Abstract
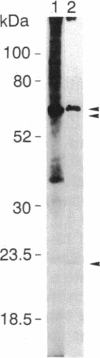
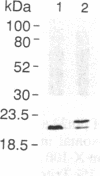
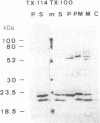
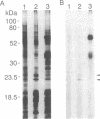
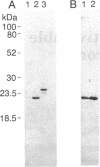
Plasma membrane vesicles were isolated from maize (Zea mays L.) coleoptile tissue by aqueous two-phase partitioning and assayed for homogeneity by the use of membrane-specific enzymatic assays. Using 5-azido-[7-3H]indole-3-acetic acid ([3H]N3IAA), we identified several IAA-binding proteins with molecular masses of 60 kDa (pm60), 58 kDa (pm58), and 23 kDa (pm23). Using Triton X-114, we were able to selectively extract pm23 from the plasma membrane. We show that auxins and functional analogues compete with [3H]N3IAA for binding to pm23. We found that PAB130, a polyclonal antibody raised against auxin-binding protein 1 (ABP-1), recognized ABP-1 as well as pm23. This suggests that pm23 shares common epitopes with ABP-1. In addition, we identified an auxin-binding protein with a molecular mass of 24 kDa (pm24), which was detected in microsomal but not in plasma membrane vesicle preparations. Like pm23 this protein was extracted from membrane vesicles with Triton X-114. We designed a purification scheme allowing simultaneous purification of pm23 and pm24. Homogeneous pm23 and pm24 were obtained from coleoptile extracts after 7000-fold purification.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Dulley J. R. Determination of inorganic phosphate in the presence of detergents or protein. Anal Biochem. 1975 Jul;67(1):91–96. doi: 10.1016/0003-2697(75)90275-4. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse T., Feldwisch J., Balshüsemann D., Bauw G., Puype M., Vandekerckhove J., Löbler M., Klämbt D., Schell J., Palme K. Molecular cloning and structural analysis of a gene from Zea mays (L.) coding for a putative receptor for the plant hormone auxin. EMBO J. 1989 Sep;8(9):2453–2461. doi: 10.1002/j.1460-2075.1989.tb08380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks G. R., Rayle D. L., Jones A. M., Lomax T. L. Specific photoaffinity labeling of two plasma membrane polypeptides with an azido auxin. Proc Natl Acad Sci U S A. 1989 Jul;86:4948–4952. doi: 10.1073/pnas.86.13.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks G. R., Rayle D. L., Lomax T. L. The diageotropica mutant of tomato lacks high specific activity auxin binding sites. Science. 1989 Jul 7;245:52–54. doi: 10.1126/science.245.4913.52. [DOI] [PubMed] [Google Scholar]
- Inohara N., Shimomura S., Fukui T., Futai M. Auxin-binding protein located in the endoplasmic reticulum of maize shoots: molecular cloning and complete primary structure. Proc Natl Acad Sci U S A. 1989 May;86(10):3564–3568. doi: 10.1073/pnas.86.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Gilbert S. F. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science. 1983 Jun 17;220(4603):1297–1300. doi: 10.1126/science.220.4603.1297. [DOI] [PubMed] [Google Scholar]
- Jones A. M., Melhado L. L., Ho T. H., Pearce C. J., Leonard N. J. Azido auxins : photoaffinity labeling of auxin-binding proteins in maize coleoptile with tritiated 5-azidoindole-3-acetic Acid. Plant Physiol. 1984 Aug;75(4):1111–1116. doi: 10.1104/pp.75.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. M., Venis M. A. Photoaffinity labeling of indole-3-acetic acid-binding proteins in maize. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6153–6156. doi: 10.1073/pnas.86.16.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leonard N. J., Greenfield J. C. Photoaffinity-labeled Auxins: Synthesis and Biological Activity. Plant Physiol. 1975 Jun;55(6):1057–1061. doi: 10.1104/pp.55.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Partial purification of a tonoplast ATPase from corn coleoptiles. Plant Physiol. 1985 Jun;78(2):327–333. doi: 10.1104/pp.78.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhado L. L., Jones A. M., Leonard N. J., Vanderhoef L. N. Azido auxins: synthesis and biological activity of fluorescent photoaffinity labeling agents. Plant Physiol. 1981 Aug;68(2):469–475. doi: 10.1104/pp.68.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K., Feldwisch J., Hesse T., Bauw G., Puype M., Vandekerchkhove J., Schell J. Auxin binding proteins from maize coleoptiles: purification and molecular characterization. Symp Soc Exp Biol. 1990;44:299–313. [PubMed] [Google Scholar]
- Palme K., Hesse T., Moore I., Campos N., Feldwisch J., Garbers C., Hesse F., Schell J. Hormonal modulation of plant growth: the role of auxin perception. Mech Dev. 1991 Feb;33(2):97–106. doi: 10.1016/0925-4773(91)90076-i. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990 Dec;15(12):483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Ripp K. G., Viitanen P. V., Hitz W. D., Franceschi V. R. Identification of membrane protein associated with sucrose transport into cells of developing soybean cotyledons. Plant Physiol. 1988 Dec;88(4):1435–1445. doi: 10.1104/pp.88.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Fluorographic detection of radioactivity in polyacrylamide gels with 2,5-diphenyloxazole in acetic acid and its comparison with existing procedures. Biochem J. 1983 Jan 1;209(1):281–284. doi: 10.1042/bj2090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann U., Viola G., Kayser B., Siemeister G., Hesse T., Palme K., Löbler M., Klämbt D. cDNA clones of the auxin-binding protein from corn coleoptiles (Zea mays L.): isolation and characterization by immunological methods. EMBO J. 1989 Sep;8(9):2463–2467. doi: 10.1002/j.1460-2075.1989.tb08381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Isolation of subcellular organelles of metabolism on isopycnic sucrose gradients. Methods Enzymol. 1974;31:734–746. doi: 10.1016/0076-6879(74)31077-4. [DOI] [PubMed] [Google Scholar]
- Zettl R., Feldwisch J., Boland W., Schell J., Palme K. 5'-Azido-[3,6-3H2]-1-napthylphthalamic acid, a photoactivatable probe for naphthylphthalamic acid receptor proteins from higher plants: identification of a 23-kDa protein from maize coleoptile plasma membranes. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):480–484. doi: 10.1073/pnas.89.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]