Abstract
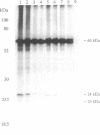
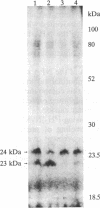
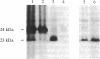
1-Naphthylphthalamic acid (NPA) is a specific inhibitor of polar auxin transport that blocks carrier-mediated auxin efflux from plant cells. To allow identification of the NPA receptor thought to be part of the auxin efflux carrier, we have synthesized a tritiated, photolabile NPA analogue, 5'-azido-[3,6-3H2]NPA ([3H2]N3NPA). This analogue was used to identify NPA-binding proteins in fractions highly enriched for plasma membrane vesicles isolated from maize coleoptiles (Zea mays L.). Competition studies showed that binding of [3H2]N3NPA to maize plasma membrane vesicles was blocked by nonradioactive NPA but not by benzoic acid. After incubation of plasma membrane vesicles with [3H2]N3NPA and exposure to UV light, we observed specific photoaffinity labeling of a protein with an apparent molecular mass of 23 kDa. Pretreatment of the plasma membrane vesicles with indole-3-acetic acid or with the auxin-transport inhibitors NPA and 2,3,5-triiodobenzoic acid strongly reduced specific labeling of this protein. This 23-kDa protein was also labeled by addition of 5-azido-[7-3H]indole-3-acetic acid to plasma membranes prior to exposure to UV light. The 23-kDa protein was solubilized from plasma membranes by 1% Triton X-100. The possibility that this 23-kDa polypeptide is part of the auxin efflux carrier system is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Feldwisch J., Zettl R., Hesse F., Schell J., Palme K. An auxin-binding protein is localized to the plasma membrane of maize coleoptile cells: identification by photoaffinity labeling and purification of a 23-kda polypeptide. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):475–479. doi: 10.1073/pnas.89.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H., Peters W., Palme K. The electrical response of maize to auxins. Biochim Biophys Acta. 1991 May 7;1064(2):199–204. doi: 10.1016/0005-2736(91)90302-o. [DOI] [PubMed] [Google Scholar]
- Hesse T., Feldwisch J., Balshüsemann D., Bauw G., Puype M., Vandekerckhove J., Löbler M., Klämbt D., Schell J., Palme K. Molecular cloning and structural analysis of a gene from Zea mays (L.) coding for a putative receptor for the plant hormone auxin. EMBO J. 1989 Sep;8(9):2453–2461. doi: 10.1002/j.1460-2075.1989.tb08380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks G. R., Rayle D. L., Jones A. M., Lomax T. L. Specific photoaffinity labeling of two plasma membrane polypeptides with an azido auxin. Proc Natl Acad Sci U S A. 1989 Jul;86:4948–4952. doi: 10.1073/pnas.86.13.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks G. R., Rayle D. L., Lomax T. L. The diageotropica mutant of tomato lacks high specific activity auxin binding sites. Science. 1989 Jul 7;245:52–54. doi: 10.1126/science.245.4913.52. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. Naturally occurring auxin transport regulators. Science. 1988 Jul 15;241(4863):346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Jones A. M., Melhado L. L., Ho T. H., Pearce C. J., Leonard N. J. Azido auxins : photoaffinity labeling of auxin-binding proteins in maize coleoptile with tritiated 5-azidoindole-3-acetic Acid. Plant Physiol. 1984 Aug;75(4):1111–1116. doi: 10.1104/pp.75.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. M., Venis M. A. Photoaffinity labeling of indole-3-acetic acid-binding proteins in maize. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6153–6156. doi: 10.1073/pnas.86.16.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhado L. L., Jones A. M., Leonard N. J., Vanderhoef L. N. Azido auxins: synthesis and biological activity of fluorescent photoaffinity labeling agents. Plant Physiol. 1981 Aug;68(2):469–475. doi: 10.1104/pp.68.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinard T., Jacobsen H. J. An inexpensive small volume equilibrium dialysis system for protein-ligand binding assays. Anal Biochem. 1989 Jan;176(1):157–160. doi: 10.1016/0003-2697(89)90286-8. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Fluorographic detection of radioactivity in polyacrylamide gels with 2,5-diphenyloxazole in acetic acid and its comparison with existing procedures. Biochem J. 1983 Jan 1;209(1):281–284. doi: 10.1042/bj2090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. R., Gardner G. Solubilization of the receptor for N-1-naphthylphthalamic Acid. Plant Physiol. 1980 Dec;66(6):1074–1078. doi: 10.1104/pp.66.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voet J. G., Howley K. S., Shumsky J. S. 5'-Azido-N-1-Napthylphthalamic Acid, a Photolabile Analog of N-1-Naphthylphthalamic Acid : Synthesis and Binding Properties in Curcurbita pepo L. Plant Physiol. 1987 Sep;85(1):22–25. doi: 10.1104/pp.85.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]