Abstract
Background
CD15, which is overexpressed on various cancers, has been reported as a cell adhesion molecule that plays a key role in non-CNS metastasis. However, the role of CD15 in brain metastasis is largely unexplored. This study provides a better understanding of CD15/CD62E interaction, enhanced by tumor necrosis factor-α (TNF-α), and its correlation with brain metastasis in non–small cell lung cancer (NSCLC).
Methods
CD15 and E-selectin (CD62E) expression was demonstrated in both human primary and metastatic NSCLC cells using flow cytometry, immunofluorescence, and Western blotting. The role of CD15 was investigated using an adhesion assay under static and physiological flow live-cell conditions. Human tissue sections were examined using immunohistochemistry.
Results
CD15, which was weakly expressed on hCMEC/D3 human brain endothelial cells, was expressed at high levels on metastatic NSCLC cells (NCI-H1299, SEBTA-001, and SEBTA-005) and at lower levels on primary NSCLC (COR-L105 and A549) cells (P < .001). The highest expression of CD62E was observed on hCMEC/D3 cells activated with TNF-α, with lower levels on metastatic NSCLC cells followed by primary NSCLC cells. Metastatic NSCLC cells adhered most strongly to hCMEC/D3 compared with primary NSCLC cells. CD15 immunoblocking decreased cancer cell adhesion to brain endothelium under static and shear stress conditions (P < .0001), confirming a correlation between CD15 and cerebral metastasis. Both CD15 and CD62E expression were detected in lung metastatic brain biopsies.
Conclusion
This study enhances the understanding of cancer cell-brain endothelial adhesion and confirms that CD15 plays a crucial role in adhesion in concert with TNF-α activation of its binding partner, CD62E.
Keywords: adhesion, brain metastasis, CD15, CD62E
Introduction
Metastasis to the Brain
Metastasis is a complex, multistep process that refers to development of distant secondary cancers from primary lesions elsewhere in the body.1–3 Metastasis to the brain occurs most commonly from lung cancer (40%–50%), breast cancer (15%–25%), and melanoma (5%–20%).4–6 Non–small cell lung cancer (NSCLC) represents the highest number of cases of brain metastases at initial presentation, and 20%–40% of NSCLC patients will develop brain metastasis during their lifetime.7,8 Overall survival of NSCLC patients with brain metastasis is extremely poor.9 The brain lacks a lymphatic vascular system and, as such, the majority of circulating cancer cells are believed to metastasize to the brain via the blood brain barrier.10 Other reported routes of entry, such as those for lymphoma, include choroid plexus and cranial nerves.11 Intravasation and extravasation through the blood-brain barrier are key steps for brain metastasis to occur; and evidence points to the important role of endothelial cells in both lymphocyte trafficking and non-CNS cancer cell metastasis.12 Moreover, astrocytes are involved in metastasis from lung to brain through expression of specific cytokines such as interleukin-8 (IL-8), interleukin-1 β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α).13 Although cancer cell extravasation has been intensively studied, the molecular mechanisms underlying metastasis to the brain are not fully understood.
CD15 and Metastasis to the Brain
CD15 or Lewis x (Lex) blood group antigen is a carbohydrate cell adhesion molecule14 expressed on glycol conjugates on various cells at different developmental stages. In particular, it is associated with human polymorphonuclear granulocytes and lung,15 breast,16 prostate, and kidney cancer cells.17 CD15 is crucial in the cell-cell recognition process;18 it has been suggested that its absence from human glioma cells might explain why brain tumors rarely metastasize extraneurally.19 CD15 has been recently identified as a potential cancer stem-like cell marker in human and murine glioma spheroids.20,21 Significantly, it is a marker for metastatic lung adenocarcinoma,15,22 being correlated with metastasis to non-CNS sites. However, the functional role of CD15 in metastasis from lung to brain remains obscure.
E-Selectin on Brain Endothelial Cells
CD62E is a cell-surface glycoprotein and CD15 ligand whose expression is induced by TNF-α, IL-1β, and lipopolysaccharide.23 Induction starts at CD62E transcription through an ATF-binding element in the CD62E promoter.23 TNF-α activates NFκB, and c-Jun NH2-terminal kinase (JNK1) and p38 signaling pathways, which are both required for CD62E expression.23 TNF-α stimulates high levels of CD62E, while IL-1β is less efficient on cultured human umbilical vascular endothelial cells (HUVECs). In addition, IL-3, TNF-α, and IL-10 stimulate CD62E expression in HUVECs, human dermal microvascular endothelial cells,24 and brain microvascular endothelial cells.25–28
Role of CD15 and E-Selectin (CD62E) in Metastasis to the Brain
Currently, no definitive model of the metastatic process of cancer entry to the brain exists; previous studies in other tissues indicate that circulating cancer cell extravasation occurs in a similar manner to that of leukocytes, with sequential steps of tethering, rolling, adhesion, and transmigration. Studies on metastasis into colon and liver have suggested that interactions between selectins and their ligands regulate cancer cell adhesion.29–31 CD62E interactions with its ligands are mediated by the N-terminus homologous to the C-type listing domain. CD62E overexpression correlates with metastatic behavior in colon cancer, while CD62E immunoblocking significantly decreases metastasis from liver to pancreas32 and lung to breast in mice33 CD15 accumulates on the invasive edges of breast carcinoma boli, suggesting its potential role in metastasis.34 Few studies have, however, focused on the role of CD15 in metastasis to the brain. CD15 is rarely expressed on primary brain tumor cells; this observation provides a plausible explanation why glioma rarely metastasizes extraneurally.19,35 Recently, the first population-based analyses of lung cancer registries in the USA and Canada, where data were collected for secondary metastasis, revealed that the brain is the most common site of metastasis at initial diagnosis of stage IV lung cancer (NSCLC), highlighting the importance of understanding entrance mechanisms of circulating lung cancer cells into the brain.7
Materials and Methods
Ethics Statement
All cell lines established “in house” were conducted in accordance with the National Research Ethics Service (NRES) instructions under Ethics permission 11/SC/0048.
Cell Culture
The human cerebral microvascular endothelial cell line (hCMEC/D3) was donated by Professor Pierre Olivier Couraud (Institute of Cochin, INSERM, Paris, France).36 hCMEC/D3 cells were cultured in endothelial basal medium-2 (EBM-2) supplemented vascular endothelial growth factor (VEGF), human epidermal growth factor (hEGF), R3-insulin-like growth factor-1 (R3-IGF-1), ascorbic acid, hydrocortisone, human fibroblast growth factor-beta (hFGF-β), heparin (all from Lonza), and 2% human serum (Biosera). Primary NSCLC cells (A549 and COR-L105) were purchased from Sigma and metastatic NSCLC cells from cervical lymph nodes (NCI-H1299) from ATCC. Low-passage brain-metastatic NSCLC cell lines (SEBTA-001 and SEBTA-005) were established in house using biopsies from patients with lung-brain secondary tumors. All NSCLCs were cultured in Dulbecco′s modified Eagle medium supplemented with 2% human serum (Biosera) and maintained in 5% CO2 and humidified atmosphere at 37°C. All cell lines were subjected to routine mycoplasma testing, utilizing a kit from Lonza. Cell authentication was conducted using a microfluidic electrophoresis system incorporating an Agilent 2100 Bioanalyzer (Agilent Technologies\) to analyze STR-PCR fragments from 10 human genomic loci of human cell lines.37
Antibodies
Primary Antibodies
Mouse monoclonal anti-CD15 (MEM-158) (Sigma) was used at the following dilutions: 1:100 for immunocytochemistry (ICC) and Western blot (WB) and 1:10 for flow cytometry and immunoblocking. Mouse monoclonal anti-CD15 (Dako) was used at 1:100 for immunohistochemistry (IHC). Mouse monoclonal anti-CD62E (Abcam) was used at the following dilutions: 1:500 for ICC, 1:10 for flow cytometry, 1:500 for WB, 1:10 for immunoblocking, and 1:150 for IHC. Rabbit polyclonal anti-ABCE1 (Novus,) was used as a loading control for WB at 1:500.
Secondary Antibodies
Fluorochrome-conjugated Alexa Flor-488 (Invitrogen) was used for ICC and flow cytometry at 1:500, and horseradish peroxidase (HRP)-conjugated IgG (Invitrogen) 1:5000 was used for chemiluminescent detection in WB.
Isotype Controls
Isotype control antibodies were used to confirm the specificity of primary antibody binding. IgM isotype antibodies (Invitrogen) were used at 1:100 for ICC, 1:10 for flow cytometry, and 1:10 for immunoblocking for CD15 isotype controls and IgG isotype antibodies (Invitrogen) at 1:50 for ICC and 1:10 for flow cytometry for CD62E controls.
Immunocytochemistry
ICC was performed according to a protocol previously established in our laboratory.38 Briefly, cells were seeded onto sterile coverslips at 1 × 105/well and incubated at 5% CO2 and humidified atmosphere at 37°C. Cells were fixed with 4% paraformaldehyde (Sigma) for 3 minutes followed by 3 washes with phosphate-buffered saline (PBS). Cells were then blocked with 10% serum in PBS (Sigma) and incubated with the primary antibody for 1 hour followed by incubation with secondary antibody. Nuclei were counterstained with Hoechst Blue (Sigma). Coverslips were mounted on slides using Vectashield (Vector Laboratories) and viewed using a Zeiss Axio Imager Z1 fluorescence microscope. Images were captured using Volocity Image Analysis Software (V 5.2, Perkin Elmer).
Flow Cytometry Analysis
Cells were harvested by gentle scraping, followed by blocking in 2% goat serum/PBS (Sigma) and then probed with primary antibody while nonspecific IgM isotype was added to the negative control. Cells were then washed with PBS and incubated with secondary antibody, resuspended in PBS, and then transferred to fluorescence-activated cell sorting (FACS) tubes (BD Biosciences). Propidium iodide (Sigma) was added to samples for extracellular antigen detection. Analysis was performed on a 4-color-multiparameter FACS Calibur (BD Biosciences). Each experiment was repeated 3 times in triplicate. The expression level was assessed by percentage of positive cell population.
Western Blotting
WB analysis was performed using cell membrane extracts, which were isolated using a cell fractionation subcellular protein fractionation kit for cultured cells (Thermo Scientific). Cell lysates were separated in 10% acrylamide SDS-PAGE gel and transferred to a PVDF high sensitivity membrane. Proteins were detected using primary antibodies and horseradish peroxidase-conjugated secondary antibodies. Immunocomplexes were revealed using an enhanced chemiluminescence reagent (Millipore). The blots were visualized and analyzed with GBOX Chemi XT16 system (Syngene).
Adhesion Assays
The CytoSelect Tumor-Endothelium Adhesion Assay Kit (Cell Biolabs) was used to evaluate the adhesion potential of tumor cells on a brain endothelial cell monolayer. Briefly, 1 × 106 brain endothelial cells (hCMEC/D3) were seeded onto fibronectin (10 µg/mL) and grown to confluency. The endothelial cell monolayer was first treated with 25 pg/mL of TNF-α overnight to activate CD62E expression. Primary and secondary lung cancer cell lines were tagged by a green fluorescent dye (Cyto Tracker, Cell Biolabs). 1 × 105 cancer cells were then seeded onto the activated hCMEC/D3 monolayer for 90 minutes. Nonadherent cells were washed with PBS, and relative cell attachment was determined using a POLARstar OPTIMA microplate reader (BMG LABTECH,). The experiment was repeated 3 times in triplicate. In a separate method to evaluate adhesion, we used ICC and confocal image analysis (see above). The same conditions that were used in the CytoSelect adhesion assay were repeated, except on coverslips, and prepared for ICC. Semiquantification of adhesion was assessed using confocal images and Zeiss ZEN software.
Viability Assays
Cells were seeded at 1 × 104 in 96-well plates and incubated at different concentrations (0, 5, 10, and 25 ng/mL) of TNF-α and TNF-β. Similar experiments were performed using CD15 monoclonal antibodies (1:100). Cell viability was measured at different time points using CellTiter 96 Aqueous One Solution cell proliferation assay (MTS) (Promega). Absorbance was measured at 490 nm using a microplate reader.
Cancer Cell Adhesion Under Shear Stress and Live Cell-imaging Microscopy
Vena8 endothelial biochips (channel volume: 2.69 µL) (CellixLtd) were used to assess tumor cells-brain endothelial adhesion under shear stress. The chips were coated with 0.5 mm of 10 µg/mL fibronectin solution (Sigma) and incubated for 1 hour. 1.5 × 106 of hCMEC/D3 cells were seeded in each channel and incubated for 2 hours. The chips were connected to a Microfluidic pump (CellixLtd,), and the whole unit was kept overnight in an incubator at 37 °C, 5% CO2 under shear stress flow on perfusion mode with a 10 mL/hour volumetric flow rate (2.5 dyn/cm2). The biochip was then connected to a Zeiss Axiovert 200 M inverted live cell (time lapse) microscope at 37°C, 5% CO2. Cancer cells (green fluorescently tagged) at 1 × 106 cells/mL were then pumped onto the hCMEC/D3 monolayer at 2.5 dyn/cm2 controlled by a Mirus Evo nanopump (CellixLtd) and analyzed via Vena Flux Assay software. Live cell images were taken once every 10 minutes over 72 hours to monitor cancer cell adhesion on the brain endothelial cell monolayer (37°C, 5% CO2). The images were collected, and movie sequences were generated using Volocity software (V5.4, Perkin Elmer). The experiment was repeated 3 independent times in triplicate.
Immunohistochemistry
Paraffin-embedded human normal brain and lung-brain metastatic biopsy tissues were immunohistochemically stained for CD15 (Dako) and CD62E (Abcam). These methods are described in Supplementary material, S3.
Confocal Microscopy
ICC images were obtained using the X40 and X100 oil immersion objectives of a Zeiss LSM 510 Meta Axioskop2 confocal microscope using lasers with excitation wave lengths of 405 nm (blue), 488 nm (green), 568 nm (red), and 674 (purple) with diode, argon, and HeNe1 lasers, respectively. Identical settings were used to image negative controls in which primary antibody was replaced by a nonspecific Isotype.
Statistical Analysis
All experiments were performed 3 times, and data are expressed as±SE. Statistical analyses were performed using 1-way ANOVA followed by Tukey's multiple comparison post hoc tests using Graph Pad Prism 6 software for analysis.
Results
CD15 Expression in Cultured Brain Endothelial and NSCLC Cell Lines
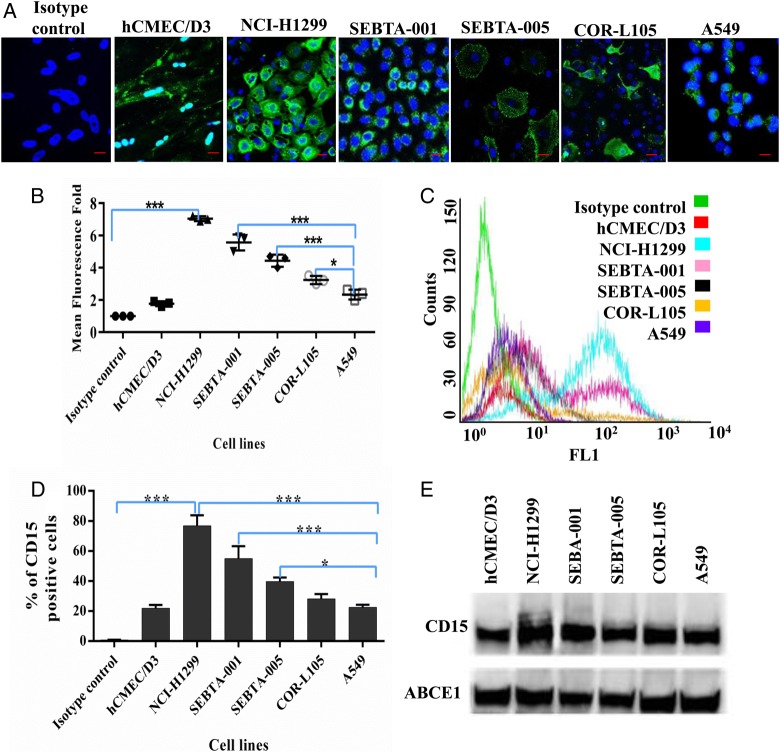
CD15 cell surface expression and localization were characterized on brain endothelial cells, primary, and metastatic NSCLC cells using ICC, flow cytometry, and WB analysis. Semiquantitation of confocal images (Fig. 1A and B) demonstrated that, when compared with isotype controls, CD15 immunoreactivity was highest on metastatic lung cancer cells NCI-H1299 (P < .0001) followed by SEBTA-001 and SEBTA-005, A549, COR-L1299, and hCMEC/D3, respectively (Fig. 1A and B). There was no significant difference between CD15 expression in hCMEC/D3 compared with isotype control and A549. There was a significant increase in CD15 expression compared with isotype control with positivity levels of NCI-H1299: 79%, SEBTA-001: 54%, SEBTA-005: 39%, COR-L105: 31%, A549: 23%, and hCMEC/D3: 19.69% (Fig. 1C and D). There were no significant differences in CD15 expression in hCMEC/D3 compared with A549 and COR-L105. Western blot results were consistent with these analyses (Fig. 1E).
Fig. 1.
Extracellular expression of CD15 in brain endothelial and lung cancer cell lines. (A) Representative immunocytochemical images showing extracellular expression of CD15 in human brain endothelial cells (hCMEC/D3), human non–small cell lung cancer cells (NSCLC) metastatic cells obtained from cervical lymph node (NCI-H1299), brain (SEBTA-001 and SEBTA-005) and in nonmetastatic NSCLC cells (A549 and COR-L105). (B) Semi-quantitative analysis of CD15 expression from confocal images (A) using Zeiss ZEN image software. (C) Representative flow cytometric histogram. (D) Flow cytometric analysis of CD15 expression on hCMEC/D3, NCI-H1299, SEBTA-001, SEBTA-005, A549, and COR-L105. CD15 was highly expressed on NCI-H1299 and SEBTA-001 with less expression on COR-L105 and SEBTA-005, which expressed relatively the same amount. N = 3, ***P < .0001, **P < .001 and *P < .01. There was also less CD15 expression on A549 and hCMEC/D3 cells. (E) Western blot of proteins from the cell lines showed highest CD15 expression in NCI-H1299, followed by SEBTA-001, SEBTA-005, COR-L105, A549, and hCMEC/D3. ABCE1 was used as a protein loading control.
TNF-α Increases CD62E Expression in Human Brain Endothelial Cells and NSCLC Cell Lines
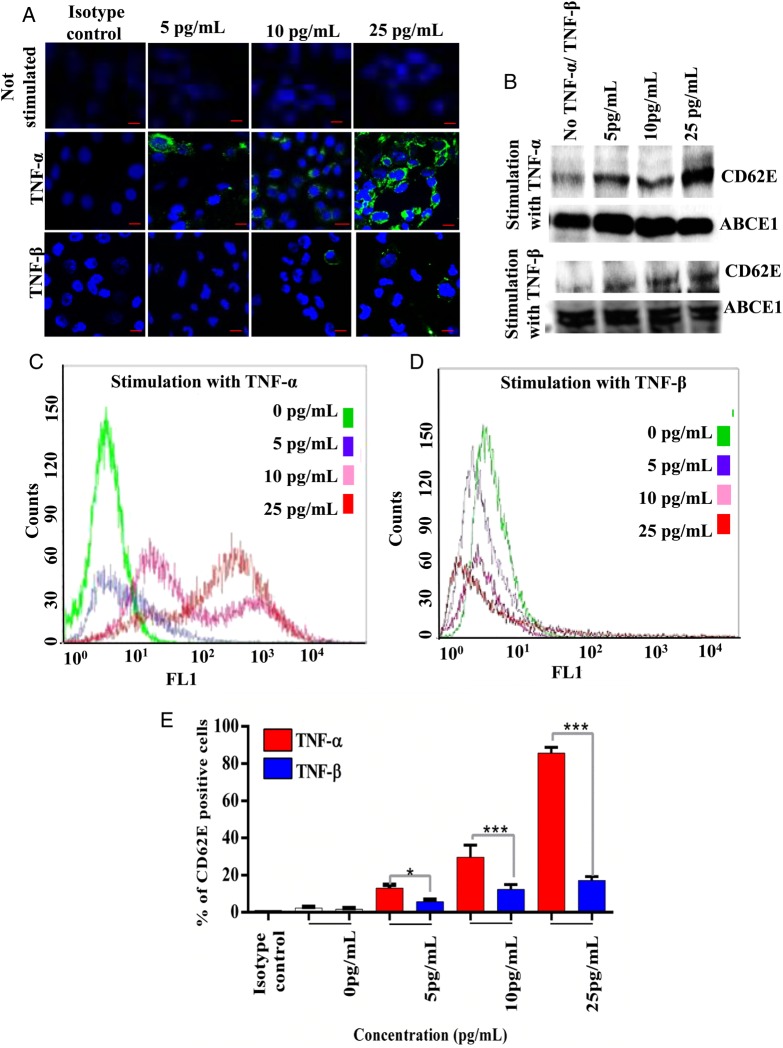
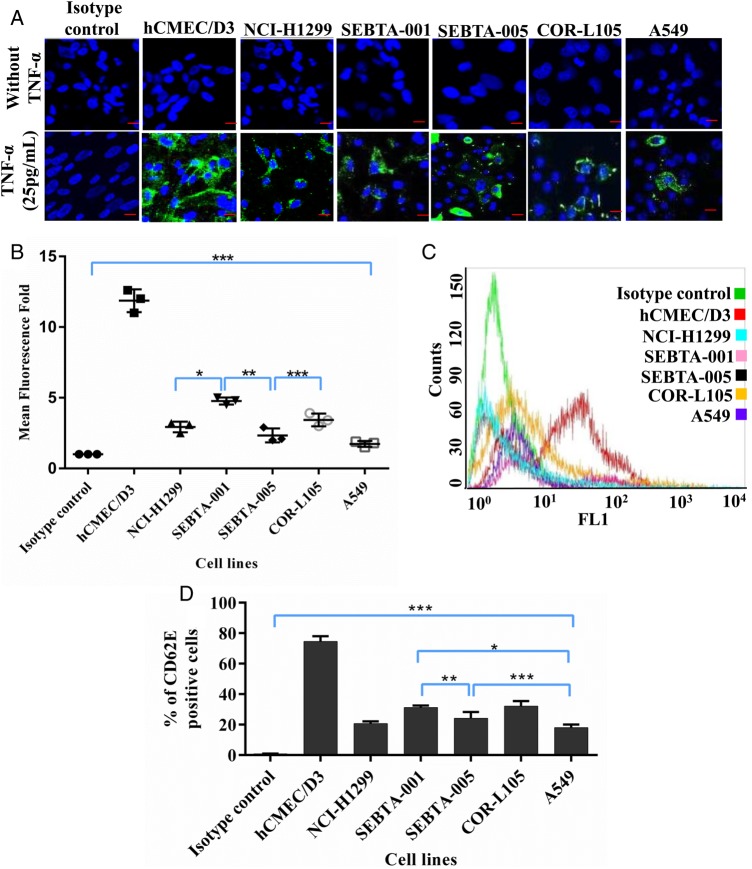
TNF-α treatment of brain-derived endothelial cells (hCMEC/D3), resulted in an increase in CD62E protein expression in a concentration-dependent manner compared with nonstimulated cells (Fig. 2A–C and E). To ensure that this was a specific effect of TNF-α, CD62E expression was further examined in TNF-β treated hCMEC/D3 cells (Fig. 2A,B and D,E). ICC, flow cytometry, and Western blotting were used to evaluate CD62E expression in brain endothelial cells cultured for 18 hours at 3 different concentrations of TNF-α and TNFβ (5 pg/mL, 10 pg/mL, and 25 pg/mL). While CD62E expression was significantly higher in TNF-α treated hCMEC/D3 cells compared with the lung cancer cell lines (P < .0001), there were also significant differences in CD62E expression within the group of lung cancer cell lines that were also treated for 18 hours with 25 pg/mL of TNF-α (Fig. 3A–D). Semiquantitation of confocal images (Fig. 3A and B) demonstrated the highest CD62E expression associated with hCMEC/D3 cells (Fig. 3B). CD62E expression in hCMEC/D3 (73.88%) cells was significantly higher than CD62E expression in all lung cancer cell lines tested (P < .0001). CD62E expression in SEBTA-001cells was significantly higher than NCI-H1299 (P < .01), SEBTA-005 (P < .001), and A549 (P < .0001). Flow cytometric analysis of lung cancer cells revealed CD62E-positive cells in SEBTA-001 (34.17%), in SEBTA-005 (27.6%), in NCI-H1299 (20.6%), in COR-L105 (32.7%), and in A549 (17.53%) (Fig. 3C and D).
Fig. 2.
CD62E expression in TNF-α treated endothelial cells. (A) hCMEC/D3 cells were positively stained for CD62E extracellular expression at different TNF-α and TNF-β concentrations (green). (B) Western blotting of hCMEC/D3 cultured in increasing concentrations of TNF-α and TNF-β. (C and D) Overlay histogram of flow cytometric analysis of CD62E expression in hCMEC/D3 cells cultured with different concentration of TNF-α and TNF-β. (E) Flow cytometric analysis of CD62E expression in hCMEC/D3. N = 3, ***P < .0001, **P < .001, *P < .01.
Fig. 3.
CD62E expression in TNF-α treated lung cancer cell lines. (A) Representative immunocytochemical images showing expression of CD62E in cancer cell lines following treatment with TNFα (25 pg/mL). CD62E was highly expressed and well distributed across cell membrane of hCMEC/D3 cells, at lower levels on non–small cell lung cancer metastatic cells (NCI-H1299 SEBTA-001 and SEBTA-005) and primary NSCLC cells (A549 and COR-L105). (B) Semiquantitation analysis of CD62E cells from confocal images (A) using Zeiss ZEN image software. (C) Overlay histogram of flow cytometric analysis of CD62E expression in cells treated with TNFα (25 pg/mL). (D) Flow cytometric analysis of CD62E expression in NCI-H1299, SEBTA-001, SEBTA-005, A549, and COR-L105. N = 3, ***P < .0001, **P < .001, *P < .01.
CD15 and CD62E Mediate Adhesion of NSCLC Cells to hCMEC/D3 Monolayer Under Static Conditions
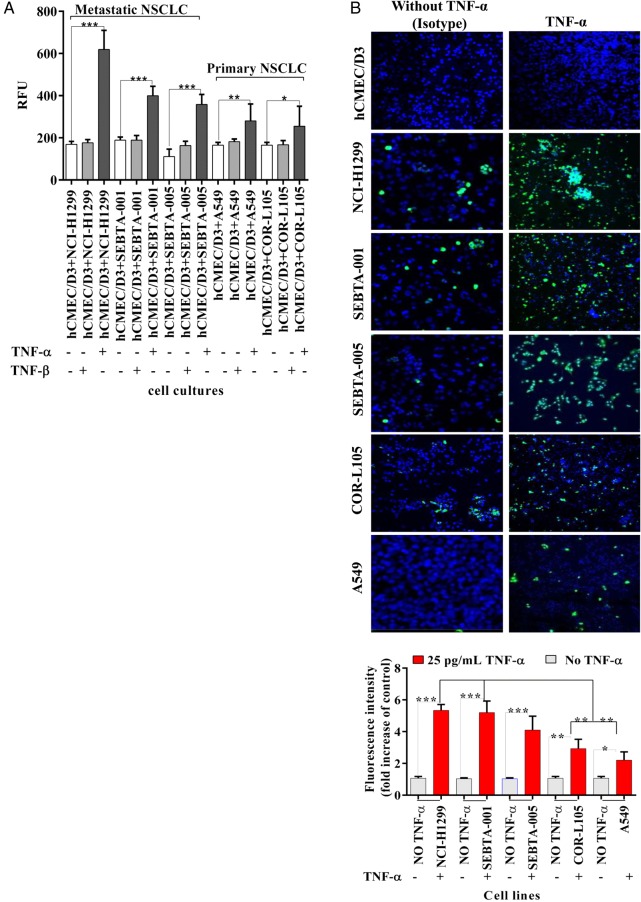
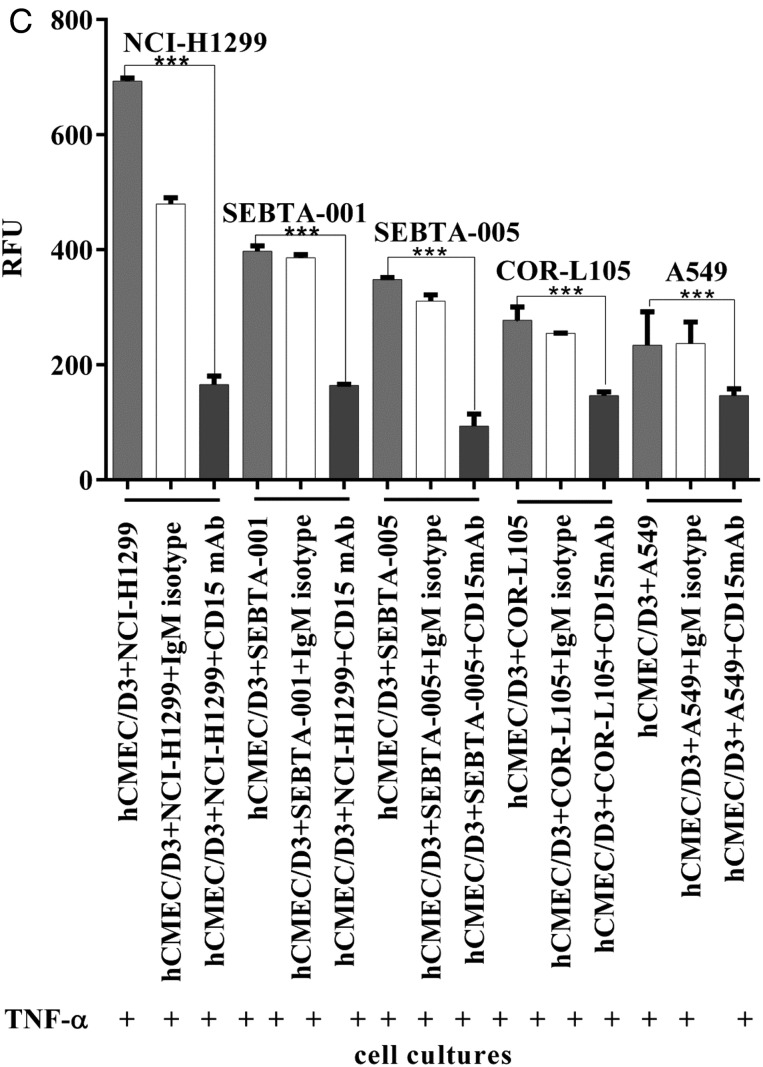
We first wanted to determine if the TNF-α increased CD62E in endothelial cells led to an increase in tumor cell adhesion. hCMEC/D3 cells were plated and treated with TNF-α or TNF-β for 18 hours, followed by several washes and the addition of serum-free medium containing lung cancer cells prelabeled with a green fluorescent dye (Cyto Tracker). Following a 90 minute incubation, co-cultures were washed, and cells were lysed and evaluated for levels of fluorescence. hCMEC/D3 cells incubated with TNF-α resulted in a significant increase in NSCLC adhesion, with the highest relative fluorescent units being associated with the NCI-H1299 cells followed by the lung cancer cells (Fig. 4A, P < .001). There was no effect on adhesion when hCMEC/D3 cells were treated with TNF-β (Fig. 4A). To rule out that a change in viability via TNF-α may have contributed to this increase in adhesion, viability assays were conducted. There was no change in hCMEC/D3 cell viability following TNF-α treatment (Supplementary material, S1). However, hCMEC/D3 cells treated with TNF-β demonstrated significantly less viability when measured over 4 days (Supplementary material, S1). The time course for the adhesion assay described above consisted of an 18 hour incubation with TNF-α or TNF-β, after which the cancer cell lines were added and allowed to adhere for 90 minutes. Therefore the change in cell viability seen with TNF-β at later time points would not have accounted for the lack of adhesion (Fig. 4A). We used confocal image analysis in a separate method to evaluate adhesion. The same conditions used in the assay above were repeated, except on coverslips, and prepared for ICC. Semiquantification of confocal images using Zeiss Zen software revealed that the metastatic cell line NCI-H1299 cells were the most adherent, followed by metastatic cells (SEBTA-001 and SEBTA-005) and primary NSCLC cells (COR-L105 and A549) (Fig. 4B). These results suggest a strong correlation between CD15 expression (Fig. 1) and NSCLC cell adhesion. To determine whether CD15 plays a key role in NSCLC cell adhesion to brain endothelium, an adhesion assay was conducted in the presence of CD15 antibodies or isotype (IgM) controls (Fig. 4C). CD15 mAb-blocking significantly decreased metastatic (NCI-H1299, SEBTA-001, and SEBTA-005) and primary NSCLC (COR-L105 and A549) adhesion compared with nontreated cells. Furthermore, there was no significant change in adhesion of cancer cells due to blocking with nonspecific isotype (IgM) (P < .0001) (Fig. 4C). There were also no observed toxic effects of antibody incubation with the various cancer cell lines or the endothelial cell line (Supplemental material, S2).
Fig. 4.
Non–small cell lung cancer (NSCLC) cell adhesion to TNF-α treated brain endothelial cells and the effect of CD15 immunoblocking. (A) Qualitative adhesion of NSCLC cells on human brain endothelial cell monolayer. Primary and metastatic NSCLCs were incubated for 90 minutes on a monolayer of activated hCMEC/D3 cells. Nonadherent cancer cells were washed away, and adherent cells were lysed and quantified via a microplate reader at 480–520 nm. N = 3, P < .0001=***. The results showed the strong effect of CD62E, once stimulated by TNF-α on lung tumor cells (red bars). Absence of TNF-α showed a significant decrease in cancer cell adhesion (white bars) (P < .001 = ***). (B) Confocal images (top panel) showing adhesion of green fluorescently labeled NSCLCs on brain endothelial cell monolayer(blue) and semiquantitation analysis of confocal images (lower panel) using Zeiss ZEN image showing a significant increase in NCI-H1299, SEBTA-001, and SEBTA-005 adhesion to TNF-α treated hCMEC/D3 cells. N = 3, ***P < .0001, **P < .001, *P < .01. (C) Quantitative adhesion of human primary and metastatic NSCLC cells blocked with CD15 mAb (red bar) and with nonspecific isotype IgM (white bar) to assess CD15 mAb-blocking efficiency and specificity. Adhesion of NSCLC cells on a monolayer of hCMEC/D3 (grey bar) acts as a negative control. N = 3, P < .0001 = ***.
CD15 mAb Blocking Decreases Adhesion of NSCLC Cells Under Shear Stress
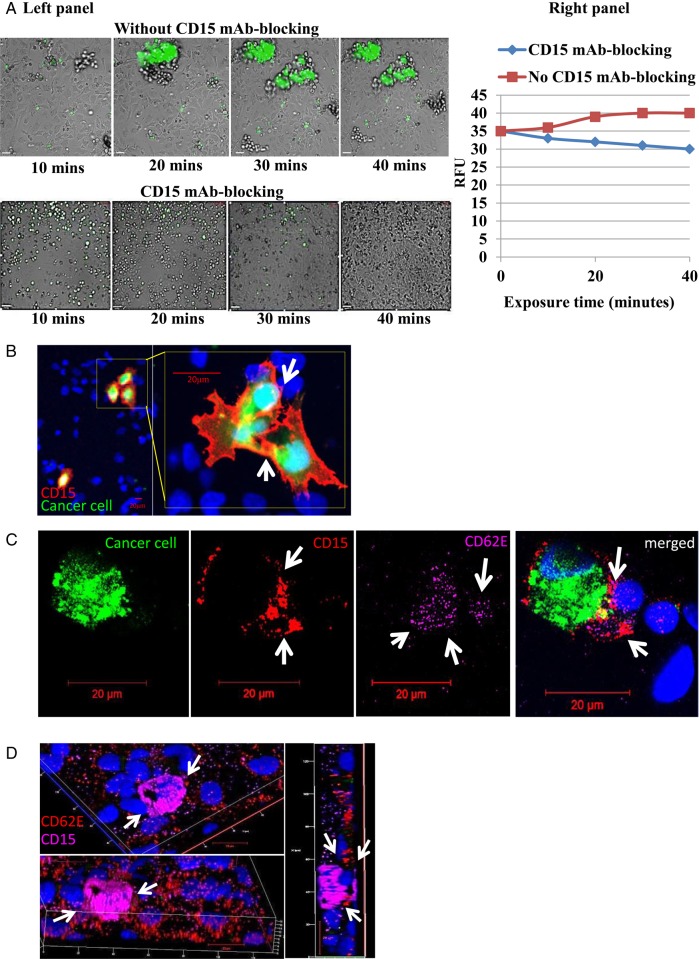
To determine if the adhesion results obtained from static experiments would hold when experimental conditions were conducted under shear stress, we used the Vena8 endothelial+biochip and micropump (Cellix) and conducted live cell microscopy to determine the effect of CD15 immunoblocking on dynamic adhesion of metastatic lung to brain cancer cells (SEBTA-001) over a 40 minute time range with a perfusion rate at 2.5 dyn/cm2 of fresh medium (Fig. 5A). CD15 mAb-blocking was shown to decrease the number of adherent cancer cells on the brain endothelial cell monolayer lining the Vena8 biochip channel compared with nonblocked cells (Fig. 5A, lower panel and side graph). The aggregation of the SEBTA-001 cells seen in Fig. 5A (arrows) without immunoblocking suggests homophilic binding of CD15 on cancer cells as well as heterophilic binding between CD15 and CD62E. This is supported by confocal images of ICC performed on co-cultures of green fluorescently tagged metastatic cancer cells (SEBTA-001) adhering on a monolayer of activated brain endothelial cells (Fig. 5B and C). Results showed prominent and condensed expression of CD15 on adherent cancer cell surface and cell processes (Fig. 5C arrows). Both CD15 and CD62E were also localized at the site of cancer cell-brain endothelial cell adhesion, and CD62E was seen distributed on the activated endothelial cells and co-localized with CD15 supporting heterophilic adhesion sites between cancer cells and brain endothelial cells (Fig. 5C arrows). To confirm CD15 and CD62E localization at the site of adhesion, 3-dimensional confocal images created from z-stacks (Fig. 5D) were analyzed in culturing SEBTA-001 on a monolayer of endothelial cells.
Fig. 5.
CD15 mAb-blocking reduces adhesion of non–small cell lung cancer (NSCLC) cells under dynamic conditions. (A, left panel) A dynamic cell adhesion assay was carried out on highly metastatic brain cells (SEBTA-001) using an AxioVert 200 M microscope (C. Zeiss) within an environmentally controlled incubator. SEBTA-001 cells were incubated with isotype control (IgM) or CD15 mAb followed by perfusion of 1 × 106 cells over a monolayer of hCMEC/D3 cells at 2.5 dyn/cm2 for 40 minutes. Phase contrast and fluorescent images were acquired at real time every 10 minutes with an X5 objective using Volocity software. Scale bar = 20 μm. (A, right panel) Representation of A, left panel in relative fluorescent units of SEBTA-001 cell adhesion with and without CD15 mAb for 10, 20, 30, and 40 minute time points. (B–D) Confocal images of green fluorescently labeled adherent brain to lung metastatic cancer (SEBT-001) cells cultured on a monolayer of hCMEC/D3 cells (blue). (B) CD15 expression (red) on the edges of SEBT-001 (green). ICC images showed expression of CD15 (red) on the adherent cancer cells (green) on a monolayer of human brain endothelial cells stained with Hoechst blue C: The merged image shows expression of CD15 (red) on adherent tumor cells (SEBTA-001) (green) on an activated monolayer of human brain endothelial cells (blue) expressing CD62E (purple). (D) Optical sections of 3-dimensional confocal image created from z-stack. The top and lower views represent one image at different angles through the z-stack, showing an adherent SEBTA-001 expressing CD15 (purple) on an activated monolayer of brain endothelial cells expressing CD62E (red). Right-side view represents an optical section through the depicted z-stack showing the precise interaction between CD15 and CD62E during NSCLC-brain endothelium cell adhesion.
CD15 and CD62E Expression in Human Biopsy of Lung-to-brain Metastasis
Immunohistochemistry was conducted on paraffin-embedded, formalin-fixed tissue sections of human lung metastasis to brain biopsies using antibodies to both CD15 and CD62E (Supplemental material Fig. S3). Both CD15- and CD62E-positive cells could be seen throughout the tumor core, whereas neither was detected in adult normal brain cortex. In patient 1, there was an area of host tumor interface where CD15-positive cells were detected within vessels, and CD62E was associated with endothelial cells lining the vessels in the same patient.
Discussion
Three-quarters of brain tumors are metastatic cancers originating from primaries in distant organs; the brain is known to be a key target of secondary NSCLC, and 20%–40% of lung cancer patients develop oligometastasis or multiple-brain metastases during their illness.8 Metastasis to the brain is a complicated, multistep process requiring interaction between metastatic cancer cells and target environment. However, these interactions are not yet fully understood, particularly the adhesion of cancer cells on brain endothelium in early stages of entry into the brain. CD15 is a cell-adhesion fucosylated carbohydrate that is expressed on leucocytes and various types of non-CNS cancer cells,39 but it is rarely expressed in the human brain.35 Moreover, CD15 overexpression has been correlated with a progression to metastatic stage. Indeed, significant correlation exists between CD15 overexpression and colorectal cancer metastasis through prominent CD15 expression on the invading edges of cancer lesions.39 CD15 is involved in the extravasation process through its interaction with selectins, particularly E-selectin (CD62E), a glycosylated transmembrane and cellular adhesion molecule crucial for homing of circulating cells through its expression on endothelial cells.39 CD62E facilitates cancer cell adhesion to endothelial cells in various cancers such as colon,39 breast,40 and lung.41 Here we hypothesized that CD15 and CD62E interaction is involved in cancer cell adhesion during metastasis to the brain. Previous studies have shown that human bone-marrow microvascular endothelial cells play an important role in metastasis to the brain via interaction between CD62E on endothelial cells and its ligands on metastatic neoplasms.42 However, most studies have employed nonspecialized experimental conditions such as human cells cultured in fetal calf serum, using nonspecific medium and nonbrain-derived endothelium. In our study, we used 2% human serum and medium supplemented with specific growth factors for each cell line to maintain cell differentiation and characteristics.43 We used human brain endothelial cells (hCMEC/D3) established from temporal lobe microvessels from an epileptic patient and then immortalized by introducing human telomerase or SV40T antigen-employing lentiviral vectors.36 HUVECs have previously been used to study metastasis to the brain;41 however, functional differences were demonstrated between leukocyte adhesion on HUVECs and brain-derived endothelium.44,45 In the present study, CD15 was overexpressed on metastatic NSCLC cell membranes (NCI-H1299, SEBTA-001, and SEBTA-005); while there was a lower expression on primary NSCLC cells (COR-L105 and A549). Moreover, CD15 was characterized on SEBTA-001 and SEBTA-005 (established in-house metastatic NSCLC cell lines obtained from brain). CD62E, the natural ligand for CD15, was upregulated on brain endothelial cells (hCMEC/D3) and cancer cells in response to a TNF-α inflammatory stimulus. Both mAb-blocking of CD15 and absence of CD62E/TNF-α correlated with significantly decreased adhesion of cancer cells on brain endothelium. Confocal microscopy revealed the expression of CD15 around adherent cancer cells and localized CD15/CD62E interaction at adhesion sites of cancer cells/brain endothelial cells; these findings suggest that CD15 and CD62E play important roles in adhesion of NSCLC cells to brain endothelium in static conditions. We then explored the possible effects of vascular blood flow by combining live-cell imaging and shear stress fluidics twinned with CD15 mAb-blocking under TNF-α immune stimulus using an in vitro model developed from human cell cultures maintained in a specialized environment to mimic in vivo environment inside blood brain microvessels. For this, a microfluidic chip (Vena8-Cellix) was used with low shear stress (2.5 dyn/cm2) and volumetric flow rates (10 mL/hour) mimicking the low flow in brain microvessels.46 Under these conditions, the adhesion of cancer cells was significantly decreased by absence of CD15 or CD62E/TNF-α, suggesting the critical role of CD15 and CD62E/TNF-α in cancer cell adhesion during early stages of cancer cell extravasation. While in vivo data are lacking for NSCLC, a recent study reinforced the importance of CD62E in breast-to-brain metastasis in mice by showing that the adhesion of breast cancer cells to brain endothelial cells was enhanced by the presence of VCAM-1/VLA-4, ALCAM-1, and Integrinβ4.47 Our in vitro study has also shed light on the functional characterization and localization of CD15 and CD62E on the site of NSCLC seeding to the brain. CD15 expression levels correlated with the adhesion of cancer cells to stimulated brain microvascular endothelial cells. NSCLC metastatic cells obtained from brain lesions (SEBTA-001 and SEBTA-005) were more adhesive than the primary NSCLC cells (COR-L105 and A549). These results are consistent with previous studies that referred to the correlation between elevated level of CD15 and metastasis in different types of non-CNS cancer.22,42 CD15 mAb-blocking resulted in a decrease in the number of adherent cells on stimulated brain microvascular endothelium (hCMEC/D3) under both static and dynamic conditions. Thus, this study indicates that CD15 is a possible target for prevention of brain metastasis in NSCLC patients. While this in vitro experimental model adds to our knowledge of metastatic cell adherence to brain endothelium, there are limitations in that it does not include other cell types that are known to be present. For example, the influence of circulating neutrophils in brain metastases and their role in this process is not known and is currently under investigation. Although little is known concerning the histopathology of CD15 expression in CNS tumors48 and human lung cancer to brain metastasis, there have been reports of CD15 expression in NSCLC49 and more recently associations with lung cancer stem-like cells.22 A more rigorous characterization of CD15 in human lung cancer to brain-derived biopsy is now in progress.
Supplementary Material
Funding
SAJ was funded by the High Committee of Education Development in Iraq (HCED-IRAQ). ZM was funded by a grant from the Dr Hadwen Trust to GJP, and HLF was supported by a grant from Brain Tumour Research to GJP.
Supplementary Material
Acknowledgments
The authors would like to thank Professor Pierre-Olivier Couraud, the Institute Cochin, INSERM in Paris for donating the human cerebral microvascular endothelial cell line (hCMEC/D3), Mrs Katie F. Loveson for conducting the human authentication for all the cell lines studied, and Dr James Brown for statistical analysis assistance.
Conflict of interest statement. None declared.
References
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. [DOI] [PubMed] [Google Scholar]
- 2.Rahmathulla G, Toms SA, Weil RJ. The molecular biology of brain metastasis. J Oncology. 2012;2012;723541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Kai K, Ueno NT, Qin L. A brief review of the biophysical hallmarks of metastatic cancer cells. Cancer Hallm. 2013;1(2–3):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney and lung and melanoma. Cancer. 2002;94(10):2698–2705. [DOI] [PubMed] [Google Scholar]
- 5.Barani IJ, Larson DA, Berger MS. Future directions in treatment of brain metastases. Surg Neurol Int. 2013;4(Suppl. 4):S220–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 7.Villano JL, Durbin EB, Normandeau C, Thakkar JP, Moirangthem V, Davis FG. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol. 2015;17(1):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. 2013;20:e300–e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12(7):884–898. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm I, Molnar J, Fazakas C, Hasko J, Krizbai IA. Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci. 2013;14:1383–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochman J, Assaf N, Deckert-Schuter M, Wiestler OD. Entry routes of malignant lymphoma into the brain and eyes in a mouse model. Cancer Res. 2001;61:5242–5247. [PubMed] [Google Scholar]
- 12.Strell S, Entschladen F. Extravasation of leukocytes in comparison to tumour cells. Cell Commun Signal. 2008;26(3–4):401–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seike T, Fujita K, Yamakawa Y et al. Interaction between Lung cancer cells and astrocytes via specific inflammatory cytokines in the microenviroment of brain metastasis. Clin Exp Metastasis. 2011;28(1):13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gooi HC, Feizi T, Kapadia A, Knowles BB, Solter D, Evans MJ. Stage-specific embryonic antigen involves alpha 1 goes to 3 fucosylated type 2 blood group chains. Nature. 1981;292(5819):156–158. [DOI] [PubMed] [Google Scholar]
- 15.Kadota A, Masutani M, Takei M, Horie T. Evaluation of expression of CD15 and sCD15 in non-small cell lung. Int J Oncol. 1999;15(6):1081–1090. [DOI] [PubMed] [Google Scholar]
- 16.Elola MT, Capurro MI, Barrio MM et al. Lewis x antigen mediates adhesion of human breast carcinoma cells to activated endothelium: Possible involvement of the endothelial scavenger receptor C-type lectin. Breast Cancer Res Treat. 2007;101(2):161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammerich KH, Ayala GE, Wheeler TM. Application of immunohistochemistry to the genitourinary system (prostate, orinary bladder, testis, and kidney). Arch Pathol Lab Med. 2008;132(3):432–440. [DOI] [PubMed] [Google Scholar]
- 18.Streit A, Stern CD. L5: A carbohydrate epitope involved in neural development. Biol Cell. 1995;84:63–67. [Google Scholar]
- 19.Martin K, Akinwunmi J, Rooprai HK et al. Nonexpression of CD15 by neoplastic glia: a barrier to metastasis? Anticancer Res. 1995;15(4):1159–1166. [PubMed] [Google Scholar]
- 20.Mao XG, Zhang X, Xue XY et al. Brain tumour stem-like cells identified by neural stem cell marker CD15. Transl Oncol. 2009;2(4):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelen S, Parm UB, Larsen A, Frokiaer J, Nielsen S, Rutzler M. AQP9 expression in glioblastoma multiforme tumors is limited to a small population of astrocytic cells and CD15(+)/CalB(+)leukocytes. PLoS One. 2013;258(9):e757–e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolte SM, Venugopal C, McFarlane N et al. A cancer stem cell model for studying brain metastases from primary lung cancer. J Natl Cancer Inst. 2013;105(8):551–562. [DOI] [PubMed] [Google Scholar]
- 23.Kaszubska W, Vanhuijsduijnen RH, Ghersa P et al. Cyclic AMP-independent ATF family members interact with NF-KappaB and function in the activation of the E-selectin promoter in response to cytokines. Mol Cell Biol. 1993;13:7180–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vora ML, Romero I, Karasek MA. Interleukin-10 induces E-selectin on small and large blood vessel endothelial cells. J Exp Med. 1996;184:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brayton J, Qing Z, Hart MN, VanGilder JC, Fabry Z. Influence of adhesion molecule expression by human brain microvessel endothelium on cancer cell adhesion. J Neuroimmunol. 1998;89(1-2):104–112. [DOI] [PubMed] [Google Scholar]
- 26.Martinez M, Joffraud M, Giraud S et al. Regulation of PSGL-1 interactions with L-selectin, P-selectin, and E-selectin: role of human fucosyltransferase-IV and -VII. J Biol Chem. 2005;280(7):5378–5390. [DOI] [PubMed] [Google Scholar]
- 27.Hess D, Thompson Y, Sprinklea A, Carroll J, Smith J. E-Selectin expression on human brain microvascular endothelial cells. Neurosci Lett. 1997;213:37–40. [DOI] [PubMed] [Google Scholar]
- 28.Wong D, Dorovini-Zis K. Regulation by cytokines and lipopolysaccharide of E-selectin expression by human brain microvessel endothelial cells in primary culture. J Neuropathol Exp Neurol. 1996;55(2):225–235. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Kubes P. Molecular mechanisms of leukocyte recruitment: organ-specific mechanisms of action. Thromb Haemost. 2003;89(2):213–220. [PubMed] [Google Scholar]
- 30.Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis . Int J Cancer. 1997;71(4):612–619. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay PL, Auger FA, Huot J. Regulation of transendothelial migration of colon cancer cells by E-selectin-mediated activation of P38 and ERK MAP kinases. Oncogene. 2006;25:6563–6573. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimoto K, Tajima H, Ohta T et al. Increased E-selectin in hepatic ischemia-reperfusion injury mediates liver metastasis of pancreatic cancer. Oncol Rep. 2012;28(3):791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laubli H, Borsig L. Selectins promote tumor metastasis . Semin Cancer Biol. 2010;20(3):169–177. [DOI] [PubMed] [Google Scholar]
- 34.Brooks SA, Leathem AJ. Expression of CD15 antigen (Lewis x) in breast cancer. Histochem J. 1995;27(9): 689–693. [PubMed] [Google Scholar]
- 35.Pilkington GJ, Bjerkvig R, De Ridder L, Kaaijk P. In vitro and in vivo models for the study of brain tumour invasion. Anticancer Res. 1997;17(6B):4107–4109. [PubMed] [Google Scholar]
- 36.Kiem VU, Weksler B, Romero I, Couraud PO, Gelli A. Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans. Eukaryotic Cell. 2009;11:1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An Q, Fillmore HL, Vouri M, Pilkington GJ. Brain tumor cell line authentication, an efficient alternative to capillary electrophoresis by using a microfluidics-based system. Neuro Oncol. 2014;16(2):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maherally Z, Smith JR, An Q, Pilkington GJ. Receptors for hyaluronic acid and poliovirus: a combinatorial role in glioma invasion? PloS ONE. 2012;7(2):e30691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gout S, Morin C, Houle F, Huot J. Death receptor-3, a new E-selectin counter-receptor that confers migration and survival advantages to colon carcinoma cells by triggering p38 and ERKMAPK activation. Cancer Res. 2006;66(18):9117–9124. [DOI] [PubMed] [Google Scholar]
- 40.Lafrenie RM, Gallo S, Podor TJ, Buchanan MR, Orr FW. The relative roles of vitronectin receptor, E-selectin and α4β1 in cancer cell adhesion to interleukin-1-treated endothelial cells. Eur J Cancer. 1994;30A(14):2151–2158. [DOI] [PubMed] [Google Scholar]
- 41.Martin-Satue M, Marrugat R, Cancelas JA, Blanco J. Enhanced expression of α (1,3)-fucosyltransferase genes correlates with E-selectin-mediated adhesion and metastatic potential of human lung adenocarcinoma cells. Cancer Res. 1998;58(7):1544–1550. [PubMed] [Google Scholar]
- 42.Fazakas C, Wilhelm I, Nagyoszi P et al. Transmigration of melanoma cells through the blood-brain barrier: Role of endothelial tight junctions and melanoma-released serine proteases. PLoS One. 2011;6:e20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatherell K, Couraud PO, Romero IA. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods. 2011;199(2):223–229. [DOI] [PubMed] [Google Scholar]
- 44.Rizzo A, Vasco C, Girgenti V et al. Melanoma cells homing to the brain: an in vitro model. BioMed Res Int. 2014;476069:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Man S, Ubogu EE, Williams KA, Tucky B, Callahan MK, Ransohoff RM. Human Brain Microvascular Endothelial Cells and Umbilical Vein Endothelial Cells Differentially Facilitate Leukocyte Recruitment and Utilize Chemokines for T Cell Migration. Clinical and Developmental Immunology. 2008;10:384982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: Theoretical modelling and experimental data. Proc Natl Acad Sci USA. 2001;98(12):6859–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soto MS, Serres S, Anthony DC, Sibson NR. Functional role of endothelial adhesion molecules in the early stages of brain metastasis. Neuro Oncol. 2014;16(4):540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reifenberger G, Sieth P, Niederhaus M, Wechsler W. Expression of CD15 in tumours of the nervous system. Histochem J. 1992;11:890–901. [DOI] [PubMed] [Google Scholar]
- 49.Kadota A, Masutani M, Takei M, Horie T. Evaluation of expression of CD15 and sCD15 in non-small cell lung cancer. Int J Oncol. 1999;15:1081–1090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.