Abstract
Objective
Little is known about the associations of serum fatty acids with lipoprotein profile and the underlying genetic and environmental etiology of these relationships. We aimed to analyze the phenotypic association of serum n-6 and n-3 polyunsaturated (PUFAs), monounsaturated (MUFAs) and saturated (SFAs) fatty acids (relative proportion to total fatty acids) with lipids and lipoproteins, and to quantify common genetic and environmental factors determining their covariation.
Methods
Two cohorts of healthy Finnish twins were assessed in young adulthood. Data were available for 1269 individual twins including 561 complete pairs. Serum metabolites were measured by nuclear magnetic resonance spectroscopy. Bivariate quantitative genetic models were used to decompose the phenotypic covariance between the pairs of traits into genetic and environmental components.
Results
Among the strongest correlations observed, serum total n-6 PUFAs and linoleic acid were inversely (max. r = −0.65) and MUFAs positively (max. r = 0.63) correlated with triglycerides and very low-density lipoprotein (VLDL) particle concentration, particularly with large VLDL (for n-6 PUFAs) and medium VLDL (for MUFAs). Genetic factors significantly contributed to their covariance with bivariate heritability estimates ranging from 44% to 56% for n-6 PUFAs and 58% to 66% for MUFAs. Genetic correlations with lipid traits were moderate to high (max. rA = −0.59 and 0.70 for n-6 PUFAs and MUFAs, respectively). Statistically significant, but substantially weaker phenotypic correlations of total n-3 PUFAs, docosahexaenoic acid (DHA) and SFAs with lipoprotein profile were not decomposed into their genetic and environmental components.
Conclusion
Shared genetic factors are important in explaining why higher concentrations of serum n-6 PUFAs and lower concentrations of serum MUFAs strongly associate with lower triglyceride and VLDL particle concentrations.
Keywords: Serum fatty acids, Lipoprotein profile, Twins, Genetic pleiotropy, Environmental factors
1. Introduction
High plasma concentrations of low-density lipoprotein cholesterol (LDL-C) and triglycerides, and low concentrations of high-density lipoprotein cholesterol (HDL-C) are known risk factors for coronary heart disease (CHD) [1,2]. Evidence from human trials suggests that polyunsaturated fatty acids (PUFAs) have the ability to alter serum lipid profile. It has been reported that n-6 polyunsaturated fatty acids (n-6 PUFAs) such as linoleic acid (LA), which comes primarily from vegetable oils, have a potential LDL-C lowering effect [3] and n-3 polyunsaturated fatty acids (n-3 PUFAs), particularly fish oil in high doses, decrease serum triglycerides and increase modestly LDL-C and HDL-C concentrations [4].
Previous observational studies have related self-reported dietary intake and serum concentrations of fatty acids (FAs) to conventionally measured lipids as well as to lipoprotein subclass profile. The number of distinct lipoprotein subclasses, as well as their average particle size, can be measured by nuclear magnetic resonance (NMR) spectroscopy [5–7]. In our earlier study in a subsample of this population, habitual fish intake was negatively associated with very low-density lipoprotein (VLDL) particle diameter, and a dietary pattern high in saturated fatty acids (SFAs) and sucrose (labeled “junk food”) was associated with an overall adverse lipoprotein subclass profile, including increased concentrations of triglycerides, small HDL and LDL and large VLDL [8]. Another study in Alaska Natives, showed that higher dietary intake of n-3 PUFAs is related to a favorable lipoprotein profile, in particular lower concentrations of large VLDL and higher concentrations of large HDL particles [9]. Concerning FAs in serum, n-6 and n-3 PUFAs have been inversely associated with triglyceride and positively with HDL-C concentrations [10,11]; however, the association with LDL-C was less consistent across populations [10–12]. In middle -aged men, serum LA was inversely associated with large VLDL, total LDL and small LDL particles as well as VLDL diameter and positively with large HDL particles and HDL diameter [13]. The same study found that serum monounsaturated fatty acids (MUFAs) were inversely related to large HDL particles and positively to large VLDL particles, while SFAs showed a positive relationship with large VLDL particles and VLDL diameter [13]. It has been demonstrated that individual serum fatty acids and lipoprotein subclasses are quite highly heritable [14], but the extent to which the associations between them are attributable to genetic and environmental factors remains largely unknown.
In the present study, we aimed to assess the relationship of serum PUFAs, MUFAs and SFAs with lipoprotein profile in young adult Finnish twins consuming their habitual diet, and to determine whether the associations are due to genetic and/or environmental factors in common.
2. Material and methods
2.1. Study population
The data were derived from FinnTwin12 (FT12) and FinnTwin16 (FT16) cohort studies, ascertained from the Population Register Centre and described in detail elsewhere [15]. Both are longitudinal studies of behavioral development and health habits of Finnish twins initially enrolled during adolescence, and repeatedly assessed by self-report questionnaires. FT12 included five consecutive birth cohorts of Finnish twins born in 1983–1987. Questionnaires were mailed to twin individuals in the autumn of the year in which their birth cohort reached age 11 (90% of the responses were received by the end of that year), and subsequent follow-up assessments were made at 14, 17.5 and ~22 years. In FT16, the baseline survey questionnaire was sent to all Finnish twins born in 1975–1979 within 2 months after their 16th birthday (response rate of 88%) and individuals were mailed three follow-up questionnaires at 17, 18.5 and ~25 years. Zygosity was determined by well-validated items on physical similarity during school age. DNA analysis confirmed questionnaire assignment of zygosity in 97% of the same-sex adolescents from FT12 (n=395 pairs).
During the fourth wave of the data collection of each cohort, a subsample of these young adult twins visited the study clinic and gave a blood sample for the NMR spectroscopy analysis. In FT12, individuals were fasting and in FT16 they were allowed to have a light breakfast (e.g. a cup of coffee or tea, bread, fruits etc.), but not anything very greasy (e.g. bacon and eggs). Preliminary analysis showed that correlations between fatty acids and lipoproteins were not significantly different for FT12 and FT16 (data not shown), and thus the two cohorts were pooled to increase statistical power. The exclusion criteria for this study were lipid-lowering medication (1 individual) and pregnancy (64 individuals), which left 1269 participants available for the analyses (57% FT12, 43% FT16). Finally, our sample included 561 complete twin pairs (for 9 of these pairs NMR data was not available in one co-twin): 221 monozygotic (MZ) (97 brother-brother and 124 sisteresister), 199 same-sex dizygotic (DZ) (100 male and 99 female) and 141 opposite sex dizygotic (OSDZ) pairs. Among these twins, at least 100 pairs were still cohabiting at the time of the examination. Data collection and analysis were approved by the ethics committee of the Department of Public Health of the University of Helsinki and the Institutional Review Board (IRB) of Indiana University. Written informed consent was obtained from all participants.
2.2. NMR-derived serum metabolites
Serum fatty acid, lipid and lipoprotein subclass concentrations were measured by proton NMR spectroscopy as previously described in detail [16,17]. This NMR platform has recently been applied in various extensive epidemiological and genetics studies [14,18,19]. The 14 lipoprotein subclass sizes determined by this methodology are as follows: chylomicrons and extremely large VLDL particles (with particle diameters from ~75 nm upwards), five different VLDL subclasses, namely, very large VLDL (average particle diameter of 64.0 nm), large VLDL (53.6 nm), medium VLDL (44.5 nm), small VLDL (36.8 nm), very small VLDL (31.3 nm); intermediate density lipoprotein (IDL) (28.6 nm), three LDL subclasses as large LDL (25.5 nm), medium LDL (23.0 nm), and small LDL (18.7); and four HDL subclasses as very large HDL (14.3 nm), large HDL (12.1 nm), medium HDL (10.9 nm), and small HDL (8.7 nm). We grouped extremely large, very large and large VLDL to “large VLDL”, small and very small VLDL to “small VLDL”, IDL and large LDL to “large LDL” and very large and large HDL to “large HDL”. Thus, 3 subclass sizes (large, medium and small) of VLDL, LDL and HDL were considered to determine the concentration of particles. The concentrations of each VLDL, LDL and HDL subclasses were summed to obtain total particle concentrations. The mean particle size for VLDL, LDL and HDL particles was calculated by weighting the corresponding subclass diameters with their particle concentrations. After measuring the lipoprotein data from native serum samples their lipids were extracted and another NMR spectrum measured (details given in Refs. [18,20]). In lipid extracts, different fatty acids, e.g., n-3 and n-6 PUFAs, give rise to a characteristic NMR resonance, the area of which is related to their concentration. The extract data were scaled according to serum total cholesterol (obtained from the native serum sample) to account for slight experimental variation in lipid acquisitions in the extraction procedure [20]; thereby the FA concentrations represent true serum concentrations. Serum fatty acids were expressed as a percentage of total fatty acids.
2.3. Statistical methods
Since the distributions of some NMR measures were highly skewed, they were first standardized by age and cohort and transformed using rank transformation methods in R statistical package. This data pre-processing achieved normality and the resulting residuals were used as input phenotypes for the following analyses. Pearson’s correlations were calculated between serum fatty acids and lipid/lipoprotein profile, and the sex-fatty acid interaction term was tested. In the analyses where twins were treated as individuals, the standard errors were corrected for clustering of twin pairs by survey methods [21]. Principal component analysis was used to determine the number of principal components that explain most part of the variance of the studied lipids and lipoproteins. As the strong correlations among these metabolites makes the traditional Bonferroni correction for multiple testing too conservative, the number of principal components provides a more permissive and correct P-value threshold. In this study, the first five principal components explained more than 95% of the variance, allowing associations to be significant at P < 0.01 after the Bonferroni correction. Statistical analyses were conducted using the Stata statistical software package (release 12.0; Stata Corporation, College Station, Texas).
To analyze the common genetic and environmental factors underlying the association between serum fatty acids and lipid/lipoprotein profile classic twin modeling was used [22]. Briefly, the analysis is based on the fact that MZ twins share the same gene sequence, whereas DZ twins share, on average, 50% of their genes identical-by-descent. On this basis, inter-individual phenotypic variance can be attributed to four sources of variation: additive genetic effects (A: correlated 1.0 for MZ and 0.5 for DZ pairs), dominance effects (D: 1.0 for MZ and 0.25 for DZ pairs), common (shared) environmental effects (C: by definition, correlated 1.0 for all pairs) and unique (non-shared) environmental effects (E: by definition, uncorrelated in all pairs). However since we had only twins reared together in our cohort, common environmental and dominance genetic effects cannot be estimated simultaneously. Genetic modeling was carried out by the Mx statistical package [23].
Bivariate Cholesky decomposition was used to examine the contribution of latent genetic and environmental factors to the covariances between serum fatty acids and lipid/lipoprotein profile. This procedure makes no assumptions on the underlying genetic architecture but simply decomposes the variation and covariation in the data into a series of uncorrelated genetic and environmental factors. The structure of the Cholesky decomposition was based on the univariate model-fitting results, which were previously published for the majority of metabolites [14], and on the chi-squared difference test between models progressed from least restrictive (full Cholesky) to more restrictive for each pair of traits. For all analyzed pairs, the AE–AE Cholesky model provided the best fit to the data. This indicates that the trait correlation between two phenotypes is due to additive genetic correlation (rA) indicating the same or closely linked genes, and unique environmental correlation (rE) indicating same or correlated environmental factors unique to each twin individual. In the present study, only phenotypic (Pearson’s) correlations with values greater than ±0.3 both in men and women were considered for Cholesky decomposition.
3. Results
3.1. Characteristics of the participants
The characteristics of the sample are reported separately by sex in Table 1. The average mean age of men and women was 24.2 years and 23.8 years, respectively. BMI was greater in males than in females. Total n-6 PUFAs, LA and MUFAs relative concentration and n-6/n-3 PUFAs ratio were also higher in males, whereas total n-3 PUFAs, DHA and SFAs showed greater values in females. Women had generally a more favorable lipid profile than men characterized by higher HDL-C, larger HDL and LDL particle diameter, and higher HDL particles concentration. However, women had higher total cholesterol, IDL-C and large LDL particle concentration.
Table 1.
Characteristics of the study participants by sex.
| Males (n = 600)
|
Females (n = 669)
|
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 24.20 | 2.17 | 23.81 | 2.07 |
| BMI (kg/m2) | 24.21 | 3.79 | 22.96 | 4.04 |
| Fatty acid proportion (% of total fatty acids) | ||||
| Total n-6 PUFAs | 34.20 | 3.91 | 34.06 | 3.86 |
| Linoleic acid | 29.48 | 3.81 | 28.99 | 4.05 |
| Total n-3 PUFAs | 3.23 | 0.80 | 3.32 | 0.91 |
| Docosahexaenoic acid | 1.31 | 0.44 | 1.66 | 0.49 |
| Monounsaturated fatty acids | 29.92 | 3.54 | 28.87 | 3.39 |
| Saturated fatty acids | 32.66 | 3.25 | 33.74 | 3.35 |
| Fatty acid ratio | ||||
| Total n-6/total n-3 PUFAs | 11.21 | 2.93 | 10.86 | 2.84 |
| Serum lipids (mmol/l) | ||||
| Total cholesterol | 4.68 | 0.87 | 4.92 | 0.87 |
| IDL cholesterol | 0.68 | 0.17 | 0.69 | 0.16 |
| LDL cholesterol | 1.83 | 0.54 | 1.69 | 0.47 |
| HDL cholesterol | 1.57 | 0.31 | 2.00 | 0.42 |
| Total triglycerides | 1.21 | 0.59 | 1.07 | 0.48 |
| Extremely large VLDL triglycerides | 0.01 | 0.02 | 0.01 | 0.01 |
| Total VLDL triglycerides | 0.83 | 0.51 | 0.66 | 0.41 |
| IDL triglycerides | 0.10 | 0.03 | 0.11 | 0.04 |
| VLDL particle concentration (nmol/l) | ||||
| Total VLDL | 76.96 | 31.04 | 68.69 | 26.69 |
| Large VLDL | 5.13 | 4.78 | 3.85 | 3.68 |
| Medium VLDL | 16.33 | 9.81 | 12.99 | 7.99 |
| Small VLDL | 55.50 | 18.36 | 51.84 | 17.22 |
| LDL particle concentration (nmol/l) | ||||
| Total LDL | 497.94 | 128.83 | 486.91 | 114.98 |
| Large LDL | 234.93 | 58.91 | 236.33 | 55.10 |
| Medium LDL | 123.80 | 33.92 | 116.82 | 29.50 |
| Small LDL | 139.21 | 38.19 | 133.76 | 32.93 |
| HDL particle concentration (μmol/l) | ||||
| Total HDL | 7.98 | 0.92 | 9.31 | 1.35 |
| Large HDL | 1.22 | 0.52 | 2.06 | 0.77 |
| Medium HDL | 2.02 | 0.33 | 2.43 | 0.47 |
| Small HDL | 4.74 | 0.41 | 4.82 | 0.54 |
| Lipoprotein particle size (nm) | ||||
| VLDL diameter | 37.09 | 1.50 | 36.45 | 1.46 |
| LDL diameter | 23.52 | 0.17 | 23.60 | 0.17 |
| HDL diameter | 9.82 | 0.21 | 10.10 | 0.24 |
3.2. Phenotypic association between fatty acids and lipid/lipoprotein profile
Pearson’s correlations between serum FAs and lipoprotein profile are shown in Figs. 1 and 2 and Supplemental Fig. 1. Since some correlations showed a significant interaction with sex, results are presented separately for men and women. The strongest associations, the majority of them ranging −0.4 to −0.6, were obtained for n-6 PUFAs (total and LA) with total triglyceride, extremely large VLDL triglyceride and total VLDL triglyceride concentrations, as well as with the number of VLDL particles and diameter (Fig. 1). Some weaker, but still important correlations (±0.2–0.3) were observed with HDL related metabolites; however, they significantly differed between sexes, with more negative associations in women than in men. The correlations between n-3 PUFAs (total and DHA) and lipid profile were weaker than ±0.3 in all cases (Fig. 2). An interaction with sex was detected for the association of total n-3 PUFAs with total triglyceride, total VLDL triglyceride and VLDL particle concentrations, in such a way that these pairs of traits showed inverse correlations in women, but were non-significant in men. The n-6/n-3 ratio presented negative correlations (~ −0.2) with triglycerides and VLDL subclasses (Supplemental Fig. 1), and very low or non-significant associations with the rest of the variables.
Fig. 1.

Phenotypic correlations between n-6 PUFAs and lipid/lipoprotein profile in men (◆) and women (◇). P value for sex interaction: *P < 0.05; **P < 0.01; ***P < 0.001. C, cholesterol; FAs, fatty acids; TG, triglycerides.
Fig. 2.
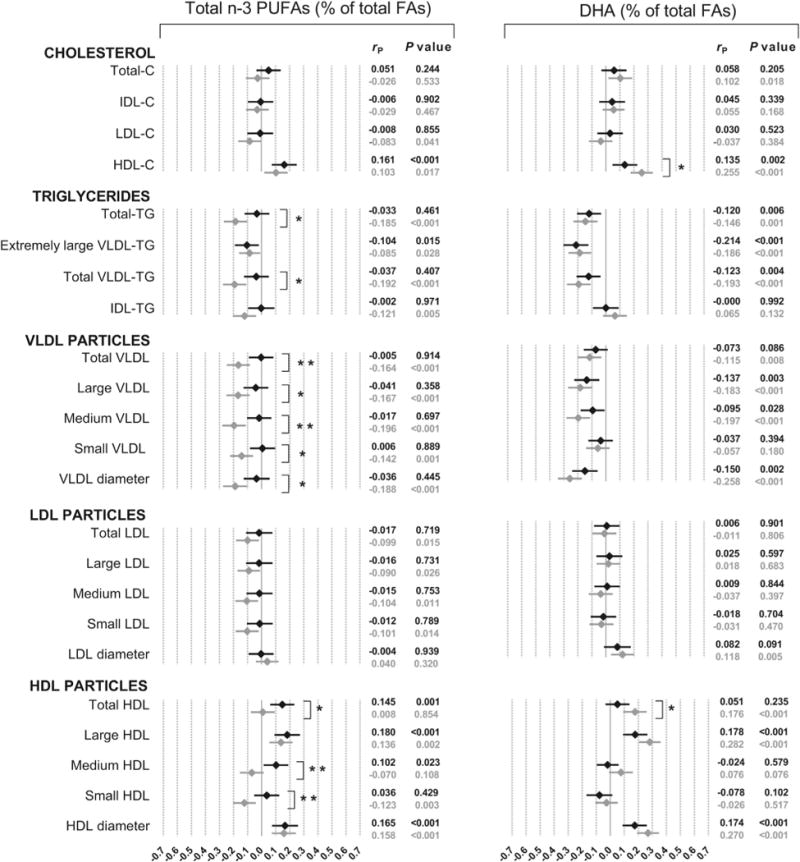
Phenotypic correlations between n-3 PUFAs and lipid/lipoprotein profile in men (◆) and women (◇). P value for sex interaction: *P < 0.05; **P < 0.01; ***P < 0.001. C, cholesterol; FAs, fatty acids; TG, triglycerides.
Phenotypic correlations for MUFAs and SFAs are presented in Fig. 3. MUFAs were highly and positively correlated with triglycerides and VLDL particle concentration (0.4–0.6). Although slightly lower, MUFAs showed substantial negative correlations with HDL-C, large HDL and HDL diameter. As for PUFAs, the weakest associations were obtained with total cholesterol, IDL-C, LDL-C and LDL particle concentrations. Finally, SFAs showed sex differences for several associations, which were in all cases more positive for women. The greatest correlations were observed with HDL-C, extremely large VLDL triglycerides and HDL particles, with maximum values of ~0.3 for females. Adjustment for BMI did not diminish these associations, showing that the differences observed between men and women were not explained by differences in BMI (data not shown).
Fig. 3.
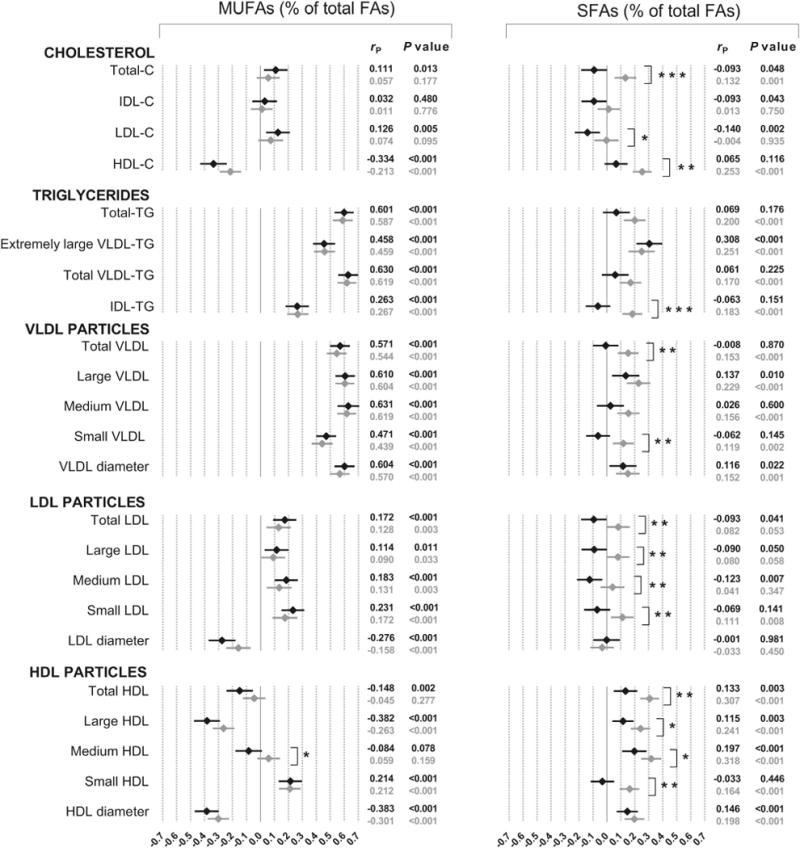
Phenotypic correlations of MUFAs and SFAs with lipid/lipoprotein profile in men (◆) and women (◇). P value for sex interaction: *P < 0.05; **P < 0.01; ***P < 0.001. C, cholesterol; FAs, fatty acids; TG, triglycerides.
3.3. Common genetic and environmental factors between fatty acids and lipid/lipoprotein profile
Results from the Cholesky decomposition parameterization are presented in Tables 2 and 3. As none of the included associations showed significant sex differences (Figs. 1 and 3), bivariate Cholesky models were carried out for men and women combined. This decision was also based on the univariate genetic analyses, in which parameter estimates could be set equal between the sexes (results not shown but are available from the corresponding author).
Table 2.
Results from the bivariate Cholesky decomposition model between n-6 PUFAs and lipid/lipoprotein profile for both sexes combined.
| PUFAs | rP (CI) | rA (CI) | rE (CI) | (CI) | (CI) | |
|---|---|---|---|---|---|---|
| Total triglycerides | Total n-6 | −0.59(−0.64,−0.53) | −0.52(−0.61,−0.42) | −0.64(−0.71,−0.57) | 0.51(0.39,0.62) | 0.49(0.38,0.61) |
| Linoleic acid | −0.50(−0.56,−0.45) | −0.42(−0.51,−0.30) | −0.59(−0.66,−0.50) | 0.49(0.35,0.61) | 0.51(0.39,0.65) | |
| Extremely large VLDL triglycerides | Total n-6 | −0.57(−0.63,−0.51) | −0.56(−0.65,−0.45) | −0.58(−0.65,−0.49) | 0.52(0.40,0.63) | 0.48(0.37,0.60) |
| Linoleic acid | −0.45(−0.51,−0.39) | −0.41(−0.52,−0.28) | −0.49(−0.58,−0.40) | 0.49(0.34,0.62) | 0.51(0.38,0.66) | |
| Total VLDL triglycerides | Total n-6 | −0.60(−0.66,−0.55) | −0.55(−0.63,−0.44) | −0.65(−0.71,−0.57) | 0.51(0.39,0.62) | 0.49(0.38,0.61) |
| Linoleic acid | −0.50(−0.55,−0.44) | −0.40(−0.51,−0.29) | −0.59(−0.66,−0.50) | 0.47(0.33,0.59) | 0.53(0.41,0.67) | |
| Total VLDL | Total n-6 | −0.52(−0.58,−0.47) | −0.47(−0.56,−0.36) | −0.57(−0.65,−0.49) | 0.53(0.40,0.64) | 0.47(0.36,0.60) |
| Linoleic acid | −0.44(−0.49,−0.39) | −0.36(−0.47,−0.25) | −0.52(−0.61,−0.43) | 0.50(0.34,0.62) | 0.50(0.38,0.66) | |
| Large VLDL | Total n-6 | −0.63(−0.69,−0.57) | −0.59(−0.68,−0.49) | −0.65(−0.71,−0.57) | 0.51(0.39,0.61) | 0.49(0.39,0.61) |
| Linoleic acid | −0.52(−0.58,−0.45) | −0.44(−0.54,−0.32) | −0.58(−0.65,−0.49) | 0.47(0.33,0.59) | 0.53(0.41,0.67) | |
| Medium VLDL | Total n-6 | −0.58(−0.64,−0.53) | −0.36(−0.47,−0.24) | −0.58(−0.66,−0.49) | 0.44(0.29,0.57) | 0.56(0.43,0.71) |
| Linoleic acid | −0.48(−0.54,−0.42) | −0.39(−0.49,−0.27) | −0.57(−0.65,−0.48) | 0.47(0.32,0.59) | 0.53(0.41,0.68) | |
| Small VLDL | Total n-6 | −0.41(−0.46,−0.36) | −0.38(−0.49,−0.26) | −0.43(−0.53,−0.33) | 0.56(0.39,0.69) | 0.44(0.31,0.61) |
| Linoleic acid | −0.35(−0.40,−0.30) | −0.30(−0.42,−0.18) | −0.39(−0.49,−0.28) | 0.53(0.35,0.69) | 0.47(0.31,0.65) | |
| VLDL diameter | Total n-6 | −0.59(−0.64,−0.53) | −0.55(−0.64,−0.44) | −0.61(−0.68,−0.54) | 0.49(0.37,0.60) | 0.51(0.40,0.63) |
| Linoleic acid | −0.48(−0.54,−0.42) | −0.39(−0.50,−0.26) | −0.56(−0.64,−0.47) | 0.44(0.29,0.57) | 0.56(0.43,0.71) | |
| Principal component | Total n-6 | −0.61(−0.67, −0.55) | −0.56(−0.64,−0.46) | −0.66(−0.72,−0.58) | 0.52(0.40,0.62) | 0.48(0.38,0.60) |
| Linoleic acid | −0.51(−0.56,−0.45) | −0.42(−0.52, 0.30) | −0.59(−0.67,−0.51) | 0.48(0.34,0.60) | 0.52(0.40,0.66) |
Phenotypic correlations (rP) were estimated using Pearson’s correlation method. Genetic (rA) and environmental (rE) correlations between PUFAs and each of the analyzed lipids and lipoproteins were obtained from the bivariate Cholesky model, which decomposes phenotypic covariation into uncorrelated genetic and environmental factors. This model additionally estimates the proportion of covariance between pairs of traits explained by the genetic component or bivariate heritability , and that explained by the unique environmental component . CI, 95% confidence interval.
Table 3.
Results from the bivariate Cholesky decomposition model between MUFAs and lipid/lipoprotein profile for both sexes combined.
| rP (CI) | rA (CI) | rE (CI) | (CI) | (CI) | |
|---|---|---|---|---|---|
| HDL cholesterol | −0.26(−0.32,−0.20) | −0.36(−0.47,−0.24) | −0.15(−0.27,−0.03) | 0.80(0.61,0.97) | 0.20(0.03,0.39) |
| Total triglycerides | 0.60(0.55,0.65) | 0.65(0.56,0.72) | 0.57(0.48,0.64) | 0.61(0.49,0.70) | 0.39(0.30,0.51) |
| Extremely large VLDL triglycerides | 0.46(0.40,0.51) | 0.51(0.39,0.62) | 0.40(0.30,0.50) | 0.60(0.45,0.72) | 0.40(0.28,0.55) |
| Total VLDL triglycerides | 0.63(0.58,0.68) | 0.69(0.60,0.76) | 0.58(0.49,0.65) | 0.61(0.50,0.70) | 0.39(0.30,0.50) |
| Total VLDL | 0.56(0.51,0.61) | 0.62(0.52,0.70) | 0.52(0.42,0.60) | 0.63(0.51,0.73) | 0.37(0.27,0.49) |
| Large VLDL | 0.61(0.56,0.66) | 0.67(0.58,0.75) | 0.55(0.46,0.63) | 0.59(0.47,0.69) | 0.41(0.31,0.53) |
| Medium VLDL | 0.63(0.58,0.68) | 0.70(0.62,0.78) | 0.56(0.47,0.64) | 0.62(0.51,0.71) | 0.38(0.29,0.49) |
| Small VLDL | 0.46(0.41,0.51) | 0.52(0.42,0.61) | 0.40(0.29,0.50) | 0.66(0.53,0.77) | 0.34(0.23,0.47) |
| VLDL diameter | 0.59(0.54,0.64) | 0.66(0.56,0.74) | 0.54(0.45,0.62) | 0.58(0.46,0.68) | 0.42(0.32,0.54) |
| Large HDL | −0.30(−0.36,−0.24) | −0.40(−0.51,−0.29) | −0.19(−0.31,−0.06) | 0.80(0.65,0.93) | 0.20(0.07,0.36) |
| HDL diameter | −0.34(−0.40,−0.28) | −0.45(−0.55,−0.34) | −0.21(−0.33,−0.09) | 0.81(0.67,0.92) | 0.19(0.08,0.33) |
Phenotypic correlations (rP) were estimated using Pearson’s correlation method. Genetic (rA) and environmental (rE) correlations between MUFAs and each of the analyzed lipids and lipoproteins were obtained from the bivariate Cholesky model, which decomposes phenotypic covariation into uncorrelated genetic and environmental factors. This model additionally estimates the proportion of covariance between pairs of traits explained by the genetic component or bivariate heritability , and that explained by the unique environmental component . CI, 95% confidence interval.
Total n-6 PUFAs and LA showed a similar pattern of genetic (−0.30 to −0.59) and environmental (−0.39 to −0.65) correlations with triglyceride and VLDL particle concentrations (Table 2). Bivariate heritability showed that overall half of the phenotypic covariation between the pairs was due to additive genetic factors (44%–56%), which was corroborated by the first principal component derived from these variables. Table 3 presents the results for MUFAs. Despite weaker than ±0.3 in women, correlations with HDL-C and large HDL were included in the quantitative genetic analysis. The relative concentration of MUFAs showed moderate genetic correlations (−0.36 to their −0.45) with HDL related metabolites and covariation was largely explained by common genetic factors (80%). Finally, MUFAs presented positive genetic (0.51–0.69) and environmental (0.40–0.58) correlations with triglycerides and VLDL subclasses, with ranging from 58% to 66%.
4. Discussion
In this cross-sectional population-based twin study, we report detailed information on the relationship of serum fatty acids with lipids and lipoprotein subclasses in healthy young adults consuming their habitual diet, as well as on the genetic and environmental factors explaining these associations.
Serum PUFA concentrations reflect the dietary lipid composition from the past few days to several weeks [24] and thus can be used as biomarkers of dietary intake of PUFAs. Since LA is an essential fatty acid that accounts for 85%–90% of the dietary total n-6 PUFAs [3], their similar pattern of association with the lipid profile was expected. Overall, we show that serum n-6 PUFAs are strongly related to a favorable serum lipoprotein subclass profile in terms of cardiovascular risk. The high negative correlation obtained between n-6 PUFAs (relative proportion to total FAs) and total triglyceride concentration in our study (−0.59) was very similar to that observed in an Italian sample (−0.58) [10], and multiple regression models from the post-World War II birth cohort (ERA-JUMP) study including 3 populations (US white, Japanese American and Japanese) of middle aged men also showed an inverse relationship [11]; however, n-6 PUFAs (relative to total PUFAs) were not associated with triglycerides in the Chinese population [12]. Explained by the fact that the majority of triglyceride molecules are carried out in VLDL particles, n-6 PUFAs were strongly correlated with VLDL subclasses. In agreement with an investigation based on 4 male populations (US white, Japanese American and Japanese and Korean), the strongest association was reported with large VLDL [13]. Regarding LDL variables, the non-significant relationship between n-6 PUFAs and LDL-C was also observed in Chinese residents [12] and in whites and Japanese Americans [11], but this association was significant and positive in the Japanese [11] and Italian population [10]. The greatest negative association in the present study was observed between serum n-6 PUFAs and concentrations of small LDL particles. Small LDL particles are considered the most atherogenic subclass of LDL, with elevated levels in patients with metabolic syndrome and subclinical atherosclerosis [25,26]. Finally, our findings agree with other studies in determining a positive relationship of serum n-6 PUFAs with HDL-C [10,11], large HDL and HDL diameter, but negative with medium and small HDL [13].
To our knowledge, the present study is the first to analyze genetic and environmental factors between fatty acids and lipoprotein profile. Family and twin studies demonstrating substantial genetic correlation between traits are important because they permit greater targeting of genetic analyses to relevant associations. In addition to the relatively high genetic correlations, we showed that around half (44%–56%) of the phenotypic association of n-6 PUFAs with triglyceride and VLDL subclass concentrations is explained by genetic factors, and that the remaining covariance is explained by shared unique environmental factors.
Although marine-derived n-3 PUFAs can reduce triglyceride concentrations, clinical benefits seem to be significant only at relatively high dose likely not to be provided by diet alone [4,27]. Dietary intake of n-3 PUFAs and a dietary pattern high in fish have, however, been related to lower concentrations of large VLDL and a reduced VLDL diameter in observational studies [8,9]. DHA can be either obtained from the diet or synthesized endogenously from alpha-linolenic acid, but since the conversion of alpha-linolenic acid to EPA is very limited (<8%) and to DHA marginal (<4%), serum concentrations of DHA can be considered as an objective biomarker of habitual fish consumption [28]. An inverse association of serum concentrations of n-3 PUFAs and DHA with triglycerides but positive with HDL-C was also observed in other populations [10–12]. We found significant inverse associations of DHA with large and medium VLDL but not with small VLDL, and positive associations with large HDL, which is in agreement with the findings of Choo et al. [13]. Our findings show that correlations with triglyceride and VLDL particle concentrations were substantially lower for n-3 PUFAs than for n-6 PUFAs. Interestingly, a recent study found that serum levels of n-6 PUFAs, but not n-3 PUFAs, were inversely related to the incidence of metabolic syndrome [29]. However, it has to be kept in mind that circulating concentrations of n-6 and n-3 PUFAs reflect both dietary intake and relevant biologic processes (e.g., elongation) and thus, validation in other populations would be required and any hypothetical benefit examined in large scale intervention studies before any dietary recommendations could be considered. Regarding the n-6/n-3 PUFAs ratio, the negative sense observed for all significant associations (except for LDL diameter in men) showed no evidence of beneficial effects of decreased n-6 PUFAs relative to n-3 PUFAs, and thus supports the idea that PUFAs alone and not their n-6/n-3 ratio should be first considered [30–32].
In the present study, higher MUFA concentrations were related to greater concentrations of triglycerides, VLDL subclasses and small HDL, and lower of HDL-C, large HDL and a smaller average HDL diameter, which agree with the findings of a previous study in middle aged men [13]. We additionally showed that more than half of the phenotypic association between MUFAs and these lipids/lipoproteins are explained by common genetic factors, being especially important for HDL related variables. Concerning SFAs, and in agreement with the results for the white population [13], we observed that the most significant associations in men were with large VLDL, VLDL diameter, and medium HDL. As suggested by Choo et al. [13], one possible explanation of the contrasted results obtained for serum MUFAs and SFAs, in comparison to those generally observed for diet, is that both are endogenously produced in the human body and thus, are unlikely to serve as biomarkers of dietary intakes. In fact, MUFAs and SFAs content of the diet have shown to correlate weaker than PUFAs with the fatty acid composition in serum [24], but other explanations are also possible. Finally, since the differences between men and women observed for some of the fatty acids-lipoproteins pairs were not explained by BMI in our study, mechanistic studies are needed to identify the biological mechanism underlying these sex differences.
Our study has some limitations. It was restricted to healthy young adults and thus, the results cannot be generalized to the entire population. We did not incorporate dietary intake in the present analysis; however, macronutrient intake and dietary patterns in relation to serum lipoproteins were analyzed in a subsample of this population and correlations were generally weak (r = −0.17–0.22) [8] as compared to those with serum FAs in the present analysis. Moreover, the use of serum PUFAs as biomarkers of dietary intake and nutritional status can be seen as a strength of this study, as they can capture dietary intake more objectively than self-reported measures, which are prone to misreporting [33]. Another limitation is that 43% of the study population was not fasting, but had a light breakfast, of which the exact composition is not known. Further, the cross-sectional nature of the study does not allow to establish the directionality of the reported associations. Finally, multiple testing can lead to potentially false positive associations among the correlations evaluated. Although the Bonferroni correction based on principal components showed a more permissive threshold (p < 0.01) than the traditional correction, it should be noted that many related lipids and lipoproteins, though not achieving p < 0.05, showed similar associations and thus support the overall conclusions. But our study has several strengths, as well. First, it is unique in that the sample includes twins and thus allows us to analyze the genetic and environmental architecture underlying the associations between serum fatty acids and lipoprotein profile. Other strengths include the relatively large sample size including both men and women, and the use of NMR technology to measure concentrations of lipoproteins.
In conclusion, higher concentrations of serum n-6 PUFAs and lower of MUFAs are strongly associated with lower triglyceride and VLDL particle concentrations. More importantly, we showed that common genetic factors are substantially relevant in determining the phenotypic covariationofn-6 PUFAs and MUFAs with triglycerides and VLDL particles, and that these genetic factors are highly correlated within pairs of traits, which have important implications for the search of pleiotropic genes. It may also have implications for interventions designed to affect concentrations of n-6-PUFAs, which would not be expected to affect fully lipid concentrations because of the underlying genetics.
Supplementary Material
Acknowledgments
Funding
This work was supported by a Postdoctoral fellowship from the Basque Government’s Department of Education, Universities and Research (DEUI) to AJ, and also by Doctoral Programs of Public Health and grants from the Juho Vainio, Yrjö Jahnsson, Jenny and Antti Wihuri and Biomedicum Helsinki Foundations. Data collection in FinnTwin12 and FinnTwin16 was supported by National Institute of Alcohol Abuse and Alcoholism (grant numbers AA08315, AA12502, AA00145, and AA09203 to RJR) and the Academy of Finland (grant numbers 205585 and 141054 to JK and 266592 to KS). This work was also supported by the Academy of Finland (grant number 137870 to PS), the Sigrid Jusélius Foundation (MAK), the Finnish Funding Agency for Technology – TEKES (MAK) and the Strategic Research Funding from the University of Oulu (MAK).
Appendix A. Supplementary material
Supplementary material related to this article can be found at http://dx.doi.org/10.1016/j.atherosclerosis.2013.12.053.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Sharrett AR, Ballantyne CM, Coady SA, et al. Atherosclerosis Risk in Communities Study Group Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–13. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 2.Breuer HW. Hypertriglyceridemia: a review of clinical relevance and treatment options: focus on cerivastatin. Curr Med Res Opin. 2001;17:60–73. [PubMed] [Google Scholar]
- 3.Harris WS, Mozaffarian D, Rimm E, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–7. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 4.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Ala-Korpela M. Critical evaluation of 1H NMR metabonomics of serum as a methodology for disease risk assessment and diagnostics. Clin Chem Lab Med. 2008;46:27–42. doi: 10.1515/CCLM.2008.006. [DOI] [PubMed] [Google Scholar]
- 6.Ala-Korpela M, Soininen P, Savolainen MJ. Letter by Ala-Korpela et al regarding article, “Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women”. Circulation. 2009;120:e149. doi: 10.1161/CIRCULATIONAHA.109.864124. author reply e150. [DOI] [PubMed] [Google Scholar]
- 7.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–70. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Bogl LH, Pietilainen KH, Rissanen A, et al. Association between habitual dietary intake and lipoprotein subclass profile in healthy young adults. Nutr Metab Cardiovasc Dis. 2013 doi: 10.1016/j.numecd.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Annuzzi G, Rivellese AA, Wang H, et al. Lipoprotein subfractions and dietary intake of n-3 fatty acid: the Genetics of Coronary Artery Disease in Alaska Natives study. Am J Clin Nutr. 2012;95:1315–22. doi: 10.3945/ajcn.111.023887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–46. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 11.Motoyama KR, Curb JD, Kadowaki T, et al. Association of serum n-6 and n-3 polyunsaturated fatty acids with lipids in 3 populations of middle-aged men. Am J Clin Nutr. 2009;90:49–55. doi: 10.3945/ajcn.2008.26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SP, Chen YH, Li H. Association between the levels of polyunsaturated fatty acids and blood lipids in healthy individuals. Exp Ther Med. 2012;4:1107–11. doi: 10.3892/etm.2012.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choo J, Ueshima H, Curb JD, et al. Serum n-6 fatty acids and lipoprotein subclasses in middle-aged men: the population-based cross-sectional ERA-JUMP study. Am J Clin Nutr. 2010;91:1195–203. doi: 10.3945/ajcn.2009.28500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kettunen J, Tukiainen T, Sarin AP, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet. 2012;44:269–76. doi: 10.1038/ng.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaprio J. Twin studies in Finland 2006. Twin Res Hum Genet. 2006;9:772–7. doi: 10.1375/183242706779462778. [DOI] [PubMed] [Google Scholar]
- 16.Soininen P, Kangas AJ, Wurtz P, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst. 2009;134:1781–5. doi: 10.1039/b910205a. [DOI] [PubMed] [Google Scholar]
- 17.Tukiainen T, Kettunen J, Kangas AJ, et al. Detailed metabolic and genetic characterization reveals new associations for 30 known lipid loci. Hum Mol Genet. 2012;21:1444–55. doi: 10.1093/hmg/ddr581. [DOI] [PubMed] [Google Scholar]
- 18.Inouye M, Kettunen J, Soininen P, et al. Metabonomic, transcriptomic, and genomic variation of a population cohort. Mol Syst Biol. 2010;6:441. doi: 10.1038/msb.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers JC, Zhang W, Sehmi J, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet. 2011;43:1131–8. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tukiainen T, Tynkkynen T, Makinen VP, et al. A multi-metabolite analysis of serum by 1H NMR spectroscopy: early systemic signs of Alzheimer’s disease. Biochem Biophys Res Commun. 2008;375:356–61. doi: 10.1016/j.bbrc.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Rao JNK, Scott AJ. On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat. 1984;12:46–60. [Google Scholar]
- 22.Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht: Kluwer; 2003. [Google Scholar]
- 23.Neale MC. Mx: statistical modeling. Richmond, VA: Department of Psychiatry, University of Virginia; 2003. [Google Scholar]
- 24.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–80. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Wurtz P, Raiko JR, Magnussen CG, et al. High-throughput quantification of circulating metabolites improves prediction of subclinical atherosclerosis. Eur Heart J. 2012;33:2307–16. doi: 10.1093/eurheartj/ehs020. [DOI] [PubMed] [Google Scholar]
- 26.Rivellese AA, Patti L, Kaufman D, et al. Lipoprotein particle distribution and size, insulin resistance, and metabolic syndrome in Alaska Eskimos: the GOCADAN study. Atherosclerosis. 2008;200:350–8. doi: 10.1016/j.atherosclerosis.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose–response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93:243–52. doi: 10.3945/ajcn.110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emken EA. Metabolism of dietary stearic acid relative to other fatty acids in human subjects. Am J Clin Nutr. 1994;60:1023S–8S. doi: 10.1093/ajcn/60.6.1023S. [DOI] [PubMed] [Google Scholar]
- 29.Vanhala M, Saltevo J, Soininen P, et al. Serum omega-6 polyunsaturated fatty acids and the metabolic syndrome: a longitudinal population-based cohort study. Am J Epidemiol. 2012;176:253–60. doi: 10.1093/aje/kwr504. [DOI] [PubMed] [Google Scholar]
- 30.Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615. doi: 10.1146/annurev.nutr.24.012003.132106. [DOI] [PubMed] [Google Scholar]
- 31.Griffin MD, Sanders TA, Davies IG, et al. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45–70 y: the OPTILIP Study. Am J Clin Nutr. 2006;84:1290–8. doi: 10.1093/ajcn/84.6.1290. [DOI] [PubMed] [Google Scholar]
- 32.Harris WS. The omega-6/omega-3 ratio and cardiovascular disease risk: uses and abuses. Curr Atheroscler Rep. 2006;8:453–9. doi: 10.1007/s11883-006-0019-7. [DOI] [PubMed] [Google Scholar]
- 33.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133(Suppl 3):895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


