22983091; http://dx.doi.org/ 10.1038/nbt.2354 [DOI] [PubMed] [Google Scholar]
- 174. Xu M, Zhao R, Zhao ZJ. Identification and characterization of leukocyte-associated Ig-like receptor-1 as a major anchor protein of tyrosine phosphatase SHP-1 in hematopoietic cells. J Biol Chem 2000; 275:17440-6; PMID:10764762; http://dx.doi.org/ 10.1074/jbc.M001313200 [DOI] [PubMed] [Google Scholar]
- 175. Eklund EA, Goldenberg I, Lu Y, Andrejic J, Kakar R. SHP1 protein-tyrosine phosphatase regulates HoxA10 DNA binding and transcriptional repression activity in undifferentiated myeloid cells. J Biol Chem 2002; 277:36878-88; PMID:12145285; http://dx.doi.org/ 10.1074/jbc.M203917200 [DOI] [PubMed] [Google Scholar]
- 176. Verbrugge A, de Ruiter T, Geest C, Coffer PJ, Meyaard L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J Leukoc Biol 2006; 79:828-36; PMID:16461736; http://dx.doi.org/ 10.1189/jlb.0705370 [DOI] [PubMed] [Google Scholar]
- 177. Nihal M. Heiba SAE. SHP-1 expression in chronic myeloid leukemia ( clinical significance and impact on response to imatinib treatment). Egypt J Haematol 2013; 38:84-9 [Google Scholar]
- 178. Tibaldi E, Brunati AM, Zonta F, Frezzato F, Gattazzo C, Zambello R, Gringeri E, Semenzato G, Pagano MA, Trentin L. Lyn-mediated SHP-1 recruitment to CD5 contributes to resistance to apoptosis of B-cell chronic lymphocytic leukemia cells. Leukemia 2011; 25:1768-81; PMID:21701493; http://dx.doi.org/ 10.1038/leu.2011.152 [DOI] [PubMed] [Google Scholar]
- 179. Tapley P, Shevde NK, Schweitzer PA, Gallina M, Christianson SW, Lin IL, Stein RB, Shultz LD, Rosen J, Lamb P. Increased G-CSF responsiveness of bone marrow cells from hematopoietic cell phosphatase deficient viable motheaten mice. Exp Hematol 1997; 25:122-31; PMID:9015212 [PubMed] [Google Scholar]
- 180. Jiao H, Yang W, Berrada K, Tabrizi M, Shultz L, Yi T. Macrophages from motheaten and viable motheaten mutant mice show increased proliferative responses to GM-CSF: detection of potential HCP substrates in GM-CSF signal transduction. Exp Hematol 1997; 25:592-600; PMID:9216734 [PubMed] [Google Scholar]
- 181. Wu C, Guan Q, Wang Y, Zhao ZJ, Zhou GW. SHP-1 suppresses cancer cell growth by promoting degradation of JAK kinases. J Cell Biochem 2003; 90:1026-37; PMID:14624462; http://dx.doi.org/ 10.1002/jcb.10727 [DOI] [PubMed] [Google Scholar]
- 182. Lorenz U, Bergemann AD, Steinberg HN, Flanagan JG, Li X, Galli SJ, Neel BG. Genetic analysis reveals cell type-specific regulation of receptor tyrosine kinase c-Kit by the protein tyrosine phosphatase SHP1. J Exp Med 1996; 184:1111-26; PMID:9064328; http://dx.doi.org/ 10.1084/jem.184.3.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Paulson RF, Vesely S, Siminovitch KA, Bernstein A. Signalling by the W/Kit receptor tyrosine kinase is negatively regulated in vivo by the protein tyrosine phosphatase Shp1. Nat Genet 1996; 13:309-15; PMID:8673130; http://dx.doi.org/ 10.1038/ng0796-309 [DOI] [PubMed] [Google Scholar]
- 184. Koyama M, Oka T, Ouchida M, Nakatani Y, Nishiuchi R, Yoshino T, Hayashi K, Akagi T, Seino Y. Activated proliferation of B-cell lymphomas/leukemias with the SHP1 gene silencing by aberrant CpG methylation. Lab Invest 2003; 83:1849-58; PMID:14691303; http://dx.doi.org/ 10.1097/01.LAB.0000106503.65258.2B [DOI] [PubMed] [Google Scholar]
- 185. Uhm KO, Lee ES, Lee YM, Park JS, Kim SJ, Kim BS, Kim HS, Park SH. Differential methylation pattern of ID4, SFRP1, and SHP1 between acute myeloid leukemia and chronic myeloid leukemia. J Korean Med Sci 2009; 24:493-7; PMID:19543515; http://dx.doi.org/ 10.3346/jkms.2009.24.3.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gauffin F, Diffner E, Gustafsson B, Nordgren A, Wingren AG, Sander B, Persson JL, Gustafsson B. Expression of PTEN and SHP1, investigated from tissue microarrays in pediatric acute lymphoblastic, leukemia. Pediat Hematol Oncol 2009; 26:48-56; PMID:19206008; http://dx.doi.org/ 10.1080/08880010802625530 [DOI] [PubMed] [Google Scholar]
- 187. Bard-Chapeau EA, Li S, Ding J, Zhang SS, Zhu HH, Princen F, Fang DD, Han T, Bailly-Maitre B, Poli V, et al. . Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell 2011; 19:629-39; PMID:21575863; http://dx.doi.org/ 10.1016/j.ccr.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Leal M, Sapra P, Hurvitz SA, Senter P, Wahl A, Schutten M, Shah DK, Haddish-Berhane N, Kabbarah O. Antibody-drug conjugates: an emerging modality for the treatment of cancer. Annal New York Acad Sci 2014; 1321:41-54; PMID:25123209; http://dx.doi.org/ 10.1111/nyas.12499 [DOI] [PubMed] [Google Scholar]
- 189. Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev 2015; 263:68-89; PMID:25510272; http://dx.doi.org/ 10.1111/imr.12243 [DOI] [PubMed] [Google Scholar]


