Supplemental Digital Content is Available in the Text.
Key Words: elastase, vonapanitase, elastin, atherosclerosis, angioplasty, peripheral artery disease
Abstract
Purpose:
This study was designed to determine whether vonapanitase (formerly PRT-201), a recombinant human elastase, treatment can fragment the protein elastin in elastic fibers and cause dilation of atherosclerotic human peripheral arteries subjected to ex vivo balloon angioplasty.
Materials and Methods:
Seven patients undergoing lower limb amputation for peripheral artery disease or who died and donated their bodies to science donated 11 tibial arteries (5 anterior, 6 posterior) for this study. All arteries were atherosclerotic by visual inspection. The arteries underwent ex vivo balloon angioplasty and thereafter were cut into rings and studied on wire myographs where the rings were stretched and tension was recorded. After treatment with vonapanitase 2 mg/mL or vehicle control, myography was repeated and the rings were then subject to elastin content measurement using a desmosine radioimmunoassay and elastic fiber visualization by histology. The wire myography data were used to derive compliance, stress-strain, and incremental elastic modulus curves.
Results:
Vonapanitase treatment reduced elastin (desmosine) content by 60% and decreased elastic fiber histologic staining. Vonapanitase-treated rings experienced less tension at any level of stretch and as a result had shifts in the compliance and stress-strain curves relative to vehicle-treated rings. Vonapanitase treatment did not alter the incremental elastic modulus curve.
Conclusions:
Vonapanitase treatment of atherosclerotic human peripheral arteries after ex vivo balloon angioplasty fragmented elastin in elastic fibers, decreased tension in the rings at any level of stretch, and altered the compliance and stress-strain curves in a manner predicting arterial dilation in vivo. Based on this result, local treatment of balloon angioplasty sites may increase blood vessel diameter and thereby improve the success of balloon angioplasty in peripheral artery disease.
INTRODUCTION
Peripheral artery disease affects approximately 8 million Americans and is associated with significant morbidity and mortality. Despite recent advances, the lower extremity arterial vasculature remains a challenging environment for endovascular therapy. Recent studies of balloon angioplasty for superficial femoral artery and popliteal artery lesions showed binary restenosis and primary patency loss rates in approximately one-third of patients at 1 year of follow-up.1,2 Restenosis is the primary chronic failure mode after balloon angioplasty and is correlated with elastic recoil, neointimal hyperplasia, and negative arterial remodeling.3,4 Elastic recoil occurs after balloon deflation when the artery wall rebounds due to arterial elasticity resulting in loss of lumen area.5,6
Arterial elasticity, the ability of the artery to expand in response to pressure and then return to the original diameter, is produced by the unique organization of vessel wall components, including smooth muscle cells and the extracellular matrix fibers elastin and collagen. Elastin is a hydrophobic, monomeric protein that is cross-linked with other elastin molecules to create a meshwork of elastic fibers and sheets that constrains vessel diameter and imparts elasticity.7 The elastic fibers are engaged and bear most of the stress during the pressure fluctuation of the cardiac cycle. At higher pressures, such as occurs during balloon angioplasty, collagen fibers are recruited and prevent vessel rupture.8,9
Vonapanitase (formerly PRT-201), is a recombinant human elastase (protease) also called as chymotrypsin-like elastase family member 1 that fragments elastin. Vonapanitase applied ex vivo for 30 minutes to the external surface of atherosclerotic tibial arteries reduced elastin content by ∼50% and increased tibial artery diameter by ∼25%.10 Local vonapanitase treatment may represent a novel strategy to prevent restenosis and patency loss after balloon angioplasty. The local administration of vonapanitase after balloon angioplasty of atherosclerotic femoral and popliteal arteries is currently being investigated in a phase 1–2 clinical trial (www.clinicaltrials.gov, NCT 01616290).
The purposes of this study was to determine whether vonapanitase could fragment elastin in elastic fibers after ex vivo balloon angioplasty of atherosclerotic human arteries and cause a compliance change that would predict a larger artery diameter and lumen area in vivo. In this study, compliance was determined using the well-accepted method of wire myography where artery rings are stretched and tension in the artery wall is recorded. The wire myography data were then used to derive compliance, stress-strain, and incremental elastic modulus curves.
MATERIALS AND METHODS
Sample Collection
Anterior and posterior tibial arteries were harvested from patients in Scotland, United Kingdom, undergoing lower limb amputation for peripheral artery disease or who died and donated their bodies to science. The study was approved by the ethics committee at each institution where their tissue was obtained. The patients undergoing amputation had given informed consent for the amputated tissue to be used for research. The deceased patients had previously made arrangements to donate their bodies to science. Arteries were removed, placed in ice-cold physiological saline solution (PSS) containing 119.0 mM NaCl, 4.7 mM KCl, 1.2 mM MgS04, 24.9 mM NaHCO3, 1.2 mM KH2PO4, 2.5 mM CaCl2, and 11.1 mM glucose in water, and transported by a courier to Biopta Ltd, Glasgow, Scotland, for use on receipt within 12–24 hours of harvest.
Angioplasty Procedure
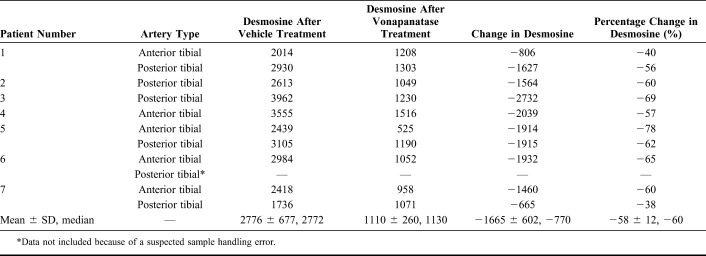
Baseline artery external and lumen diameters were measured using the graticule eyepiece of a microscope (Fig. 1). Lumen diameter was defined as the distance between the opposing internal elastic lamina (ie, ignored the presence of atherosclerotic plaque in the lumen). After passage of a guidewire through the artery, an angioplasty balloon (Fox sv; Abbot Vascular Inc, Santa Clara, CA) was inserted into the lumen of the artery and inflated to 14 atmospheres of pressure. This pressure was maintained for 1 minute. A previous pilot study had shown that 14 atmospheres of pressure inflation for 1 minute was effective at crushing atherosclerotic plaque in tibial arteries. The balloon has a rated burst pressure of 18 atmospheres. Balloon diameter was chosen to match the artery lumen diameter, resulting in the use of 2, 3, or 4-mm diameter balloons. In some cases where there was a very severe lumen narrowing, the artery was angioplastied using a smaller diameter balloon followed by a larger balloon. After angioplasty, the artery was cut into 6 rings each 1.5 mm long. Two rings were immediately placed into 10% neutral-buffered formalin as baseline samples for desmosine radioimmunoassay and histology. The remaining 4 rings (2 assigned to vehicle treatment and 2 assigned to vonapanitase treatment) were studied on a large vessel wire myography device (Panlab, Spain).
FIGURE 1.
Section of tibial artery before (left) and after (right) angioplasty.
Wire Myography
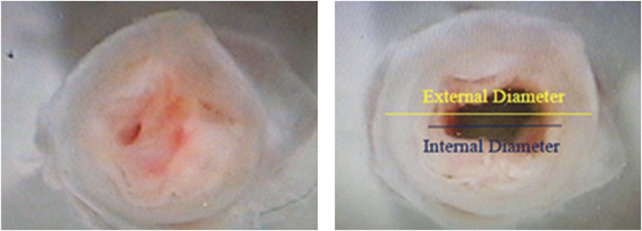
Wire myography was used to determine the effect of elastase treatment on the compliance of the angioplastied arterial segment. In the experiments, stretch was the independent variable and tension in the artery ring wall was the dependent variable. Each artery ring was mounted onto 2 wires and placed in a glass organ bath. A length of nylon thread was attached to one of the wires directly connected to a force transducer to allow continuous recording of tension (Fig. 2). The other wire was in a fixed position. The distance between the wires was adjustable using a micrometer. Each organ bath contained 25 mL of PSS aerated with 95% O2 and 5% CO2 and maintained at a temperature of 37°C. The rings were allowed to equilibrate for 30 minutes. The bath solution was replaced with calcium-free PSS containing 50 mM caffeine for 5 minutes to deplete the intracellular calcium stores. Then the rings were washed 3 times with calcium-free PSS.
FIGURE 2.
Schematic of wire myograph.
The rings were stretched in successive increments, waiting 1 minute between stretches, to create an initial compliance curve. Stretch resulted in a change in distance between the 2 wires that approximated the change in lumen circumference divided by 2 because the artery rings became oblong during the stretch as shown in the schematic in Figure 2. At each increment of stretch, the tension in the tissue was measured. Transmural pressure was calculated using Laplace's law, wherein effective pressure in mm Hg = 7.5 × wall tension × 2π/internal circumference. The rings were stretched to a tension of approximately 2.4 kgf (80 mm Hg). For the higher pressure, experiment rings were stretched up to 5 kgf (140 mm Hg).
Sample Treatment
After initial compliance testing, the tension on rings was reduced to a level corresponding to a pressure of 40 mm Hg. The bath solution was replaced with phosphate-buffered saline with 0.01% polysorbate 80 (PBSP) or vonapanitase 2 mg/mL in PBSP for 60 minutes. The vonapanitase concentration was chosen based on the results of pilot studies that showed a vonapanitase concentration >1 mg/mL applied for 60 minutes was necessary to remove >50% of the elastin from atherosclerotic human tibial arteries after angioplasty, the amount of elastin removal associated with arterial dilation. After treatment, the rings were washed 3 times with calcium-free PSS and the stretch on the rings was reduced to the starting tension for the first compliance curve. Rings were then stretched in successive increasing increments, waiting 1 minute between stretches, until the force was increased to a level corresponding to a pressure of 80 mm Hg. In a single experiment with PBSP or vonapanitase, 2 mg/mL in PBSP for 60 minutes, forces were increased during the posttreatment compliance testing to a level corresponding to a pressure of 120–140 mm Hg. At the end of the experiments, the rings were removed from the bath solution and stored in 10% neutral-buffered formalin.
Quantitative Desmosine Content Analysis and Histology
After all experiments were completed, 1 of the 2 rings from each compliance experiment was shipped to the University of Texas, Tyler, TX, for analysis of desmosine. Desmosine is a protein cross-link unique to elastin. Desmosine levels in the rings from the experiments were determined by radioimmunoassay and reported as picomoles of desmosine per mg of protein.11 Protein content of the sample was measured using a ninhydrin-based protein assay.12 The remaining ring was shipped to Histologix Ltd, Nottingham, United Kingdom, for histology using a Verhoeff Van-Gieson (VVG) stain to identify elastic fibers.
Data Analysis and Statistics
Compliance data were imported into GraphPad Prism (GraphPad Software; San Diego, CA), version 4 and plotted. The pre and posttreatment curves were compared using 2-way analysis of variance. Compliance is defined as the change in lumen area for a given change in pressure. Stress is the intensity of force acting over a given area. Circumferential wall stress is defined as P × r/h, where P, pressure, r, vessel radius, and h is wall thickness. Strain is the percent change in length when a vessel is exposed to a stress. Circumferential wall strain is equal to (2πr2−2πr1)/2πr1 or (r2−r1)/r1. Incremental elastic modulus is used to describe the tangent or first derivative of the stress-strain curve. Vehicle-treated and vonapanitase-treated rings were compared for desmosine content using a paired t test.
RESULTS
Sample Collection, Angioplasty Procedure, and Wire Myography
The 7 patients (5 undergoing amputation and 2 recently deceased) donated 11 tibial arteries (5 anterior, 6 posterior) for this study. All patients were white, 5 were male, and ages ranged from 49 to 84 years, all had hypertension, and 4 of 7 had diabetes mellitus. Smoking history was unknown.
All arteries were atherosclerotic by visual inspection. The artery walls were thickened with yellow atherosclerotic plaque. The texture of the vessels was mainly firm with interspersed softer areas. The average baseline external diameter of the arteries was 4.1 ± 1.5 (median 4 mm, range 2.5–8 mm). The average baseline lumen diameter was 2.4 ± 0.7 (median 2 mm, range 2–4 mm). All arteries were patent but contained areas of stenosis >50% of the lumen diameter.
The arteries were subjected to balloon angioplasty. Table 1 summarizes the artery lumen and balloon diameters recorded. On 3 occasions, the artery was dilated initially with a smaller diameter balloon, followed by a larger diameter balloon. Angioplasty resulted in an increase in lumen diameter by visual inspection (Fig. 1). After the arteries were sectioned into rings as described in methods, the compliance testing, drug treatment, biochemical testing, and histology was completed. Figure 3 shows how the rings were used in the various analyses. Figure 4 displays the calculated transmural pressure in response to increasing stretch of the artery rings by the wire myograph (presented as calculated diameter). The relationship of stretch and calculate diameter to pressure was unaffected by vehicle treatment (Fig. 4, panels A and C). In contrast, vonapanitase treatment altered the relationship such that at any diameter the transmural pressure was reduced (Fig. 4, panels B and D). Figure 5 displays the compliance curves of anterior (A) and posterior (B) tibial artery rings before and after treatment with vonapanitase. Vonapanitase increased compliance at low pressures. Treatment with vehicle did not alter compliance (see Figure 1S, panels A and B, Supplemental Digital Content 1, http://links.lww.com/JCVP/A211). Supplemental Digital Content 2 (see Figure 2S, http://links.lww.com/JCVP/A212) displays the stress-strain curve of anterior (A) and posterior (B) tibial artery rings before and after treatment with vonapanitase. Vonapanitase treatment shifted the curve to the right indicating that an increase in circumferential stress would result in a larger increase in circumference. Treatment with vehicle did not alter the stress-strain curves (see Figure 2S, panels C and D, Supplemental Digital Content 2, http://links.lww.com/JCVP/A212). Einc as a function of calculated transmural pressure of anterior and posterior tibial artery rings before and after treatment with vonapanitase did not show a difference (data not shown).
TABLE 1.
Artery Lumen and Angioplasty Balloon Diameters
FIGURE 3.
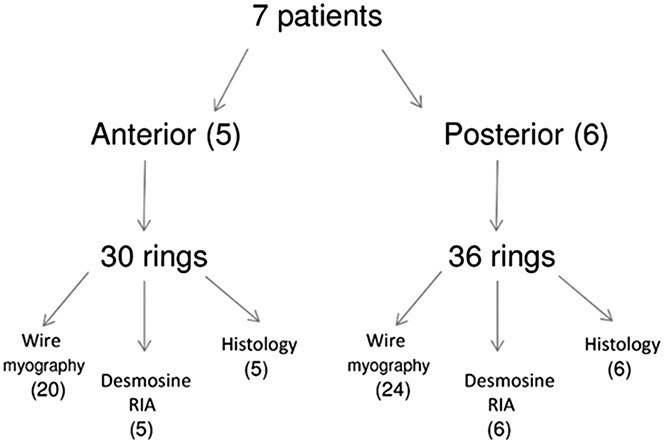
Flowchart describing use of the artery rings in the study.
FIGURE 4.
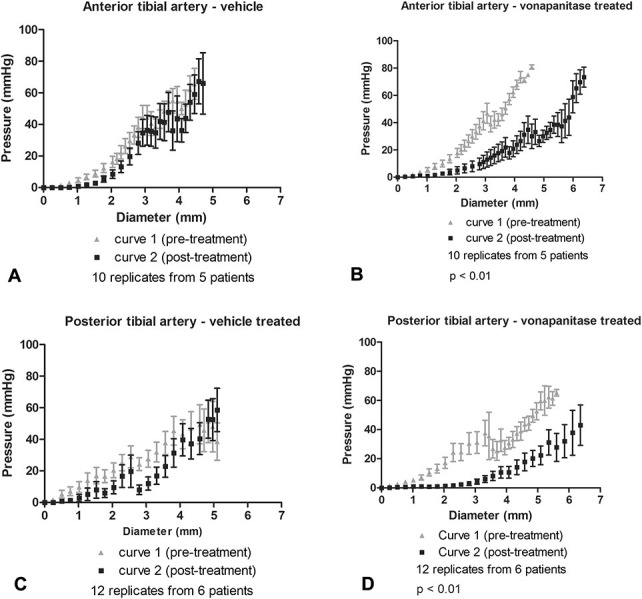
Diameter-pressure curves of atherosclerotic anterior and posterior tibial arteries before and after treatment with vehicle PBSP or vonapanitase. Panel A–anterior tibial artery treated with vehicle, panel B–anterior tibial artery treated with vonapanitase, panel C–posterior tibial artery treated with vehicle, panel D–posterior tibial artery treated with vonapanitase. Error bars represent standard error of the mean.
FIGURE 5.
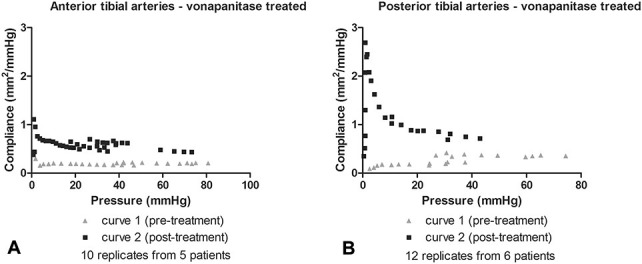
Pressure-compliance curves of atherosclerotic anterior (A) and posterior (B) tibial arteries before and after treatment with vonapanitase.
In the arteries of 1 of the 7 patients, the compliance protocol was modified to explore greater stretch resulting in higher tension and higher calculated transmural pressures during posttreatment compliance testing in an attempt to match pressures likely to be encountered in vivo. Vehicle treatment did not alter the relationship between stretch and calculated diameter and calculated pressure (see Figure 3SA, Supplemental Digital Content 3, http://links.lww.com/JCVP/A213), or the compliance (see Figure 3SC, Supplemental Digital Content 3, http://links.lww.com/JCVP/A213), stress-strain (see Figure 3SE, Supplemental Digital Content 3, http://links.lww.com/JCVP/A213), or Einc curves (data not shown). In contrast, vonapanitase treatment altered the relationship such that at any diameter the tension and pressure was reduced, again indicating that vonapanitase-treated rings had a larger lumen circumference, diameter, and area across the full range of calculated pressures tested (see Figure 3SB, Supplemental Digital Content 3, http://links.lww.com/JCVP/A213). Vonapanitase treatment again increased compliance (see Figure 3SD, Supplemental Digital Content 3, http://links.lww.com/JCVP/A213) at low pressures, shifted the stress-strain (see Figure 3SF, Supplemental Digital Content 3, http://links.lww.com/JCVP/A213) curve to the right, and had no impact on Einc (data not shown). No rings tore during the compliance testing.
Quantitative Desmosine Content Analysis and Histology
Table 2 shows the desmosine (elastin) content of rings treated with vehicle or vonapanitase. Treatment of rings with vonapanitase resulted in a mean reduction in desmosine content of 60% relative to vehicle treatment. The mean desmosine content of vehicle-treated and vonapanitase-treated rings were significantly different (P < 0.0001).
TABLE 2.
Desmosine Content (Picomole Desmosine/Milligram Protein) of Arteries Treated With Vehicle or Vonapanitase
VVG staining of sections of the rings demonstrated extensive loss of elastic fiber staining after vonapanitase treatment. Figure 6 displays representative images of rings from a single donor after compliance testing after vehicle treatment (Fig. 6, panel A) or vonapanitase treatment (Fig. 6, panel B). VVG stains elastic fibers a blue-black color. As shown in the representative images, elastic fibers were observed in rings treated with vehicle, whereas a substantial reduction in elastic fibers was observed in vonapanitase-treated rings.
FIGURE 6.
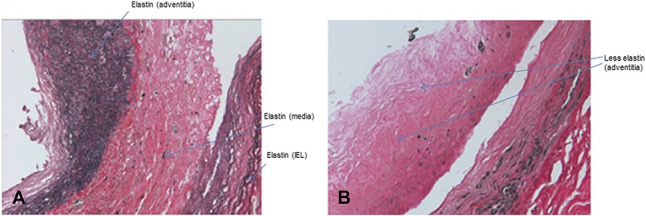
VVG histology staining of anterior tibial artery ring segments after angioplasty and treatment with vehicle (panel A) or vonapanitase (panel B). Elastin fibers stain blue-black. Images are ×20 magnification.
DISCUSSION
The results of this study demonstrate that removal of elastic fibers from human atherosclerotic tibial artery rings with vonapanitase, a recombinant human elastase, after ex vivo balloon angioplasty alters artery compliance, resulting in a substantial increase in artery diameter across a range of transmural pressures. The methods in this study avoided forces corresponding to pressures higher than 80 mm Hg to avoid plastic changes to the rings. Arteries are normally subjected to very brief increases in stretching forces at 60–100 cycles per minute in vivo, whereas the wire myography stretches the rings successively waiting 1 minute between stretches. In the rings from the final donor, force was increased in a stepwise fashion up to a level corresponding to a pressure of 120–140 mm Hg after vonapanitase treatment demonstrating that the compliance curve remained shifted, suggesting that vonapanitase-treated arteries would have a greater lumen area throughout the entire cardiac cycle in vivo.
The pharmacologic treatment of severely atherosclerotic human arteries subjected to ex vivo balloon angioplasty has not been previously reported in the literature. The use of diseased human tissue minimizes the use of laboratory animals for research purposes. Researchers interested in understanding the effects of therapeutic agents in atherosclerotic arteries after balloon angioplasty could benefit from using this model.
The mechanisms for increasing vessel lumen diameter by angioplasty and elastase treatment are complementary. Balloon dilation increases lumen diameter by crushing plaque, whereas vonapanitase increases lumen diameter by dilating the entire blood vessel wall through removal of elastic fibers. The potential complementary mechanisms of angioplasty and elastase are explored in this current nonclinical study and in a recently completed clinical study of vonapanitase treatment after angioplasty of the superficial femoral and popliteal arteries in patients with intermittent claudication or ischemic rest pain (clinicaltrials.gov, NCT 01616290). The results of the clinical study were recently presented at a scientific meeting showing vonapanitase was safely administered to the adventitia of femoral and popliteal arteries after angioplasty with no vonapanitase-related adverse effects and no aneurysms.
Angioplasty of tibial arteries has been reported to be followed by elastic recoil of on average 30% lumen diameter loss immediately after the angioplasty balloon is deflated.13 Vonapanitase treatment after angioplasty could maintain or perhaps even increase blood flow by maintaining or increasing blood vessel diameter. Because blood flow is related to the fourth power of the lumen radius, even small changes in the diameter of a blood vessel will significantly increase or decrease flow. For instance, a 5% increase in radius theoretically could increases blood flow by 22%. A 20% increase in radius could theoretically increase blood flow by approximately 100%. Furthermore, retaining a larger lumen diameter after angioplasty could potentially counteract the negative effects of neointimal hyperplasia on lumen diameter and potentially prolong patency and reduce the need for stenting.14 In this way, vonapanitase treatment could be seen as a “biological stent,” providing the benefits of a stent but without the need for a permanent metal implant.
For this new treatment to become widely accepted, a number of questions regarding the safety of this approach will need to be addressed. Elastin is a relatively weak fiber and can be removed from tissues without an effect on tissue strength.9 However, it has been reported that porcine type I pancreatic elastases applied in the lumen of arteries in animals can create aneurysms.15 With the benefit of hindsight, it is now known that preparations of elastase used in those reports likely contained contaminants that promoted inflammation, collagen degradation, and uncontrolled dilation.15 Vonapanitase is a highly purified formulation and does not cause progressive aneurysmal dilation. In clinical trials, in hemodialysis patients undergoing placement of arteriovenous fistulas and grafts, vonapanitase applied to the external surface of blood vessels at the time of surgery was well tolerated without a significant increase in adverse effects over placebo.16–18 Vonapanitase is inactivated by alpha 1-antitrypsin and alpha 2-macroglobulin, which are abundant in blood; therefore, vonapanitase must be applied to the external surface of blood vessels or administered into the vessel wall with a drug-delivery catheter. Any vonapanitase administered intraluminally would be immediately inactivated by antiproteases.19
The major limitation of this study is that ex vivo treatments cannot replicate in vivo effects, especially the longer term effect of the treatment on the success of angioplasty and safety. Toxicology studies in appropriate models and clinical trials will need to establish the therapeutic value of perivascular elastase treatment after balloon angioplasty.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Barry Starcher who measured the desmosine content of the artery rings.
Footnotes
Supported by Proteon Therapeutics, Inc.
K. S. Bland and F. N. Franano were former employees of Proteon Therapeutics, Inc who are consultants and own stock or stock options. M. D. Wong and S. K. Burke and D. P. Gottlieb are current employees and own stock or stock options in Proteon Therapeutics, Inc. The remaining authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jcvp.org).
REFERENCES
- 1.Rocha-Singh KJ, Jaff MR, Crabtree TR, et al. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv. 2007;69:910–919. [DOI] [PubMed] [Google Scholar]
- 2.Laird JR, Katzen BT, Scheinert D, et al. ; RESILIENT Investigators. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010;3:267–276. [DOI] [PubMed] [Google Scholar]
- 3.Serruys PW, Luijten HE, Beatt KJ, et al. Incidence of restenosis after successful coronary angioplasty: a time-related phenomenon. A quantitative angiographic study in 342 consecutive patients at 1, 2, 3, and 4 months. Circulation. 1988;77:361–371. [DOI] [PubMed] [Google Scholar]
- 4.Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. [DOI] [PubMed] [Google Scholar]
- 5.Gardiner GA, Jr, Bonn J, Sullivan KL. Quantification of elastic recoil after balloon angioplasty in the iliac arteries. J Vasc Interv Radiol. 2001;12:1389–1393. [DOI] [PubMed] [Google Scholar]
- 6.Isner JM, Rosenfield K, Losordo DW, et al. Combination balloon-ultrasound imaging catheter for percutaneous transluminal angioplasty. Validation of imaging, analysis of recoil, and identification of plaque fracture. Circulation. 1991;84:739–754. [DOI] [PubMed] [Google Scholar]
- 7.Haas KS, Phillips SJ, Comerota AJ, et al. The architecture of adventitial elastin in the canine infrarenal aorta. Anat Rec. 1991;230:86–96. [DOI] [PubMed] [Google Scholar]
- 8.Dobrin PB, Baker WH, Glay WC. Elastolytic and collagenolytic studies of arteries. Arch Surg. 1984;119:405–409. [DOI] [PubMed] [Google Scholar]
- 9.Dobrin PB, Canfield TR. Elastase, collagenase, and the biaxial elastic properties of dog carotid artery. Am J Physiol. 1984;247(1 pt 2):H124–H131. [DOI] [PubMed] [Google Scholar]
- 10.Burke SK, Macdonald K, Moss E, et al. Effects of recombinant human type I pancreatic elastase on human atherosclerotic arteries. J Cardiovasc Pharmacol. 2014;64:530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starcher BC, Mecham RP. Desmosine radioimmunoassay as a means of studying elastogenesis in cell culture. Connect Tissue Res. 1981;8:255–258. [DOI] [PubMed] [Google Scholar]
- 12.Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem. 2001;292:125–129. [DOI] [PubMed] [Google Scholar]
- 13.Baumann F, Fust J, Engelberger RP, et al. Early recoil after balloon angioplasty of tibial artery obstructions in patients with critical limb ischemia. J Endovasc Ther. 2014;21:44–51. [DOI] [PubMed] [Google Scholar]
- 14.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol. 2011;31:2391–2396. [DOI] [PubMed] [Google Scholar]
- 15.Dobrin PB. Animal models of aneurysms. Ann Vasc Surg. 1999;13:641–648. [DOI] [PubMed] [Google Scholar]
- 16.Peden EK, Leeser DB, Dixon BS, et al. A multi-center, dose-escalation study of human type I pancreatic elastase (PRT-201) administered after arteriovenous fistula creation. J Vasc Access. 2013;14:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dwivedi AJ, Roy-Chaudhury P, Peden EK, et al. Application of human type I pancreatic elastase (PRT-201) to the venous anastomosis of arteriovenous grafts in patients with chronic kidney disease. J Vasc Access. 2014;15:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hye RJ, Peden EK, O'Connor TP, et al. Human type 1 pancreatic elastase treatment of arteriovenous fistulas in patients with chronic kidney disease. J Vasc Surg. 2014;60:454–461. [DOI] [PubMed] [Google Scholar]
- 19.Qamar AA, Burke SK, Lafleur JD, et al. The ability of serum from alpha 1-antitrypsin-deficient patients to inhibit PRT-201, a recombinant human type I pancreatic elastase. Biotechnol Appl Biochem. 2012;59:22–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.