Abstract
Purpose
Immune responses to antigens originating in the CNS are generally attenuated, since collateral damage can have devastating consequences. The significance of this finding for the efficacy of tumor-targeted immunotherapies is largely unknown.
Experimental Design
The B16 murine melanoma model was used to compare cytotoxic responses against established tumors in the CNS and in the periphery. Cytokine analysis of tissues from brain tumor-bearing mice detected elevated TGF-β secretion from microglia and in the serum and TGF-β signaling blockade reversed tolerance of tumor antigen-directed CD8 T cells. Additionally, a treatment regimen using focal radiation therapy and recombinant Listeria monocytogenes was evaluated for immunologic activity and efficacy in this model.
Results
CNS melanomas were more tolerogenic than equivalently progressed tumors outside the CNS as antigen-specific CD8 T cells were deleted and exhibited impaired cytotoxicity. Tumor-bearing mice had elevated serum levels of TGF-β; however, blocking TGF-β signaling with a small molecule inhibitor or a monoclonal antibody did not improve survival. Conversely, tumor antigen-specific vaccination in combination with focal radiation therapy reversed tolerance and improved survival. This treatment regimen was associated with increased polyfunctionality of CD8 T cells, elevated T effector to T regulatory cell ratios and decreased TGF-β secretion from microglia.
Conclusions
These data suggest that CNS tumors may impair systemic antitumor immunity and consequently accelerate cancer progression locally as well as outside the CNS while antitumor immunity may be restored by combining vaccination with radiation therapy. These findings are hypothesis-generating and warrant further study in more contemporary melanoma models as well as human trials.
Introduction
Brain metastases afflict 20% to 40% of patients with advanced cancer and represent a major source of morbidity and mortality (1). The basic mechanisms constraining immune responses against CNS tumor antigens, however, remain poorly defined (2). Patients receiving chemotherapy and radiation for high-grade gliomas exhibit impaired T cell homeostasis (3), and lymphopenia has been identified as a negative prognostic indicator in these patients (4). While it is unclear if these observations represent sequelae of pathology and/or treatment effect, location in the immunologically distinct CNS may play an important role in clinical outcome.
Although the presence of the blood-brain barrier, lack of conventional lymphatics, paucity of antigen presenting cells, and low basal expression of Major Histocompatibility Complex (MHC) molecules qualify the CNS as immunologically unique, peripheral leukocytes access the brain and orchestrate robust immune responses under inflammatory conditions (5). Unlike peripheral lymphocytes, however, these cells interact with a variety of tissue-resident cells, including astrocytes, neurons, and microglia, which modulate lymphocyte function in a highly context-dependent manner (6). Location within the CNS may be important for immunosuppression in animal models as extracranially implanted gliomas are characterized by lower levels of Tumor Growth Factor (TGF)-β transcription, increased infiltration by CD4 and CD8 T cells, decreased T regulatory cell (Treg) accumulation, and slower growth as compared with intracranial gliomas (7).
To investigate the effects of tumor location on immune function we used B16, a poorly immunogenic murine melanoma cell line that expresses no MHC II and low levels of MHC I (8) and/or a more immunogenic variant which expresses a class I (H-2Kb) restricted epitope of ovalbumin. We found that brain tumors are more tolerogenic than equivalently advanced tumors located outside the CNS and that mice harboring brain tumors have higher local and circulating levels of TGF-β, although blocking TGF-β failed to mediate tumor regression. Having established intracranial B16 as a challenging model, we tested the induction / expansion of anti-tumor T cells by vaccination. Vaccination alone had a modest effect on survival, but combination immunotherapy using a recombinant Listeria monocytogenes (LM) based vector and focal radiation therapy (RT) significantly prolonged survival.
Although previous studies demonstrated glioma regression after treatment with RT and PD-1 blocking antibodies (9), we found that LM and RT were superior to anti-PD-1 and RT against established intracranial melanoma. Mechanistically, vaccination combined with focal RT significantly decreased secretion of TGF-β1 from microglia and increased intratumoral polyfunctional CD8 T cell density. Based on these data, we propose a mechanism by which microglia in the brain tumor microenvironment mediate systemic immune tolerance and describe how appropriately primed T cells can reverse this effect. These findings may have implications for systemic disease control as well as designing and implementing effective immunotherapies for patients with metastatic brain tumors.
Materials and Methods
Mice, cell lines, antibodies, and vaccines
Female C57BL/6 (Jackson Laboratory) or LY5.2 (NCI) mice (6–8 weeks) were housed in pathogen-free conditions under approved animal protocols (Institutional Animal Care and Use Committee of Johns Hopkins University). OT-1/CD45.2/Rag−/− and Pmel/CD45.2 mice were used as donors for adoptive transfer experiments. Recombinant LM-OVA was constructed in the Lm ΔactA ΔinlB ΔuvrAB background by integrating pPL2-OVA as described (10,11) (Aduro Biotech, Berkeley, CA). LM-OVA was grown in BHI to mid-log, washed, and stored in PBS/8% glycerol at −80°C in single-use aliquots. For vaccination, LM-OVA was thawed, diluted in PBS to 1×107 cfu per mouse (0.1 LD50), and administered by intraperitoneal injection. For Vac-OVA or Vac-GP100 mice received 1×106 pfu (0.1 LD50). The G4 hybridoma was used to produce hamster antimurine PD-1 monoclonal antibodies as described at 10 mg/kg (12).
Tumor models
B16-OVA cells were maintained in culture under continuous selection. For intracranial implantation, cells were resuspended at either 1,000 cells/µL for survival experiments or 5,000 cells/µL for immunology experiments. For flank tumor and lung tumor implantation, cells were resuspended at 50 cells/µL and 500 cells/µL, respectively. Flank tumors were established by injecting 200 µL subcutaneously in the right flank. Lung tumors were established by injecting 200 µL by tail vein injection. Intracranial tumors were established as previously described (9). No cell line authentication was done.
Flow cytometry
Flow cytometry was carried out on a FACSCalibur or LSR II (BD Biosciences). For adoptive transfer experiments, the following antibodies were used: CD45.2 PE, PB (Biolegend), CD8a PerCP, Pac Orange (Invitrogen), CD4 PerCP (BD), IFN-γ APC, PE-Cy7 (Biolegend), Granzyme B PE (eBio), TNF-α PE (BD), IL-2 APC (BioLegend), FoxP3 AF700 (BioLegend), CD11b AF700, PE (eBio), and IL-17 PerCP/Cy5.5 (BioLegend). Data were analyzed using FlowJo software (Tree Star).
Adoptive transfer experiments
Spleens and lymph nodes from OT-1, Rag−/− or Pmel mice were collected and homogenized. For wild-type Pmel mice, CD8 T cells were isolated by positive selection (Miltenyi Biotech) and labeled with CFSE (Invitrogen). Cells were resuspended in PBS at 1.25×107 cells/mL then transferred by retro-orbital injection (2.5×106 cells) 12 days after implantation of 1×104 F10 B16-OVA cells in the brain or flank of mice expressing the congenic marker CD45.1 (LY5.2). Five days after adoptive transfer, brains, draining lymph nodes, and spleens were collected and homogenized. Brain and flank tumors were excised and TIL were isolated using Percoll (Sigma) density gradient centrifugation per manufacturer instructions. Cells were isolated and stimulated with 2 µM H-2Kb restricted class I epitope SIINFEKL (OVA257–264) or the human epitope KVPRNQDWL (gp10025–33) in the presence of GolgiStop (BDBiosciences), then analyzed by FACS.
In vivo CTL assays
Assays were performed as previously described (13). Splenocytes from wild-type C57BL/6 mice were isolated and divided into two groups. One group was labeled CFSElo (0.5 µM), while a second group was labeled CFSEhi (5 µM) and loaded with SIINFEKL peptide at a concentration of 2 µM. Cells were combined and transferred by retro-orbital injection. For tumor-bearing mice, 1×104 F10 B16-OVA cells were implanted in the brain or flank 17 days prior to target transfer. Vac-OVA or LM-OVA were administered as described above. Spleens were harvested from recipient mice 18 hours after target transfer and splenocytes analyzed by FACS.
Radiation therapy
16 Gy was delivered using the small animal radiation research platform (SARRP) (Xstrahl, Suwanee, GA) as previously described (9).
Tumor-infiltrating lymphocyte immunophenotyping, pathology, and immunohistochemistry
2,000 F10 B16-OVA cells were implanted in the left hemisphere of C57BL/6 mice. RT was delivered on day 7, LM-OVA was administered on day 10, and mice were sacrificed on day18. Tumors were excised from surrounding brain tissue and homogenized. TIL were isolated using density gradient centrifugation (Percoll). Cells were stimulated for 4 hours with PMA/Ionomycin, washed, stained for CD8, CD4, IFN-γ, TNF-α, IL-2, Granzyme B, FoxP3, and IL-17, and analyzed by flow cytometry. For immunohistochemistry, mice underwent transcardial perfusion with 10 mL PBS followed by 4% paraformaldehyde/PBS. Brains were removed and cryoprotected in 30% sucrose/PBS for 48 hours at 4°C, snap frozen, and stored at −80°C prior to sectioning. H&E staining was performed by the histology core facility. For immunostaining, slides were washed twice for 5 minutes in PBS and blocked in 5% NGS/PBS for 1 hour. Tissues were incubated with anti-CD3ε antibody (Dako, Carpinteria, CA) (1:10 diluted in 3% NGS/PBS) overnight at 4°C and washed in PBS before incubating with goat anti-rabbit secondary (1:1000) for one hour at room temperature.
APC co-culture experiments
1×104 F10 B16-OVA cells were implanted and RT and LM-OVA were administered as described (day 10). On day 17, mice were sacrificed and serum, brains, spleens and tumor draining lymph nodes were collected. Red blood cells were lysed in spleens and CD11c+ cells were isolated by positive selection (Miltenyi). Monocytes were isolated from brains by density gradient centrifugation, stained for CD11b AF700 (eBio) and CD45 PE (BioLegend), and sorted using a FACSAria (BD). CD11b+/CD45-mid cells, as well as CD11c+ splenocytes and unsorted DLN cells were plated in a 96-well plate (1×104 cells/well). OT-1 CD8 cells were CFSE-labeled (0.5 µM) and plated with APCs at a ratio of 1:5 for CD11b+/CD45 microglia, a ratio of 1:5 for CD11c+ splenocytes, and a 1:1 ratio for DLNs. SIINFEKL peptide was added to the wells (2 µM) and plates were maintained in an incubator for 48 hours. GolgiStop (BDBiosciences) was added for the last 6 hours and supernatants were collected and stored at −80°C. Cells were collected and stained for CD8, CD45.2, and IFN-γ and analyzed by FACS. Supernatants and serum samples were analyzed for concentrations of IFN-γ, IL-2, IL-12, GM-CSF, IL-10, and TGF-β1 by multiplex (Luminex, Austin, TX).
Survival experiments and tumor volume analysis
2,000 F10 B16-OVA cells were implanted in the left hemisphere of C57BL/6 mice. RT was delivered (16 Gy) 7 days after tumor implantation and LM-OVA was administered on day 10. Mice received a LM-OVA boost on day 31. Mice were sacrificed according to protocol upon development of motor deficits or sustained hunched posture. In studies involving post-mortem tumor volume assessment, brains were removed at the time of death and stored in 4% paraformaldehyde/PBS. Tissues were transferred to PFPE (Fomblin®, Sigma-Aldrich) and MR images were acquired by the Small Animal Imaging Resource Program at Johns Hopkins. Tumor borders were delineated slice-by-slice using ImageJ software and tumor volumes were calculated.
Statistics
Data were analyzed by 2-tailed Student’s T-test or ANOVA using GraphPad Prism software.
Results
CD8 T cells recognizing an endogenous tumor-associated melanoma antigen are deleted, while CD8 T cells recognizing a tumor-restricted neo-antigen persist
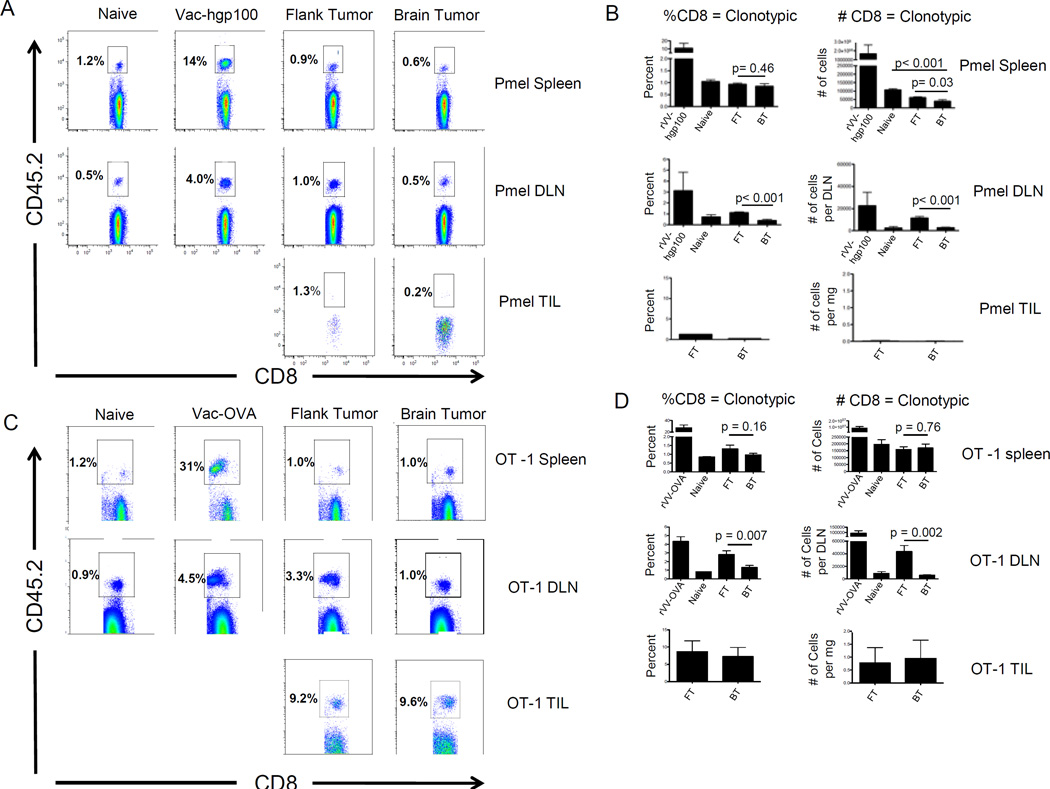
Antigen-specific tolerance is an early event in tumor progression (14), and prior studies have shown that naïve CD8 T cells specific for the endogenous self/tumor antigen gp100 (Pmel) are rapidly tolerized upon adoptive transfer into C57BL/6 mice with established B16 flank tumors (15). To test whether tumor location affects antigen-specific tolerance, we adoptively transferred Pmel CD8 T cells into mice bearing equivalently sized (Supplemental Figure 1) B16 flank or brain tumors. Five days post-transfer very few tumor-infiltrating Pmel CD8 T cells persisted in brain or flank tumors (Figure 1A). However, there was a significantly lower percentage (p < 0.001) and number (p < 0.001) of adoptively transferred cells in the cervical lymph nodes (brain tumor DLN) compared with flank tumor DLN (Figure 1B). This effect was systemic, as there were also fewer Pmel CD8 T cells in the spleens of brain tumor-bearing mice as compared with cancer-free (p < 0.001) or flank tumor-bearing mice (p = 0.03).
Figure 1. Adoptively transferred tumor antigen-specific CD8 T cells are tolerized by CNS melanoma.
(A) Representative FACS plots of Pmel (CD45.2+) CD8 T cells isolated from spleens, brain tumor DLN, and TIL. (B) Summary graphs showing percentages and numbers of Pmel CD8 T cells. C) Representative FACS plots of the percentage of OT-1 (CD45.2+) CD8+ T cells isolated from spleens, brain tumor DLN, and TIL, animals bearing B16-OVA tumors. (D) Summary graphs showing percentages and numbers of CD8 T cells represented by the adoptively transferred OT-1 population. N = 5 mice / group, repeated × 3.
To extend these results to a tumor-restricted antigen, we implanted B16-OVA brain and flank tumors. Here, ovalbumin models a mutated neo-antigen, to which pre-existing tolerance is not expected. This model has the advantage that tolerance is unlikely to be primarily deletional, as suggested by previous studies (16). We found a significant decrease in OT-1 percentage (p = 0.007) and number (p = 0.002) in brain tumor DLNs compared with flank tumor DLNs (Figure 1 C, D). Unlike the Pmel model, however, differences in the spleens did not reach statistical significance (Figure 1D).
Immunological tolerance to CNS melanoma antigens is not the result of ignorance
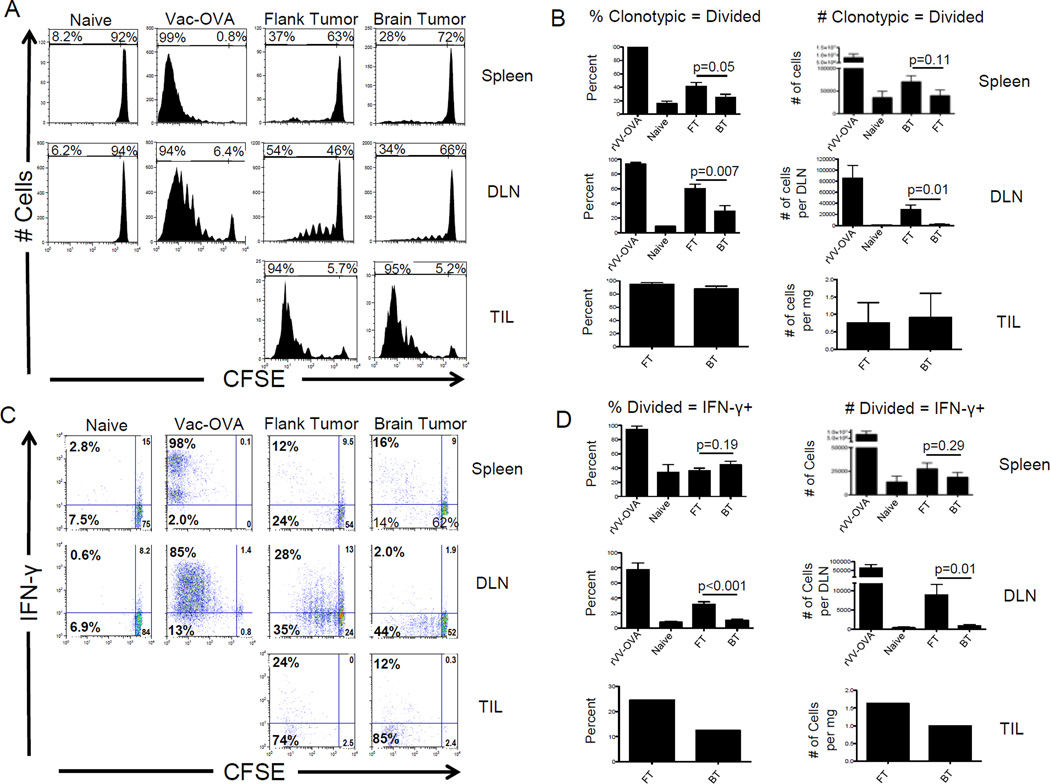
Antigens exit the CNS via cerebrospinal fluid (CSF) drainage along olfactory nerves passing through the cribriform plate and along perivascular spaces, including channels associated with dural venous sinuses(17), en route to the cervical lymph nodes (2). Given that antigens in the CNS parenchyma are poorly recognized by naïve lymphocytes (18), we hypothesized that differences in tumor antigen recognition might underlie the observed differences in brain and flank tumor-bearing animals. To test this hypothesis, we adoptively transferred CFSE-labeled OT-1 CD8 T cells to mice bearing B16-OVA brain or flank tumors. These studies could not be performed with Pmel cells, since so few cells escape deletional tolerance (Figure 1B). As shown in Figure 2A, the majority of tumor-infiltrating lymphocytes (TIL) were divided in both brain and flank tumors, consistent with antigen recognition. OT-1 CD8 T cells in the brain tumor DLN also showed clear evidence of division, with a significant fraction of cells undergoing greater than 4 divisions (Figures 2A, B). Although division was attenuated in brain tumor DLNs as compared with flank tumor DLNs, these results provide clear evidence of CNS tumor antigen recognition, though we cannot determine whether initial recognition occurred in the DLN or upon entry into the tumor itself. To determine if brain tumor antigen recognition impaired acquisition of CD8 T cell effector function, we analyzed interferon (IFN)-γ production by antigen-specific CD 8 T cells (Figure 2C) and found a significantly lower percentage (p < 0.001) and number (p = 0.01) of divided, IFN-γ+ CD8 T cells in the DLNs of brain tumor-bearing mice compared with flank tumor-bearing mice (Figure 2D). Extending these data to a third tumor site, we found that established lung tumors also stimulated IFN-γ secretion more readily than brain tumors (p < 0.001) and similar to flank tumors (p = 0.56) (Supplemental Figure 2).
Figure 2. Tumor-specific CD8 T cells undergo fewer divisions and produce less IFN-γ in response to CNS melanoma as compared to equivalent flank tumors.
(A) Representative histograms of CFSE in cancer-specific T cells (B) Summary graphs showing percentages and numbers of specifc T cells undergoing ≥ 1 division. (C) Representative FACS plots of division vs. IFN-γ. (D) Summary graphs showing percentages and numbers of divided cells producing IFN-γ. N = 5 mice/group, repeated × 3 with similar results.
Antigen-specific cytotoxicity is systemically impaired in animals with CNS melanoma
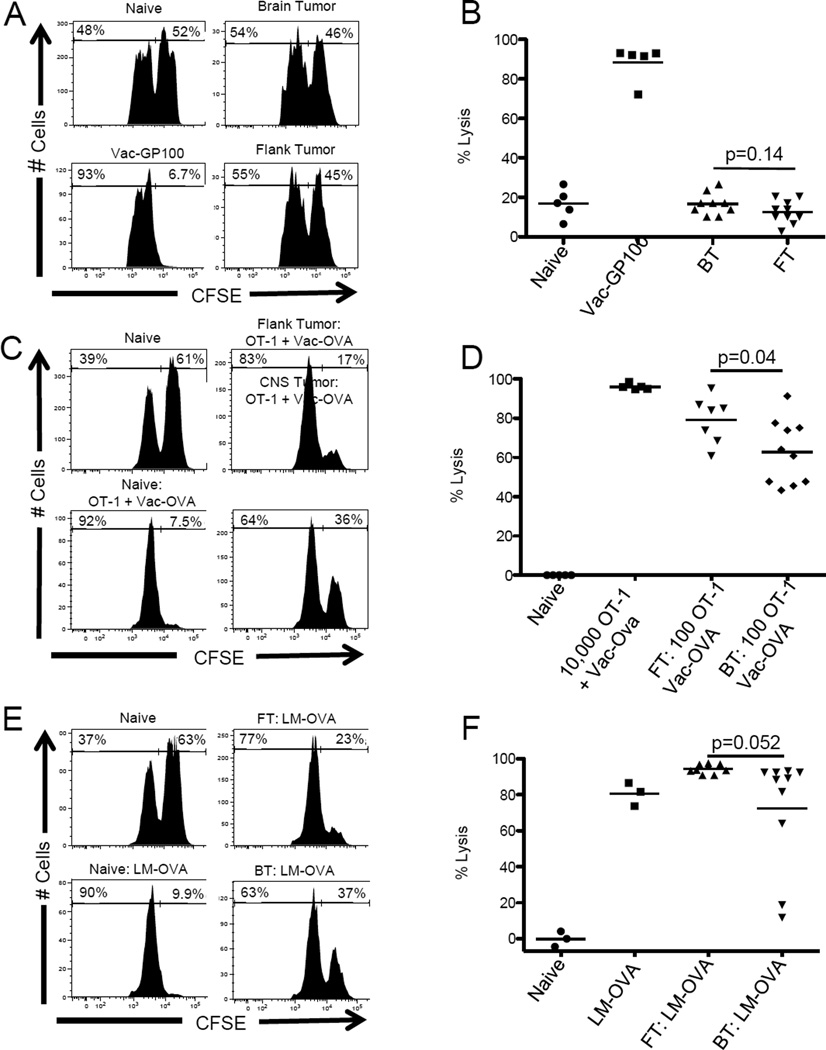
We next tested the effects of CD8 T cell priming in the context of a brain tumor or flank tumor on in vivo effector function by performing a series of cytotoxic T lymphocyte (CTL) assays in mice bearing either B16-OVA brain or flank tumors. In the absence of tumor antigen-specific vaccination or adoptive T cell transfer, recognition of tumor antigen was insufficient to confer effector function (p = 0.14) (Figure 3A and 3B). To test whether adoptively transferred, vaccine-primed antigen-specific T cells respond differentially in the context of a brain or flank tumor, we adoptively transferred a physiologically relevant number (approximately 100) OT-1 CD8 T cells (19) to mice bearing established B16-OVA tumors, then vaccinated with a recombinant OVA-expressing vaccinia-based vaccine (Vac-OVA) (15). In cancer-free mice targets persisted in untreated animals, while essentially all targets were lysed after adoptive transfer of antigen-specific CD8 T cells plus vaccination (Figure 3C and 3D). Lysis was attenuated in tumor-bearing mice, and CNS melanoma attenuated CD8 T cell function to a greater degree than flank tumors (Figure 3D). We next examined whether this relative resistance to vaccination applied to therapy with a novel listeria-based vaccine (20), and found that even in the absence of adoptive transfer, this vaccine strain mediated lysis with a non-significant trend towards decreased killing in mice bearing CNS melanoma as compared to mice bearing flank tumors (p = 0.052) (Figure 3E, F).
Figure 3. CNS melanoma impairs a systemic tumor-antigen directed lytic response.
(A) Representative histograms showing numbers of peptide-pulsed (CSFE-high) and control (CFSE-low) cells recovered from unvaccinated mice bearing B16-OVA brain or flank tumors. (B) Summary graphs showing percent target lysis in unvaccinated mice with brain or flank tumors. Each data point represents one animal. (C) Representative histograms showing OVA-pulsed and control peaks in mice with B16-OVA brain or flank tumors after adoptive transfer of 100 OT-1 cells and vaccination with Vac-OVA. (D) Summary graphs showing percent target lysis in mice receiving adoptive transfer of 100 OT-1 cells and vaccination with Vac-OVA. (E) Representative histograms showing OVA-pulsed and control peaks in mice with B16-OVA brain and flank tumors after vaccination with LM-OVA. (F) Summary graphs showing percent target lysis in mice with B16-OVA brain and flank tumors after vaccination with LM-OVA. Experiments repeated × 2 with similar results. N = 3–10 animals per group.
TGF-β is elevated in the serum of mice with CNS melanoma
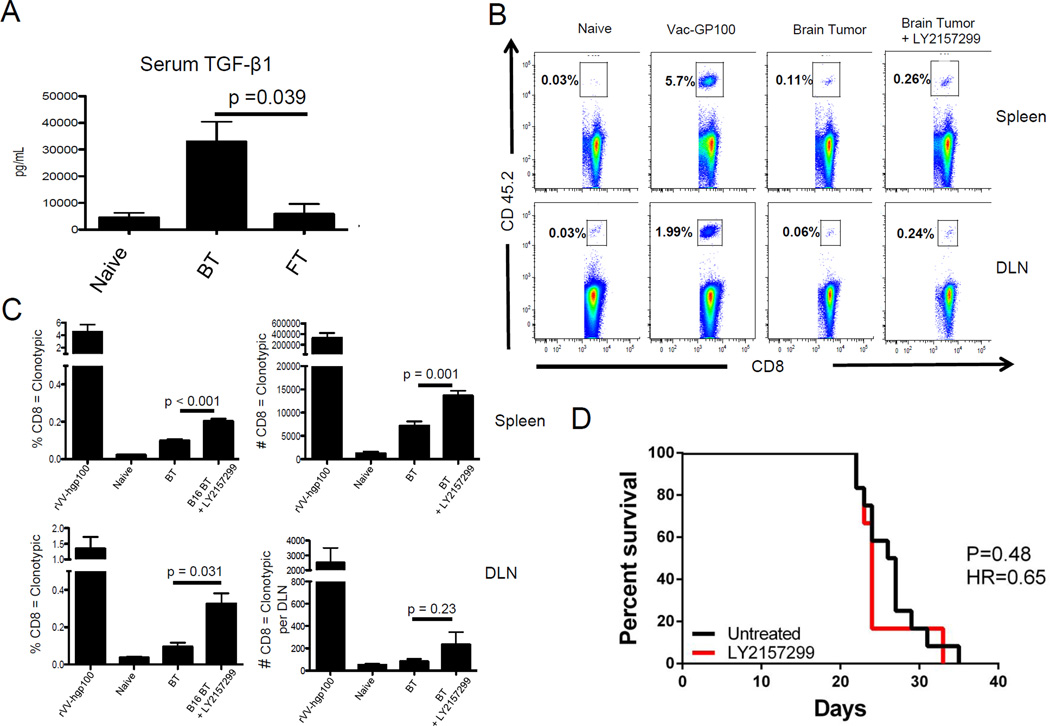
TGF-β family cytokines are pluripotent molecules involved in regulating tissue homeostasis (21) and TGF-β1 has been implicated as a critical driver of melanoma progression (22). To determine whether CNS melanoma is associated with systemically elevated levels of TGF-β, we measured serum levels of TGF-β1. As shown in Figure 4A, mice with B16-OVA brain tumors had significantly higher levels of serum TGF-β1 compared to mice with B16-OVA flank tumors (p = 0.039). Based on these data, we hypothesized that blocking TGF-β signaling might reverse brain tumor-mediated tolerance. To test this hypothesis, we returned to the Pmel adoptive transfer model and treated brain tumor-bearing mice with a small molecule TGF-β signaling inhibitor (LY2157299) (23). We found that inhibiting TGF-β signaling significantly increased the percentage and number of Pmel CD8 T cells in the spleens of brain tumor-bearing mice (p < 0.001, p = 0.001, respectively) and significantly increased the percentage of Pmel CD8 T cells in the DLN (p = 0.031) (Figure 4 B, C). Somewhat surprisingly, TGF-β blockade did not increase the number of TIL, nor did treatment affect overall survival (Figure 4D). Nearly complete blockade of TGF-β signaling was confirmed via western blotting (data not shown). Treatment with the pan TGF-β neutralizing antibody 1D11 also did not improve overall survival in these animals (data not shown). Taken together, these results show that CNS melanoma is associated with elevated TGF-β levels, but that blockade alone is not sufficient to alter pre-clinical outcome.
Figure 4. Elevated TGF-β1 is associated with systemic tolerance in animals with CNS melanoma.
A) Concentration of TGF-β1 in serum of mice with B16 brain or flank tumors. (B) Representative FACS plots demonstrating the percentage of CD8 T cells represented by the adoptively transferred Pmel population in the spleen and cervical lymph nodes after treatment with LY2157299. (C) Summary graphs showing the percentage and number of adoptively transferred Pmel cells recovered from animals with CNS melanoma after treatment with LY2157299. D) Survival of animals with CNS melanoma treated with the TGF-β signaling inhibitor LY2157299. A–C = 5 animals / group, repeated × 2. D = 10 animals / group, repeated × 1.
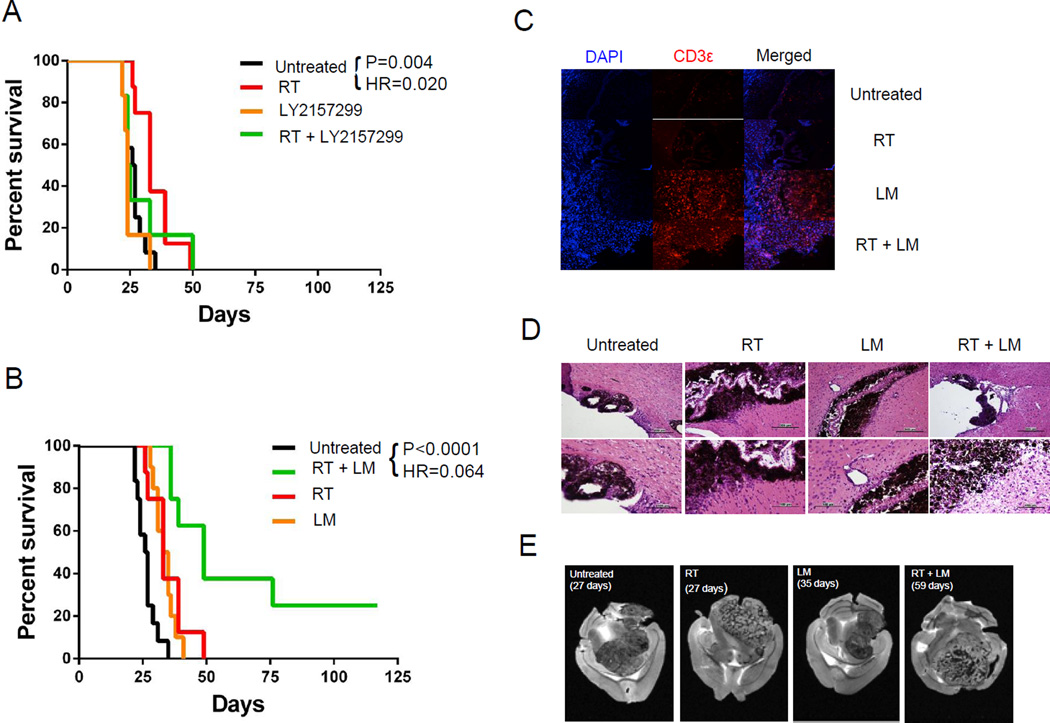
Combination treatment regimens in animals with CNS melanoma
RT is a standard treatment modality for CNS lesions, and in previous studies using an orthotopic glioma model we found that combining RT with other immunotherapies had a synergistic effect on OS (9). We thus tested whether the combination of TGF-β signaling inhibition + focal RT delivered using the small animal radiation research platform (SARRP) could mediate treatment effects in this CNS melanoma model. Unfortunately, this was not the case; although RT enhanced survival significantly (HR = 0.02, p = 0.004), TGF-β inhibition did not add to this effect (Figure 5). Since LM-based vaccination alone appeared to partially reverse brain tumor-mediated tolerance (Figure 3), we next tested whether LM-based vaccination could prolong survival. Indeed, the LM-based vaccine mediated a relatively modest increase in OS, but the combination of vaccination + RT significantly increased survival, with a number of animals showing long-term non-progression (Figure 5B). The addition of TGF-beta blockade to this combination regimen (using either 1D11 or LY2157299) did not extend survival compared with LM-OVA + RT (Supplemental Figure 3), nor did the addition of PD-1 blockade (Supplemental Figure 4). To explore the mechanism of action of this efficacious combination regimen, we performed immunostaining with anti-CD3ε. The results confirmed the presence of an intratumoral T-cell infiltrate in mice receiving LM alone or LM in combination with radiation therapy (Figure 5C). Corresponding H&E staining demonstrated a lack of inflammation in untreated tumors and minimal inflammation in tumors treated with RT alone (Figure 5D). LM vaccination, by contrast, was associated with increased perivascular inflammation, and combination therapy was associated with a marked peritumoral lymphocytic infiltrate. To test whether combination immunotherapy altered patterns of brain tumor progression, brains from representative animals (3 per group) were imaged ex vivo with magnetic resonance imaging (MRI). Tumor borders were delineated in each slice and volumes were calculated using ImageJ software by a blinded observer (Figure 5E). To test for differences in morphology, we identified the dorsal-ventral midplane of each tumor and calculated the ratio of tumor volumes superior and inferior to this plane. Although no differences were observed with RT, LM vaccination appeared to constrain tumor growth, with untreated tumors and tumors treated with RT alone exhibiting more variable morphology at the time of death (Supplemental Figure 5A). Additionally, we found that there was no difference in tumor volume between groups at the time of death (Supplemental Figure 5B).
Figure 5. Combining focal RT with vaccination improves survival in mice with established CNS melanoma.
(A) Combination treatment with focal RT + TGF-β1 signaling inhibition (B) Combination treatment with focal RT + LM-based vaccine (C) CD3 immunofluorescence in brain sections from treated mice at 20×. (D) Representative H&E micrographs of brain tissue from mice with treated brain tumors at 20× (top row) and 40× (bottom row). (E) Representative ex vivo MRI slices from mice with treated brain tumors. Experiments performed × 3 with 10 mice/group, typical results shown.
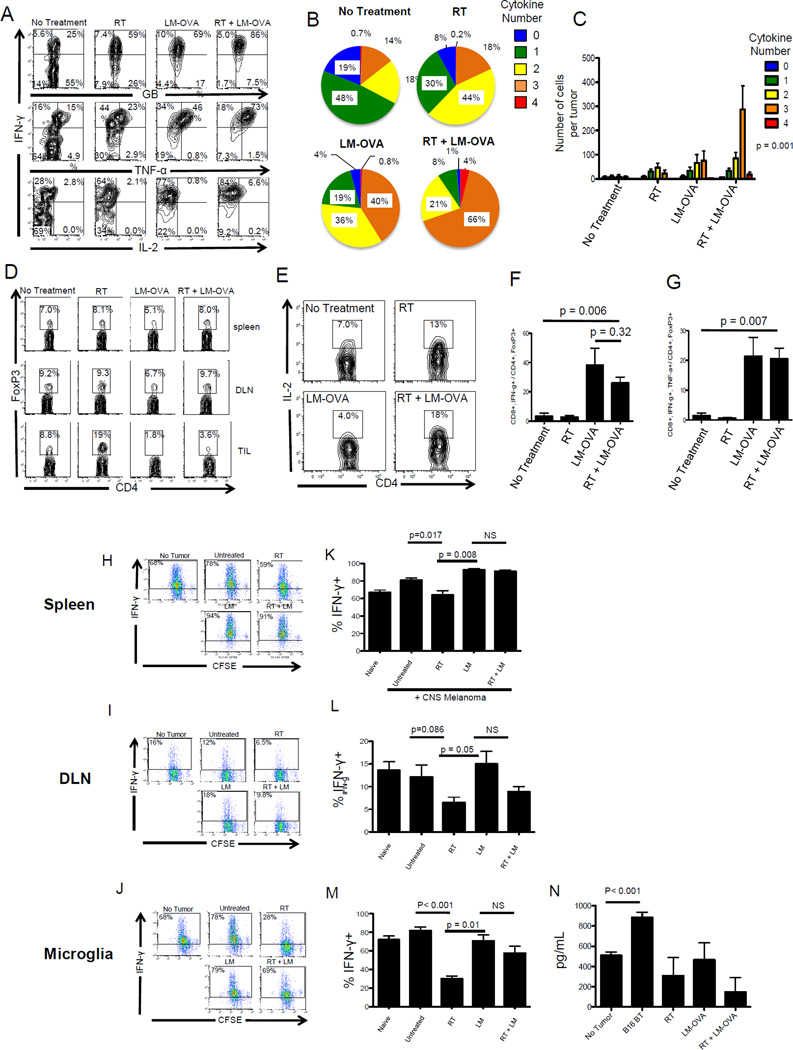
Combination therapy is associated with polyfunctional CD8 T cells, an increased Teff to Treg ratio, and increased APC function
To elucidate the immunological mechanisms by which focal RT and systemic LM mediate treatment effects in CNS melanoma, mice with established CNS disease were treated with RT, LM or the combination of RT and LM. Tumors were harvested 17 days after implantation and endogenous TIL were analyzed for cytokine production by flow cytometry. As show in in figure 6A, RT or LM alone generated a modest increase in Granzyme B, IFN-γ and TNF-α production, whilst with combination therapy, the majority of cells produced Granzyme B (GB), IFN-γ and Tumor Necrosis Factor (TNF)-α (Figure 6B). In addition to stimulating cytokine production, combination therapy increased the density of tumor-infiltrating polyfunctional CD8 T cells (p = 0.001 by two-way ANOVA) (Figure 6C).
Figure 6. Combination therapy with LM-based vaccination and focal RT is associated with polyfunctional CD8 T cells, an increased Teff to Treg ratio, and improved APC function.
(A) FACS plots demonstrating the effects of combination therapy on cytokine production from adoptively transferred, tumor-infiltrating CD8 T cells. (B,C) Percentages and numbers, respectively, of CD8 TIL producing single and multiple cytokines (D) Quantification of Treg (FoxP3+,CD4+) in treated animals E) Quantification of CD4 TIL producing IL-2. (F,G) Teff to Treg ratios in TIL, with effectors defined as IFNγ+, or IFNg TNFα double positive respectively. H-J) CFSE dilution of OT-1 T cells cultured with pulsed APC from Spleen, DLN and microglia (CD11b+ CD45-mid). (K-M) Summary graphs of the percentage of OT-1 responders producing IFN-γ when co-cultured with the indicated APC populations. (N) TGF-β1 concentrations in supernatants from J, M. A-G, N repeated × 2, N = 3–5 animals, group, H-M repeated × 1, N = 10 animals / group.
Accumulation of Tregs is an important mechanism of immunosuppression in cancer (24), so we next evaluated the effects of this regimen on peripheral and intratumoral Tregs. In the periphery, we observed a modest decrease in the percentage of CD4 T cells expressing FoxP3 with LM vaccination (Figure 6D). Similarly, LM vaccination decreased the percentage of Tregs within the tumor. Conversely, RT approximately doubled the percentage of CD4 TIL expressing FoxP3, consistent with prior data (25). Combination therapy resulted in an intratumoral Treg profile similar to LM alone, while favorably increasing the percentage of CD4 T cells producing IL-2 compared with either RT or LM monotherapy (Figure 6E). In several human caner types, the intratumoral Teff/Treg ratio correlates with clinical outcome. Indeed, we found that LM-based vaccination significantly increased this ratio, but RT did not add further to this, suggesting that increases in Teff / Treg were not the sole mechanism explaining the efficacy of the combination regimen. To further elucidate the mechanism(s) underlying the combination treatment effect, we tested whether the combination regimen affected the ability of various APC populations to present relevant tumor antigens ex vivo. We also quantified concentrations of the pro-inflammatory cytokines IFN-γ, IL-2, GM-CSF, and IL-12 as well as the inhibitory cytokines IL-10 and TGF-β in the supernatants of OT-1 CD8 T cells co-cultured with either splenic dendritic cells, tumor DLNs, or microglia (Supplemental Figure 6). We found that microglia isolated from mice with brain tumors secreted significantly more TGF-β compared with microglia from naïve animals and that combination therapy significantly decreased TGF-β secretion from microglia (Figure 6). Interestingly, focal RT alone decreased antigen presentation by microglia and splenic APCs, with a trend towards decreased presentation noted in the tumor DLN as well (Figure 6), whereas LM-based vaccination largely corrected this APC defect in both distant (splenic) and local sites.
Discussion
Brain metastases are a negative prognostic indicator in patients with metastatic melanoma (26) despite the fact that most patients succumb to systemic disease progression rather than neurologic compromise (10). One potential explanation is that metastasis to the brain is a relatively late event and thus a harbinger for widely disseminated disease. This hypothesis is challenged, however, by the finding that patients presenting with isolated melanoma brain metastases have shorter life expectancies than patients presenting with visceral metastases or synchronous brain and visceral lesions (27). A hypothesis consistent with these clinical data is that brain metastases accelerate systemic disease progression, potentially through an immune-mediated mechanism. Immunosuppression has been extensively studied in patients with high-grade gliomas, but little is known about the systemic immunologic effects of metastatic brain tumors.
Using a B16 model we demonstrated that brain tumors inhibit cellular immunity to a greater degree than flank or lung tumors in mice. Pmel CD8 T cells were more readily deleted in mice with brain tumors compared with equivalent B16 flank tumors, OT-1 T cells underwent fewer divisions, and daughter cells produced less IFN-γ in response to brain tumors compared with flank tumors. Of note, OT-1 TIL were divided in both brain and flank tumors, consistent with the hypothesis that naïve T cells have limited access to the CNS in the absence of inflammation (6) and that tumor antigens originating in the CNS are presented in secondary lymphoid organs. Furthermore, although re-stimulation of OVA-directed cells in the CNS is sufficient to restore effector function in a CD8-mediated EAE model (28), our data indicate that dividing TIL produce less IFN-γ in brain tumors than in flank tumors. Although caution is warranted in interpreting these pooled TIL data; this observation indicates that tolerance acquired upon priming in secondary lymphoid organs may persist in the brain tumor microenvironment.
Impaired cellular immunity has long been suspected in GBM patients and may be compounded by interventions such as chemotherapy and steroids (4,29). Our data demonstrate that tumor location within the CNS may be an independent mediator of systemic immunosuppression. We used a series of in vivo CTL experiments to explore the functional significance of this finding. Tumor-reactive lymphocytes characteristically express the exhaustion markers PD-1, LAG-3 and TIM-3 (30), so it was not surprising that tumor antigen processing was insufficient to stimulate a cytotoxic response. This finding is important, however, as it demonstrated that antigen recognition on B16 tumors was insufficient to mediate significant cytotoxicity. We next applied immunologic pressure by adoptively transferring a physiologically relevant number of OT-1 cells and vaccinating with Vac-OVA. Here, we found that mice with B16-OVA brain tumors exhibited impaired target lysis, indicating that the presence of a CNS tumor may blunt systemic responses to some immunotherapies.
Dysfunctional myeloid cells have been identified as key mediators of immunosuppression in cancer patients (31) and M2-differentiated microglia may be drivers of glioma progression (32). TGF-β is a pleotropic cytokine that induces immune suppression and drives tumor progression in several solid tumors, including melanoma and glioma (8,33). TGF-β has also been shown to enhance IL-4 mediated, M2 microglial activation (34). Our data indicate that microglia isolated from mice with B16-OVA brain tumors express significantly higher levels of TGF-β than microglia from naïve mice. We found that this elevation in TGF-β was also systemic, as B16-OVA brain tumor bearing mice had significantly elevated serum levels of TGF-β1 compared with tumor-free or flank tumor-bearing mice. Further, we found that TGF-β signaling blockade rescued deletional tolerance in the Pmel adoptive transfer model. Based on these data, we suspect that CNS melanoma may drive microglia into an alternatively activated phenotype characterized by TGF-β expression. However, TGF-beta blockade in this model was unable to mediate a significant anti-tumor effect, either alone, combined with RT or with LM-based vaccination. Those data are perhaps somewhat contradictory to recent studies demonstrating that blocking TGF-β prior to hypofractionated radiation enhances preclinical responses (35), and additional work will be required to determine whether these differences reflect the use of different TGF-β blocking agents, the location of the tumor, or the cancer model under study. While our studies focused on microglia and antigen-presenting cells in the tumor draining lymph nodes and spleen, it is clear that tolerance to tumor antigens is mediated by a number of additional myeloid cell types, particularly myeloid derived suppressor cells (MDSC) (31). MDSC have been described in a CNS glioma model (36); and additional work will be required to address the role of MDSC populations in this model, both in the systemic tolerance mediated by implanted CNS tumors, as well as in the response of those tumors to RT, vaccination, or combination regimens.
Live-attenuated LM vaccines have demonstrated efficacy in several preclinical cancer models (37,38) and safety in Phase I and II clinical trials (39,40). The ability of LM to generate adaptive T cell-mediated immunity is based on its intracellular lifecycle and propensity to infect CD8+ dendritic cells (DCs), where bacterial antigens are processed through both MHC class I and class II pathways (40). Liau and colleagues have previously reported that a different strain of LM delays progression of intracranial B16 tumors (41). In our studies, LM-based vaccination restored CTL activity in a majority of B16 brain tumor-bearing mice; however, a trend remained toward impaired CTL function compared with flank tumor-bearing animals. Interestingly, treatment with LM vaccines increased lysis in mice with brain or flank tumors as compared to tumor-free mice. This effect was not observed with a vaccinia-based vaccine and suggests that LM may be have been superior to vaccinia in boosting a low level of T cell priming that occurs upon recognition of antigen on tumor cells. These data are consistent with the notion that brain tumors may be more systemically tolerogenic than flank tumors, but also show that a potent vaccine may reverse CTL tolerance. Both vaccine platforms, however, failed to completely abrogate brain tumor-mediated tolerance, indicating that combination therapy may be required to achieve maximum efficacy.
RT has been associated with a mix of pro-inflammatory and inhibitory immunologic effects (15,42), but may have particular utility in potentiating the activity of immunotherapy (43). Demaria and colleagues showed that RT in combination with FLt3-ligand impairs growth of irradiated tumors as well as tumors outside the radiation field (44,45) and that local RT combined with cytotoxic T lymphocyte antigen-4 (CTLA-4) blockade inhibits metastasis in a breast cancer model (46). Newcomb and colleagues showed that radiation therapy combined with GVAX generates long-term survival and protective immunity in an orthotopic glioma model (47) and our group demonstrated that combining focal RT with PD-1 blockade prolongs survival of mice with orthotopic gliomas and protects against flank tumor re-challenge (9). Consistent with these data, in some studies we found that, while anti-PD-1 alone or in combination with LM-OVA vaccination had no effect on survival, the combination of RT + anti-PD-1 produced a small percentage of long-term survivors (data not shown). The translational relevance of these findings should be interpreted with caution given that PD-1 or CTLA-4 blockade alone have a modest effect on B16 progression (48), while multiple clinical trials have proven efficacy of checkpoint blockade in human melanoma. Further, immune checkpoint blockade may represent a form of vaccination in humans; CTLA-4 blockade has been shown to boost T cell responses to shared tumor antigens (49), and responses to PD-1 blockade in humans may represent recognition of mutated tumor antigens (50). Additional experiments in other melanoma models are warranted to more clearly delineate the role of checkpoint blockade in the setting of tumor antigen-specific vaccination.
We found that combining focal RT with LM-OVA vaccination significantly prolonged survival over either monotherapy. Combination therapy stimulated tumor infiltration by polyfunctional CD8 T cells and increased Teff to Treg ratios, an immune profile that has been associated with tumor regression (24,51). Furthermore, combination treatment with focal RT and LM-OVA altered the cytokine profile of microglia, reducing TGF-β1 secretion to levels indistinguishable from naïve microglia. Consistent with previous reports, RT increased intratumoral Treg density (52). Conversely, LM-OVA decreased the percentage of Tregs both within the tumor and peripherally. Combination therapy had mixed effects: focal RT abrogated the systemic reduction in Tregs stimulated by LM-OVA vaccination while maintaining a favorable intratumoral Teff to Treg ratio. These data suggest a therapeutic mechanism by which LM vaccination decreased the number of Tregs locally and systemically and bolstered APC function, while focal RT promoted polyfunctionality of CTLs and increased intratumoral Teff density. Translation of these studies to patients with CNS melanoma should be tempered by two additional caveats: First, vaccine monotherapy has generally not been as clinically effective as predicted by animal models. Second, although cancer vaccines can add to or synergize with immune checkpoint blockade in several animal models (53), human studies combining either CTLA-4 blockade (54) or PD-1 blockade (55) with peptide vaccines have not clearly shown additional clinical benefit.
Yet, based on these data, it is reasonable to hypothesize that development of a melanoma brain metastasis curbs immunologic pressure against tumor antigens and accelerates systemic disease progression. This model affords a plausible explanation for why brain metastases carry a grave prognosis, even in the setting of stable CNS disease. Going forward, it will be important to verify the relevance of these findings in other, more contemporary, melanoma models as well as in human disease and determine if CNS tumor burden correlates with the degree of immune suppression. If so, prompt and aggressive treatment of metastatic brain lesions with immunotherapy, or possibly other modalities, may not only be critical for preserving neurologic function, but also for restoring cellular immunity and delaying systemic disease progression.
Supplementary Material
Statement of Translational Relevance.
Brain metastases are a significant source of morbidity and mortality for patients with advanced melanoma, yet little is known about the effects of tumor location on antitumor immunity. The data presented here indicate that B16 melanoma brain tumors are more systemically immunosuppressive than equivalently advanced flank and lung tumors, and that focal radiation therapy and tumor antigen-specific vaccination can restore immune function and mediate tumor regression. Based on these data, we hypothesize that patients harboring CNS malignancies may exhibit accelerated systemic disease progression. Furthermore, these findings suggest that combination immunotherapy regimens involving specific vaccination and focal radiation therapy may be active against CNS melanoma.
Acknowledgments
We would like to thank Jonathan M. Yingling (Bristol-Myers Squibb, NJ) for generously providing LY2157299 for these experiments and Dr. Nicholas P. Restifo (NCI, Bethesda, MD) for generously providing Vac-GP100.
Grant Support:
CGD is supported by National Institutes of Health R01 CA127153, the Patrick C. Walsh Fund, the OneInSix Foundation the Prostate Cancer Foundation and the Melanoma Research Association. CMJ was funded by the Howard Hughes Medical Fellows Research Program. CP was supported by The Bart McLean Fund for Neuroimmunology Research-Project Restore. ML is supported by the WW Smith Foundation.
Conflicts of Interest: C.G. Drake has received sponsored research funding from Aduro Biotech and Bristol Myers Squibb under agreements overseen by Johns Hopkins University. T. Dubensky, P. Lauer, and D. Brockstedt are employees of Aduro Biotech.
References
- 1.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 2.Jackson CM, Lim M, Drake CG. Immunotherapy for Brain Cancer: Recent Progress and Future Promise. Clinical Cancer Research. 2014;20:3651–3659. doi: 10.1158/1078-0432.CCR-13-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellsworth S, Balmanoukian A, Kos F, Nirschl CJ, Nirschl TR, Grossman SA, et al. Sustained CD4(+) T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology. 2014;3:e27357. doi: 10.4161/onci.27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in Patients with High-Grade Gliomas Treated with Radiation and Temozolomide. Clinical Cancer Research. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nature Reviews Immunology. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 6.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biollaz G, Bernasconi L, Cretton C, Püntener U, Frei K, Fontana A, et al. Site-specific anti-tumor immunity: Differences in DC function, TGF-β production and numbers of intratumoral Foxp3+ Treg. Eur J Immunol. 2009;39:1323–1333. doi: 10.1002/eji.200838921. [DOI] [PubMed] [Google Scholar]
- 8.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer Research. 2001;61:1095–1099. [PubMed] [Google Scholar]
- 9.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 Blockade and Stereotactic Radiation Produce Long-Term Survival in Mice With Intracranial Gliomas. International Journal of Radiation Oncology*Biology*Physics. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 11.Lauer P, Chow MYN, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol. 2002;184:4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Research. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 13.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- 14.Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of"self-”reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobrzanski MJ, Reome JB, Hollenbaugh JA, Dutton RW. Tc1 and Tc2 effector cell therapy elicit long-term tumor immunity by contrasting mechanisms that result in complementary endogenous type 1 antitumor responses. J Immunol. 2004;172:1380–1390. doi: 10.4049/jimmunol.172.3.1380. [DOI] [PubMed] [Google Scholar]
- 17.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 19.Blattman JN, Antia R, Sourdive DJD, Wang X, Kaech SM, Murali-Krishna K, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brockstedt DG, Bahjat KS, Giedlin MA, Liu W, Leong M, Luckett W, et al. Killed but metabolically active microbes: a new vaccine paradigm for eliciting effector T-cell responses and protective immunity. Nat Med. 2005;11:853–860. doi: 10.1038/nm1276. [DOI] [PubMed] [Google Scholar]
- 21.Jakowlew SB. Transforming growth factor-β in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Valdes N, Basagoiti M, Dotor J, Aranda F, Monreal I, Riezu-Boj JI, et al. Induction of Monocyte Chemoattractant Protein-1 and Interleukin-10 by TGF 1 in Melanoma Enhances Tumor Infiltration and Immunosuppression. Cancer Research. 2011;71:812–821. doi: 10.1158/0008-5472.CAN-10-2698. [DOI] [PubMed] [Google Scholar]
- 23.Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, et al. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008;44:142–150. doi: 10.1016/j.ejca.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Curiel TJ. Regulatory T cells and treatment of cancer. Current Opinion in Immunology. 2008;20:241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Persa E, Balogh A, Sáfrány G, Lumniczky K. The effect of ionizing radiation on regulatory T cells in health and disease. Cancer Letters. 2015 doi: 10.1016/j.canlet.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Weide B, Richter S, Büttner P, Leiter U, Forschner A, Bauer J, et al. Soyer HP, editor. Serum S100B, Lactate Dehydrogenase and Brain Metastasis Are Prognostic Factors in Patients with Distant Melanoma Metastasis and Systemic Therapy. PLoS ONE. 2013;8:e81624. doi: 10.1371/journal.pone.0081624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma MW, Qian M, Lackaye DJ, Berman RS, Shapiro RL, Pavlick AC, et al. Challenging the current paradigm of melanoma progression: brain metastasis as isolated first visceral site. Neuro-Oncology. 2012:849–858. doi: 10.1093/neuonc/nos113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Na S-Y, Hermann A, Sanchez-Ruiz M, Storch A, Deckert M, Hünig T. Oligodendrocytes Enforce Immune Tolerance of the Uninfected Brain by Purging the Peripheral Repertoire of Autoreactive CD8+ T Cells. Immunity. 2012;37:134–146. doi: 10.1016/j.immuni.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Lesniak MS, Gabikian P, Tyler BM, Pardoll DM, Brem H. Dexamethasone mediated inhibition of local IL-2 immunotherapy is dose dependent in experimental brain tumors. J Neurooncol. 2004;70:23–28. doi: 10.1023/b:neon.0000040821.50347.c5. [DOI] [PubMed] [Google Scholar]
- 30.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124:2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature Reviews Immunology. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 33.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X, Spittau B, Krieglstein K. TGFβ signalling plays an important role in IL4-induced alternative activation of microglia. J Neuroinflammation. 2012;9:210. doi: 10.1186/1742-2094-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young KH, Newell P, Cottam B, Friedman D, Savage T, Baird JR, et al. TGF Inhibition Prior to Hypofractionated Radiation Enhances Efficacy in Preclinical Models. Cancer Immunology Research. 2014;2:1011–1022. doi: 10.1158/2326-6066.CIR-13-0207. [DOI] [PubMed] [Google Scholar]
- 36.Jia W, Jackson-Cook C, Graf MR. Journal of Neuroimmunology. Journal of Neuroimmunology Elsevier B.V. 2010;223:20–30. doi: 10.1016/j.jneuroim.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura K, Jain A, Allen HE, Laird LS, Chia CY, Ravi S, et al. Selective targeting of antitumor immune responses with engineered live-attenuated Listeria monocytogenes. Cancer Research. 2006;66:1096–1104. doi: 10.1158/0008-5472.CAN-05-2307. [DOI] [PubMed] [Google Scholar]
- 38.Pan ZK, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 39.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: A Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 40.Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, et al. A Live-Attenuated Listeria Vaccine (ANZ-100) and a Live-Attenuated Listeria Vaccine Expressing Mesothelin (CRS-207) for Advanced Cancers: Phase I Studies of Safety and Immune Induction. Clinical Cancer Research. 2012;18:858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prins RM, Bruhn KW, Craft N, Lin JW, Kim C-H, Odesa SK, et al. Central Nervous System Tumor Immunity Generated by a Recombinant Listeria monocytogenes Vaccine Targeting Tyrosinase Related Protein-2 and Real-Time Imaging of Intracranial Tumor Burden. Neurosurgery. 2006;58:169–178. doi: 10.1227/01.neu.0000192367.29047.64. [DOI] [PubMed] [Google Scholar]
- 42.Drake CG. In: Molecular Determinants of Radiation Response. DeWeese TL, Laiho M, editors. New York, NY: Springer New York; 2011. pp. 251–263. [Google Scholar]
- 43.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker JJ, Jones JC, Strober S, Knox SJ. Characterization of direct radiation-induced immune function and molecular signaling changes in an antigen presenting cell line. Clinical Immunology. 2013;148:44–55. doi: 10.1016/j.clim.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 47.Newcomb EW, Demaria S, Lukyanov Y, Shao Y, Schnee T, Kawashima N, et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12:4730–4737. doi: 10.1158/1078-0432.CCR-06-0593. [DOI] [PubMed] [Google Scholar]
- 48.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, et al. Chatenoud L, editor. Immune Monitoring of the Circulation and the Tumor Microenvironment in Patients with Regionally Advanced Melanoma Receiving Neoadjuvant Ipilimumab. PLoS ONE. 2014;9:e87705. doi: 10.1371/journal.pone.0087705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perret R, Sierro SR, Botelho NK, Corgnac S, Donda A, Romero P. Adjuvants That Improve the Ratio of Antigen-Specific Effector to Regulatory T Cells Enhance Tumor Immunity. Cancer Research. 2013;73:6597–6608. doi: 10.1158/0008-5472.CAN-13-0875. [DOI] [PubMed] [Google Scholar]
- 52.Formenti SC, Demaria S. Systemic effects of local radiotherapy. The Lancet Oncology. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li B, Simmons A, Du T, Lin C, Moskalenko M, Gonzalez-Edick M, et al. Clinical Immunology. Vol. 133. Elsevier Inc; 2009. Allogeneic GM-CSF-secreting tumor cell immunotherapies generate potent anti-tumor responses comparable to autologous tumor cell immunotherapies; pp. 184–197. [DOI] [PubMed] [Google Scholar]
- 54.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, et al. Safety, Correlative Markers, and Clinical Results of Adjuvant Nivolumab in Combination with Vaccine in Resected High-Risk Metastatic Melanoma. Clinical Cancer Research. 2015;21:712–720. doi: 10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.