Abstract
Background
The aim of the study was to examine the efficacy of a collaborative care intervention to reduce depression, pain and fatigue and improve quality of life.
Participants
A total of 261 patients with advanced cancer and 179 family caregivers were randomized to a web-based collaborative care intervention or enhanced usual care. The intervention included (1) a website with written and audiovisual self-management strategies, bulletin board, and other resources; (2) visits with a care coordinator during physician appointment every two months; and (3) telephone follow up every two weeks. Primary patient outcomes included measures of depression, pain, fatigue, and health related quality of life. Secondary outcomes included Interleukin (IL)-1α, IL-1β, IL-6, IL-8, Natural Killer (NK) cell numbers, and caregiver stress and depression.
Results
At baseline, 51% of patients reported one or more symptoms in the clinical range. For patients who presented with clinical levels of symptoms, and were randomized to the intervention, reductions in depression (Cohen’s d=0.71), pain (Cohen’s d=0.62), and fatigue (Cohen’s d=0.26) and improvements in quality of life (Cohen’s d =0.99) were observed when compared to the enhanced usual care arm at 6-months. Reductions in IL-6 (phi=0.18), IL-1β (phi=0.35); IL-1α (phi=0.19); IL-8 (phi=15) and increases in NK cell numbers (phi=0.23) were observed when compared to enhanced usual care arm at 6-months. Reductions in caregiver stress (Cohen’s d=0.75) and depression (Cohen’s d=0.37) were observed at 6-months for caregivers whose loved one was randomized to the intervention arm.
Conclusions
Integration of screening and symptom management into cancer care is recommended.
Introduction
Symptom management is critical to maintain quality of life in patients with life limiting conditions. In 2002, the National Institute of Health State of the Science Consensus conference concluded that the three most common and debilitating symptoms associated with cancer were depression, pain and fatigue and this remains true today despite the advances that have been made in symptom management.1–9 To date, several interventions have been tested to reduce individual symptoms with success.10–20 However, because these symptoms are often comorbid and can exacerbate one another, interventions that target multiple symptoms are warranted. An intervention designed to treat these symptoms simultaneously is warranted to improve quality of life of patients with advanced cancer.
Stepped collaborative care interventions have been widely employed in the primary care setting for the treatment of depression and more recently have been utilized to treat other symptoms (e.g., pain) in a variety of settings.21,22,23 A recent meta-analyses concluded that collaborative care interventions were superior to usual care and are more cost-effective than face to face and pharmacological treatment for depression.24
Collaborative care interventions have begun to be extended to oncology settings. Dwight-Johnson and colleagues (2005) tested a collaborative care intervention in patients with cancer and comorbid depression.25 Compared to usual care, the patients randomized to the intervention arm experienced reductions in depression and pain.25,14,26 deRaaf and colleagues also tested non-pharmacological strategies administered by a nurse, in collaboration with the oncologist, to reduce fatigue.27 These investigators demonstrated that the intervention was effective in reducing fatigue in patients with cancer.27
Recent advances in technology have made the scalability and cost-effectiveness of collaborative care interventions even greater. Collaborative care interventions reduce barriers associated with face to face treatment including the need for additional appointments while undergoing treatment for cancer; the stigma associated with seeking mental health or services from a pain center; and the costs associated with treatment, transportation, and absenteeism for the patient and/or caregiver. Furthermore, the web-based collaborative care interventions permits the dissemination of a standardized empirically based treatments that are individually tailored and are available to patients who may not have otherwise had access to treatment for their cancer-related symptoms. The use of a “stepped” care approach permits the individualization of treatment based on the patients’ needs and provide care for patients who require varying levels of care in a cost-effective manner.28,29
Advanced cancer patients in the palliative care setting and those in underserved areas report the most severe symptoms due to the tumor burden, limited access to treatment due to the advanced stage of disease, and the least frequent contact with health care professionals, particularly once treatment has ended. It has also been demonstrated that those from socioeconomically disadvantaged background have higher symptom burden and less access to palliative care services.30
The aims of the present study were to: (1) understand the prevalence of patient report of individual and multiple symptoms (e.g., pain, fatigue, and depression) in the context of advanced cancer; (2) test the efficacy of a web-based stepped collaborative care intervention in a predominantly underserved advanced cancer patient population; and (3) test the efficacy of the web-based stepped collaborative care intervention on biomarkers of inflammation and caregiver stress and depression.
Methods
Design
The study was a randomized controlled trial of a web-based collaborative care intervention for patients diagnosed with cancer. The study consisted of two arms (1) web-based stepped collaborative care intervention, and (2) enhanced usual care. Patients and family caregivers were enrolled in the study and they were assessed at the time of randomization and then at 6-months. Please see Figure 1 for the CONSORT flow diagram with regard to enrollment and follow-up.
Figure 1.
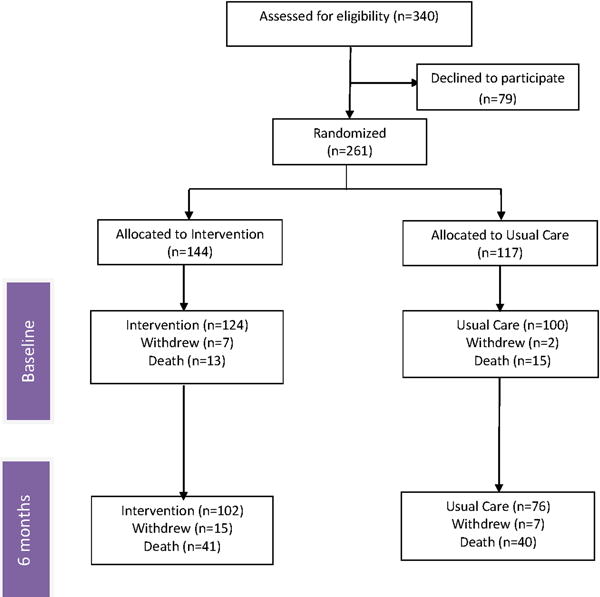
CONSORT diagram of enrollment and 6-months follow up
Sample
Patients diagnosed with hepatocellular, cholangiocarcinoma, gallbladder, neuroendocrine, and pancreatic carcinoma or other primary cancers that have metastasized to the liver (e.g., ovarian, breast, colorectal) were enrolled in the study. The majority of patients received regional chemotherapy (transarterial chemoembolization) or radiation (90 yttrium) or surgery. The primary side effect of these treatments is fatigue. A minority of patients develop nausea and vomiting or other symptoms. Chronic pain is also not common as a result of the treatment. For those who underwent surgery, the side effects were pain and fatigue after surgery but last approximately 2 weeks. The interviews and blood draws to evaluate the efficacy of this intervention were performed 7 weeks after the cancer-related treatment to avoid capturing treatment-related side effects. The inclusion criteria were: (1) biopsy and/or radiograph proven diagnosis of cancer; and (2) age greater than or equal to 21 years. The exclusion criteria included: (1) less than 21 years of age, (2) lack of fluency in English, (3) evidence of thought disorder, delusions, hallucinations, or suicidal ideation (these patients were referred for immediate face to face and/or psychotropic medication). The inclusion criteria did not include a specific level for any of the three target symptoms as we expected that patients would experience one or more of these symptoms over the course of the intervention.
Data Collection
We used baseline and 6-month data for the purposes of this study. All data was collected by trained interviewers using a structured computerized interview. The interviewers were blinded to the study arm assignment. The primary outcomes to assess the efficacy of the intervention included the Center for Epidemiological Studies-Depression31, the Brief Pain Inventory (BPI)32, the Functional Assessment of Cancer Therapy – Anemia (FACT-AN)33, and the Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep).24 Secondary outcomes included serum cytokines levels and Natural Killer Cell (NK) cell numbers. Although the caregivers were not the target of the intervention; caregiver stress and depression were also assessed using the Caregiver Quality of Life Index-Cancer Scale (CQOLC)34 and the Center for Epidemiological Studies-Depression scale.31
Randomization to Study Arms
The patients were randomly assigned to one of two arms of the study (web-based stepped collaborative care intervention versus enhanced usual care) based on a block randomization design according to gender and vascular invasion (gender: male/female; macroscopic vascular invasion of the portal or hepatic veins as determined by imaging and/or histology: yes/no).
Allocation Concealment
Allocation concealment was achieved through the use of a random number table that assigned consecutive patients across group. A research assistant who was not part of the study placed the trial assignments in opaque envelopes consecutively by group. These opaque envelopes were sealed, numbered serially, and placed into four numbered containers, one for each stratification subgroup (male and vascular invasion; male and no vascular invasion; female and vascular invasion; female and no vascular invasion). The statistician put the randomization table in a sealed envelope to be opened only at the end of the trial.
Web-based Collaborative Care Intervention
The web-based collaborative care intervention included access to a psycho-educational website (see Figure 2) and to a collaborative care coordinator with training and experience with cognitive-behavioral therapy (CBT) and psycho-oncology. The patient had telephone contact with the care coordinators approximately every 2 weeks and face to face contact with the care coordinator in the oncology outpatient clinic and/or hospital approximately every 2 months. The care coordinator would provide CBT and/or recommendations for pharmacological management of symptoms if the patient preferred medication to CBT or in addition to CBT. For example, the care coordinator would recommend to the medical team or the patients’ primary care physician (PCP) if an antidepressant was indicated or if the patients’ pain was not well managed (e.g., addition of a long acting pain medication, referral to pain center).
Figure 2.
Home page of website
More frequent contact occurred when a symptom was more severe and/or needed multiple contacts to manage. The dose and active ingredients of the intervention was recorded using an ACCESS database. The objective of the intervention was to treat cancer-related symptoms including depression, pain, and fatigue. The presence and severity of these symptoms varied over the course of treatment and patients were treated as symptoms were reported to the clinician. A stepped approach was used so if the patient’s symptoms did not improve increasingly intensive strategies were used to decrease symptom burden.
The website included (1) psycho-educational information with regard to depression, pain, fatigue, nausea and vomiting and sleep; (2) a self-management area where the patient could record their symptoms and monitor changes through graphical depictions; (3) an area for journaling; (4) a chat room that connected the patient to other patients enrolled in the study, (5) an audiovisual library that included relaxation techniques and educational videos by the patient’s nurse coordinators; and (6) resource library. The website also includes a text to voice option to address issues of literacy and larger font for older adults.
Care Coordinators
The care coordinators were Master’s level or Ph.D. therapists trained in cognitive-behavioral therapy and experienced in evaluating and treating patients diagnosed with cancer. The care coordinators would have face to face contact with the patient in the outpatient cancer clinic or in the hospital when the patient visited the hospital for follow up or treatment. The care coordinators contacted the patients by phone but were also available as needed to the patient for questions and concerns. The care coordinator provided information to the medical team about any changes in the patient’s symptoms that may warrant changes in treatment (e.g., increasing the current dose of SSRI, change medication, add psychotherapy). However the medical team may or may not accept the care coordinators recommendations. For example, if a patient is being treated with an selective serotonin reuptake inhibitor (SSRI) and the care coordinated confirmed with the patient s/he had been adherent to the medication regimen, treated for the appropriate duration (e.g., at least 6–8 weeks), and the patient continued to meet the DSM 5 diagnostic criteria for depression; the care coordinator would discuss with the patient if s/he was interested also in changing their treatment. If so, the care coordinator would then relay the patient’s desires to the medical team.
Fidelity of the Intervention
The therapists were trained using a 300 page intervention manual which included the evaluation of depression, pain, and fatigue and cognitive-behavioral treatment of these symptoms. The care coordinators were supervised by a clinical health psychologist who has received additional training and certification by the American Psycho-Oncology Society for maintaining the fidelity of psychosocial interventions. The clinical psychologist provided training and weekly supervision of the care coordinators and to assess and maintain adherence to the intervention protocol. If the care coordinator was not adhering to the study protocol, s/he would receive additional training and supervision.
Enhanced Usual Care
The enhanced usual care arm of the study was “usual care” provided by the medical team in addition to the assessment of symptoms and blood draws at the same time as the patients randomized to the intervention arm to assess the efficacy of the intervention. For ethical reasons, if a patient or caregiver scored high on the CES-D (≥16) or the BPI average pain score (>5) the patient was contacted by a care coordinator and provided education about their symptoms and referrals to a mental health professional in their community or to their Primary Care Physician (PCP) for pharmacological treatment for depression. If the patient scored high on the BPI average pain item (≥5), s/he was referred to a pain center or an expert in symptom management if it was cancer related. Of the patients who were referred for treatment, <1% pursued any type of psychotherapy and/or treatment with medication. Despite the lack of patients following through with treatment for their symptoms, we provided patients with elevated scores in the usual care arm “attention” and it is plausible that providing such contact may have sensitized these participants to pursue additional clinical care at a later point in time.”
Procedure
The study did not commence until the University of Pittsburgh’s Institutional Review Board approved the study. The medical team referred the patient and family member and if the patient agreed, the study team member would explain the risks and benefits of the study. If the patient provided written informed consent, s/he was then randomly assigned to the intervention or enhanced usual care arm. A psychiatric intake was then conducted by the care coordinator either the day of consent or by phone to determine the patient needs. If the patient was randomly assigned to the intervention arm, the intervention commenced after the baseline telephone interview was completed.
Data Analysis
The data was entered and verified in SPSS version 21. Descriptive statistics were performed to characterize the sample and examine the distribution of data. The number of visits to each page of the website and duration was recorded in real time. A 2×4 mixed analysis of variance (ANOVA) was performed on CESD, FACT-Fatigue, BPI average pain score, and FACT-Hepatobiliary as a function of treatment group and time using General Linear Mixed Models (GLMM) approach. The within-subjects IV is time (Baseline and 6 months). The between-subjects IV is treatment group (usual, intervention). Evidence became available over the course of the trial by Hart and colleagues (2012) that suggested that two separate GLMM analyses be performed, first with all participants and then with patients with clinically significant symptoms. Clinically levels of symptoms a CES-D score ≥16, average pain ≥3 or a FACT-Fatigue score ≥26. Prior research has also shown that a 5–6 point difference in the overall FACT-G is clinically meaningful.35 The effect size measures the magnitude of the relationship and was defined using a Cohen’s d for the psychological and physical outcomes (small effect <0.20; medium effect size=0.30–0.70; and a large effect size >0.80) and a phi was used for the biological outcomes (small effect<0.10; medium effect<0.30; large effect<0.50).
Results
A total of 340 participants were approached for enrollment in the study. Of the 340 participants, 261 (77%) agreed to participate. A CONSORT flow diagram (Figure 1) depicts the number of patients approached for enrollment, randomization, attrition due to death and follow up of patients from baseline to 6-months.
Sociodemographic, disease, and treatment related characteristics can be found in Table 1. Of the 261 participants enrolled in the study, the majority of patients were male (73%). The mean age was 61 years (SD=11). No significant difference between the two arms of the study were observed on age, gender, ethnicity, diagnosis, treatment, or baseline scores on the CES-D, FACT-Fatigue, BPI average pain score (0–10), or overall quality of life (FACT-G).
Table 1.
Sociodemographic and disease specific characteristics (exact p-values are reported for two-way chi-square tests between the intervention and usual care arms).
| Entire Sample (N = 261) |
Clinically Significant Symptoms (N = 132) |
|||
|---|---|---|---|---|
| Variable | n (%) | Intervention vs Usual Care p-value |
n (%) | Intervention vs Usual Care p-value |
| Gender (n, % male) | 190 (73) | 0.577 | 94 (71) | 0.128 |
| Age (Mean, SD) | 61 (11) | 0.751 | 60 (11) | 0.707 |
| Race (n, %) | 0.779 | 0.188 | ||
| Caucasian | 224 (86) | 111 (84) | ||
| African American | 24 (9) | 16 (12) | ||
| Asian-American | 3 (1) | 1 (1) | ||
| Hispanic | 1 (0) | |||
| Other | 1 (0) | |||
| Diagnosis | 0.897 | 0.211 | ||
| HCC and CCC | 167 (64) | 80 (61) | ||
| Other Primary Cancers with Liver Metastases | 94 (36) | 52 (39) | ||
| Treatment (n, %) | 0.145 | 0.505 | ||
| Regional Therapy | 133 (51) | 68 (52) | ||
| Surgery | 54 (21) | 29 (22) | ||
HCC=Hepatocellular carcinoma; CCC=Cholangiocarcinoma
Website Usage
Figure 2 depicts a screen shot of the home page of the website. The website contained over 500 pages of content.
Ninety-two percent of the participants who participated reported that they had access to a computer at home or work. Of the 8% who did not have access to a computer, 6% borrowed a laptop computer from the study team and 2% preferred printed information from the website as needed. The patients were provided the website username and password if they were randomized to the intervention arm of the study. The patients were not directed to use the website over the course of the intervention. Table 2 depicts the number of visits and duration on each of the major sections of the website.
Table 2.
Presentation of symptoms at diagnosis
| Symptom(s) | Prevalence at Diagnosis |
|---|---|
| Depression only (≥16) | 6.5% |
| Pain only (≥3) | 6.1% |
| Fatigue only (≥26) | 8.8% |
| Depression (≥16) + Fatigue (≥26) | 8.0% |
| Depression (≥16) + Pain (≥3) | 3.4% |
| Pain (≥3) + Fatigue (≥26) | 4.2% |
| Pain (≥3), Fatigue (≥26), + Depression (≥16) | 13.4% |
| Total Cancer-Related Symptoms | 50.4% |
Prevalence of cancer-related symptoms
At the time of randomization, a total of 132 patients (51%) reported one or more symptoms in the clinical range. Eighty-two of the 261 patients (31%) reported depressive symptoms in the clinical range of the CES-D (>16); 71 (27%) patients reported an average level of pain in the last week to be greater than or equal to 3; and 90 patients (35%) reported moderate to severe fatigue. Of these patients who reported any of the three symptoms, 6.5% of patient reported only depression, 6.1% only having pain, 8.8% of patient reported fatigue only, 8.0% of patient reported depression and fatigue, and 3.4% reported depression and pain, 4.2% reported pain and fatigue, and 13.4% reported all three symptoms at baseline. See Table 3.
Table 3.
Website usage by patients
| Section of Website | Page Views | Total Duration (in mins) | Section of Website | Page Views | Total Duration (in mins) |
|---|---|---|---|---|---|
| Home page | 143 | 142.9 | My Journal | 49 | 88.7 |
| The Liver Cancer Center | 89 | 196.1 | End of Life | 29 | 33.4 |
| Managing Symptoms | 115 | 179.7 | Diagnosis & Tx | 147 | 275 |
| Staying Healthy | 100 | 100 | What Others Say | 482 | 381.7 |
| How Am I Doing? | 86 | 102.9 | Family Caregivers | 84 | 77.3 |
| Resources | 58 | 67.7 | Total | 1491 | 1813.9 |
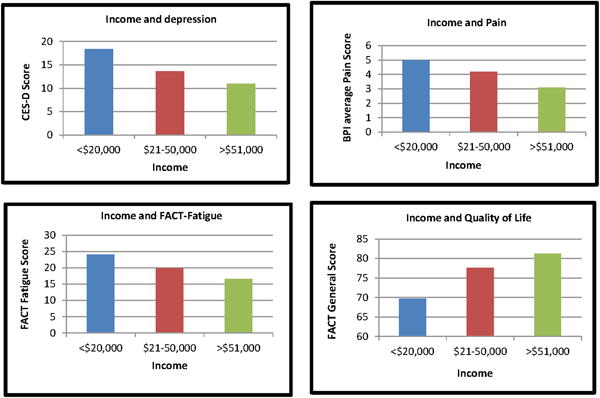
The website was developed for patients who may have low literacy or sensory deficits. Patients who were from a socioeconomically disadvantaged background had higher levels of depression, pain, and fatigue. Patients from socioeconomically disadvantaged backgrounds reported significantly higher levels of depression [F(2,211)=8.3, p<0.001]; pain [F(2, 136)=6.9, p<0.001]; fatigue [F(2,209)=5.3, p=0.006]; and poorer quality of life [F(2,170)=7.3, p<0.001].
Intervention versus enhanced Usual Care
For the patients who had clinical levels of depressive symptoms measured by the CES-D≥16; a medium effect size (Cohen’s d= 0.71) was observed at 6-months follow up [t (14) =1.41, p=0.182]. The second symptom that was targeted as part of the collaborative care intervention was pain. At 6 months, a reduction of pain, with a medium effect size of (Cohen’s d=0.62) was observed between the intervention and enhanced usual care arm [t (9) =1.76, p=0.112]. We observed the intervention group was superior to the enhanced usual care arm in that reductions of fatigue were observed at 6 months with an effect size of 0.26 [t(15)=1.80, p=0.09]. Statistically and clinically significant changes in overall quality of life were observed with an effect size of 0.99 from baseline to 6-months follow-up [t (16) =−2.19, p=0.05]. No significant difference was observed by treatment type for patients in the intervention versus the usual care arm of the study (Chi-square=0.792, p=0.851; intervention arm = 62.5% underwent regional chemotherapy or radiation and the remaining 37.5% surgery and usual care arms= 71.4% underwent regional chemotherapy or radiation and the remaining 28.6% surgery).
Using an intent-to-treat analyses the effect size (phi) was small to medium effect size was observed at 6-months for IL-6 (Phi=0.337; Chi-square=1.59, p=0.207); IL-1β (Phi=0.378; Chi-square=2.0, p=0.149); and IL-8 (Phi=0.304; Chi-square=1.30, p=0.304). A medium effect size was observed for NK cell number (Phi=0.491) at 6-months [Chi-square=3.62, p=0.057]. See Table 5.
Table 5.
Effects of the intervention on biological outcomes in patients reporting clinical levels of symptoms at baseline
| Biomarker | Baseline | 6-months | Effect Size (Phi) | ||
|---|---|---|---|---|---|
| Intervention (Mean, SD) |
Usual Care (Mean, SD) |
Intervention (Mean, SD) |
Usual Care (Mean, SD) |
||
| n=42 | n=39 | n=15 | n=12 | ||
| IL-6 pg/ml | 197.3 (989) | 57.5 (132.3) | 35.1 (39.7) | 28.7 (27.9) | 0.178 |
| IL-1β pg/ml | 573.9 (1618) | 184.8 (367.7 | 186.5 (363.5) | 243.6 (560.2) | 0.353ˆ |
| IL-1α pg/ml | 575.6 (3233) | 149.7 (710) | 89.7 (188.2) | 33.0 (50.8) | 0.194 |
| IL-8 pg/ml | 169.1 (225) | 217.3 (371) | 132.2 (81.4) | 75.7 (55.2) | 0.153 |
| NK cell number | 70.9 (71.6) | 75.4 (80.1) | 191.8 (77.2) | 54.8 (44.3) | 0.227ˆ |
Phi=small effect<0.10; medium effect<0.30; large effect<0.50
p<0.10;
p=0.05;
p=0.01;
p<0.001
Post-hoc analyses using Mann Whitney U demonstrated that clinical levels of fatigue were associated with Natural Killer cells [Mann Whitney U=10, p=0.066] with patients reporting greater levels of fatigue having lower Natural Killer cell numbers (Mean rank=5.33) when compared to those with reported lower levels of fatigue (Mean rank=13.52). Clinical levels of pain were not found to be significantly associated with any of the biomarkers measured. A trend toward significance was observed for depression and IL-8 [Mann Whitney U=3.385, p=0.184] with clinical levels of depression being associated with a higher levels of IL-8 (Mean rank=12.92) when compared to patients without clinical levels of depression (Mean rank=8.14). A trend toward significance was observed with regard to the association between overall quality of life, which may reflect improvement in all symptoms, and IL-1β [Spearman rho=0.357, p=0.103] and IL-1α [Spearman rho=0.339, p=0.168].
Although not a target of the intervention, caregivers with patients randomized to the intervention arm had reductions in stress and depression. When only caregiver with patients who had clinically significant symptoms were included in the analyses, an effect size of 0.748, was observed for caregiver stress at 6-months follow up and 0.372 for depression. See Table 6 for means and standard deviations.
Table 6.
Effects of the intervention on caregiver outcomes in patients reporting clinical levels of symptoms at baseline
| Secondary Outcomes | Baseline | 6-months | Cohen’s d | ||
|---|---|---|---|---|---|
| Intervention Mean (SD) |
Usual Care Mean (SD) |
Intervention Mean (SD) |
Usual Care Mean (SD) |
||
| Caregiver Stress | 63.0 (9.0) | 64.4 (13.4) | 58.8 (7.2) | 68.0 (15.9) | 0.748* |
| Caregiver CES-D | 29.1 (10.0) | 26.1 (7.3) | 24.8 (5.9) | 27.0 (0.0) | 0.372ˆ |
Cohen’s d= Small Effect=0.30; Medium Effect=0.50; Large effect=0.80
p=0.10;
p=0.05;
p=0.01;
p<0.001
Demographic characteristics such as age, gender, and educational level as well as disease specific variables of the patient (e.g., tumor size, lesion number) were not found to be significant predictors of the patients’ reported depressive symptoms. Cross-lagged panel analyses, while adjusting for gender, yielded the findings that patients’ baseline depressive symptoms significantly predicted the caregivers’ reported stress at baseline and 4 months follow up. The caregivers’ reported stress level at 2-months was also predicted by the patients’ level of depressive symptoms. [Chi-square (9, N = 119) = 5.561, p = .783; RMSEA = .000, CFI = 1.000, SRMR = .031]. See Figure 4.
Figure 4.
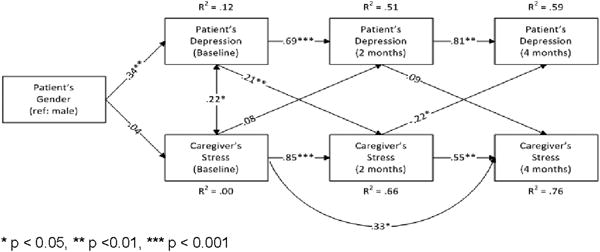
Patients’ depressive symptoms and caregiver stress from baseline and 2- and 4-months follow-Up
At baseline, the patients’ quality of life also significantly predicted the caregivers’ depressive symptoms and a trend was observed between patient quality of life and the caregivers’ level of depressive symptoms at 2-months follow-up. [Chi-square (10, N = 120) = 7.986, p = .630; RMSEA = .000, CFI = 1.000, SRMR = .037]. See Figure 5.
Figure 5.
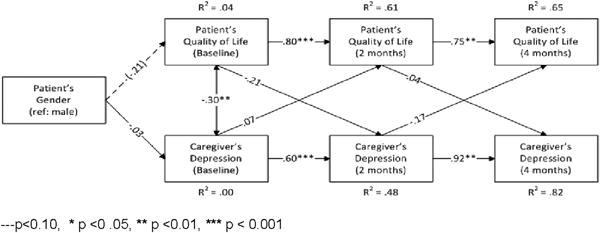
Patient health-related quality of life and the caregiver depressive symptoms from baseline and 2- and 4-months follow-Up
Discussion
Once used only in primary care settings, collaborative care interventions are becoming increasingly utilized in other medical settings, including oncology, to treat depression and other symptoms. The findings of this study demonstrate that a web-based stepped collaborative care intervention is feasible, acceptable, and efficacious in the reduction of depressive symptoms, pain and improving quality of life in advanced cancer patients. Based on a recent meta-analysis, this is the first web-based collaborative care intervention to target and reduce three of the most distressing symptoms to cancer patients.36 Furthermore, in addition to reducing symptoms, the intervention reduced inflammation, improved caregiver outcomes such as stress and depression, and demonstrated clinically meaningful changes in patients’ quality of life.36
The access to computers and the internet was relatively high despite the age and socioeconomic status of the patients. Nearly half of the patients enrolled reported a family income of $30,000 or less yet the majority had access to a computer at home or work. Patients primarily used the website at the time of diagnosis and then when they were confronted with a recurrence and/or new treatment. Post-hoc analysis of the timing of website usage showed that patients tended to use the website after office hours (between 5–9pm after access to care coordinators and nurses was less available).
The findings of this study also suggest that more than one symptom can be targeted and treated at the same time and that the reduction of depression and pain also improved overall quality of life. Our findings were consistent with a systematic review by Uitterhoeve and colleagues (2004) which found that psychosocial interventions were effective in improving quality of life in patients with advanced cancer.37 Nonetheless, these interventions have not become standard of care in the oncology setting despite the call for screening and treatment of distress (IOM, American College of Surgeons, ASCO).
A survey in 2005 found that only 53% of NCCN centers who responded to the survey (83% response rate) performed screenings for distress and/or depression and only three performed routine screenings.38,39 Recently, the Institute of Medicine (IOM) and the NCCN published guidelines for the screening and treatment of distress40 and in the last two years the American College of Surgeons (ACoS) and the American Society for Clinical Oncology (ASCO) have included screening for distress/depression as a quality indicator.41 The quality indicators stipulate that the oncologist provide: (1) evidence in the medical record that the patient’s emotional well-being was assessed within 1 month of their first visit, and (2) if an emotional problem exists that there was action taken to address the problem or there was an explanation provided for why no action was taken. Despite these guidelines, and introduction of these quality indicators; routine screening and treatment for distress in the context of oncology settings still remains rare.42 Jacobsen has suggested, that in part, this is the result of inconsistent findings that have been reported with regard to the efficacy of psychosocial interventions in the treatment of depression.43 Jacobsen recently concluded that the adoption of routine screening may be greater if there was stronger evidence that screening and treatment of patients predicted better clinical outcomes and that randomized controlled trials were needed.42
The inconsistencies in the efficacy of psychosocial interventions have been in part due to the inclusion of patients in interventions studies with and without clinical levels of depression or other symptoms. A recent meta-analysis reviewed more than 7700 studies and found that only 10 randomized controlled trials have been performed testing the efficacy of depression treatment for cancer patients with clinical levels of depression.44 Of the 1362 participants included in the meta-analyses, the random effects model showed interventions using CBT and/or SSRIs to be superior to control conditions in reducing depressive symptoms in people diagnosed with cancer.44 Although the interventions were found to be effective (e.g., statistically significant differences between the intervention and control arms, the effect sizes for the majority of the interventions remain modest. Although the sample size was small, we observed small to medium effect sizes for primary and secondary outcomes.
The web-based collaborative care intervention was not shown to be particularly effective in reducing fatigue. However, the effect sizes for our study were similar to other interventions targeting fatigue alone. However, Kangas and colleagues (2008) concluded from their meta-analytic review, that patients randomized to a psychosocial or physical activity interventions when compared to patients randomized to the standard of care arms was effective at reducing fatigue.45 Multimodal exercise and walking programs, restorative approaches, supportive-expressive, and cognitive-behavioral psychosocial interventions showed the most potential for ameliorating cancer-related fatigue.45 Although cognitive behavioral strategies and restorative approaches were employed in the present study, the advancing disease in this sample may have impacted the lack of improvement in this symptom.
Not only were psychological and physical symptoms reduced but we observed reductions in pro-inflammatory cytokines as well as increases in the number of NK cells in those who were randomized to the intervention arm of the study. These changes in biomarkers were also compared to that of normal controls and found to be clinically meaningful as a greater number of patients in the intervention arm achieved “normal” levels (when compared to healthy controls) of these cytokines and NK cell numbers. Further research is warranted to determine if these changes in inflammation and tumor surveillance translate into slowed tumor growth or development of metastases.
In a recent meta analyses, a positive association between depression and pro-inflammatory cytokines including IL-6, TNFα, and IL-1β, in the serum and cerebrospinal fluid, and NK cell numbers was observed.46,47,48,49 Similar associations between depression and biomarkers of inflammation and NK cells have also been reported in those diagnosed with cancer.50,51 We included six cytokines and NK cell numbers secondary to their link with the targeted symptoms and with survival across cancer types.52–56
In the present study we observed a link between fatigue and lower Natural Killer cell numbers. Natural Killer cells have been extensively studied in chronic fatigue and low numbers associated with higher levels of fatigue.57 Depression has been linked to higher levels of IL-6 and lower NK cell numbers in prior studies; however in the present study higher levels of depression were associated with higher serum levels of IL-8. Interleukin-8 is important in the context of cancer as it induces phagocytosis, promotes angiogenesis, increases the proliferation and survival of tumor cells, and potentiates the migration of tumor cells.58 Intereukin-8 also is considered a pro-inflammatory mediator and secretion of this protein is associated with increased oxidant stress and recruitment of inflammatory cells resulting in localized inflammation.58 Finally, improvements in overall quality of life were associated with decreased serum levels of IL-1α and IL-1β. Interleukin-1 is important in the regulation of immune and inflammatory responses and these cytokines are associated with tumorigenesis, tumor invasion, metastases across cancer types.59,60
Although less studied, evidence is mounting with regard to the effectiveness of psychosocial or pharmacological interventions in the modulation of ANS and HPA hormonal activity.61 A meta-analysis concluded that pharmacological treatments for depression reduced depressive symptoms when compared to controls but were not found to reduce TNFα in a sample from the general population. Treatment did reduce IL-1β and in most studies IL-6 but SSRIs appeared to have the greatest effects on changes of IL-6.62 Only a few studies have examined the reduction of biomarkers after behavioral or pharmacological treatments in cancer patients despite the plethora of evidence that depression increases the risk of mortality in the context of cancer.63,64
The reduction of patients’ symptoms also effectively reduced the caregivers’ stress and depression. Although prior research has demonstrated the effect of patient functioning on the caregiver, this is the first study to observe that with improved patients’ symptoms, the family caregivers’ quality of life was improved.65–68 The reduction of stress and depression may also have effects on the caregivers’ health by reducing the risk of cardiovascular disease which has been shown to be associated with caregiving.69–77
One of the major challenges with psychosocial intervention trials in general is the low enrollment rates and high attrition. While we observed adequate enrollment rates, particularly considering the advanced stage of cancer in which patients presented and the inclusion of family caregivers, attrition particularly due to death was high in this trial. However, we found that most patients remained active in the intervention but may have not completed outcomes measures. Only 8% withdrawal from the intervention was observed over the course of 6 months. The high rate of attrition in the trial was secondary to death, which is reflective of the palliative care setting. Attrition was also affected by the length of the battery of questionnaires used to measure the efficacy of the interventions as well as the frequency of the questionnaires. It would be recommended in future studies to reduce the number of questionnaires as well as the frequency of administration to decrease attrition in the assessment of outcomes.
Screening for distress has now been recommended by several organizations (e.g., National Comprehensive Cancer Network, Institute of Medicine, College of Surgeons, American Society for Clinical Oncology) yet screening for distress and treatment remains rare in the oncology setting. Several barriers exist with regard at the patient and provider level as well as at the macrolevel (e.g., institutions, insurers). Until these barriers can be overcome, it is likely that despite the recommendations that screening of symptoms and treatment will remain rare in the context of cancer.
Figure 3.
Socioeconomic disadvantage and symptom burden
Table 4.
Effects of the intervention from baseline to 6-months with patients reporting clinical levels of symptoms
| Measure | Baseline | 6-months | Effect Size | ||
|---|---|---|---|---|---|
| Intervention (Mean, SD) |
Usual Care (Mean, SD) |
Intervention (Mean, SD) |
Usual Care (Mean, SD) |
||
| n=42 | n=39 | n=15 | n=12 | ||
| CES-D (0–60) | 25.9 (9.8) | 25.49 (6.9) | 15.3 (10.5) | 24.7 (15.07) | 0.71ˆ |
| BPI (0–10) | 5.8 (1.2) | 6.1(1.6) | 4.7 (1.5) | 6.1 (2.6) | 0.62ˆ |
| FACT-Fatigue (0–52) | 36.8 (7.9) | 35.4 (7.1) | 28.3 (9.4) | 31.1 (11.4) | 0.26+ |
| FACT-General (0–100) | 67.2 (17.1) | 65.8 (16.9) | 82.4 (15.2) | 63.2 (21.5) | 0.99** |
Cohen’s d= Small Effect=0.30; Medium Effect=0.50; Large effect=0.80
Clinical levels of the symptom is indicated with CES-D ≥ 16; BPI≥3; FACT-Fatigue≥26
p=0.20;
p<0.10;
p=0.05;
p=0.01;
p<0.001
Acknowledgments
We would like to thank the nursing staff and Physician Assistants who worked closely with the care coordinators (Jackie Barnes, Cindy Wiltsie, Gretchen Foster, Susan Pugliese, Victoria Hyatt, Ann Pitcairn, Nicole Bonacci, Roberta Gillespie; Brittany Halladay) and the nurses and phlebotomist who assisted with blood draws (Zak Lanir, Valerie Switzer, Elaine Farmer, Judy Dick, Laura Stergis, and Pat Boden), the therapists (Ryan Hunt, Maranda Friday, Kendal Kingsley; Leigh Gemmel) and to the Project Managers (Sonja Likumahuwa, Deborah Brower, and Andrea Dunlavy, Koty Nadeau, Justin Lazaroff).
Funding: National Cancer Institute K07CA118576; R21CA127046; P30CA047904
Footnotes
Financial Disclosures and Conflicts of Interest: The authors have no financial disclosures or conflicts of interest
References
- 1.NIH State-of-the-Science Statement on symptom management in cancer: pain, depression, and fatigue. NIH Consens State Sci Statements. 2002;19:1–29. [PubMed] [Google Scholar]
- 2.Atesci FC, Baltalarli B, Oguzhanoglu NK, Karadag F, Ozdel O, Karagoz N. Psychiatric morbidity among cancer patients and awareness of illness. Support Care Cancer. 2004;12:161–7. doi: 10.1007/s00520-003-0585-y. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Zheng Y, Zheng W, et al. Prevalence of depression and its related factors among Chinese women with breast cancer. Acta oncologica (Stockholm, Sweden) 2009;48:1128–36. doi: 10.3109/02841860903188650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 5.Lynch BM, Steginga SK, Hawkes AL, Pakenham KI, Dunn J. Describing and predicting psychological distress after colorectal cancer. Cancer. 2008;112:1363–70. doi: 10.1002/cncr.23300. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros M, Oshima CT, Forones NM. Depression and Anxiety in Colorectal Cancer Patients. J Gastrointest Cancer. 2010 doi: 10.1007/s12029-010-9132-5. [DOI] [PubMed] [Google Scholar]
- 7.Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. European Journal of Cancer. 2006;42:846–63. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Theobald DE. Cancer pain, fatigue, distress, and insomnia in cancer patients. Clin Cornerstone. 2004;6(Suppl 1D):S15–21. doi: 10.1016/s1098-3597(05)80003-1. [DOI] [PubMed] [Google Scholar]
- 9.Bell CL, K M, Fischberg D. Pain outcomes of inpatient pain and palliative care consultations: Differences by race and diagnosis. J Palliat Med. 2011;14:1142–8. doi: 10.1089/jpm.2011.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Depression and pain. Hurting bodies and suffering minds often require the same treatment. Harv Ment Health Lett. 2004;21:4–5. [PubMed] [Google Scholar]
- 11.Crul BJ, Blok LM, van Egmond J, et al. The present role of percutaneous cervical cordotomy for the treatment of cancer pain. Journal of Headache & Pain. 2005;6:24–9. doi: 10.1007/s10194-005-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng G, Cassileth BR. Integrative oncology: complementary therapies for pain, anxiety, and mood disturbance. CA: a Cancer Journal for Clinicians. 2005;55:109–16. doi: 10.3322/canjclin.55.2.109. [DOI] [PubMed] [Google Scholar]
- 13.Doorenbos AZ, Given CW, Given B, et al. Symptom experience in the last year of life among individuals with cancer. Journal of Pain & Symptom Management. 2006;32:403–12. doi: 10.1016/j.jpainsymman.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ell K, Xie B, Quon B, Quinn DI, Dwight-Johnson M, Lee PJ. Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. J Clin Oncol. 2008;26:4488–96. doi: 10.1200/JCO.2008.16.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719–26. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 16.Hopko DR, Bell JL, Armento M, et al. Cognitive-behavior therapy for depressed cancer patients in a medical care setting. BEHAV THER. 2008;39:126–36. doi: 10.1016/j.beth.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Kaya E, Feuer D. Prostate cancer: palliative care and pain relief. Prostate Cancer & Prostatic Diseases. 2004;7:311–5. doi: 10.1038/sj.pcan.4500747. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz ME, Kurtz JC, Given CW, Given B. A randomized, controlled trial of a patient/caregiver symptom control intervention: effects on depressive symptomatology of caregivers of cancer patients. J Pain Symptom Manage. 2005;30:112–22. doi: 10.1016/j.jpainsymman.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loscalzo M. Psychological approaches to the management of pain in patients with advanced cancer. Hematol Oncol Clin North Am. 1996;10:139–55. doi: 10.1016/s0889-8588(05)70331-2. [DOI] [PubMed] [Google Scholar]
- 20.Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE. Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain. 1995;63:189–98. doi: 10.1016/0304-3959(95)00039-U. [DOI] [PubMed] [Google Scholar]
- 21.Dobscha SK, C K, Perrin NA, Hanson GC, Leibowitz RQ, Doak MN, Dickinson KC, Sullivan MD, Gerrity MS. Collaborative care for chronic pain in primary care: a cluster randomized trial. Journal of the American Medical Association. 2009;301:1242–52. doi: 10.1001/jama.2009.377. [DOI] [PubMed] [Google Scholar]
- 22.Lin EH, K W, Von Korff M, Tang L, Williams JW, Jr, Kroenke K, Hunkeler E, Harpole L, Hegel M, Arean P, Hoffing M, Della Penna R, Langston C, Unützer J, IMPACT Investigators Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. Journal of the American Medical Association. 2003;290:2428–9. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 23.Dickinson KC, S R, Duckart JP, Corson K, Gerrity MS, Dobscha SK. VA healthcare costs of a collaborative intervention for chronic pain in primary care. Med Care. 2010;48:38–44. doi: 10.1097/MLR.0b013e3181bd49e2. [DOI] [PubMed] [Google Scholar]
- 24.Gilbody S, B P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Archives of Internal Medicine. 2006;166:2314–21. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 25.Dwight-Johnson M, E K, Lee PJ. Can collaborative care address the needs of low-income Latinas with comorbid depression and cancer? Results from a randomized pilot study. Psychosomatics. 2005;46:224–32. doi: 10.1176/appi.psy.46.3.224. [DOI] [PubMed] [Google Scholar]
- 26.Ell K, X B, Wells A, Nedjat-Haiem F, Lee P-J, Vourlekis B. Economic stress among low-income women with cancer: Effects on quality of life. Cancer. 2008;112:616–25. doi: 10.1002/cncr.23203. [DOI] [PubMed] [Google Scholar]
- 27.deRaaf PJ, D C, Timman R, Busschback JJ, Oldenmmenger WH, van der Rjit Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: a randomized controlled trial. Journal of Clinical Oncology. 2013;31:716–23. doi: 10.1200/JCO.2012.44.4216. [DOI] [PubMed] [Google Scholar]
- 28.Katon WJ, Schoenbaum M, Fan MY, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Archives of General Psychiatry. 2005;62:1313–20. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- 29.MA IJ, Huijbregts KM, van Marwijk HW, et al. Cost-effectiveness of collaborative care including PST and an antidepressant treatment algorithm for the treatment of major depressive disorder in primary care; a randomised clinical trial. BMC Health Serv Res. 2007;7:34. doi: 10.1186/1472-6963-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis JM, D M, Currow DC, Davidson PM. Dying in the margins: understanding palliative care and socioeconomic deprivation in [Review] Journal of Pain & Symptom Management. 2011;42:105–18. doi: 10.1016/j.jpainsymman.2010.10.265. [DOI] [PubMed] [Google Scholar]
- 31.Radoff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 32.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 33.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain & Symptom Management. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 34.Weitzner MA, McMillan SC. The Caregiver Quality of Life Index-Cancer (CQOLC) Scale: revalidation in a home hospice setting. J Palliat Care. 1999;15:13–20. [PubMed] [Google Scholar]
- 35.Steel JL, E D, Cella D, Olek M, Carr BI. Measuring health related quality of life in patients with hepatobiliary carcinoma. Annals of Oncology. 2006;17:304–217. doi: 10.1093/annonc/mdj072. [DOI] [PubMed] [Google Scholar]
- 36.Agboola So, J W, Elefiky A, Kvedar JC, Jethwani K. The effect of technology based interventions on pain, depression, and quality of life in patients with cancer: A systematic review of randomized controlled trials. Journal of Medical Internet Research. 2015;17:e65. doi: 10.2196/jmir.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uitterhoeve RJ, V M, Litjens M, Potting K, Bensing J, De Mulder P, van Achterberg T. Psychosocial interventions for patients with advanced cancer – a systematic review of the literature. British Journal of Cancer. 2004;91:1050–62. doi: 10.1038/sj.bjc.6602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobsen PB, R S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Cancer Network. 2007;5:99–103. doi: 10.6004/jnccn.2007.0010. [DOI] [PubMed] [Google Scholar]
- 39.Network NCC. Guidelines for Supportive Care: Distress [Google Scholar]
- 40.A E, A NP. Cancer Care for the Whole Patient: Meeting the Pschosocial Health Needs. The Institute of Medicine of the National Academies The National Academies Press; Washington DC: 2007. [Google Scholar]
- 41.P J. Measuring and improving quality of cancer care. Psycho-Oncology. 2010:19. [Google Scholar]
- 42.Jacobsen PB. Screening for psychological distress in cancer patients: challenges and opportunities. Journal of Clinical Oncology. 2007;25:4526–7. doi: 10.1200/JCO.2007.13.1367. [DOI] [PubMed] [Google Scholar]
- 43.Jacobsen PB, J HS. Psychosocial Interventions for Anxiety and Depression in Adult Cancer Patients: Achievenments and Challenges. CA Cancer J Clin. 2008;58:214–30. doi: 10.3322/CA.2008.0003. [DOI] [PubMed] [Google Scholar]
- 44.Hart SL, H M, Diefenbach M, Anderson DR, Kilbourn KM, Craft LL, Steel JL, Cuijpers P, Mohr DC, Berendsen M, Spring B, Stanton AL. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. Journal of the National Cancer Institute. 2012;104:990–1004. doi: 10.1093/jnci/djs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kangas M, B D, Montgomery GH. Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychological Bulletin. 2008;134:700–14. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 46.Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychol Bull. 1993;113:472–86. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 47.M I. Effects of sleep and sleep loss on immunity and cytokines. Brain, Behavior, and Immunity. 2002;16:503–12. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 48.Zorrilla EP, Luborsky L, McKay JR, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 49.Dowlati Yekta, H N, Swardfager Walter, Liu Helena, Sham Lauren, Reim Elyse K, Lanctôt Krista L. A Meta-Analysis of Cytokines in Major Depression. Biological Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 50.Musselman DL, Miller AH, Porter MR, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158:1252–7. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 51.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–25. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 52.Evans C, M I, Heriot AG, FInalayson C, Dalgleish AG, Kumar D. The correlation between colorectal cancer rates of proliferation and apoptosis and system cytokines levels;plus their influence on survival. British Journal of Cancer. 2006;94:1412–9. doi: 10.1038/sj.bjc.6603104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 54.D HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. Nature Reviews. 2008;8:887–99. [Google Scholar]
- 55.Trompet S, de Craen AJ, Mooijaart S, et al. High Innate Production Capacity of Proinflammatory Cytokines Increases Risk for Death from Cancer: Results of the PROSPER Study. Clin Cancer Res. 2009;15:7744–8. doi: 10.1158/1078-0432.CCR-09-2152. [DOI] [PubMed] [Google Scholar]
- 56.Taketomi A, Shimada M, Shirabe K, Kajiyama K, Gion T, Sugimachi K. Natural killer cell activity in patients with hepatocellular carcinoma: a new prognostic indicator after hepatectomy. Cancer. 1998;83:58–63. doi: 10.1002/(sici)1097-0142(19980701)83:1<58::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 57.Brenu EW, H S, Atkinson GM, van Driel ML, Kreijkamp-Kaspers S, Ashton KJ, Staines DR, Marshall-Gradisnik SM. Natural killer cells in patients with severe chronic fatigue syndrome. Auto Immun Highlights. 2013;16:69–80. doi: 10.1007/s13317-013-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waugh DJ, W C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 59.Apte RN1, D S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 60.Voronov E, S D, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apt RN1. IL-1 is required for tumor invasiveness and angiogenesis. Journal of Translational Medicine. 2006;4:48. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.A MH. Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain Behav Immun. 2013;30:S88–S98. doi: 10.1016/j.bbi.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dowlati Y, H N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A Meta-Analysis of Cytokines in Major Depression. Biological Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 63.Lisa M, Thornton P, Andersen, PhD Barbara L, Schuler, MA Tammy A, William E, Carson, MD A Psychological Intervention Reduces Inflammatory Markers by Alleviating Depressive Symptoms: Secondary Analysis of a Randomized Controlled Trial. Psychosomatic Med. 2009;71:715–24. doi: 10.1097/PSY.0b013e3181b0545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steel JL, Geller DA, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. Journal of Clinical Oncology. 2007;25:2397–405. doi: 10.1200/JCO.2006.06.4592. [DOI] [PubMed] [Google Scholar]
- 65.Bevans M, Sternberg E. Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA. 2012;307:398–403. doi: 10.1001/jama.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weitzner MA, McMillan SC, Jacobsen PB. Family caregiver quality of life: differences between curative and palliative cancer treatment settings. J Pain Symptom Manage. 1999;17:418–28. doi: 10.1016/s0885-3924(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 67.Chentsova-Dutton Y, Shuchter S, Hutchin S, Strause L, Burns K, Zisook S. The psychological and physical health of hospice caregivers. Ann Clin Psychiatry. 2000;12:19–27. doi: 10.1023/a:1009070826012. [DOI] [PubMed] [Google Scholar]
- 68.Milne DJ, Mulder LL, Beelen HC, Schofield P, Kempen GI, Aranda S. Patients’ self-report and family caregivers’ perception of quality of life in patients with advanced cancer: how do they compare? Eur J Cancer Care (Engl) 2006;15:125–32. doi: 10.1111/j.1365-2354.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 69.Ji J, Zoller B, Sunquist K, Sunquist J. Increased risks of coronary heart disease and stroke among spousal caregivers of cancer patients. Circulation Journal. 2012;125:1742–7. doi: 10.1161/CIRCULATIONAHA.111.057018. [DOI] [PubMed] [Google Scholar]
- 70.Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S. women: a prospective study. American Journal of Preventive Medicine. 2003;24:113–9. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 71.von Kanel R, Mills P, Mausbach B, et al. Effect of Alzheimer caregiving on circulating levels of C-reactive protein and other biomarkers relevant to cardiovascular disease risk: a longitudinal study. Gerontology. 2012;58:354–65. doi: 10.1159/000334219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rumsfeld JS, Ho M. Depression and cardiovascular disease: A call for recognition. Circulation. 2005;111:250–3. doi: 10.1161/01.CIR.0000154573.62822.89. [DOI] [PubMed] [Google Scholar]
- 73.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk of cardiovascular diseases: systematic review and meta analysis. J Geriatr Psychiatry. 2007;22:613–26. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 74.Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. Journal of Psychosomatic Research. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 75.Mausbach BT, Patterson TL, Rabinowitz YG, et al. Depression and distress predict time to cardiovascular disease in dementia caregivers. Health Psychology. 2007;26:539–44. doi: 10.1037/0278-6133.26.5.539. [DOI] [PubMed] [Google Scholar]
- 76.Orth-Gomer K, S N, Wang H-X, Walldin C, Blom M, Jernberg T. Stress reduction prolongs life in women with coronary disease. Circulation: Cardiovascular Quality and Outcomes. 2009;2:25–32. doi: 10.1161/CIRCOUTCOMES.108.812859. [DOI] [PubMed] [Google Scholar]
- 77.Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nature Reviews Cardiology. 2012;9:360–70. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]


