Abstract
Peripheral inflammation can trigger a number of neuroinflammatory events in the CNS, such as activation of microglia and increases of proinflammatory cytokines. We have previously identified an interesting phenomenon, termed “euflammation”, which can be induced by repeated subthreshold infectious challenges. Euflammation causes innate immune alterations without overt neuroimmune activation. In the current study, we examined the protective effect of euflammation against peripheral inflammation-induced neuroinflammation and the underlying mechanisms. When E.coli or lipopolysaccharide (LPS) was injected inside or outside the euflammation induction locus (EIL), sickness behavior, global microglial activation, proinflammatory cytokine production in the brain, expression of endothelial cyclooxygenase II and induction of c-fos expression in the paraventricular nucleus of the hypothalamus were all attenuated in the euflammatory mice compared with those in the control unprimed mice. Euflammation also modulated innate immunity outside the EIL by upregulating receptors for pathogen-associated molecular patterns in spleen cells. In addition, euflammation attenuated CNS activation in response to an intra-airpouch (outside the EIL) injection of LPS without suppressing the cytokine expression in the airpouch. Collectively, our study demonstrates that signaling of peripheral inflammation to the CNS is modulated dynamically by peripheral inflammatory kinetics. Specifically, euflammation can offer effective protection against both bacterial infection and endotoxin induced neuroinflammation.
Keywords: Cytokines, Neuroimmune, Lipopolysaccharide, Microglia
1. INTRODUCTION
Peripheral inflammation triggers the production and release of inflammatory cytokines, which propagate peripheral inflammatory signaling to the brain (Galic et al., 2012), causing inflammatory cytokine expression in the brain that mediates neuronal excitability and ultimately behavioral abnormalities (Dantzer et al., 2008). This neuroimmune activation theory has been well-supported by mounting evidence demonstrating signal transduction at the brain endothelium, activation of glial cells and increases of proinflammatory cytokines in the brain after peripheral immune challenges (Laye et al., 1994; Pyter et al., 2009; Quan et al., 1999; Reyes et al., 1999; Riazi et al., 2008). Such neuroinflammatory manifestation, however, is not the only consequence of peripheral inflammation. In our previous studies, we found subthreshold levels of localized infection may not cause the central consequences of peripheral inflammation. Further, a prior subthreshold infectious challenge can prime the host to more effectively combat a subsequent larger infectious load while maintaining neuroimmune quiescence. We have termed the inflammation induced by repeated subthreshold infectious challenges “euflammation” (Chen et al., 2013; Tarr et al., 2014). Although the altered innate immunity caused by euflammation has been previously characterized, it remains to be determined whether priming the host with euflammation could alleviate neuroinflammation induced by peripheral immune challenges.
In the current study, results showed that euflammation effectively attenuated microglial activation and cytokine expression in the brain in response to E.coli or LPS challenges. Mechanistically, these protective effects were associated with mitigated immune-to-brain signaling, enhanced immune surveillance and local inflammatory responses. Collectively, our data indicate that neuroinflammation induced by peripheral immune challenges can be mitigated by euflammation.
2. METHODS
2.1. Animals
Male FVB (6-8 weeks) mice were obtained from Charles River Laboratories (Wilmington, MA). They were group housed in polypropylene cages, with food and water available ad libitum, in rooms maintained at 21°C under a 12 hrs light/dark cycle. All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2. Euflammation Induction
A 3-day euflammation induction procedure was adopted from a protocol described previously (Chen et al., 2013). Briefly, mice received 3 consecutive intraperitoneal (IP) injections of E.coli (strain LT004): 2.0×107 CFUs on day 1, 25×107 CFUs on day 2, and 100×107 CFUs on day 3. E.coli was suspended in 100 μl sterile PBS for injection.
2.3. Airpouch Creation and Maintenance
Dorsal airpouch was created as described previously (Tarr et al., 2014). Briefly, mice were anesthetized on day 1 with inhalation of 2.5% isoflurane and a 1 cm2 area of the back was shaved. Subsequently, 1.5 ml of filtered air was injected into the subcutaneous space under the shaved area to create a bulging empty pouch using a 26 ½ gauge needle. On days 2 and 3, airpouches were maintained by injecting 0.5 ml of filtered air to compensate for spontaneous deflation. Euflammation induction or control PBS injection was started on the same day as the beginning of the creation of the airpouch.
2.4. Acute Inflammation Induction and Experimental Design
Mice received four different types of acute inflammatory challenges. They were IP injection of 100x107 CFU of E.coli LT004 (IP E.coli), IP injection of 250 μg/kg LPS (IP LPS), intra-airpouch (IA) injection of 100×107 CFU of E.coli LT004 (IA E.coli) and IA injection of 250 μg/kg LPS (IA LPS). In the euflammation (EU) groups, mice underwent the 3-day euflammation induction from day 1 to day 3 and received an acute inflammation challenge on day 4. For comparison, the unprimed (UP) groups received IP PBS injections for 3 days and an acute inflammation challenge on day 4. An additional control group (Ctrl) received IP PBS injections for 4 days but no inflammatory challenge. We also examined an EU-only group in which mice received the 3-day euflammation induction without further immune challenges. For immunohistochemical (IHC) labeling of Iba-1, mice were sacrificed at 24 hrs after the last injection because brain microglial activation has been reported to occur at 24, not at 3 or 6, hrs after acute peripheral immune challenges (Liu et al., 2015; Proescholdt et al., 2002). For IHC labeling of COX-2/c-fos, ELISA analyses of blood cytokines/corticosterone, and qRT-PCR analyses of brain/spleen cytokine mRNAs, mice were sacrificed at 3 hrs after the last injection in the IP E.coli, IP LPS and the Ctrl groups, but at 6 hrs after the last injection in the IA E.coli and IA LPS groups. The above mentioned parameters have been reported previously to show peak responses at 3 and 6 hrs following IP and IA immune challenges, respectively (Gaspar et al., 2014; Tarr et al., 2014). All the mice were sacrificed between 2 to 4 pm to avoid the influence of circadian rhythm. Spleen mRNAs of pathogen recognition receptors at 24 hrs after the last euflammation injection were also analyzed in the EU-only group. The experimental design is graphically illustrated in Table 1.
Table 1.
Schematic illustration of the experimental design. Acute inflammatory challenges include IP E.coli, IP LPS, IA E.coli and IA LPS injections. Mice in the UP group received IP PBS injections for 3 days followed by an acute inflammatory challenge on day 4. Mice in the EU groups received increasing doses of IP E.coli injections for 3 days followed by an acute inflammatory challenge on day 4. Mice in the Ctrl group received 4 days of IP PBS injections. Mice in the EU-only group received euflammation induction for 3 days only. For circulating cytokine ELISA, brain and spleen cytokine qRT-PCR, IHC labeling of c-fos and COX-2, mice were sacrificed at 3 hrs after the IP immune challenges or 6 hrs after the IA immune challenges. For spleen pathogen recognition receptor qRT-PCR and IHC labeling of Iba-1, mice were sacrificed at 24 hrs after the last injection.
| Treatment group | Euflammation induction (IP) | Acute inflammation | Time point for sacrifice | ||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |||
| IP E.coli | UP | PBS | PBS | PBS | IP E.coli 100×107 | Sac 3 hrs post injection | Or Sac 24 hrs post injection for IHC of Iba-1 |
| EU | E.coli 2×107 | E.coli 25×107 | E.coli 100×107 | IP E.coli 100×107 | |||
| IP LPS | UP | PBS | PBS | PBS | IP LPS | ||
| EU | E.coli 2×107 | E.coli 25×107 | E.coli 100×107 | IP LPS | |||
| IA E.coli | UP | PBS | PBS | PBS | IA E.coli 100×107 | Sac 6 hrs post injection | |
| EU | E.coli 2×107 | E.coli 25×107 | E.coli 100×107 | IA E.coli 100×107 | |||
| IA LPS | UP | PBS | PBS | PBS | IA LPS | ||
| EU | E.coli 2×107 | E.coli 25×107 | E.coli 100×107 | IA LPS | |||
| CTRL | PBS | PBS | PBS | IP PBS | Sac 3 hrs post injection | ||
| EU-only | E.coli 2×107 | E.coli 25×107 | E.coli 100×107 | Sac 24 hrs post injection | |||
2.5. Open Field Test
Sickness behavior was determined using the open field test as described previously (Tarr et al., 2014). Mice were placed individually in the corner of the open field apparatus (40 × 40 × 25 cm Plexiglas box) and activity was recorded for 10 min using an automated digital beam break system attached to a computer containing an open field software template (Omnitech Electronics; Columbus, OH). Following testing, the open field boxes were cleaned with H2O and 70% ethanol to reduce odor cues. Mice with sickness behavior traveled less in the open field apparatus.
2.6. RNA Isolation and qRT-PCR
Brains and spleens were collected from different groups of mice (n = 5/group). Hippocampus, hypothalamus and prefrontal cortex were further dissected from the brains on ice. Tissues were transferred to sterile Eppendorf tubes, suspended in the TRIzol reagent (Invitrogen; Carlsbad, CA), and homogenized with a sonicator (Misonix; Farmingdale, NY). Total RNA was isolated following the manufacture's instruction. RNA concentration and purity were determined with a Nanodrop spectrophotometer (Denville; S. Plainfield, NJ). RNAs were reversed transcribed into cDNAs using a Reverse Transcription Kit (Promega; Madison, WI). qRT-PCR was performed using the Assay-on-Demand Gene Expression protocol as previously described (Tarr et al., 2014). In brief, the amount of cDNA was measured on an ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster, CA) by real-time PCR and normalized based on reference cDNA levels (GAPDH). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold difference from GAPDH normalized PBS treated controls for each specific tissue type.
2.7. Immunohistochemistry
For Iba-1 labeling, mice were perfusion-fixed with 4% formaldehyde. Brains were dissected, postfixed in 4% formaldehyde for 24 hrs and immersed in 20% sucrose for another 24 hrs at 4°C. Brains were frozen at −80°C. Later, 40-μm-thick coronal sections were cut and collected with a cryostat. Sections were then incubated in 1% sodium borohydride and 0.5% hydrogen peroxide to reduce background. They were incubated with anti-Iba1 primary antibody (1:1000; rabbit anti-mouse, Cat# 019-19741; Wako Chemicals, Richmond, VA), followed by Alexa Fluor 594 conjugated secondary antibody (1:500; ThermoFisher Scientific, Grand Island, NY).
For COX-2 and c-fos labeling, fresh brains were rapidly frozen in −80°C isopentane for 20 sec and 20-μm-thick coronal sections were later generated with a cryostat. Sections were fixed in an acetone/alcohol mixture, followed by incubation in a glucose oxidase and sodium azide solution. They were incubated with anti-COX-2 (1:200; rabbit-anti-mouse, catalog #160106; Cayman Chemical, Ann Arbor, MI) or anti-c-fos (1:1000; rabbit-anti-mouse, catalog #sc-52; Santa Cruz, Dallas, TX) primary antibody and anti-rabbit/anti-rat secondary antibody. The labeling was amplified using an ABC solution (Vector Laboratories, Burlingame, CA) and visualized with a diaminobenzidine (DAB) peroxidase substrate kit (Vector Laboratories, Burlingame, CA).
2.8. ELISA for cytokines/corticosterone
Whole blood samples were collected from mice by cardiac puncture. Serum samples were collected after centrifugation of whole blood at 4000 rpm for 20 min and were transferred to −20 °C until use. ELISA for serum interleukin (IL)-1β, IL-6 and corticosterone levels were conducted using the commercially available mouse IL-1β and IL-6 kits (BD Biosciences, San Jose, CA) and the corticosterone kit (Abcam, Cambridge, MA).
Cytokine levels in the airpouch fluid in the IA LPS group (n = 6-8/group) were determined. In these mice, 6 hrs after the LPS administration, 1 ml sterile PBS was injected into the airpouch followed by gentle massage. An incision on the airpouch was made with sterile scissors and lavage contents were carefully removed with a micropipette. Samples were centrifuged at 1500 rpm for 8 min and supernatants were removed for further analysis of IL-1β, IL-6 and tumor necrosis factor (TNF)-α with ELISA kits (BD Biosciences, San Jose, CA).
2.9. Quantification of COX-2/c-fos-labeled cells
Bright field photomicrographs were acquired with a digital camera connected to an optic microscope (DXM 1200, Nikon Eclipse E600, Melville, NY). Pictures were taken from coronal sections between bregma 0.62 and 0.38 mm for caudate putamen (striatum) and between bregma −0.70 and −0.94 mm for paraventricular nucleus (PVN). Selected areas of the pictures were outlined using known anatomical landmarks. COX-2/c-fos positive cells were identified and quantified by light thresholding with automated ImageJ software program (National Institutes of Health, Bethesda, MD). The average number of labeled cells from a total of 12 pictures was used for statistical analysis.
2.10. Proportional and Cell Body Area Analysis of Microglia
For Iba-1 IHC labeling, confocal images were acquired using a Zeiss LSM510. Images were optimized for color, brightness, and contrast for best clarity. Three-dimensional reconstruction of images was performed using ImageJ. For proportional area analysis of microglia, a protocol from a previous study (Liu et al., 2015) was performed. In brief, representative images were obtained at 20 × magnification. A threshold for positive staining was determined for each image after it was processed by densitometric scanning using ImageJ. Proportional area was reported as average percentage area under the positive threshold for all representative pictures. For cell body area analysis, individual cell body size was calculated, summed and averaged. For each cell, the appropriate threshold value was set up manually at the level at which the binary overlay covered cell body without branching processes.
2.11. Statistical Procedures
Standard one-way ANOVA was used to analyze data. When appropriate, significant main and interaction effects were subjected to Fisher's PLSD post hoc analyses for further comparison. Observations more than three interquartile ranges from the first and third quartile were excluded from analyses. An alpha level of p ≤ 0.05 was used as the criterion for the rejection of the null hypothesis. All data were analyzed using StatView statistical software (SAS Institute Inc., Cary, NC). Results are reported as treatment means ± standard error of the mean (SEM).
3. RESULTS
3.1. Euflammation attenuated acute peripheral inflammation-induced sickness behavior
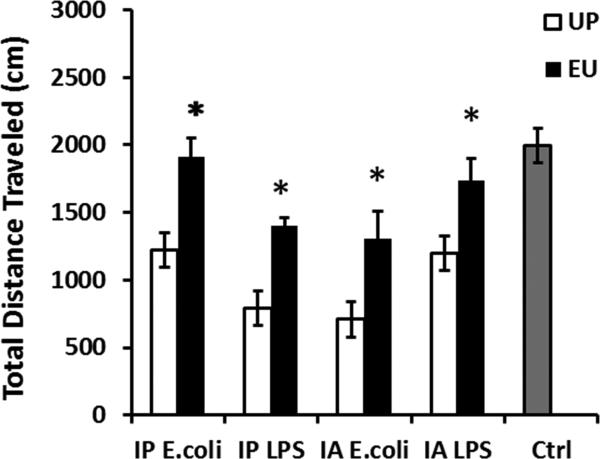
In the UP groups, mice after IP injection of either E.coli or LPS showed decreased total distance traveled compared with the Ctrl group (F(4,27) = 13.706, post hoc analyses for all the comparisons with the Ctrl group, p's < 0.001) (Fig.1). Similarly, IA E.coli or IA LPS also caused reduced total distance traveled in the open field in the UP groups (post hoc analyses for all the comparisons with the Ctrl group, p's < 0.001). In contrast, mice in the EU groups exhibited significantly higher locomotor activities than mice in the corresponding UP groups (Fisher's PLSD post hoc analyses, in IP E.coli, p's < 0.01; in IA E.coli, IP LPS and IA LPS, p's < 0.05).
Figure 1.
Results of open field tests following peripheral immune challenges. Data are presented as means ± SEM. *Represents significant differences between the UP and EU groups following the same treatment (p < 0.05).
3.2. Euflammation attenuated acute peripheral inflammation-induced brain proinflammatory cytokine expression
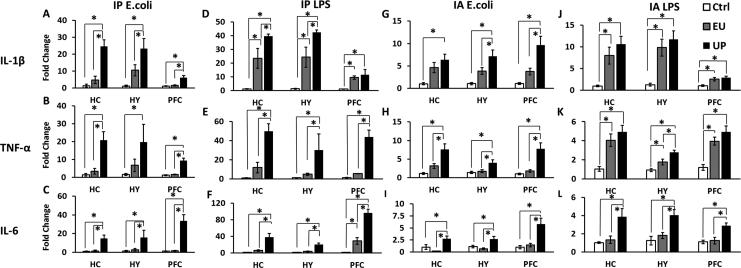
We then examined brain proinflammatory cytokine levels after the acute immune challenges. Results from IP E.coli challenged animals are shown in Figs.2A-C. Compared with the unchallenged control group (Ctrl), mice in the UP group exhibited robust increases of IL-1β in hippocampus (HC), hypothalamus (HY) and prefrontal cortex (PFC) (p < 0.001), increases of TNF-α (in HC and PFC, p < 0.0001; in HY, p < 0.05) and increases of IL-6 mRNA (p < 0.01). Mice in the EU group, levels of IL-1β, TNF-α or IL-6 in all the three brain regions were not significantly different from the Ctrl group, but were significantly reduced compared with the corresponding UP groups (Fisher's PLSD post hoc analyses for all comparisons, p's < 0.05).
Figure 2.
IL-1β, TNF-α, and IL-6 mRNA levels in the hippocampus (HC), hypothalamus (HY), and prefrontal cortex (PFC) after IP E.coli (A-C), IP LPS (D-F), IA E.coli (G-I) or IA LPS (J-L) injections. Data are presented as means ± SEM. *Represents significant differences between the UP, EU and the Ctrl groups (p < 0.05).
After IP LPS injection, elevated IL-1β (in HC and HY, p < 0.001; in PFC, p < 0.05), TNF-α (in HC and PFC, p < 0.0001; in HY, p < 0.01) and IL-6 mRNA (p < 0.001) were similarly detected in the UP group (Figs.2D-F). Fisher's PLSD post hoc analyses revealed that all three cytokine mRNA levels in the EU group showed increases of IL-1β in all three brain regions and IL-6 in PFC compared with the Ctrl group (IL-1β in HC, HY and PFC, p's < 0.01; IL-6 in PFC, p's < 0.05), while these cytokines in the EU group displayed significant reductions in all three brain regions compared with the UP group except for IL-1β in the PFC region (IL-1β in HC and HY, p's < 0.05; TNF-α and IL-6 in HC, HY and PFC, p's < 0.01).
Similar results were obtained after IA E.coli injections. Mice in the UP group showed significantly higher IL-1β (p < 0.05), TNF-α (p < 0.01) and IL-6 mRNA levels (in HC and PFC, p < 0.05; in HY, p < 0.01) in all the three brain regions than the Ctrl group (Figs.2G-I). Fisher's PLSD post hoc analyses revealed that TNF-α and IL-6 mRNA level in all three regions (in HC and HY, p's < 0.01; in PFC, p's < 0.005), IL-1β mRNA level in HY (p's < 0.05) and PFC (p's < 0.05) in the EU group were significantly reduced compared to the UP group. Data showed no statistical difference in all three cytokines between the EU group and the Ctrl group in HC, HY or PFC.
After IA LPS challenge, small increases of cytokine mRNA levels were observed. Again, IL-1β (in HC and HY, p < 0.001; in PFC, p < 0.05), TNF-α (p < 0.01) and IL-6 mRNA (in HC and HY, p < 0.01; in PFC, p < 0.05) were elevated in the UP group above the Ctrl group in all the three brain regions (Figs.2J-L). In the EU group, only IL-1β and TNF-α levels were elevated above the Ctrl group (in all three brain regions, p's < 0.05), but IL-6 levels were not increased. In addition, all three cytokines in the EU group were lower compared with those in the UP group, although only the decrease in the IL-6 levels reached statistical significance (p's < 0.01).
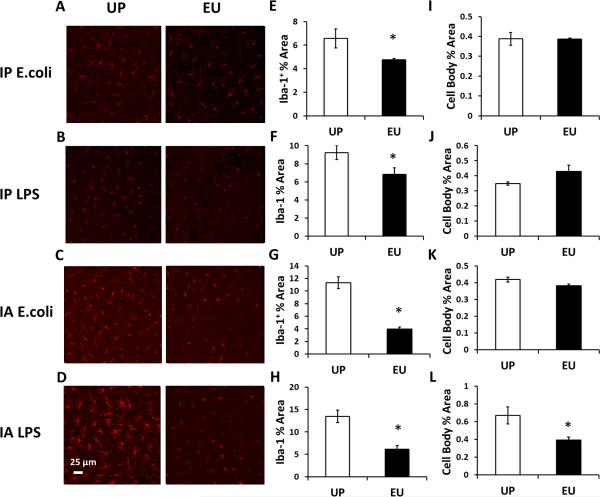
3.3. Euflammation ameliorated acute peripheral inflammation-induced microglial activation
In previous studies, peripheral immune challenge was shown to induce altered microglial morphology (Imai and Kohsaka, 2002). Altered microglial morphology can be detected by IHC labeling of Iba-1 protein. In this study, following peripheral immune challenges, the proportional area and cell body area of Iba-1 labeling were determined. After IP E.coli, IP LPS or IA E.coli challenges, microglial cells in the UP groups showed more Iba-1+ areas than that in the corresponding EU groups (p < 0.05) (Figs.3A-C, E-G), although no difference was found in the Iba-1 labeled cell body area between the UP and the corresponding EU groups (Figs.3I-K). The most significant contrast was found after the IA LPS challenge: microglial cells in the UP group showed clear de-ramified morphology with shortened processes whereas microglial cells in the EU group showed ramified morphology with smaller cell bodies, indicating distinct morphological phenotypes (Fig.3D). Quantitative analyses also confirmed both Iba-1+ area and the size of Iba-1 labeled microglial cell body were smaller in the EU group compared with the UP group (total area, p < 0.001; cell body area, p < 0.005) (Figs.3H&L). Interestingly, IA LPS challenge induced higher Iba-1 labeled area compared with IP LPS (p < 0.05), suggesting the IA challenges are more potent in inducing microglial activation (Fig.3A D).
Figure 3.
IHC labeling of Iba-1+ microglia 24 hrs after IP E.coli (A), IP LPS (B), IA E.coli (C), or IA LPS injections (D). Quantification of the proportional area of Iba-1 labeling and cell body area of Iba-1+ microglia following IP E.coli (E, I), IP LPS (F, J) IA E.coli (G, K) or IA LPS (H, L) injections. Data are presented as means ± SEM. *Represents significant differences between the UP and EU groups (p < 0.05).
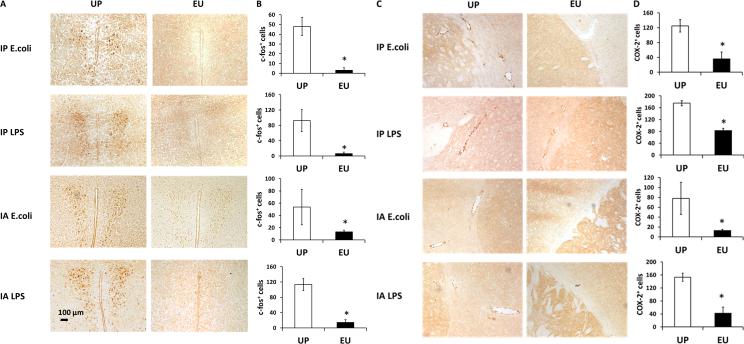
3.4. Euflammation reduced acute peripheral inflammation-induced endothelial COX-2 induction and prevented neuronal c-fos activation in the brain
We speculated that blockade of immune-to-brain afferent signaling could be a potential mechanism by which euflammation attenuates peripheral inflammation-induced neuroinflammation. Previous reports showed that peripheral LPS injection can induce activation of PVN neurons (Quan et al., 2003). Therefore, we examined the expression of c-fos, an immediate early gene, in the PVN neurons. All the peripheral immune challenges in the current study were sufficient to trigger robust c-fos expression in PVN in the UP groups, but not in the EU groups (Fig.4A). Quantitative analyses of c-fos-labeled cells in the PVN are shown in Figure 4B. The number of c-fos+ cells in the EU groups were drastically reduced compared with the UP groups (p < 0.05).
Figure 4.
IHC labeling of PVN c-fos (A) and endothelial COX-2 (C) following peripheral immune challenges in the UP and EU groups. Results of c-fos+ and COX-2+ cell quantification are shown in (B) and (D). Data are presented as means ± SEM. *Represents significant differences between the UP and EU groups (p < 0.05).
Since endothelial COX-2 expression is critical in immune-to-brain signaling (Minghetti, 2004; Quan et al., 2003), IHC labeling of COX-2 was performed. Robust endothelial COX-2 labeling was observed after all four types of peripheral immune challenges in the UP groups. Reduced COX-2 labeling was found in the corresponding EU groups (Fig.4C). Results of quantitative analyses are shown in Fig.4D.
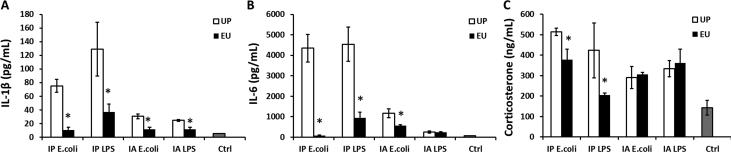
3.5. Euflammation reduced acute peripheral inflammation-induced cytokine and corticosterone in the circulation
Inflammatory cytokines in the circulation have been considered as the critical mediators of immune-to-brain communication (Quan and Herkenham, 2002). We examined the influence of euflammation on serum proinflammatory cytokines induced by acute inflammatory challenges. In the UP groups, increased expression of IL-1β and IL-6 were found to be robustly induced by each acute peripheral inflammatory challenge compared with the Ctrl group (IL-1β, F(4, 13) = 10.597; IL-6, F(4, 13) = 26.427; post hoc analyses for all the comparisons with the Ctrl group, p's < 0.01) (Figs.5A-B). Overall, IP injections of E.coli or LPS induced higher cytokine levels in the circulation than those in IA challenged mice (for IL-1β and IL-6, p's < 0.01). In the EU groups, the induced IL-1β and IL-6 levels were significantly lower than the corresponding UP groups (p < 0.01) except for the IL-6 levels after IA LPS injection.
Figure 5.
Protein levels of IL-1β (A), IL-6 (B) and corticosterone (C) in the serum following peripheral immune challenges in the UP, EU and the Ctrl groups. Data are presented as means ± SEM. *Represents significant differences between the UP and EU groups (p < 0.05).
Fig.5C shows circulating corticosterone levels in the UP group were increased after all the inflammatory challenges compared with the Ctrl group (F(4, 13) = 3.14, p < 0.05). EU mice showed reduced increase of circulating corticosterone levels than those in the UP groups following IP E.coli or IP LPS injections (p's < 0.05) but not following IA E.coli or IA LPS injections (Fig.5C).
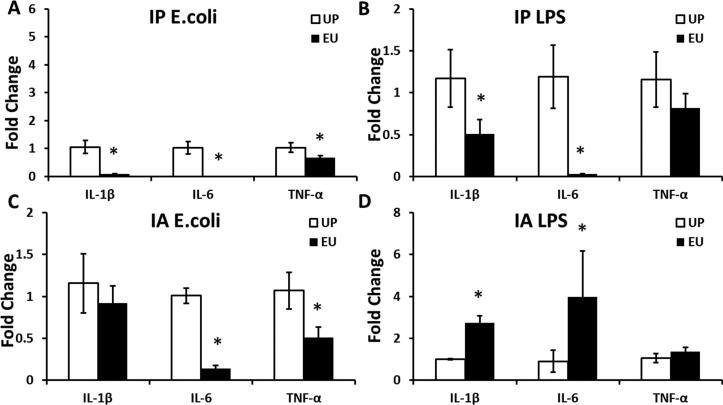
3.6. Euflammation dynamically regulated proinflammatory cytokine expression outside the Euflammation Induction Locus (EIL)
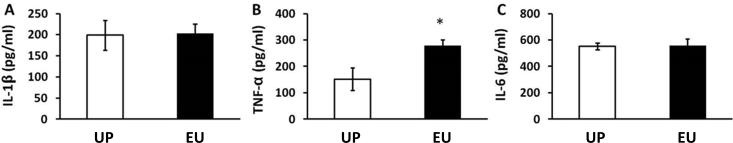
Our previous studies showed induction of euflammation by IP injection of E. coli dose not induce endotoxin tolerant cells in spleen and blood, but induces endotoxin tolerance in peritoneal macrophages (Tarr et al., 2014). We therefore surmised that cells outside of the topographic space of the peritoneum, including spleen, could exhibit different phenotypes from those inside the EIL (peritoneum). In the present study, spleen and airpouch (outside the EIL) cytokine responses to the different types of peripheral immune challenges were assessed (Figs.6&7). In spleen cells, IL-1β, IL-6, and TNF-α induced by IP E.coli were all reduced by EU (IL-1β and IL-6, p < 0.01; TNF-α, p < 0.05) (Fig.6A); IL-1β and IL-6, but not TNF-α induced by IP LPS were significantly reduced by EU (IL-1β, p < 0.05; IL-6, p < 0.01) (Fig.6B). After IA E.coli IL-6 and TNF-α, but not IL-1β, were significantly reduced by EU (IL-6, p < 0.01; TNF-α, p < 0.05) (Fig.6C). In contrast, IA LPS induced significantly higher splenic IL-1β and IL-6 levels in the EU group compared with the UP group. (IL-1β, p < 0.01; IL-6, p < 0.05) (Fig.6D). In addition, airpouch TNF-α, but not IL-1β and IL-6, was higher following the IA LPS injection (p < 0.05) (Fig.7).
Figure 6.
IL-1β, TNF-α, and IL-6 mRNA levels in the splenic cells after IP E.coli (A), and IP LPS (B), IA E.coli (C) and IA LPS (D) injections in the UP and EU groups. Data are presented as group means ± SEM. *Represents significant differences between the UP and EU groups (p < 0.05).
Figure 7.
Protein levels of IL-1β (A), TNF-α (B), and IL-6 (C) in the lavage fluid of the airpouch after IA LPS injections in the UP and EU groups. Data are presented as means ± SEM. *Represents significant differences between UP and EU groups (p < 0.05).
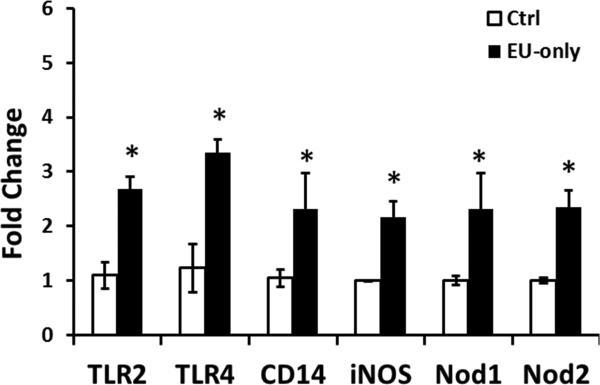
3.7. Euflammation promoted enhanced pathogen recognition outside the EIL
Previous reports indicated that immune cells outside the EIL became significantly more effective in bacterial clearance following euflammation induction (Tarr et al., 2014). To further explore the impact of euflammation on the regulation of innate immune cells outside the EIL, mRNA of pathogen recognition molecules in the spleen was examined. Results show that following euflammation induction, spleen cells expressed higher mRNA levels of TLR2, TLR4, CD14, iNOS, Nod1 and Nod2 than the Ctrl mice (TLR2, p < 0.01; others, p < 0.05) (Fig.8).
Figure 8.
Pathogen recognition receptors (TLR2, TLR4, CD14, Nod1, and Nod2) and iNOS mRNA levels in the spleen after the 3 day euflammation induction or PBS injections. *Represents significant differences between the Ctrl and the EU-only group (p < 0.05).
4. DISCUSSION
In the present study, we examined the protective effects of euflammation against neuroinflammation induced by injections of large doses of LPS or bacteria. Euflammation effectively reduced peripheral inflammation-induced brain and circulating cytokine expressions and microglial activation, attenuated immune-to-brain signaling including endothelial COX-2 and PVN neuronal c-fos induction. Corticosterone release, as an indicator of HPA axis activation, was also modulated by euflammation. In addition, euflammation upregulated pathogen recognition molecules outside the EIL, probably contributed to the enhanced local inflammatory responses in the EU mice. The modulatory effects of euflammation on the abovementioned neuroimmune events in all the peripheral and central compartments are summarized in the Table 2.
Table 2.
Summary of CNS and peripheral neuroimmune events examined in the current study following four different acute immune challenges.
| Relative to UP mice | IP E.coli-EU | IP LPS-EU | IA E.coli-EU | IA LPS-EU | |
|---|---|---|---|---|---|
| CNS | Microglial activation | ↓ | ↓ | ↓ | ↓ |
| COX-2 induction | ↓ | ↓ | ↓ | ↓ | |
| c-fos expression | ↓ | ↓ | ↓ | ↓ | |
| Cytokine mRNA expression | IL-1β↓ TNF-α↓ IL-6↓ |
IL-1β↓ (except in PFC) TNF-α↓ IL-6↓ |
IL-1β↓ (except in HC) TNF-α↓ IL-6↓ |
IL-1β (n.s.) TNF-α↓(in HY) IL-6↓ |
|
| Sickness level | ↓ | ↓ | ↓ | ↓ | |
| Periphery | Blood cytokine and corticosterone expression | IL-1β↓ IL-6↓ Corticosterone ↓ |
IL-1β↓ IL-6↓ Corticosterone↓ |
IL-1β↓ IL-6↓ Corticosterone (n.s.) |
IL-1β↓ IL-6 (n.s.) Corticosterone (n.s.) |
| Spleen cytokine mRNA expression | IL-1β↓ TNF-α↓ IL-6↓ |
IL-1β↓ TNF-α (n.s.) IL-6↓ |
IL-1β (n.s.) TNF-α ↓ IL-6↓ |
IL-1β ↑ TNF-α (n.s.) IL-6↑ |
|
| Air Pouch cytokine protein expression | N/A | N/A | N/A | IL-1β (n.s.) TNF-α↑ IL-6 (n.s.) |
|
All comparisons were relative to the UP mice. “↑” or “↓” indicated an increase/decrease in the expression levels, IHC labeled percentage areas, or sickness levels. n.s., no statistical difference. N/A, not applicable.
An important finding of the present study was that mRNAs of multiple pathogen recognition receptors in the spleen were increased following euflammation induction. This is consistent with our previous observation that increased expression of innate activation markers were detected outside the EIL in euflammatory mice (Tarr et al., 2014). Increased expression of pathogen recognition receptors in the host after an innate immune stimulation has been described in the literature as a part of innate immune training, which is known to confer stronger immune protection against a later re-infection (Netea et al., 2011). Because euflammation induced increased expression of multiple pattern recognition receptors (TLR2, TLR4, Nod1, Nod2 and CD14), which covers a broad spectrum of immune responses, it is possible that euflammation, induced in the present study by a single strain of bacteria, could potentially provide cross-protection against other infections.
It has been well accepted that peripheral inflammation causes a “mirror” inflammatory response in the brain, probably due to transportation of peripheral cytokines into the CNS and additional synthesis of cytokines within the CNS (Dantzer et al., 2008; Galic et al., 2012; Riazi et al., 2008). This concept, however, is not absolute because several reports showed that the brain cytokine can be induced by peripheral LPS without significant systemic cytokine expression under certain specific conditions (Chakravarty and Herkenham, 2005; Murray et al., 2011). Following the euflammation induction, all four immune challenges induced lower inflammatory cytokine expressions in the CNS (Fig.2), but in the spleen and in the airpouch, IA LPS challenge induced higher cytokine expression in the EU mice compared with UP mice (Fig.6&7). These results suggest that the brain cytokine induction could be attenuated without dampening the localized peripheral inflammation. Thus, CNS cytokine profiles do not always mirror peripheral cytokine profiles; they can also be modulated by the inflammatory responses in the recent past.
The reduction in the brain and circulating proinflammatory cytokines in the EU mice was not simply caused by endotoxin tolerance. In the literature, in vivo endotoxin tolerance is characterized by systemic downregulation of inflammatory cytokines and co-stimulatory molecules for antigen presentation (Biswas and Lopez-Collazo, 2009). In our previous study, we found myeloid cells inside the EIL exhibiting reduced MHCII, TLR4 and CD86 expression but increased phagocytic activities, indicating a classical endotoxin tolerance phenotype. The expressions of these molecules, however, were increased in the myeloid cells outside the EIL. Moreover, the circulating myeloid cells (outside the EIL) were found to produce higher pro- and anti-inflammatory cytokines after ex vivo LPS challenges, indicating myeloid cells outside the EIL exhibit reactive, rather than tolerant, phenotype (Tarr et al., 2014). In the current study, IA LPS induced higher cytokine expression in both spleen and airpouch in the EU mice than that in the naïve mice (Fig.6&7), again, confirming that immune cells outside the EIL in the EU mice exhibit the reactive phenotype. Interestingly, IA E.coli, another inflammatory challenge outside the EIL, did not induce heightened inflammatory cytokines in spleen cells. This apparent inconsistency may be explained by the fact that EU mice are known to eliminate IA administered E.coli orders more efficiently than naïve mice (Tarr et al., 2014), thereby significantly reduced the overall inflammatory load. Unexpectedly, IA LPS did not induce higher cytokine expressions in the circulation in the EU mice (Fig.5). Previous reports have shown that bacterial infections could elicit circulating molecules for the recognition and clearance of endotoxin, such as LPS binding protein and soluble CD14 (Bannerman et al., 2003; Bannerman et al., 2004). Therefore, LPS injected into the airpouch of EU mice might have been better cleared and/or contained, such that less LPS leaked outside the site of local inflammation to stimulate the expression of circulating cytokines. Collectively, while the detailed regulation of circulating cytokine expression by euflammation remains to be elucidated, the complexity of peripheral inflammatory profiles outside the EIL demonstrates euflammation does not simply produce a state of global endotoxin tolerance.
Our c-fos results showed that after peripheral inflammatory challenges, activities of PVN neurons were substantially reduced in the EU mice. PVN has been considered as a key brain region which mediates numerous neuroendocrine, behavioral, and physiological responses to peripheral immune challenges (Quan and Herkenham, 2002; Sternberg, 1998). Our finding, therefore, indicates that euflammation may prevent CNS activation by blocking immune-to-brain communication. One major outcome of peripheral inflammation-triggered CNS activation is the activation of HPA axis and the subsequent corticosterone release in the circulation. The observation that corticosterone levels were reduced in the EU mice after IP E.coli and IP LPS injections probably reflected a reduction of HPA axis activation following these two challenges by euflammation. Unexpectedly, euflammation had no impact on IA E.coli or IA LPS induced corticosterone levels. These results may be explained by the possibility that corticosterone release under these conditions are dissociated from PVN activation, similar to other reported cases of corticosterone induction by certain inflammations (Harbuz and Jessop, 1999).
Endothelial COX-2 induction and circulating inflammatory cytokines have been shown to be the key steps in blood brain barrier (BBB)-dependent humoral pathway of immune-to-brain signaling pathways (Quan et al., 2003). Our results show that the number of COX-2 labeled endothelial cells and circulating cytokines were reduced in the EU mice in response to peripheral inflammatory challenges. Therefore, euflammation mitigates humoral immune-to-brain signaling. It should be noted that in addition to humoral immune-to-brain signaling, neural pathways, such as somatosensory nerves and vagal sensory nerves, contribute significantly to local inflammation induced CNS activation (Belevych et al., 2010; Quan, 2014). In fact, CNS activation induced by IA immune challenges is known to be mediated, at least in part, by these neural pathways (Roth and De Souza, 2001). Indeed, the present results show IA LPS induced more significant microglial morphological changes than IP LPS (Fig.3A-D), although higher circulating cytokines were induced by IP LPS (Fig.5), indicating circulating cytokines are not the dominant factors in IA LPS-induced microglial activation. In addition, euflammation reduced PVN c-fos activation induced by IA E.coli and IA LPS, indicating that euflammation may also inhibit neural immune-to-brain signaling; the mechanism for this phenomenon, however, remains to be elucidated.
The current study examined the potential salubrious effects of euflammation on attenuating peripheral inflammation-induced neuroinflammation. Many pathological processes in the brain have been associated with excessive cytokine production in the CNS. These include traumatic brain injury (Kadhim et al., 2008), ischemia (Denes et al., 2010), epileptic seizures (Vezzani et al., 2011), and neurodegenerative diseases, such as Alzheimer's Disease (Eikelenboom et al., 2011). These conditions have also been attributed to the activation of microglia, a main source of brain cytokines (Imai and Kohsaka, 2002; Riazi et al., 2008). In the past, non-euflammatory peripheral immune challenges, which induce significant sickness behavior, were shown to exacerbate chronic neurodegenerative pathology and cognitive dysfunction by enhancing neuroinflammation (Cunningham et al., 2005). In the present study, we found euflammation ameliorated morphological alteration of microglial cells and reduced cytokine expressions induced by peripheral immune challenges. Therefore, our findings suggest that euflammation, in contrast to non-euflammatory inflammation, could inhibit rather than enhance neuroinflammation.
Highlights.
➢ This paper demonstrates signaling of peripheral inflammation to the CNS is modulated dynamically by peripheral inflammatory kinetics
➢ Euflammation can offer effective protection against both bacterial infection and endotoxin induced neuroinflammation
➢ Euflammation enhanced local inflammation outside euflammation induction locus by upregulating inflammation-associated genes and increasing local TNF-α production
ACKNOWLEDGEMENTS
This work was supported in full by the NIH grants: R21 MH099482 to NQ and College of Dentistry Intramural Seed Grant Program at the Ohio State University to AJT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
References
- Bannerman DD, Paape MJ, Hare WR, Sohn EJ. Increased levels of LPS-binding protein in bovine blood and milk following bacterial lipopolysaccharide challenge. Journal of dairy science. 2003;86:3128–3137. doi: 10.3168/jds.S0022-0302(03)73914-9. [DOI] [PubMed] [Google Scholar]
- Bannerman DD, Paape MJ, Lee JW, Zhao X, Hope JC, Rainard P. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clinical and diagnostic laboratory immunology. 2004;11:463–472. doi: 10.1128/CDLI.11.3.463-472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych N, Buchanan K, Chen Q, Bailey M, Quan N. Location-specific activation of the paraventricular nucleus of the hypothalamus by localized inflammation. Brain, behavior, and immunity. 2010;24:1137–1147. doi: 10.1016/j.bbi.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends in immunology. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Tarr AJ, Liu X, Wang Y, Reed NS, Demarsh CP, Sheridan JF, Quan N. Controlled progressive innate immune stimulation regimen prevents the induction of sickness behavior in the open field test. Journal of inflammation research. 2013;6:91–98. doi: 10.2147/JIR.S45111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain, behavior, and immunity. 2010;24:708–723. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Veerhuis R, van Exel E, Hoozemans JJ, Rozemuller AJ, van Gool WA. The early involvement of the innate immunity in the pathogenesis of late-onset Alzheimer's disease: neuropathological, epidemiological and genetic evidence. Current Alzheimer research. 2011;8:142–150. doi: 10.2174/156720511795256080. [DOI] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Frontiers in neuroendocrinology. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar EB, Sakai YI, Gaspari ED. A mouse air pouch model for evaluating the immune response to Taenia crassiceps infection. Experimental parasitology. 2014;137:66–73. doi: 10.1016/j.exppara.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Jessop DS. Dissociation between c-fos mRNA in the paraventricular nucleus and corticosterone secretion in rats with adjuvant-induced arthritis. The Journal of endocrinology. 1999;163:107–113. doi: 10.1677/joe.0.1630107. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40:164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- Kadhim HJ, Duchateau J, Sebire G. Cytokines and brain injury: invited review. Journal of intensive care medicine. 2008;23:236–249. doi: 10.1177/0885066608318458. [DOI] [PubMed] [Google Scholar]
- Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain research. Molecular brain research. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Yamashita T, Chen Q, Belevych N, McKim DB, Tarr AJ, Coppola V, Nath N, Nemeth DP, Syed ZW, Sheridan JF, Godbout JP, Zuo J, Quan N. Interleukin 1 type 1 receptor restore: a genetic mouse model for studying interleukin 1 receptor-mediated effects in specific cell types. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:2860–2870. doi: 10.1523/JNEUROSCI.3199-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. Journal of neuropathology and experimental neurology. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- Murray CL, Skelly DT, Cunningham C. Exacerbation of CNS inflammation and neurodegeneration by systemic LPS treatment is independent of circulating IL-1beta and IL-6. Journal of neuroinflammation. 2011;8:50. doi: 10.1186/1742-2094-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell host & microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Proescholdt MG, Chakravarty S, Foster JA, Foti SB, Briley EM, Herkenham M. Intracerebroventricular but not intravenous interleukin-1beta induces widespread vascular-mediated leukocyte infiltration and immune signal mRNA expression followed by brain-wide glial activation. Neuroscience. 2002;112:731–749. doi: 10.1016/s0306-4522(02)00048-9. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Pineros V, Galang JA, McClintock MK, Prendergast BJ. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9069–9074. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N. In-depth conversation: spectrum and kinetics of neuroimmune afferent pathways. Brain, behavior, and immunity. 2014;40:1–8. doi: 10.1016/j.bbi.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, He L, Lai W. Endothelial activation is an intermediate step for peripheral lipopolysaccharide induced activation of paraventricular nucleus. Brain research bulletin. 2003;59:447–452. doi: 10.1016/s0361-9230(02)00951-6. [DOI] [PubMed] [Google Scholar]
- Quan N, Herkenham M. Connecting cytokines and brain: a review of current issues. Histology and histopathology. 2002;17:273–288. doi: 10.14670/HH-17.273. [DOI] [PubMed] [Google Scholar]
- Quan N, Stern EL, Whiteside MB, Herkenham M. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. Journal of neuroimmunology. 1999;93:72–80. doi: 10.1016/s0165-5728(98)00193-3. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Fabry Z, Coe CL. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain research. 1999;851:215–220. doi: 10.1016/s0006-8993(99)02189-7. [DOI] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17151–17156. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J, De Souza GE. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica ... [et al.] 2001;34:301–314. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Overview of the conference and the field. Annals of the New York Academy of Sciences. 1998;840:1–8. doi: 10.1111/j.1749-6632.1998.tb09543.x. [DOI] [PubMed] [Google Scholar]
- Tarr AJ, Liu X, Reed NS, Quan N. Kinetic characteristics of euflammation: the induction of controlled inflammation without overt sickness behavior. Brain, behavior, and immunity. 2014;42:96–108. doi: 10.1016/j.bbi.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nature reviews. Neurology. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]