This study confirms that the measure of lymph node involvement transformed by the log odds ratio is a suitable predictor of 5-year overall survival in stage III rectal cancer. The log odds of positive lymph nodes may be applied to stratify high-risk patients in the management of adjuvant therapy.
Keywords: Prognosis, Lymph node, Log odds, Stage III rectal cancer, Survival stratification
Abstract
Introduction.
The log odds of positive lymph nodes (LODDS) is an empiric transform formula that incorporates positive and negative lymph node data into a single ratio for prognostic utility. We sought to determine the value of the log odds ratio as a prognostic indicator compared with established lymph node indices in advanced-stage rectal cancer patients who have undergone curative resection.
Methods.
Retrospective analysis of rectal cancer operations from 1995 to 2013 identified all stage III cancer patients who underwent curative resection. Patients were stratified into three groups according to calculated lymph node ratios (LNRs) and log odds ratios (LODDS). The relationship between LNR, LODDS, and 5-year overall survival (OS) were assessed.
Results.
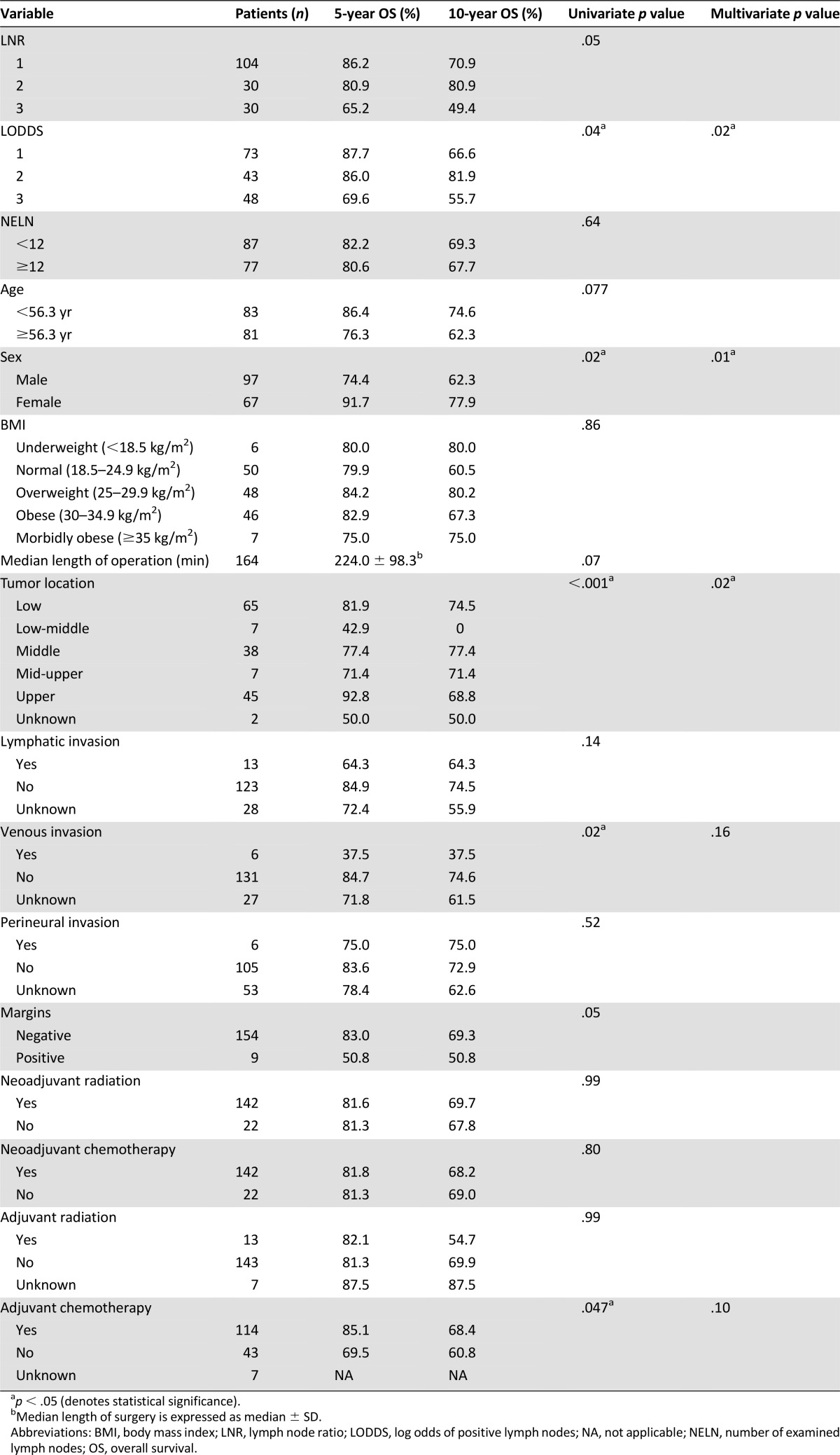
OS for all patients was 81.4%. Both LNR and LODDS stratifications identified differences in 5-year OS. LODDS stratification was significantly associated with OS (p = .04). Additional significant clinicopathologic demographic variables included sex (p = .02), venous invasion (p = .02), tumor location (p < .001), and receipt of adjuvant chemotherapy (p = .047). LODDS separated survival among patients in the low LNR group (LNR1).
Conclusion.
This study confirms that the measure of lymph node involvement transformed by the log odds ratio is a suitable predictor of 5-year overall survival in stage III rectal cancer. LODDS may be applied to stratify high-risk patients in the management of adjuvant therapy.
Implications for Practice:
Traditionally, clinicians have relied solely on the total number of positive lymph nodes affected when determining patient prognosis in rectal cancer. However, the current staging strategy does not account for “high-risk,” biologically aggressive tumors that fall into the same risk categories as less clinically aggressive tumors. The log odds of positive lymph nodes is a logistic transform formula that uses pathologic lymph node data to stratify survival differences among patients within a single stage of disease. This formula allows clinicians to identify whether patients with clinically aggressive tumors fall into higher-risk groups, providing additional insight into how to better counsel patients and manage postoperative therapies.
Introduction
Colon and rectal cancers remain one of the leading causes of cancer morbidity in the Western and European worlds. The spread of cancerous cells to lymph nodes (LNs) is the predominant distinguishing feature of stage III tumors in rectal cancer; these cells harbor distinct differences in cancer biology and survival. Use of LNs as a prognostic tool and indicator is continually being evaluated for adequacy and appropriateness of application.
Because of the divergence in management strategies between colon and rectal tumors, such as neoadjuvant chemoradiation, and standards of surgical resection margins, prognosis in rectal cancer now supersede those of colon cancer [1–3]. Despite this, only 15%–27% of patients achieve a complete pathological response following neoadjuvant therapy [4]. A possible explanation for poor survival outcomes is the compounded complexity from heterogeneous tumor biology. It is not surprising that a subset of patients remain high risk among all stages of disease, raising the question of whether all patients within the same stage of disease ought to be treated similarly.
Lymph node involvement in advanced rectal cancer is particularly relevant because of the standard of care involving neoadjuvant radiation therapy, which affects the course of disease. Prior data identified an association between a more extensive lymph node resection with improved survival in patients with colon cancer; however, the evidence is not uniform [5–7]. Several multidisciplinary organizations and consensus panels have established a minimum recommendation of 12 harvested lymph nodes to constitute adequate staging and surveillance for colorectal cancer [8–11]. Much emphasis was then directed to the qualitative and quantitative aspects of lymph node resection as prognostic markers. Alternate LN parameters include the total number of examined lymph nodes (NELN) and the lymph node ratio (LNR). Some studies have indicated superiority of LNR compared with pathologic nodal stage and NELN in colon cancer [12, 13]. The log odds of positive lymph nodes (LODDS) was defined as the probability of a positive lymph node when it is harvested [14]. LODDS has shown significant predictive impact in stage III colon cancer, but study design and analyses have varied. Only a few investigators have applied the LODDS models in rectal cancer.
The aim of this study was to evaluate the prognostic effect of LODDS in patients with advanced-stage rectal cancer (stage III) compared with NELN and LNR within a single institution experience. We sought to determine whether LODDS could improve survival stratification and isolate high-risk patients and delineate differences in prognosis within the same tumor, node, metastasis (TNM) classification.
Materials and Methods
This study was retrospectively analyzed through evaluation of patient charts from the University of Wisconsin. Patients older than age 18 years who were preoperatively diagnosed with primary, node-positive, advanced-stage (American Joint Committee on Cancer [AJCC] stage III) rectal cancer between January 1995 and July 2013 were included in the study. Preoperative tumor staging was coded according to the TNM system, as described by the AJCC seventh edition and extracted from preoperative imaging records. Patients presenting with primary malignancies other than rectal carcinoma, negative node status, stage IV disease, recurrent disease, and any operation that was not an initial attempt at curative resection were excluded from the study. This retrospective analysis was approved by the institutional review board.
Demographic and cancer-specific data were collected for each patient. These included age; sex; heritage; tumor location; lymph nodes examined; histologic features; lymphatic, venous, and perineural involvement; and margins. Tumor location was reported according to the distance from the anal verge in centimeters and grouped into low-, middle-, and upper-rectum categories. Tumors reported to extend into two regions of the rectum were classified into low-mid and mid-upper groups. Tumor stage was reported in the pathology report by an independent pathologist at the time of clinical diagnosis and staged according to the AJCC seventh edition. Pathologic T, N, and M stages were also collected after curative resection per pathology report. LNR and LODDS ratios were computed via postsurgical pathological data, whereas patients were included by clinical staging criteria.
LODDS was calculated by the empirical logistic formula:
LODDS was calculated for each subject, and patients were divided by tertiles into three groups designated LODDS1 (<−1.2788), LODDS2 (−1.2788 to −0.7105), and LODDS3 (>−0.7105). LNR was defined as the number of positive lymph nodes divided by the total number of examined lymph nodes. Patients were divided into three LNR groups as follows: LNR1 (0), LNR2 (0.01–0.176), and LNR3 (>0.176). The median LNR of all patients with LNR > 0 was used to divide patients into LNR2 and LNR3. Patients were also evaluated according the threshold recommendation by the AJCC. High NELN included 12 or more LNs harvested from surgery, whereas the low NELN group had fewer than 12 LNs resected.
Patient demographic and tumor characteristics were evaluated with descriptive statistics. Five- and 10-year overall survival (OS) were reported for the different levels of each factor studied. The overall survival was estimated with the Kaplan-Meier estimator and compared between groups using a log-rank test. The Cox proportional hazards model was used to further investigate associations between variables of interest and survival after adjustment for other factors. Pearson correlation was used to evaluate the relationship between LNR and LODDS. Statistical significance was designated by a p value of <.05. All statistical analyses were conducted by using SPSS software, version 22 (IBM, Chicago, IL, http://www-01.ibm.com).
Results
Patient’s Clinicopathological Features
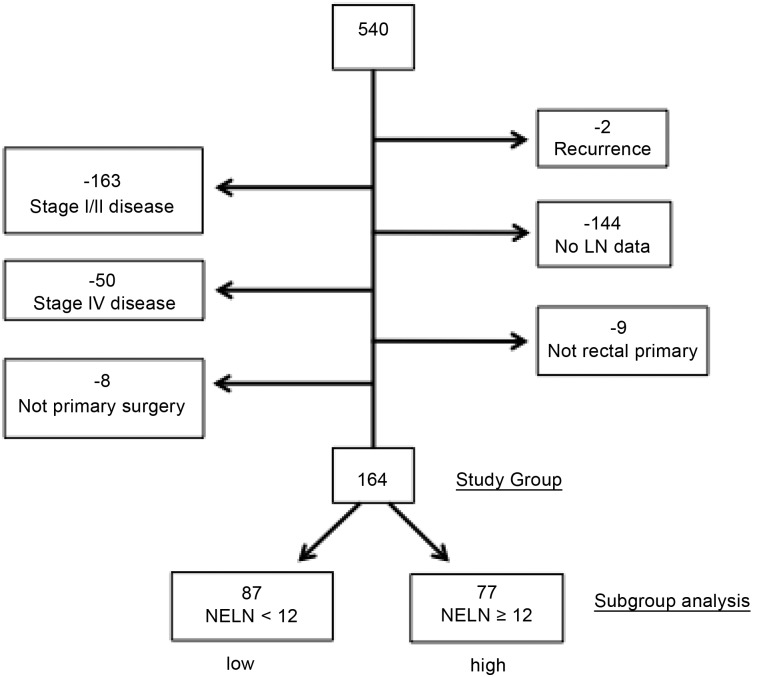
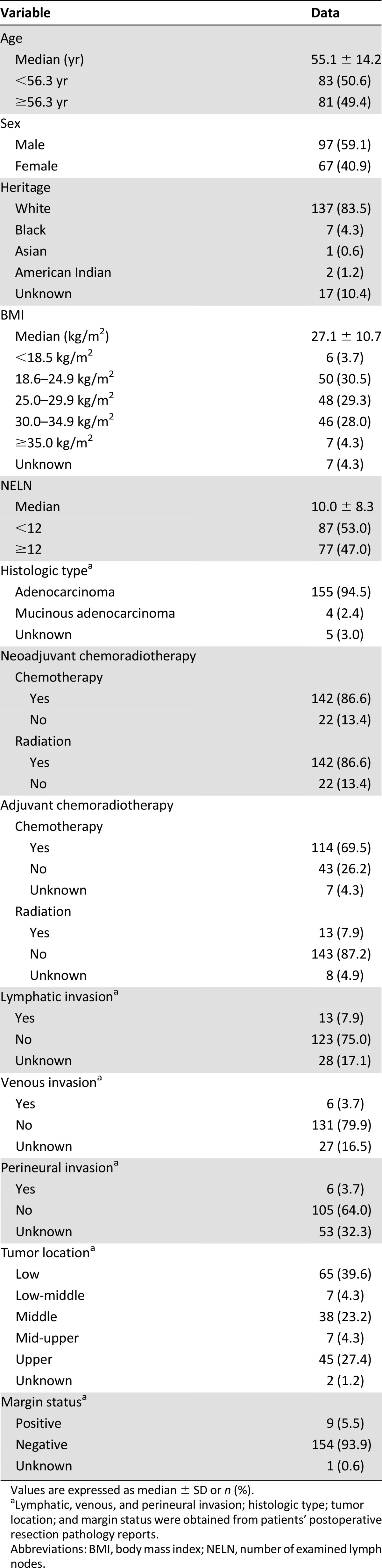
Five hundred forty patients with rectal cancer underwent curative resection at our institution between December 1995 and September 2013. The predominant mode of clinical staging was via rectal ultrasonography. After the exclusion of patients with stage I or II disease (n = 163), recurrent disease (n = 2), incomplete LN data (n = 144), metastatic disease (n = 50), other primary carcinoma (n = 9), and nonprimary attempt at surgery (n = 8), 164 patients comprised the final study group (Fig. 1). Within this group, 97 patients (59.1%) were male and 67 (40.9%) were female. The median age was 55 years (range, 25–95 years). The racial distribution reflected the state in which we live, and whites made up at least 82.5% (n = 137) of the study group. The dominant histopathological diagnosis was adenocarcinoma (95.4%). One hundred forty-two (86.6%) patients in this cohort received neoadjuvant chemotherapy and radiation therapy. The median number of LNs resected, including in patients who underwent neoadjuvant radiation therapy, was 10 (range, 0–64). The 5- and 10-year estimated Kaplan-Meier mortality rates were 18.6% and 31.5%, respectively.
Figure 1.
Study group selection criteria (n = 164).
Abbreviations: LN, lymph node; NELN, number of examined lymph nodes.
LODDS groups were created by using tertiles: They comprised 73 patients in the LODDS1 group (44.5%), 43 in the LODDS2 group (26.2%), and 48 in the LODDS3 group (29.3%). The cutoff values were −1.279 for the lower LODDS tertile (LODDS1) and 0.710 for the upper tertile (LODDS3). Stratification of patients into tertiles according to LNR did not yield similar numbers of patients into each group because of the large number of patients with LNR of 0. Therefore, LNR1 consisted of all patients with LNR of 0 (n = 104), and the remaining patients were divided into LNR2 and LNR3 by the median value (LNR of 0.176). There were 30 patients in LNR2 and 30 in LNR3. Patient clinicopathological data are depicted in Table 1.
Table 1.
Patient demographic and tumor characteristics
Analysis of the Prognostic Effect of LNR and LODDS on OS
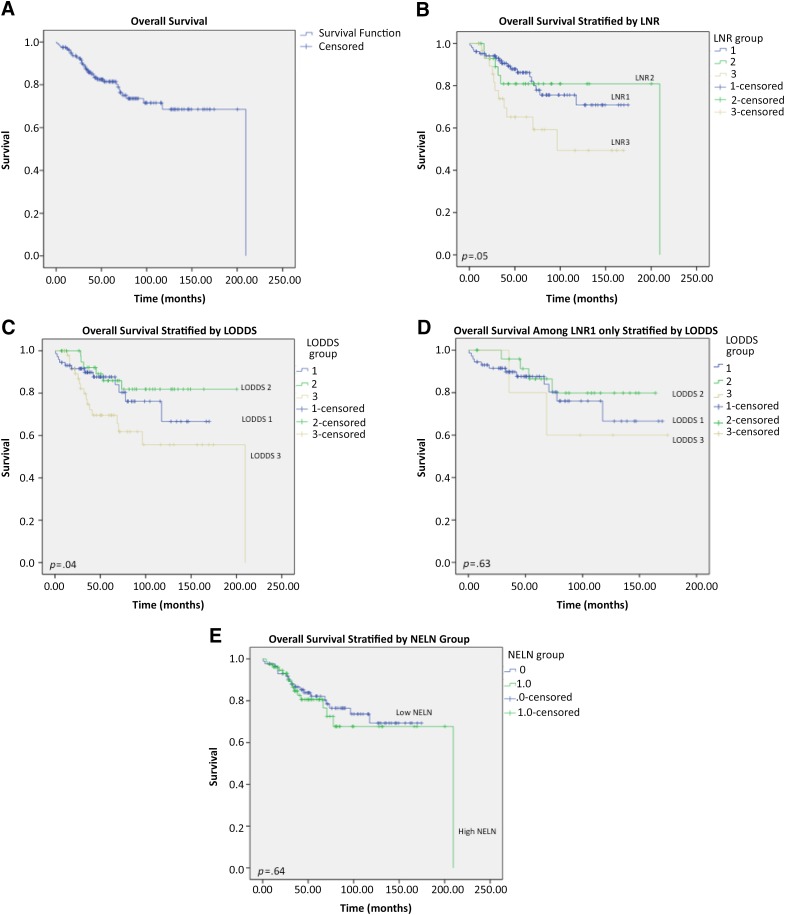
The observed 5-year survival rate in the entire cohort study was 81.4% (Fig. 2A). Survival analysis revealed 5-year OS rates of 86.2%, 80.9%, and 65.2% in the LNR1, LNR2, and LNR3 groups, respectively. The OS rate decreased with increasing LNR but was not significantly different between groups (p = .05) (Fig. 2B). LODDS revealed significantly different survival rates between groups: 87.7%, 86.0%, and 69.6% for LODDS1, LODDS2, and LODDS3, respectively (p = .04) (Fig. 2C). The highest LNR and LODDS stratifications, LNR3 and LODDS3, demonstrated the worst survival rates among the entire cohort. One hundred four patients were stratified into LNR1 (63.4%). Within this group, LODDS stratification separated patients without reaching statistical significance (p = .63) (Fig. 2D).
Figure 2.
Overall 5-year survival of study patients. (A): All patients (n = 164). (B): All patients stratified by LNR. (C): All patients stratified by LODDS. (D): All patients in LNR1 stratified by LODDS. (E): Patients stratified into high and low NELN.
Abbreviations: LNR, lymph node ratio; LNR1, lymph node ratio of 0; LNR2, lymph node ratio of 0.01–0.176; LRN3, lymph node ratio >0.176; LODDS, log odds of positive lymph nodes; NELN; number of examined lymph nodes.
Analysis of Prognostic Factors for OS
Univariate Cox proportional hazards modeling identified LODDS (p = .04), sex (p = .02), tumor location (p < .001), venous invasion (p = .02), and adjuvant chemotherapy (p = .047) as significant predictors of OS. After adjustment for other factors, multivariable logistic regression identified sex (p = .01), tumor location (p = .02), and LODDS (p = .02) as independent prognostic factors (p < .05) (Table 2).
Table 2.
Univariate and multivariable analyses of potential prognostic indicators in rectal cancer survival following curative resection
Analysis on NELN
Subgroup analysis was performed on patient groups distinguished by the total number of lymph nodes examined per the AJCC guidelines’ designation of achieving an adequate lymph node resection (LN ≥ 12). Eighty-seven patients (53.0%) had fewer than 12 LN examined (low NELN).
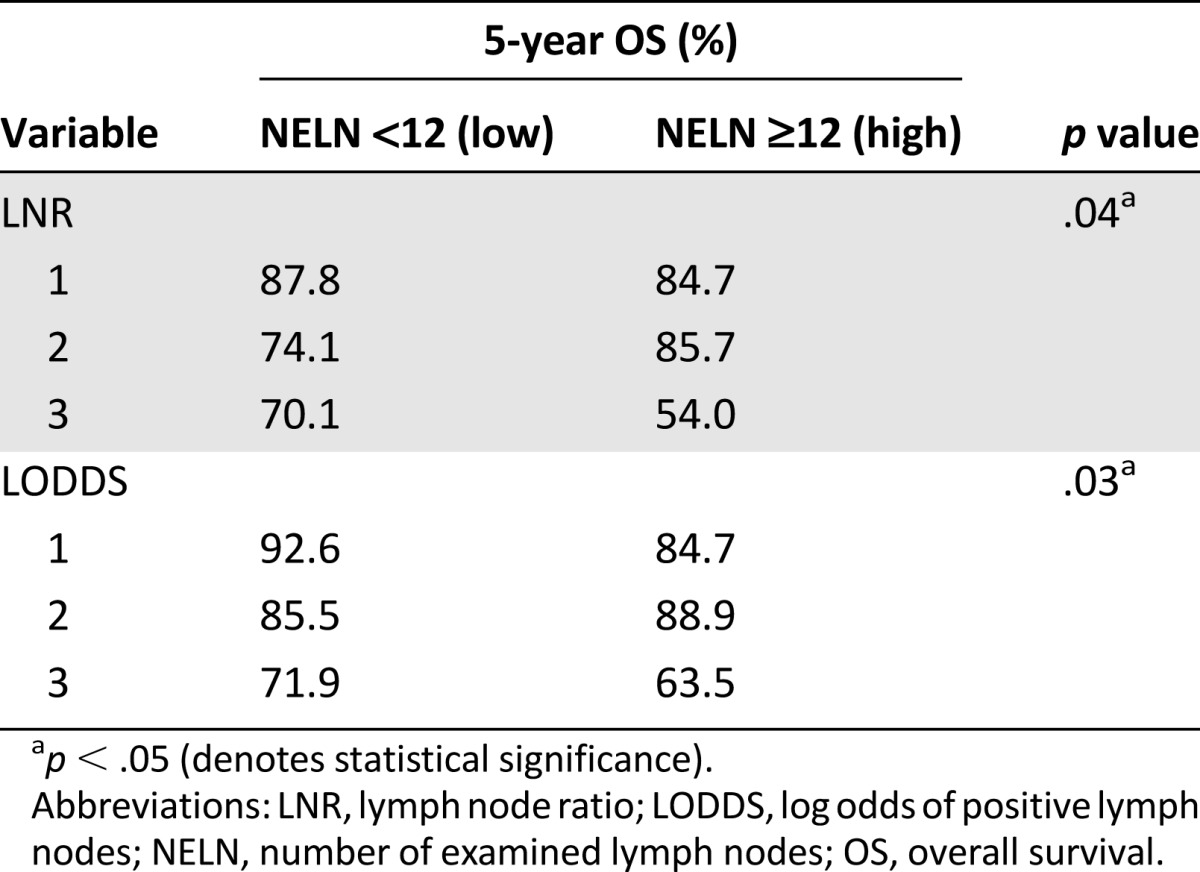
The observed 5-year OS for low NELN (82.2%) and high NELN (80.6%) were not significantly different (p = .64) (Fig. 2E; Table 3). LNR and LODDS stratifications affected survival when evaluated for the effects on NELN groupings (p = .04 and 0.03, respectively) (Table 4).
Table 3.
Five-year overall survival for patients stratified by high and low number of examined lymph nodes
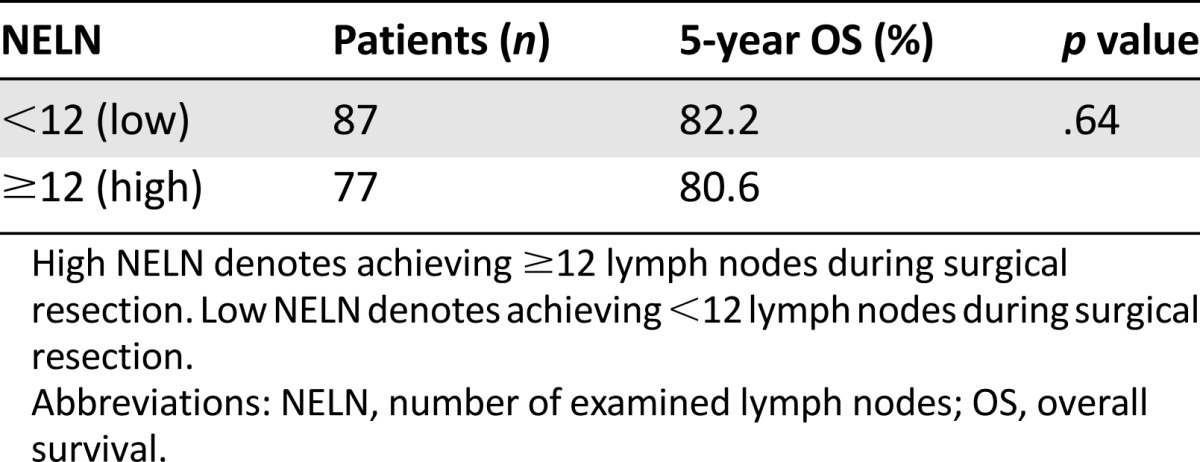
Table 4.
Subgroup analysis: effect of stratification by lymph node ratio and log odds of positive lymph nodes on number of examined lymph node groups in evaluation of overall survival
Discussion
Nodal status is the most important prognostic factor for colon and rectal cancer survival. Various studies have shown that the involvement of regional lymph nodes strongly affects long-term survival, resulting in continuous scrutiny over its role as a survival indicator. Rectal cancer is unique among other malignancies because of the changes in lymph node status and disease behavior secondary to neoadjuvant radiation, rendering LN involvement particularly difficult to study. Some authors argue that the current LN parameters used to determine prognosis are no longer appropriate from a quality measure perspective, hence the emergence of new LN indices, such as LNR and LODDS. The principle reason lies in incomplete or perhaps inadequate patient stratification that takes into account heterogeneous disease behavior among advanced-stage individuals. In this study, we confirmed that both LNR and LODDS are predictive of OS; however, LODDS further stratified select patients from low LNR groups into higher LODDS groups, identifying patients as high-risk.
The principal treatment modality for locally advanced rectal tumors remains neoadjuvant chemoradiation therapy with radical resection and/or postoperative chemotherapy or radiation therapy. This commonly results in tumor and LN regression, rendering some resections more difficult, and evaluation by the pathologist even more so. In a Dutch study, Mekenkamp et al. identified age older than 60 years, obesity, small LN size, preoperative radiotherapy, and poor histologic grade as key characteristics in rectal cancer associated with lower LN yield [15]. Strikingly, there were large variations between pathologists and laboratories, suggesting that establishing guidelines with minimum numbers of LNs was perceived to be ideal but not necessarily realizable. In the Clinical Outcomes of Surgical Therapy trial, Mathis and colleagues failed to demonstrate the total LN count as predictive of OS or disease-free survival [5]. Increasing evidence suggests that LNR and LODDS are superior prognostic indicators compared with standard LN status and NELN [14, 16–23]. However, the question still stands as to whether LODDS is superior to LNR, and which model should be used. Data in this study revealed clinically relevant trends in OS under both LNR and LODDS stratifications. We found that LODDS could further delineate survival differences within LNR1. Wang and colleagues first demonstrated the superiority of LODDS to LNR in a retrospective study of more than 20,000 patients with stage III colon cancer derived from the Surveillance, Epidemiology, and End Results Program population-based registry [19]. The authors reported that LODDS could be used to distinguish heterogeneous patients within standard stage IIIB and IIIC classifications (p < .0001) [19]. More recently, Makkai-Popa et al. revealed LODDS was best correlated with the risk for distant metastases while stratifying patients according to their risk for recurrent disease [24]. Persiani et al. [20] found that only LODDS was able to discriminate between subsets of patients with significantly different survival rates, whereas those exact patients were grouped into the same nodal group for NELN and LNR stratifications. As in the current study, LODDS further stratified patients into distinct survival groups within an individual LNR group (LNR1) (Fig. 2D). This suggests that LODDS may capture subtleties inherently missed when other LN indicators are used, rendering LODDS as more useful.
The LNR is limited when the ratio results in null values (e.g., 0, 1). LNR relies on the assumption that patients within the same stratification carry the same prognosis regardless of the NELN. Patients who are clinically different would otherwise be indistinguishable within a single LNR stratification. To illustrate this conundrum, consider two patients with clear prognostic differences (5/5 positive LNs vs. 30/30 positive LNs). Both patients fall into the same LNR stratification (LNR of 1), potentially introducing false prognostic representation of true events. LODDS obviates the inevitable product of singularities in the instance that none or all harvested lymph nodes are involved, and survival can still be estimated irrespective of the NELN. As a result, LODDS has been associated with the lower risk for stage migration in various malignancies [20].
Sex, tumor location, venous invasion, receipt of adjuvant chemotherapy, and LODDS were significant predictors of OS. After adjustment for confounders, multivariable regression analysis revealed independent prognostic capacity in sex, tumor location, and LODDS (Table 2). In this model, LODDS was consistently significantly associated with survival under multivariable analysis. In subgroup analysis, LNR and LODDS were both statistically significant predictors of OS when analyzed in conjunction with NELN (Table 4). Although univariate analysis did not demonstrate significant differences between NELN groups alone (p = .64) (Table 3), the added LNR and LODDS stratifications were more informative by identifying survival differences when patients were already grouped by NELN criteria. Patients in LNR3 and LODDS3 demonstrated the worst OS, regardless of high or low NELN group classifications. This argues that NELN designation in itself is insufficient to provide prognostic information; rather, the application of LNR or LODDS produces a significant survival effect among patients in NELN groups.
These comparisons have shown that LODDS may exhibit improved prognostic significance over LNR under univariate modeling, and both LNR and LODDS help distinguish OS differences in evaluations of the effect on NELN. Given these data, it is not possible to say whether LODDS is superior to LNR; however, LODDS is more robust in that it can stratify high-risk patients within LNR1 and LNR2 (supplemental online Table 1). Correlation analysis showed a Pearson coefficient of r = 0.799 (p < .001), confirming agreement between LNR and LODDS as mathematical constructs. The fact that the LN indices did not achieve perfect correlation (r = 1.0) indicates inherent differences in prognostic capacity despite the similarities. The influence of neoadjuvant chemotherapy and radiation therapy on LNR or LODDS is not known. Eighty-seven percent of patients (n = 142) in our study underwent neoadjuvant therapy, with similar 5-year OS rates, and neither chemotherapy nor radiation was significantly associated with OS. Although neoadjuvant therapy is well known to have considerable effect on survival in rectal cancer patients, our analysis did not reveal significant associations on survival outcomes estimates. Thus, a unified approach or evaluation of solely those who received neoadjuvant therapy was not conducted.
The greatest limitations in this study included retrospective data analysis and small sample size, reflecting any inherent bias and type II error. Nearly 200 patients were excluded by stage alone, and the second greatest deterrent to patient inclusion was the lack of raw data available in patient records. Before 2008, the number of lymph nodes examined was not routinely reported in pathology reports. As a tertiary care center, our institution accepts complicated cases from referring institutions where patients have already established a team of outside providers for workup and follow-up. The absence of collectible data was probably the result of information lost in transfer and follow-up.
Conclusion
Both LNR and LODDS are capable of predicting survival differences among stage III rectal cancer patients. LODDS may be an improved prognostic indicator because it is able to isolate the highest-risk individuals within low-LNR groups. The superiority of LODDS or LNR cannot be ascertained because of the limitations previously mentioned; however, it is clear that LODDS offers greater refinement in stratification of individuals suspected to be at higher risk. Such high-risk, advanced-stage rectal cancer patients may be targeted for novel multidisciplinary therapies, such as expanded chemotherapy and biologics. Further investigation in the form of a large, prospective multicenter investigation is necessary to validate whether LODDS should be incorporated into the rectal cancer staging system.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Author Contributions
Conception/Design: Christina W. Lee, Katheryn H. Wilkinson, Adam C. Sheka, Glen E. Leverson, Gregory D. Kennedy
Provision of study material or patients: Christina W. Lee, Katheryn H. Wilkinson, Adam C. Sheka, Glen E. Leverson, Gregory D. Kennedy
Collection and/or assembly of data: Christina W. Lee, Katheryn H. Wilkinson, Adam C. Sheka, Glen E. Leverson, Gregory D. Kennedy
Data analysis and interpretation: Christina W. Lee, Glen E. Leverson,
Manuscript writing: Christina W. Lee, Glen E. Leverson, Gregory D. Kennedy
Final approval of manuscript: Christina W. Lee, Katheryn H. Wilkinson, Adam C. Sheka, Glen E. Leverson, Gregory D. Kennedy
Disclosures
The authors indicated no financial relationships.
References
- 1.van de Velde CJ, Boelens PG, Borras JM, et al. EURECCA colorectal: Multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014;50:e1–1e34. doi: 10.1016/j.ejca.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 2.Veen T, Nedrebø BS, Stormark K, et al. Qualitative and quantitative issues of lymph nodes as prognostic factor in colon cancer. Dig Surg. 2013;30:1–11. doi: 10.1159/000349923. [DOI] [PubMed] [Google Scholar]
- 3.Nedrebø BS, Søreide K, Eriksen MT, et al. Survival effect of implementing national treatment strategies for curatively resected colonic and rectal cancer. Br J Surg. 2011;98:716–723. doi: 10.1002/bjs.7426. [DOI] [PubMed] [Google Scholar]
- 4.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 5.Mathis KL, Green EM, Sargent DJ, et al. Surgical quality surrogates do not predict colon cancer survival in the setting of technical credentialing: a report from the prospective COST trial. Ann Surg. 2013;257:102–107. doi: 10.1097/SLA.0b013e318260a8e6. [DOI] [PubMed] [Google Scholar]
- 6.Parsons HM, Tuttle TM, Kuntz KM, et al. Association between lymph node evaluation for colon cancer and node positivity over the past 20 years. JAMA. 2011;306:1089–1097. doi: 10.1001/jama.2011.1285. [DOI] [PubMed] [Google Scholar]
- 7.Vather R, Sammour T, Kahokehr A, et al. Lymph node evaluation and long-term survival in Stage II and Stage III colon cancer: a national study. Ann Surg Oncol. 2009;16:585–593. doi: 10.1245/s10434-008-0265-8. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 9.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen LB, Wille-Jørgensen P. National and international guidelines for rectal cancer. Colorectal Dis. 2014;16:854–865. doi: 10.1111/codi.12678. [DOI] [PubMed] [Google Scholar]
- 11.Chen SL, Bilchik AJ. More extensive nodal dissection improves survival for stages I to III of colon cancer: A population-based study. Ann Surg. 2006;244:602–610. doi: 10.1097/01.sla.0000237655.11717.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg R, Friederichs J, Schuster T, et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: A single-center analysis of 3,026 patients over a 25-year time period. Ann Surg. 2008;248:968–978. doi: 10.1097/SLA.0b013e318190eddc. [DOI] [PubMed] [Google Scholar]
- 13.Lu YJ, Lin PC, Lin CC, et al. The impact of the lymph node ratio is greater than traditional lymph node status in stage III colorectal cancer patients. World J Surg. 2013;37:1927–1933. doi: 10.1007/s00268-013-2051-4. [DOI] [PubMed] [Google Scholar]
- 14.Arslan NC, Sokmen S, Canda AE, et al. The prognostic impact of the log odds of positive lymph nodes in colon cancer. Colorectal Dis. 2014;16:O386–O392. doi: 10.1111/codi.12702. [DOI] [PubMed] [Google Scholar]
- 15.Mekenkamp LJ, van Krieken JH, Marijnen CA, et al. Lymph node retrieval in rectal cancer is dependent on many factors--the role of the tumor, the patient, the surgeon, the radiotherapist, and the pathologist. Am J Surg Pathol. 2009;33:1547–1553. doi: 10.1097/PAS.0b013e3181b2e01f. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Hassett JM, Dayton MT, et al. Lymph node ratio: Role in the staging of node-positive colon cancer. Ann Surg Oncol. 2008;15:1600–1608. doi: 10.1245/s10434-007-9716-x. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg R, Engel J, Bruns C, et al. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg. 2010;251:1070–1078. doi: 10.1097/SLA.0b013e3181d7789d. [DOI] [PubMed] [Google Scholar]
- 18.Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Hassett JM, Dayton MT, et al. The prognostic superiority of log odds of positive lymph nodes in stage III colon cancer. J Gastrointest Surg. 2008;12:1790–1796. doi: 10.1007/s11605-008-0651-3. [DOI] [PubMed] [Google Scholar]
- 20.Persiani R, Cananzi FC, Biondi A, et al. Log odds of positive lymph nodes in colon cancer: A meaningful ratio-based lymph node classification system. World J Surg. 2012;36:667–674. doi: 10.1007/s00268-011-1415-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Kulaylat M, Rockette H, et al. Should total number of lymph nodes be used as a quality of care measure for stage III colon cancer? Ann Surg. 2009;249:559–563. doi: 10.1097/SLA.0b013e318197f2c8. [DOI] [PubMed] [Google Scholar]
- 22.Telian SH, Bilchik AJ. Significance of the lymph node ratio in stage III colon cancer. Ann Surg Oncol. 2008;15:1557–1558. doi: 10.1245/s10434-008-9862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HY, Choi HJ, Park KJ, et al. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14:1712–1717. doi: 10.1245/s10434-006-9322-3. [DOI] [PubMed] [Google Scholar]
- 24.Makkai-Popa ST, Luncă S, Târcoveanu E, et al. Lymph node status assessed through the log odds ratio - a better tool in the prognosis of colorectal cancer relapse. Rom J Morphol Embryol. 2014;55:97–102. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.