Abstract
Purpose
The purpose of this study was to assess the breast-specific gamma imaging (BSGI) in Breast Imaging Reporting and Data System (BI-RADS) 4 lesions on mammography and/or ultrasound.
Methods
We performed a retrospective review of 162 patients who underwent BSGI in BI-RADS 4 lesions on mammography and/or ultrasound.
Results
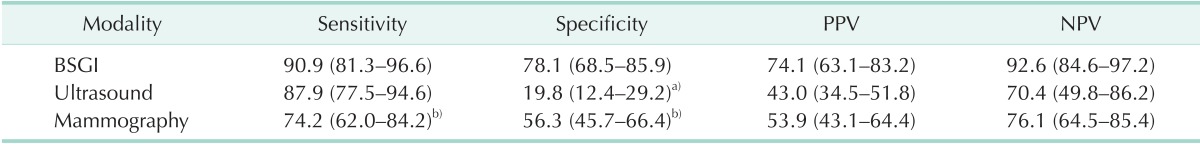
Of the 162 breast lesions, 66 were malignant tumors and 96 were benign tumors. Sensitivity and specificity of BSGI were 90.9% and 78.1%, and positive predictive value and negative predictive value were 74.1% and 92.6%. The sensitivity or specificity of mammography and ultrasound were 74.2% and 56.3% and 87.9% and 19.8%, respectively. The sensitivity and specificity of BSGI for breast lesions ≤1 cm were 88.0% and 86.8%, while the values of beast lesions >1 cm were 92.7% and 61.5%. The sensitivity or specificity of BSGI and mammography for patients with dense breasts were 92.0% and 81.3% and 72.0% and 50.0%, respectively. 26 patients showed neither a nodule nor microcalcification on ultrasound, but showed suspicious calcification on mammography. The sensitivity and specificity of BSGI with microcalcification only lesion were 75.0% and 94.4%.
Conclusion
This study demonstrated that BSGI had shown high sensitivity and specificity, as well as positive and negative predictive values in BI-RADS 4 lesions on ultrasound and/or mammography. BSGI showed excellent results in dense breasts, in lesions that are less than 1 cm in size and lesions with suspicious microcalcification only.
Keywords: Breast neoplasms, Breast specific gamma imaging, Mammography, Ultrasonography
INTRODUCTION
Breast cancer is one of the most common cancers among women with 16,000 new breast cancer cases reported in Korea every year. The prevalence rate of breast cancer has rapidly risen every year. Fortunately, the early detection rate of cases with stage II or earlier has also risen. Active participation in early detection, diagnosis and treatment has contributed to improvement of the survival rate [1].
Mammography is currently method of choice for early detection of breast cancer. The sensitivity of mammography has been reported to be 78%–85%, but the sensitivity decreases to 42%–68% in women with radiographically-dense breasts [2,3]. Compared with mammography, ultrasound has a sufficiently high sensitivity to detect breast cancer regardless of breast density. Malignancy was correctly reported in 84.7% of breast cancer patients with dense breasts [4]. However, the specificity of ultrasound was low due to higher false-positive detection rates, as mentioned in previous studies [4,5].
Since the 1990s, scintimammography with γ-emitting technetium labeled radio-tracers was utilized as another adjunctive imaging modality. However, over the years, the role of scintimammography has gradually decreased given the low sensitivity (generally <50%) demonstrated in the detection of small size (<1 cm) carcinomas [6,7]. This makes the procedure unreliable in both screening and diagnosis of tumors at a very early stage. In an effort to overcome the limitations of a traditional gamma camera for breast cancer detection, a high-resolution, small-field-of-view breast-specific gamma imaging (BSGI) was developed. This allows reliable detection of tumors smaller than 1 cm [4,8].
Clinical and research indications, suggested by the Society of Nuclear Imaging, include (1) evaluation of recently diagnosed malignancy, (2) high risk breast malignancy, (3) indeterminate breast abnormalities with remaining diagnostic concern, (4) technically-difficult breast imaging, (5) contraindicated MRI, and (6) monitoring of patients' response to preoperative neoadjuvant chemotherapy [9].
This study has focused on the Breast Imaging Reporting and Data System (BI-RADS) category 4 lesion with respect to mammography and/or ultrasound. The range of malignancy risk of BI-RADS 4 (with subcategories of A through C) is wide with a figure showing 3%–95%, in which biopsy should be considered in this category. The positive biopsy rate of BI-RADS 4 is about 15%–85%, while that of BI-RADS 4A subcategory is less than 20%. An adjunctive imaging modality for malignancy diagnosis may be helpful in risk stratification of BI-RADS 4 lesion, and expectedly may increase the positive biopsy rate. The purpose of this study was to make assessment of BSGI in BI-RADS 4 lesion on mammography and/or ultrasound, and to make comparative analysis with mammography and ultrasound, in consideration of clinical significance of the effectiveness of BSGI.
METHODS
This retrospective study included 162 patients who had been diagnosed as having BI-RADS 4 category lesions on mammography and/or ultrasound and have also undergone BSGI at the Konkuk University Medical Center from December, 2009 through September, 2013. Patients who had previously undergone surgery for breast cancer were excluded. All patients' charts were retrospectively reviewed, while all results and data were obtained from medical records. Histologic diagnosis was not available at the time of imaging study for all of these patients. Histopathologic confirmation was made by use of a core needle biopsy or surgical excision after performing the imaging studies. The Institutional Review Board in the Konkuk University Medical Center approved this retrospective study (KUH1020053).
Imaging and interpretation
Patients were administered 925–1,110 MBq of 99mTc-sestamibi through the antecubital vein contralateral to the breast lesion. BSGI was performed 10 minutes after injection of the radioisotope. The patients were seated for the procedure, and craniocaudal and mediolateral oblique images were obtained of the breasts bilaterally using a high-resolution breast-specific gamma camera (6800 Gamma Camera; Dilon Technologies Inc., Newport News, VA, USA). The acquisition time for each image was approximately 5 minutes and 100,000 counts per image were defined as the minimal range. BGSI images were interpreted by 2 nuclear medicine physician. The lesions detected by BSGI were classified as positive and negative according to visual interpretation. Lesions lacking focal uptake and those with diffuse heterogeneous or minimal patchy uptake were interpreted as negative, while those which showed scattered patchy uptake with partly focal uptake or any other focal uptake lesions were interpreted as positive. The lesions that were detected with either BSGI or conventional imaging were considered to be the same lesion when they were located in the same quadrant or distance from the nipple.
Mammography and ultrasound images were interpreted in accordance with the BI-RADS classification system by 2 radiologists. Regarding BI-RADS reporting for breast density, BI-RADS 3 or 4 on mammogram, interpreted as having dense breasts, means that fibrous and glandular tissues of the breast make up greater than 50% of the breast.
Biopsy results were classified as positive (i.e., malignancy or carcinoma in situ) or negative (i.e., benign conditions not requiring additional intervention). With respect to the size of breast lesion, the lesion size from pathologic results was used in cases of surgical excision. In breasts having both invasive ductal carcinoma (IDC) and background ductal carcinomas in situ (DCIS), the size of breast lesion was based on background DCIS that would be generally greater. In cases where only a core needle biopsy was performed, the longest diameter on ultrasound was used. Accurate size could not be measured in 16 patients with a nonmalignant calcification-only lesion on mammogram and they were excluded from statistical analysis.
Statistical analysis
Tumor sizes and patient's age are presented as the mean ± standard deviation (range) and analyzed using the independent t-test. A McNemar test was utilized for comparisons of sensitivity, specificity of BSGI, mammography, and ultrasound. Statistical significance was defined as being with in the 95% confidence interval (CI) and P-values of <0.05.
RESULTS
Of the total of 162 patients, 136 (84%) were diagnosed as having BI-RADS 4 lesions on ultrasound. 91 of 162 patients (56.2%) were diagnosed as having BI-RADS 4 lesions with findings of either microcalcification or a suspicious mass on mammography. 64 patients (39.5%) showed BI-RADS category 4 lesions simultaneously on mammography and ultrasound.
Malignant and benign lesion
Of the 162 undetermined breast lesions, 66 lesions (40.7%) proved to be malignant tumors and the remaining 96 lesions (59.3%) were diagnosed as benign tumors based on the biopsy-confirmed pathologic evaluation. Malignancies included IDCs (n = 39) (Fig. 1), invasive lobular carcinomas (n = 2), mucinous carcinoma (n = 1), adenoid cystic carcinoma (n = 1), DCIS (n = 17), lobular carcinomas in situ (LCIS, n = 4). Malignancies ranged from 2 to 77 mm in size, with 12 being less than 10 mm. The sizes of malignant and benign breast lesions were 23.3 ± 18.3 (2–77) mm and 14.4 ± 19.4 (3–145) mm, respectively. The sensitivity and specificity of BSGI, mammography, and ultrasound were shown in Table 1.
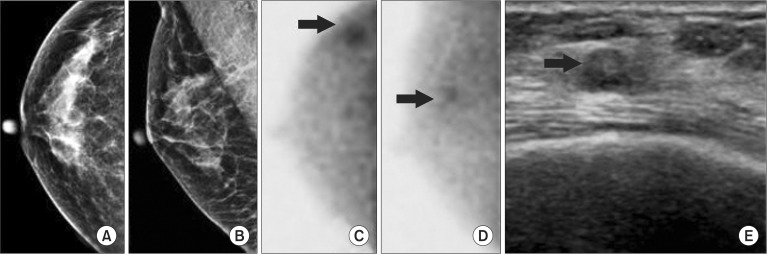
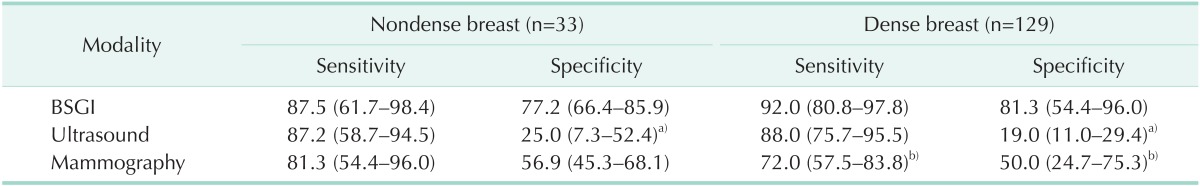
Fig. 1. Breast image of a 53-year-old woman. (A, B) Mammography did not demonstrate any abnormal lesions in the heterogeneously dense breast. (C, D) Breast specific gamma imaging demonstrated focal increased radiotracer uptake in the upper outer quadrant area (arrow) of the right breast. (E) Ultrasound demonstrated an ill-defined hypoechoic nodule at 9 h of the right breast (arrow). Pathology demonstrated an 8-mm focus of invasive ductal carcinoma.
Table 1. Sensitivity and specificity of breast specific gamma imaging (BSGI) and conventional imaging for Breast Imaging Reporting and Data System 4 lesions.
Values are presented as percentage (95% confidence interval).
PPV, positive predictive value; NPV, negative predictive value.
a)BSGI to ultrasound, McNemar P < 0.05. b)BSGI to mammography, McNemar P < 0.05.
False-positive/negative lesions
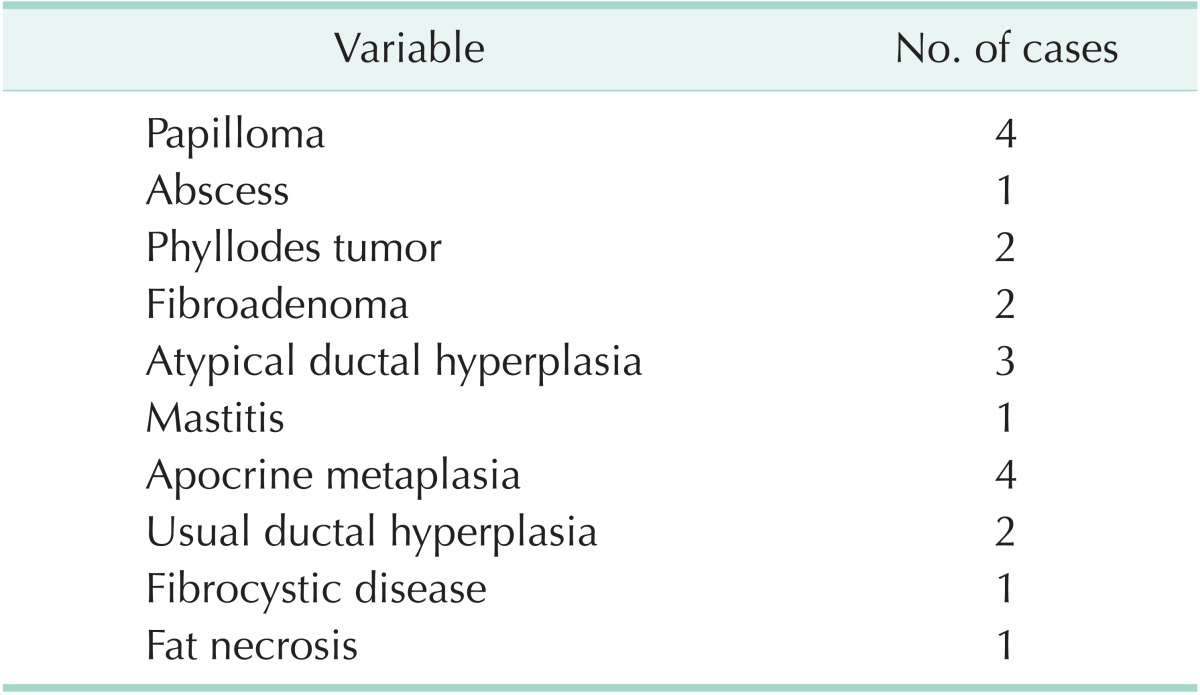
Twenty-one out of 96 benign lesions show a false positive lesion of BSGI as seen in Table 2. The mean size of these 20 false-positive lesions was 26.2 ± 31.3 (5–145) mm (with exception of 1 patient with a calcification-only lesion). Of those 66 malignant lesions, 6 showed a false-negative lesion of BSGI that included 1 IDC (18.0 mm), 3 DCIS (2.0 mm, 8.0 mm, 10.0 mm), and 2 LCIS (6.0 mm, 12.0 mm). The mean size of 6 false-negative lesions was 9.3 ± 5.5 (2–18) mm.
Table 2. Pathology of breast specific gamma imaging in false positive results.
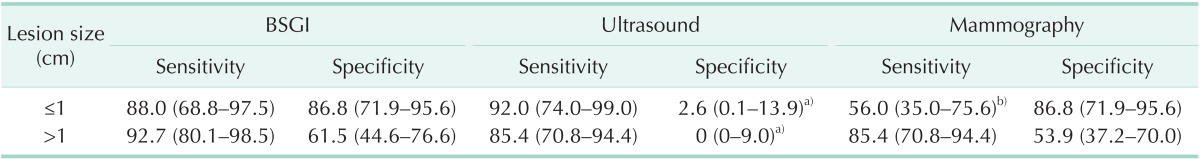
The results of BSGI, mammography and ultrasound were analyzed in accordance with lesion size (Table 3). The sensitivity and specificity of BSGI for these 68 breast lesions with a size of ≤1 cm were 88.0% and 86.8%, respectively. The sizes of malignant and benign breast masses of 68 breast lesions with a size of ≤1 cm were 8.1 ± 2.0 (4–10) mm and 7.2 ± 2.1 (3–9) mm, respectively (P = 0.080). The sensitivity and specificity of BSGI for those 75 breast lesions with a size of >1 cm were 92.7% and 61.5%, respectively. The sizes of malignant and benign breast masses of those 75 breast lesions were 30.9 ± 18.0 (12–62) mm and 25.2 ± 27.2 (11–145) mm, respectively (P = 0.278).
Table 3. Sensitivity and specificity of breast specific gamma imaging (BSGI), mammography, and ultrasound as per the breast lesion size.
Values are presented as percentage (95% confidence interval).
a)BSGI to ultrasound, McNemar P < 0.05. b)BSGI to mammography, McNemar P < 0.05.
Patients with dense breast
Of these 162 subjects, 129 (79.6%) were shown to have dense breasts with a BI-RADS density category of 3 or 4 on mammography. Fifty out of these 129 patients had a malignant lesion. The sensitivity or specificity of BSGI performed for patients with dense breasts were 92.0% and 81.3%, respectively. The sensitivity and specificity of BSGI, mammography, and ultrasound for patients with/without dense breasts were shown in Table 4.
Table 4. Sensitivity and specificity of breast specific gamma imaging (BSGI), mammography, and ultrasound in patients with/without dense breast.
Values are presented as percentage (95% confidence interval).
a)BSGI to ultrasound, McNemar P < 0.05. b)BSGI to mammography, McNemar P < 0.05.
Suspicious microcalcification on mammography
Twenty-six patients showed neither a nodule nor microcalcification on ultrasound, but showed suspicious microcalcification belonging to BI-RADS category 4 only on mammography. Eight out of 26 patients (30.8%) were confirmed to have malignancy after needle-localized surgical excision (Fig. 2). Malignancies included DCIS (n = 6) and LCIS (n = 2). The mean size of these malignant masses was 21.8 ± 12.9 (2–56) mm. The sensitivity and specificity of BSGI were 75.0% and 94.4%, respectively. BSGI showed 2 false-negative lesions that included a 2.0-mm DCIS and a 12.0-mm LCIS.
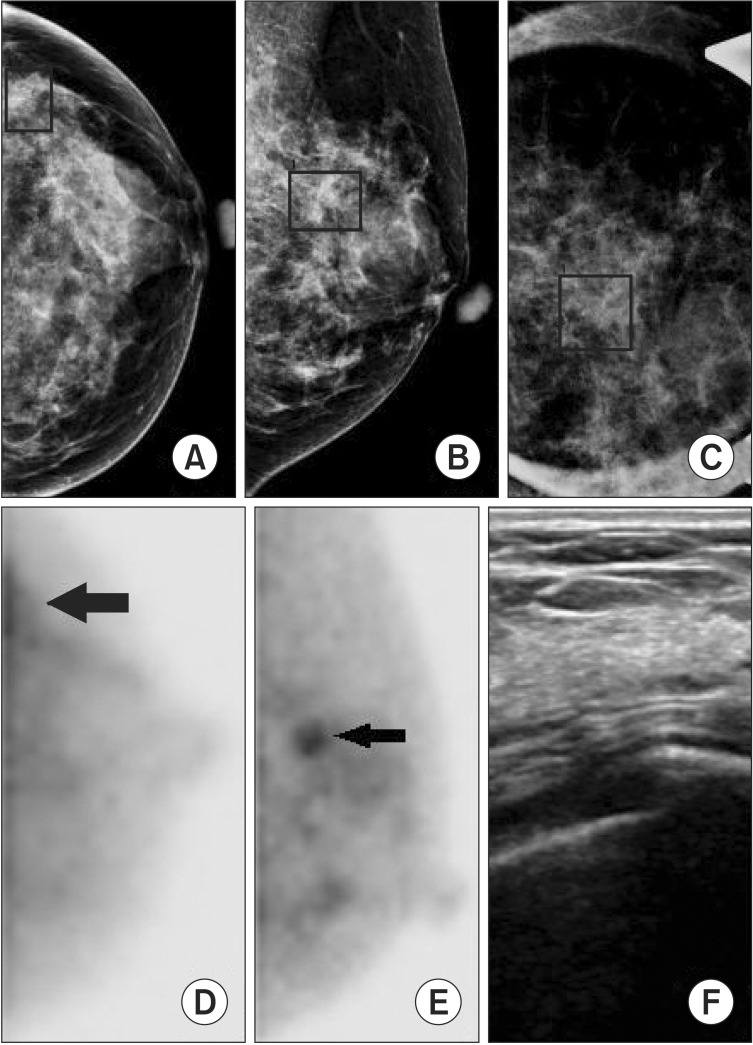
Fig. 2. A 47-year-old woman with left breast microcalcifications. (A–C) Mammography (left craniocaudal, left mediolateral oblique, left mediolateral oblique magnification) demonstrated cluster of amorphous microcalcifications at the upper out quadrant area. (D, E) Breast specific gamma imaging demonstrated focal increased radiotracer uptake in the upper outer quadrant area (arrow) of the left breast. (F) Ultrasound was negative and is not shown. Pathology demonstrated a 9-mm focus of lobular carcinoma in situ.
DISCUSSION
BI-RADS 4 category lesions may not have all the morphological characteristics of a typical cancer on mammography and ultrasound, but these lesions have a high probability of being malignant. The probability of malignancy of these lesions can vary from 3% to 94%. Furthermore, there may still be rather significant interobserver variations in the classification of categories 3 or 4. The lesions in between the BI-RADS categories 3 and 4 may improve the specificity of biopsy, and reduce useless invasive procedure when they are categorized into BI-RADS 3. In reality, observers however tend to classify these lesions into category 4 if there is the slightest uncertainty due to the possibility of malpractice. Thus, the BI-RADS category 4 would have nothing but a wide range, while these lesions are subcategorized into 4A, 4B, and 4C. However, differentiating these three subcategories would not be easy either. In this study, the validity of BSGI was assessed in patients with the BI-RADS category 4 lesions on mammography and/or ultrasound. The sensitivity and specificity of BSGI were 90.9% and 78.1%, respectively. The positive and negative predictive values were 74.1% and 92.6%, respectively. These results demonstrated the potential validity of BSGI. Not only the sensitivity and specificity were high, but also the negative predictive value of BSGI was elevated. This demonstrates sufficient potential as an adjunctive imaging method that improves the specificity of category 4 on mammography and/or ultrasound.
BSGI provides physiologic data in breast cancer imaging via two mechanisms. First, the radioactive tracer sestamibi is distributed evenly throughout the circulatory system. However, because these malignant tumors induce neoangiogenesis to support their hyperproliferation, pharmaceutical delivery to these lesions are enhanced [10]. Second, sestamibi specifically binds to mitochondria within cells, and because cancer cells have a higher cytoplasmic mitochondrial density than the surrounding breast tissue, they further retain radio-pharmaceutical [11]. These two mechanisms make BSGI highly sensitive and specific while BSGI is unaffected by breast density [12]. Mammography and ultrasound convey largely anatomic data (abnormalities). On the other hand, MRI relies on physiologic changes for the evaluation of breast cancer. On this aspect, MRI is similar to BSGI in providing physiologic data. However, MRI costs three times more than BSGI. Its poor specificity and high false-positive rate result in numerous unnecessary biopsies, additional imaging, and patient anxiety. Furthermore, BSGI can be performed easily in patients with obesity, claustrophobia, metal implants, and other conditions that limit the use of MRI [13,14]. Owing to insurance-related issues largely among patients, MRI was not included in this study.
In this study, the proportion of patients with dense breasts on mammography, corresponding to the BI-RADS density categories 3 and 4, was 79.6%. The dense breast rate of Korean women was higher than that of western women. Kim et al. [15] reported that the rates of dense breasts of Korean women at the age of 40–44 years and of 45–49 years had been 78.3% and 61.1%, respectively. Patients of dense breasts in this study showed decreased sensitivity and specificity on mammography. But the results of ultrasound and BSGI of these patients showed that is unaffected by breast density.
BSGI has consistently shown to detect breast cancers including subcentimeter cancers and difficult-to-detect cancers, such as DCIS and invasive lobular cancers [14,16,17]. This investigation also showed detection with BSGI of the smallest malignant mass of 6 mm and revealed similar BSGI results as that of other previous reports. In comparison of the group having a lesion size of 1 cm or larger with another group having a lesion size of less than 1 cm, the sensitivity of BSGI of the former was higher but the specificity was low at 61.5%. The mean size of the false-positive lesions on BSGI was 26.2 ± 34.3, which was larger than the average size. Pathologic findings of the false-positive lesions included a fibrocystic change (with and without sclerosing adenosis), papilloma, abscess and huge phyllodes tumor. Increased 99mTc sestamibi activities, often seen in patients with proliferative breast lesions likely reflect increased mitochondrial activities and mitochondrial density [18]. In addition, radiotracer uptake increased in direct proportion to the degree of regional blood flow [19]. This might be the cause of false-positive findings in nonmalignant proliferative or large-sized lesions which resulted in increased vascularity and/or mitochondrial activity (Fig. 3).
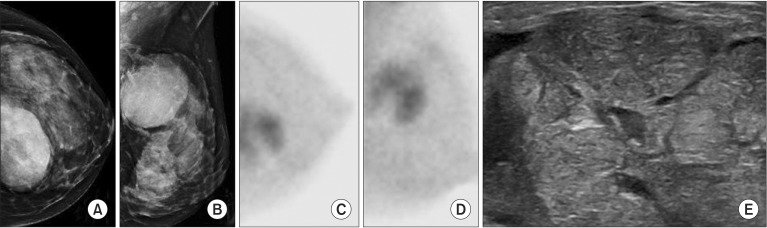
Fig. 3. A 37-year-old woman with a palpable lump in the left breast. (A, B) Mammography demonstrated a large mass at 12 h of the left breast. (C, D) Breast specific gamma imaging demonstrated increased radiotracer uptake in the upper inner quadrant area of the left breast. (E) Ultrasound demonstrated 70-mm hypoechoic mass in the left breast. Pathology demonstrated a 56-mm benign phyllodes tumor.
This study revealed a total of 6 patients with a false-negative finding on BSGI. Of those 39 patients with IDC, 1 patient with a lesion size of 18 mm showed a false negative result (with a sensitivity of 97.5%). The cause of false-negative finding would not be found. However, the possibility of technical inadequacy could not be excluded since the test was first carried out for this patient in the earliest phase of this study. Of those 17 patients with DCIS, 3 showed a false-negative finding on BSGI (with a sensitivity of 85.4%). All three of these patients had a lesion of less than 10 mm in size. Keto et al. [13] reported the BSGI sensitivity of 89% among 17 patients with DCIS lesions, while Brem et al. [14] reported the BSGI sensitivity of 91% in their DCIS patients. Conventional imaging methods demonstrated that the sensitivity of diagnosing DCIS had been lower than that of diagnosing IDC in a previous study [4]. BSGI has better sensitivity for the detection of DCIS than any other imaging method. However, BSGI has a lower sensitivity for detecting DCIS than it does for detecting IDC (85.4% vs. 97.5 %). The result of detecting DCIS from this study was similar to that of previous investigations. Ling et al. [20] reported that the BSGI sensitivity for the diagnosis of lobular neoplasm had been 100% while the sensitivity of BSGI for the detection of LCIS was 50% in this study. Nevertheless, the number of LCIS patients was too small to show any statistical significance, further study would be necessary.
In this investigation, 26 patients were diagnosed, on only mammography, as belonging to BI-RADS category 4, having only microcalcification lesion. The sensitivity and specificity of BSGI were 75.0% and 94.4%, respectively, revealing relatively good results. A finding of suspicious microcalcification on mammography alone necessitates a stereotatic biopsy or a needle-localized surgical excision. The reported false negative rates were 0.3% to 8% for stereotactic biopsy performed with a 14-gauge needle [21,22]. However, stereotatic biopsy has not commonly been performed for women in Korea and the rest of Asia, in whom their breasts are small and thin. Hahn et al. [23] reported a false negative rate of 17.6% ascertained from stereotactic guided vacuum-assisted biopsies among Asian women. In this study, the number of subjects with microcalcification - only lesion was 26, which was rather a small number. However, the false negative rate of BSGI was low at 10.5%. As compared with stereotatic biopsy, owing to the aspect that BSGI is noninvasive and requires neither compression nor positioning, BSGI has an advantage of patients feeling comfortable. If an investigation of many patients were conducted in the future, BSGI would assist in the decision-making process of whether or not a surgical excision could be performed in patients with suspicious microcalcification - only lesion.
There are limitations to this study. First, it is a retrospective study which could lead to selection bias. Second, this study analyzed a relatively small number of patients. This could lead to reduced statistical power. Third, we enrolled patients without the exclusion of the menstruation factor which may have impacted radiotracer uptake.
In conclusion, this study demonstrated that BSGI had shown high sensitivity and specificity, as well as positive and negative predictive values in patients with BI-RADS 4 lesions on ultrasound and/or mammography. Similarly, BSGI showed excellent results in dense breasts, in lesions that are less than 1 cm in size and lesions with suspicious microcalcification only. The results show that BSGI may be useful as an adjunctive diagnostic modality for detecting suspicious lesions. Furthermore, the anticipation is that a prospective study enrolling many patients may prove high negative predictive values and elevated specificity of BSGI, with which unnecessary biopsy and surgical procedure may be reduced.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Kim KS, Kim Z, Shim EJ, Kim NH, Jung SY, Kim J, et al. The reality in the followup of breast cancer survivors: survey of Korean Breast Cancer Society. Ann Surg Treat Res. 2015;88:133–139. doi: 10.4174/astr.2015.88.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg RD, Hunt WC, Williamson MR, Gilliland FD, Wiest PW, Kelsey CA, et al. Effects of age, breast density, ethnicity, and estrogen replacement therapy on screening mammographic sensitivity and cancer stage at diagnosis: review of 183,134 screening mammograms in Albuquerque, New Mexico. Radiology. 1998;209:511–518. doi: 10.1148/radiology.209.2.9807581. [DOI] [PubMed] [Google Scholar]
- 4.Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 5.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Bohm-Velez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberman M, Sampalis F, Mulder DS, Sampalis JS. Breast cancer diagnosis by scintimammography: a meta-analysis and review of the literature. Breast Cancer Res Treat. 2003;80:115–126. doi: 10.1023/A:1024417331304. [DOI] [PubMed] [Google Scholar]
- 7.Howarth D, Sillar R, Clark D, Lan L. Technetium-99m sestamibi scintimammography: the influence of histopathological characteristics, lesion size and the presence of carcinoma in situ in the detection of breast carcinoma. Eur J Nucl Med. 1999;26:1475–1481. doi: 10.1007/s002590050481. [DOI] [PubMed] [Google Scholar]
- 8.Brem RF, Rapelyea JA, Zisman G, Mohtashemi K, Raub J, Teal CB, et al. Occult breast cancer: scintimammography with high-resolution breast-specific gamma camera in women at high risk for breast cancer. Radiology. 2005;237:274–280. doi: 10.1148/radiol.2371040758. [DOI] [PubMed] [Google Scholar]
- 9.Goldsmith SJ, Parsons W, Guiberteau MJ, Stern LH, Lanzkowsky L, Weigert J, et al. SNM practice guideline for breast scintigraphy with breast-specific gammacameras 1.0. J Nucl Med Technol. 2010;38:219–224. doi: 10.2967/jnmt.110.082271. [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Sharma MC, Sarkar C. Morphology of angiogenesis in human cancer: a conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005;46:481–489. doi: 10.1111/j.1365-2559.2005.02142.x. [DOI] [PubMed] [Google Scholar]
- 11.Delmon-Moingeon LI, Piwnica-Worms D, Van den Abbeele AD, Holman BL, Davison A, Jones AG. Uptake of the cation hexakis (2-methoxyisobutylisonitrile)-technetium-99m by human carcinoma cell lines in vitro. Cancer Res. 1990;50:2198–2202. [PubMed] [Google Scholar]
- 12.Sampalis FS, Denis R, Picard D, Fleiszer D, Martin G, Nassif E, et al. International prospective evaluation of scintimammography with (99m)technetium sestamibi. Am J Surg. 2003;185:544–549. doi: 10.1016/s0002-9610(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 13.Keto JL, Kirstein L, Sanchez DP, Fulop T, McPartland L, Cohen I, et al. MRI versus breast-specific gamma imaging (BSGI) in newly diagnosed ductal cell carcinoma-insitu: a prospective head-to-head trial. Ann Surg Oncol. 2012;19:249–252. doi: 10.1245/s10434-011-1848-3. [DOI] [PubMed] [Google Scholar]
- 14.Brem RF, Fishman M, Rapelyea JA. Detection of ductal carcinoma in situ with mammography, breast specific gamma imaging, and magnetic resonance imaging: a comparative study. Acad Radiol. 2007;14:945–950. doi: 10.1016/j.acra.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Kim MH, Oh KK. Analysis and comparison of breast density according to age on mammogram between Korean and western women. J Korean Radiol Soc. 2000;42:1009–1014. [Google Scholar]
- 16.Brem RF, Floerke AC, Rapelyea JA, Teal C, Kelly T, Mathur V. Breast-specific gamma imaging as an adjunct imaging modality for the diagnosis of breast cancer. Radiology. 2008;247:651–657. doi: 10.1148/radiol.2473061678. [DOI] [PubMed] [Google Scholar]
- 17.Brem RF, Ioffe M, Rapelyea JA, Yost KG, Weigert JM, Bertrand ML, et al. Invasive lobular carcinoma: detection with mammography, sonography, MRI, and breast-specific gamma imaging. AJR Am J Roentgenol. 2009;192:379–383. doi: 10.2214/AJR.07.3827. [DOI] [PubMed] [Google Scholar]
- 18.Waxman AD. The role of (99m)Tc methoxyisobutylisonitrile in imaging breast cancer. Semin Nucl Med. 1997;27:40–54. doi: 10.1016/s0001-2998(97)80035-9. [DOI] [PubMed] [Google Scholar]
- 19.Cutrone JA, Khalkhali I, Yospur LS, Diggles L, Weinberg I, Pong EM, et al. Tc-99m Sestamibi Scintimammography for the Evaluation of Breast Masses in Patients with Radiographically Dense Breasts. Breast J. 1999;5:383–388. doi: 10.1046/j.1524-4741.1999.98086.x. [DOI] [PubMed] [Google Scholar]
- 20.Ling CM, Coffey CM, Rapelyea JA, Torrente J, Teal CB, McSwain AP, et al. Breast-specific gamma imaging in the detection of atypical ductal hyperplasia and lobular neoplasia. Acad Radiol. 2012;19:661–666. doi: 10.1016/j.acra.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Elvecrog EL, Lechner MC, Nelson MT. Nonpalpable breast lesions: correlation of stereotaxic large-core needle biopsy and surgical biopsy results. Radiology. 1993;188:453–455. doi: 10.1148/radiology.188.2.8327696. [DOI] [PubMed] [Google Scholar]
- 22.Gisvold JJ, Goellner JR, Grant CS, Donohue JH, Sykes MW, Karsell PR, et al. Breast biopsy: a comparative study of stereotaxically guided core and excisional techniques. AJR Am J Roentgenol. 1994;162:815–820. doi: 10.2214/ajr.162.4.8140997. [DOI] [PubMed] [Google Scholar]
- 23.Hahn SY, Shin JH, Han BK, Ko EY. Sonographically-guided vacuum-assisted biopsy with digital mammography-guided skin marking of suspicious breast microcalcifications: comparison of outcomes with stereotactic biopsy in Asian women. Acta Radiol. 2011;52:29–34. doi: 10.1258/ar.2010.100289. [DOI] [PubMed] [Google Scholar]