The results of a preplanned cardiac safety analysis of global longitudinal strain (GLS), and troponin-I (TnI) and brain natriuretic peptide (BNP) levels in the phase II study of paclitaxel, trastuzumab, and pertuzumab (THP) for metastatic HER2-positive breast cancer are reported. There were no statistically significant changes in GLS, and TnI and BNP levels. The finding supports the cardiac safety of THP in this group of patients.
Keywords: Cardiotoxicity, Heart failure, Trastuzumab, Pertuzumab, Imaging, Biomarkers
Abstract
Introduction.
Myocardial strain imaging and blood biomarkers have been proposed as adjuncts to left ventricular ejection fraction (LVEF) monitoring for the early detection of cardiotoxicity during cancer therapy. We report the results of a preplanned cardiac safety analysis of global longitudinal strain (GLS), and troponin-I (TnI) and brain natriuretic peptide (BNP) levels in the phase II study of paclitaxel, trastuzumab, and pertuzumab (THP) for metastatic HER2-positive breast cancer.
Patients and Methods.
Patients with 0–1 lines of prior therapy were treated with weekly paclitaxel (80 mg/m2) plus trastuzumab (8 mg/kg loading dose followed by 6 mg/kg) and pertuzumab (840 mg loading dose followed by 420 mg) every 3 weeks. Exploratory endpoints were GLS measured with speckle-tracking echocardiography every 3 months and TnI and BNP levels measured every 6 weeks (immediately pre- and postchemotherapy infusion) at 6 time points.
Results.
Sixty-seven of 69 enrolled patients were treated with THP: 19 (28%) had hypertension, 8 (12%) had diabetes, 11 (16%) had hyperlipidemia, and 26 (38%) had smoking history. After a median follow-up of 21 months (range: 3–38 months), no patients developed symptomatic heart failure. Two patients (3.0%) experienced asymptomatic LVEF decline (grade 2). The mean GLS (±SD) was 19% ± 2% (baseline), 19% ± 2% (month 6), and 19% ± 3% (month 12). Detectable TnI (>0.06 ng/mL) and elevated BNP (>100 pg/mL) levels were observed in 3 (4.3%) and 2 (3.0%) patients, respectively, but were not associated with LVEF decline.
Conclusion.
The absence of any significant changes in GLS and cardiac biomarkers (TnI and BNP) further support the cardiac safety of THP in patients with metastatic HER2-positive breast cancer.
Implications for Practice:
Dual anti-HER2 therapy with trastuzumab and pertuzumab in combination with taxane-based chemotherapy improves overall survival in patients with metastatic HER2-positive breast cancer. There is a critical need to investigate the potential cardiotoxicity of dual anti-HER2 blockade, given the importance of HER2 signaling in cardiac homeostasis and stress response. Global longitudinal strain and cardiac biomarkers have been proposed as adjuncts to left ventricular ejection fraction for the early detection of cardiotoxicity. In this phase II study of combination trastuzumab and pertuzumab with paclitaxel, no clinically significant change was observed in global longitudinal strain or cardiac biomarkers. These results further support the cardiac safety of dual anti-HER2 blockade previously reported in the CLEOPATRA study. The findings in the current study also call into question the role of intensive cardiac monitoring among patients treated with anti-HER2 therapy in the absence of anthracyclines. Less frequent cardiac assessments could lead to a reduction in unnecessary treatment interruption and is an important consideration given the rise in medical expenditures, but this requires further investigation.
Abstract
摘要
引言. 有人提出使用心肌应变成像和血液生物标记物作为左心室射血分数 (LVEF) 监测的辅助检查, 用于早期发现癌症治疗过程中的心脏毒性。本研究报告了紫杉醇、曲妥珠单抗和帕妥珠单抗 (THP) 治疗 HER2 阳性转移性乳腺癌的II期研究中, 对整体纵向应变 (GLS)、 肌钙蛋白-I (TnI) 以及大脑钠尿肽 (BNP) 水平进行的预先计划的心脏安全性分析结果。
患者与方法. 初治和曾接受过一线治疗的患者在本研究中接受紫杉醇 (80 mg/m2) 每周一次联合曲妥珠单抗 (8 mg/kg负荷剂量后6 mg/kg) 和帕妥珠单抗 (840 mg 负荷剂量后 420 mg) 每 3 周一次治疗。探索性终点为每 3 个月一次使用斑点追踪超声心动图测量的 GLS, 以及每 6 周测定一次 TnI 和 BNP 水平 (化疗输注前后即刻), 共 6 个时间点。
结果. 67/69 例纳入的患者接受了 THP 治疗, 其中 19 例 (28%) 有高血压, 8 例 (12%) 有糖尿病, 11例 (16%) 有高脂血症, 26 例 (38%) 有吸烟史。中位随访 21 个月 (范围: 3∼38 个月) 后, 没有患者发生症状性心力衰竭。2 例 (3.0%) 患者发生了无症状的 LVEF 下降 (2 级)。平均 GLS (±SD) 为 19%±2% (基线)、 19%±2% (6 个月) 和 19%±3% (12 个月)。3 例 (4.3%) 患者 TnI 可测 (> 0.06 ng/mL), 2 例 (3.0%) 患者 BNP 水平升高 (> 100 pg/mL), 但均与 LVEF 下降无关。
结论. GLS和心脏标记物 (TnI和BNP) 未发生任何显著变化进一步支持了THP在HER2阳性转移性乳腺癌患者中的心脏安全性。The Oncologist 2016;21:418–424
对临床实践的提示: 曲妥珠单抗与帕妥珠单抗的二联抗 HER2 治疗, 与以紫杉烷类为基础的化疗联用时, 能够改善 HER2 阳性的转移性乳腺癌患者的总生存。鉴于 HER2 信号转导在心脏稳态和应激反应中具有重要作用, 因此非常有必要对 HER2 双重阻断是否有潜在的心脏毒性进行研究。整体纵向应变和心脏生物标记物可作为左心室射血分数的辅助检查, 用于早期发现心脏毒性。本项 II 期研究中, 曲妥珠单抗、帕妥珠单抗与紫杉醇联合治疗后整体纵向应变和心脏生物标记物水平均无具有临床意义的改变。这些结果进一步支持了之前 CLEOPATRA 研究中报告的 HER2 双重阻断的心脏安全性。本研究的发现同时也对密切心脏监测在接受抗 HER2 治疗而未接受蒽环类治疗的患者中的作用提出了疑问。降低心脏评估的频率可减少不必要的治疗中断, 并且在医疗成本不断增加的大环境下也是一个值得考虑的重要问题, 但这需要开展进一步研究。
Introduction
Trastuzumab in combination with chemotherapy has reduced disease recurrence and overall mortality in patients with early and metastatic human epidermal growth factor receptor 2 (HER2)-positive breast cancer [1–3]. Left ventricular systolic dysfunction (LVSD) is the most concerning cardiac toxicity associated with trastuzumab, particularly when it is administered as part of an anthracycline-containing regimen. In adjuvant clinical trials of trastuzumab, 2%–4% of patients experienced severe heart failure (HF) and 14%–19% of patients developed a significant decline in left ventricular ejection fraction (LVEF) [3, 4]. The underlying mechanism of trastuzumab-induced LVSD has been attributed to the blockade of HER2 signaling that impairs the normal stress response and cellular repair mechanisms of cardiomyocytes [5].
Preclinical and clinical studies demonstrate that dual anti-HER2 therapy with trastuzumab and pertuzumab provides more complete blockade of HER2 signaling and improves tumor shrinkage and cell death [6–8]. Pertuzumab is a humanized monoclonal antibody that targets HER2 at a different epitope than trastuzumab and prevents HER2 heterodimerization [9]. The CLEOPATRA trial was a phase III trial of combination pertuzumab and trastuzumab with docetaxel as first-line therapy in HER2-positive metastatic breast cancer; study results showed significant improvement in progression-free survival and overall survival [10]. However, given the important role of HER2 signaling in cardiomyocytes, there has been a concern that more complete HER2 blockade with trastuzumab and pertuzumab may increase the risk for LVSD.
In the cardiac safety analysis of the CLEOPATRA trial, dual anti-HER2 therapy did not increase the incidence of LVSD compared with the control arm [11]. A significant decline in LVEF by ≥10 absolute percentage points to <50% was observed in 3.8% of patients in the pertuzumab and trastuzumab arm versus 6.6% of patients in the trastuzumab arm, and the incidence of symptomatic LVSD was low in both groups (1.0% vs. 1.8%, respectively). However, cardiac monitoring in the CLEOPATRA trial consisted of LVEF assessments using two-dimensional echocardiography (ECHO), which lacks the sensitivity to detect early and mild subclinical changes in left ventricular (LV) systolic function [12]. After taking into consideration the temporal and observer variability of ECHO, the smallest change in LVEF that can be considered significantly different is approximately 10% [13, 14]. Furthermore, a reduction in LVEF of this magnitude is often considered a late manifestation of cardiotoxicity, with failure to recover normal LV systolic function in many patients despite pharmacologic therapy [15].
There has been growing interest in the use of more sensitive and specific markers of early LVSD (i.e., before an overt decline in LVEF) so that cardioprotective strategies or modification of cancer treatment can be implemented to mitigate the risk for cardiac toxicity related to cancer therapy. Myocardial strain indices such as global longitudinal strain (GLS) can be measured with speckle-tracking echocardiography and provide a more sensitive and quantitative assessment of LV systolic function than LVEF. Studies have shown that a decrease in GLS precedes a decrease in LVEF in patients with breast cancer treated with adjuvant chemotherapy and trastuzumab [16, 17]. Cardiac biomarkers (e.g., troponin-I [TnI] and brain natriuretic peptide [BNP]) can also identify patients at increased risk for cardiotoxicity [18, 19]. To our knowledge, no prior study has investigated the use of speckle-tracking echocardiography or cardiac biomarkers for the cardiac monitoring of patients treated with dual anti-HER2 therapy.
We previously reported that weekly paclitaxel in combination with trastuzumab and pertuzumab (THP) is an effective alternative to docetaxel-based combination therapy for the treatment of metastatic HER2-positive breast cancer with a favorable cardiac toxicity profile and no significant change in LVEF [20]. Here, we report on the exploratory cardiac substudy of this regimen using speckle-tracking echocardiography and cardiac biomarkers.
Patients and Methods
Patients and Study Design
The full methods of this study have been reported [20]. In brief, eligible patients had metastatic HER2-positive breast cancer with 0–1 prior lines of therapy and LVEF of ≥50% determined by ECHO or multigated acquisition scan (MUGA). Major exclusion criteria included prior pertuzumab use, history of prior cardiac morbidities within 12 months (i.e., myocardial infarction, congestive heart failure, or uncontrolled ventricular arrhythmias), grade 2 or higher neuropathy, or prior history of grade 3 or higher toxicity to trastuzumab or paclitaxel resulting in permanent cessation of treatment. The study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center, and written informed consent was obtained from each participant.
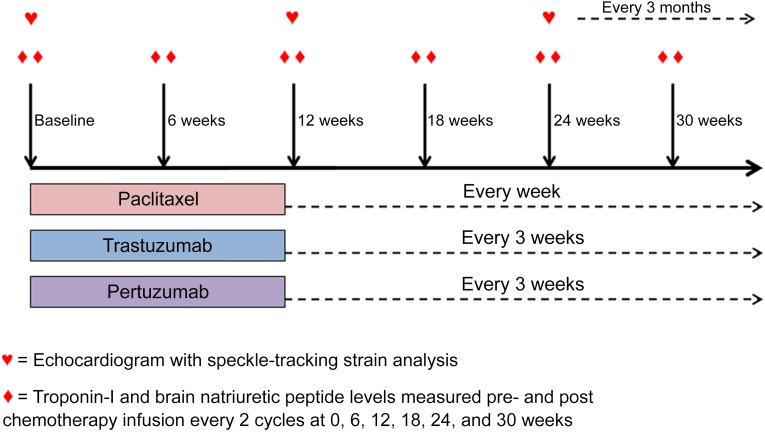
Study drugs were administered intravenously on a 3-week schedule until disease progression. Paclitaxel (80 mg/m2) was administered weekly. Trastuzumab was given at an initial loading dose of 8 mg/kg followed by 6mg/kg every 3 weeks. Pertuzumab was given at an initial dose of 840 mg followed by 420 mg every 3 weeks. One cycle consisted of 3 doses of weekly paclitaxel with trastuzumab and pertuzumab on day 1 (Fig. 1).
Figure 1.
Treatment schema. Participants were studied at baseline and at standardized intervals every 3 months during paclitaxel, trastuzumab, and pertuzumab therapy, using two-dimensional echocardiograms with speckle-tracking strain analysis. Biomarker levels were measured before and after treatment infusion at baseline (cycle 1) and every other cycle until week 30 (cycle 11).
Cardiac Monitoring
LVEF was assessed by ECHO at baseline and every fourth cycle (every 3 months). Major criteria for removal from study included unacceptable treatment-related toxicity or disease progression. ECHO was performed using the Vivid 7 or E9 system (GE Healthcare, Milwaukee, WI, http://www3.gehealthcare.com). LVEF was calculated from the apical 4- and 2-chamber views using a modified Simpson biplane method, and the lower limit for LVEF by ECHO at our institution is 55%. GLS was measured using speckle-tracking echocardiography. Three apical views were used to calculate peak systolic GLS offline, using standard, commercially available software (EchoPAC SW BT13; GE Healthcare). GLS measures were reported in absolute values. Abnormal GLS was defined as <16%, which represents 2 standard deviations below the mean in healthy adults [21, 22]. The reproducibility of GLS measurements was assessed in 20 randomly selected ECHOs by 2 observers.
Biomarker Testing
Blood was collected before and after chemotherapy infusion at baseline and every other cycle for up to 6 time points. TnI was determined by a fluorometric enzyme immunoassay analyzer (Tosoh Bioscience, San Francisco, CA, http://www.tosohbioscience.com) with a low end sensitivity of 0.06 ng/mL. BNP was measured on a Biosite Triage analyzer (Alere, San Diego, CA, http://www.alere.com) using a fluorescence immunoassay. All values of BNP >100 pg/mL were considered elevated [23].
Interruption and Discontinuation of Therapy
LVSD was defined per National Cancer Institute Common Toxicity Criteria Adverse Event version 4.0. Grade 3 or 4 LVSD (symptomatic HF) resulted in permanent discontinuation of therapy. For an asymptomatic LVEF decline, defined as a decrease from baseline by ≥16 absolute percentage points or a decrease of 10–15 absolute percentage points to below the lower limit of normal, therapy was temporarily withheld, with LVEF reassessment within 3 weeks. If the LVEF remained decreased upon reassessment, requiring another hold, therapy was permanently discontinued. The cardiac safety endpoint was defined as the incidence of grade 3 or 4 LVSD, non-LVSD cardiac death, or probable cardiac death. Based on prior cardiac safety data with targeted anti-HER2 therapy, a rate of significant asymptomatic LVEF decline ≤20% and grades 3 or 4 LVSD (HF) ≤4% were deemed acceptable [24–26].
Statistical Analysis
Patient characteristics including LVEF, GLS, and biomarker values were summarized using descriptive statistics. Exploratory cardiac endpoints were summarized for descriptive purposes. GLS was compared at baseline, and at 3 and 6 months using a 1-way analysis of variance for repeated measures. For GLS, the inter- and intraobserver variabilities were measured by the intraclass correlation coefficient (ICC). The intraobserver ICC for GLS was 0.91 (95% confidence interval [CI]: 0.78–0.96). The corresponding interobserver ICC was 0.86 (95% CI: 0.68–0.94). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, https://www.sas.com).
Results
Baseline Characteristics
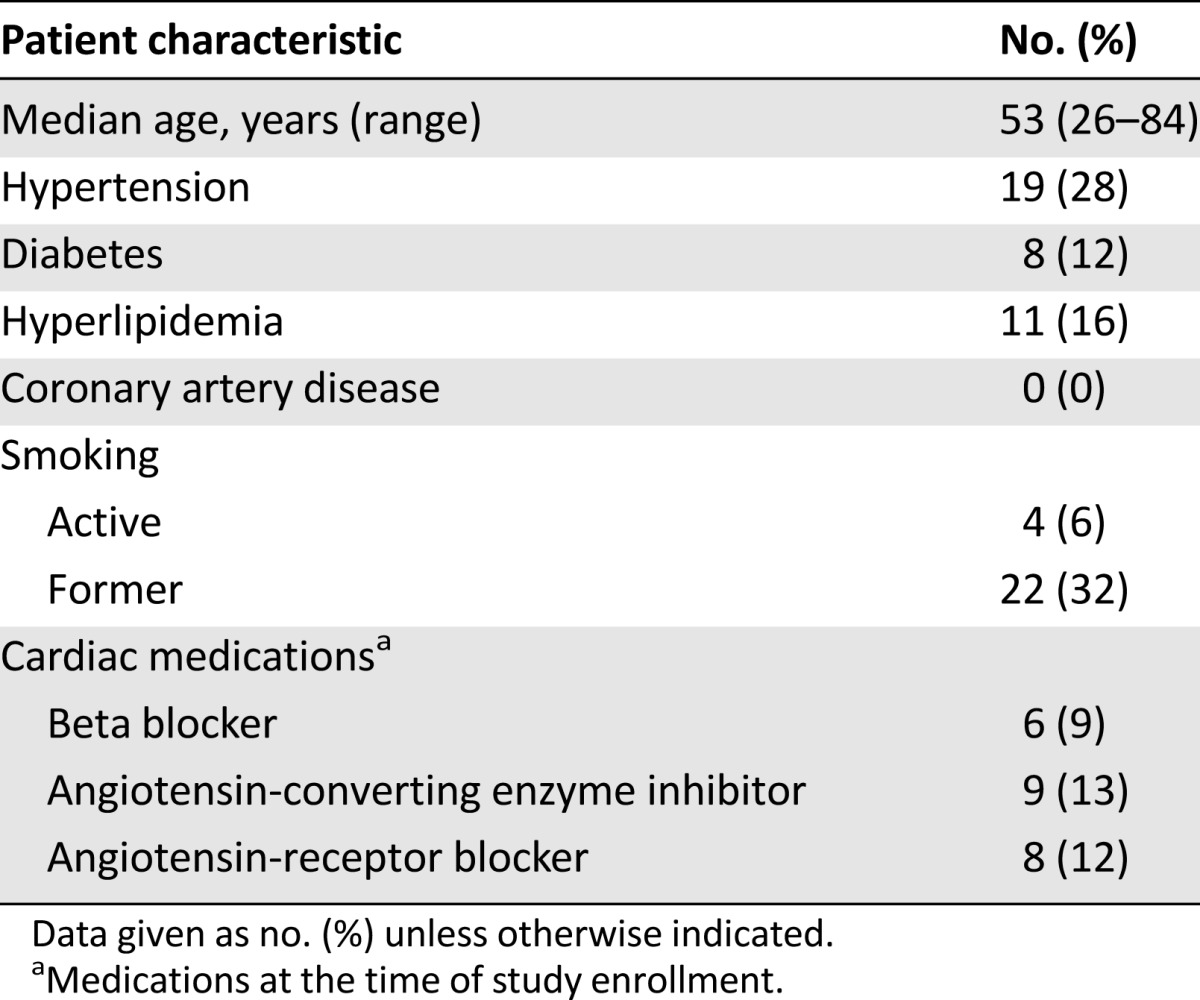
From January 2011 to December 2013, 69 patients were enrolled. Baseline characteristics have been previously reported (supplemental online Table 1) [20]. The median follow-up was 21 months (range: 3–38 months); the median age was 53 years (range: 26–84 years). Overall, 42 patients (61%) had at least one cardiac risk factor at baseline: 19 (28%) with hypertension, 8 (12%) with diabetes, 11 (16%) with hyperlipidemia, and 26 (38%) with smoking history (Table 1). Nineteen (28%) had received anthracycline chemotherapy and 22 (32%) had received trastuzumab as neoadjuvant therapy.
Table 1.
Patient characteristics (N = 69)
Cardiac Events
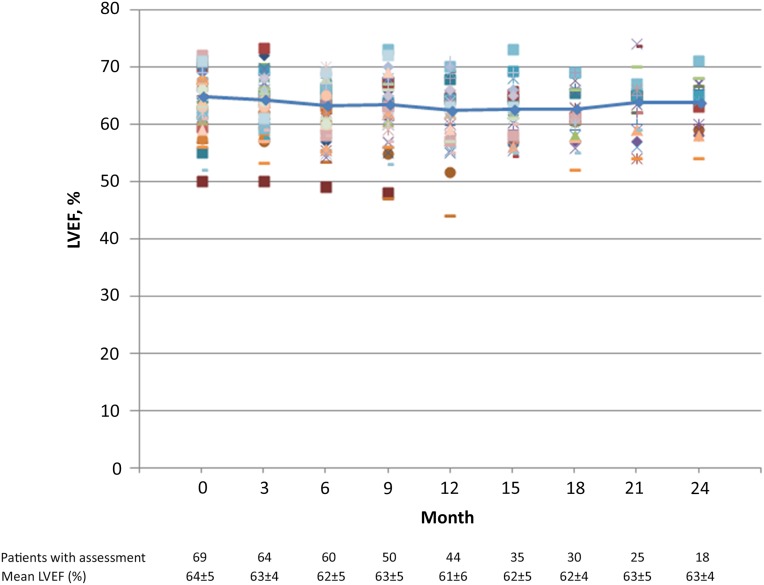
Two patients did not receive any anti-HER2 antibody because of immediate hypersensitivity to paclitaxel and came off the study on the same day of enrollment. Therefore, the cardiac safety population comprised 67 patients (Table 2). No patients developed LVSD (grades 3 or 4). The mean LVEF at baseline was 64% (range: 50%–72%), and overall mean LVEF values remained stable throughout the study (Fig. 2) [20]. The incidence of asymptomatic LVEF decline (both grade 2) was 3.0% (n = 2). One patient developed an LVEF decline of ≥16% (a decrease from 72% at baseline to 55% at month 15), and 1 patient developed an LVEF of <50% with a decline of 10%–15% from baseline (57% at baseline to 47% at month 9).
Table 2.
Baseline LVEF and LVEF decline during trastuzumab/pertuzumab treatment
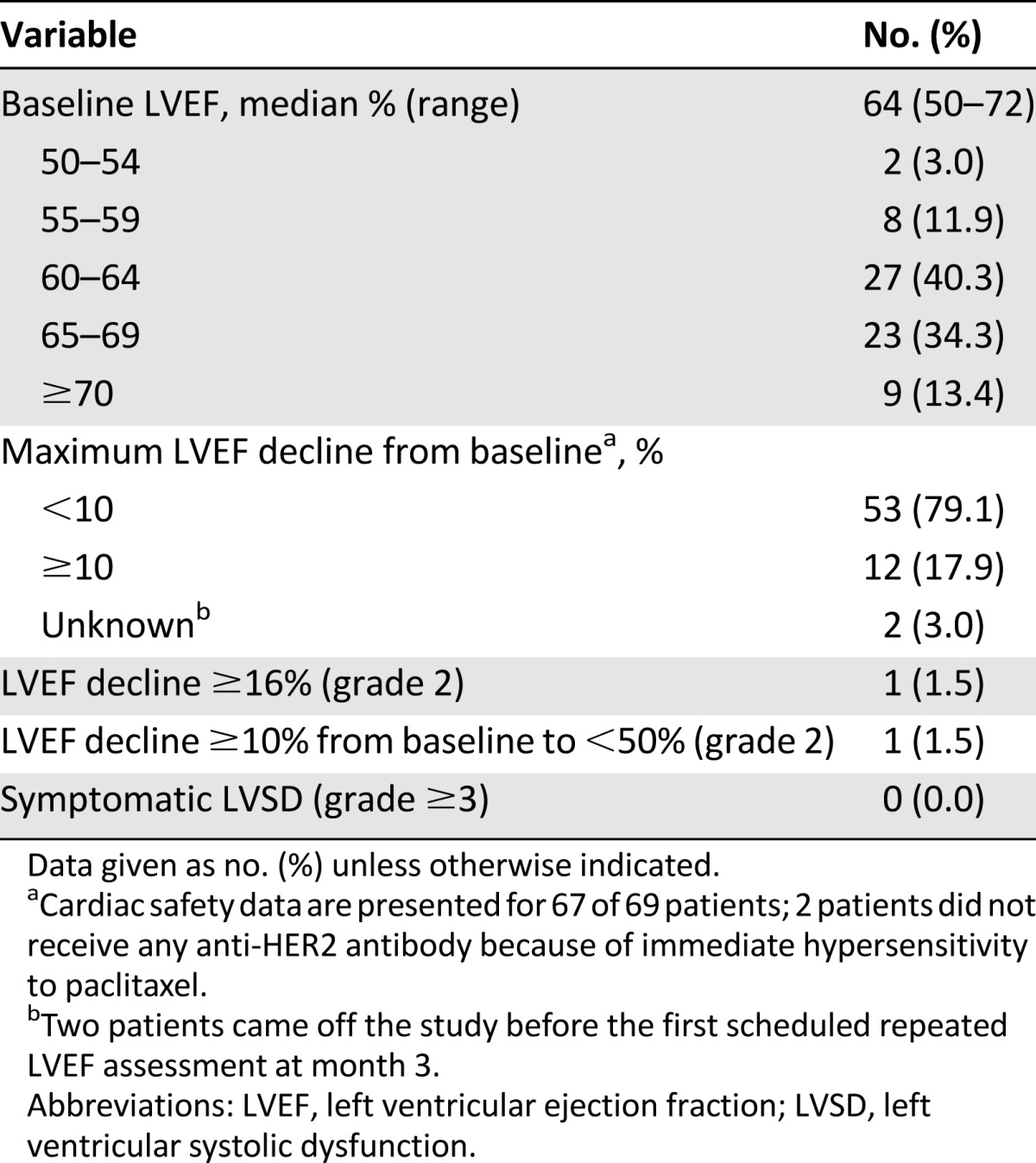
Figure 2.
Time course of LVEF. Trend line represents mean LVEF during treatment with paclitaxel, trastuzumab, and pertuzumab. Individual patients’ results are shown in different colors.
Abbreviation: LVEF, left ventricular ejection fraction.
The first patient was a 61-year-old woman with history of hypertension, left bundle branch block, nonischemic cardiomyopathy (LVEF of 20%) 8 years before study enrollment, and no history of anthracycline exposure. Her LVEF had normalized before study enrollment while receiving carvedilol (25 mg twice daily) and lisinopril (20 mg once daily) therapy, and these medications were continued during the treatment period. Her initial LVEF values were 57% (baseline), 59% (3 months), and 54% (6 months). LVEF declined to 47% (at 9 months) and repeated ECHO 2 weeks later showed an LVEF of 37% (still asymptomatic), after which anti-HER2 therapy was discontinued per study criteria. GLS decreased from 19.4% (3 months) to 17.4% (6 months) to 13.7% (9 months). She was followed by a cardiologist but no adjustments in her medications were made because she was asymptomatic and already taking optimal doses of heart failure medications. Subsequent follow-up LVEF values were 44% (12 months), 54% (18 months), and 45% (24 months). She is doing well with optimal cardiac medications without signs or symptoms of HF, as of this writing.
The second patient with asymptomatic LVEF decline was a 38-year-old woman with no significant cardiovascular risk factors and no prior anthracycline exposure. Her baseline LVEF was 72% and that decreased to 55% at month 15. She remained asymptomatic with no signs or symptoms of HF, and the decision was made to continue anti-HER2 therapy (a study violation because her LVEF declined ≥16% from baseline). However, she remained asymptomatic and subsequent follow-up LVEF was 57% at month 18 and 62% at month 21.
Global Longitudinal Strain
Speckle-tracking strain analysis was performed and interpretable in 316 of 395 (80%) scheduled LVEF assessments per protocol. Reasons for lack of speckle-tracking strain analysis included the following: MUGA (n = 2), outside ECHO (n = 8), poor image quality (n = 27), no LVEF assessment performed at indicated study time point (n = 6), and speckle-tracking images not obtained (n = 36).
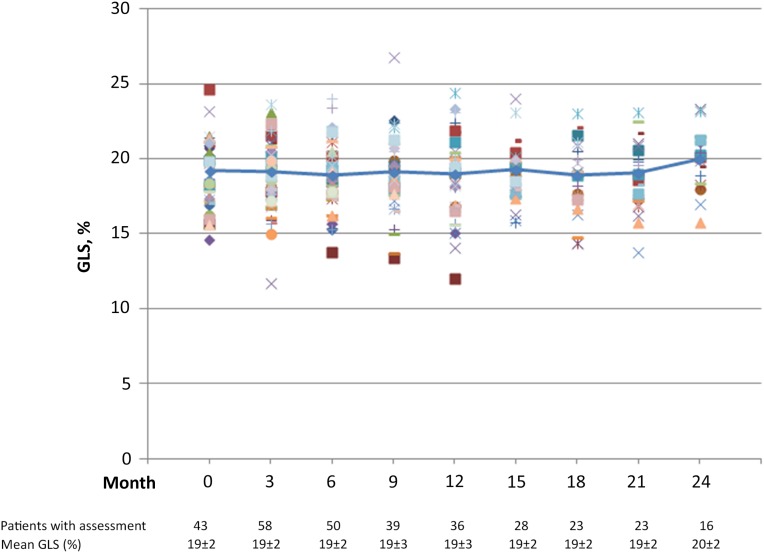
GLS did not decrease during the first 6 months of treatment (from 19% ± 2% to 19% ± 2%; p = .27). Overall, there was no clinically significant change in mean GLS throughout the study period (Fig. 3). Abnormal GLS <16% was observed in 17 of 67 patients (25%) during the treatment period. Only 1 of the 17 patients developed an asymptomatic LVEF decline that resulted in treatment discontinuation. This patient had a previous history of cardiomyopathy.
Figure 3.
Time course of GLS. Trend line represents mean GLS during treatment with paclitaxel, trastuzumab, and pertuzumab. Individual patients’ results are shown in different colors. GLS is reported for 316 of 395 (80%) scheduled LVEF assessments. Reasons for lack of speckle-tracking strain analysis included the following: multigated acquisition scan (n = 2), outside two-dimensional echocardiography (n = 8), poor image quality (n = 27), no left ventricular ejection fraction assessment performed at indicated study time point (n = 6), and images for speckle tracking not obtained (n = 36).
Abbreviation: GLS, global longitudinal strain.
Cardiac Biomarkers
Overall, 1,381 of 1,656 TnI and BNP measurements (83%) were performed per protocol. Across all time points, TnI was detectable in 3 patients (4.5%). Specifically, the TnI level was normal at the preinfusion time point but became elevated postinfusion in 2 patients—1 at cycle 1 (0.07 mg/dL) and the other at cycle 3 (0.10 mg/dL). In 1 patient, the TnI level was elevated before infusion at cycle 3 (0.13 mg/dL) but was subsequently undetectable postinfusion. All TnI measurements performed at cycles 5, 7, 9, and 11 were undetectable.
BNP levels did not change significantly throughout the study period. During cycle 1, 2 patients (3.0%) had an elevated BNP level (110 and 131 pg/mL) that remained persistently elevated postinfusion (126 and 146 pg/mL). In these 2 patients, BNP was normal (<100 pg/mL) for all subsequent measurements. BNP levels were normal in all other patients throughout the study period. Among the five patients with detectable TnI or elevated BNP levels during the study period, none developed a significant asymptomatic LVEF decline (as defined by the study) or symptomatic HF.
Discussion
The results of this study confirm the favorable cardiac safety profile of combination treatment with weekly paclitaxel and dual anti-HER2 therapy with trastuzumab and pertuzumab. The overall incidence of significant asymptomatic grade 2 LVEF decline was 3.0% and, importantly, no patients developed symptomatic HF. Here, for the first time, to our knowledge, the use of novel methods for cardiac monitoring was analyzed in patients treated with dual anti-HER2 therapy, including speckle-tracking echocardiography and blood-based biomarkers. Results of this analysis further support the cardiac safety of THP despite a more complete blockade of anti-HER2 signaling.
Interestingly, nearly 25% of patients developed an abnormal GLS during therapy; however, these changes were transient and reversed despite continuation of anti-HER2 therapy. It is important to note that only 28% of patients in our study were previously treated with anthracyclines, most several years before beginning dual anti-HER2 therapy. The use of GLS to predict subsequent LVEF decline or heart failure was previously demonstrated in studies of patients treated with both anthracyclines and trastuzumab, and, therefore, cannot be generalized to patients treated with THP [12, 16, 27, 28]. Our results do not support the role of GLS measurement in patients treated with dual anti-HER2 therapy without the anthracyclines.
Biomarkers have been shown to detect subclinical cardiac toxicity during cancer therapy. In a study of 251 patients with breast cancer treated with trastuzumab in the adjuvant and metastatic setting (78% of patients were previously exposed to anthracycline chemotherapy), Cardinale et al. demonstrated that an elevated TnI level was predictive of LVEF decline [18]. A study by Ky et al. demonstrated that an elevation of ultrasensitive TnI after anthracycline chemotherapy, as well as the change in the concentration of ultrasensitive TnI after anthracycline chemotherapy from baseline, was associated with subsequent LVEF decline in patients undergoing doxorubicin and trastuzumab therapy [29]. In contrast, a prospective study by Morris et al. showed that the majority of patients (67%) with early breast cancer treated with an anthracycline-based regimen followed by paclitaxel, trastuzumab, and lapatinib had a detectable TnI level during the anti-HER2 phase; however, levels of TnI did not correlate with significant asymptomatic LVEF decline or predict symptomatic HF [30]. Similarly, studies on the value of BNP level to predict subsequent LVEF decline or HF have been inconsistent [31–33].
In our study, cardiac biomarker testing with TnI and BNP (before and after chemotherapy infusion) failed to show any evidence suggestive of clinically significant myocardial injury or volume or pressure overload. None of the five patients with elevated cardiac biomarkers developed significant LVEF decline or symptomatic HF. Based on our results, there is insufficient evidence to support the use of TnI or BNP testing in patients with metastatic breast cancer treated with paclitaxel and dual anti-HER2 therapy.
The optimal timing, frequency, and modality of cardiac monitoring during anti-HER2 therapy remain an area of uncertainty. Baseline LVEF assessment before initiation of cancer therapy serves an important role to evaluate for structural heart disease that would preclude the administration of potentially cardiotoxic anti-HER2 therapy. Subsequent serial cardiac monitoring during cancer therapy has allowed for the detection of LVEF decline (mostly asymptomatic); however, there is the potential harm that this is leading to unnecessary discontinuation of lifesaving anti-HER2 therapy. In addition, the long-term significance of an asymptomatic LVEF decline in this population remains unknown, and studies (with or without intervention with cardiac medications) are needed to elucidate this matter. Measurement of GLS can be used as an adjunctive prognostic measure to routine LVEF, particularly among patients treated with anthracyclines. However, there remain several limitations to the widespread application of GLS, including the lack of availability or expertise outside of specialized academic medical centers, the need to validate the role of GLS in larger and multicenter prospective studies, and intervendor variability in strain measurements. Efforts are underway to harmonize techniques for strain measurement and reduce vendor differences [34].
The optimal approach for monitoring and treatment of cardiotoxicity must take into consideration several factors, including the potential clinical benefit of a specific treatment regimen, cardiovascular risk associated with therapy, pre-existing cardiovascular disease (e.g., prior history of cardiomyopathy), availability of effective preventive strategies, and overall prognosis of the patient. In patients with metastatic HER2-positive breast cancer, the substantial survival benefit of continuous dual anti-HER2 therapy combined with the low observed cardiotoxicity rate (mostly asymptomatic) may justify a modification to the standard cardiac monitoring schedule. Per package inserts for both trastuzumab and pertuzumab, the “standard” LVEF monitoring is every 3 months while on therapy [35, 36]. However, with a low incidence of cardiotoxicity in our study, perhaps less frequent LVEF monitoring should be considered for most patients receiving trastuzumab and pertuzumab without the anthracyclines.
There are limitations to this study. First, patients who were considered at a high risk for cardiotoxicity, such as those with a history of myocardial infarction or HF within 12 months, were excluded from this study. Second, data for other cardiovascular risk factors (i.e., cerebrovascular disease, prior coronary revascularization, or arrhythmias) were inconsistently collected. Therefore, study findings and recommendations for a reduced number of LVEF assessments cannot be generalized to such patients. Furthermore, our findings should not be extended to patients being actively treated with an anthracycline-containing regimen, and routine cardiac monitoring may still be performed for these patients. Third, statistical analysis of changes in LVEF and GLS is limited because of the variable duration of cancer treatment and number of cardiac assessments performed per patient. A prospective trial that incorporates uniform assessment and management of cardiac risk factors with a central review of LVEF data in patients receiving anti-HER2 therapy (without an anthracycline) is needed to help further define which patients can safely undergo less intensive LVEF monitoring. Continued efforts to eliminate unnecessary medical testing remain critically important in the current era of increasing medical expenditures.
Conclusion
The combination treatment of a taxane with trastuzumab and pertuzumab in the metastatic setting has led to significant improvements in outcomes with a very low cardiac toxicity profile [10, 20]. The absence of any significant changes of LVEF, global longitudinal strain, or cardiac biomarkers (TnI or BNP) in our study further supports the excellent cardiac safety of this treatment regimen.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This work was supported by Roche/Genentech and funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Editor's Note: See the related commentary, “Enhanced Cardiac Testing in a Dual Anti-HER2 Regimen: What Have We Learned?” by Michael S. Ewer and Sandra M. Swain, on page 399 of this issue.
Author Contributions
Conception/Design: Jennifer E. Liu, Clifford A. Hudis, Chau T. Dang
Provision of study material or patients: Chau T. Dang
Collection and/or assembly of data: Anthony F. Yu, Carlos Manrique, Shawn Pun, Elton Mara, Martin Fleisher, Chau T. Dang
Data analysis and interpretation: Anthony F. Yu, Jennifer E. Liu, Sujata Patil, Lee W. Jones, Richard M. Steingart, Clifford A. Hudis, Chau T. Dang
Manuscript writing: Anthony F. Yu, Jennifer E. Liu, Sujata Patil, Lee W. Jones, Richard M. Steingart, Clifford A. Hudis, Chau T. Dang
Final approval of manuscript: Anthony F. Yu, Jennifer E. Liu, Sujata Patil, Richard M. Steingart, Clifford A. Hudis, Chau T. Dang
Disclosures
Clifford A. Hudis: Roche, Genentech (C/A, RF); Chau T. Dang: Genentech, GlaxoSmithKline (funding to MSKCC) (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. N Engl J Med. 2012;367:2150–2153. doi: 10.1056/NEJMcibr1203156. [DOI] [PubMed] [Google Scholar]
- 6.Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 7.Phillips GD, Fields CT, Li G, et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: Critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res. 2014;20:456–468. doi: 10.1158/1078-0432.CCR-13-0358. [DOI] [PubMed] [Google Scholar]
- 8.Ahn ER, Vogel CL. Dual HER2-targeted approaches in HER2-positive breast cancer. Breast Cancer Res Treat. 2012;131:371–383. doi: 10.1007/s10549-011-1781-y. [DOI] [PubMed] [Google Scholar]
- 9.Agus DB, Gordon MS, Taylor C, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–2543. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 10.Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swain SM, Ewer MS, Cortés J, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: A randomized, double-blind, placebo-controlled phase III study. The Oncologist. 2013;18:257–264. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Thavendiranathan P, Grant AD, Negishi T, et al. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Otterstad JE, Froeland G, St John Sutton M, et al. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18:507–513. doi: 10.1093/oxfordjournals.eurheartj.a015273. [DOI] [PubMed] [Google Scholar]
- 15.Thavendiranathan P, Poulin F, Lim KD, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J Am Coll Cardiol. 2014;63(25 pt A):2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 16.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negishi K, Negishi T, Hare JL, et al. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–498. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 19.Skovgaard D, Hasbak P, Kjaer A. BNP predicts chemotherapy-related cardiotoxicity and death: Comparison with gated equilibrium radionuclide ventriculography. PLoS One. 2014;9:e96736. doi: 10.1371/journal.pone.0096736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang C, Iyengar N, Datko F, et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33:442–447. doi: 10.1200/JCO.2014.57.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizariene V, Bucyte S, Zaliaduonyte-Peksiene D, et al. Left ventricular mechanics in asymptomatic normotensive and hypertensive patients with aortic regurgitation. J Am Soc Echocardiogr. 2011;24:385–391. doi: 10.1016/j.echo.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Reckefuss N, Butz T, Horstkotte D, et al. Evaluation of longitudinal and radial left ventricular function by two-dimensional speckle-tracking echocardiography in a large cohort of normal probands. Int J Cardiovasc Imaging. 2011;27:515–526. doi: 10.1007/s10554-010-9716-y. [DOI] [PubMed] [Google Scholar]
- 23.Maisel A. B-type natriuretic peptide levels: Diagnostic and prognostic in congestive heart failure: What’s next? Circulation. 2002;105:2328–2331. doi: 10.1161/01.cir.0000019121.91548.c2. [DOI] [PubMed] [Google Scholar]
- 24.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portera CC, Walshe JM, Rosing DR, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14:2710–2716. doi: 10.1158/1078-0432.CCR-07-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–2270. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 28.Hare JL, Brown JK, Leano R, et al. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J. 2009;158:294–301. doi: 10.1016/j.ahj.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris PG, Chen C, Steingart R, et al. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011;17:3490–3499. doi: 10.1158/1078-0432.CCR-10-1359. [DOI] [PubMed] [Google Scholar]
- 31.Okumura H, Iuchi K, Yoshida T, et al. Brain natriuretic peptide is a predictor of anthracycline-induced cardiotoxicity. Acta Haematol. 2000;104:158–163. doi: 10.1159/000046508. [DOI] [PubMed] [Google Scholar]
- 32.Nousiainen T, Jantunen E, Vanninen E, et al. Natriuretic peptides as markers of cardiotoxicity during doxorubicin treatment for non-Hodgkin’s lymphoma. Eur J Haematol. 1999;62:135–141. doi: 10.1111/j.1600-0609.1999.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 33.Daugaard G, Lassen U, Bie P, et al. Natriuretic peptides in the monitoring of anthracycline induced reduction in left ventricular ejection fraction. Eur J Heart Fail. 2005;7:87–93. doi: 10.1016/j.ejheart.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Herceptin prescribing information. Available at http://www.gene.com/download/pdf/herceptin_prescribing.pdf. Accessed April 13, 2015.
- 36.Perjeta prescribing information. Available at http://www.gene.com/download/pdf/perjeta_prescribing.pdf. Accessed April 13, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.