Abstract
Using laboratory mouse models, the molecular pathways responsible for the metabolic benefits of endurance exercise are beginning to be defined. The most common method for assessing exercise endurance in mice utilizes forced running on a motorized treadmill equipped with a shock grid. Animals who quit running are pushed by the moving treadmill belt onto a grid that delivers an electric foot shock; to escape the negative stimulus, the mice return to running on the belt. However, avoidance behavior and psychological stress due to use of a shock apparatus can interfere with quantitation of running endurance, as well as confound measurements of postexercise serum hormone and cytokine levels. Here, we demonstrate and validate a refined method to measure running endurance in naïve C57BL/6 laboratory mice on a motorized treadmill without utilizing a shock grid. When mice are preacclimated to the treadmill, they run voluntarily with gait speeds specific to each mouse. Use of the shock grid is replaced by gentle encouragement by a human operator using a tongue depressor, coupled with sensitivity to the voluntary willingness to run on the part of the mouse. Clear endpoints for quantifying running time-to-exhaustion for each mouse are defined and reflected in behavioral signs of exhaustion such as splayed posture and labored breathing. This method is a humane refinement which also decreases the confounding effects of stress on experimental parameters.
Keywords: Behavior, Issue 90, Exercise, Mouse, Treadmill, Endurance, Refinement
Introduction
Obesity, insulin resistance, and type 2 diabetes are interrelated metabolic disorders that exert profound effects on the health of both the U.S. and worldwide populations 1-4. Endurance exercise can prevent, as well as treat, these conditions5,6. Moreover, assessment of gait speed and endurance are utilized clinically as diagnostic tests for frailty, sarcopenia, and the consequences of other disorders such as chronic obstructive pulmonary disease, in human subjects 7.
The biochemical pathways underlying the beneficial effects of endurance exercise on body composition and insulin sensitivity are beginning to be elucidated, using genetically or pharmacologically modified mice that display enhanced or reduced exercise capacity 8-11. However, many such studies have utilized motorized treadmills equipped with shock grids to force mice to run 8-11. Animals who quit running are pushed by the moving treadmill belt onto a grid that delivers an electric foot shock; to escape the negative stimulus, the mice return to running on the belt. Such procedures may introduce psychological stress and avoidance behavior as confounding factors affecting experimental parameters12. Other methods of measuring endurance, such as quantitation of ambulatory activity using a beam-break apparatus or quantitation of in-cage wheel running, may be confounded by modulation of circadian cycles, anxiety, inadvertent training, or food seeking behavior12-16. Moreover, these procedures require single housing, another source of psychological stress for mice. Therefore, a direct measurement of exercise endurance not confounded by stress is needed.
To obviate these concerns, our laboratory has developed and validated a method to assess maximal running capacity and sustained running speed in untrained, naïve mice using a motorized treadmill not equipped with a shock grid. The space at the end of the running belt, where the shock grid usually resides, then becomes a platform for mice to rest, and provides a place for mice that refuse to run to sit until removed from the apparatus. Mice are encouraged to run by a human observer using gentle tapping or touching with a tongue depressor, coupled with sensitivity to the voluntary willingness to run on the part of the mouse. This method has been used to quantify differences in exercise endurance between genetically-modified and control C57BL/6 mice that differ in expression of the muscle derived cytokine interleukin-15 (IL-15)16,17. This method is a humane refinement that decreases the confounding effects of negative reinforcement caused by use of a shock grid.
Protocol
The procedure described here was approved by the VA Puget Sound Institutional Animal Care and Use Committee, and complies with the ILAR Guide for the Care and Use of Laboratory Animals.
1. Experimental Preparation
Decide beforehand if blood or tissue collection soon after exercise is necessary, and if so, determine the post-exercise interval for conduction of such procedures. Examples include determination of exercise induced hormone or cytokine release into the circulation (necessitating blood collection), determination of exercise induced expression of mRNA species of interest (necessitating euthanasia followed by tissue collection). If immediate post exercise blood or tissue collection is indicated, have materials for these procedures on hand in the procedure room. Receive institutional review board approval and perform according to regulatory guidelines. Not all experiments require postexercise blood or tissue collection; a simple comparison of running endurance may be all that is needed.
Decide in advance on experimental groups and postexercise procedures for mice (see description above).
Place the treadmill on a sturdy table in a quiet room dedicated during the running trials to this procedure.
Set the treadmill for mouse-sized lanes and at an inclination appropriate to the protocol. The inclination of +5o is used here to obviate muscle damage due to eccentric exercise (lengthening the muscle at the same time as contraction) that occurs during downhill running.
Place an absorbent pad below the treadmill belt to catch feces.
2. Acclimation of Mice to the Treadmill
Decide if a “sedentary” (no exercise) group of mice is necessary, and expose sedentary mice to the treadmill without running as outlined in Steps 2.1 - 2.5. Determine if 2 or 4 experimental groups are required, e.g., Sedentary/Exercise (2 groups); Exercised Controls/Exercised Treatment (2 groups, no sedentary animals); or, Sedentary/Exercised x Control/Treatment (4 groups). The “treatment” may be a transgenic or knockout genotype, a pharmacological regimen, special diet, or other experimental intervention.
Allow the mouse to acclimate to the procedure room in its transported home cage, with cage mates, for 1 - 2 hr.
Select a single mouse and record tag number; weigh mouse and record weight.
Place mouse in the treadmill without the motor turned on for acclimation, about 5 min. Turn power on, without the treadmill belt running, and let the mouse acclimate to the machine noise for another 5 min.
Remove mice in the “sedentary” groups (if used; see Note 1) from the treadmill at this point and return them to their cage or conduct post-exercise procedures as per the experimental design (see Note 2).
3. Starting the Run-to-Exhaustion test
After acclimation, turn the belt to a low speed (10 m/min) when the animal is exploring the belt and not on the platform.
Gently tap or lift the hindquarters of the mouse with a tongue depressor, one to three times, to encourage reluctant animals to stay on the treadmill and run. NOTE: Most mice will run readily, but they sometimes stop for short periods. When this occurs, use the tongue depressor to gently tap or lift the rear of the animal to encourage resumption of running. Often, the mice do not need to be touched with the tongue depressor; noticing the hand with the tongue depressor coming towards them causes the mice to run some more.
Start a laboratory timer once the mouse is running at the initial speed of 10 m/min.
Turn the speed up slowly when the mouse is at the top (uphill end) of the belt, at increments of 1 m/min, about every 2 min.
Turn the speed down to the previous setting if the mouse repeatedly runs in short bursts and not at a steady pace.
Determine the maximal sustained running speed of each mouse (usually 14 - 17 m/min for C57BL/6 mice) by adjusting the speed and watching the mouse. Record this speed. NOTE: The location of the animal on the treadmill cues the investigator as to the willingness of the animal to run at a higher speed. If the mouse is at the far (uphill) end and about to run into the wall, it is ready to run at a higher speed. If the mouse is running at a steady pace in the middle of the treadmill it may be at its maximal speed, or may need further adjustment, depending on the quality of the run.
4. Endpoints and Mice that Stop
Use the tongue depressor to gently tap or lift the hindquarters of the animals that stop, to encourage resumption of running (see Note 3.2). Do not stop the timer for temporary interruptions in running.
Identify and remove mice that refuse to run . Note this on the data sheet. Depending on your experimental design, mice that refuse can be retested on a different day NOTE: Some mice will completely refuse to run. Such mice will run in short bursts, then stop to groom or “pedal”, that is, sit on the platform and use their front legs on the belt. The posture of these mice is hunched, with all four feet planted under the animal to keep from being pushed back onto the treadmill by the tongue depressor. Occasionally a mouse becomes aggressive towards the tongue depressor when unwilling to run, biting the tongue depressor or climbing up it as a way of escape. It is clear within the first 5 min of the trial whether the mouse will, or will not, run. Remove mice that refuse to run from the analysis, but do not count them as “sedentary” mice.
Identify exhausted mice and stop the timer. NOTE: Exhaustion is defined by three successive stops and refusal to resume running despite gentle encouragement, plus physical signs of exhaustion such as labored breathing and splayed posture. Mice recover from exhaustion within 30 to 60 min.
Record the total amount of time spent running at all speeds.
Depending on the experimental design, either return the mouse is to its cage, or conduct immediate postexercise procedures such as blood or tissue collection as per institutional guidelines.
Wipe the belt clean with a germicidal towelette after testing each individual mouse so that the scent of the previous mouse does not influence behavior of subsequent mice tested.
Test the next mouse. Use a fresh tongue depressor for each mouse. Mice that reside in the same cage can be tested sequentially on the same day without additional acclimation to the procedure room.
Representative Results
This procedure for measuring exercise endurance accurately reflects the molecular and metabolic profile of different strains of C57BL/6 mice that differ in expression of the cytokine IL-15 16,17. Transgenic mice that overexpress IL-15 (IL-15 TG mice) exhibit significantly increased run-to-exhaustion times compared to littermate controls, while mice that lack IL-15 (IL-15 KO mice) exhibit significantly reduced run times prior to reaching exhaustion (Figure 1A). Published studies have shown that muscles are shifted towards a more oxidative phenotype in IL-15 TG mice16; conversely, muscles in IL-15 KO mice are shifted to a less oxidative phenotype17.These muscle phenotypes correlate with levels of spontaneous physical activity in a beam-break apparatus, and with exercise endurance using the voluntary run-to-exhaustion test. Wild-type control C57BL/6 mice from two different sources (in-house colony and a commercial supplier) do not exhibit significantly different run times (Figure 1A).
Maximal sustained running speed differed only in IL-15 KO mice (Figure 1B). Distance run (not shown) can also be calculated and analyzed statistically; however, separation of the two parameters (time-to-exhaustion and speed) yields more information.
An unavoidable limitation of this technique is that some mice refuse to run voluntarily. The percentage of young wild-type control mice that refuse to run is around 10% at 4 months of age. The percentage of refusals increases to 20% at 8 months of age, and increases to more than 60% by 16 months of age (Figure 2). However, the percentage of refusals in the older age group is significantly lower in IL-15 TG mice (Figure 2).
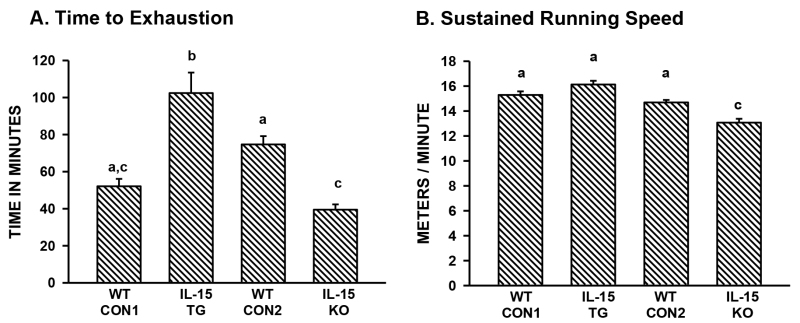 Figure 1. Representative data from the voluntary run-to-exhaustion test. (A) Time to exhaustion; (B) Sustained running speed. For both panels, 4 strains of C57BL/6 male mice were tested: WT CON1 (wild-type littermate controls from in-house transgenic colony); IL-15 TG (interleukin-15 overexpressing transgenic mice16, littermates of WT CON1); WT CON2 (wild-type control mice, obtained commercially); IL-15 KO (interleukin-15 knockout mice17, obtained commercially). Bars represent mean + SEM; n = 7 - 29 mice per group. Data were analyzed by Kruskal-Wallis one-way ANOVA on ranks; bars with different superscripts are significantly different at P <0.05. Data are reprinted from previous publications16,17, with permission from the journal.
Figure 1. Representative data from the voluntary run-to-exhaustion test. (A) Time to exhaustion; (B) Sustained running speed. For both panels, 4 strains of C57BL/6 male mice were tested: WT CON1 (wild-type littermate controls from in-house transgenic colony); IL-15 TG (interleukin-15 overexpressing transgenic mice16, littermates of WT CON1); WT CON2 (wild-type control mice, obtained commercially); IL-15 KO (interleukin-15 knockout mice17, obtained commercially). Bars represent mean + SEM; n = 7 - 29 mice per group. Data were analyzed by Kruskal-Wallis one-way ANOVA on ranks; bars with different superscripts are significantly different at P <0.05. Data are reprinted from previous publications16,17, with permission from the journal.
 Figure 2. Percentage of mice refusing to run voluntarily increases with age. Male interleukin-15 overexpressing transgenic mice (IL-15 TG) and littermate controls, both on a C57BL/6 background, were tested in the voluntary run-to-exhaustion protocol at 4, 8, 12, and 16 months of age. Bars represent percentage of mice in each age group that refused to run (n = 11 - 29 mice per group). Data were analyzed by Fisher’s Exact Test; asterisk denotes a significant difference at P <0.05 between control and IL-15 TG at 16 months of age. Please click here to view a larger version of this figure.
Figure 2. Percentage of mice refusing to run voluntarily increases with age. Male interleukin-15 overexpressing transgenic mice (IL-15 TG) and littermate controls, both on a C57BL/6 background, were tested in the voluntary run-to-exhaustion protocol at 4, 8, 12, and 16 months of age. Bars represent percentage of mice in each age group that refused to run (n = 11 - 29 mice per group). Data were analyzed by Fisher’s Exact Test; asterisk denotes a significant difference at P <0.05 between control and IL-15 TG at 16 months of age. Please click here to view a larger version of this figure.
Discussion
Described here is a method to assess voluntary running endurance in laboratory mice using a motorized treadmill without the use of a shock grid. This method can reveal differences among sub-lines of C57BL/6 mice that differ in expression of the cytokine IL-15, which in turn causes differences in expression of factors that underlie exercise endurance16,17. In keeping with the principles of the “Three R’s” in laboratory animal science, this method can be used as a more humane alternative, or refinement, to forced exercise protocols that utilize a shock grid8-11. Forced exercise can also introduce the confounding effects of psychological stress and avoidance behavior12 on both running parameters and post-exercise measurements such as serum cytokines and hormones.
A limitation of the technique is that determination of sustained maximal running speed, refusal to run, and the end point of exhaustion are each assessed subjectively by the human observer. However, most young mice will run voluntarily if acclimated slowly to the apparatus; moreover, mice generally exhibit clear behavioral signs of refusal to run and of having reached exhaustion after running. Nevertheless, successful conduction of this technique requires some observer sensitivity to the animals. Therefore, it is recommended that a single observer, preferably treatment-blinded, be used for all of the run-to-exhaustion tests within a study to minimize observer bias in determination of these parameters. Despite the somewhat subjective nature of the endpoints for determination of running speed and time-to-exhaustion, this methodology is robust and can detect clear differences in running endurance due to upregulation16 or deletion17 of a single gene, IL-15.
This protocol was demonstrated with different sub-lines of C57BL/6 mice, and was not tested with other strains of laboratory mice. C57BL/6 mice were utilized because this is the most common genetic background utilized for transgenic and knockout mice18. A study by Lightfoot et. al.19 indicated that C57BL/6 mice performed in the low-medium range of exercise endurance among inbred strains of laboratory mice. However, that study utilized forced treadmill running with a shock grid and much higher running speeds, whereas in this technique, both the maximal sustained speed and the duration of running are individual to each mouse. Additionally, data solely from male mice was shown here. While the technique works with female mice (not shown), these are not generally utilized in studies of transgenic and knockout mice.
Since running in this protocol is voluntary, loss of data from some mice that refuse to run is unavoidable, and should be taken into account when estimating group sizes necessary for statistical significance. Additionally, representative data show that the tendency to refuse to run increases with age in wild-type C57BL/6 mice. Therefore this technique may not be suitable for determination of exercise endurance in very old mice. However, genetic manipulation to increase IL-15 expression significantly lowered the percentage of refusals in older mice, suggesting that refusal to run may also reflect decreased exercise capacity in older animals. Voluntary exercise frequency, as well as gait speed, decline with age in both rodents and humans, and can be reflective of declines in metabolic parameters and muscle mass 20-22. In this regard, the parameters of time-to-exhaustion and maximum sustained running speed were presented separately in the representative data. Distance run (m) can be calculated by multiplying time-to-exhaustion by maximum sustained running speed; however, this measure is somewhat inaccurate due to the initial phase of the run at slightly slower speeds. Some models of rodent treadmills have digital recording features that can be set to calculate distance run precisely; however, presenting time-to-exhaustion and speed separately may yield more information.
The reduced precision of endpoints, differences in running speed, and the complication of mice that refuse to run in the technique described here, are balanced by the consideration that the technique is more humane and less stressful to mice. However, blood markers of murine stress such as corticosterone levels were not determined to demonstrate rigorously that this technique is less stressful than forced exercise using a shock grid. Such determinations are technically difficult in acute exercise experiments because glucocorticoid levels are confounded by the anesthetics used in obtaining blood samples in mice23 and by exercise itself 24. One study25 indicated that chronic forced exercise using a shock grid induced both psychological and immune stress in mice, while voluntary wheel running (in which mice could control both the duration and speed of running) had the opposite effect. Close behavioral examination of the mice, combined with their demonstrated willingness to run and the long durations of voluntary running bouts, suggest mice are not unduly stressed by the protocol described here. Moreover, representative data indicate this protocol can reveal clear differences in running endurance due to introduction or deletion of a single gene product within an inbred strain 16,17. Therefore, this protocol represents a more humane and scientifically useful alternative to forced exercise for determination of exercise endurance in murine models.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgments
Supported by Merit Review #BX001026 from the Department of Veterans Affairs (LSQ), and use of resources and facilities at VA Puget Sound Health Care System, the Transgenic Resource Core at the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging (NIA #5P30AG-013280), and the University of Washington Diabetes Endocrinology Research Center (NIH #P30 DK-17047). We thank Cynthia Pekow DVM and Kari L. Koszdin DVM, VA Puget Sound, provided helpful comments on the manuscript.
References
- Hill JA, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Natl Acad Sci USA. 2008;67(2):128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- Benton CR, Wright DC, Bonen A. PGC-1α-mediated regulation of gene expression and metabolism: Implications for nutrition and exercise prescriptions. Appl. Physiol. Nutr. Metab. 2008;33(5):843–862. doi: 10.1139/H08-074. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S, et al. Use of the Short Physical Performance Battery score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J. Gerontol. A Biol. Med. Sci. 2009;64(2):223–229. doi: 10.1093/gerona/gln022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, et al. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Bio. 2004;2(10):e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrasseur NK, et al. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J. Gerontol. A Biol. Med. Sci. 2009;64(9):940–948. doi: 10.1093/gerona/glp068. [DOI] [PubMed] [Google Scholar]
- Burch N, et al. Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle. PLoS ONE. 2010;5(6):e10970. doi: 10.1371/journal.pone.0010970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. Mitochondrial biogenesis and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation by physical activity. Diabetes. 2011;60(1):157–167. doi: 10.2337/db10-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knab AM, et al. Repeatability of exercise behaviors in mice. Physiol. & Behavior. 2009;98(4):433–440. doi: 10.1016/j.physbeh.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, et al. IL-15 Receptor deletion results in circadian changes of locomotor and metabolic behavior. J. Mol. Neurosci. 2010;41(2):315–321. doi: 10.1007/s12031-009-9319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Hsuchou H, Kastin AJ, Rood JC, Pan W. Essential role of interleukin-15 receptor in normal anxiety behavior. Brain Behav. Immun. 2010;24(8):1340–1346. doi: 10.1016/j.bbi.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli EE, et al. Loss of IL-15 receptor α alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J. Clin. Invest. 2011;121(8):3120–3132. doi: 10.1172/JCI44945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn LS, Anderson BG, Conner JD, Wolden-Hanson TW. IL-15 overexpression promotes endurance, oxidative energy metabolism, and muscle PPARδ, SIRT1, PGC-1α, and PGC-1β expression in male mice. Endocrinology. 2013;154(1):232–245. doi: 10.1210/en.2012-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn LS, Anderson BG, Conner JD, Wolden-Hanson T, Marcell TJ. IL-15 is required for post-exercise induction of the pro-oxidative mediators PPARdelta and SIRT1. Endocrinology. 2013. [DOI] [PMC free article] [PubMed]
- Ward JM, Anver MR, Mahler JF, Devor-Henneman DE. Chapter 13, Pathology of mice commonly used in genetic engineering (C57BL/6; 129; B6,129; and FVB/N) In: Ward JM, Mahler JF, Maronpot RR, Sundberg JP, editors. The Pathology of Genetically Engineered Mice. Ames, IA, Chapter: Iowa State University Press; 2000. pp. 161–179. [Google Scholar]
- Lightfoot JT, Turner MJ, Debate KA, Kleeberger SR. Interstrain variation in murine aerobic capacity. Med. Sci. Sports Exerc. 2001;33(12):2053–2057. doi: 10.1097/00005768-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Turner MJ, Kleeberger SR, Lightfoot JT. Influence of genetic background on daily running-wheel activity differs with aging. Physiol. Genomics. 2005;22(1):76–85. doi: 10.1152/physiolgenomics.00243.2004. [DOI] [PubMed] [Google Scholar]
- Haight TJ, van der Laan MJ, Manini T, Tager IB. Direct effects of leisure-time physical activity on walking speed. J. Nutr. Health Aging. 2013;17(8):666–673. doi: 10.1007/s12603-013-0024-9. [DOI] [PubMed] [Google Scholar]
- Studenski S. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen KR, Kalliokoski O, Teilmann AC, Hau J, Abelson KS. The effect of isoflurane anaesthesia and vasectomy on circulating corticosterone and ACTH in BALB/c mice. Gen. Comp. Endocrinol. 2012;179(3):406–413. doi: 10.1016/j.ygcen.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Sellers TL, Jaussi AW, Yang HT, Heninger RW, Winder WW. Effect of the exercise-induced increase in glucocorticoids on endurance in the rat. J. Appl. Physiol. 1988;65(1):173–178. doi: 10.1152/jappl.1988.65.1.173. [DOI] [PubMed] [Google Scholar]
- Cook MD, et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav. Immun. 2013;33:46–56. doi: 10.1016/j.bbi.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


