Abstract
Important interactions between female reproduction and autoimmunity are suggested by the female-predominance of systemic lupus erythematosus (SLE) and other autoimmune diseases and the amelioration of certain autoimmune diseases during pregnancy. Sexually dimorphic risk of developing SLE involves modulation of genetic risk by environmental factors, sex hormones and non-hormonal factors encoded on the sex chromosomes. In some lupus models, estrogen, via estrogen receptor alpha (ER-α), enhances production of highly pathogenic IgG2a/c autoantibodies (autoAbs). Some studies indicate that treatment with progesterone, a chief female reproductive steroid, can suppress IgG2a/2c autoAb production. Little is known about how endogenous progesterone impacts lupus autoimmunity. To investigate this, we introduced a disruptive progesterone receptor (PR) gene mutation into lupus-prone mice and tracked the development of spontaneous IgG autoAbs. Here, we present evidence that PR can suppress the emergence of class-switched IgG2c autoAbs, suggesting that PR and ER-α counter-regulate a critical step in lupus autoimmunity. PR's control of IgG2c autoAb production correlates with alterations in the relative abundance of splenic T follicular helper (TFH) cells and non-TFH CD4+ T cells, especially regulatory T cells (TREGS). Surprisingly, PR also appears to help maintain sexually dimorphic abundance of splenic leukocytes, a feature common to many mouse models of SLE. Together our results identify a novel molecular link between female reproduction and lupus autoimmunity. Further investigation into the immunomodulatory functions of PR promises to inform reproductive health care in women and offer mechanistic insight into important immunologic phenomena of pregnancy.
Introduction
At least 9 in 10 people with SLE are female. Sexual dimorphism in risk of developing SLE remains incompletely explained but likely involves complex interplay between genetic risk, environmental triggers, pregnancy-specific factors, sex hormones and non-hormonal factors encoded on the X and Y chromosomes (1-4). Results from large cohort studies indicate that exposure to estrogen in the form of birth control or hormone replacement therapy increases a woman's risk of developing SLE (5, 6). Limited evidence suggests that exposure to progesterone could be protective in this regard (7). Female-predominant disease is recapitulated in several mouse models of SLE. For example, female NZB × New Zealand White (NZW) F1 (NZB/W) mice develop higher levels of IgG autoantibodies (autoAbs) and more frequent glomerulonephritis (GN) when compared to age-matched male controls (8, 9). These differences appear to involve disease-enhancing effects of estrogen in females and protective actions of gonadal testosterone in males (10-12). Important estrogen effects are mediated by estrogen receptor alpha (ER-α) (13, 14), a nuclear receptor critically involved in female reproductive physiology. Estrogen enhances lupus autoimmunity through several immunologic nodes including activation of type 1 and type 2 interferon (IFN), increased T helper type 1 (TH1) cell differentiation, enhanced survival of autoreactive B cell clones and their production of class-switched IgG autoAbs, particularly those of the pathogenic IgG2a/2c subclass (reviewed in references (4, 15, 16)). The mechanisms by which testosterone and other male factors suppresses lupus autoimmunity remain poorly understood.
There is a similar lack of knowledge regarding progesterone, a chief female reproductive steroid with immunomodulatory properties distinct from those of estrogen and testosterone (17). Early studies by Roubinian et al. using the NZB/W model showed that treatment of castrated female mice with progesterone resulted in modest increases in both mortality and production of anti-DNA Abs; in castrated male mice, the same treatment decreased mortality, despite increasing anti-DNA Abs (10). Two subsequent studies examined the effects of chronic medroxyprogesterone acetate (MPA, a synthetic form of progesterone used for contraception) in gonadally intact female NZB/W mice. In one study, treatment with MPA resulted in decreased serum IgG autoAb levels, GN and death (18). In the other study, however, MPA had little effect on these parameters (19). Together, these results suggest that the effects of progesterone on lupus autoimmunity are complex and depend on hormone dose, its timing, and interactions with other gonadal factors.
An additional layer of complexity arises from the fact that progesterone can signal through at least three different receptor types: PR, the glucocorticoid receptor (GR) and membrane progesterone receptors (mPRs) (20-22). PR, GR and ER-α are ligand-activated transcription factors belonging to the nuclear receptor (NR) family of proteins. At low physiologic concentrations, progesterone can bind and activate PR and mPRs (22). At high physiologic concentrations (e.g., during pregnancy), progesterone can also bind and activate GR (20). Synthetic forms of progesterone used in birth control and hormone replacement therapy vary widely in their binding to PR, GR and mPRs (23). A critical role of PR in reproduction was demonstrated by generation of mice with a disruption in the PR gene mutation (PR−/− mice). Female PR−/− mice have multiple reproductive abnormalities and are infertile (24). Male PR−/− are virile but demonstrate abnormalities in behavior related to reproduction (25). This is not entirely surprising, since outside of pregnancy and the ovulatory cycle male and female rodents (and humans) show similar levels of circulating progesterone (26-28). Moreover, PR can regulate target gene transcription in the absence of ligand (29). Finally, PR, like other NRs, can regulate different sets of genes in different tissues depending on the presence of various NR co-factors (30).
The immunologic functions of PR are poorly understood. Using PR−/− mice, we recently showed that PR specifically suppresses thymus-dependent (TD) IgG antibody (Ab) responses to immunization via effects in antigen (Ag)-specific CD4+ T cells in vivo (28). Also using PR−/− mice, Lee et al. recently showed that progesterone, via PR, supports the development and function of TREGS in vitro (31), potentially linking high progesterone states (e.g., pregnancy) to expansion of TREGS (4), important suppressors of autoimmunity (32). Together, these results suggested that progesterone might also regulate lupus autoimmunity via effects on CD4+ T cells and autoAb production.
To study the role of progesterone in lupus autoimmunity, we introduced the PR−/− mutation into C57Bl/6 (B6) mice homozygous for the NZB autoimmunity locus 2 (Nba2) on distal chromosome 1 (B6.Nba2, hereafter simply Nba2). Female Nba2 mice, like female NZB/W mice, spontaneously develop splenomegaly and IgG anti-nuclear Abs much earlier than age-matched male mice (33, 34), but despite deposition of immune complexes in renal glomeruli do not develop significant glomerulonephritis (GN) -- suggesting that additional genetic factors are required to couple these processes and cause kidney disease (34). In this study, we assessed the effects of PR deficiency on spontaneous development of autoAbs, GN and splenic leukocyte expansion in age-matched female and male Nba2 mice. Our results indicate that in female Nba2 mice, PR suppresses the emergence of class-switched IgG2c autoAbs, an effect that correlates with alterations in the relative abundance of splenic TFH and non-TFH CD4+ T cells. By comparing female and male mice, we also identify an unanticipated role for PR in generating and/or maintaining sexual dimorphism in splenic leukocyte abundance in this model.
Results
PR deficiency increases IgG autoAb production in aged female, but not male, Nba2 mice
Because we had previously observed that PR deficiency in non-autoimmune mice resulted in enhanced IgG Ab responses to TD immunization (28), we hypothesized that we would observe increased IgG, but not IgM, autoAb levels in Nba2.PR−/− mice. B6.PR−/− mice, generated in our lab and described in ref. (28), were crossed Nba2 mice to generate Nba2.PR+/− breeding pairs. Subsequent litters showed Mendelian or near-Mendelian distribution PR−/− mutation among male and female pups (data not shown). We then observed PR+/+ and PR−/− mice from several litters for 10 mo., measuring serum autoAb levels every 2 mo. beginning at age 4 mo. We chose to measure serum anti-chromatin reactivity because of relatively high penetrance of this phenotype among aged female Nba2 mice (34). The assay we used detected Ig with reactivity to chromatin and its individual components, histones and DNA (35). Among the 25 animals observed, only one spontaneous death occurred: a 9-mo-old male PR+/+ mouse that was found dead in its cage from unknown causes.
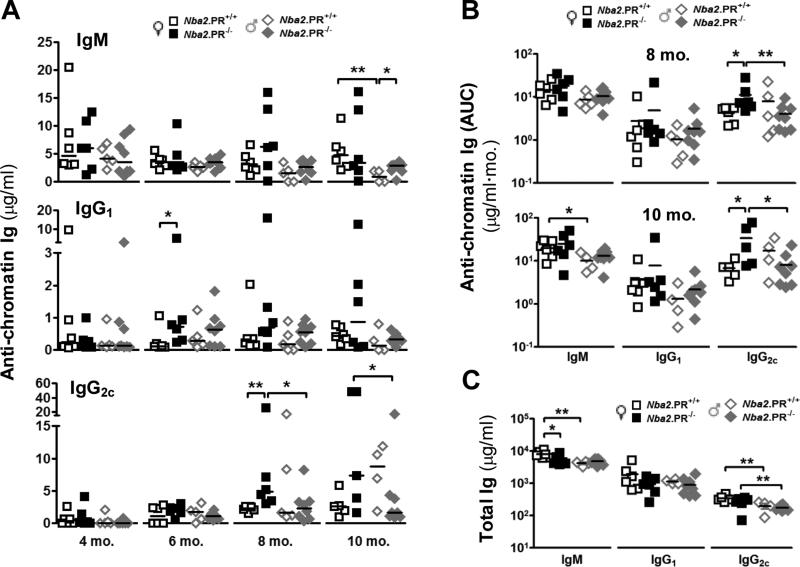
Among female mice, loss of PR did not appreciably affect median serum anti-chromatin IgM levels at any time point, although two PR−/− mice showed high levels at 8 and 10 mo. (Fig. 1A). In contrast, from 6 mo. onward, most PR−/− female mice showed higher IgG1 and IgG2c autoAb levels than the median value for sex-matched controls. The differences in median values were statistically significant at 6 mo. (IgG1) and 8 mo. (IgG2c) (Fig. 1A). To better estimate autoAb production throughout the life of each animal, we calculated cumulative autoAb production by summing all values for each animal up to 6, 8 or 10 mo. and calling this sum area under curve (AUC). PR deficiency had no statistically significant effect on mean IgM or IgG1 autoAb AUC at any time point (Fig. 1B and data not shown). However, PR deficiency increased IgG2c autoAb AUC levels among female mice at 8 and 10 mo. (Fig. 1B). These increases could not be explained by increased total serum IgG2c levels (Fig. 1C). PR deficiency did, however, lead to a slight but statistically significant decrease in mean total serum IgM levels in female mice at 10 mo. Together, these results indicate that PR can suppress or delay the emergence of class-switched IgG2c and IgG1 autoAbs in aged female Nba2 mice. Similar effects of PR on IgG autoAb production were not observed in aged male mice (Figs. 1A – 1C). However, at 10 mo., male PR−/− showed higher median IgM autoAb levels than did sex-matched controls (Fig. 1A).
Figure 1.
PR deficiency leads to increased serum IgG1 and IgG2c autoAb levels in aged female Nba2 mice. (A) Serum anti-chromatin IgM, IgG1 and IgG2c levels in female and male mice at indicated ages were determine by ELISA. Shown are medians (lines) and individual values (symbols). *, p < 0.05, **, p < 0.01 Mann-Whitney test. (B) Cumulative IgM, IgG1 and IgG2c in female and male mice at ages 8 and 10 mo. as calculated by area under curve (AUC, see text). Shown are means (lines) and individual values (symbols). *, p < 0.05, **, p < 0.01 unpaired t test of log-transformed data. (C) Total serum IgM, IgG1 and IgG2c levels at 10 mo. Shown are means (lines) and individual values (symbols). *, p < 0.05, **, p < 0.01 unpaired t test.
Among PR+/+ controls, female mice showed higher anti-chromatin and total IgM than male mice, and these differences were statistically significant at 10 mo. (Figs. 1A – 1C). Similar differences were not observed for either total or anti-chromatin IgG1 and IgG2c.
PR deficiency may impact glomerular IC deposition in aged Nba2 mice
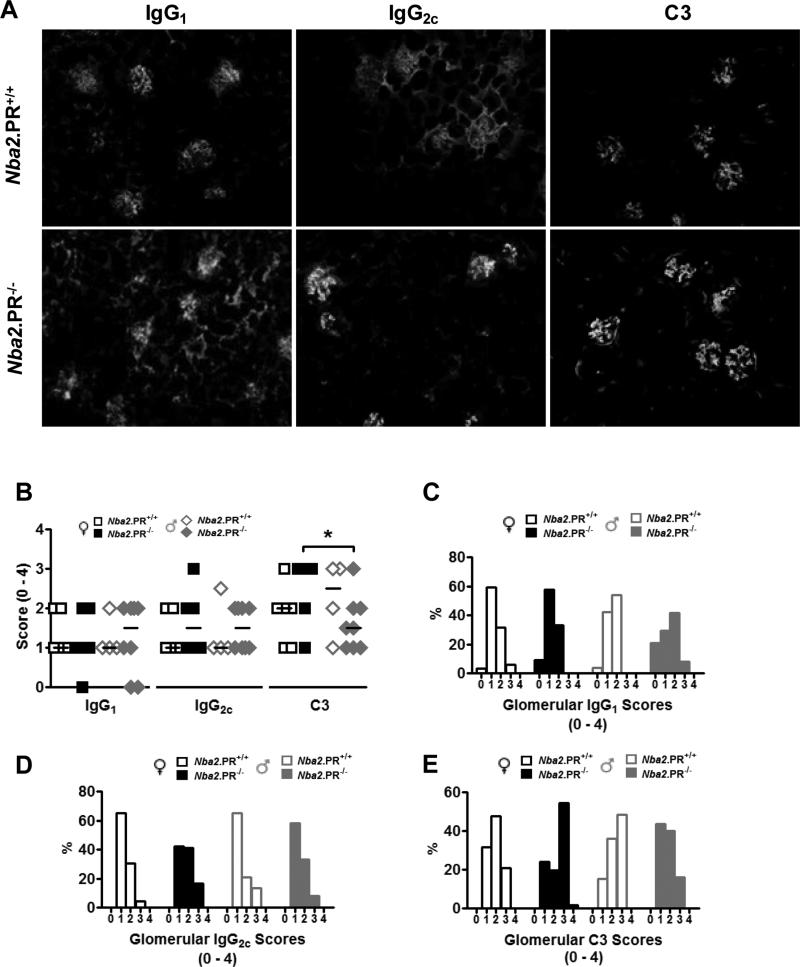
In female NZB/W mice, the Nba2 locus is critical for full expression of anti-chromatin Abs and IC-mediated GN (34, 36). Because PR loss increased levels of circulating IgG autoAbs in female mice (Figs. 1A and 1B), we examined kidneys at 10 mo. for increased IC deposition, as determined by IgG and C3 immunoreactivity in freshly frozen kidney sections (Fig. 2A). Despite clear effects on serum autoAb production, PR loss did not markedly impact glomerular IgG1, IgG2c or C3 staining in either sex (Fig. 2B). However, the number of animals observed might have been insufficient to detect subtle differences in median staining scores. Therefore, we also examined the distributions of scores (Figs. 2C – 2E). In this analysis, the most pronounced effects of PR loss were on glomerular C3 scores. In female mice, PR loss resulted in an increase in mode C3 score from 2 to 3. In contrast, loss of PR in male mice resulted in a decrease in mode C3 score from 3 to 1. Combined, this differential effect of PR loss on C3 scores resulted in significantly different median C3 scores between female and male PR−/− mice (Fig. 2B).
Figure 2.
Effects of PR deficiency on glomerular IgG and C3 deposition in 10 mo.-old Nba2 mice. (A) representative IgG1, IgG2c and C3 staining of kidney sections from female mice of indicated PR genotype, 40x. (B) Shown are median glomerular IgG1, IgG2c and C3 scores for individual mice (symbols) and groups (lines). *, p < 0.05 Mann-Whitney test. (C – D) Distributions of IgG1 (C), IgG2c (D) and C3 (E) scores from 10 glomeruli per animal.
Effects of PR deficiency on glomerular inflammation and damage in aged Nba2 mice
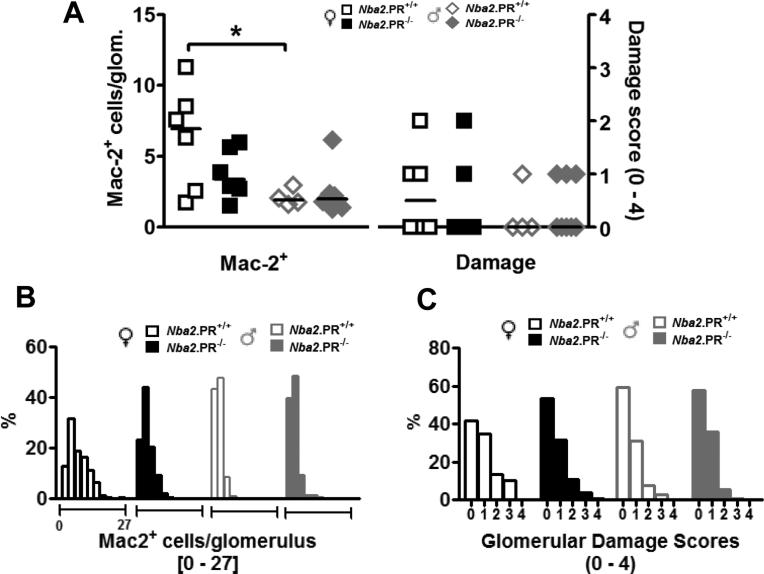
C3 fixation and subsequent downstream inflammatory events are believed to be important mechanisms leading to IC-mediated glomerular injury (37). Therefore, we asked if changes in serum autoAb levels and glomerular complement fixation were associated with changes in glomerular inflammation or damage. Glomerular inflammation was determined by quantifying numbers of cells per glomerulus expressing Mac-2 (galectin-3), a monocyte/macrophage marker, as previously described (18). Damage was assessed by examination of glomeruli under light microscopy (Supplementary Fig. 1). Despite increased serum IgG2c autoAbs (Fig. 1) and marginally increased glomerular IC deposition (Fig. 2), aged female Nba2.PR−/− mice had fewer mean Mac2+ cells per glomerulus than did controls, although this difference was not statistically significant (Fig. 3A). This effect reflected a restriction in of Mac-2 scores to lower values (Fig. 3B). Similarly, PR deficiency in female Nba2 mice led to a decrease in median damage scores (though not statistically significant) (Fig. 3A) and a subtle shift in distribution toward lower damage scores (Fig. 3C). In aged male Nba2 mice, PR loss had little appreciable effect on either Mac-2 or damage scores, which were minimal to begin with – this despite increased in glomerular C3 scores (Fig. 2E). Together, these data are consistent with previously reported observations that in female Nba2 mice, IC deposition is not tightly linked to inflammation and damage. They further suggest that PR independently regulates IC deposition and glomerular inflammation.
Figure 3.
Effects of PR deficiency on glomerular Mac-2+ cell infiltration and structural damage in 10 mo.-old Nba2 mice. (A) Glomerular Mac-2 and damage scores. Shown are mean Mac-2+ cells/glomerulus for individual mice (symbols) and groups (lines), and median damage score for individual mice (symbols) and groups (lines). *, p < 0.05, unpaired t test. (B and C) Distributions of glomerular Mac-2 (B) and damage (C) scores from 30 glomeruli per animal.
To investigate the possibility that PR was acting at the level of the glomerulus in female Nba2 mice to limit inflammation, we measured expression of PR protein in freshly frozen kidney sections from female Nba2.PR+/+ mice. While we observed the expected presence or absence of nuclear PR protein in positive control tissue (B6.PR+/+ uterus) and negative control tissue (B6.PR−/− uterus) (Supplementary Figs. 2A – 1D), we could not detect PR protein in Nba2.PR+/+ kidneys using this technique (Supplementary Figs. 2E and 1F).
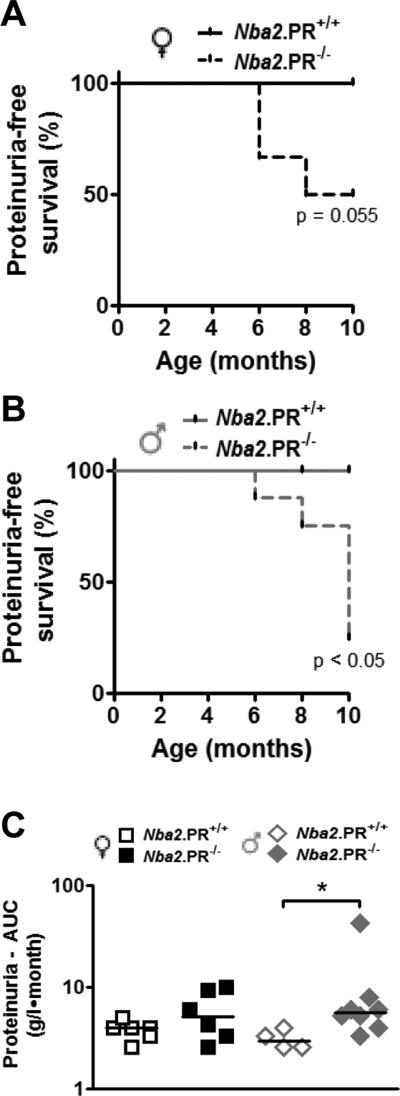
Proteinuria is often used as a surrogate marker of glomerular injury in mouse models of SLE. We measured proteinuria levels every 2 mo. beginning at age 4 mo. Surprisingly, PR deficiency led to increased frequencies of moderate (3+) or higher proteinuria in both female and male Nba2 mice (Figs. 4A and 4B), despite marginally diminished or unchanged glomerular damage scores (Fig. 3). The effects on proteinuria were only apparent after 6 mo. No PR+/+ animals exhibited moderate or higher proteinuria. We also calculated AUC for proteinuria between 2 and 10 mo. (Fig. 4C), estimating protein concentration based on proteinuria scores. In male Nba2 mice, PR deficiency led to significantly increased mean AUC proteinuria levels; a much more subtle effect in the same direction was observed among female Nba2 mice. Importantly, the animals showing moderate or higher proteinuria were not necessarily the same ones with the highest glomerular damage scores (data not shown), suggesting that the observed effects of PR on proteinuria were mediated through mechanisms other than GN.
Figure 4.
Increased frequency of moderate proteinuria in aged PR−/− mice. Shown are frequencies of moderate proteinuria-free survival in female (A) and male (B) Nba2 mice between 2 and 10 mo. age. Depending on the animal, observation began at either 2 or 4 mo. P values determined by log-rank test. (C) Estimated cumulative proteinuria between 4 and 10 mo. as determine by AUC (see text). Shown are medians (lines) and individual values (symbols). *, p < 0.05 Mann-Whitney test.
PR deficiency minimizes or abrogates sexual dimorphism in splenic leukocyte abundance in aged Nba2 mice
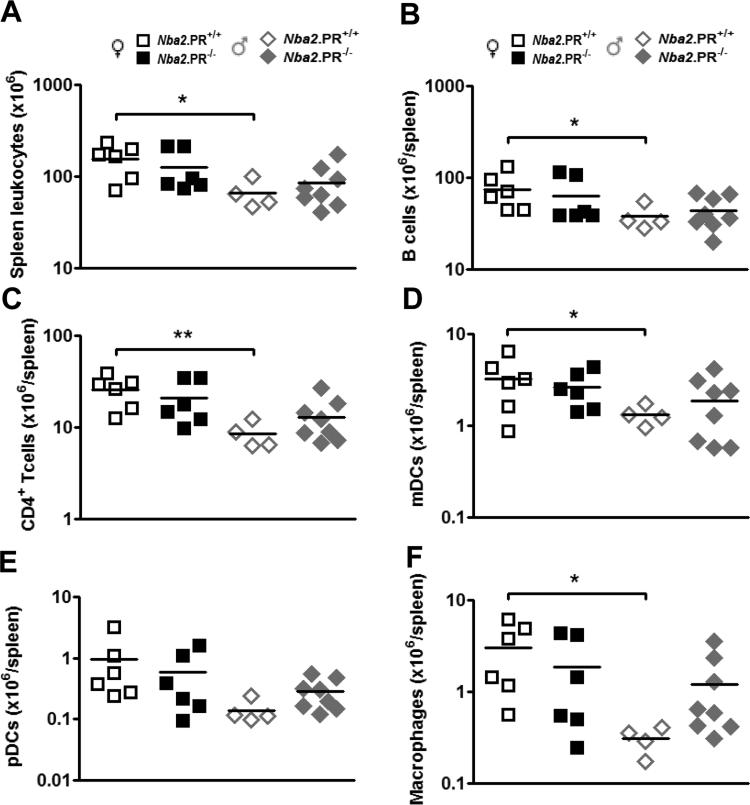
To investigate potential cellular mechanisms underlying increased or accelerated IgG2c autoAb production in female Nba2.PR−/− mice, we examined the spleens of 10 mo.-old mice. We observed no appreciable effects of PR loss on abundance of total splenic leukocytes, B cells, CD4+ T cells, myeloid DCs (mDCs), plasmacytoid DCs (pDCs) or macrophages, in either sex (Figs. 5A – 5F). Consistent with previous reports of this model, aged female Nba2.PR+/+ mice had significantly more massive spleens with more leukocytes per spleen than aged male Nba2.PR+/+ mice (Supplementary Fig. 3A and Fig. 5A). A similar pattern was seen for all major subsets (Figs. 5B – 5F). Interestingly, most of these differences were minimized or completely abrogated after PR loss due to subtle but opposing effects in each sex. In male mice, subtle variations in the degree to which major subsets followed this pattern resulted in increased proportions of CD4+ T cells, pDCs and macrophages after PR loss (Supplementary Figures 3B – 2F). Together, these results indicate that previously observed sexual dimorphism in splenic leukocyte abundance in aged Nba2 mice involves specific effects of PR in both female and male animals.
Figure 5.
PR deficiency minimizes sexual dimorphism in splenic leukocyte abundance in 10 mo.-old Nba2 mice. Shown are means (lines) and individual values (symbols) for splenic abundance of leukocytes (A), B cells (B220hiCD11c−mPDCA1−) (B), CD4+ T cells (CD3+CD4+) (C), mDCs (B220−CD11chimPDCA-1−) (D), pDCs (B220+CD11clomPDCA1+) (E), and macrophages (F4/80hi) (F). *, p < 0.05; **, p < 0.01, unpaired t test.
PR deficiency reduces splenic TREG abundance and increases TFH proportions in aged female Nba2 mice
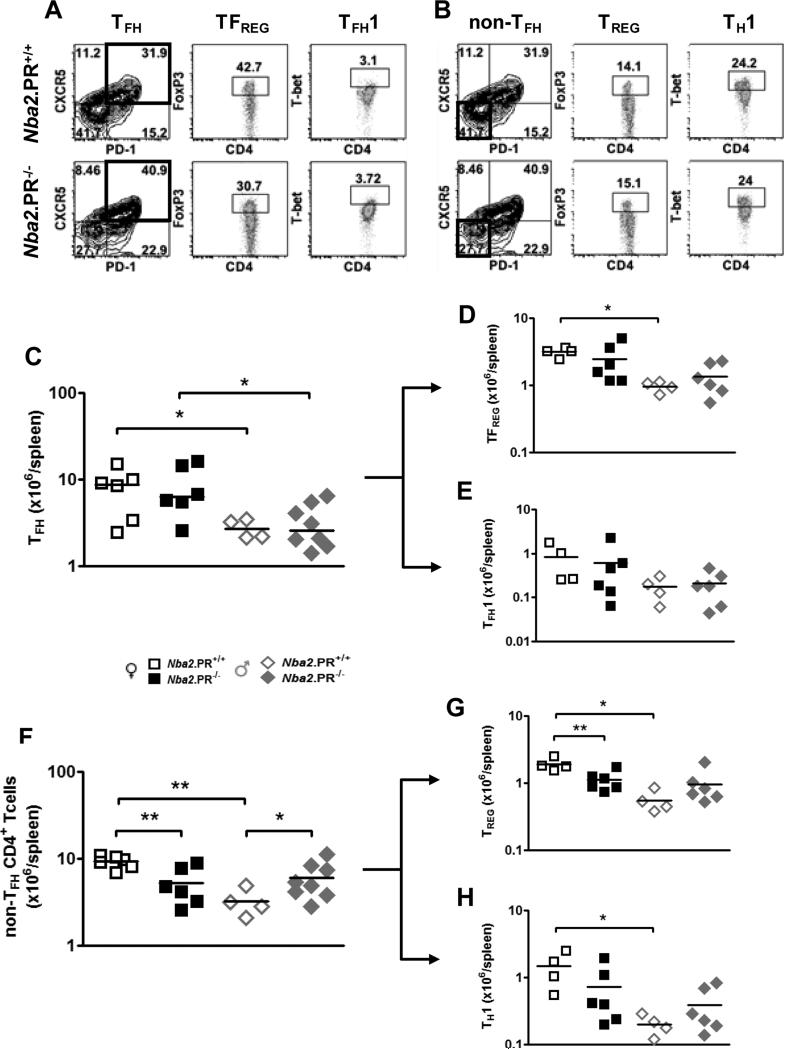
Since we had previously observed that PR suppressed TD IgG Ab responses via effects in CD4+ T cells, we focused our attention on this cell type, particularly four subsets potentially regulated by PR and involved in IgG2c autoAb production. T follicular helper (TFH) cells drive TD IgG responses by inducing germinal center (GC) B cell Ig class switch recombination (CSR) (38). Deregulated TFH differentiation and/or survival can lead to lupus-like autoimmunity in mice, including anti-nuclear Abs and IC-mediated GN (39). FoxP3-expressing TREGS are important suppressors of autoimmunity (32) whose induction and activity are enhanced by PR in vitro (31). FoxP3-expressing T follicular regulatory (TFREG) cells are a subset of TFH cells that suppress GC reactions and subsequent Ab production (38). Finally, T-bet-expressing TH1 and T follicular helper type 1 (TFH1) cells produce IFN-γ (17), an important inducer of IgG2c CSR in mouse B cells whose expression may be suppressed by PR (28). The surface and intracellular markers used to identify these CD4+ T cell subsets are displayed in Figs. 6A and 6B.
Figure 6.
PR deficiency leads to decreased splenic TREG abundance in 10 mo.-old female Nba2 mice. Representative FACS plots show gating scheme used to sort CD3+CD4+ cells into TFH cells and their TFREG, and TFH1 subsets (A), and non-TFH CD4+ T cells into their TREG and TH1 subsets (B); TFH = CD3+CD4+CXCR5+PD-1+; TFREG = CD3+CD4+CXCR5+PD-1+FoxP3+; TFH1 = CD3+CD4+CXCR5+PD-1+Tbet+; non-TFH = CD3+CD4+CXCR5−PD-1−; TREG = CD3+CD4+CXCR5−PD-1−FoxP3+; TH1 = CD3+CD4+CXCR5−PD-1−Tbet+. (C – H) Shown are means (lines) and individual values (symbols) for splenic abundance of TFH cells (C) and their TFREG (D) TFH1 (E) subsets, and non-TFH CD4+ T cells (F) and their TREG (G) and TH1 (H) subsets. *, p < 0.05, **, p < 0.01, unpaired t test.
PR loss did not appreciably impact the abundance of TFH cells or their TFREG and TFH1 subsets in either sex (Figs. 6C – 6E). In female mice, however, PR loss significantly reduced abundance non-TFH CD4+ T cells and their TREG and Th1 (not statistically significant) subsets (Figs. 6F – 6H). In male mice, PR loss had the opposite effect, increasing abundance of non-TFH CD4+ T cells (statistically significant) and their TREG and in TH1 subsets (not statistically significant). As a result, sex differences in non-TFH CD4+ T cell abundance among PR+/+ mice were abrogated after PR loss. Together, these data indicate that in female Nba2 mice, PR supports the development or survival of non-TFH CD4+ T cells, particularly TREGS, but that in male mice, PR has the opposite effect. These sex-specific effects contribute to sexually dimorphic abundance of spleen CD4+ T cell subsets, including TREGS.
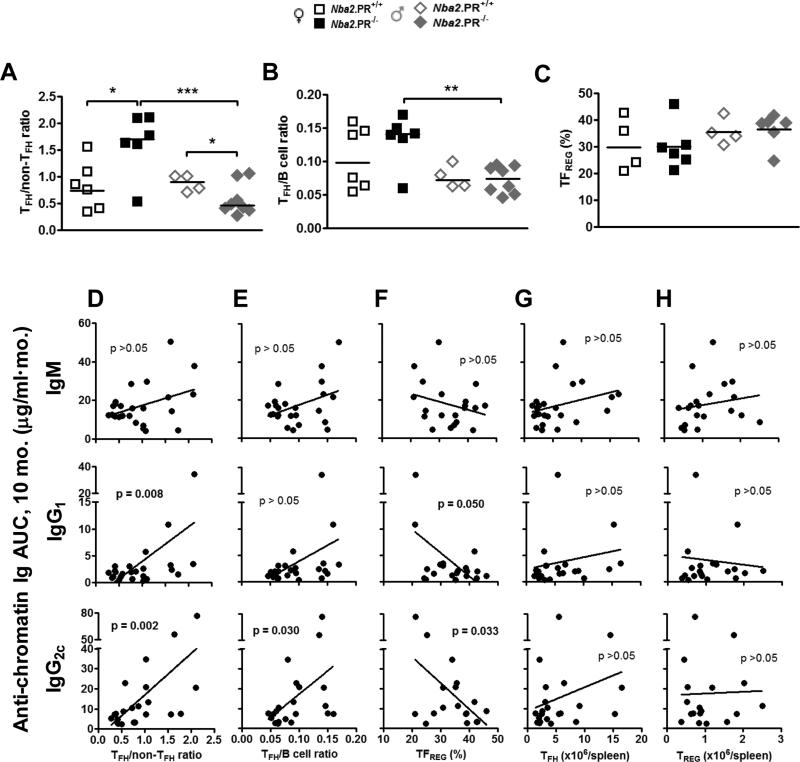
In female PR−/− mice, TFH abundance was preserved in the face of reduced non-TFH CD4+ T cell abundance, suggesting that PR was influencing the relative abundance (proportion) of TFH cells within the splenic CD4+ T cell compartment. This is potentially important because abundance of TFH cells relative to other splenic subsets can determine GC responses and subsequent Ab production (40). In female Nba2 mice, PR deficiency significantly increased TFH/non-TFH CD4+ T cell ratios (Fig. 7A); the opposite effect was observed in male mice – consistent with effects on IgG2c autoAb levels (Fig. 1). The same relationships were observed when we compared percentage of CD4+ T cells expressing TFH markers (Supplementary Fig. 4A). There was a parallel effect of PR deficiency on TFH/B cell ratios in female mice, but these differences were not statistically significant (Fig. 7B).
Figure 7.
PR deficiency increases the ratio of TFH cells to non-TFH CD4+ T cells in 10 mo.-old female Nba2 mice but decreases this ratio in age-matched male Nba2 mice. Shown are medians (lines) and individual values (symbols) for TFH/non-TFH CD4+ T cell ratios (A), TFH/B cell ratios (B) and TFREG percentage of TFH cells (C). *, p < 0.05; **, p < 0.01 unpaired t test of log-transformed data (A and B) or untransformed data (C). (D – H) Linear regression analysis of serum anti-chromatin Ig AUC levels at 10 mo. vs. TFH/non-TFH CD4+ T cell ratio (D), TFH/B cell ratio (E), TFREG percentage of TFH cells (F), TFH cells/spleen (G), and TREG cells/spleen (H) in all mice. P values for linear regression analyses are shown within each graph.
To investigate the possibility that PR's effects on TFH/non-TFH CD4+ T ratios were involved in dysregulation of serum IgG autoAb responses, we performed linear regression analysis on serum autoAb AUC at 8 and 10 mo. vs. various splenic CD4+ T cell indices (Figs. 7D – 7H). Serum IgG1 and IgG2c autoAb AUC levels at 10 mo., but not those of IgM, showed highly significant, positive correlation with splenic TFH/non-TFH CD4+ T cell ratios (Fig. 7D), consistent with an effect on GC reactions and associated B cell CSR. Similar, statistically significant correlations were also observed between splenic TFH/non-TFH CD4+ T cell ratios and autoAb levels at 10 mo. and autoAb AUC at 8 mo. (Supplementary Figs. 5A and 5C). We also observed statistically significant, positive correlations between splenic TFH/B cell ratios and serum IgG2c autoAb levels at 10 mo. (Fig. 7E and Supplementary Fig. 5B). In GC reactions, the balance of TFH and TFREG is an important determinant of IgG Ab responses. Not surprisingly, we observed statistically significant, negative correlations between 10 mo. IgG autoAb AUC (but not IgM) and the percentage of TFH cells with a regulatory (TFREG) phenotype (Fig. 7F), even though TFREG percentages were not appreciably impacted by PR loss in either sex (Supplementary Fig. 4B). Finally, we observed no significant correlations between 10 mo. IgG autoAb AUC and abundance of splenic TFH cells (Figs. 7G) or TREG cells (Fig. 7H), despite clear effects of PR on the latter (Fig. 6G). Together, these results indicate that in aged Nba2 mice, sex-specific effects of PR on the emergence of class-switched IgG autoAbs positively correlate with PR's effects on the proportion of splenic CD4+ T cells expressing TFH markers.
Effects of PR deficiency on splenic expression of IFN-γ and ER-α gene mRNA levels and activation of antigen presenting cells in aged Nba2 mice
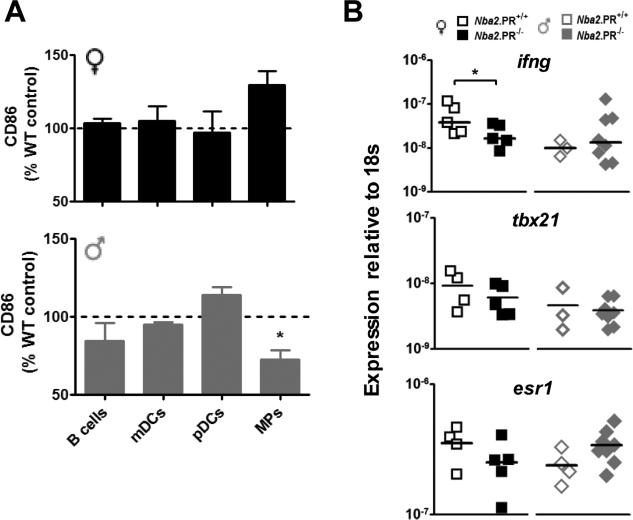
We also examined the effects of PR loss on the activation of splenic antigen presenting cells (APCs) as determined by expression of the co-stimulatory molecule, CD86. In general, CD86 expression on APCs was unaffected by PR loss, except in the case of macrophages (Fig. 8A), where in male mice it was decreased. The opposite effect (not statistically significant) was observed in female mice.
Figure 8.
PR deficiency alters splenic APC activation and ifng and esr1 mRNA expression in aged Nba2 mice. A, CD86 expression on splenic APC subsets from female (black) and male (grey) PR−/− mice relative to PR+/+ controls assessed on the same day. Shown are means ± standard error, n = 3 per group. *, p < 0.05 one sample t test. B, IFN-γ (ifng), T-bet (tbx21) and ER-α (esr1) gene mRNA levels in freshly frozen splenic leukocytes from 10 mo.-old mice. Shown are means (lines) and individual values (symbols). *, p < 0.05 unpaired t test.
We next investigated possible molecular mechanisms linking altered IgG2c autoAb production to loss of PR, a ligand-activated transcription factor. We had previously observed that splenic CD4+ T cells from non-autoimmune adult female PR−/− mice over-express IFN-γ gene (ifng) mRNA and protein in vitro, despite normal expression of the T-bet gene (tbx21), a transcriptional regulator of IFN-γ expression and TH1 differentiation (28). Unexpectedly, PR deficiency resulted in significantly decreased ifng expression in total splenic leukocytes from female mice (Fig. 8B); in male mice there was little effect. Decreased ifng expression in female PR−/− mice may relate to reduced (T-bet+) TH1 cell abundance (Fig. 6H).
In uterine tissue, PR can repress the expression of the ER-α gene (esr1); and in female NZB/W mice, esr1 gene deficiency causes selective reduction in serum autoAbs of the IgG2a subclass (equivalent to IgG2c subclass in B6 strains) (15). Nevertheless, PR loss resulted in reduced esr1 mRNA levels in splenic leukocytes from female mice (Fig. 8B), suggesting that PR does not repress IgG2c autoAb production via effects on esr1 expression. Interestingly, PR loss had the opposite effect on splenic esr1 expression in male mice. As a result, sex-dependent differences in splenic esr1 expression among PR+/+ mice were reversed after PR loss.
Discussion and Conclusions
Here we present evidence indicating that PR, a nuclear receptor essential to female reproduction, is involved in regulating splenic CD4+ T cell populations and IgG autoAb production in aged lupus-prone Nba2 mice of both sexes. By comparing female and male mice, we also identify an unsuspected role for PR in generating and/or maintaining sexual dimorphism in abundance of splenic leukocyte subsets.
In many aged female mice, PR deficiency was associated with markedly increased levels of class-switched IgG2c autoAbs. While some female PR−/− mice also showed abnormally high levels of class-switched IgG1 autoAbs, this phenotype appeared to be less penetrant than the IgG2c phenotype. IgM autoAbs were the least impacted. Together, these results suggest an effect on pathways leading to IgG CSR in autoreactive B cells. We did not observe major effects on B cell abundance or activation, but it is possible that there were important effects on the GC B cell subset, particularly its expression of activation-induced deaminase, which is required in B cells for CSR and may be under the transcriptional control of PR (15, 41). Consistent with this idea, PR loss resulted in lower levels of total IgM in aged female mice (Fig. 1C), suggesting deregulation of CSR may have been more generalized. The relatively strong effect on IgG2c autoAbs remains unexplained, but likely involves interactions of PR with female-restricted factors, since in male mice, IgG2c autoAbs were not increased, but slightly decreased. Female-restricted factors might include endogenous estrogen, a known enhancer of both PR expression and IgG2a/c autoAbs, elevated progesterone levels during the estrous cycle, and PR co-factors unique to females. Finally, it is possible that PR was simply delaying the emergence of IgG2c autoAbs in female mice. Arguing against this possibility is the fact that PR's effects only became apparent from 6 mo. onward, the age at which Nba2 mice typically begin to develop significant autoAbs (33, 34). Thus, it seems that PR's main effect on IgG2c and maybe IgG1 autoAb levels in female mice was to suppress them.
In association with altered autoAb production, we observed prominent PR effects on the composition of splenic CD4+ T cell populations. In aged female Nba2 mice, for example, PR deficiency led to significant reductions in splenic TREG abundance (Fig. 6G). TREG deficiency can cause systemic autoimmunity in mice and humans, and TREG dysfunction has been linked to several autoimmune diseases, including SLE (32). Thus, increased autoAb production in female PR−/− mice may have involved reduced splenic TREG numbers. However, we were unable to demonstrate significant correlation between TREG abundance (Fig. 7H) or proportions (data no shown) and autoAb levels, suggesting that any effect was indirect. It is possible that PR's effects on TREG abundance are important for pathways upstream of tolerance loss in these mice, e.g. macrophage activation (Fig. 8A). Regardless, the observed connection between PR and TREG abundance may be important for other reasons. Both PR and TREGS are required for successful allogeneic pregnancy in mice, and possibly humans as well (42). During normal murine pregnancy, systemic maternal TREG populations (including splenic TREGS) expand significantly, a phenomenon that can be mimicked in non-pregnant mice by progesterone treatment (43) and that appears to involve PR (our unpublished observations). In vitro, ligand-bound PR can induce FoxP3 expression in CD4+ T cells and stabilize their regulatory phenotype (31). Thus, PR signaling in CD4+ T cells appears to support regulatory phenotypes associated with successful pregnancy. This offers intriguing mechanistic links between PR gene polymorphisms, maternal TREG abnormalities (44) and recurrent pregnancy loss (45). Similar progesterone effects on TREG stability may also contribute to amelioration of rheumatoid arthritis during pregnancy (4).
PR appears also to regulated the abundance of non-TFH CD4+ T cells other than TREGS and TH1 cells, since these two cell types comprised only about 40% of the non-CD4+ T cell population (Supplementary Figs. 4B and 4C). Whether these were naïve or memory CD4+ T cells remains to be determined. Nevertheless, prominent sex-specific PR effects on non-TFH CD4+ T cell abundance, coupled with preserved TFH abundance, resulted in significant changes in TFH/non-TFH CD4+ T cell ratios in both sexes (Fig. 7A), which correlated well with serum IgG1 and IgG2c autoAb AUC at 10 mo. (Fig. 7D). This correlation is likely to be mechanistically relevant, because the abundance of TFH cells relative to other splenic subsets is a major determinant of GC reactions and subsequent IgG Ab production (40). How PR influences the survival and development of CD4+ T cells in vivo is unknown. In reproductive tissues, PR is known to interact with STAT3 (46), an important inducer of development and survival in TREG cells. It may also be that PR influences CD4+ T cell development through effects in the thymus (47), or that PR influences the differentiation of naïve CD4+ T cells via effects in mDCs or macrophages (48), as suggested by selective sex-specific effects of PR loss on macrophage activation (Fig. 8A). Moreover, we have observed pgr mRNA levels in purified splenic macrophages that are much higher than in other splenic leukocyte subsets (our unpublished observations).
Because our previously published results (28) indicated that PR could regulate TD Ab responses via effects in CD4+ T cells, we focused the present study on this cell type. However, as mentioned above, it is possible that PR also was acting in B cells to regulate autoAb responses. While we noted only marginally significant correlations between TFH/B cell ratios and serum IgG autoAbs (Fig. 7E), we did not analyze PR effects on B cell subsets, such as GC B cells, in vivo. This will be an important line of future investigation. It will also be important to determine which of the above PR effects require ligand activation, because these effects are most likely engaged during pregnancy and could be important for limiting certain, harmful maternal Ab responses such as those targeting fetal red blood cell antigens (hemolytic disease of the newborn).
Our results in female Nba2 mice contrast sharply with reported results from similar studies examining ER-α deficiency in related lupus models. In female NZB/W mice, for example, ER-α deficiency results in selective reduction of serum IgG2a anti-dsDNA levels (equivalent of IgG2c in B6 mice) (14) – the opposite of what we observed in female PR−/− mice. These contrasting results suggest that ER-α and PR (and their ligands) counter-regulate the emergence of pathogenic, class-switched IgG2a/c autoAbs. Thus, the balance of estrogen vs. progesterone effects could be an important determinant of disease expression in genetically prone individuals. These results also lend new significance to observed progesterone deficiency in young women presenting with SLE (49) and further suggest that certain forms of hormonal contraception and replacement therapy (which often contain both estrogen and progesterone) could be either harmful or beneficial depending on their relative activation of ER-α, PR and other hormone receptors.
In our study, conspicuous glomerular IC deposition was not accompanied by prominent glomerular damage, consistent with previous observations in the Nba2 model (34). Moreover, modestly increased IC deposition in female PR−/− mice was insufficient to induce significant glomerular damage, consistent with the idea that in this model IC deposition and glomerular injury are not linked. Nevertheless, PR appeared to be facilitating glomerular macrophage accumulation independently of its effects on IC deposition, and only in female mice. This may have involved the ability of ligand-bound PR to induce vascular permeability (50), even though we could not detect high-level PR protein expression in glomeruli of PR+/+ mice. Finally, the sex-specific effects of PR loss on median C3 scores and their distributions (Fig. 2E) remain unexplained but may reflect PR effects on deposition of unmeasured, complement-fixing IgG subclasses such as IgG2b.
The effects of PR loss on frequencies of moderate or higher proteinuria could not be explained by effects on glomerular damage, an important cause of proteinuria. It is possible, however, that PR was regulating other factors contributing to proteinuria, such as glomerular podocyte function, blood pressure, or protein metabolism. An effect on metabolism is at least suggested by increased body mass in male PR−/− mice (Supplementary Fig. 6).
Our comparison of female and male mice revealed an unsuspected role for PR in generating or maintaining sex differences in spleen mass and splenic leukocyte abundance, and these effects were most prominent for non-TFH CD4+ T cell abundance and their TREG and TH1 subsets (Figs. 6F - 6G). Thus, sexual dimorphism in abundance of splenic TREGS appeared to involve sex-specific effects of PR. In addition to mechanisms mentioned above, female-restricted PR effects may involve PR's interactions with lupus susceptibility genes more highly expressed in female Nba2 mice, e.g. ifi202 (51). Male-restricted effects of PR might involve male-specific PR co-factors or ligand-independent transcriptional regulation by PR, which might be more prominent in male mice. Not only do these results suggest that PR is involved in immunologic sexual dimorphism, but they also highlight the importance of accounting for sex when studying gene function in vivo, an issue that was recently recognized by the U.S. National Institutes of Health (see ref. (52) for commentary).
In summary, our results demonstrate that PR, a NR critical for female reproduction, suppresses the emergence of class-switched IgG autoAbs in aged female lupus-prone Nba2 mice. This appears to occur at the level of splenic CD4+ T cell and alterations in the relative abundances of TFH and non-TFH subsets. Interestingly, PR appears to be involved in maintaining normal numbers of splenic TREG in female Nba2 mice, a mechanism that could relate to TREG functions during pregnancy. Our comparison of female and male mice indicates that PR has sex-specific immunologic effects, which together help shape sexual dimorphism in splenic leukocyte abundance, a well described feature of this and other lupus models. To our knowledge, this is the first report linking PR to regulation of autoimmunity and immunologic sexual dimorphism in vivo. Further investigation of PR's immunomodulatory roles should offer important insight into poorly understood immunologic phenomena of pregnancy, including immunologic tolerance of the fetus, pregnancy-associated infections and amelioration of certain autoimmune diseases.
Materials and Methods
Generation of B6.Nba2.PR KO mice
C57BL/6 (B6) homozygous for the NZB-derived Nba2 locus on chromosome 1 (B6.Nba2) were provided by Daniel Stetson, Ph.D., University of Washington. The generation of the B6.Nba2 strain is described in reference (53). B6 mice bearing a targeted disruption of the PR gene (B6.PR KO) were generated by our laboratory (28) using mice received from John Lydon, Ph.D., Baylor College of Medicine. The PR KO mutation was back-crossed 3 generations onto the B6.Nba2 genetic background to generate PR heterozygous breeding pairs. Homozygosity for the Nba2 locus was confirmed by PCR using the following primer pairs, as previously described (34, 53):
| Forward (5’→3’) | Reverse (5’→3’) | |
|---|---|---|
| D1Mit166 | GAT GAA GTG AAA ATG ATC CTT GC | TAT CTT TTG TGG ACT CGG GG |
| D1Mit196 | AAA AAT GAG GTG CTA TTG AAA AGC | TTA TGC ATC AAA CCA AAA TCT CA |
| D1Mit201 | TCC ATC CAT ACT CCT GTC TGC | CAA GGA CTA GGG CTG TCA CTG |
| D1Mit36 | GAG GAA TGT AGA GTC CAA CCT GG | TGA ATA GAT TAA GAG CCT GGA AGC |
| D1Mit47 | CTG ACC TCC ACA CGA CCC | GCT TGG GAA ACT GGA TGA AAA |
| D1Mit510 | TTC AGG TTA ATT CTA CAA ACA AGC A | TCA AAA TAT CAT GCT GTA TAC CAT G |
All mice were housed in the University of Washington's animal facilities under SPF conditions, and all experiments with them were performed in compliance with the University of Washington Institutional Animal Care and Use Committee.
Detection of serum autoAbs and Ig levels
Serum anti-chromatin Ig was measured by sandwich enzyme-linked immunosorbent assay (ELISA) as previously described (35). Briefly, polystyrene microtiter plates were coated overnight with calf-thymus DNA (Sigma-Aldrich, St. Louis, MO, USA) in DNA-coating buffer (Thermo Scientific, Logan, UT, USA) followed by a second overnight coating with purified histone protein (Sigma-Aldrich). Plates were blocked with bovine serum albumin (Sigma-Aldrich). Sera were diluted 1/50 – 1/2000 before being assayed. To estimate anti-histone Ig levels in sera for each experiment, portions of assay plates were coated overnight with goat anti-mouse Ig capture Ab (Southern Biotech, Birmingham, AL, USA) instead of calf thymus DNA or histones, similarly blocked and used to detect known quantities of purified mouse IgM, IgG1 or IgG2c (Southern Biotech, Birmingham, AL, USA). After extensive washing, bound Ig was detected using goat HRP-conjugated goat anti-mouse IgM, IgG1 or IgG2c (Southern Biotech). Total serum Ig levels were determined as above, except sera were diluted 1/50,000 – 1/500,000.
Immunohistochemistry, glomerular pathology scores and proteinuria scores
At age 10 mo., mice were killed and kidneys and spleens were isolated. Left kidneys were preserved in 10% formalin and embedded in paraffin. Right kidneys were snap-frozen in liquid nitrogen and TissueTek OCT compound (Sakura Finetek, Torrance, CA, USA) and stored at −80°C. All histology scoring was performed on actual tissue sections in a blinded manner. Histopathology assessments were performed on 4 μm sections of formalin-fixed kidneys subjected to periodic acid-Schiff and methenamine–silver stain (Jones stain). A total of 30 glomeruli from superior (10), inferior (10) and hilar (10) regions of a section from each mouse were assessed in a blinded fashion by GH for glomerular damage using the following 0 – 4 scoring scheme: 0 = normal appearing glomerulus; 1 = mesangial expansion or glomerular hypercellularity; 2 = mesangial expansion and glomerular hypercellularity; 3 = a score of 2 plus segmental hyaline deposits; 4 = crescents or global hyaline deposits or fibrinoid necrosis or sclerosis. For Mac-2 staining, 4 μm sections from formalin-fixed kidneys were incubated sequentially with normal horse serum, rat anti-mouse Mac-2 antibody (Cedarlane, Burlington, NC, USA), and horseradish peroxidase–conjugated rat anti-mouse polymer components (Biocare, Concord, CA, USA). The immunoreaction was visualized using the NovaRed kit (Vector, Burlingame, CA, USA). 30 glomeruli from each mouse kidney section were blindly scored for number of Mac-2+ cells per glomerulus. Kidney and uterus cryosections (4 μm) were fixed in ice-cold acetone, washed with phosphate buffered saline (PBS) and blocked with normal goat serum. Kidney sections were then incubated with fluorescein-conjugated goat (control) IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA), or identical concentrations of fluorescein-conjugated goat anti-mouse IgG1, IgG2c (both SantaCruz Biotechnology) or C3 (Cappel, West Chester, PA, USA). Kidney and uterus cryosections were also incubated with rabbit polyclonal IgG against mouse PR (Abcam, Cambridge, UK) followed by either fluorescein-conjugated goat-anti-rabbit IgG (Jackson ImmunoResearch, PA, USA) or biotin-conjugated goat anti-rabbit (Jackson ImmunoResearch) then Streptavidin-conjugated to Alexa Fluor® 555 (Invitrogen, Carlsbad, CA, USA). Stained sections were visualized and images recorded using either a Nikon Eclipse E 400 Epi-Fluorescence microscope equipped with a Photometrics CoolSNAP FX camera, an EVOS FL cell imaging system (AMG, Bothell, WA, USA) or an EVOS XL Core cell imaging system (AMG). 10 – 20 glomeruli from each section were blindly assessed for IgG or C3 fluorescence intensity on a 0 – 4 scale based on the following reference standards: 0 = typical fluorescence intensity (none to minimal) observed in similar stained kidney sections from 6-mo-old female B6 mice; 4 = typical fluorescence intensity (bright) observed in similarly stained sections from 6-mo-old female B6.Sle1/2/3 lupus mice with IC-mediated GN. For conglomerate analysis of glomerular IgG1, IgG2c and C3 scores, only the first 10 glomeruli scored from each animal were included in order to eliminate bias toward animals with higher numbers of scored glomeruli.
Splenic leukocyte isolation and flow cytometry analysis
Spleens from 10-mo-old mice were freshly isolated and minced extensively. Red blood cells were lysed using hypertonic solution (BioLegend, San Diego, CA, USA). Live splenic leukocytes were stained with fluorochrome-labeled monoclonal Abs (moAbs) recognizing CD4, CD40, CD86, and CD11c (BD Biosciences, San Jose, CA, USA), PD-1, CXCR5 (eBioscience, San Diego, CA, USA), F4/80, I-AB, and B220 (BioLegend, San Diego, CA, USA), mPDCA1 (Miltenyi Biotec, Germany). For intracellular staining, splenic leukocytes were fixed and permeabilized using a proprietary Fix/Perm buffer set (BioLegend) and stained with moAbs recognizing FoxP3 (BioLegend) and T-bet (BD Biosciences). Flow cytometry data were acquired with FACScan Aria and Canto machines (BD Biosciences) and analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
RNA isolation and quantitative PCR
Freshly isolated splenic leukocytes were pelleted and immediately frozen/stored at −80°C. Total RNA was isolated using RNeasy Mini Kits (Qiagen, Valencia, CA, USA). After enzymatic degradation of DNA, reverse transcription was performed using the SuperScript III First-Strand synthesis system (Invitrogen). From cDNA isolates, specific sequences were amplified using Absolute Blue Q-PCR SYBR Green ROX Mix (Thermo Scientific). The results were normalized to 18S RNA levels in each sample. Forward/reverse primer sequences used: ifng (GCTTTGCAGCTCTTCCTCAT/GTCACCATCCTTTTGCCAGT); tbx21 (GGTGTCTGGGAAGCTGAGAG/CCACATCCACAAACATCCTG); and esr1 (TTCTTCTCAAGCAGGTGGCCC/GCGAGTTACAGACTGGCTCC). The results were normalized to 18S RNA levels in each sample.
Measurement of proteinuria
Urine protein was measure by dipstick (Multistix 9; Bayer, Bury St. Edmunds, UK) and quantified on a 0 – 4 scale per manufacturer's instruction. Approximate protein concentration correlating with each score were 0 (0 g/L), 1 trace (0.3 g/L), 2 (1 g/L), 3 (3 g/L) and 4 (>20 g/L).
Statistical analysis
Student's t tests, Mann-Whitney tests, linear regression, log transformations, and log-rank analyses were performed using Prism 5 software (GraphPad, San Diego, CA, USA).
Supplementary Material
Acknowledgements
This work was supported by U.S.A. National Institutes of Health grants AI101564, AI73739, an American Recovery and Reinvestment Act supplement to AI73739, and support from the Robert F. and Betty Snead Fund for Innovation in Lupus Research. We thank the laboratories of Edward Clark, Ph.D. and Keith Elkon, M.D. for sharing their expertise, resources and constant encouragement.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Mohan C. Environment versus genetics in autoimmunity: a geneticist's perspective. Lupus. 2006;15:791–793. doi: 10.1177/0961203306070005. [DOI] [PubMed] [Google Scholar]
- 2.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 3.Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho TJ, Koutseva N, Zaghlul S, Graves T, Rock S, Kremitzki C, Fulton RS, Dugan S, Ding Y, Morton D, Khan Z, Lewis L, Buhay C, Wang Q, Watt J, Holder M, Lee S, Nazareth L, Rozen S, Muzny DM, Warren WC, Gibbs RA, Wilson RK, Page DC. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014;508:494–499. doi: 10.1038/nature13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat Rev Rheumatol. 2014;10:740–751. doi: 10.1038/nrrheum.2014.144. [DOI] [PubMed] [Google Scholar]
- 5.Costenbader K, Feskanich D, Stampfer M, Karlson E. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007;56:1251–1262. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- 6.Bernier M, Mikaeloff Y, Hudson M, Suissa S. Combined oral contraceptive use and the risk of systemic lupus erythematosus. Arthritis Rheum. 2009;61:476–481. doi: 10.1002/art.24398. [DOI] [PubMed] [Google Scholar]
- 7.Petri M, Thompson E, Abusuwwa R, Huang J, Garrett E. BALES: the Baltimore lupus environmental study [abstract]. Arhtritis Rheum. 2001;44:S331. [Google Scholar]
- 8.Aarons I. Renal immunofluoresncence in NZB/NZW mice. Nature. 1964;203:1080–1081. doi: 10.1038/2031080a0. [DOI] [PubMed] [Google Scholar]
- 9.Steward M, Hay F. Changes in immunoglobulin class and subclass of anti-DNA antibodies with increasing age in N/ZBW F1 hybrid mice. Clin Exp Immunol. 1976;26:363–370. [PMC free article] [PubMed] [Google Scholar]
- 10.Roubinian J, Talal N, Siiteri P, Sadakian J. Sex hormone modulation of autoimmunity in NZB/W mice. Arthritis Rheum. 1979;22:1162–1169. doi: 10.1002/art.1780221102. [DOI] [PubMed] [Google Scholar]
- 11.Roubinian J, Talal N, Greenspan J, Goodman J, Siiteri P. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roubinian JR, Papoian R, Talal N. Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. J Clin Invest. 1977;59:1066–1070. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40:66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- 14.Bynote KK, Hackenberg JM, Korach KS, Lubahn DB, Lane PH, Gould KA. Estrogen receptor-alpha deficiency attenuates autoimmune disease in (NZB x NZW)F1 mice. Genes Immun. 2008;9:137–152. doi: 10.1038/sj.gene.6364458. [DOI] [PubMed] [Google Scholar]
- 15.Incorvaia E, Sicouri L, Petersen-Mahrt SK, Schmitz KM. Hormones and AID: balancing immunity and autoimmunity. Autoimmunity. 2013;46:128–137. doi: 10.3109/08916934.2012.748752. [DOI] [PubMed] [Google Scholar]
- 16.Hughes GC, Clark EA. Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity. 2007;40:470–481. doi: 10.1080/08916930701464764. [DOI] [PubMed] [Google Scholar]
- 17.Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev. 2012;11:A502–514. doi: 10.1016/j.autrev.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes GC, Martin D, Zhang K, Hudkins KL, Alpers CE, Clark EA, Elkon KB. Decrease in Glomerulonephritis and Th1-Associated Autoantibody Production After Progesterone Treatment in NZB/NZW Mice. Arthritis Rheum. 2009;60:1775–1784. doi: 10.1002/art.24548. [DOI] [PubMed] [Google Scholar]
- 19.Keisler L, Kier A, Walker S. Effects of prolonged adminstration of the 19-nortestosterone derivatives norethindrone and norgestrel to female NZB/W mice: comparison with medroxyprogesterone and ehtinyl estradiol. Autoimmunity. 1991;9:21–32. doi: 10.3109/08916939108997120. [DOI] [PubMed] [Google Scholar]
- 20.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqalini JR, Schweppe KW, Thijssen JHH. Reprint of classification and pharmacology of progestins. Maturitas. 2008;61:171–180. doi: 10.1016/j.maturitas.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Ellman S, Sticht H, Thiel F, Beckmann M, Strick R, Strissel P. Estrogen and progesterone receptors: from molecular structures to clinical targets. Cell Mol Life Sci. 2009;66:2405–2426. doi: 10.1007/s00018-009-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas P. Characteristics of membrane progestin receptor alpha (mPR-alpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Africander D, Verhoog N, Hapgood J. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76:636–652. doi: 10.1016/j.steroids.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Shymala G, Coneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 25.Schneider JS, Stone MK, Wynne-Edwards KE, Horton TH, Lydon J, O'Malley B, Levine JE. Progesterone receptors mediate male aggression toward infants. Proc Natl Acad Sci U S A. 2003;100:2951–2956. doi: 10.1073/pnas.0130100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Döhler KD, Wuttke W. Serum LH, FSH, prolactin and progesterone from birth to puberty in female and male rats. Endrocrinology. 1974;94:1003–1008. doi: 10.1210/endo-94-4-1003. [DOI] [PubMed] [Google Scholar]
- 27.Oettel M, Mukhopadhyay AK. Progesterone: the forgotten hormone in men? The Aging Male. 2004;7:236–257. doi: 10.1080/13685530400004199. [DOI] [PubMed] [Google Scholar]
- 28.Hughes GC, Clark EA, Wong AH. The intracellular progesterone receptor regulates CD4+ T cells and T cell-dependent antibody responses. J Leukoc Biol. 2013;93:369–375. doi: 10.1189/jlb.1012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glass C, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol. 2006;6:44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- 30.Lonard DM, O'Malley BW. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012;8:598–604. doi: 10.1038/nrendo.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JH, Lydon JP, Kim CH. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J Immunol. 2012;42:1–14. doi: 10.1002/eji.201142317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choubey D. Interferon-inducible Ifi200-family genes as modifiers of lupus susceptibility. Immunol Lett. 2012;147:10–17. doi: 10.1016/j.imlet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gubbels M, Jørgensen T, Metzger T, Menze K, Steele H, Flannery S, Rozzo S, Kotzin B. Effects of MHC and gender on lupus-like autoimmunity in Nba2 congenic mice. J Immunol. 2005;175:6190–6196. doi: 10.4049/jimmunol.175.9.6190. [DOI] [PubMed] [Google Scholar]
- 35.Sobel ES, Mohan C, Morel L, Schiffenbauer J, Wakeland EK. Genetic dissection of SLE pathogenesis: adoptive transfer of Sle1 mediates the loss of tolerance by bone marrow-derived B cells. J Immunol. 1999;162:2415–2421. [PubMed] [Google Scholar]
- 36.Vyse TJ, Rozzo SJ, Drake CG, Izui S, Kotzin BL. Control of multiple autoantibodies linked with a lupus nephritis susceptibility locus in New Zealand black mice. J Immunol. 1997;158:5566–5574. [PubMed] [Google Scholar]
- 37.Bagavant H, Fu SM. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr Opin Rheumatol. 2009;21:489–494. doi: 10.1097/BOR.0b013e32832efff1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, Copley RR, Bell JI, Cornall RJ, Goodnow CC. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 40.Pratama A, Vinuesa CG. Control of TFH cell numbers: why and how? Immunol Cell Biol. 2014;92:40–48. doi: 10.1038/icb.2013.69. [DOI] [PubMed] [Google Scholar]
- 41.Pauklin S, Petersen-Mahrt S. Progesterone inhibits activation-induced deaminase by binding to the promoter. J Immunol. 2009;183:1238–1244. doi: 10.4049/jimmunol.0803915. [DOI] [PubMed] [Google Scholar]
- 42.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 43.Mao G, Wang J, Kang Y, Tai P, Wen J, Zou Q, Li G, Ouyang H, Xia G, Wang B. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endrocrinology. 2010;151:5477–5488. doi: 10.1210/en.2010-0426. [DOI] [PubMed] [Google Scholar]
- 44.Lee SK, Kim JY, Lee M, Gilman-Sachs A, Kwak-Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol. 2012;67:311–318. doi: 10.1111/j.1600-0897.2012.01116.x. [DOI] [PubMed] [Google Scholar]
- 45.Manuck TA, Lai Y, Meis PJ, Dombrowski MP, Sibai B, Spong CY, Rouse DJ, Durnwald CP, Caritis SN, Wapner RJ, Mercer BM, Ramin SM. Progesterone receptor polymorphisms and clinical response to 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2011;205:135, e131–139. doi: 10.1016/j.ajog.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Ogle TF. Signal transducer and activator of transcription 3 is expressed in the decidualized mesometrium of pregnancy and associates with the progesterone receptor through protein-protein interactions. Biol Reprod. 2002;67:114–118. doi: 10.1095/biolreprod67.1.114. [DOI] [PubMed] [Google Scholar]
- 47.Tibbetts T, Conneely O, O'Malley B. Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol. Reprod. 1999;60:1158–1165. doi: 10.1095/biolreprod60.5.1158. [DOI] [PubMed] [Google Scholar]
- 48.Jones LA, Kreem S, Shweash M, Paul A, Alexander J, Roberts CW. Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone. J Immunol. 2010;185:4525–4534. doi: 10.4049/jimmunol.0901155. [DOI] [PubMed] [Google Scholar]
- 49.Medeiros PB, Febronio MV, Bonfa E, Borba EF, Takiuti AS, Silva CAA. Menstrual and hormonal alterations in juvenile systemic lupus erythematosus. Lupus. 2009;18:38–43. doi: 10.1177/0961203308094652. [DOI] [PubMed] [Google Scholar]
- 50.Goddard LM, Murphy TJ, Org T, Enciso JM, Hashimoto-Partyka MK, Warren CM, Domigan CK, McDonald AI, He H, Sanchez LA, Allen NC, Orsenigo F, Chao LC, Dejana E, Tontonoz P, Mikkola HK, Iruela-Arispe ML. Progesterone receptor in the vascular endothelium triggers physiological uterine permeability preimplantation. Cell. 2014;156:549–562. doi: 10.1016/j.cell.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panchanathan R, Shen H, Bupp MG, Gould K, Choubey D. Female and male sex hormones differentially regulate expression of ifi202, an interferon-inducible lupus susceptibility gene within the Nba2 interval. J Immunol. 2009;183 doi: 10.4049/jimmunol.0802665. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clayton JA, Collins FS. NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rozzo S, Allard J, Choubey D, Vyse T, Izui S, Peltz G, Krotzin B. Evidence for an interferon-inducible gene, ifi202, in the suceptibility to systemic lupus. Immunity. 2001;15:435–443. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.